Abstract
Objective
Studies in mice suggest that cilostazol, an FDA-approved phosphodiesterase 3 (PDE3) inhibitor, might have a contraceptive effect within the approved dose range. We sought to evaluate the potential contraceptive effects of cilostazol in a non-human primate model.
Study Design
Adult female rhesus macaques were stimulated to develop multiple preovulatory follicles by administering human recombinant gonadotropins and oocytes were collected by follicle aspiration 36 hours after an ovulatory stimulus (human chorionic gonadotropin; hCG). Monkeys received no further treatment (controls), or the PDE3 inhibitor cilostazol at the maximum approved human dose of 100 mg twice daily starting 6 days prior to follicle aspiration. Recovered oocytes were scored for meiotic stage [germinal vesicle (GV) intact, germinal vesicle breakdown (GVDB)], and metaphase II stage oocyte were fertilized in vitro and observed for normal embryo development.
Results
Similar proportions of GV stage oocytes were recovered from control (27% ± 4) and cilostazol (27% ± 9) treated females, and the proportion of embryos that developed into blastocysts was also similar for both groups (7% ± 5 control vs 15% ± 8 cilostazol).
Conclusion
Oral dosing of cilostazol tablets during controlled ovarian stimulation protocols did not prevent oocyte maturation or embryo development in macaques.
Implications
Since administration of the maximum approved human dose of cilostazol (an FDA-approved PDE3 inhibitor) to macaques did not prevent oocyte maturation or fertilization, it is not likely that this dose would be contraceptive in women.
Keywords: non-hormonal contraception, cAMP, germinal vesicle, non-human primate, meiosis
1. INTRODUCTION
Interference with the regulation of meiosis represents one strategy for non-hormonal contraception. Cyclic adenosine 3′,5′-monophosphate (cAMP) has been established as a critical second messenger that, when elevated, maintains the immature oocyte in prophase I arrest of meiosis [1]. Within a few hours after the luteinizing hormone (LH) surge, gap junctions between cumulus granulosa cells surrounding the oocyte close, depleting the supply of cyclic guanosine 3′,5′-monophosphate (cGMP) to the oocyte [2]. With intra-oocyte levels of cGMP low, phosphodiesterase (PDE) 3A selectively targets cAMP for hydrolytic reduction, lowering its concentration [3] and this initiates a series of events that results in breakdown of the germinal vesicle (GVBD) and progression to metaphase II (MII) of meiosis, a stage where the mature ova arrests until fertilization [4].
Specific PDE3 inhibitors have been shown to prevent the resumption of meiosis both in vitro and in vivo in a number of species including humans [5], without affecting ovulation or the development of the corpus luteum [6, 7]. Socially-housed breeding groups of adult female cynomolgus monkeys treated with the specific PDE3 inhibitor ORG9935, did not become pregnant when the serum level of the drug exceeded 300 nmol/L [8]. However, poor bioavailability of this compound and non-target side effects (hypotension, nausea) were limiting factors preventing development of ORG9935 as a contraceptive.
Recently, Li et al [9] compared the FDA approved PDE3 inhibitor cilostazol (Pletal®) to ORG9935 in a rodent model. CD-1 mice received a single 300 mg/kg oral dose of either cilostazol or ORG9935 agent during gonadotropin-stimulated cycles and oocytes were recovered from the ampulla 2 hours after an ovulatory stimulus. Results with cilostazol treatment (100% germinal vesicle (GV) intact) were superior to those observed with ORG9935 (83.5% GV) [9]. In a subsequent study by Albarzanchi et al, mice administered cilostazol failed to become pregnant but maintained normal blood pressure values throughout treatment [9, 10].
Although an infertility effect is not described in the labeling for Pletal®, given the reported block in mouse oocyte meiosis, we evaluated cilostazol as a potential inhibitor of meiosis in rhesus monkey oocytes. The findings using rhesus macaques were compared to a parallel cilostazol and control treatment study using mice.
