Abstract
The present study sought to evaluate whether white matter microstructure abnormalities observed in a cohort of adolescents with attention-deficit/hyperactivity disorder (ADHD) have specific relationships with either or both Hyperactivity/Impulsivity and Inattentive ADHD symptom domains that would support a dimensional view of ADHD as adopted in the DSM-V. Diffusion tensor imaging (DTI) data were acquired on 22 adolescents diagnosed with ADHD. Multiple regression analyses were performed to determine whether scalar DTI measures in 13 tracts-of-interest demonstrated meaningful associations with Hyperactivity/Impulsivity or Inattentive symptom severity. Fractional anisotropy and radial diffusivity measures of white matter integrity exhibited significant linear relationships with Hyperactivity/Impulsivity and Inattentive symptom severity. However, only radial diffusivity in the right superior longitudinal fasciculus was specifically linked to Inattentive symptom severity and not Hyperactivity/Impulsivity symptom severity. Our results provide preliminary evidence that symptom domains in ADHD are linked to neuroanatomical substrates and confirm the value in examining ADHD from a dimensional perspective.
Keywords: ADHD, Adolescents, Dimensionality, DTI, Superior longitudinal fasciculus
1. Introduction
The unresolved and long-debated (Lahey and Carlson, 1991; Cantwell, 1996; Sherman et al., 1997; Hudziak et al., 1998; Gomez et al., 1999; Neuman et al., 1999; Lahey et al., 2005; Nigg et al., 2005; Woo and Rey, 2005; Baeyens et al., 2006; Larsson et al., 2006; Volk et al., 2006) question whether the Inattention and Hyperactivity/Impulsivity symptoms of attention-deficit/hyperactivity disorder (ADHD) have shared versus distinct etiologies took a new turn when the fifth edition of the Diagnostic and Statistical Manual for Mental Disorders (American Psychiatric Association, 2013) replaced categorically distinct ADHD clinical subtypes with clinical “Presentations.” This decision was based in large part on evidence that the subtypes have notable similarities (e.g., cognitive and academic dysfunction, treatment response), as well as the fact it better accounts for the within-patient instability of ADHD symptom expression over time (Willcutt et al., 2012). Although this diagnostic change seemingly endorses the idea that the different ADHD symptom types stem from common causal factors, there is as yet insufficient evidence for either distinctiveness or similarity of pathophysiology underlying the two ADHD symptom types to conclude they are the same or different. Some recent genetic evidence is strongly suggestive of differing ADHD symptom etiologies (e.g., Nigg et al., 2004; Larsson et al., 2006; Swanson et al., 2007). It is not yet known, however, what specific biological mechanisms might be linked to these differing genetic profiles. For instance, neuropsychological test performance, often useful to infer dysfunction in specific brain regions, shows more similarities than differences across hundreds of ADHD studies (Willcutt et al., 2012), differing perhaps only in a matter of degree of specific cognitive impairments.
One approach to identify distinct neurobiological abnormalities in ADHD subtypes would be to directly contrast Predominantly Inattentive and Predominantly Hyperactive/Impulsive DSM-IV-defined (American Psychiatric Association, 2000) ADHD patients using structural or functional neuroimaging. To date, however, there have been no studies that have directly compared brain structures in non-comorbid ADHD subtypes and only four studies that have directly compared brain function in non-comorbid ADHD subtypes (Solanto et al., 2009; Edel et al., 2013; McCarthy et al., 2013; Rodrak and Wongsawat, 2013). Functional studies typically demonstrate the existence of diffuse brain activity differences between Combined (ADHD-C) and Inattentive (ADHD-I) subtypes, almost entirely ignoring the Hyperactive-Impulsivity (ADHD-H) subtype. This strategy has an important drawback, because ADHD patients commonly present with a mix of the two symptoms types, frequently missing meeting ADHD “Combined Subtype” criteria by only one or two symptoms in either symptom domain. A useful way forward can be seen in another recent DSM-5 conceptual change that recognizes the importance of a dimensional perspective in psychiatry in general (e.g., McHugh and Slavney, 1998; Maser and Patterson, 2002; Haslam, 2003; Helzer et al., 2006a; Helzer et al., 2006b; Helzer et al., 2006c), and ADHD in particular. According to a dimensional perspective, differing profiles of severity (e.g., based either on the severity or the frequency of ADHD symptoms (Swanson et al., 2009) might reflect important etiological differences. Clear depiction of these abnormalities would be missed if one only considered the presence or absence of an arbitrary-numbered constellation of symptoms. A handful of promising studies already have attempted to link dimensions of ADHD symptom severity to brain abnormalities. For instance, in one study, higher ADHD Inattention predicted lower ADHD brain activation during all conditions of a Stroop Color-Word functional magnetic resonance imaging (fMRI) task (Depue et al., 2010). In contrast, there was no association of brain activation levels with Hyperactivity/Impulsivity symptom severity. Several studies of brain structure have linked Hyperactivity/Impulsivity severity to lower right ventral striatum volume (Carmona et al., 2009), greater amygdala volume (Frodl et al., 2010), and smaller posterior thalamus volume (Ivanov et al., 2010). Greater Inattention was associated with other brain region abnormalities, e.g., smaller right amygdala (Frodl et al., 2010) or larger right medial thalamus volume (Ivanov et al., 2010). While this limited evidence suggests that some forms of neurobiological impairment are linked to a specific dimensional severity continuum in ADHD, more work is needed – both to replicate initial findings and to extend them to other measures of brain structure and function. Moreover, dimensional studies of ADHD have yet to clearly identify shared neurobiological abnormalities between the two symptom types.
