Abstract
Objective
To examine the association between previous cesarean delivery and subsequent placenta previa while distinguishing cesarean delivery prior to onset of labor from intrapartum cesarean delivery.
Study Design
Retrospective cohort study of electronic medical records from 20 Utah hospitals (2002–2010) with restriction to the first two singleton deliveries of women nulliparous at study entry (n=26,987). First pregnancy delivery mode was classified as 1) vaginal (reference); 2) cesarean delivery prior to labor onset (prelabor); or 3) cesarean delivery after labor onset (intrapartum). Risk of second delivery previa was estimated by prior delivery mode using logistic regression and adjusted for maternal age, insurance, smoking, co-morbidities, prior pregnancy loss, and history of previa.
Results
The majority of first deliveries were vaginal (82%, n=22,142), followed by intrapartum cesarean delivery (14.6%, n=3,931), or prelabor cesarean delivery (3.4%, n=914). Incidence of second delivery previa was 0.29% (n=78) and differed by prior delivery mode: vaginal, 0.24%; prelabor cesarean delivery, 0.98%; intrapartum cesarean delivery, 0.38% (P<0.001). Relative to vaginal delivery, prior prelabor cesarean delivery was associated with an increased risk of second delivery previa (adjusted odds ratio, 2.62 [95% confidence interval, 1.24–5.56]). There was no significant association between prior intrapartum cesarean delivery and previa [adjusted odds ratio, 1.22 (95% confidence interval, 0.68–2.19)].
Conclusion
Prior prelabor cesarean delivery was associated with a more than two-fold significantly increased risk of previa in the second delivery, while the approximately 20% increased risk of previa associated with prior intrapartum cesarean delivery was not significant. Although rare, the increased risk of placenta previa after prior prelabor cesarean delivery may be important when considering non-medically indicated prelabor cesarean delivery.
Keywords: Cesarean delivery, intrapartum, placenta previa, prelabor
INTRODUCTION
Placenta previa is observed in as many as 20% of transabdominal and 5% of transvaginal ultrasounds before 20 weeks gestational age,1,2 but the majority (approximately 90%) resolve by term.3 Approximately one in 200 pregnancies are complicated by persistent placenta previa at delivery, which is associated with medically indicated late-preterm and early term delivery, increased risk of maternal intrapartum and postpartum hemorrhage, need for blood transfusion, sepsis, and hysterectomy.3–7 Placenta previa is also associated with prematurity, low Apgar scores and fetal, and neonatal death.5,8,9
The exact etiology of placenta previa is unknown, but previous uterine surgery, including cesarean delivery (CD), is associated with an increased risk.10 Uterine scaring has been suggested to interfere with the process of natural growth of the placenta at more vascular sites and atrophy of the placental attachment site in the relatively less vascular lower uterus. Impaired migratory function has been postulated to result in decreased likelihood of resolution of placenta previa prior to delivery.3,8,10,11 Importantly, the incidence of placenta previa has been rising in parallel with the increasing rate of cesarean delivery in the U.S.12 Cesarean delivery suture type and closure method have been identified as modifiable characteristics of the surgery that may alter the risk of previa in subsequent pregnancies.13,14 Similarly, labor may be an additional risk factor that could modify previa risk associated with prior cesarean delivery since intrapartum factors may affect uterine repair after cesarean delivery.
The purpose of this study was to examine the association between the timing of prior cesarean delivery relative to labor onset (prelabor versus intrapartum) and the risk for placenta previa.
MATERIALS AND METHODS
The Consecutive Pregnancies Study was a retrospective cohort study conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health. Detailed information on 114,679 pregnancies from 51,086 women with at least two deliveries after 20 weeks of gestation at 20 Utah hospitals from 2002–2010 were extracted from the maternal and infant electronic medical records and supplemented with International Classification of Diseases, ninth revision (ICD-9) discharge codes. Pregnancies were linked across an individual woman using a unique maternal ID. Institutional review board approval was obtained for all participating institutions and granted an exemption by the Office of Human Subjects Research (OHSR) at the National Institute of Health as all of the data transferred were de-identified.