2. MATERIALS AND METHODS
2.1 Animals and Controlled Ovarian Stimulation
All study protocols and experiments were performed after approval from and in strict accordance to the Oregon Health & Science University Institutional Animal Care and Use Committee and followed the National Institute of Health’s Guide for the Care and Use of Laboratory Animals.
Monkeys
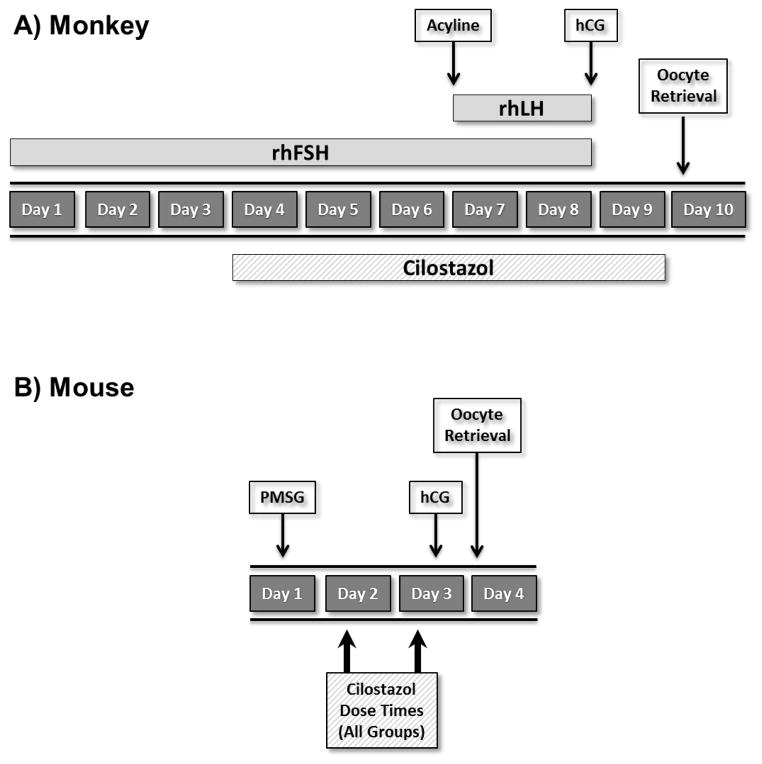
The general care and housing of rhesus monkeys at the Oregon National Primate Research Center (ONPRC) has been previously described [11]. Beginning days 1–4 of menses, adult females rhesus macaques (n=6) received 30 IU recombinant human FSH intramuscularly (IM) twice daily (BID) for 8 days (Organon, West Orange, NJ) with 30 IU recombinant human LH IM BID added on days 7 and 8 (Serono Reproductive Biology Institute, Rockland, MA). On day 7 animals also received 0.1 mg/kg of the gonadotropin releasing hormone antagonist, acyline (NICHD Contraceptive Discovery and Development Branch, Bethesda, MD) subcutaneously to prevent an endogenous LH surge (Figure 1A). Response to gonadotropins was monitored daily by measuring serum concentrations of estradiol (E2) and progesterone (P4) using the Roche E-170 clinical electrochemiluminescent immunoassay platform (Roche Diagnostics, Indianapolis, IN, USA). The assay sensitivity is 5 pg/mL for E2 and 0.03 ng/mL for P4; inter- and intra-assay variation is less than 6%. On day 8, monkeys received 1000 IU recombinant human chorionic gonadotropin (hCG: Ovidrel®, EMD Serono, Rockland, MA) IM as an ovulatory stimulus.
Figure 1.
Experimental design used in A) monkeys and B) mouse. PMSG = pregnant mare serum gonadotropin; rhFSH = recombinant human FSH; rhLH = recombinant human LH; hCG = human chorionic gonadotrophin.
