Abstract
The telencephalic allocortex develops protein inclusions before the neocortex in many age-related proteinopathies. One major defense mechanism against proteinopathic stress is the heat shock protein (Hsp) network. We therefore contrasted Hsp defenses in stressed primary neo- and allocortical cells. Neocortical neurons were more resistant to the proteasome inhibitor MG132 than neurons from three allocortical subregions: entorhinal cortex, piriform cortex, and hippocampus. However, allocortical neurons exhibited higher MG132-induced increases in Hsp70 and Hsc70. MG132-treated allocortical neurons also exhibited greater levels of protein ubiquitination. Inhibition of Hsp70/Hsc70 activity synergistically exacerbated MG132 toxicity in allocortical neurons more than neocortical neurons, suggesting that the allocortex is more reliant on these Hsp defenses. In contrast, astrocytes harvested from neo- or allocortex did not differ in their response to Hsp70/Hsc70 inhibition. Consistent with the idea that chaperones are maximally engaged in allocortical neurons, an increase in Hsp70/Hsc70 activity was protective only in neocortical neurons. Finally, the levels of select Hsps were altered in neocortex and allocortex in vivo with aging.
Keywords: Alzheimer’s disease, Parkinson’s disease, archicortex, paleocortex, heme oxygenase 1, Hsp70, Braak, entorhinal cortex, piriform cortex, hippocampus
1. Introduction
The modern neocortex or isocortex of the telencephalon can readily be distinguished at the structural and functional levels from the phylogenetically primitive allocortex (comprised of paleocortex and archicortex). In Parkinson’s disease (PD) and Alzheimer’s disease (AD), the neocortex is affected by α-synuclein and tau inclusions after the allocortex (Braak et al., 1993; Braak et al., 2003a; Braak et al., 2003b; Braak et al., 2006; Duyckaerts et al., 2009). The reason underlying this striking difference is still unknown. One explanation may be that protein-misfolding stress (proteotoxicity) travels through circuits in a staggered fashion, so that regions are sequentially collared into bearing inclusions depending upon their individual projection patterns. A second possibility is that brain regions exhibit differences in their vulnerability to proteotoxicity and can thus keep inclusions at bay for varying periods of time. We propose that these two scenarios occur simultaneously and have examined the second possibility here.
The concept that pathology is transmitted from cell to cell has gained considerable traction in recent years (Desplats et al., 2009; Hansen et al., 2011; Luk et al., 2012; Volpicelli-Daley et al., 2011). However, transmissibility cannot fully explain the topography of inclusions in PD and AD, because not all the areas connected to an affected region develop protein inclusions and die. For this reason, it has been hypothesized that inherent differences in vulnerability also help determine cell fate (Braak et al., 2006; Dickson, 2007). It is important to identify the natural defenses against proteotoxic stress in resilient brain regions because they may explain why neurodegenerative diseases usually do not appear until old age and are slow to progress.
The major form of stress in neurodegenerative disorders is thought to be proteotoxic stress and one of the main lines of defense against these conditions is the heat shock protein (Hsp) family of chaperones, part of the essential vitagene network (Cornelius et al., 2013). For example, Hsp70 refolds denatured proteins and guides irreparably damaged proteins to the proteasome or lysosome for degradation (Aridon et al., 2011; Kalia et al., 2010; Lanneau et al., 2010). Hsp70 also offsets apoptosis. Hsp70 ameliorates MPTP, α-synuclein, and β-amyloid toxicity (Dong et al., 2005; Klucken et al., 2004; Magrane et al., 2004; Moloney et al., 2014; Nagel et al., 2008). However, the impact of Hsp70 on regionally selective vulnerability to proteotoxicity is poorly understood.
We recently gathered evidence that the neurons of the neo- and allocortex have different intrinsic vulnerabilities to oxidative and proteotoxic stress (Posimo et al., 2013). Primary neocortical cultures were more resistant to proteasome inhibitors such as MG132 but more vulnerable to hydrogen peroxide. It is possible that the increased susceptibility of allocortex to proteotoxicity may partly underlie the spatiotemporal pattern of cortical inclusions in PD and AD. Relative to allocortex, neocortex cultures also exhibited lower levels of ubiquitinated proteins and less loss of proteasome activity in response to MG132. However, allocortex cultures exhibited greater increases in Hsp70 upon MG132 treatment. To determine the functional consequences of these regional differences in Hsps, we first reestablished the neo/allocortical culture model with higher neuronal purity, improved basal survival, and more robust regional differences in MG132 vulnerability than previously reported (Posimo et al., 2013). Second, we compared the vulnerability of neocortex to that of three allocortical subregions: entorhinal cortex, piriform cortex, and hippocampus. Piriform cortex is comprised of three layers, meeting the classic definition of trilaminar allocortex. Entorhinal cortex is a transitional zone and composed of more than three layers, but it is closely associated with the allocortex and classified as peri-archicortex (Braak, 1980; Creutzfeldt, 1995; Filimonoff, 1947; Peters and Jones, 1984). Hippocampus is also considered part of archicortex. All three regions are more susceptible to tau inclusions than neocortex (Braak et al., 1993; Braak and Braak, 1995; Braak et al., 2006; Duyckaerts et al., 2009). Third, we examined the effects of MG132 and the oxidative toxin paraquat on Hsps in primary cortical cultures and the impact of Hsp70/Hsc70 inhibition and activation on neo/allocortical vulnerability to these compounds. Finally, we measured the impact of aging on Hsp expression in primary sensorimotor neocortex and entorhinal allocortex in vivo, as aging is a major risk factor for neurodegeneration and can be leveraged as a natural model of proteotoxic and oxidative stress (Keller et al., 2000b).
2. Methods
2.1 Animals
Animal use was approved by the Duquesne University Institutional Animal Care and Use Committee and carried out in accordance with the principles outlined in the NIH Guide. Female Sprague Dawley rats were fed ad libitum and singly housed in a room maintained at a constant temperature with a 12 hr light/dark cycle. Rats were sacrificed at 2–3.9 (n=6), 4–6 (n=6), 8–9 (n=6), 16–18.9 (n=5), and 19–22 (n=6) months of age for the aging study. These rats formed part of an in-house breeding colony designed to generate rat pup tissue for postnatal cultures (see Section 2.3). Tissue was dissected according to the definitions in the Paxinos rat atlas (Paxinos and Watson, 1998).
2.2 Antibodies and Chemicals
Primary and secondary antibodies are listed in Supplementary Table 1 and Table 2. The proteasome inhibitor MG132 was purchased from EMD Millipore (Billerica MA, Cat. no. 474790). Hsp70/Hsc70 activity was inhibited with the previously characterized compounds VER155008 (R&D Systems, Minneapolis, MN; Chatterjee et al., 2013; Massey et al., 2010; Saykally et al., 2012; Schlecht et al., 2013) and MAL3-101 (Adam et al., 2014; Braunstein et al., 2011; Hatic et al., 2012; Huryn et al., 2011; Kilpatrick et al., 2013). Hsp70 activity was enhanced with 115-7c (MAL1-271) (Kilpatrick et al., 2013; Wisen et al., 2010). Heme oxygenase 1 was inhibited with tin protoporphyrin (SnPP) (Drummond and Kappas, 1981). All toxins and inhibitors were stored at −80 °C as a 10 mM stock solution in dimethyl sulfoxide (DMSO), except for the oxidative toxin paraquat (Sigma-Aldrich, St. Louis, MO), which was dissolved in PBS (100 mM).
2.3 Primary Cultures
Neocortical tissue and tissue from entorhinal allocortex, piriform allocortex, and hippocampal allocortex was dissected from the brains of postnatal day 1 or 2 Sprague Dawley rats (Charles River, Wilmington, MA). Cells were dissociated and plated as previously described (Posimo et al., 2013), with the exception that cultures were incubated in Neurobasal-A media (Gibco, Life Technologies) supplemented with 2% v/v serum-free B27 (Gibco, Life Technologies) and 2 mM L-glutamine. Cultures were treated with an equal v/v of vehicle or toxins on day in vitro 2 (DIV2). On DIV3, a full media exchange was performed. On DIV4, cell viability assays were performed as described below. In our experience, cells treated with proteasome inhibitors do not respond with much cell loss until 2d after treatment. Thus, a 48h time point was used for the viability assays.
Primary astrocytes were also harvested from neocortex and allocortex. For the astrocyte cultures, entorhinal cortex was combined with piriform cortex to generate sufficient astrocytic material. Briefly, tissue was dissociated in Dulbecco’s modified eagle medium (Gibco, Life Technologies) supplemented with 10% fetal clone III (Hyclone, Thermo Scientific) and 1% penicillin/streptomycin (Gibco, Life Technologies) after incubation with 0.25% trypsin with EDTA (Invitrogen, Life Technologies). After 7–9 days, cultures were placed overnight on an orbital shaker at 260 rpm. Two to three days later, astrocytes were passaged and seeded onto plates. Astrocyte cultures were treated with toxins on DIV5 and assayed on DIV7.
2.4 Viability Assays
Cell viability in neuronal cultures was assessed by quantifying ATP levels using the Cell Titer Glo assay (Promega, Madison, WI) and by quantifying levels of the neuronal marker microtubule associated protein 2 (MAP2) using an In-Cell Western assay, as described (Posimo et al., 2013; Posimo et al., 2014). The infrared In-Cell Western assay for MAP2 was imaged on an Odyssey Imager and analyzed with Odyssey software (Version 3.0, Li-COR). Astrocyte cultures were stained with Hoechst (10 μg/ml Hoechst 33258, bisBenzimide) in phosphate-buffered saline with 0.3% Triton-X for 15 min for blinded cell counts. Astrocytes were also stained with the infrared nuclear stain DRAQ5 (1:10,000, 700 nm; Biostatus, Shepshed, Leicestershire, UK) and assayed on the Odyssey Imager to validate the Hoechst cell count data. In addition, cultures were immunocytochemically stained for MAP2, the astrocyte marker glial fibrillary acidic protein (GFAP), and/or the synaptic protein synaptophysin in the visible wavelengths as described (Posimo et al., 2013). MAP2+ neurons, GFAP+ astrocytes, and/or Hoechst-stained nuclei were then counted by a blinded observer at 200× magnification in a 0.213 mm2 field of view (three fields per well).