Analyses were restricted to women who were nulliparous at study entry (n=27,741) due to the established relationship between increasing parity and placenta previa risk.8 The cohort was further limited to women with consecutive singleton pregnancies, as multiple gestation is also an established risk factor for placenta previa (n=27,062).3 We performed a complete-case analysis and excluded a total of 75 women with missing data on one of the following: ethnicity (n=56, 0.21%), marital status (n=2, 0.01%), or smoking status (n=18, 0.07%). The final sample consisted of the 26,987 women with consecutive, singleton pregnancies.
First pregnancy delivery mode was categorized as vaginal or cesarean delivery. We further categorized cesarean delivery into either 1) cesarean delivery performed prior to labor onset (prelabor cesarean delivery) or 2) cesarean delivery performed after labor onset (intrapartum cesarean delivery). Prelabor cesarean delivery was designated if a trial of labor was absent and none of the following were documented: induction of labor, augmentation of labor, episiotomy, intrapartum tocolytics, shoulder dystocia, vaginal lacerations, a cesarean indication noting failure to progress or failed induction, or a date/time of full cervical dilation or onset of spontaneous labor. All other cesarean deliveries not meeting the above conditions were classified as intrapartum. There were 102 women with cervix dilated six cm or greater at the time of the first examination following admission to labor and delivery and we considered them to have presented in active labor, so they were classified as intrapartum cesarean.
Cases of placenta previa in the second pregnancy were identified through the diagnosis documented in the prenatal and labor and delivery medical records. No additional cases were detected by ICD-9 codes (641.00–641.03; 641.10–641.13). Women with a diagnosis of placenta previa that delivered vaginally (n=26) were not considered as cases for the purposes of this study as partial and complete placenta previa require cesarean delivery.3,6
Maternal race/ethnicity and second pregnancy age at delivery, insurance type, marital status, smoking during pregnancy, and gravidity were obtained from the medical record. Due to the homogenous nature of this cohort (>87% white), race/ethnicity was classified as white vs. non-white. Maternal medical history of asthma, anemia, pregestational diabetes, chronic hypertension, kidney disease, and thyroid disease, as well as chorioamnionitis in the second pregnancy were obtained from the medical record and supplemented with ICD-9 codes. Once classified with a chronic condition women were considered to have the condition at all subsequent pregnancies. Given the reported association between a history of placenta previa and risk of subsequent placenta previa,6 we also included diagnosis of placenta previa occurring in the first pregnancy using the case definition described above. Lastly, gestational age was determined as recorded in the medical record according to the best obstetrical estimate. Consistency checks were performed using repeated pregnancy data on all relevant covariates and conditions.
Differences in participant characteristics at the second pregnancy according to prior delivery mode were determined using Chi-square, Fisher’s exact or Student’s t-test. The risk of placenta previa was estimated according to prior delivery mode using multivariable logistic regression with likelihood ratio tests. Serial models were constructed to explore the potential different confounders. Potential covariates for the adjusted models were explored based on prior literature, use of a directed acyclic graph and evaluation of individual regression models with a p value <0.10 considered significant.15,16
The first model (Model A) was a multivariable logistic regression model with first pregnancy delivery mode as the independent variable and second pregnancy previa as the dependent variable adjusted for demographic and known baseline risk factors including maternal age, insurance status, smoking status, history of pregnancy loss, and history of placenta previa. The second logistic regression model (Model B) included all of the covariates in Model A and further adjusted for maternal co-morbidities (anemia, pregestational diabetes, and thyroid disease) selected according to the above criteria. For the model interpretation, interest is focused on assessing the risk of previa and not on the risk of known confounding factors.
In order to examine the potential impact of cesarean incision type at first pregnancy on second pregnancy placenta previa occurrence, sensitivity analyses were performed by removing all first pregnancies having a transverse fetal position delivered via cesarean (n=80, 0.3%) or cesarean prior to 36 weeks gestation (n=369, 1.4%), both conditions which are indicative of a higher probability of classical, instead of a low transverse, uterine incision.17 Furthermore, it was possible that cases of placenta previa at the second pregnancy delivered preterm may have spontaneously resolved had the pregnancy been allowed to progress. To address this potential competing risk, another set of sensitivity analyses were performed excluding 1,897 (7.0%) pregnancies delivered prior to 37 weeks. All analyses were conducted using SAS 9.3 software (SAS Institute Inc., Cary, North Carolina, US) and two-tailed P-values <0.05 were considered statistically significant.
