Abstract
Objective
The pathophysiological response to burn injury disturbs the balance between skeletal muscle protein synthesis and breakdown, resulting in severe muscle wasting. Muscle loss after burn injury is related to increased mortality and morbidity. Consequently, mitigation of this catabolic response has become a focus in the management of these patients. The aim of this review is to discuss the literature pertaining to pharmacological interventions aimed at attenuating skeletal muscle catabolism in severely burned patients.
Data selection
Review of the literature related to skeletal muscle protein metabolism following burn injury was conducted. Emphasis was on studies utilizing stable isotope tracer kinetics to assess the impact of pharmacological interventions on muscle protein metabolism in severely burned patients.
Conclusion
Data support the efficacy of testosterone, oxandrolone, human recombinant growth hormone, insulin, metformin, and propranolol in improving skeletal muscle protein net balance in patients with severe burns. The mechanisms underlying the improvement of protein net balance differ between types and dosages of drugs, but their main effect is on protein synthesis. Finally, the majority of studies have been conducted during the acute hypermetabolic phase of the injury. Except for oxandrolone, the effects of drugs on muscle protein kinetics following discharge from the hospital are largely unknown.
Keywords: burn injury, hypermetabolism, catabolism, muscle, protein, stable isotopes
1. Introduction
Severe thermal injury, defined as wounds compromising more than 40% of the patient’s total body surface area (TBSA), is followed by a severe hypermetabolic response [1] and dramatic elevations in the protein synthesis and breakdown rates [2]. The efflux of amino acids from skeletal muscle in response to burn injury is thought to supply substrate for vital processes such as wound healing, immune function and hepatic protein synthesis [3, 4]. Although necessary for recovery, this pathophysiologic response leads to an extensive loss of skeletal muscle protein that is not easily reversed by aggressive nutritional support alone [5].
Prolonged catabolism of lean body mass (LBM) has been associated with detrimental outcomes such as muscle weakness, immune-suppression, impaired wound healing [6], severe growth arrest [7], delayed recovery and rehabilitation, and even decreased survival rates [8]. Thus, amelioration of skeletal muscle loss to improve strength and function and to decrease physical and functional impairment is of major importance for enhancing the recovery process and improving survival rates in severe burn victims.
The purpose of this review is to discuss the current data pertaining to the effects of pharmacological interventions on muscle protein synthesis, breakdown and net balance (synthesis minus breakdown) following a major thermal injury.
2. Acute Burn-Induced Changes in Skeletal Muscle Protein Kinetics
In otherwise healthy humans, muscle mass is maintained through a dynamic balance between protein accretion and protein degradation [9]. To evaluate the impact of the burn injury on muscle protein metabolism, Biolo and colleagues [5] determined protein synthesis and breakdown rates and also transmembrane transport of amino acids in 18 normal volunteers and 19 acutely burned adult patients. In the post-absorptive state, the absolute rates of muscle protein synthesis and breakdown were elevated in the burn group by 50% and 83%, respectively. Thus, the marked increase in muscle protein breakdown rate was not matched by a synthetic response of the same magnitude, leading to a significantly more negative net protein balance in the burn group.
Further, the rate at which amino acids were delivered to the leg was 2–3 times greater in the burn cohort due to a two-fold elevation in the rate of leg blood flow. However, absolute inward amino acid transport into the muscle was not significantly different in patients compared to controls. In fact, after normalizing for the delivery rate to the leg, the capacity of the transport systems was found to be attenuated by 50–63% in the burn group. On the other hand, the efflux of amino acids being traced was 50% greater in the burn patients compared to the control subjects.
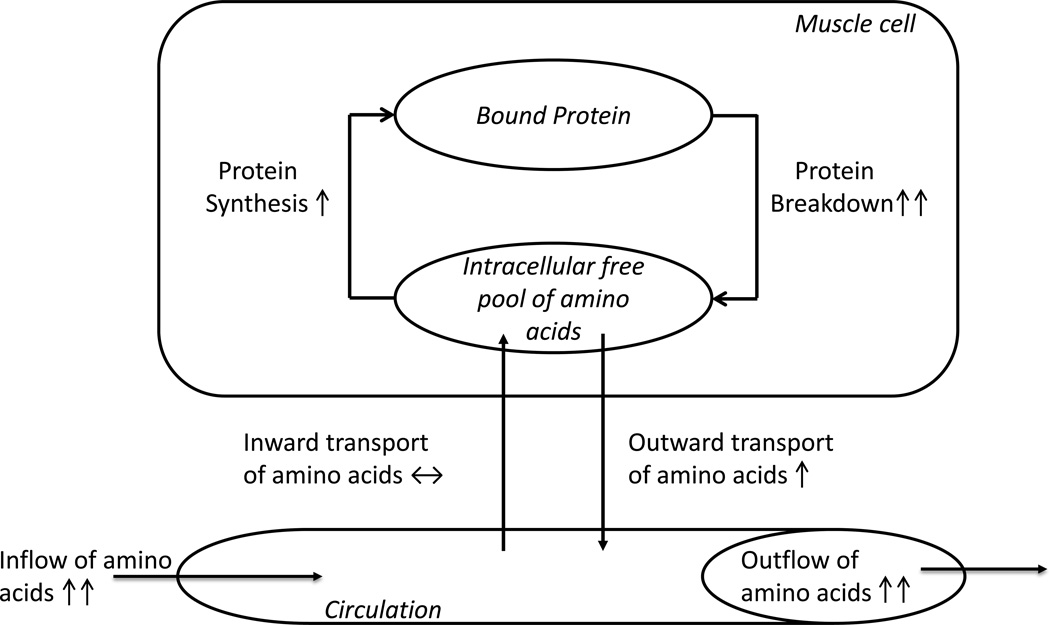
Under normal circumstances, amino acids in the free intracellular pool can derive either from the breakdown of proteins or from the inward transport of amino acids. In the aforementioned study, the rate of intracellular appearance of essential amino acids increased following burn trauma. For example, the intracellular concentration of leucine was 1.7- fold greater in burn patients than controls (358 vs. 206 nmol/ml). Interestingly, this occurred despite an impaired amino acid transport system, suggesting that amino acids in the intracellular free pool may derive primarily from the breakdown of muscle protein (figure 1).