2.5 Western Blots
Lysates were subjected to Western blotting 24h after toxin treatments, as described (Posimo et al., 2013). We did not wait for 48h to assess protein changes as done for the viability assays (see above) because only the surviving cells that are least vulnerable to MG132 would then be assessed. Thus, we chose to examine Hsps and co-chaperones at the 24h timepoint, while the cells most affected by the toxin were still attached to the plate and viable. Membranes were either blocked in Odyssey block (LI-COR) or 5% milk in Tris buffered saline (TBS). Membranes were then incubated in primary antibodies with 0.1% Tween in either 1:1 Odyssey block/PBS or 5% bovine serum albumin in TBS (overnight at 4°C). The membranes were next washed with PBS or TBS with 0.1% Tween for 3 × 10 min and secondary antibodies were applied in the same buffers for 1 h at room temperature. Proteins were quantified on the Odyssey Imager. We used rabbit anti-GAPDH, mouse anti-α-tubulin, or mouse anti-β-actin to normalize for protein content depending on the species of the primary antibodies and the expected molecular weights. As multiple proteins were probed on the same blots, the identical loading controls are presented twice in some figures.
2.6 Statistical Analyses
Data are presented as the mean and SEM from 3–6 animals in vivo or a minimum of 3 independently run experiments in vitro. Western blot data were analyzed by two-way or three-way ANOVA followed by the LSD post hoc correction (SPSS Version 20, Armonk, NY). All remaining data were analyzed by the Bonferroni post hoc correction following two or three-way ANOVA. For data with only two groups, the two-tailed Student’s t-test was employed. The Grubb’s outlier test was performed once on all the data and bands with fluorescent lint or air bubbles were excluded from the Western blot analyses. Differences were deemed significant only when p ≤ 0.05.
3. Results
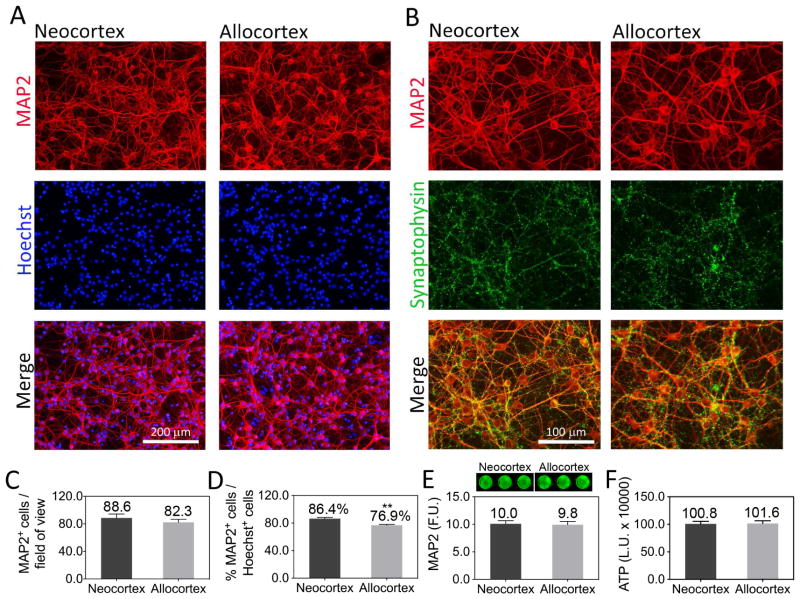
We chose to dissect neo- and allocortical tissue on postnatal days 1 and 2, as the telencephalic edifice is well developed by this age and cortical neurogenesis is complete (Bayer and Altman, 1991; Gotz, 2001; Okano and Temple, 2009; Rice and Barone, 2000). Furthermore, primary cortical neurons harvested on postnatal days 1–2 can still survive dissociation and in vitro culturing. In our previous report, we found that neocortical neurons did not survive basal culturing conditions as well as neurons harvested from the allocortex (Posimo et al., 2013). We therefore switched to Neurobasal-A media, specifically designed for postnatal neurons. In this media, neurons from primary sensorimotor neocortex and entorhinal allocortex exhibited equivalent survival rates in vitro (Fig. 1). Both types of cultures also expressed dense synaptophysin+ puncta (Fig. 1B). Allocortical cultures exhibited slightly lower levels of neuronal purity [86% MAP2+ for neocortex and 76% MAP2+ for allocortex; Fig. 1D], as determined by expression of the specific neuron marker MAP2. Neocortical cultures were less vulnerable to MG132 than cultures from entorhinal allocortex, as assessed by quantifying MAP2 and ATP levels (Fig. 2A, B). At the highest MG132 concentration, allocortex exhibited less loss of ATP levels (Fig. 2B). However, this difference was slight and might be attributable to non-neuronal elements such as glia because it was absent with the MAP2 assay.
Figure 1. Primary postnatal cultures of neo- and allocortex.
(A, B) Primary sensorimotor neocortex and entorhinal allocortex cells were harvested from 1 or 2 day-old rat pups and stained for the neuronal marker MAP2, the synaptic marker synaptophysin, and with the nuclear Hoechst stain on DIV4. (C, D) MAP2+ neurons and Hoechst-stained nuclei were counted by a blinded observer to assess the neuronal density and purity of the cultures. Neocortex had slightly higher levels of MAP2+ neurons as a percentage of total cell numbers. (E, F) No difference in overall MAP2 content or ATP levels was observed between neo- and allocortical cultures on DIV4, according to the In-Cell Western assay for MAP2 and the Cell Titer Glo assay for ATP. A representative In-Cell Western image is shown above panel E. Shown are the mean ± SEM of at least 3 independent experiments. ** p ≤ 0.01 vs neocortex, two-tailed Student’s t-test. F.U.=fluorescence units. L.U.=luminescence units.
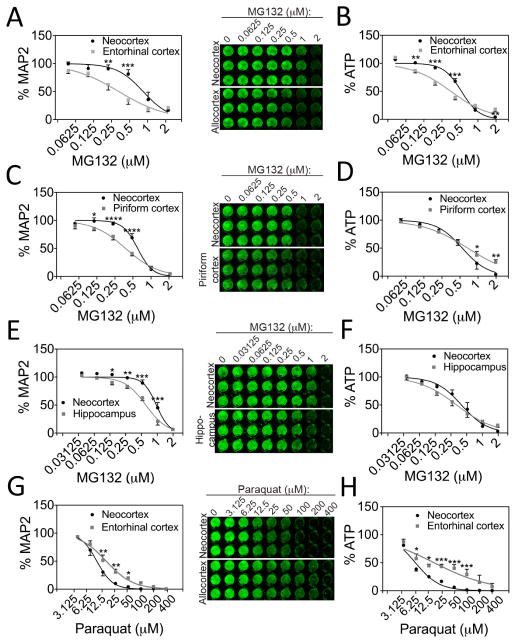
Figure 2. Regional differences in vulnerability to cellular stress.
MAP2 content (A, C, E, G) and ATP levels (B, D, F, H) in neocortex cultures and cultures harvested from entorhinal, piriform, and hippocampal allocortex 48h after treatment with the proteasome inhibitor MG132 or the oxidative toxin paraquat. Fitted curves were extrapolated from non-linear regression analyses after conversion of the X-axis to a log scale. MAP2 and ATP levels at 0 μM MG132 were set to 100% (not shown in log scale graphs). For ease of presentation, concentrations of MG132 are listed in micromolar units rather than in log units on the X-axis. Neocortical neurons were more resistant to proteotoxic stress from MG132 than neurons from three allocortical subregions according to the MAP2 assay, but less resistant to oxidative stress from paraquat. The ATP metabolic assay agreed with some, but not all of the MAP2 In-Cell Westerns. Shown are the mean ± SEM of 3–4 independent experiments. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001 neo- vs allocortex, Bonferroni post hoc correction following two-way ANOVA.
In addition to centering dissections in entorhinal allocortex, we also contrasted neo- and paleocortical vulnerability by centering dissections in piriform allocortex. Paleocortex was more vulnerable to MG132 than neocortex according to the MAP2 but not the ATP assay (Fig. 2C, D). Thus, the ATP results may reflect metabolic similarities in neo- and paleocortical glia. Neocortical cultures were also more resistant to MG132 than hippocampal cultures from archicortex in the MAP2 but not the ATP assay (Fig. 2E, F). Consistent with our previous findings on hydrogen peroxide toxicity (Posimo et al., 2013), neocortical cultures were more vulnerable to paraquat toxicity than allocortical cultures in both assays (Fig. 2G, H). These findings suggest that MAP2+ neocortical neurons are less vulnerable to proteotoxicity than neurons from the allocortex, but more vulnerable to reactive oxygen species. Furthermore, the increased neuronal vulnerability to proteotoxicity appears to be generalizable to multiple subregions of allocortex.