RESULTS
Women were mostly white (87%), married (89%) and had private insurance (73%). The average maternal age at the second pregnancy was 26.2 years (range 14 to 46 years). The majority of deliveries at the first pregnancy were vaginal (82%, n=22,142), followed by intrapartum CD (14.6%, n=3,931), or prelabor CD (3.4%, n=914). Distributions of race/ethnicity, maternal age, history of prior pregnancy loss, and occurrence of multiple maternal co-morbidities were significantly different between the prior delivery types (Table 1). Compared to women who previously had a vaginal delivery or prelabor cesarean delivery, women who previously underwent an intrapartum cesarean delivery were significantly more likely to be non-white. Women who previously had any type of cesarean delivery were significantly older and more likely to have chronic hypertension compared to women who previously delivered vaginally. Compared to women who previously had a vaginal delivery or an intrapartum cesarean delivery, women who previously underwent a prelabor cesarean delivery had significantly higher prevalence of pregestational diabetes, kidney disease, and thyroid disease and were more likely to have a history of pregnancy loss.
Table 1.
Second Pregnancy Characteristics According to First Pregnancy Delivery Mode
| Second Pregnancy | First Pregnancy Delivery Mode | |||
|---|---|---|---|---|
| Sample Characteristics | Vaginal | Prelabor CD | Intrapartum CD | |
| (n=22,142) | (n=914) | (n=3,931) | ||
| n (%) | n (%) | n (%) | p valuea | |
| Race/Ethnicity | ||||
| White | 19,327 (87.3) | 813 (88.9) | 3,368 (85.7) | 0.005 |
| Non-White | 2,815 (12.7) | 101 (11.1) | 563 (14.3) | |
| Insurance Type | ||||
| Private | 16,058 (72.5) | 682 (74.6) | 2,892 (73.6) | 0.17 |
| Public | 6,084 (27.5) | 232 (25.4) | 1,039 (26.4) | |
| Marital Status | ||||
| Married | 19,741 (89.2) | 820 (89.7) | 3,500 (89.0) | 0.84 |
| Not Married | 2,401 (10.8) | 94 (10.3) | 431 (11.0) | |
| Maternal Age, mean (SD) | 25.9 (4.0) | 27.4 (4.8) | 27.3 (4.5) | <0.001 |
| Smoked during pregnancy | 632 (2.9) | 35 (3.8) | 118 (3.0) | 0.21 |
| Maternal Co-Morbidities | ||||
| Asthma | 1,803 (8.1) | 81 (8.9) | 385 (9.8) | 0.002 |
| Anemia | 1,230 (5.6) | 77 (8.4) | 331 (8.4) | <0.001 |
| Pregestational Diabetes | 289 (1.3) | 44 (4.8) | 118 (3.0) | <0.001 |
| Chronic Hypertension | 226 (1.0) | 19 (2.1) | 103 (2.6) | <0.001 |
| Kidney Disease | 203 (0.9) | 19 (2.1) | 40 (1.0) | 0.002 |
| Thyroid Disease | 1,060 (4.8) | 79 (8.6) | 242 (6.2) | <0.001 |
| Chorioamnionitis | 81 (0.4) | 4 (0.4) | 29 (0.7) | 0.004 |
| Prior Pregnancy Loss | ||||
| 0 | 16,995 (76.8) | 672 (73.5) | 2,924 (74.4) | <0.001 |
| 1 | 3,949 (17.8) | 166 (18.2) | 740 (18.8) | |
| 2 | 848 (3.8) | 46 (5.0) | 196 (5.0) | |
| ≥3 | 350 (1.6) | 30 (3.3) | 71 (1.8) | |
| Prior Placenta Previa | 0 (0%) | 16 (1.8) | 7 (0.2) | <0.001 |
Abbreviations: CD, cesarean delivery; SD, standard deviation
First pregnancy delivery mode was classified as vaginal (reference), cesarean delivery performed prior to labor onset (prelabor CD), or cesarean delivery performed after onset of labor (intrapartum CD).