Mice
Mice were maintained on a 12 hour light/dark cycle in conventional polycarbonate shoebox caging with access to food and water ad libitum. B6;129 female mice (n=12) were given a single intraperitoneal dose of 5 IU pregnant mare serum gonadotropin (PMSG: National Hormone and Peptide Program, Torrance, CA) to stimulate ovarian follicular development. An ovulatory dose of 5 IU hCG (intraperitoneal; Sigma-Aldrich, St. Louis, MO) was administered 48 hours later to promote maturation of follicular cells and induce ovulation (Figure 1B).
2.2 Treatment with the PDE3 inhibitor cilostazol
Monkeys
During the gonadotropin stimulation protocol, monkeys either received no further treatment (controls, n=3), or oral administration of the PDE3 inhibitor (n=3) cilostazol (Pletal®, Otsuka Pharmaceutical, Princeton, NJ) in a 100 mg tablet twice daily for 6 days beginning on day 4 of the COS protocol. This represents the maximum total dose approved for use in humans. For the approximately 6.3 kg monkeys used in our study this dose was 15.9 mg/kg, more than 10 fold greater by weight than the equivalent dose for a 75 kg woman (1.3 mg/kg). The last dose of cilostazol was within 12 hours of follicle aspiration and oocyte recovery. Oral dosing was performed under light sedation with 40–50 mg of ketamine delivered intramuscularly by ONPRC clinical staff to facilitate assisted swallowing of the cilostazol tablet while rinsing down the back of the throat with juice or water.
Mice
Animals were treated as described by Li et al. [9] and given an oral dose of 50 (n=3) or 300 (n=3) mg/kg cilostazol (Sigma-Aldrich) in DMSO 46 hours after administration of PMSG (2 hours prior to hCG administration). To insure that timing of drug administration was not a factor in our results, we included an additional treatment group dosing animals twice with 300 mg/kg cilostazol, once at 24 hours and again at 46 hours post PMSG (n=3).
2.3 Oocyte collection, culture, and in vitro fertilization
Monkeys
Anesthetized monkeys underwent follicle aspiration via dual puncture laparoscopy 36 hours after the administration of hCG. Large (> 4 mm) antral follicles on both ovaries were aspirated using a 22 gauge needle into a physiologic buffered solution of TALP-HEPES with 0.3% bovine serum albumin (BSA). Oocytes were sorted from pooled follicular contents and remaining cumulus cells were removed by transient exposure to 1% hyaluronidase (Sigma-Aldrich) in TALP-HEPES with 0.3% BSA[12]. Cumulus-free oocytes were immediately categorized into the following meiotic stages: germinal vesicle (GV; immature, meiosis not resumed), metaphase I (MI; maturing), or metaphase II (MII; mature, 2nd meiotic arrest and fertilization competent). Oocytes with degenerated cytoplasm or evidence of vacuole formation were not included in the results. Following the initial assessment, all oocytes were washed in TALP+0.3% BSA medium [12] and maintained for 4 hours at 38°C with 5% CO2 in humidified air. Standard in vitro fertilization (IVF) was performed as previously described [11] and all mature MII ova were inseminated in 100 μl drops of TALP+0.3% BSA with fresh collected sperm provided through the ONPRC ART Core and cultured for 18 hours. Following IVF, presumed zygotes were washed in HECM9 medium and cultured in a humidified 6/5/89 proportional mixture of CO2/O2/N2 gas [12]. At 36 hours post insemination, embryos were observed for evidence of fertilization indicated by the first mitotic cell division to the 2-cell stage. The embryos were cultured for 7–8 days until blastocyst formation (blastocoel clearly visible) or developmental arrest.