Figure 1.
Schematic representation of the pathophysiologic findings in skeletal muscle protein kinetics contributing to muscle wasting following a major burn injury. Increased protein breakdown and decreased inward transport of amino acids relative to the inflow of amino acids are two important mechanisms.
The profoundly elevated muscle protein breakdown would saturate the intracellular free pool of amino acids leading to a concurrent elevation in the rate of muscle protein synthesis [5], since the availability of free amino acids is an important limiting step in this process [10]. However, this elevation in protein synthesis is not adequate to compensate for the increased rate of protein breakdown, which over time results in muscle wasting.
3. Prolonged Muscle Catabolism in Patients with Severe Burns
Muscle protein loss is a characteristic feature of the post-burn hypermetabolic stress response. However, alterations in muscle protein kinetics continue long after wound healing and discharge, as demonstrated by Hart et al. [11]. Twenty five children with >40% TBSA burns underwent protein kinetic studies, body composition analyses and metabolic rate measurements at 6, 9 and 12 months post-burn. Skeletal muscle protein net balance was dramatically negative even at 6 and 9 months post-burn [11]. However, at 12 months, amelioration of the rate of protein breakdown was related to an improvement in net balance, which was not significantly different from that of unburned young adults, who served as reference group, in the post-absorptive state. Although limited by the absence of reference values for healthy children, this study suggests that severely burned patients approach normal protein kinetics over time, but that this may take a significant amount of time.
Interestingly, the changes in muscle protein net balance matched the reductions in resting energy expenditure (REE) and improvements in body composition over time. Whole body DEXA scans showed a progressive decline in lean body mass from the discharge time-point until 6 and 9 months post-burn, but accretion at 12 months post-burn. On the other hand, REE decreased from a striking 179% of age-predicted REE at baseline to a still significantly elevated 115% at 12 months post-burn.
Although accretion of lean body mass has been reported at 1 year post-burn, burn survivors may not reach normal values even three years after injury [12]. This may be related to relentless disturbances in the growth hormone axis, reduced insulin sensitivity and prolonged inflammation, all of which contribute to up-regulation of proteolytic pathways and muscle atrophy [13].
4. Pharmacological Interventions and Muscle Protein Kinetics in Patients with Severe Burns
4.1 Testosterone and Oxandrolone
Testosterone, an anabolic steroid, increases lean body mass in healthy young adults in a dose dependent manner [14]. It also stimulates muscle protein synthesis without affecting amino acid transport into the skeletal muscle, thus, suggesting that the anabolic effects of this drug in healthy subjects is mediated through an increased efficiency in the utilization of amino acids deriving from protein breakdown [15]. However, the hepatotoxic and masculinizing effects of testosterone may limit its therapeutic use. On the other hand, oxandrolone, a synthetic testosterone analogue, has minimal virilizing effects, is cleared by the kidneys, and has been approved for and successfully used in chronic wasting syndromes, including burn trauma [16].
Acute Effects of Testosterone and Oxandrolone on Muscle Protein Kinetics
The decreased androgen environment found in severe burns [17] is a component of the hormonal cascade that favors the persistent loss of skeletal muscle protein. Interestingly, oral treatment with oxandrolone in burned adults during the acute hospitalization can reduce nitrogen and weight loss [18]. Moreover, Ferrando et al. [19], showed that intramuscular administration of testosterone enanthate (200 mg/wk) for two weeks in severely burned adult patients improved muscle protein retention. In this study, protein accretion was mainly the result of a 45% decrease in the rate of muscle protein breakdown (Table 1) which was also accompanied by decreased outward transport of amino acids from skeletal muscle. Although no changes in muscle protein synthesis were seen, testosterone improved protein synthesis efficiency (PSE), meaning that there was an increase in the fraction of amino acids from the intracellular pool that were incorporated into bound muscle protein.
Table 1.