Significance values for the three group comparison
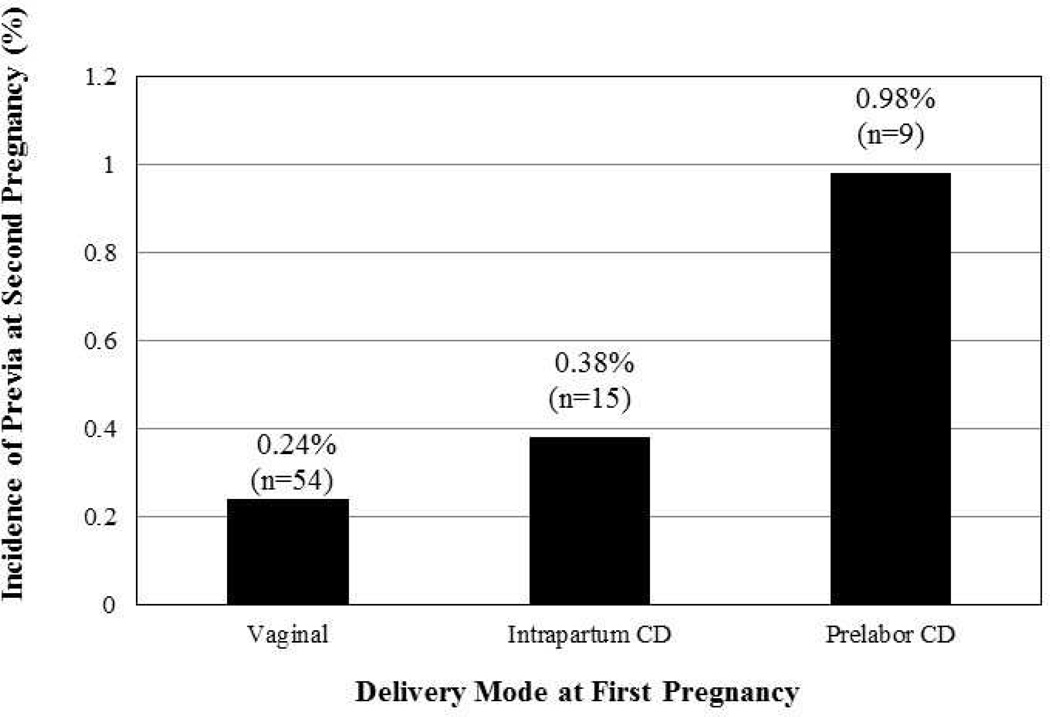
The overall incidence of second pregnancy placenta previa was 0.29% and varied significantly by prior delivery mode (Figure 1). The greatest risk of placenta previa was observed among women with a first pregnancy prelabor cesarean delivery both before adjustment [odds ratio (OR), 4.07 (95% confidence interval (CI) 2.00–8.26)] and after adjustment for maternal age, insurance status, smoking, prior pregnancy loss, and history of placenta previa (Table 2). This association remained statistically significant after further adjustment for maternal co-morbidities including anemia, pregestational diabetes, and thyroid disease [adjusted odds ratio (aOR), 2.62 (95% CI 1.24–5.56)]. Intrapartum cesarean delivery was associated with a lower, non-significant increase in risk in all models.
Figure 1.
Percentage and frequency of placenta previa in second pregnancy by first pregnancy delivery mode (Vaginal, Intrapartum Cesarean Delivery (CD), and Prelabor Cesarean Delivery (CD); p<0.001).
Table 2.
Risk of Placenta Previa in Second Pregnancy According to Prior Delivery Mode
| First Pregnancy Delivery Mode | |||
|---|---|---|---|
| Vaginal | Prelabor CD | Intrapartum CD | |
| (n=22,142) | (n=914) | (n=3,931) | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Unadjusted | 1.00 (Reference) | 4.07 (2.00–8.26) | 1.57 (0.88–2.78) |
| Model Aa | 1.00 (Reference) | 3.13 (1.49–6.56) | 1.37 (0.77–2.45) |
| Model Bb | 1.00 (Reference) | 2.62 (1.24–5.56) | 1.22 (0.68–2.19) |
Abbreviations: CD, cesarean delivery; CI, confidence interval; OR, odds ratio.
Model A adjusted for maternal age, pregnancy loss, history of previa, insurance, and smoking
Model B adjusted for all variables from model A and anemia, pregestational diabetes, and thyroid disease.