One aspect of brain structure that has not yet been examined from a dimensional perspective in ADHD is white matter microstructure, as measured by diffusion tensor imaging (DTI). DTI is sensitive to white matter axonal density, diameter, and organization and myelination (Gong et al., 2009). Characteristic changes, including increased axonal diameter and density and ongoing myelination, have been well documented in normal child and adolescent brain development (Snook et al., 2005; Eluvathingal et al., 2007; Lebel et al., 2008; Perrin et al., 2009), making DTI measures of white matter microstructure ideal for investigating how ADHD symptoms may manifest via abnormal development of white matter. To date, at least 15 studies have been published describing white matter connectivity abnormalities in ADHD (for review, see van Ewijk et al., 2012). However, this ever-growing growing number of studies has not yielded a consistent set of white matter tracts that are abnormal in ADHD. Of all available studies, ADHD abnormalities have been most often reported in the middle cerebellar peduncle, corpus callosum, internal capsule, corona radiata, cingulum bundle, superior longitudinal fasciculus, and inferior longitudinal fasciculus. These previous studies typically examined modest sample sizes, often of only a single DSM-IV subtype (typically Combined ADHD), or failed to distinguish among subtypes in their analyses. Only one DTI study to date has specifically examined dimensional white matter abnormalities in a mixed sample of Inattentive, Hyperactive-Impulsive, or Combined subtype ADHD children and adolescents related to the severity of Hyperactivity/Impulsivity (Hamilton et al., 2008), finding decreased fractional anisotropy in the bilateral corticospinal tract and superior longitudinal fasciculus in the ADHD cohort compared with the healthy controls. This study found no association between DTI measures and symptom scores as measured on the SNAP-IV symptom rating scales (Swanson, 1992), but the authors noted that their small sample size meant that the study was most likely unpowered to note anything other than the strongest effects. Another study examined a cohort of 96 neurologically normal 6-year-old boys and found that higher inattentive and hyperactivity/impulsivity ratings (as assessed using the Conners' Parent Rating Scale; Conners et al., 1998) were associated with reduced fractional anisotropy in a number of major white matter tracts including the right and left sagittal stratums, right posterior thalamic radiation, and the body and splenium of the corpus callosum (Qiu et al., 2012). While this latter study did not directly examine ADHD-diagnosed children, it linked white matter microstructure to ADHD symptoms and adds evidence for the validity of a dimensional framework for ADHD neurobiology.
The purpose of the present study was to evaluate whether ADHD white matter microstructure abnormalities have specific relationships with either (or both) major clinical domains of ADHD symptom severity (i.e., Hyperactivity/Impulsivity [ADHD-HI] vs. Inattention [ADHD-I]). Because so few previous DTI studies have considered neurobiological abnormality in ADHD from the perspective of dimensional severity, any evidence for unique relationships between white matter tract abnormality and severity of ADHD symptoms in either of the two DSM-IV ADHD clinical symptom domains would provide much needed biological validation of the proposed dimensional framework for ADHD. This would be a productive step in the direction of identifying any specific biological correlates shared by (or unique to) each symptom domain. Inherent in this argument is that the notion of severity – either ADHD or white matter microstructure – indexes the degree of severity or expression of a neurobiological abnormality unique to that symptom domain. Our approach was to examine a sample of youth representing a range of symptom severities (i.e., some without Hyperactive/Impulsivity, some without Inattentive, and others with varying degrees of each type of symptoms). Our analyses sought to show whether relationships between DTI-measured white matter microstructure and ADHD symptoms were either unique to one presentation or shared across both Hyperactivity/Impulsivity and Inattention. Although DTI fractional anisotropy (FA) is most commonly examined because it represents an effective summary measure of overall water diffusivity around white matter, recent research (e.g., Alexander et al., 2007; Thomason and Thompson, 2011) has indicated the value of concurrently examining additional scalar DTI measures (radial diffusivity, mean diffusivity, and axial diffusivity) that are believed to capture more specific aspects of white matter microstructural abnormalities.
2. Methods
2.1. Participants
Participants included 22 adolescents (18 males; 4 females) ages 12-18 (mean/SD age 15.0/1.9) diagnosed with ADHD (DSM-IV 314.00 or 314.01) who were recruited via community and physician referral as part of a National Institute of Mental Health (NIMH)-funded study of brain structure and function (K23MH070036). Because the purpose of this study was to examine dimensional relationships between ADHD symptom severity and brain structure and not to replicate the diverse previously identified ADHD white matter abnormalities, a non-ADHD comparison group was not included. The ADHD sample was specifically chosen because the study's recruitment criteria permitted a range of ADHD symptom severity. DSM-IV diagnoses were evaluated by the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (KSADS-PL; Kaufman et al., 1997) administered by experienced staff. The KSADS-PL was chosen over more typical measures, such as the SNAP or Conners' Parent Rating Scale, as while the latter two measures code symptoms using a summary score (e.g., 0 = definitely absent, …, 3 = definitely threshold) which arguably better captures conjoint measures of both number of symptoms and their degree of expression, such summary measures are typically highly correlated. Mean/SD ADHD Hyperactivity/Impulsivity symptoms were 6.1/2.3 (range 1-8); mean/SD ADHD Inattention symptoms were 7.2/1.8 (range 1-9). Roughly one-third of the sample were high on ADHD-I but low on ADHD-HI symptoms, another third was high on ADHD-HI but low on ADHD-I, and the final third was high on both ADHD-I and ADHD-HI symptoms. As per study criteria, none of the ADHD-diagnosed participants had any other current DSM IV-defined psychiatric comorbidity. Additionally, approximately one-third of participants reported regularly taking some sort of ADHD medication. As the DTI data were acquired as part of a larger study examining brain function in addition to brain structure, those participants currently taking ADHD medication were requested to withhold medication for 24 h before their magnetic resonance imaging (MRI) scan. All study procedures were explained in detail to potential participants and a parent or legal guardian during an informed consent/assent protocol approved by Hartford Hospital's Institutional Review Board. Research procedures adhered to ethical standards. Participants received monetary compensation for their time.