Effects of pharmacological interventions on muscle protein kinetics across the leg.
| Testosterone a Adults [19] |
Oxandroloneb Children [20] |
rhGHc Children [31] |
Insulind Adults [37] |
Insuline Adults and children [36] |
Propranololf Children [50] |
Metforming Adults [40] |
Ketoconazoleh Children [51] |
|
|---|---|---|---|---|---|---|---|---|
| The three pool model | ||||||||
| Amino acid inflow into the leg via femoral artery | -- | -- | -- | ↑193 | -- | -- | -- | |
| Amino acid outflow from leg via femoral vein | -- | -- | -- | ↑175 | -- | -- | -- | |
| Inward transport into muscle | ↓33 | -- | -- | ↑535 | -- | |||
| Outward transport from muscle | ↓53 | -- | -- | ↑349 | -- | |||
| AV shunt past muscle | -- | -- | -- | -- | ||||
| Muscle protein synthesis (PS) | -- | ↑140 | ↑165* | ↑384 | -- | ↑25 | -- | |
| Muscle protein breakdown (PB) | ↓45 | -- | -- | ↑157 | -- | -- | -- | |
| Protein synthesis efficiency | ↑114 | ↑151 | ↑57* | |||||
| Net Balance | ↑87 | ↑107 | ↑67*□ | ↑75* | ↑120□ | ↑183* | ↑59 | -- |
| Fractional synthetic rate | -- | ↑107 | ↑93* | ↑62 | ↑42* | ↑46□ | ||
| The Two Pool Model | ||||||||
| Rate of appearance (PB) | -- | -- | ||||||
| Rate of disappearance (PS) | ↑138* | ↑74* |
Not different.
Percent decrease from baseline when pre and post intervention data available.
Percent increase from baseline when pre and post intervention data available.
Percent difference in treated vs. control group.
Estimated from figures when values not available.
= 200 mg/wk for two weeks
= 0.1 mg /kg twice a day for 5 days
= 0.2 mg/kg once a day for 19 days
= 2.6 mU∙kg−1∙min−1 for 3.5 days
= 7.2 mU∙kg−1∙min−1 for 7 days
= 6.3 mg∙kg−1∙day−1 divided in four doses for two weeks
= 850 mg three times a day for 7 days
= 5 mg/kg twice a day for two weeks
In healthy volunteers, testosterone improves muscle protein accretion by increasing protein synthesis without significantly affecting protein breakdown. The different effects of testosterone in burned and healthy subjects may be because protein synthesis, although insufficient to counter-balance protein balance, is already nearing maximum rates in burned adults. On the other hand, in children the acute anabolic effects of oxandrolone seem to be achieved through different mechanisms. Fourteen severely burned children with delayed admissions (>7 days post-burn) received 0.1 mg/kg of oxandrolone twice a day for seven days [20]. Contrary to burned adults, the improvement in protein net balance over the intervention period was due to an elevation in muscle protein synthesis rate rather than a decrease in protein breakdown. The treated group exhibited a 140% increase in protein synthesis whereas the placebo group showed a slight, but not significant, decrease. The inward transport of amino acids into the muscle did not change with oxandrolone, whereas PSE increased by 151%.
The authors suggested that the discrepancy in the mechanism by which oxandrolone exerts favorable outcomes on muscle protein metabolism in children and adults may be related to a greater capacity for protein synthesis during childhood. Indeed, it has previously been shown that children are less catabolic than adults following burn injury as a result of a greater synthetic capacity of skeletal muscle [21].
Long Term Effects of Oxandrolone on Muscle Protein Kinetics
When administered in a dose of 10 mg twice a day, oxandrolone has been shown to decrease weight loss and improve muscle strength and endurance in burn adults [16]. Patients (n=7) were started on oxandrolone once their REE was <130% of age predicted, at which point they were considered to be in the recovery phase of the injury. After 3 weeks of intervention, maximal effects were seen in the group receiving a combination of oxandrolone, exercise and a high protein diet (2 g∙kg−1∙day−1) vs. high protein intake plus exercise alone. In children, prolonged administration of oxandrolone yields similar results [22]. The addition of three months of exercise to 12 months of oxandrolone therapy resulted in significant accretion of lean body mass and bone mineral content which were still evident up to five years post-burn. Oxandrolone-treated children also improved muscle strength and were able to overcome the severe growth arrest associated with burn trauma even after discontinuation of treatment.
How the long term effects of oxandrolone on lean body mass and muscle strength relate to changes in skeletal muscle protein kinetics have not been assessed in burn adults. In children on the other hand, stable isotope studies have been conducted by our group [23]. The administration of oxandrolone at 0.1 mg/kg twice a day from admission until 6 months post-injury did not lead to differences in leg muscle protein kinetics compared to a control group. However, adding a 3-hour continuous infusion of mixed amino acids resulted in improvement in protein net balance in the treated group. Although protein synthesis increased in both groups during the infusion of amino acids, net gain of muscle protein was significant only in oxandrolone-treated kids. This was mainly due to changes in the rate of protein breakdown which increased by 36% in the control group, whereas it decreased by 4% in the intervention group in the amino acid period.
Thus, it seems like the long term accrual of muscle protein with oxandrolone treatment is due to an increased response of skeletal muscle to anabolic stimuli (e.g., amino acids and exercise), rather than an elevated net balance in the basal state. This suggests that adequate dietary protein quality and quantity, as well as exercise, should be paired with oxandrolone treatment for months following burn trauma in order to achieve further deposit of muscle protein [24]. While a limitation of this study was that muscle protein synthesis and breakdown were estimated as rate of disappearance and appearance of an amino acid tracer out of and into the circulation, and no incorporation into the bound protein from the intracellular free amino acid pool was assessed, it still demonstrates a positive impact of oxandrolone treatment on protein kinetics, even long time after discharge.