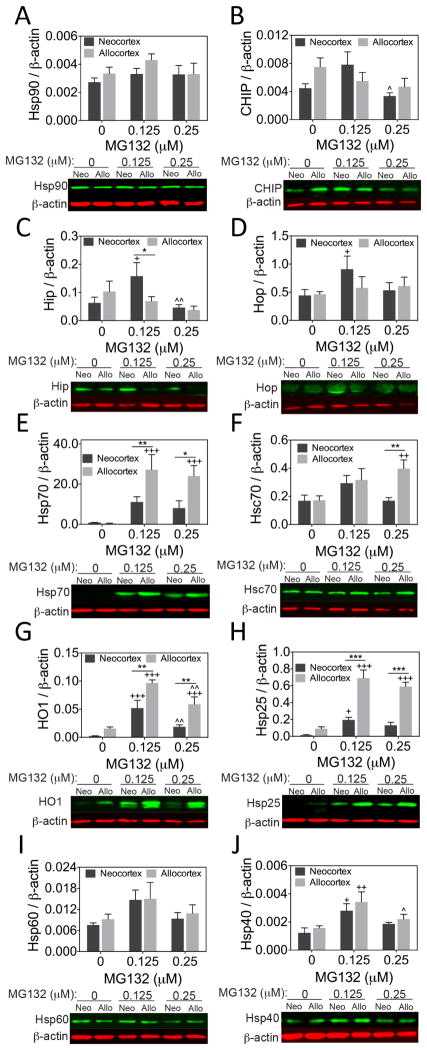
Next, we measured the impact of MG132 and paraquat on Hsp and co-chaperone levels in neocortex and entorhinal allocortex cultures. There was no statistical difference in Hsp90 or CHIP levels as a function of the brain region of origin or MG132 treatment, with the exception of a decrease in CHIP levels between 0.125 and 0.25 μM MG132 (Fig. 3A, B). MG132 increased levels of the co-chaperones Hip and Hop in neocortical cultures at low concentrations (0.125 μM; Fig. 3C, D). Furthermore, Hip was found to be lower in MG132-treated allocortex than neocortex. In contrast, Hsp70 rose to much higher levels in allocortex than neocortex at both MG132 concentrations (Fig. 3E). A similar pattern was evident for heat shock cognate 70 (Hsc70) at the higher concentration of MG132 (Fig. 3F) and for heme oxygenase 1 (HO1 or Hsp32) and Hsp25 at both concentrations of MG132 (Fig. 3G, H). Finally, Hsp40 levels were increased in both neo- and allocortex at low concentrations of MG132 and Hsp60 was unaffected (Fig. 3I, J). Notably, several proteins examined (CHIP, Hip, HO1, and Hsp40) exhibited biphasic responses to stress, a phenomenon known as hormesis (Calabrese, 2013). Taken together, these findings reveal robust stress-induced increases in Hsp70, Hsc70, HO1, and Hsp25, especially in cultures harvested from allocortex. In contrast, β-actin or GAPDH levels were unaffected by any of the treatments and were independent of brain region of harvest.
Figure 3. Heat shock protein and co-chaperone expression in neo- and allocortical cultures.
Levels of the heat shock proteins Hsp90, Hsp70, Hsc70, HO1, Hsp25, Hsp60, and Hsp40 (A, E, F, G, H, I, J) and the co-chaperones CHIP, Hip, and Hop (B, C, D) in neo- and allocortical (entorhinal) cultures 24h after treatment with MG132, as measured by infrared two-color Western blotting. Allocortex exhibited greater MG132-induced increases in Hsp70, Hsc70, HO1, and Hsp25, but neocortex exhibited greater stress-induced increases in Hip and Hop. Shown are the mean ± SEM of 6 independent experiments. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 vs neocortex, + p ≤ 0.05, ++ p ≤ 0.01, +++ p ≤ 0.001 vs 0 μM MG132, ^ p ≤ 0.05, ^^ p ≤ 0.01 vs 0.125 μM MG132, LSD post hoc following two-way ANOVA.
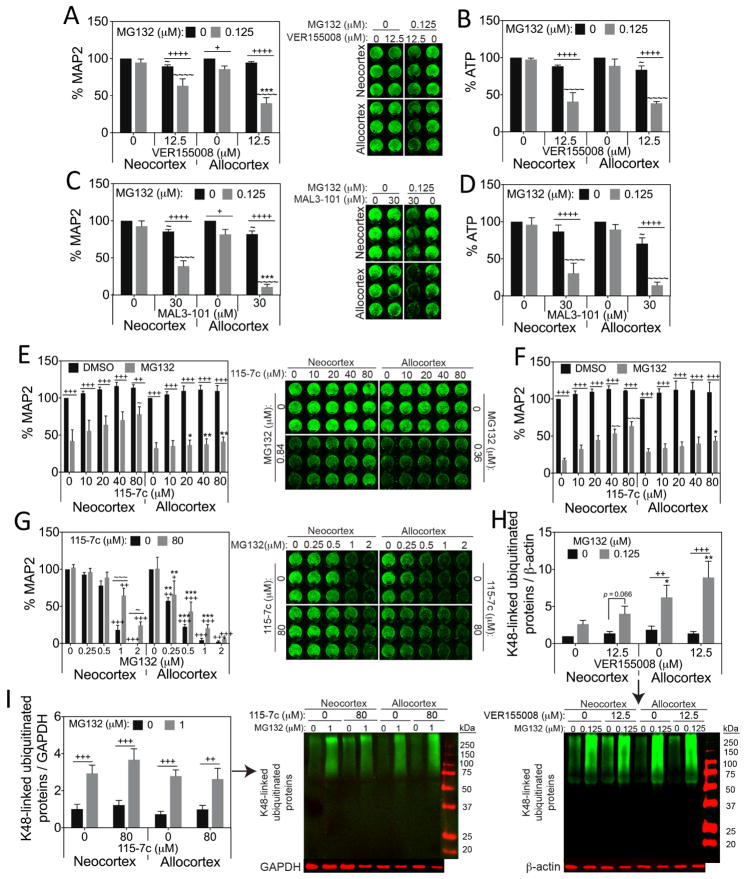
In order to examine the functional consequences of the MG132-induced increase in Hsp70 and Hsc70, we treated neo- and allocortical cultures with the Hsp70/Hsc70 inhibitors VER155008 (Chatterjee et al., 2013; Macias et al., 2011; Massey et al., 2010; Saykally et al., 2012; Williamson et al., 2009) and MAL3-101 (Adam et al., 2014; Braunstein et al., 2011; Hatic et al., 2012; Huryn et al., 2011; Kilpatrick et al., 2013). MG132 toxicity was synergistically exacerbated by these two mechanistically different Hsp70/Hsc70 inhibitors in both neo- and allocortical neurons, but the effect sizes were consistently larger for allocortex according to the MAP2 neuron viability assay (Fig. 4A, C). On the other hand, neo- and allocortex were equally vulnerable to Hsp70/Hsc70 activity loss according to the ATP assay (Fig. 4B, D). These findings support the view that both types of neurons rely on Hsp70 defenses, but that the allocortex may raise Hsp70 and Hsc70 to higher levels than the neocortex because it is in greater need of these defenses.
Figure 4. Allocortical neurons rely more on Hsp70/Hsc70 than neocortical neurons under proteotoxic conditions.
MAP2 and ATP levels in neo- and allocortical (entorhinal) cultures treated with MG132 and the Hsp70/Hsc70 inhibitors VER155008 (A, B) or MAL3-101 (C, D), and the Hsp70 activity enhancer 115-7c for 48h (E–G). See text for details on MG132 concentrations in E & F. Loss of Hsp70/Hsc70 activity with VER155008 or MAL3-101 synergistically exacerbated cell loss in response to MG132 according to the MAP2 assay, especially in allocortical neurons. Enhancement of Hsp70 activity with 115-7c protected neocortical neurons against proteotoxicity better than allocortical neurons. (H, I) K48-linked ubiquitinated proteins in neo- and allocortical cultures treated 24h with MG132 and VER155008 or MG132 and 115-7c. Ubiquitinated protein levels were higher in allocortical cultures than neocortical cultures after treatment with low concentrations of MG132 (0.125 μM). Hsp70 activity manipulation with VER155008 and 115-7c exerted no effects on ubiquitinated proteins. Shown are the mean ± SEM of 3–7 independent experiments. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 vs neocortex, + p ≤ 0.05, ++ p ≤ 0.01, +++ p ≤ 0.001, ++++ p ≤ 0.0001 vs 0 μM MG132, ~ p ≤ 0.05, ~~ p ≤ 0.01, ~~~ p ≤ 0.001, ~~~~ p ≤ 0.0001 vs 0 μM VER155008, MAL3-101, or 115-7c, Bonferroni post hoc correction following three-way ANOVA for MAP2 and ATP assays or LSD post hoc correction following three-way ANOVA for immunoblotting data.
In order to verify that Hsp70/Hsc70 activity is protective in this model, we treated neo- and allocortical cultures with the Hsp70/Hsc70 activator 115-7c (Kilpatrick et al., 2013; Wisen et al., 2010). 115-7c was significantly protective against MG132 in neocortical, but not allocortical cultures (Fig. 4E). In Figure 4E, IC50 concentrations of MG132 from Fig. 2A were applied (0.36 μM for allocortex and 0.84 μM for neocortex from a non-linear regression analysis). However, MG132 was less toxic to neocortex in 2/5 experiments (~20% MAP2 loss) and highly toxic in the remaining 3 experiments (~80% MAP2 loss). When we examined the 115-7c data without the two former experiments (Fig. 4F) we found that Hsp70/Hsc70 activation protects neocortical neurons even when the toxicity of MG132 is severe (~80% MAP2 loss). To further validate these results, we conducted a second set of experiments with a range of concentrations of MG132 and one concentration of 115-7c (Fig. 4G). In neocortex, 115-7c was significantly protective only at high concentrations of MG132 (1–2 μM). Further, as expected, 115-7c failed to significantly protect allocortical cultures at any concentration, although there was a trend towards 115-7c-mediated allocortical protection at 0.5 μM MG132 (p = 0.059). These findings reveal that enhancing Hsp70/Hsc70 activity is less effective in allocortex, perhaps because Hsp70 molecules in allocortex are already maximally engaged. In other words, Hsp70 chaperone responses may already be fully upregulated in the allocortex by potentially greater numbers of misfolded proteins.
To test the hypothesis that allocortex exhibits higher levels of misfolded proteins, we examined the effects of MG132, VER155008, and 115-7c on the K48-linked ubiquitinated protein pool (Fig. 4H, I), which is known to be targeted to the proteasome for degradation (Sadowski and Sarcevic, 2010). As expected, allocortex exhibited higher levels of ubiquitinated proteins than neocortex when treated with 0.125 μM MG132, either in the absence or presence of VER155008 (Fig. 4H). However, at higher concentrations of MG132 (1 μM), there was no difference between neo- and allocortical protein ubiquitination (Fig. 4I). It is worth noting that neo- and allocortex cultures were equally vulnerable to this concentration of MG132 according to both the MAP2 and ATP viability assays (see Fig. 2A, B). Thus, the lack of a difference in K48-linked ubiquitin here was not surprising. In addition, neither VER155008 nor 115-7c significantly impacted K48-linked ubiquitination, suggesting that Hsp70/Hsc70 activity is not a significant rate-limiting modulator of this measure in neo- and allocortical cultures.