2.2. Diffusion tensor imaging
Diffusion data were acquired on a Siemens 3T Allegra head-only scanner located at the Olin Neuropsychiatry Research Center at The Institute of Living/Hartford Hospital. The pulse sequence was a single-shot spin echo EPI sequence (repetition time/echo time = 6300/82 ms, field of view = 200 mm, matrix = 128, averages = 8, diffusion-sensitizing orientations = 12, b = 0 and 1000 s/mm2) that effectively covered the whole brain in 45 slices with 1.6 × 1.6 × 3.0 mm resolution. To minimize blood flow and cerebrospinal fluid pulsation effects, scanning was gated with peripheral arterial pulse. Total scan time was approximately 11 min. An initial DTI data quality check ensured that any gradient directions with excessive motion artifacts or noise were identified and removed. Motion and eddy corrections were done by registering the diffusion-weighted images to a common non-diffusion-weighted image using a mutual information cost function as employed in the FLIRT toolbox (Jenkinson and Smith, 2001; Jenkinson et al., 2002; Greve and Fischl, 2009) provided in the FSL software suite (Smith et al., 2004; Woolrich et al., 2009; Jenkinson et al., 2012). We noted that only four participants (three with high ADHD-I and ADHD-HI symptom counts and one with a high ADHD-I symptom count) exhibited head motion excessive enough to warrant removing one gradient direction from all further analyses. No other relationship between head motion and ADHD symptom counts was noted. The diffusion tensor and scalar fractional anisotropy (FA), radial diffusivity (RD), mean diffusivity (MD), and axial diffusivity (AD) measures for voxels were calculated using tract-based spatial statistics (TBSS; Smith et al., 2007) within a mask created from a B0 image. These data were spatially normalized to a common Montreal Neurological Institute (MNI) space template using the FNIRT toolbox (Andersson et al., 2008). Resulting images were smoothed with a 4-mm full width at half-maximum Gaussian kernel. A mean FA image calculated from all 22 participants was subsequently used to mask single subject maps (FA > 0.20) to focus analysis on white matter regions.
2.3. Region-of-interest analysis
Thirteen tracts of interest were selected based on a review of the current ADHD DTI literature (Ashtari et al., 2005; Hamilton et al., 2008; Makris et al., 2008; Bechtel et al., 2009; Chao et al., 2009; Castellanos et al., 2009; Pavuluri et al., 2009; Silk et al., 2009; Cao et al., 2010; Davenport et al., 2010; Kobel et al., 2010; Konrad et al., 2010; Konrad et al., 2012; Tamm et al., 2012), including a recent meta-analysis (van Ewijk et al., 2012). We focused on the most replicated findings. Tracts were selected if ADHD-related FA differences were reported in at least three of the available studies (maximum number of studies reporting differences in a given region was five). A white matter parcellation map (Mori et al., 2008) was used to identify which aspects of our sample's TBSS skeleton were localized to our 13 white matter tracts of interest: middle cerebellar peduncle, corpus callosum-genu, corpus callosum-body, corpus callosum-splenium, right anterior limb of internal capsule, left anterior limb of internal capsule, right anterior corona radiata, left anterior corona radiata, right cingulum, right superior longitudinal fasciculus, left superior longitudinal fasciculus, right inferior fronto-occipital fasciculus, and left inferior fronto-occipital fasciculus. Mean and standard deviation values of the overlap between a given tract and a subject's TBSS skeleton were computed for each tract and each scalar DTI map (FA, RD, MD, and AD) for all subjects, individually.
2.4. Hypothesis testing
To test study aims, a series of multivariate general linear models (i.e., multiple regression) was used. This was a useful approach that asked whether the DTI data were generally associated in meaningful ways with ADHD-HI or ADHD-I symptom severity, which then prompted post hoc univariate tests to determine which of the relationships between either ADHD-HI and/or ADHD-I symptom severity and the 13 tracts examined produced that omnibus effect. We note, as a brief aside, that we also performed additional t-tests to insure that our measure of symptom severity was not in some way related to medication status (e.g., that those participants who reported taking ADHD medication had consistently higher or lower symptom severity than those who did not report taking medication). No such relationships were noted between either Inattentive or Hyperactive/Impulsive symptom counts and medication status (p > 0.1). The mean scalar DTI values for each tract (i.e., FA, RD, MD, and AD in separate analyses) were predicted by both Inattention and Hyperactivity/Impulsivity symptom counts. Each model included an age covariate. However, because the ADHD study population was highly skewed towards males and our sample was mostly male, no statistical correction was attempted for gender. Our primary goal using this approach was to identify specific white matter tracts whose microstructural integrity had a unique association with either Inattentive or Hyperactivity/Impulsivity symptom counts, or a shared association with both. The former would suggest specific tract abnormalities might underlie unique neurobiological basis for specific ADHD dimensions. For the latter, any significant statistical interactions within the regression framework might indicate that the expression of a shared neural liability for ADHD is greater for one of the two symptom domains. Significant multivariate omnibus F results (p < 0.05) prompted univariate post-hoc tests (p < 0.05, uncorrected) to determine which of the 13 tracts of interest was most responsible for any linear or interaction effects. Because our sample size is such that our results could be construed as preliminary, we chose to report results as being statistically significant even in the absence of either correcting for the number of DTI scalar measures at the multivariate level or the number of tracts of interest at the univariate level. Given that this is one of the first studies to consider the relationship between ADHD symptom severity and brain structure abnormalities, we felt it was important to be as inclusive as possible in reporting our results. As such, we also reported non-significant post hoc results having at least medium effect sizes (Cohen, 1988). We note, though, that we took care to ensure that the interpretation of results focused only on the findings supported by convincing statistical evidence.
3. Results
3.1. Fractional anisotropy
A significant multivariate interaction effect for Inattentive and Hyperactivity/Impulsivity symptom counts on FA measurements (F(20,5) = 9.026; p < 0.019) indicated that there were overall differences in how white integrity within the 13 tracts of interest was associated with ADHD clinical profile. Post-hoc tests revealed that this multivariate effect was due to symptom severity associations only with the right superior longitudinal fasciculus (F(4,5) = 10.072; p < 0.013; ηp2 = 0.890) (see Table 1 for summary of univariate interaction effects for all 13 tracts of interest). Fig. 1A depicts this statistical interaction graphically, where FA value versus Inattentive symptom count is shown in black and FA value versus Hyperactivity/Impulsivity symptom count is shown in gray. The best linear fit lines for each symptom count are also depicted. Because graphical depictions of these results raised the possibility that the effects might be due to data from a few subjects with more extreme ADHD symptom counts, we re-did the analyses after selectively omitting any seeming outliers, with no change in results.