4.2 Recombinant Human Growth Hormone (rhGH)
Following a major burn, dramatic changes occur in the growth hormone/IGF-1/IGFBP-3 hormonal axis. In children, serum levels of IGF-1 and its binding protein IGFBP-3 are persistently low for up to three years post-burn [25]. Further, these findings are associated with severe growth arrest, as evidenced by dramatic height velocity delays up to three years post-burn [7]. Exogenous administration of rhGH, however, can successfully revert this condition [26], promote accretion of lean body mass [27], decrease wound healing time [28], and improve nitrogen balance [29] in the burn population.
It is believed that the anabolic effects of rhGH are mediated through increased stimulation of IGFBP-3 and IGF-1 synthesis in the liver [30]. In addition, IGF-1 is thought to regulate muscle proteolysis by preventing the overexpression of muscle specific E3 ubiquitin ligases atrogin-1 and MuRF-1 [13]. The effects of rhGH on muscle protein kinetics after discharge from the hospital have not been assessed. However, in acutely ill burned children, rhGH improves protein net balance (Table 1) [31]. When administered from admission to discharge from the ICU at a dose of 0.2 mg∙kg−1∙day−1, muscle protein synthesis increased by 2.4-fold, whereas protein breakdown did not change. The studies using 15N-lysine as tracer were conducted between the second and third week post-burn and were complemented with a hyperinsulinemic-euglycemic clamp during the last two hours of the infusion protocol.
Insulin at a dose of 250 mU/m2 did not further stimulate protein synthesis, breakdown or net balance in the rhGH-treated patients. On the other hand, protein synthesis and net balance were significantly improved in the control group during the insulin clamp. These findings suggest that insulin and rhGH may work via a common mechanism as they do not have additive or synergistic effects on muscle protein synthesis. The fact that levels of IGF-1 were two times higher with rhGH in the treated group supports the argument that beneficial effects of rhGH on skeletal muscle are in part mediated by IGF-1 [32]. In adddition, glucagon and insulin plasma levels increased with rhGH treatment. Thus, it is also possible that the anabolic effects of rhGH on muscle protein may be mediated in part through an elevation in plasma insulin concentrations.
In the long term, rhGH administration promotes accretion of lean body mass. Analysis of body composition in children receiving rhGH from discharge to 12 months post-injury showed significant and sustained improvement in lean body mass even one year after discontinuation of treatment. Whether accretion of skeletal muscle in chronically treated burn patients is achieved through sole stimulation of protein synthesis, as seen in the acute state, remains unknown [30].
The use of rhGH in acute severely ill patients has been limited due to reports of increased morbidity and mortality (40%) after treatment in critically ill nonburned adults [33]. In burn adults, administration of rhGH has been linked to hyperglycemia and increased hypermetabolism [18], wheras in children administration of rhGH attenuated the hypermetabolic response [27]. Indeed, no significant adverse effects were reported when rhGH was used in the acute hypermetabolic phase of the injury in burn children [28]. The different responses evoked by rhGH in children and adults suggests that age may be a predictive variable of outcome in this patient population and that, as for oxandrolone and testosterone, the effects of rhGH on skeletal muscle protein synthesis and breakdown may also exhibit variations with age.
4.3 Insulin
Severe burn injury causes insulin resistance and profound changes in glucose metabolism which contribute to adverse outcomes [34]. Yet, despite the decreased effects of insulin on peripheral glucose metabolism, muscle cells retain some insulin sensitivity which is evidenced by supression (although not normalization) of muscle protein breakdown in response to insulin [35]. Indeed, exogenous administration of insulin in severely burn patients results in improvement of skeletal muscle net protein balance. Interestingly, amelioration of muscle catabolism is achieved through different mechanisms depending on insulin dosage.
Effects of High Doses of Insulin on Muscle Protein Kinetics
Sakurai and colleagues [36] determined protein kinetics in nine severely burned patients before and after seven days of continuous insulin infusion (7.5 mU∙kg−1∙min−1; Table 1). Patients received enteral nutrition (82% carbohydrates, 15% protein and 3% fat) throughout the stable isotope infusion studies. Despite adequate nutritional intake, there was a large negative balance between protein synthesis and breakdown. Conversely, after the insulin treatment period, the rate of phenylalanine inward transport increased by 535%. Likewise, protein synthesis rate exhibited a dramatic 384% increase when compared to the pre-insulin period. Even though protein breakdown also increased with insulin infusion, the elevation was less than two-fold leading to improved protein net balance.
In conclusion, insulin in a high dosage ameliorates mucle protein loss predominantly by stimulating protein synthesis, possibly through an elevated inward transport of amino acids. However, the increased synthetic rate may in turn stimulate protein breakdown in order to preserve the intracellular pool of amino acids, which makes protein synthesis sustainable. Of importance is the finding that high doses of insulin appear to counteract the severe deficiency in the inward amino acid transport associated with burn injury.
Effects of Submaximal Doses of Insulin on Muscle Protein Kinetics
Administration of insulin requires close clinical monitoring due to the risk of hypoglycemia. Subsequently, using lower dosages of insulin to improve muscle protein kinetics may be a more preferable strategy. To investigate the effects of a submaximal dose of insulin on amino acid kinetics and protein net balance, patients with ≥60% TBSA burns were randomly assigned to receive standard care (n=5) or exogenous insulin (n=8) at an average of 2.6 mU∙kg−1∙min−1 [37]. Protein synthesis, measured using leucine tracer, was 165% greater in the treated group (347±64 vs. 131±25 mmol∙min−1∙100 ml of leg−1) (Table 1). On the other hand, protein breakdown, as well as inward and outward transport of amino acids, were not different between groups, suggesting that insulin at a low dose increases protein synthesis by promoting a more efficient utilization of amino acids derived from protein breakdown.