Mice
Fifteen hours after the hCG injection, mice were sacrificed by CO2 inhalation and cervical dislocation. Ovulated oocytes were immediately recovered from the ampulla region of the fallopian tube into EmbryoMax® M2 buffered collection medium (M2: EMD Millipore, Temecula, CA) with 200 μM 1-methyl-3-(2-methylpropyl)-7H-purine-2,6-dione (IBMX: Sigma-Aldrich) to prevent any recovered GV stage oocytes from resuming meiosis before evaluation. Cumulus cells were removed by quickly pipetting the oocytes through a small bore (100 μm) glass pipette in M2+IBMX and 300 μg/ml hyaluronidase (Sigma-Aldrich) and then immediately washed into fresh M2+IBMX. Oocytes were evaluated for meiotic resumption using the same criteria as the monkey and those with MI and MII configuration were reported as a combined GVBD rate, comparable to the reporting format in Li, et al. [9].
2.4 Statistical Analysis
Descriptive statistics are reported for the maturation status of oocytes recovered after follicle aspiration, and for the proportion that underwent normal fertilization and embryo development. For cilostazol to be an effective contraceptive, treatment would need to inhibit GVBD in all oocytes and/ or prevent fertilization. Therefore the anticipated effect size would be very large. For this pilot study, a sample size of 3 animals per treatment group was selected as the minimum needed to exclude biologic variability in the effect. No hypothesis tests were performed.
3. RESULTS
3.1 Macaque experiments
The effects of in vivo exposure to cilostazol on oocyte maturation in macaques during controlled ovarian stimulation cycles are summarized in Table 1. Although variability in in the number of recovered oocytes and maturation stage was seen between animals, most had progressed to GVBD, with the mean (±SEM) proportion arrested at the GV stage similar between control (27% ±4) and cilostazol (27% ±9) treated females. Although the proportion of oocytes collected at the MII stage (53% cilostazole vs. 28% control), that reached the two-cell stage following fertilization (45% vs. 60%), and that progressed to blastocyst (15% vs 7%) differed between groups, there was no suggestion of a major effect of cilostazol treatment.
Table 1.
Assessment of the stage of meiosis [GV: germinal vesicle; MI: metaphase I; MII: metaphase II] and embryo development [Fert: fertilized embryo (at least 2-cell stage); Blast: blastocyst] of rhesus monkey oocytes aspirated from follicles after a controlled ovarian stimulation. The treatment group received the PDE3 inhibitor cilostazol during the last 6 days of the stimulation protocol while the Control group received no additional treatment. The total number of oocytes recovered from each animal (# recovered) and fertilized (# fertilized) differs as only those oocytes at MII underwent in vitro fertilization.
| Animal ID | Group | # recovered | %GV | %MI | %MII | # fertilized | %Fert | %Blast |
|---|---|---|---|---|---|---|---|---|
| 001 | Control | 26 | 23 | 54 | 23 | 20 | 55 | 0 |
| 002 | Control | 84 | 36 | 19 | 45 | 60 | 52 | 17 |
| 003 | Control | 47 | 23 | 60 | 17 | 34 | 74 | 3 |
|
| ||||||||
| mean | 27 | 44 | 28 | 60 | 7 | |||
| SEM | ±4 | ±13 | ±9 | ±7 | ±5 | |||
|
| ||||||||
| 004 | CIL | 23 | 35 | 26 | 39 | 15 | 40 | 27 |
| 005 | CIL | 44 | 36 | 25 | 39 | 22 | 27 | 18 |
| 006 | CIL | 11 | 9 | 9 | 82 | 9 | 67 | 0 |
|
| ||||||||
| mean | 27 | 20 | 53 | 45 | 15 | |||
| SEM | ±9 | ±5 | ±14 | ±12 | ±8 | |||
3.2 Effects of cilostazol on in vivo oocyte maturation – mouse studies
There was no evidence of an effect of cilostazol treatment on in vivo oocyte maturation in B6;129 mice; 100% (90/90) of oocytes recovered from the ampulla of control mice were GVBD, comparable to the proportions recovered from mice treated with 50 (109/111, 98%) or 300 (116/116, 100%) mg/kg of cilostazol administered 46 hours after PMSG, or 300 mg/kg administered twice (24 and 46 hours after PMSG) (98% GVBD, 100/102).