To address the impact of first pregnancy classical uterine incision cesarean delivery and second pregnancy gestational age at the time of delivery on the incidence of second pregnancy placenta previa, we conducted two sensitivity analyses with model B adjustments. Excluding women with a higher probability of having a classical uterine incision cesarean delivery did not have a meaningful impact on the adjusted (model B) association between delivery mode and risk of placenta previa [prelabor cesarean delivery aOR, 2.82 (95% CI, 1.27–6.27); Intrapartum cesarean delivery aOR, 1.29 (95% CI, 0.72–2.32)] indicating that our results were not likely to be biased by inclusion of cesarean deliveries with possible classical incisions. Excluding deliveries prior to 37 weeks at the second pregnancy strengthened the adjusted (model B) association between delivery mode and placenta previa [prelabor cesarean delivery aOR, 3.50 (95% CI, 1.29–9.48); intrapartum cesarean delivery aOR, 1.28 (95% CI, 0.55–3.00)], suggesting that the relationship between prior delivery mode and risk of subsequent placenta previa was not the result of including preterm cases that may have spontaneously resolved.
COMMENT
In this large, longitudinal cohort of first and second consecutive pregnancies, we found that first pregnancy prelabor cesarean delivery was associated with more than a two-fold increased risk of placenta previa in the second pregnancy. In contrast, prior intrapartum cesarean delivery was associated with a non-significant 20% increased risk of placenta previa. Timing of cesarean delivery relative to labor onset is an important distinction, especially given the high percentages of primary and repeat cesarean delivery that occur in the U.S.18,19 These findings highlight the importance of labor in relation to cesarean delivery for the development of future placenta previa and, therefore, may inform clinical decision-making related to primary cesarean delivery especially in cases of maternal request or non-medically indicated cesarean delivery.20,21
The association between cesarean delivery and risk of placenta previa is well-documented, but has remained poorly understood.10 One recent case-control study explored placenta previa risk and timing of prior cesarean delivery relative to labor stage and, similar to our study, found significantly increased risk of placenta previa among women who previously had a prelabor cesarean delivery compared to women with a previous cesarean delivery in the first stage of labor.13 However, that study included multiparous women and those with multiple prior cesarean deliveries and did not include a vaginal delivery reference group. It is plausible that the mechanisms underlying the association between primary prelabor cesarean delivery and increased risk of placenta previa in a second pregnancy may be due to changes in uterine structure and immune function that occur with onset of labor. It is known that the uterus expands throughout pregnancy to accommodate the growing fetus and that there are changes in the amount and organization of collagen fibers in the uterus in preparation for and with onset of labor.22–24 It is possible that cesarean delivery performed after the onset of labor, when the uterine tissue is at its thinnest, minimizes the amount of tissue damaged by the incision or that these structural changes associated with labor render the tissue more resilient to damage. A second hypothesis is that the immune processes which occur with the onset of labor, supported by an increase in macrophages, t-lymphocytes, neutrophils and leukocytes, stimulate uterine restructuring and healing after cesarean incision.23,25,26 Thus, interference with this healing process by cesarean incision prior to labor may increase the risk of subsequent placenta previa. Alternatively, it has been suggested that cesarean incisions made after labor onset are more likely to occur in the upper portion of the cervix rather than the lower uterine segment, thereby decreasing the likelihood of future placenta previa.27 It is also not clear whether these results would also translate to women considering a trial of labor after cesarean (TOLAC) and this should be evaluated in future studies.
A challenge in evaluating the association between timing of primary cesarean delivery and risk of second pregnancy placenta previa is that prevalence of placenta previa varies by gestational age. Thus it is possible that some cases among women who delivered preterm would have resolved had the pregnancy progressed. Furthermore, as the lower uterine segment begins to develop placenta previa can result in bleeding early in pregnancy, leading to preterm labor and delivery.9 In a sensitivity analysis limited to second pregnancies delivered after 37 completed weeks, we found that the association between prelabor cesarean delivery and placenta previa appeared to increase rather than decrease, suggesting that our findings were not due to inclusion of preterm cases that may have spontaneously resolved.