Preservation of the anabolic response to insulin in the presence of affected peripheral glucose uptake is in agreement with other studies showing that protein catabolism is not completely mediated through a defect in glucose metabolism [35]. Further, the effects of insulin on protein kinetics are dose dependent with high dosages of insulin resulting in increased amino transport and muscle protein turnover, and lower doses improving protein synthesis efficiency.
4.4 Metformin
Insulin insensitivity and hyperglycemia have been associated with increased muscle catabolism in severely burned individuals [38]. Metformin, an anti-hyperglycemic drug in the biguanide family, lowers blood glucose levels by reducing hepatic glucose production, reducing glucose absorption by the intestines, and by increasing glucose uptake and utilization by peripheral tissues. In contrast to insulin, the risk of hypoglycemia is minimal with metformin [39], making it an attractive glucose lowering agent.
In an attempt to assess the effects of metformin on glucose and muscle protein metabolism following burn injury, Gore and colleagues [40] studied severely burned adults in the acute period post-injury. Patients were randomized to receive placebo (n=5) or metformin (n=8). Stable isotope infusion studies were conducted before and after the intervention in both groups. After seven days of treatment, endogenous glucose production decreased by 51% with metformin treatment. In addition, glucose clearance and oxidation increased by 87% and 56%, respectively. As a result, serum glucose levels were significantly lower in the metformin group vs. placebo (139±25 vs. 184±56 mg/dl) after the intervention.
Interestingly, despite decreased endogenous glucose production and increased glucose clearance and oxidation, metformin treatment did not decrease the rate of muscle protein breakdown. Certainly, it would be expected that a reduction in gluconeogenesis would lead to a decreased demand of substrate (amino acids) from skeletal muscle, and thus reduced protein breakdown rates. Similarly, a shift in the utilization of amino acids as fuel toward a greater oxidation of glucose should primarily decrease muscle protein breakdown. However, the improvement in net balance reported in the intervention group was mainly due to an elevation in the rate of protein synthesis and not to changes in protein breakdown. This suggests that the anabolic effect of metformin in burn patients is mediated by improving the state of insulin resistance associated with the stress response to burn injury. Indeed, the infusion of insulin after seven days of metformin treatment further increased the rate of muscle protein synthesis [40], supporting the notion that metformin enhances the tissue response to insulin.
Metformin provides beneficial effects on glucose metabolism and skeletal muscle protein kinetics. However, lactic acidosis, a rare but severe complication has been linked to metformin treatment particularly in acutely ill subjects with some degree of renal dysfunction [41]. As burn patients are prone to multiorgan dysfunction including acute kidney injury [42], caution of use and close monitoring are warranted.
Insulin resistance and muscle wasting are chronic complications of burn trauma [11, 43]. Metformin not only targets both complications, but is also economically beneficial and can easily be administered orally. These advantages make it an attractive alternative for the long term treatment of insulin resistance and skeletal muscle loss. Although no severe complications have been reported in the burn population, larger studies are needed to assess safety and efficacy in both the acute and long term setting.
4.5 Propranolol
Hypermetabolism is a hallmark of the metabolic response that follows severe thermal injuries [1], and occurs in concert with hyperactivity of the sympathetic system [44]. This stress response has been associated with altered lipid [45, 46] and protein [5] metabolism, resulting in the erosion of lean body mass [45]. To attenuate the pathophysiologic response to burn injury and decrease muscle wasting, blockage of the adrenergic stimulation has been studied extensively in this patient population.
Propranolol, a nonselective β-blocker, successfully decreases heart rate, myocardial oxygen consumption [47, 48] and resting energy expenditure [1, 49] in severely burned patients. To further study the effects of propranolol on hypermetabolism and muscle protein net balance, 25 pediatric patients with burns encompassing >40% of the TBSA were enrolled in a prospective randomized trial during the acute hospital stay [50]. Thirteen children received oral propranolol for two weeks, and 12 served as controls. To achieve the study goal of a 20% decrease in heart rate from baseline, propranolol was given in a daily dose of 2 mg∙kg−1∙day−1 divided into four doses, and adjusted to a total average of 6.3 mg∙kg−1∙day−1.
Propranolol treatment did not alter skeletal muscle proteolysis (Table 1). The intracellular appearance of phenylalanine, which represents protein breakdown, was not different from that of controls. On the other hand, the mean rate of intracellular utilization of phenylalanine, i.e., protein synthesis, was greater in propranolol treated patients. Because protein synthesis increased with propranolol, muscle protein net balance was positive in the intervention group after two weeks of treatment, while it remained negative in the control group (0.035 ± 0.011 vs. −0.042 ± 0.016, µmol∙min−1∙100 ml of leg volume−1; P=0.001). This improvement was not accompanied by significant changes in the inward and outward transport of phenylalanine from skeletal muscle. However, protein synthesis efficiency at post-treatment was greater in the propranolol group compared to control (61 ± 3% vs.39 ± 6%, P=0.03). Further, changes in skeletal muscle protein kinetics with propranolol treatment occurred concomitantly with a decline in the resting energy expenditure and better preservation of the fat free mass and lean body mass, as demonstrated by analyses of body composition.