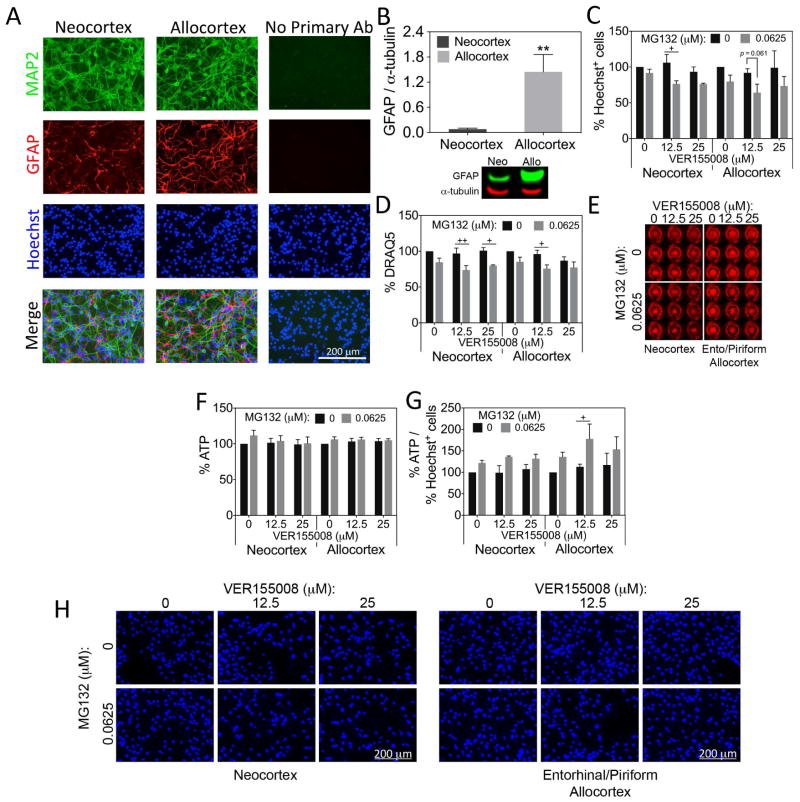
We chose to harvest cultures from postnatal rat pups because neo- and allocortex are easier to distinguish in older animals. However, our postnatal cultures are not purely neuronal and contain some GFAP+ astrocytes (Fig. 5A), consistent with observations that astroglial genesis peaks around birth (Bayer and Altman, 1991; Gotz, 2001; Okano and Temple, 2009; Rice and Barone, 2000). Thus, it is conceivable that some of the effects of MG132 and the Hsp70 modulators may have been indirectly mediated by astrocytic responses to proteotoxic stress. Although GFAP expression appeared to be somewhat lower in neocortical cultures than allocortical cultures, the number of cells expressing GFAP was similar (9.5% GFAP+ in neocortex and 13.2% GFAP+ in allocortex, p = 0.064, two-tailed t-test). Nonetheless, GFAP protein content was significantly higher in allocortical cultures in Western blots (Fig. 5B). No evidence was found for an impact of brain region on α-tubulin levels in these Western blots. The GFAP findings raise the possibility that some of the vulnerability differences between neo- and allocortex are indirectly attributable to differences in non-neuronal elements such as astrocytes. Thus, in order to specifically examine the responses of neo- and allocortical astrocytes to Hsp70 activity loss, we cultured astrocytes harvested from neo- and allocortex. Preliminary data show that the neocortical glia cultures are 92% GFAP+ and allocortical glia cultures are 95% GFAP+ (Weilnau et al., in preparation). We treated astrocyte cultures from both brain regions with subtoxic MG132 in the absence or presence of the Hsp70/Hsc70 inhibitor VER155008 (Fig. 5C–H). As in the experiments on neurons, low concentrations of MG132 were used in order to be able to resolve any synergistic potentiation of MG132 toxicity with VER155008. Blinded counts of Hoechst+ nuclei revealed slight synergy between MG132 and VER155008 in neocortical astrocytes, as shown in Figures 5C and H. That is, MG132 was slightly more toxic when Hsp70/Hsc70 activity was inhibited. Because of higher variability in the allocortical data, MG132 was not significantly toxic in the presence of VER155008, although there was a trend towards this result (see Fig. 5C). The DRAQ5 assay for nuclei, which samples the entire well at once, revealed that MG132 was toxic in the presence of VER155008 in both allocortical and neocortical astrocytes (Fig. 5D, E). However, the synergy of MG132 and VER155008 was not very robust with either assay. Furthermore, the ATP assay showed no effect of either MG132 or VER155008 in neo- or allocortical astrocytes (Fig. 5F). Importantly, no difference between neo- and allocortical astrocytes was observed with any of the three assays, supporting the view that the neo- versus allocortical differences in Figure 4 are not attributable to astrocytes. However, when we expressed the data on ATP levels as a function of cell number, we discovered that MG132 elicited an increase in ATP output per cell in allocortical cultures treated with VER155008, a sign of greater metabolic stress (Fig. 5G). The smaller increase in ATP output per cell in MG132-treated neocortical cultures was not significant but, once again, there was no statistical difference between neo- and allocortex. These findings support the interpretation that the neo/allocortical differences in reliance on Hsp70 defenses are largely attributable to neurons.
Figure 5. Neo- and allocortical astrocytes do not differ in their reliance on Hsp70/Hsc70 defenses.
(A) Neo- and allocortical (entorhinal) neuronal cultures were stained for MAP2, GFAP, and Hoechst. Some GFAP+ astrocytes were visible in both cultures. Omission of primary antibodies led to loss of fluorescent signal. (B) Western blots for GFAP verify the presence of this protein in cultures from both brain regions and reveal that GFAP protein levels are higher in allocortex cultures. Shown are the mean ± SEM of 6 independent experiments. ** p ≤ 0.01 vs neocortex, two-tailed Student’s t-test. (C–H) Astrocyte cultures harvested from neo- and allocortex were treated with MG132 and the Hsp70/Hsc70 inhibitor VER155008 for 48h and the number of Hoechst+ cells, DRAQ5 nuclear staining, and ATP levels were assessed. ATP levels were also expressed as a function of Hoechst+ cells in panel G. Representative DRAQ5 and Hoechst images from the data in panels C and D are shown (E, H). Unlike in neurons, there was no robust exacerbation of MG132 toxicity in neo- or allocortical astrocytes after loss of Hsp70/Hsc70 activity, even at higher concentrations of VER155008. Shown are the mean ± SEM of 3 independent experiments. + p ≤ 0.05, ++ p ≤ 0.01 vs 0 μM MG132, Bonferroni post hoc correction following three-way ANOVA.
Our Western blotting data thus far suggested that allocortex neurons raise Hsp70/Hsc70 defenses more than neocortical neurons. Similar to the Hsp70 expression pattern, HO1 (also known as Hsp32) was also much higher in stressed allocortical cultures (see Fig. 3G). This finding suggested that allocortex may also rely more on HO1 than neocortex to defend against proteotoxicity. Consistent with this view, inhibition of HO1 activity with tin protoporphyrin (SnPP) synergistically exacerbated MG132 toxicity in allocortex more than neocortex according to the MAP2 assay (Fig. S1). However, ATP levels were higher in MG132-treated allocortical cultures than neocortical cultures in the presence of SnPP.
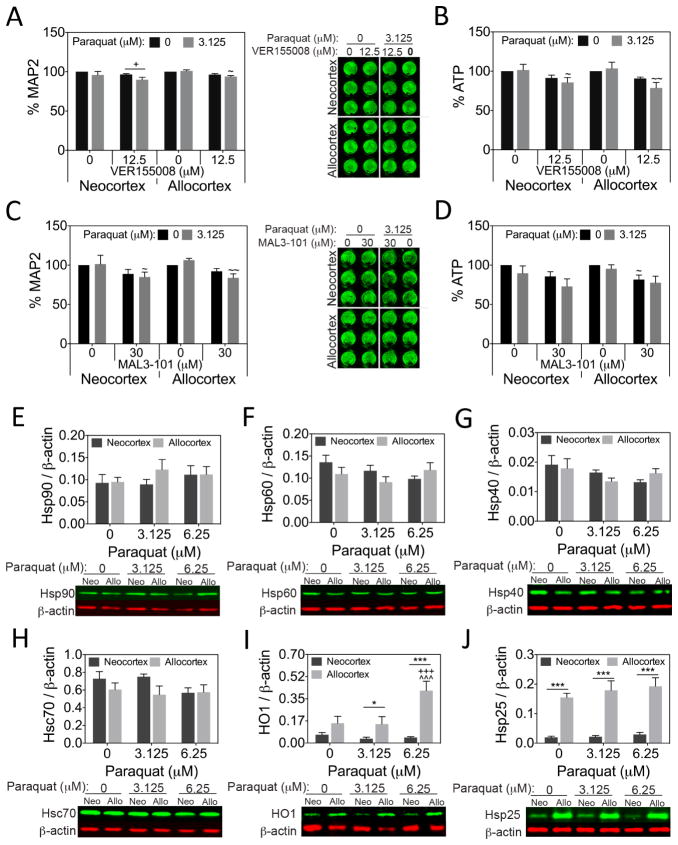
Although Hsp70 induction is critical when protein homeostasis is compromised, Hsp70 is less likely to protect neurons against oxidative toxicity (Crum et al., in press). To test this hypothesis in the current model, we also treated neo/allocortical neuron cultures with paraquat in the presence or absence of VER115005 and MAL3-101. Neither Hsp70/Hsc70 inhibitor elicited a robust effect when combined with paraquat (Fig. 6A–D), suggesting that these chaperones do not greatly mitigate oxidative toxicity in this model. Furthermore, select Hsp proteins were unchanged after paraquat treatment (Fig. 6E–H). Notably, Hsp70 levels were undetectable in paraquat-treated neo- and allocortical cultures (not shown), suggesting a lack of Hsp70 induction with oxidative stress. Hsp25 levels were higher in allocortical cultures in the absence or presence of paraquat (Fig. 6J) and HO1 levels were higher in allocortical cultures in the paraquat-treated groups, as one might expect from a protein involved in antioxidant defense (Fig. 6I). These data are consistent with greater Hsp responses to oxidative injury in allocortical cultures.