Although a rare condition, given its association with serious maternal and neonatal morbidities,5,9 we cannot underscore the clinical importance of better understanding the etiology of previa. While the small number of previa cases may be considered a study limitation, this is a consequence of the rarity of the disease and not the size of the cohort. The NICHD Consecutive Pregnancies Study is unique to have such large numbers with detailed clinical information on repeat pregnancies. Also we detected a significant association between prelabor cesarean delivery and placenta previa, suggesting that there was adequate statistical power for the main analysis despite the relatively small number of cases. Ideally, future prospective studies collecting detailed information on consecutive pregnancies should be conducted to confirm the reported risk estimates. One limitation of this study is that misclassification of exposure (prelabor vs. intrapartum cesarean) is possible, but this is unlikely given the detailed labor information recorded in the medical record. Also, the population captured in this study was predominantly white. It is not clear whether the association between cesarean delivery and subsequent previa varies by race. We limited this analysis to women’s first and second pregnancies so that we could certain about the type of prior delivery method. While our data are not necessarily generalizable to deliveries beyond parity 1, it is plausible that risk of previa may worsen with additional cesarean deliveries prior to the onset of labor.
In conclusion, the findings of this study add to the evidence that there are aspects of cesarean delivery that could be altered to potentially reduce the frequency of subsequent cases of placenta previa, particularly when considering cesarean delivery on maternal request and non-medically indicated cesarean delivery. Much recent focus has been on preventing primary cesarean delivery due to its association with maternal and neonatal morbidities.19 Our study highlights additional information regarding cesarean delivery and its timing in relation to labor onset that could be used for clinical decision making by patients and their physicians regarding delivery planning.
Acknowledgments
Financial Support: This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Contract numbers: HHSN275200800002I, HHSN27500004).
Role of the Funding Source: Upon approval of the study protocol, the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development as a funding source did not influence the design and conduct of the current study nor the collection, management, analysis, and interpretation of the data nor the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors report no conflict of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Disclaimer: P.S. Albert, L.A. Sjaarda and K.L. Grantz are employees of the federal government; please see accompanying cover sheet.
This research was presented at the 34th Annual Meeting of the Society for Maternal-Fetal Medicine in New Orleans, LA, February 3–8th, 2014.
REFERENCES
- 1.Taipale P, Hiilesmaa V, Ylostalo P. Diagnosis of placenta previa by transvaginal sonographic screening at 12–16 weeks in a nonselected population. Obstet Gynecol. 1997;89:364–367. doi: 10.1016/S0029-7844(96)00503-0. [DOI] [PubMed] [Google Scholar]
- 2.Oyelese Y. Placenta previa: The evolving role of ultrasound. Ultrasound Obstet Gynecol. 2009;34:123–126. doi: 10.1002/uog.7312. [DOI] [PubMed] [Google Scholar]
- 3.Oyelese Y, Smulian JC. Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol. 2006;107:927–941. doi: 10.1097/01.AOG.0000207559.15715.98. [DOI] [PubMed] [Google Scholar]
- 4.Crane JM, Van den Hof MC, Dodds L, Armson BA, Liston R. Maternal complications with placenta previa. Am J Perinatol. 2000;17:101–105. doi: 10.1055/s-2000-9269. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg T, Pariente G, Sergienko R, Wiznitzer A, Sheiner E. Critical analysis of risk factors and outcome of placenta previa. Arch Gynecol Obstet. 2011;284:47–51. doi: 10.1007/s00404-010-1598-7. [DOI] [PubMed] [Google Scholar]
- 6.Rao KP, Belogolovkin V, Yankowitz J, Spinnato JA. Abnormal placentation: Evidence-based diagnosis and management of placenta previa, placenta accreta and vasa previa. Obstet Gynecol Surv. 2012;67:503–519. doi: 10.1097/OGX.0b013e3182685870. [DOI] [PubMed] [Google Scholar]