Table 1. Summary of univariate interaction effects for all thirteen tracts of interest with both ADHD-I and ADHD-HI symptom counts.
Results are shown only for fractional anisotropy and radial diffusivity, as neither mean diffusivity nor axial diffusivity demonstrated any overall significant multivariate effect with ADHD symptom counts. The only tract with a significant post-hoc univariate interaction, the right superior longitudinal fasciculus, is highlighted in bold text.
| White matter tract | F | p | ηp2 | F | p | ηp2 |
|---|---|---|---|---|---|---|
| FA | RD | |||||
| Middle cerebellar peduncle | 0.453 | -- | -- | 0.000 | -- | -- |
| Corpus callosum – genu | 1.430 | -- | 0.534 | 1.981 | -- | 0.613 |
| Corpus callosum – body | 0.436 | -- | -- | 0.522 | -- | -- |
| Corpus callosum – splenium | 2.410 | -- | 0.658 | 2.283 | -- | 0.646 |
| Right anterior internal capsule | 0.357 | -- | -- | 0.057 | -- | -- |
| Left anterior internal capsule | 1.725 | -- | 0.580 | 2.014 | -- | 0.617 |
| Right anterior corona radiata | 0.475 | -- | -- | 0.386 | -- | -- |
| Left anterior corona radiata | 1.585 | -- | 0.559 | 0.820 | -- | 0.396 |
| Right cingulum | 1.067 | -- | 0.461 | 1.981 | -- | 0.613 |
| Right superior longitudinal fasciculus | 10.072 | 0.013 | 0.890 | 8.375 | 0.019 | 0.870 |
| Left superior longitudinal fasciculus | 3.101 | -- | 0.713 | 2.884 | -- | 0.698 |
| Right inferior fronto-occipital fasciculus | 1.960 | -- | 0.611 | 1.322 | -- | 0.514 |
| Left inferior fronto-occipital fasciculus | 1.989 | -- | 0.614 | 1.191 | -- | 0.488 |
Fig. 1.
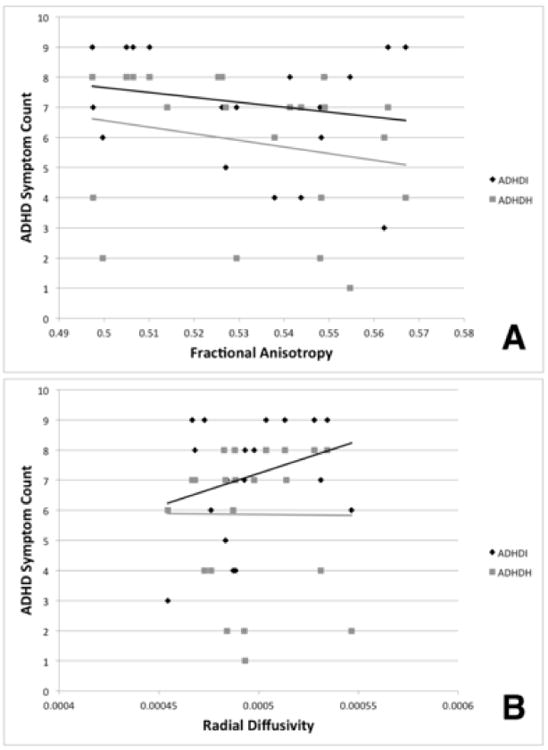
Interaction effects of the right superior longitudinal fasciculus white matter microstructure integrity with both ADHD symptom domain counts. A. Fractional anisotropy. B. Radial diffusivity. ADHD-I symptom counts are shown in black; ADHD-HI symptom counts are shown in gray. The solid lines depict the respective linear best fit lines for each DTI scalar measure and ADHD symptom count relationship.
Multivariate effects also were observed for the simple linear relationships of Inattentive symptom count (F(30,6) = 7.296; p < 0.010) and Hyperactivity/Impulsivity symptom count (F(25,5) = 10.413; p < 0.007). However, post-hoc tests did not identify any individual tracts that significantly accounted for this omnibus effect at conventional p < 0.05 significance thresholds when considering each symptom dimension separately. However, because it may be of interest to future studies, we note that almost all of the 13 tracts of interest had at least moderate-sized FA effect sizes when considering Inattentive symptoms alone or Hyperactivity/Impulsivity symptoms alone (Table 2).
Table 2. Summary of the univariate linear effects for all 13 tracts of interest.
As with Table 1, results are only shown for fractional anisotropy and radial diffusivity. Despite the medium to large effect sizes, no tract demonstrated a significant post-hoc linear association with either ADHD-I or ADHD-HI symptom counts.