In summary, short term propranolol treatment attenuates muscle wasting in severely burned patients mainly by increasing protein synthesis and muscle protein synthesis efficiency, while protein breakdown remains unchanged. The effects of long term propranolol treatment on muscle protein metabolism in patients with large burns remain unknown.
4.6 Ketoconazole
The stress response triggered by burn injury is associated with elevated levels of cortisol [12]. Ketoconazole, an imidazole antifungal agent, diminishes the synthesis of cortisol by blocking the P450-dependent enzyme system. It was hypothesized that ketoconazole would mitigate muscle protein wasting by decreasing cortisol secretion in severely burned children [51]. To test this hypothesis, patients were randomized to receive either placebo (n=32) or ketoconazole (n=17; 5 mg/kg every 12 hours) throughout their initial admission. Protein kinetic studies were performed five days after the first excision surgery and again two weeks later.
Ketoconazole decreased urinary cortisol to normal values in the treatment group by post-burn day eight, whereas urinary catecholamines, serum cytokines and resting energy expenditure remained unchanged. When protein synthesis and protein breakdown were assessed, no difference between placebo and ketoconazole treated subjects were found (Table 1). Interestingly, this study suggests that cortisol may not regulate muscle protein degradation post-burn, instead, catecholamines, cytokines, and other stress hormones may be more potent regulators of the hypercatabolic response triggered by burn injury.
5. Summary and Conclusions
The pathophysiologic stress response induced by major thermal trauma is characterized by profound and long lasting inflammatory, metabolic, and hormonal changes. Under these circumstances, skeletal muscle supplies other tissues with amino acids in order to ensure survival. However, this compensatory response eventually results in muscle wasting and increased morbidity and mortality. As a result, pharmacological strategies have been implemented in an attempt to attenuate the burn-induced catabolic state.
Stable isotope tracer kinetic measurements across the leg have been successfully used to determine the burn-induced changes in skeletal muscle protein kinetics. These analytical tools have yielded better understanding of the effects of different pharmacological interventions on muscle metabolism, whereas future genomics and proteomics approaches will more fully elucidate their mechanistic actions.
Most pharmacological agents reviewed here improve muscle protein net balance, and their effects on protein kinetics seem to depend on the patient’s age, dosage, and probably also time post-burn. Certainly, improvement in net balance with these drugs is mainly due to increments in protein synthesis and/or protein synthesis efficiency, with minimal or no effect on protein breakdown. For instance, acute administration of testosterone in adults improves PSE, whereas in children oxandrolone improves both PSE and protein synthesis rates. Six months of administration of oxandrolone in children increases the response of the skeletal muscle to anabolic stimuli such as amino acids and exercise. The responsiveness of skeletal muscle protein synthesis to these drugs is facilitated by the increased availability of intracellular amino acids derived from the elevated protein breakdown. Except for oxandrolone, the effect of other drugs on muscle protein kinetics following discharge from the hospital is largely unknown. Finally, multicenter trials would provide more solid evidence of the efficacy of these drugs, and potentially lead to improved treatment of this patient population.
Highlights.
We discuss data pertaining the effects of drugs on skeletal muscle metabolism
Protein synthesis is the most responsive parameter to pharmacological interventions
Patient’s age, drug dosage and time post burn affect the response to these drugs
The effects of most drugs following discharge from the hospital is largely unknown
Acknowledgements
The work was supported by NIH H133A070026, H133A70019, RO1-HD049471, RO1-GM056687, T32-GM008256, P50-GM060338 and Shriners of North America 84090, 84080, 85310, 71006, and 71008, 71009. CP is partly supported by an Interdisciplinary Rehabilitation Research Postdoctoral Training Grant (H133P110012) from the National Institute of Disability and Rehabilitation Research and Department of Education. EB and ECD are supported by funding from the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000.
Abbreviations
- TBSA
Total body surface area
- LBM
Lean body mass
- REE
Resting energy expenditure
- PSE
Protein synthesis efficiency
- rhGH
Recombinant human growth hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest Statement
Eva C. Diaz, David N. Herndon, Craig Porter, Labros S. Sidossis, Oscar Suman and Elisabet Børsheim have neither financial relationships nor conflict of interests relevant to this article to disclose.
Contributor Information
Eva C. Diaz, Email: ecdiazfuentes@uams.edu.
David N. Herndon, Email: dherndon@utmb.edu.
Craig Porter, Email: cr2porte@UTMB.EDU.
Oscar E. Suman, Email: oesuman@utmb.edu.
Elisabet Børsheim, Email: EBorsheim@uams.edu.