Figure 6. Paraquat toxicity in neo- and allocortical neurons is not exacerbated by Hsp70/Hsc70 inhibition.
MAP2 and ATP levels in neo- and allocortical (entorhinal) cultures 48h after treatment with paraquat or vehicle in the absence or presence of the Hsp70/Hsc70 inhibitors VER155008 (A, B) or MAL3-101 (C, D). Paraquat-induced cell loss was not robustly exacerbated by inhibition of Hsp70/Hsc70 activity in either neo- or allocortical neurons according to the MAP2 and ATP assays. Expression of heat shock proteins Hsp90, Hsp60, Hsp40, Hsc70, HO1, and Hsp25 in neo- and allocortical cultures treated with paraquat (E–J). Of all the proteins shown, paraquat only increased levels of the antioxidant defense protein HO1 in allocortical cultures. Allocortical cultures had higher levels of HO1 and Hsp25 than neocortical cultures. Shown are the mean ± SEM of 3–6 independent experiments. * p ≤ 0.05, *** p ≤ 0.001 vs neocortex, + p ≤ 0.05, +++ p ≤ 0.001 vs 0 μM paraquat, ~ p ≤ 0.05, ~~ p ≤ 0.01 vs 0 μM VER155008 or MAL3-101, ^^^ p ≤ 0.001 vs 3.125 μM paraquat, Bonferroni post hoc correction following three-way ANOVA for MAP2 and ATP data or LSD post hoc correction following two-way ANOVA for immunoblotting data.
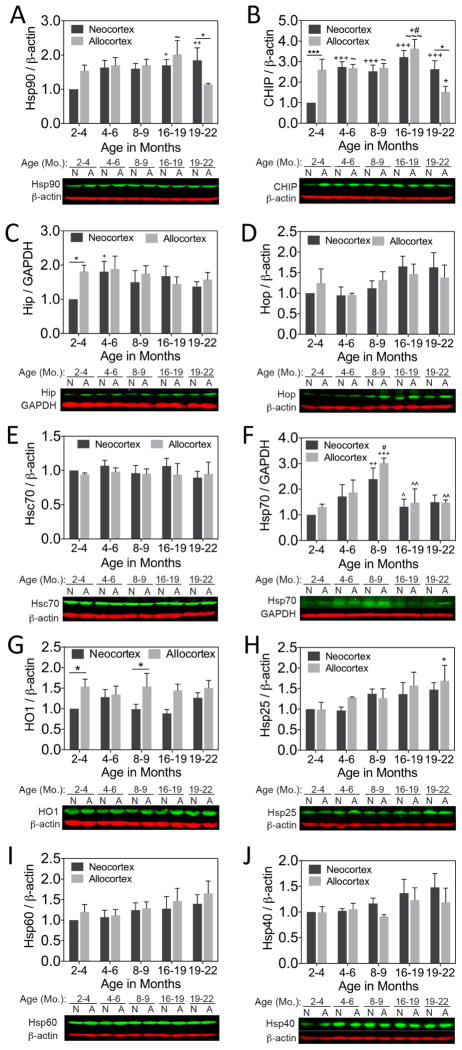
Finally, we examined how aging affects Hsp expression in neo- and allocortical tissue from female rats. Hsp90 and the E3 ubiquitin ligase CHIP were higher in primary sensorimotor neocortex than entorhinal allocortex in the oldest animals (Fig. 7A, B). Neocortical levels of Hsp90 were higher in the 16–19 month old rats than in the youngest, 2–4 month-old group. Neocortical levels of CHIP were higher at all ages relative to the 2–4 month group. The co-chaperone Hip was higher in allocortex than neocortex in the youngest animals and rose from 2–4 to 4–8 months of age in the neocortex (Fig. 7C). In contrast, Hop and Hsc70 showed no regional or age-related differences (Fig. 7D, E). Hsp70 rose to high levels at 8–9 months of age but declined to control values thereafter (Fig. 7F). There was no neo- versus allocortical difference in Hsp70 with aging. However, HO1 was higher in allocortex than neocortex at 2–4 and 8–9 months of age (Fig. 7G), and allocortical Hsp25 was higher in 19–22 month old rats than in the 2–4 month old group (Fig. 7H). Hsp60 and Hsp40 exhibited no significant age or regional differences. No evidence of any significant effect of age or brain region on β-actin or GAPDH levels in vivo was gathered.
Figure 7. Heat shock proteins and co-chaperones in neo- and allocortex as a function of age in vivo.
Female rats were sacrificed at 2–3.9, 4–6, 8–9, 16–18.9, and 19–22 months of age. Heat shock proteins Hsp90, Hsc70, Hsp70, HO1, Hsp25, Hsp60, and Hsp40 (A, E, F, G, H, I, J) and co-chaperones CHIP, Hip, and Hop (B, C, D) were measured in sensorimotor neocortex and entorhinal allocortex by infrared immunoblotting. Allocortical tissue exhibited higher levels of HO1 at multiple ages and higher levels of Hip at the youngest age. Age-related changes (usually increases) in Hsp90, CHIP, Hip, Hsp25 and Hsp70 were also apparent. Allocortical tissue exhibited lower levels of Hsp90 and CHIP than neocortical tissue in the oldest group, but allocortex and neocortex expressed equivalent levels of Hsp70 at all ages. n= 3–6 animals per group. * p ≤ 0.05, *** p ≤ 0.001 vs neocortex, + p ≤ 0.05, ++ p ≤ 0.01, +++ p ≤ 0.001 vs 2–4 mo., # p ≤ 0.05 vs 4–6 mo., ^ p ≤ 0.05, ^^ p ≤ 0.01 vs 8–9 mo., ~ p ≤ 0.05, ~~~ p ≤ 0.001 vs 19–22 mo., LSD post hoc correction following two-way ANOVA.
4. Discussion
The present study is the first to report regional variations in Hsp defenses in the neo- versus allocortex in the context of proteinopathic stress and aging. Injured allocortical neurons mounted a greater increase in Hsp70/Hsc70 defenses, were significantly more vulnerable to Hsp70/Hsc70 loss, and less able to recover from MG132 toxicity upon Hsp70 activation. These findings suggest that boosting Hsp70 activity through genetics or pharmacotherapies will have a greater protective impact on the neocortex. However, the allocortex may be in greater danger when Hsp defenses are compromised. The allocortex may require other protective mechanisms to enhance its stress response as it appears to already have high levels of chaperone activities in response to protein misfolding. These findings of topographical differences in reliance on Hsp70 defenses strongly support the view that brain region and intrinsic vulnerability differences must be taken into consideration when designing new therapies. There is precedence in the literature to expect topographic differences in vulnerability in the central nervous system. For example, the CA1 and CA3 subregions are less resistant than other hippocampal areas (Mattson et al., 1989). Dopaminergic neurons of the caudal, ventrolateral substantia nigra are more vulnerable than more rostral, dorsomedial neurons and neurons in the neighboring ventral tegmental area (Braak et al., 2003a; Damier et al., 1999). Furthermore, dopaminergic neurons in the retina are more resistant to parkinsonian neurotoxins than the dopamine neurons of the ventral mesencephalon and do not degenerate in PD (Nagel et al., 2009). Our study adds to this body of literature by showing that allocortical neurons are more vulnerable to proteotoxic stress, but are less vulnerable to oxidative stress than their neocortical neighbors.
The proteasome normally degrades misfolded proteins and is a primary defense mechanism against proteotoxic stress. During this process, misfolded proteins are tagged with ubiquitin and can be escorted to the proteasome by heat shock proteins. Proteasome inhibition therefore increases the burden of misfolded, ubiquitinated proteins and elicits protein aggregation and cell death (Rideout et al., 2001; Rideout and Stefanis, 2002; Sun et al., 2006). For example, inclusion body formation and neurodegeneration are known to follow stereotaxic infusions of proteasome inhibitors into the brain but not the periphery (Fornai et al., 2003; Kordower et al., 2006; Paz Gavilan et al., 2006; Sun et al., 2006). As with any toxin model, cell death with proteasome inhibitors is acute compared to the protracted neurodegeneration that occurs in patients. Nevertheless, proteasome inhibition is relevant to AD and PD because proteotoxicity is widely considered to be a central feature of these disorders. For example, both disorders are linked to the formation of protein aggregates (Braak et al., 1993; Braak and Braak, 1995; Braak et al., 2000a; Braak et al., 2000b; Braak et al., 2002; Braak et al., 2003a) and proteasome function is significantly reduced in postmortem brain tissue from both PD and AD subjects (Keller et al., 2000a; McNaught and Jenner, 2001; McNaught et al., 2002). These observations support the use of proteasome inhibitors to model neurodegeneration in vitro and in vivo.
In neurodegenerative diseases, Hsps are often induced as a compensatory defense mechanism or disrupted (Di Domenico et al., 2010; Schipper et al., 1998; Schipper, 2000; Schipper et al., 2006; Uryu et al., 2006; Zhang et al., 2005). For example, Hsp70 is higher in the inferior parietal lobule in patients with mild cognitive impairment (Di Domenico et al., 2010). Hsp70 protects neurons in many experimental models of neurodegeneration (Kalia et al., 2010; Sherman and Goldberg, 2001). In addition, amyloid plaques and Lewy bodies colocalize with Hsp70, perhaps following an attempt to prevent proteotoxicity (Kalia et al., 2010; Sherman and Goldberg, 2001). Hsc70 is a constitutively expressed member of the same chaperone family and can guide damaged proteins containing a C-terminal KFERQ motif to the lysosome for degradation (Arias and Cuervo, 2011). Hsc70 levels decline in the temporal cortex in AD (Yoo et al., 2001) and fall in the substantia nigra in PD (Alvarez-Erviti et al., 2010; Chu et al., 2009; Mandel et al., 2005). The synergistic potentiation of cell loss when Hsp70/Hsc70 activity was inhibited in the present study supports the view that diseased tissue would benefit from Hsp gene induction and that this natural defense could slow the demise of additional neurons. Accordingly, Hsp70/Hsc70-based therapies may be promising candidates for proteinopathic conditions such as PD and AD.