| White matter tract | F | p | ηp2 | F | p | ηp2 | F | p | ηp2 | F | p | ηp2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | RD | |||||||||||
| ADHD-I | ADHD-H | ADHD-I | ADHD-H | |||||||||
| Middle cerebellar peduncle | 0.592 | -- | 0.415 | 0.490 | -- | 0.329 | 2.004 | -- | 0.706 | 3.110 | -- | 0.757 |
| Corpus callosum – genu | 1.656 | -- | 0.665 | 0.737 | -- | 0.424 | 2.627 | -- | 0.759 | 1.225 | -- | 0.550 |
| Corpus callosum – body | 0.442 | -- | 0.346 | 0.320 | -- | -- | 0.549 | -- | 0.397 | 0.286 | -- | -- |
| Corpus callosum – splenium | 0.673 | -- | 0.447 | 1.318 | -- | 0.569 | 0.874 | -- | 0.512 | 0.963 | -- | 0.490 |
| Right anterior internal capsule | 0.456 | -- | 0.354 | 1.259 | -- | 0.557 | 0.760 | -- | 0.477 | 1.787 | -- | 0.641 |
| Left anterior internal capsule | 0.614 | -- | 0.424 | 0.315 | -- | -- | 1.110 | -- | 0.571 | 0.557 | -- | 0.358 |
| Right anterior corona radiata | 0.404 | -- | 0.326 | 1.263 | -- | 0.558 | 0.852 | -- | 0.505 | 1.906 | -- | 0.656 |
| Left anterior corona radiata | 0.743 | -- | 0.471 | 0.658 | -- | 0.397 | 0.643 | -- | 0.435 | 0.394 | -- | -- |
| Right cingulum | 1.150 | -- | 0.580 | 0.875 | -- | 0.467 | 2.117 | -- | 0.718 | 1.421 | -- | 0.587 |
| Right superior longitudinal fasciculus | 2.717 | -- | 0.765 | 2.186 | -- | 0.686 | 1.123 | -- | 0.574 | 1.414 | -- | 0.586 |
| Left superior longitudinal fasciculus | 1.369 | -- | 0.622 | 0.393 | -- | -- | 0.877 | -- | 0.513 | 0.660 | -- | 0.398 |
| Right inferior fronto-occipital fasciculus | 0.828 | -- | 0.499 | 0.306 | -- | -- | 1.002 | -- | 0.546 | 0.642 | -- | 0.391 |
| Left inferior fronto-occipital fasciculus | 0.392 | -- | 0.320 | 0.160 | -- | -- | 0.352 | -- | -- | 0.309 | -- | -- |
3.2. Radial diffusivity
The radial diffusivity (RD) results largely paralleled those observed for FA. An overall multivariate effect of symptom counts and RD was noted for the interaction of Inattentive and Hyperactivity/Impulsivity symptoms (F(20,4) = 8.473; p < 0.021). Post-hoc tests revealed that only the right superior longitudinal fasciculus exhibited a significant univariate interaction effect (F(4,5) = 8.375; p < 0.019; ηp2 = 0.870) (Table 1). Fig. 1B depicts the statistical interaction of RD versus the two ADHD symptom counts. The RD value versus Inattentive symptom count is shown in black, while RD versus Hyperactivity/Impulsivity count is shown in gray. As with Fig. 1A, best linear fit lines are also shown to better illustrate the relationship. Again, re-evaluation after selectively omitting cases with extreme values did not alter the findings.
Overall multivariate simple linear effects of symptom counts on RD for all 13 tracts of interest were again observed for Inattentive (F(30,6) = 9.955; p < 0.004) and Hyperactivity/Impulsivity symptom domains (F(25,5) = 10.643; p < 0.006). As seen for FA, the majority of individual tracts exhibited at least medium effect sizes for linear associations with Inattentive symptom counts alone or Hyperactivity/Impulsivity symptom counts alone (Table 2).
3.3. Mean diffusivity
No overall multivariate effects were observed for the mean diffusivity data. Therefore, no post-hoc univariate tests were examined.
3.4. Axial diffusivity
No overall multivariate effects were observed for the axial diffusivity data. No post-hoc univariate tests were examined.
4. Discussion
The purpose of this study was to ask whether examining DTI-measured white matter miscrostructure in ADHD from a dimensional perspective might identify abnormalities specific to either Inattention or Hyperactivity/Impulsivity severity. The study produced several preliminary but meaningful findings. First, our analyses showed that FA and RD measures of white matter integrity were linearly linked to ADHD symptom severity. Moreover, the relationships between white matter microstructure and symptom severity appeared to differ for ADHD-I and ADHD-HI. Second, although the medium effect sizes seen for most measurements suggested this was a general phenomenon, univariate testing evidence was strong enough only to confirm these multivariate relationships specifically involved right superior longitudinal fasciculus. The right superior longitudinal fasciculus connects the superior/inferior parietal lobe with several regions in the frontal lobe, including the dorsolateral prefrontal cortex (Makris et al., 2005), making it an important anatomical connection among brain regions thought to constitute the dorsal visual attention stream (Ptak, 2012). Normal development of the right superior longitudinal fasciculus has been linked to better working memory performance (Petrides, 2005), reduced inattentiveness (Frye et al., 2010), better performance on tests of visual attention (Konrad et al., 2010), and better performance on tasks requiring sustained attention (Klarborg et al., 2013). Studies have also noted abnormal microstructural properties (e.g., reduced FA, increased MD, increased RD) in this tract in patients with schizophrenia (Guo et al., 2012; Nakamura et al., 2012), first episode psychosis patients (Colombo et al., 2012; Ruef et al., 2012), adults with childhood ADHD (Makris et al., 2008), children with ADHD (Hamilton et al., 2008; Silk et al., 2009), and the unaffected siblings of adults with ADHD (Lawrence et al., 2013). There is considerable evidence, then, to support an argument that abnormal development of the right superior longitudinal fasciculus may be a general predictor of ADHD, as overall integrity of this tract appears to support many of the cognitive functions observed to be deficient in ADHD (McLean et al., 2004; Martinussen et al., 2005; Willcutt et al., 2005).
The current study also sought to extend beyond simply considering measures of FA, showing that RD measurements -- possibly indicative of abnormal myelination as seen in animal models (Song et al., 2002) -- are specifically linked to ADHD-I symptom severity. ADHD-HI symptoms, in contrast, showed no relationship to RD in this tract (Fig. 1B). Myelin integrity is known to be important for the transmission of neural signals along axons. Therefore, it is possible that the relationship between Inattention symptoms and abnormal right superior longitudinal fasciculus microstructure might underlie many of the cognitive problems observed in ADHD, a hypothesis that can be directly tested in future research. Although the interpretation is speculative, it comports with observations (Willcutt et al., 2012) that patients diagnosed with Predominantly Hyperactive/Impulsive ADHD have been sometimes seen to exhibit fewer cognitive deficiencies than those patients diagnosed with either ADHD-C or ADHD-I (i.e., those displaying a greater number of Inattentive symptoms). Although FA also was linked to ADHD symptom dimensions in both multivariate tests and specifically to the right superior longitudinal fasciculus, interaction tests for specific tracts revealed a different type of relationship between white matter and ADHD-I and ADHD-HI symptoms. In contrast to RD measurements, both ADHD-HI and ADHD-I symptom severity was linked to less coherent white matter microstructure, with this relationship being slightly stronger for ADHD-HI symptoms. This underscores the differences among the commonly examined DTI metrics, arguing for the specificity of examining RD, AD, and MD values in addition to FA in order to uncover more specific and meaningful findings. Such detail will become increasingly more important as greater specificity of the genetic and neurophysiological underpinnings of ADHD becomes clearer with future study.