4. DISCUSSION
Interference with the regulation of oocyte maturation has the potential to be a highly effective method of non-hormonal contraception. A variety of PDE3 inhibitors have been shown to inhibit GVBD both in vitro and in vivo in a number of species [6, 7, 13]. Furthermore, proof of concept for this contraceptive strategy in a nonhuman primate has been established; female macaques social housed in breeding groups failed to become pregnant during treatment with the PDE3 inhibitor ORG9935 when drug levels exceeded300 nmol/L [8].. Unfortunately, poor solubility and bioavailability of the ORG9935 compound led to adverse effects with high drug levels, and pregnancy when levels became low
Recently the PDE3 inhibitor cilostazol (Pletal®), which is FDA-approved for treatment of intermittent claudication, was reported to have a contraceptive effect in rodents with no reported unfavorable side effects [9, 10]. However, these data conflict with information from the prescribing information for Pletal® detailing pregnancy results from developmental toxicity studies in rats (1000 mg/kg/day) and rabbits (150 mg/kg/day) at doses far above those approved in humans. Given that cilostazol has a favorable pharmacokinetic profile and is FDA-approved, we performed this pilot study to explore its ability to inhibit meiosis in a nonhuman primate to resolve these conflicting reports and evaluate its contraceptive potential for women.
We followed the same controlled ovarian stimulation protocols we used in our prior studies evaluating ORG9935 in rhesus macaques [7]. We treated rhesus monkeys with cilostazol 100 mg (the maximum FDA-approved human dose) tablets twice daily for 6 days prior to follicle aspiration and observed no effect on oocyte maturation, fertilization, or embryo development. Although a major weakness of this pilot study is small sample size and insufficient power to demonstrate a statistically significant difference between treatment and control, to be effective as a contraceptive, the inhibition of oocyte maturation or fertilization would need to be close to 100%. Since we observed no trend toward meiotic inhibition in the cilostazol-treated macaques, further replications at this dose are not justified given the limited availability and expense of the nonhuman primate model.
Although we did not obtain plasma levels of cilostazol in this study, we do not believe that our dose or dosing schedule was inadequate. In adult humans, receiving 100 mg oral doses of cilostazol twice daily, steady state blood levels reach about 800 ng/mL (2162 nmol/L) [14], well above the levels of the PDE 3 inhibitor ORG9935 (300 nmol/L) found to be contraceptive in non-human primates [8]. We administered the maximum human dose to rhesus monkeys, and did not adjust the dose for weight; cilostazol was administered to the monkeys at over 10-times the recommended therapeutic dose for humans.
Given the lack of an observed effect on oocyte maturation and blastocyst formation in macaques, we treated mice using the same cilostazol dosing strategy reported by Li et al. [9] and Albarzanchi et al. [10] to prevent in vivo oocyte maturation. In contrast to the results reported by these groups, we did not observe meiotic inhibition with cilostazol. One possible explanation for the difference in results is that the strain of mouse used in our experiments (B6;129) differed from those used in the previous reports (CD-1 and Swiss Webster). However, our results in monkeys provide additional evidence that the observed effect of the studies by Li et al. [9] and Albarzanchi et al. [10] are neither generalizable nor relevant to primates.
Taken together, these data demonstrate that at currently-approved maximal therapeutic concentrations, cilostazol is not likely to result in a contraceptive effect in women. While PDE3 inhibition has great potential as a potent non-hormonal contraceptive strategy, further research toward improving the bioavailability and selectivity of candidate agents is needed to advance the field.
Acknowledgments
The authors would like to thank the ONPRC Surgical Services Team, Division of Comparative Medicine, and the Behavioral Services Unit. This research was funded by NICHD grant U54HD055744 and a NIH core grant P51OD011092. The content is the responsibility of the authors and does not reflect the official views of the National Institutes of Health.