We have discovered that proteotoxic stress increases Hsp70 and Hsc70 to higher levels in allocortical cultures. Given the well-known stress-inducible nature of Hsps, higher levels of Hsps in allocortex might reflect higher proteinopathic stress. The viability data support the view that the allocortex is under greater proteotoxic stress, as defined by 1) the propensity to die when the proteasome is inhibited, and 2) enhanced sensitivity to cell loss in response to loss of chaperone activity. Furthermore, if we define proteotoxic stress as a higher burden of misfolded proteins, the K48-linked ubiquitin pool should reflect at least some of that stress (Sadowski and Sarcevic, 2010) and is also higher in allocortex, at least at lower MG132 concentrations. The potentially greater burden of misfolded proteins in allocortex is also associated with a lack of significant protection with the Hsp70 activator 115-7c. We therefore speculate that Hsp70 molecules in allocortical cultures may be already maximally engaged and cannot be stimulated any further to elicit more neuroprotection. In sum, we interpret the in vitro findings to support the view that Hsp70 levels are higher in MG132-treated allocortical neurons because these cells are in greater need of endogenous defenses against protein misfolding, but that this response is insufficient to render them as resistant as neocortical neurons.
Similar to Hsp70, therapies that boost the HO1 system of antioxidant defenses are also likely to protect against proteinopathic degeneration by modulating the redox environment. HO1 levels are higher in hippocampal and cortical neurons and astrocytes in AD patients (Schipper, 2000; Schipper et al., 2006). Furthermore, HO1 is increased in astrocytes in PD and is found in Lewy bodies (Schipper et al., 1998). Many experimental studies demonstrate that HO1 is highly protective (Calabrese et al., 2009), consistent with our observations on the effects of SnPP. We found that allocortical cultures may rely more on HO1 as well as Hsp70 defenses than neocortical cultures when subjected to proteotoxicity. Based on similarities in the patterns of expression of Hsp70, Hsc70, HO1, and Hsp25, we expect that allocortical neurons are also more reliant on Hsp25 defenses than neocortical neurons. Studies in which Hsp25 is silenced are warranted to investigate this possibility. It is noteworthy that many of the in vivo Hsp expression patterns were different from the in vitro patterns, perhaps as a result of greater astrocyte numbers in vivo, the postnatal time of harvest for in vitro cultures, and/or differences in applied stresses (MG132 versus aging). For example, the higher expression of Hsp70 in MG132-stressed allocortical neurons was not apparent in aged animals. Other conflicting findings include higher levels of Hip in allocortex at the youngest age, but higher levels of Hip in MG132-treated neocortex. Nevertheless, HO1 levels were higher at several ages in allocortical tissue in vivo and in paraquat and MG132-treated allocortex in vitro. Notably, aged allocortex exhibited lower levels of Hsp90 and CHIP than aged neocortex, an observation that supports further examination of these proteins in relation to diseases associated with aging. Finally, the age- and MG132-related increase in Hsp25 in allocortex is consistent with previous work that this Hsp is higher in the cortex, striatum, substantia nigra, and the olfactory bulb of aged animals relative to young controls (Crum et al., in press; Dickey et al., 2009; Gleixner et al., 2014; Gupte et al., 2010; Schultz et al., 2001).
If allocortex cultures raise Hsps to higher levels than neocortex cultures, then why are allocortex neurons more vulnerable to MG132? Braak has hypothesized that allocortical neurons exhibit less myelination and more lipofuscin (a sign of compromised autophagy) and are therefore subject to greater oxidative stress (Braak et al., 2003b). However, myelination is largely lost in neuron culture models and our allocortical cultures are more, not less resistant to oxidative stress. We speculate that the allocortex may have evolved greater antioxidant and chaperone defenses because it is subjected to a greater risk of proteotoxicity as animals age. Although the root cause of higher levels of protein misfolding and greater vulnerability to proteotoxic stress in allocortex is still elusive, our experiments suggest that the co-chaperones Hip and Hop might be upregulated more readily by MG132 in neocortical cultures. Furthermore, our previous work suggested that neocortical cultures have higher levels of the critical antioxidant glutathione, the ferroxidase ceruloplasmin, and may engage autophagic defenses more readily (Posimo et al., 2013). Of course, differences in the neo/allocortical afferent and efferent connections may also readily explain the topographic pattern of spread of telencephalic inclusions in PD and AD.
In neurodegenerative disorders, astrocytes are also subjected to proteotoxicity, as they remove α-synuclein (Lee et al., 2010) and β-amyloid (Wyss-Coray et al., 2003) from the extracellular space and upregulate Hsps (Dabir et al., 2004; Durrenberger et al., 2009; Renkawek et al., 1999; Seidel et al., 2012). Astrocytes develop α-synuclein inclusions in PD in a manner that parallels the staging of intraneuronal pathology (Braak et al., 2007). However, we failed to note robust differences in reliance on Hsp70 defenses between neo- and allocortical astrocytes. These data support the notion that the differences between neo- and allocortical cultures in the VER155008 and MAL3-101 experiments are largely attributable to neurons.
In multiple experiments, our ATP data and MAP2 analyses were discordant. We have validated both types of assays in several recent publications (Posimo et al., 2013; Posimo et al., 2014; Unnithan et al., 2012). However, as discussed previously, ATP levels are often upregulated in injured cells. Thus, higher levels of ATP in allocortical neurons do not necessarily reflect higher cell numbers but may reflect potentially greater metabolic stress. As ATP levels likely reflect both neuronal and non-neuronal elements in the postnatal cultures, we did not express ATP as a function of MAP2 content, as done for ATP/Hoechst+ nuclear counts in the relatively pure astrocyte cultures. Future studies to determine ATP content per cell in the neuron models would help to address this confound.
Additional limitations of our experimental design that are common in in vitro studies include a possible selection for cells that are resistant to the preparation procedure. If cell selection affected neo- and allocortex differently, it would not explain why the cells survived equally well under baseline conditions but might explain differences in vulnerability to subsequent MG132 application. On the other hand, if neo- or allocortex were differentially damaged during dissociation and culturing, it would not explain the switch in relative neo/allocortical vulnerabilities between MG132 and paraquat. Another potential limitation is that neo- and allocortex might be at different developmental stages at the time of harvest. For example, the neocortex is thought to develop after the allocortex during embryonic life and may survive the dissociation process better if fewer axonal projections are torn apart (Kostovic, 1990; Kostovic et al., 1993; Prayer, 2011). However, it must be noted again that such a difference would not explain why allocortex is less resistant to MG132 but more resistant to paraquat. Finally, varying quantities of non-neuronal elements in the in vitro models may also drive vulnerability differences, although the astrocyte culture data suggest that the defining elements would probably not be astrocytic in nature.
5. Conclusions
In conclusion, we have demonstrated that allocortical neurons rely more on Hsp70/Hsc70 defenses than neocortical neurons, perhaps because they are more vulnerable to cell loss from proteotoxic stress. However, therapies that depend upon activation of endogenous Hsp70 molecules may be less effective in allocortical neurons. Future therapies that boost stress responses in the cerebral pallium must take regional variations in endogenous Hsp defenses into account.
Supplementary Material
Supplementary Figure 1: Allocortical neurons rely on HO1 more than neocortical neurons under proteotoxic conditions. (A, B) MAP2 and ATP levels were assessed in neo- and allocortical cultures 48h after treatment with the HO1 activity inhibitor tin protoporphyrin (SnPP) and MG132. Loss of HO1 activity synergistically exacerbated MG132 toxicity according to the MAP2 assay, especially in allocortical neurons. However, loss of ATP in response to VER155008 and MG132 co-treatment was not as severe in allocortical cultures as in neocortical cultures. Shown are the mean ± SEM of 3–4 independent experiments. ** p ≤ 0.01, **** p ≤ 0.0001 vs neocortex, + p ≤ 0.05, ++ p ≤ 0.01, ++++ p ≤ 0.0001 vs 0 μM MG132, ~~ p ≤ 0.01, ~~~~ p ≤ 0.0001 vs 0 μM SnPP, Bonferroni post hoc correction following three-way ANOVA.
Highlights.
Allocortical neurons are more vulnerable to proteotoxicity than neocortical neurons
Allocortical neurons are more reliant on heat shock proteins Hsp70/Hsc70 and Hsp32 (HO1)
Allocortical astrocytes and neocortical astrocytes are equally reliant on Hsp70/Hsc70
Stressed allocortical neurons have higher levels of ubiquitinated proteins
Boosting Hsp70 activity is less effective in allocortical neurons than neocortical neurons
Acknowledgments
We are grateful to Mary Caruso, Deborah Willson, and Jackie Farrer for excellent administrative support and to Denise Butler-Bucilli and Christine Close for outstanding animal care. This study was supported by the Hillman Foundation (RKL), the Pennsylvania Department of Health (C.U.R.E. award to RKL), NIH grant DK79307 (JLB), and NIGMS grant GM067082 (PW).
Abbreviations
- AD
Alzheimer’s disease
- DMSO
dimethyl sulfoxide
- GFAP
glial fibrillary acidic protein
- Hsc70
heat shock cognate 70
- Hsp
heat shock protein
- HO1
heme oxygenase 1
- MAP2
microtubule associated protein 2
- PD
Parkinson’s disease
- PBS
phosphate-buffered saline
- TBS
Tris-buffered saline
Footnotes
Designed the experiments: RKL. Wrote the paper: RKL and JMP. Conducted the experiments and analyzed the data: JMP, JNW, AMG, MTB, NW. Generated the figures: JMP. Provided advice on data interpretation and edited the manuscript: JLB and PW. Provided the Hsp70 modulators: PW.