Although this study is an important step forward for efforts to understand dimensional conceptualization of neurobiological abnormality in ADHD and provides preliminary results on which future studies can be based, it has limitations, most notably the small sample size available for this initial inquiry. Although we could confirm that the major tracts most frequently found to be abnormal in previous ADHD studies were linearly associated with ADHD symptom severity – and crucially, that these associations differed for ADHD-I and ADHD-HI – statistical power was inadequate to have confidence in interpreting any but the findings for the right longitudinal fasciculus. However, we did observe a number of additional tracts that showed medium to large linear and interaction effects. These findings confirm the basic idea of a dimensional relationship between brain structure and symptom severity in ADHD, a relationship in need of further exploration. For instance, findings of increased FA in several of these tracts, including the right cingulum, corpus callosum, and left internal capsule, have also been linked to better performance on a number of commonly administered cognitive tasks, such as the continuous performance test (Mabbott et al., 2006; Takahashi et al., 2010). Given the importance of understanding dimensional models of psychiatric disorders with respect to their implications for genetic and physiological etiology, we believe that these findings are meaningful and will serve to guide future research. They confirm that using DTI scalar measures to examine the neurobiological differences underlying ADHD symptom dimensions may be a fruitful area of future study. Also, the effect sizes and relationships reported in this study can be used to plan more effective studies that provide clearer answers about the relationships among brain structure, cognition, and ADHD symptom expression. Another limitation of the study is our inability to examine gender effects. Because our patients were predominantly male, we were unable to determine whether our results could be driven by the small subset of female participants, or if the lack of apparent gender effects was simply due to a lack of statistical power to observe anything but large effects. Although understandably difficult in this population, future studies should specifically look at gender effects in white matter microstructure abnormalities as related to ADHD symptomology in more gender-balanced samples. Our small sample size also meant that we were under-powered to provide any conclusive observations on how maturational effects, IQ effects, or long-term effects of ADHD medications might have affected these relationships. Arguably, these topics are far beyond the scope of this initial effort and would require a much larger study to provide clear answers. However, our approach to analyses gave confidence that the current findings were unrelated to age, gender, or current medication status, all factors that have been implicated in previous neurocognitive research to be important in understanding these complex relationships.
FA measures of white matter showed linear relationship with ADHD symptom severity.
RD measures of white matter showed linear relationship with ADHD symptom severity.
RD in superior longitudinal fasciculus was linked to Inattentive symptoms.
RD in superior longitudinal fasciculus not linked to Hyperactivity/Impulsivity symptoms.
Acknowledgments
This work was funded by the National Institute of Mental Health grant K23MH070036.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Publishing; Washington, DC: 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed (DSM-5) American Psychiatric Publishing; Washington, DC: 2013. [Google Scholar]
- Andersson J, Smith S, Jenkinson M. FNIRT -- FMRIB's non-linear registration tool. Proceedings of the 14th Annual Meeting of the Organization for Human Brain Mapping; Melbourne, Australia. 2008. p. 496. [Google Scholar]
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, Rhinewine J, Kane JM, Adesman A, Milanaik R, Maytal J, Diamond A, Szeszko P, Ardekani BA. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biological Psychiatry. 2005;57:448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Baeyens D, Roeyers H, Walle JV. Subtypes of attention-deficit/hyperactivity disorder (ADHD): distinct or related disorders across measurement levels? Child Psychiatry & Human Development. 2006;36:403–417. doi: 10.1007/s10578-006-0011-z. [DOI] [PubMed] [Google Scholar]
- Bechtel N, Kobel M, Penner IK, Klarhofer M, Scheffler K, Opwis K, Weber P. Decreased fractional anisotropy in the middle cerebellar peduncle in children with epilepsy and/or attention deficit/hyperactivity disorder: a preliminary study. Epilepsy and Behavior. 2009;15:294–298. doi: 10.1016/j.yebeh.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Cantwell DP. Attention deficit disorder: a review of the past 10 years. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35:978–987. doi: 10.1097/00004583-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Cao Q, Sun L, Gong G, Lv Y, Cao X, Shuai L, Zhu C, Zang Y, Wang Y. The macrostructural and microstructural abnormalities of corpus callosum in children with attention deficit/hyperactivity disorder: a combined morphometric and diffusion tensor MRI study. Brain Research. 2010;1310:172–180. doi: 10.1016/j.brainres.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Carmona S, Proal E, Hoekzema EA, Gispert JD, Picado M, Moreno I, Soliva JC, Bielsa A, Rovira M, Hilferty J, Bulbena A, Casas M, Tobena A, Vilarroya O. Ventro-striatal reductions underpin symptoms of hyperactivity and impulsivity in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;66:972–977. doi: 10.1016/j.biopsych.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Kelly C, Milham MP. The restless brain: attention-deficit hyperactivity disorder, resting-state functional connectivity, and intrasubject variability. Canadian Journal of Psychiatry. 2009;54:665–672. doi: 10.1177/070674370905401003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao TC, Chou MC, Yang P, Chung HW, Wu MT. Effects of interpolation methods in spatial normalization of diffusion tensor imaging data on group comparison of fractional anisotropy. Magnetic Resonance Imaging. 2009;27:681–690. doi: 10.1016/j.mri.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge Academic; New York: 1988. [Google Scholar]