References
- 1.Wilmore DW, Long JM, Mason AD, Jr, Skreen RW, Pruitt BA., Jr Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974 Oct;180:653–669. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kien CL, Young VR, Rohrbaugh DK, Burke JF. Increased rates of whole body protein synthesis and breakdown in children recovering from burns. Ann Surg. 1978 Apr;187:383–391. doi: 10.1097/00000658-197804000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe RR. Regulation of skeletal muscle protein metabolism in catabolic states. Curr Opin Clin Nutr Metab Care. 2005 Jan;8:61–65. doi: 10.1097/00075197-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Gore DC, Chinkes DL, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Quantification of protein metabolism in vivo for skin, wound, and muscle in severe burn patients. JPEN J Parenter Enteral Nutr. 2006 Jul-Aug;30:331–338. doi: 10.1177/0148607106030004331. [DOI] [PubMed] [Google Scholar]
- 5.Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002 Jul;87:3378–3384. doi: 10.1210/jcem.87.7.8699. [DOI] [PubMed] [Google Scholar]
- 6.Pereira C, Murphy K, Jeschke M, Herndon DN. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol. 2005 Oct;37:1948–1961. doi: 10.1016/j.biocel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg. 1990 Mar;125:392–395. doi: 10.1001/archsurg.1990.01410150114021. [DOI] [PubMed] [Google Scholar]
- 8.Pereira CT, Barrow RE, Sterns AM, Hawkins HK, Kimbrough CW, Jeschke MG, et al. Age-dependent differences in survival after severe burns: a unicentric review of 1,674 patients and 179 autopsies over 15 years. J Am Coll Surg. 2006 Mar;202:536–548. doi: 10.1016/j.jamcollsurg.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Waterlow JC. Protein turnover with special reference to man. Q J Exp Physiol. 1984 Jul;69:409–438. doi: 10.1113/expphysiol.1984.sp002829. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe R, Ferrando A, Sheffield-Moore M, Urban R. Testosterone and muscle protein metabolism. Mayo Clin Proc. 2000 Jan;75(Suppl):S55–S59. discussion S59–60. [PubMed] [Google Scholar]
- 11.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000 Aug;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 12.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heszele MF, Price SR. Insulin-like growth factor I: the yin and yang of muscle atrophy. Endocrinology. 2004 Nov;145:4803–4805. doi: 10.1210/en.2004-1037. [DOI] [PubMed] [Google Scholar]
- 14.Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, et al. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002 Jul;283:E154–E164. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- 15.Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998 Nov;275:E864–E871. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- 16.Demling RH, DeSanti L. Oxandrolone, an anabolic steroid, significantly increases the rate of weight gain in the recovery phase after major burns. J Trauma. 1997 Jul;43:47–51. doi: 10.1097/00005373-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Parker CR, Jr, Baxter CR. Divergence in adrenal steroid secretory pattern after thermal injury in adult patients. J Trauma. 1985 Jun;25:508–510. doi: 10.1097/00005373-198506000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Demling RH. Comparison of the anabolic effects and complications of human growth hormone and the testosterone analog, oxandrolone, after severe burn injury. Burns. 1999 May;25:215–221. doi: 10.1016/s0305-4179(98)00159-4. [DOI] [PubMed] [Google Scholar]
- 19.Ferrando AA, Sheffield-Moore M, Wolf SE, Herndon DN, Wolfe RR. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med. 2001 Oct;29:1936–1942. doi: 10.1097/00003246-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Hart DW, Wolf SE, Ramzy PI, Chinkes DL, Beauford RB, Ferrando AA, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001 Apr;233:556–564. doi: 10.1097/00000658-200104000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuvdendorj D, Chinkes DL, Zhang XJ, Ferrando AA, Elijah IE, Mlcak RP, et al. Adult patients are more catabolic than children during acute phase after burn injury: a retrospective analysis on muscle protein kinetics. Intensive Care Med. 2011 Aug;37:1317–1322. doi: 10.1007/s00134-011-2223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porro LJ, Herndon DN, Rodriguez NA, Jennings K, Klein GL, Mlcak RP, et al. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. J Am Coll Surg. 2012 Apr;214:489–502. doi: 10.1016/j.jamcollsurg.2011.12.038. discussion 502–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuvdendorj D, Chinkes DL, Zhang XJ, Suman OE, Aarsland A, Ferrando A, et al. Long-term oxandrolone treatment increases muscle protein net deposition via improving amino acid utilization in pediatric patients 6 months after burn injury. Surgery. 2011 May;149:645–653. doi: 10.1016/j.surg.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Przkora R, Herndon DN, Suman OE. The effects of oxandrolone and exercise on muscle mass and function in children with severe burns. Pediatrics. 2007 Jan;119:e109–e116. doi: 10.1542/peds.2006-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008 Sep;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low JF, Herndon DN, Barrow RE. Effect of growth hormone on growth delay in burned children: a 3-year follow-up study. Lancet. 1999 Nov 20;354:1789. doi: 10.1016/s0140-6736(99)02741-5. [DOI] [PubMed] [Google Scholar]