None of the authors have any conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam C, Baeurle A, Brodsky JL, Wipf P, Schrama D, Becker JC, Houben R. The HSP70 Modulator MAL3-101 Inhibits Merkel Cell Carcinoma. PLoS One. 2014;9:e92041. doi: 10.1371/journal.pone.0092041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH. Chaperone-mediated autophagy markers in Parkinson disease brains. Archives of neurology. 2010;67:1464–72. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- Arias E, Cuervo AM. Chaperone-mediated autophagy in protein quality control. Current opinion in cell biology. 2011;23:184–9. doi: 10.1016/j.ceb.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridon P, Geraci F, Turturici G, D’Amelio M, Savettieri G, Sconzo G. Protective role of heat shock proteins in Parkinson’s disease. Neuro-degenerative diseases. 2011;8:155–68. doi: 10.1159/000321548. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Neocortical Development. Raven Press; New York: 1991. [Google Scholar]
- Braak H. Architectonics of the human telencephalic cortex. Springer-Verlag; Berlin; New York: 1980. [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–8. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–8. doi: 10.1016/0197-4580(95)00021-6. discussion 278–84. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Bohl J, Bratzke H, Braak E. Pathological changes in the parahippocampal region in select non-Alzheimer’s dementias. Ann N Y Acad Sci. 2000a;911:221–39. doi: 10.1111/j.1749-6632.2000.tb06729.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Schultz C, Braak E. Vulnerability of select neuronal types to Alzheimer’s disease. Ann N Y Acad Sci. 2000b;924:53–61. doi: 10.1111/j.1749-6632.2000.tb05560.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249(Suppl 3):III/1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003a;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003b;110:517–36. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Schultz C, Del Tredici K. Vulnerability of cortical neurons to Alzheimer’s and Parkinson’s diseases. J Alzheimers Dis. 2006;9:35–44. doi: 10.3233/jad-2006-9s305. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta neuropathologica. 2007;114:231–41. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- Braunstein MJ, Scott SS, Scott CM, Behrman S, Walter P, Wipf P, Coplan JD, Chrico W, Joseph D, Brodsky JL, Batuman O. Antimyeloma Effects of the Heat Shock Protein 70 Molecular Chaperone Inhibitor MAL3-101. Journal of oncology. 2011;2011:232037. doi: 10.1155/2011/232037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ. Biphasic dose responses in biology, toxicology and medicine: accounting for their generalizability and quantitative features. Environmental pollution. 2013;182:452–60. doi: 10.1016/j.envpol.2013.07.046. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Mancuso C, Barone E, Calafato S, Bates T, Rizzarelli E, Kostova AT. Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Frontiers in bioscience. 2009;14:376–97. doi: 10.2741/3250. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Andrulis M, Stuhmer T, Muller E, Hofmann C, Steinbrunn T, Heimberger T, Schraud H, Kressmann S, Einsele H, Bargou RC. The PI3K/Akt signaling pathway regulates the expression of Hsp70, which critically contributes to Hsp90-chaperone function and tumor cell survival in multiple myeloma. Haematologica. 2013;98:1132–41. doi: 10.3324/haematol.2012.066175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35:385–98. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Cornelius C, Perrotta R, Graziano A, Calabrese EJ, Calabrese V. Stress responses, vitagenes and hormesis as critical determinants in aging and longevity: Mitochondria as a “chi”. Immunity & ageing: I & A. 2013;10:15. doi: 10.1186/1742-4933-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. Cortex cerebri: performance, structural, and functional organization of the cortex. Oxford University Press; Oxford; New York: 1995. [Google Scholar]
- Crum TS, Gleixner AM, Posimo JM, Mason DM, Broeren MT, Heinemann SD, Wipf P, Brodsky JL, Leak RK. Heat shock protein responses to aging and proteotoxicity in the olfactory bulb. Journal of Neurochemistry. 2015 doi: 10.1111/jnc.13041. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabir DV, Trojanowski JQ, Richter-Landsberg C, Lee VM, Forman MS. Expression of the small heat-shock protein alphaB-crystallin in tauopathies with glial pathology. The American journal of pathology. 2004;164:155–66. doi: 10.1016/s0002-9440(10)63106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122 (Pt 8):1437–48. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–5. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico F, Sultana R, Tiu GF, Scheff NN, Perluigi M, Cini C, Butterfield DA. Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: an investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain Res Brain Res Protoc. 2010;1333:72–81. doi: 10.1016/j.brainres.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey C, Kraft C, Jinwal U, Koren J, Johnson A, Anderson L, Lebson L, Lee D, Dickson D, de Silva R, Binder LI, Morgan D, Lewis J. Aging analysis reveals slowed tau turnover and enhanced stress response in a mouse model of tauopathy. The American journal of pathology. 2009;174:228–38. doi: 10.2353/ajpath.2009.080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. Linking selective vulnerability to cell death mechanisms in Parkinson’s disease. Am J Pathol. 2007;170:16–9. doi: 10.2353/ajpath.2007.061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Wolfer DP, Lipp HP, Bueler H. Hsp70 gene transfer by adeno-associated virus inhibits MPTP-induced nigrostriatal degeneration in the mouse model of Parkinson disease. Molecular therapy: the journal of the American Society of Gene Therapy. 2005;11:80–8. doi: 10.1016/j.ymthe.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Drummond GS, Kappas A. Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc Natl Acad Sci U S A. 1981;78:6466–70. doi: 10.1073/pnas.78.10.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrenberger PF, Filiou MD, Moran LB, Michael GJ, Novoselov S, Cheetham ME, Clark P, Pearce RK, Graeber MB. DnaJB6 is present in the core of Lewy bodies and is highly up-regulated in parkinsonian astrocytes. Journal of neuroscience research. 2009;87:238–45. doi: 10.1002/jnr.21819. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- Filimonoff IN. A rational subdivision of the cerebral cortex. Arch Neurol Psychiatry. 1947;58:296–311. doi: 10.1001/archneurpsyc.1947.02300320047002. [DOI] [PubMed] [Google Scholar]
- Fornai F, Lenzi P, Gesi M, Ferrucci M, Lazzeri G, Busceti CL, Ruffoli R, Soldani P, Ruggieri S, Alessandri MG, Paparelli A. Fine structure and biochemical mechanisms underlying nigrostriatal inclusions and cell death after proteasome inhibition. J Neurosci. 2003;23:8955–66. doi: 10.1523/JNEUROSCI.23-26-08955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleixner AM, Pulugulla SH, Pant DB, Posimo JM, Crum TS, Leak RK. Impact of aging on heat shock protein expression in the substantia nigra and striatum of the female rat. Cell Tissue Res. 2014;357:43–54. doi: 10.1007/s00441-014-1852-6. [DOI] [PubMed] [Google Scholar]
- Gotz M. Cerebral Cortex Development. Encyclopedia of Life Sciences. 2001:1–7. [Google Scholar]
- Gupte AA, Morris JK, Zhang H, Bomhoff GL, Geiger PC, Stanford JA. Age-related changes in HSP25 expression in basal ganglia and cortex of F344/BN rats. Neuroscience letters. 2010;472:90–3. doi: 10.1016/j.neulet.2010.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. The Journal of clinical investigation. 2011;121:715–25. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatic H, Kane MJ, Saykally JN, Citron BA. Modulation of transcription factor Nrf2 in an in vitro model of traumatic brain injury. Journal of neurotrauma. 2012;29:1188–96. doi: 10.1089/neu.2011.1806. [DOI] [PubMed] [Google Scholar]
- Huryn DM, Brodsky JL, Brummond KM, Chambers PG, Eyer B, Ireland AW, Kawasumi M, Laporte MG, Lloyd K, Manteau B, Nghiem P, Quade B, Seguin SP, Wipf P. Chemical methodology as a source of small-molecule checkpoint inhibitors and heat shock protein 70 (Hsp70) modulators. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6757–62. doi: 10.1073/pnas.1015251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia SK, Kalia LV, McLean PJ. Molecular chaperones as rational drug targets for Parkinson’s disease therapeutics. CNS & neurological disorders drug targets. 2010;9:741–53. doi: 10.2174/187152710793237386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer’s disease. J Neurochem. 2000a;75:436–9. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech Ageing Dev. 2000b;113:61–70. doi: 10.1016/s0047-6374(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Kilpatrick K, Novoa JA, Hancock T, Guerriero CJ, Wipf P, Brodsky JL, Segatori L. Chemical induction of Hsp70 reduces alpha-synuclein aggregation in neuroglioma cells. ACS chemical biology. 2013;8:1460–8. doi: 10.1021/cb400017h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 Reduces alpha-Synuclein Aggregation and Toxicity. The Journal of biological chemistry. 2004;279:25497–502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Kanaan NM, Chu Y, Suresh Babu R, Stansell J, 3rd, Terpstra BT, Sortwell CE, Steece-Collier K, Collier TJ. Failure of proteasome inhibitor administration to provide a model of Parkinson’s disease in rats and monkeys. Ann Neurol. 2006;60:264–8. doi: 10.1002/ana.20935. [DOI] [PubMed] [Google Scholar]
- Kostovic I. Structural and histochemical reorganization of the human prefrontal cortex during perinatal and postnatal life. Prog Brain Res. 1990;85:223–39. doi: 10.1016/s0079-6123(08)62682-5. discussion 239–40. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Petanjek Z, Judas M. Early areal differentiation of the human cerebral cortex: entorhinal area. Hippocampus. 1993;3:447–58. doi: 10.1002/hipo.450030406. [DOI] [PubMed] [Google Scholar]