- Colombo RR, Schaufelberger MS, Santos LC, Duran FL, Menezes PR, Scazufca M, Busatto GF, Zanetti MV. Voxelwise evaluation of white matter volumes in first-episode psychosis. Psychiatry Research: Neuroimaging. 2012;202:198–205. doi: 10.1016/j.pscychresns.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Karatekin C, White T, Lim KO. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Research: Neuroimaging. 2010;181:193–198. doi: 10.1016/j.pscychresns.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue BE, Burgess GC, Willcutt EG, Bidwell LC, Ruzic L, Banich MT. Symptom-correlated brain regions in young adults with combined-type ADHD: their organization, variability, and relation to behavioral performance. Psychiatry Research: Neuroimaging. 2010;182:96–102. doi: 10.1016/j.pscychresns.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edel MA, Enzi B, Witthaus H, Tegenthoff M, Peters S, Juckel G, Lissek S. Differential reward processing in subtypes of adult attention deficit hyperactivity disorder. Journal of Psychiatric Research. 2013;47:350–356. doi: 10.1016/j.jpsychires.2012.09.026. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cerebral Cortex. 2007;17:2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Stauber J, Schaaff N, Koutsouleris N, Scheuerecker J, Ewers M, Omerovic M, Opgen-Rhein M, Hampel H, Reiser M, Moller HJ, Meisenzahl E. Amygdala reduction in patients with ADHD compared with major depression and healthy volunteers. Acta Psychiatrica Scandinavica. 2010;121:111–118. doi: 10.1111/j.1600-0447.2009.01489.x. [DOI] [PubMed] [Google Scholar]
- Frye RE, Hasan K, Malmberg B, Desouza L, Swank P, Smith K, Landry S. Superior longitudinal fasciculus and cognitive dysfunction in adolescents born preterm and at term. Developmental Medicine & Child Neurology. 2010;52:760–766. doi: 10.1111/j.1469-8749.2010.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Harvey J, Quick C, Scharer I, Harris G. DSM-IV AD/HD: confirmatory factor models, prevalence, and gender and age differences based on parent and teacher ratings of Australian primary school children. Journal of Child Psychology and Psychiatry. 1999;40:265–274. [PubMed] [Google Scholar]
- Gong G, Rosa-Neto P, Carbonell F, Chen ZJ, He Y, Evans AC. Age- and gender-related differences in the cortical anatomical network. Journal of Neuroscience. 2009;29:15684–15693. doi: 10.1523/JNEUROSCI.2308-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Liu Z, Gao K, Xiao C, Chen H, Zhao J. Right lateralized white matter abnormalities in first-episode, drug-naive paranoid schizophrenia. Neuroscience Letters. 2012;531:5–9. doi: 10.1016/j.neulet.2012.09.033. [DOI] [PubMed] [Google Scholar]
- Hamilton LS, Levitt JG, O'Neill J, Alger JR, Luders E, Phillips OR, Caplan R, Toga AW, McCracken J, Narr KL. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 2008;19:1705–1708. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam N. Categorical versus dimensional models of mental disorder: the taxometric evidence. Australian & New Zealand Journal of Psychiatry. 2003;37:696–704. doi: 10.1080/j.1440-1614.2003.01258.x. [DOI] [PubMed] [Google Scholar]
- Helzer JE, Bucholz KK, Bierut LJ, Regier DA, Schuckit MA, Guth SE. Should DSM-V include dimensional diagnostic criteria for alcohol use disorders? Alcoholism: Clinical and Experimental Research. 2006a;30:303–310. doi: 10.1111/j.1530-0277.2006.00028.x. [DOI] [PubMed] [Google Scholar]
- Helzer JE, Kraemer HC, Krueger RF. The feasibility and need for dimensional psychiatric diagnoses. Psychological Medicine. 2006b;36:1671–1680. doi: 10.1017/S003329170600821X. [DOI] [PubMed] [Google Scholar]
- Helzer JE, van den Brink W, Guth SE. Should there be both categorical and dimensional criteria for the substance use disorders in DSM-V? Addiction. 2006c;101(Suppl 1):17–22. doi: 10.1111/j.1360-0443.2006.01587.x. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Heath AC, Madden PF, Reich W, Bucholz KK, Slutske W, Bierut LJ, Neuman RJ, Todd RD. Latent class and factor analysis of DSM-IV ADHD: a twin study of female adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37:848–857. doi: 10.1097/00004583-199808000-00015. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L, Sanchez-Pena J, Miller AM, Chakravarty MM, Klahr K, Durkin K, Greenhill LL, Peterson BS. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. The American Journal of Psychiatry. 2010;167:397–408. doi: 10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of Abnormal Child Psychology. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Klarborg B, Skak Madsen K, Vestergaard M, Skimminge A, Jernigan TL, Baare WF. Sustained attention is associated with right superior longitudinal fasciculus and superior parietal white matter microstructure in children. Human Brain Mapping. 2013;34(12):3216–3232. doi: 10.1002/hbm.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobel M, Bechtel N, Specht K, Klarhofer M, Weber P, Scheffler K, Opwis K, Penner IK. Structural and functional imaging approaches in attention deficit/hyperactivity disorder: does the temporal lobe play a key role? Psychiatry Research: Neuroimaging. 2010;183:230–236. doi: 10.1016/j.pscychresns.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Konrad A, Dielentheis TF, El Masri D, Bayerl M, Fehr C, Gesierich T, Vucurevic G, Stoeter P, Winterer G. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. European Journal of Neuroscience. 2010;31:912–919. doi: 10.1111/j.1460-9568.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- Konrad A, Dielentheis TF, El Masri D, Dellani PR, Stoeter P, Vucurevic G, Winterer G. White matter abnormalities and their impact on attentional performance in adult attention-deficit/hyperactivity disorder. European Archives of Psychiatry and Clinical Neuroscience. 2012;262:351–360. doi: 10.1007/s00406-011-0251-1. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Carlson CL. Validity of the diagnostic category of attention deficit disorder without hyperactivity: a review of the literature. Journal of Learning Disabilities. 1991;24:110–120. doi: 10.1177/002221949102400208. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSM-IV Subtypes of ADHD from preschool through elementary school. Archives of General Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- Larsson H, Lichtenstein P, Larsson JO. Genetic contributions to the development of ADHD subtypes from childhood to adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:973–981. doi: 10.1097/01.chi.0000222787.57100.d8. [DOI] [PubMed] [Google Scholar]