- 27.Branski LK, Herndon DN, Barrow RE, Kulp GA, Klein GL, Suman OE, et al. Randomized controlled trial to determine the efficacy of long-term growth hormone treatment in severely burned children. Ann Surg. 2009 Oct;250:514–523. doi: 10.1097/SLA.0b013e3181b8f9ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herndon DN, Barrow RE, Kunkel KR, Broemeling L, Rutan RL. Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann Surg. 1990 Oct;212:424–429. doi: 10.1097/00000658-199010000-00005. discussion 430–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilmore DW, Moylan JA, Jr, Bristow BF, Mason AD, Jr, Pruitt BA., Jr Anabolic effects of human growth hormone and high caloric feedings following thermal injury. Surg Gynecol Obstet. 1974 Jun;138:875–884. [PubMed] [Google Scholar]
- 30.Przkora R, Herndon DN, Suman OE, Jeschke MG, Meyer WJ, Chinkes DL, et al. Beneficial effects of extended growth hormone treatment after hospital discharge in pediatric burn patients. Ann Surg. 2006 Jun;243:796–801. doi: 10.1097/01.sla.0000219676.69331.fd. discussion 801–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gore DC, Honeycutt D, Jahoor F, Wolfe RR, Herndon DN. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg. 1991 Jan;126:38–43. doi: 10.1001/archsurg.1991.01410250042006. [DOI] [PubMed] [Google Scholar]
- 32.Klein GL, Wolf SE, Langman CB, Rosen CJ, Mohan S, Keenan BS, et al. Effects of therapy with recombinant human growth hormone on insulin-like growth factor system components and serum levels of biochemical markers of bone formation in children after severe burn injury. J Clin Endocrinol Metab. 1998 Jan;83:21–24. doi: 10.1210/jcem.83.1.4518. [DOI] [PubMed] [Google Scholar]
- 33.Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999 Sep 9;341:785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 34.Gauglitz GG, Herndon DN, Jeschke MG. Insulin resistance postburn: underlying mechanisms and current therapeutic strategies. J Burn Care Res. 2008 Sep-Oct;29:683–694. doi: 10.1097/BCR.0b013e31818481ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jahoor F, Shangraw RE, Miyoshi H, Wallfish H, Herndon DN, Wolfe RR. Role of insulin and glucose oxidation in mediating the protein catabolism of burns and sepsis. Am J Physiol. 1989 Sep;257:E323–E331. doi: 10.1152/ajpendo.1989.257.3.E323. [DOI] [PubMed] [Google Scholar]
- 36.Sakurai Y, Aarsland A, Herndon DN, Chinkes DL, Pierre E, Nguyen TT, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995 Sep;222:283–294. 294–297. doi: 10.1097/00000658-199509000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999 Jan;229:11–18. doi: 10.1097/00000658-199901000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gore DC, Chinkes DL, Hart DW, Wolf SE, Herndon DN, Sanford AP. Hyperglycemia exacerbates muscle protein catabolism in burn-injured patients. Crit Care Med. 2002 Nov;30:2438–2442. doi: 10.1097/00003246-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Tornio A, Niemi M, Neuvonen PJ, Backman JT. Drug interactions with oral antidiabetic agents: pharmacokinetic mechanisms and clinical implications. Trends Pharmacol Sci. 2012 Jun;33:312–322. doi: 10.1016/j.tips.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Gore DC, Wolf SE, Sanford A, Herndon DN, Wolfe RR. Influence of metformin on glucose intolerance and muscle catabolism following severe burn injury. Ann Surg. 2005 Feb;241:334–342. doi: 10.1097/01.sla.0000152013.23032.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen YK. Impact of acute kidney injury on metformin-associated lactic acidosis. Int Urol Nephrol. 2009 Dec;41:967–972. doi: 10.1007/s11255-009-9549-6. [DOI] [PubMed] [Google Scholar]
- 42.Palmieri T, Lavrentieva A, Greenhalgh D. An assessment of acute kidney injury with modified RIFLE criteria in pediatric patients with severe burns. Intensive Care Med. 2009 Dec;35:2125–2129. doi: 10.1007/s00134-009-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gauglitz GG, Herndon DN, Kulp GA, Meyer WJ, 3rd, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009 May;94:1656–1664. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodall M, Stone C, Haynes BW., Jr Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann Surg. 1957 Apr;145:479–487. doi: 10.1097/00000658-195704000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason AD., Jr Weight loss in burned patients. J Trauma. 1979 Nov;19:903–904. [PubMed] [Google Scholar]
- 46.Herndon DN, Nguyen TT, Wolfe RR, Maggi SP, Biolo G, Muller M, et al. Lipolysis in burned patients is stimulated by the beta 2-receptor for catecholamines. Arch Surg. 1994 Dec;129:1301–1304. doi: 10.1001/archsurg.1994.01420360091012. discussion 1304–5. [DOI] [PubMed] [Google Scholar]
- 47.Williams FN, Herndon DN, Kulp GA, Jeschke MG. Propranolol decreases cardiac work in a dose-dependent manner in severely burned children. Surgery. 2011 Feb;149:231–239. doi: 10.1016/j.surg.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minifee PK, Barrow RE, Abston S, Desai M, Herndon DN. Improved myocardial oxygen utilization following propranolol infusion in adolescents with postburn hypermetabolism. J Pediatr Surg. 1989 Aug;24:806–810. doi: 10.1016/s0022-3468(89)80541-x. discussion 810–1. [DOI] [PubMed] [Google Scholar]
- 49.Breitenstein E, Chiolero RL, Jequier E, Dayer P, Krupp S, Schutz Y. Effects of beta-blockade on energy metabolism following burns. Burns. 1990 Aug;16:259–264. doi: 10.1016/0305-4179(90)90136-k. [DOI] [PubMed] [Google Scholar]
- 50.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001 Oct 25;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 51.Jeschke MG, Williams FN, Finnerty CC, Rodriguez NA, Kulp GA, Ferrando A, et al. The effect of ketoconazole on post-burn inflammation, hypermetabolism and clinical outcomes. PLoS One. 2012;7:e35465. doi: 10.1371/journal.pone.0035465. [DOI] [PMC free article] [PubMed] [Google Scholar]