Footnotes
Disclosures: Dr. Jensen has received payments for consulting from Agile Pharmaceuticals, Abbvie Pharmaceuticals, Bayer Healthcare, ContraMed, Evofem Inc, HRA Pharma, Merck Pharmaceuticals, Teva Pharmaceuticals and the Population Council, and for giving talks for Bayer and Merck. He has also received research funding from Abbvie, Bayer, the Population Council, and the Bill & Melinda Gates Foundation. These companies and organizations may have a commercial or financial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU. The other authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sun Q-Y, Miao Y-L, Schatten H. Towards a new understanding on the regulation of mammalian oocyte meiosis resumption. Cell Cycle. 2009;8:2741–7. doi: 10.4161/cc.8.17.9471. [DOI] [PubMed] [Google Scholar]
- 2.Norris RP, Freudzon M, Mehlmann LM, et al. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–38. doi: 10.1242/dev.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conti M, Andersen CB, Richard F, et al. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187:153–9. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 4.Norris RP, Ratzan WJ, Freudzon M, et al. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–78. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nogueira D, Albano C, Adrianenssens T, et al. Human oocytes reversibly arrested in prophase I by phosphodiesterase type 3 inhibitor in vitro. Biology of reproduction. 2003;69:1042–52. doi: 10.1095/biolreprod.103.015982. [DOI] [PubMed] [Google Scholar]
- 6.Jensen JT, Zelinski MB, Stanley JE, Fanton JW, Stouffer RL. The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation in the naturally selected dominant follicle in rhesus macaques. Contraception. 2008;77:303–7. doi: 10.1016/j.contraception.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen JT, Zelinski-Wooten MB, Schwinof KM, Vance JE, Stouffer RL. The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation during gonadotropin-stimulated ovarian cycles in rhesus macaques. Contraception. 2005;71:68–73. doi: 10.1016/j.contraception.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Jensen JT, Stouffer RL, Stanley JE, Zelinski MB. Evaluation of the phosphodiesterase 3 inhibitor ORG 9935 as a contraceptive in female macaques: initial trials. Contraception. 2010;81:165–71. doi: 10.1016/j.contraception.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Yu Y, Yan J, et al. The role of cilostazol, a phosphodiesterase 3 inhibitor, on oocyte maturation and subsequent pregnancy in mice. PloS one. 2012;7:e30649. doi: 10.1371/journal.pone.0030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albarzanchi AM, Sayes CM, Ridha Albarzanchi MT, Fajt VR, Dees WL, Kraemer DC. Cilostazol blocks pregnancy in naturally cycling mice. Contraception. 2013;87:443–8. doi: 10.1016/j.contraception.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Wolf DP, Thomson JA, Zelinski-Wooten MB, Stouffer RL. In vitro fertilization-embryo transfer in nonhuman primates: the technique and its applications. Mol Reprod Dev. 1990;27:261–80. doi: 10.1002/mrd.1080270313. [DOI] [PubMed] [Google Scholar]
- 12.Tachibana M, Sparman M, Mitalipov S. Chromosome transfer in mature oocytes. Nature protocols. 2010;5:1138–47. doi: 10.1038/nprot.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen JT, Schwinof KM, Zelinski-Wooten MB, Conti M, DePaolo LV, Stouffer RL. Phosphodiesterase 3 inhibitors selectively block the spontaneous resumption of meiosis by macaque oocytes in vitro. Human reproduction (Oxford, England) 2002;17:2079–84. doi: 10.1093/humrep/17.8.2079. [DOI] [PubMed] [Google Scholar]
- 14.Suri A, Forbes WP, Bramer SL. Pharmacokinetics of multiple-dose oral cilostazol in middle-age and elderly men and women. Journal of clinical pharmacology. 1998;38:144–50. doi: 10.1002/j.1552-4604.1998.tb04403.x. [DOI] [PubMed] [Google Scholar]