- Lawrence KE, Levitt JG, Loo SK, Ly R, Yee V, O'Neill J, Alger J, Narr KL. White matter microstructure in subjects with attention-deficit/hyperactivity disorder and their siblings. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:431–440 e434. doi: 10.1016/j.jaac.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Noseworthy M, Bouffet E, Laughlin S, Rockel C. White matter growth as a mechanism of cognitive development in children. Neuroimage. 2006;33:936–946. doi: 10.1016/j.neuroimage.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, Brown AB, Bush G, Monuteaux MC, Caviness VS, Kennedy DN, Seidman LJ. Attention and executive systems abnormalities in adults with childhood ADHD: a DT-MRI study of connections. Cerebral Cortex. 2008;18:1210–1220. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Maser JD, Patterson T. Spectrum and nosology: implications for DSM-V. Psychiatric Clinics of North America. 2002;25:855–885. viii–ix. doi: 10.1016/s0193-953x(02)00022-9. [DOI] [PubMed] [Google Scholar]
- McCarthy H, Skokauskas N, Frodl T. Identifying a consistent pattern of neural function in attention deficit hyperactivity disorder: a meta-analysis. Psychological Medicine. 2013:1–12. doi: 10.1017/S0033291713001037. [DOI] [PubMed] [Google Scholar]
- McHugh PR, Slavney PR. The Perspectives of Psychiatry. 2nd. The Johns Hopkins University Press; Baltimore, MD: 1998. [Google Scholar]
- McLean A, Dowson J, Toone B, Young S, Bazanis E, Robbins TW, Sahakian BJ. Characteristic neurocognitive profile associated with adult attention-deficit/hyperactivity disorder. Psychological Medicine. 2004;34:681–692. doi: 10.1017/S0033291703001296. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kawasaki Y, Takahashi T, Furuichi A, Noguchi K, Seto H, Suzuki M. Reduced white matter fractional anisotropy and clinical symptoms in schizophrenia: a voxel-based diffusion tensor imaging study. Psychiatry Research: Neuroimaging. 2012;202:233–238. doi: 10.1016/j.pscychresns.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Neuman RJ, Todd RD, Heath AC, Reich W, Hudziak JJ, Bucholz KK, Madden PA, Begleiter H, Porjesz B, Kuperman S, Hesselbrock V, Reich T. Evaluation of ADHD typology in three contrasting samples: a latent class approach. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:25–33. doi: 10.1097/00004583-199901000-00016. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Stawicki JA, Sachek J. Evaluating the endophenotype model of ADHD neuropsychological deficit: results for parents and siblings of children with ADHD combined and inattentive subtypes. Journal of Abnormal Psychology. 2004;113:614–625. doi: 10.1037/0021-843X.113.4.614. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Yang S, Kamineni K, Passarotti AM, Srinivasan G, Harral EM, Sweeney JA, Zhou XJ. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;65:586–593. doi: 10.1016/j.biopsych.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Sex differences in the growth of white matter during adolescence. Neuroimage. 2009;45:1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist. 2012;18:502–515. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- Qiu A, Rifkin-Graboi A, Tuan TA, Zhong J, Meaney MJ. Inattention and hyperactivity predict alterations in specific neural circuits among 6-year-old boys. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51:632–641. doi: 10.1016/j.jaac.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Rodrak S, Wongsawat Y. EEG brain mapping and brain connectivity index for subtypes classification of attention deficit hyperactivity disorder children during the eye-opened period; Conference Proceedings: 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2013. pp. 7400–7403. [DOI] [PubMed] [Google Scholar]
- Ruef A, Curtis L, Moy G, Bessero S, Badan Ba M, Lazeyras F, Lovblad KO, Haller S, Malafosse A, Giannakopoulos P, Merlo M. Magnetic resonance imaging correlates of first-episode psychosis in young adult male patients: combined analysis of grey and white matter. Journal of Psychiatry & Neuroscience. 2012;37:305–312. doi: 10.1503/jpn.110057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DK, Iacono WG, McGue MK. Attention-deficit hyperactivity disorder dimensions: a twin study of inattention and impulsivity-hyperactivity. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:745–753. doi: 10.1097/00004583-199706000-00010. [DOI] [PubMed] [Google Scholar]
- Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Human Brain Mapping. 2009;30:2757–2765. doi: 10.1002/hbm.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, Behrens TE. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nature Protocols. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Schulz KP, Fan J, Tang CY, Newcorn JH. Event-related FMRI of inhibitory control in the predominantly inattentive and combined subtypes of ADHD. Journal of Neuroimaging. 2009;19:205–212. doi: 10.1111/j.1552-6569.2008.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Swanson JM. School-based Assessments and Interventions for ADD Students. KC Publishing; Irvine, CA: 1992. [Google Scholar]
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, Taylor E, Casey BJ, Castellanos FX, Wadhwa PD. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychology Review. 2007;17:39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Wigal T, Lakes K. DSM-V and the future diagnosis of attention-deficit/hyperactivity disorder. Current Psychiatry Reports. 2009;11:399–406. doi: 10.1007/s11920-009-0060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Iwamoto K, Fukatsu H, Naganawa S, Iidaka T, Ozaki N. White matter microstructure of the cingulum and cerebellar peduncle is related to sustained attention and working memory: a diffusion tensor imaging study. Neuroscience Letters. 2010;477:72–76. doi: 10.1016/j.neulet.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Tamm L, Barnea-Goraly N, Reiss AL. Diffusion tensor imaging reveals white matter abnormalities in attention-deficit/hyperactivity disorder. Psychiatry Research: Neuroimaging. 2012;202:150–154. doi: 10.1016/j.pscychresns.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM. Diffusion imaging, white matter, and psychopathology. Annual Review of Clinical Psychology. 2011;7:63–85. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews. 2012;36:1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Volk HE, Henderson C, Neuman RJ, Todd RD. Validation of population-based ADHD subtypes and identification of three clinically impaired subtypes. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2006;141B:312–318. doi: 10.1002/ajmg.b.30299. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, Loo SK, Carlson CL, McBurnett K, Lahey BB. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. Journal of Abnormal Psychology. 2012;121:991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo BS, Rey JM. The validity of the DSM-IV subtypes of attention-deficit/hyperactivity disorder. Australian & New Zealand Journal of Psychiatry. 2005;39:344–353. doi: 10.1080/j.1440-1614.2005.01580.x. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]