- Lanneau D, Wettstein G, Bonniaud P, Garrido C. Heat shock proteins: cell protection through protein triage. The Scientific World Journal. 2010;10:1543–52. doi: 10.1100/tsw.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. The Journal of biological chemistry. 2010;285:9262–72. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–53. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias AT, Williamson DS, Allen N, Borgognoni J, Clay A, Daniels Z, Dokurno P, Drysdale MJ, Francis GL, Graham CJ, Howes R, Matassova N, Murray JB, Parsons R, Shaw T, Surgenor AE, Terry L, Wang Y, Wood M, Massey AJ. Adenosine-derived inhibitors of 78 kDa glucose regulated protein (Grp78) ATPase: insights into isoform selectivity. Journal of medicinal chemistry. 2011;54:4034–41. doi: 10.1021/jm101625x. [DOI] [PubMed] [Google Scholar]
- Magrane J, Smith RC, Walsh K, Querfurth HW. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:1700–6. doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel S, Grunblatt E, Riederer P, Amariglio N, Jacob-Hirsch J, Rechavi G, Youdim MB. Gene expression profiling of sporadic Parkinson’s disease substantia nigra pars compacta reveals impairment of ubiquitin-proteasome subunits, SKP1A, aldehyde dehydrogenase, and chaperone HSC-70. Annals of the New York Academy of Sciences. 2005;1053:356–75. doi: 10.1196/annals.1344.031. [DOI] [PubMed] [Google Scholar]
- Massey AJ, Williamson DS, Browne H, Murray JB, Dokurno P, Shaw T, Macias AT, Daniels Z, Geoffroy S, Dopson M, Lavan P, Matassova N, Francis GL, Graham CJ, Parsons R, Wang Y, Padfield A, Comer M, Drysdale MJ, Wood M. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer chemotherapy and pharmacology. 2010;66:535–45. doi: 10.1007/s00280-009-1194-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Guthrie PB, Kater SB. Intrinsic factors in the selective vulnerability of hippocampal pyramidal neurons. Prog Clin Biol Res. 1989;317:333–51. [PubMed] [Google Scholar]
- McNaught KS, Jenner P. Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci Lett. 2001;297:191–4. doi: 10.1016/s0304-3940(00)01701-8. [DOI] [PubMed] [Google Scholar]
- McNaught KS, Belizaire R, Jenner P, Olanow CW, Isacson O. Selective loss of 20S proteasome alpha-subunits in the substantia nigra pars compacta in Parkinson’s disease. Neurosci Lett. 2002;326:155–8. doi: 10.1016/s0304-3940(02)00296-3. [DOI] [PubMed] [Google Scholar]
- Moloney TC, Hyland R, O’Toole D, Paucard A, Kirik D, O’Doherty A, Gorman AM, Dowd E. Heat shock protein 70 reduces alpha-synuclein-induced predegenerative neuronal dystrophy in the alpha-synuclein viral gene transfer rat model of Parkinson’s disease. CNS Neurosci Ther. 2014;20:50–8. doi: 10.1111/cns.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel F, Falkenburger BH, Tonges L, Kowsky S, Poppelmeyer C, Schulz JB, Bahr M, Dietz GP. Tat-Hsp70 protects dopaminergic neurons in midbrain cultures and in the substantia nigra in models of Parkinson’s disease. Journal of neurochemistry. 2008;105:853–64. doi: 10.1111/j.1471-4159.2007.05204.x. [DOI] [PubMed] [Google Scholar]
- Nagel F, Bahr M, Dietz GP. Tyrosine hydroxylase-positive amacrine interneurons in the mouse retina are resistant against the application of various parkinsonian toxins. Brain Res Bull. 2009;79:303–9. doi: 10.1016/j.brainresbull.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Okano H, Temple S. Cell types to order: temporal specification of CNS stem cells. Curr Opin Neurobiol. 2009;19:112–9. doi: 10.1016/j.conb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press, Elsevier Science; San Diego, CA: 1998. [Google Scholar]
- Paz Gavilan M, Vela J, Castano A, Ramos B, del Rio JC, Vitorica J, Ruano D. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiology of aging. 2006;27:973–82. doi: 10.1016/j.neurobiolaging.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Peters A, Jones EG. Cerebral cortex. Plenum Press; New York: 1984. [Google Scholar]
- Posimo JM, Titler AM, Choi HJ, Unnithan AS, Leak RK. Neocortex and allocortex respond differentially to cellular stress in vitro and aging in vivo. PLoS One. 2013;8:e58596. doi: 10.1371/journal.pone.0058596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posimo JM, Unnithan AS, Gleixner AM, Choi HJ, Jiang Y, Pulugulla SH, Leak RK. Viability assays for cells in culture. Journal of visualized experiments: JoVE. 2014 doi: 10.3791/50645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prayer D. Fetal MRI. Springer; Heidelberg: 2011. [Google Scholar]
- Renkawek K, Stege GJ, Bosman GJ. Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson’s disease. Neuroreport. 1999;10:2273–6. doi: 10.1097/00001756-199908020-00009. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 (Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout HJ, Larsen KE, Sulzer D, Stefanis L. Proteasomal inhibition leads to formation of ubiquitin/alpha-synuclein-immunoreactive inclusions in PC12 cells. J Neurochem. 2001;78:899–908. doi: 10.1046/j.1471-4159.2001.00474.x. [DOI] [PubMed] [Google Scholar]
- Rideout HJ, Stefanis L. Proteasomal inhibition-induced inclusion formation and death in cortical neurons require transcription and ubiquitination. Mol Cell Neurosci. 2002;21:223–38. doi: 10.1006/mcne.2002.1173. [DOI] [PubMed] [Google Scholar]
- Sadowski M, Sarcevic B. Mechanisms of mono- and poly-ubiquitination: Ubiquitination specificity depends on compatibility between the E2 catalytic core and amino acid residues proximal to the lysine. Cell division. 2010;5:19. doi: 10.1186/1747-1028-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykally JN, Rachmany L, Hatic H, Shaer A, Rubovitch V, Pick CG, Citron BA. The nuclear factor erythroid 2-like 2 activator, tert-butylhydroquinone, improves cognitive performance in mice after mild traumatic brain injury. Neuroscience. 2012;223:305–14. doi: 10.1016/j.neuroscience.2012.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper HM, Liberman A, Stopa EG. Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Experimental neurology. 1998;150:60–8. doi: 10.1006/exnr.1997.6752. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Heme oxygenase-1: role in brain aging and neurodegeneration. Exp Gerontol. 2000;35:821–30. doi: 10.1016/s0531-5565(00)00148-0. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Bennett DA, Liberman A, Bienias JL, Schneider JA, Kelly J, Arvanitakis Z. Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiology of aging. 2006;27:252–61. doi: 10.1016/j.neurobiolaging.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Schlecht R, Scholz SR, Dahmen H, Wegener A, Sirrenberg C, Musil D, Bomke J, Eggenweiler HM, Mayer MP, Bukau B. Functional analysis of Hsp70 inhibitors. PLoS One. 2013;8:e78443. doi: 10.1371/journal.pone.0078443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz C, Dick EJ, Cox AB, Hubbard GB, Braak E, Braak H. Expression of stress proteins alpha B-crystallin, ubiquitin, and hsp27 in pallido-nigral spheroids of aged rhesus monkeys. Neurobiology of aging. 2001;22:677–82. doi: 10.1016/s0197-4580(01)00229-9. [DOI] [PubMed] [Google Scholar]
- Seidel K, Vinet J, Dunnen WF, Brunt ER, Meister M, Boncoraglio A, Zijlstra MP, Boddeke HW, Rub U, Kampinga HH, Carra S. The HSPB8-BAG3 chaperone complex is upregulated in astrocytes in the human brain affected by protein aggregation diseases. Neuropathol Appl Neurobiol. 2012;38:39–53. doi: 10.1111/j.1365-2990.2011.01198.x. [DOI] [PubMed] [Google Scholar]
- Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29:15–32. doi: 10.1016/s0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- Sun F, Anantharam V, Zhang D, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Proteasome inhibitor MG-132 induces dopaminergic degeneration in cell culture and animal models. Neurotoxicology. 2006;27:807–15. doi: 10.1016/j.neuro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Unnithan AS, Choi HJ, Titler AM, Posimo JM, Leak RK. Rescue from a two hit, high-throughput model of neurodegeneration with N-acetyl cysteine. Neurochemistry international. 2012;61:356–368. doi: 10.1016/j.neuint.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Uryu K, Richter-Landsberg C, Welch W, Sun E, Goldbaum O, Norris EH, Pham CT, Yazawa I, Hilburger K, Micsenyi M, Giasson BI, Bonini NM, Lee VM, Trojanowski JQ. Convergence of heat shock protein 90 with ubiquitin in filamentous alpha-synuclein inclusions of alpha-synucleinopathies. The American journal of pathology. 2006;168:947–61. doi: 10.2353/ajpath.2006.050770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DS, Borgognoni J, Clay A, Daniels Z, Dokurno P, Drysdale MJ, Foloppe N, Francis GL, Graham CJ, Howes R, Macias AT, Murray JB, Parsons R, Shaw T, Surgenor AE, Terry L, Wang Y, Wood M, Massey AJ. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. Journal of medicinal chemistry. 2009;52:1510–3. doi: 10.1021/jm801627a. [DOI] [PubMed] [Google Scholar]
- Wisen S, Bertelsen EB, Thompson AD, Patury S, Ung P, Chang L, Evans CG, Walter GM, Wipf P, Carlson HA, Brodsky JL, Zuiderweg ER, Gestwicki JE. Binding of a small molecule at a protein-protein interface regulates the chaperone activity of hsp70-hsp40. ACS chemical biology. 2010;5:611–22. doi: 10.1021/cb1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–7. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Yoo BC, Kim SH, Cairns N, Fountoulakis M, Lubec G. Deranged expression of molecular chaperones in brains of patients with Alzheimer’s disease. Biochemical and biophysical research communications. 2001;280:249–58. doi: 10.1006/bbrc.2000.4109. [DOI] [PubMed] [Google Scholar]
- Zhang Y, James M, Middleton FA, Davis RL. Transcriptional analysis of multiple brain regions in Parkinson’s disease supports the involvement of specific protein processing, energy metabolism, and signaling pathways, and suggests novel disease mechanisms. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2005;137B:5–16. doi: 10.1002/ajmg.b.30195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Allocortical neurons rely on HO1 more than neocortical neurons under proteotoxic conditions. (A, B) MAP2 and ATP levels were assessed in neo- and allocortical cultures 48h after treatment with the HO1 activity inhibitor tin protoporphyrin (SnPP) and MG132. Loss of HO1 activity synergistically exacerbated MG132 toxicity according to the MAP2 assay, especially in allocortical neurons. However, loss of ATP in response to VER155008 and MG132 co-treatment was not as severe in allocortical cultures as in neocortical cultures. Shown are the mean ± SEM of 3–4 independent experiments. ** p ≤ 0.01, **** p ≤ 0.0001 vs neocortex, + p ≤ 0.05, ++ p ≤ 0.01, ++++ p ≤ 0.0001 vs 0 μM MG132, ~~ p ≤ 0.01, ~~~~ p ≤ 0.0001 vs 0 μM SnPP, Bonferroni post hoc correction following three-way ANOVA.