- 7.Spong CY, Mercer BM, D'Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118:323–333. doi: 10.1097/AOG.0b013e3182255999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faiz AS, Ananth CV. Etiology and risk factors for placenta previa: An overview and meta-analysis of observational studies. J Matern Fetal Neonatal Med. 2003;13:175–190. doi: 10.1080/jmf.13.3.175.190. [DOI] [PubMed] [Google Scholar]
- 9.Norgaard LN, Pinborg A, Lidegaard O, Bergholt T. A Danish national cohort study on neonatal outcome in singleton pregnancies with placenta previa. Acta Obstet Gynecol Scand. 2012;91:546–551. doi: 10.1111/j.1600-0412.2012.01375.x. [DOI] [PubMed] [Google Scholar]
- 10.Gurol-Urganci I, Cromwell DA, Edozien LC, et al. Risk of placenta previa in second birth after first birth cesarean section: A population-based study and meta-analysis. BMC Pregnancy Childbirth. 2011;11:95. doi: 10.1186/1471-2393-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall NE, Fu R, Guise JM. Impact of multiple cesarean deliveries on maternal morbidity: A systematic review. Am J Obstet Gynecol. 2011;205:262, e261–e268. doi: 10.1016/j.ajog.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 12.Osterman MJK, Martin JA. Changes in Cesarean Delivery Rates by Gestational Age: United States, 1996–2011. NCHS data brief. 2013;124:1–8. (June 2013) [PubMed] [Google Scholar]
- 13.Chiu TL, Sadler L, Wise MR. Placenta praevia after prior caesarean section: An exploratory case-control study. Aust N Z J Obstet Gynaecol. 2013;53:455–458. doi: 10.1111/ajo.12098. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa H, Itakura A, Mitsui T, et al. Methods for myometrium closure and other factors impacting effects on cesarean section scars of the uterine segment detected by the ultrasonography. Acta Obstet Gynecol Scand. 2006;85:429–434. doi: 10.1080/00016340500430436. [DOI] [PubMed] [Google Scholar]
- 15.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 16.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22:745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 17.Bethune M, Permezel M. The Relationship Between Gestational Age and the Incidence of Classical Caesarean Section. Aust N Z J Obstet Gynaecol. 1997;37:153–155. doi: 10.1111/j.1479-828x.1997.tb02243.x. [DOI] [PubMed] [Google Scholar]
- 18.Solheim KN, Esakoff TF, Little SE, Cheng YW, Sparks TN, Caughey AB. The effect of cesarean delivery rates on the future incidence of placenta previa, placenta accreta, and maternal mortality. J Matern Fetal Neonatal Med. 2011;24:1341–1346. doi: 10.3109/14767058.2011.553695. [DOI] [PubMed] [Google Scholar]
- 19.Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the First Cesarean. Obstet Gynecol. 2012;120:1181–1193. doi: 10.1097/aog.0b013e3182704880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuglenes D, Aas E, Botten G, Oian P, Kristiansen IS. Maternal preference for cesarean delivery: Do women get what they want? Obstet Gynecol. 2012;120:252–260. doi: 10.1097/AOG.0b013e3182605b1a. [DOI] [PubMed] [Google Scholar]
- 21.Ecker J. Elective cesarean delivery on maternal request. JAMA. 2013;309:1930–1936. doi: 10.1001/jama.2013.3982. [DOI] [PubMed] [Google Scholar]
- 22.Granstrom L, Ekman G, Ulmsten U, Malmstrom A. Changes in the connective tissue of corpus and cervix uteri during ripening and labour in term pregnancy. Br J Obstet Gynaecol. 1989;96:1198–1202. doi: 10.1111/j.1471-0528.1989.tb03196.x. [DOI] [PubMed] [Google Scholar]
- 23.Winkler M, Oberpichler A, Tschesche H, Ruck P, Fischer DC, Rath W. Collagenolysis in the lower uterine segment during parturition at term: Correlations with stage of cervical dilatation and duration of labor. Am J Obstet Gynecol. 1999;181:153–158. doi: 10.1016/s0002-9378(99)70452-7. [DOI] [PubMed] [Google Scholar]
- 24.Ginsberg Y, Goldstein I, Lowenstein L, Weiner Z. Measurements of the lower uterine segment during gestation. J Clin Ultrasound. 2013;41:214–217. doi: 10.1002/jcu.22023. [DOI] [PubMed] [Google Scholar]
- 25.Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20:154–167. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 26.Winkler M, Fischer DC, Hlubek M, van de Leur E, Haubeck HD, Rath W. Interleukin-1beta and interleukin-8 concentrations in the lower uterine segment during parturition at term. Obstet Gynecol. 1998;91:945–949. doi: 10.1016/s0029-7844(98)00098-2. [DOI] [PubMed] [Google Scholar]
- 27.Berube L, Arial M, Gagnon G, Brassard N, Boutin A, Bujold E. Factors associated with lower uterine segment thickness near term in women with previous caesarean section. J Obstet Gynaecol Can. 2011;33:581–587. doi: 10.1016/s1701-2163(16)34906-4. [DOI] [PubMed] [Google Scholar]