Abstract
Maternal diabetes-induced birth defects occur in 6-10% of babies born to mothers with pregestational diabetes, representing a significant maternal-fetal health problem. Currently, these congenital malformations represent a significant maternal-fetal medicine issue, but are likely to create an even greater public health threat as 3 million women of reproductive age (19-44 years) have diabetes in the United States alone, and this number is expected to double by 2030. Neural tube defects (NTDs) and congenital heart defects are the most common types of birth defects associated with maternal diabetes. Animal studies have revealed that embryos under hyperglycemic conditions exhibit high levels of oxidative stress resulting from enhanced production of reactive oxygen species and impaired antioxidant capability. Oxidative stress activates a set of pro-apoptotic kinase signaling intermediates leading to abnormal cell death in the embryonic neural tube, which causes NTD formation. Work in animal models also has revealed that maternal diabetes triggers a series of signaling intermediates: protein kinase C (PKC) isoforms, PKCα, βII and δ; apoptosis signal-regulating kinase 1 (ASK1), c-Jun-N-terminal kinase 1/2 (JNK1/2), caspase and apoptosis. Specifically, maternal diabetes in rodent models activates the pro-apoptotic unfolded protein response and endoplasmic reticulum (ER) stress. A reciprocal causation between JNK1/2 activation and ER stress exists in diabetic embryopathy. Molecular studies further demonstrate that deletion of the genes for PKCα, Ask1, Jnk1 or Jnk2 abolishes maternal diabetes-induced neural progenitor apoptosis and ameliorates NTD formation. Similar preventive effects are also observed when ASK1, JNK1/2 or ER stress is inhibited. Cell membrane stabilizers and antioxidant supplements are also effective in prevention of diabetes-induced birth defects. Mechanistic studies have revealed important insights into our understanding the cause of diabetic embryopathy and have provided a basis for future interventions against birth defects or other pregnancy complications associated with maternal diabetes. The knowledge of a molecular pathway map identified in animal studies has created unique opportunities to identify molecular targets for therapeutic intervention.
Keywords: diabetic embryopathy, pro-apoptotic kinase signaling, oxidative stress hypothesis, neural tube defects, protein kinase C, apoptosis signal-regulating kinase 1, c-Jun-N-terminal kinase 1/2, endoplasmic reticulum stress
Introduction
Each year in the United States, about 150,000 babies—3% of all live births—are born with at least one major congenital malformation1, 2. The Prevalence of birth defects is significantly worse in offspring of women who have type 1 or 2 diabetes. In these cases, 6%–10% of babies are born with a major congenital malformation3, 4. Based on the National Health and Nutrition Examination Survey, conducted from 1988–1994, 1.1% of women 20–39 years of age have type 1 or 2 diabetes5, and the incidence of diabetes among women of childbearing age has increased over the past four decades3. It is projected that the number of women of childbearing age with type 2 diabetes will double by 2010 3, suggesting that approximately 8,000 babies will be born each year in the United States with a congenital malformation in pregestational type 1 or 2 diabetic pregnancies.
Observational studies in humans have demonstrated a strong link between the extent of a mother's glycemic control and the incidence of congenital malformations in her offspring6-11. The putative teratogenic effects of hyperglycemia are supported by studies which demonstrate that clinical intervention targeted at achieving euglycemia can reduce the incidence of diabetes-associated birth defects12. When euglycemia is successfully maintained periconceptionally and during the first trimester, the prevalence of malformations is reduced to a level comparable to that of the general population13-15. However, even with excellent compliance and clinical care, euglycemia may be difficult to achieve and maintain. In addition, it is possible that organogenesis can be affected by short periods of hyperglycemia that are not reflected in the averaged values of glycosylated hemoglobin levels used to monitor glucose levels. A further obstacle is that most women with diabetes do not seek preconceptional care and most have unplanned pregnancies16.
Hence, a very important public health goal is to develop and implement new and easily accessible intervention strategies to decrease the occurrence of diabetes-induced congenital anomalies. To achieve this goal, we need a thorough understanding of the biochemical and molecular mechanisms underlying diabetic embryopathy. Although we are still far from reaching this goal, one area where we have made progress is in our understanding of the link between maternal hyperglycemia and oxidative stress. The molecular pathways involved in the cellular response to stress are potential therapeutic targets to prevent diabetes-induced embryonic malformations.
Excess apoptosis is a causal event in the induction of malformations
Diabetes-associated malformations may involve one or more organs and frequently result in significant disability or death12, 17. Adverse effects of maternal hyperglycemia have been documented in the yolk sac of diabetic animal models and in cultured murine embryos17-20. Studies with in vivo and in vitro models have determined that the stages of embryogenesis vulnerable to hyperglycemia-induced malformations comprise the critical period of organogenesis between 8.5-11.5 and 9–12 days of gestation in the mouse and rat, respectively, which is equivalent to gestational weeks 3–5 in humans21, 22.
Both clinical cases and animal studies have clearly demonstrated that the main characteristics of maternal hyperglycemia-associated defects are organ agenesis and underdevelopment17, 23. The organ systems most commonly affected include the central nervous, cardiovascular, gastrointestinal, craniofacial, genitourinary, and skeletal systems1, 23, 24. Because the neural folds and the heart develop early during embryogenesis, a higher incidence of malformations is observed in these organs. In the central nervous system, abnormalities can be categorized as underdevelopment of the midbrain and hindbrain, and failure of the neural tube to close at both anterior (rostral) and posterior (caudal) ends of the neural axis12, 17, 23. The failure of posterior neural tube closure results in spina bifida, one of the common birth defects among offspring of diabetic mothers24, 25.
Multiple studies have confirmed that excessive cell death, at least in the central nervous system, contributes to the abnormal development of structures in the embryos of diabetic animals17, 26-29. These observations strongly suggest that high concentrations of glucose cause damage to the neural progenitor cells, leading to apoptosis and, ultimately, abnormal organogenesis. However, the mechanisms by which hyperglycemia triggers cell death in the embryonic cells are largely unknown.
Programmed cell death is a precisely controlled cellular event that can be triggered by extracellular signals or other stimuli under normal and pathological conditions30-33. In most cases, apoptosis is characterized by the condensation of chromatin, degradation (fragmentation) of DNA, and formation of apoptotic bodies31, 34, 35, 36. The intracellular factors activated during apoptosis are the members of the Bcl-2 family36, notably Bax and Bim. When apoptosis initiates, Bax and Bim become activated37, 38. Activated Bax moves to the mitochondrion to form a transmembrane channel with Bak, another Bcl-2 family member. Bim is phosphorylated and translocates to the mitochondria to help open the Bax/Bak channel, resulting in cytochrome C release into the cytosol39, 40. Cytochrome C binds to apoptosis protease-activating factor-1, and the resulting complex activates Caspase-9. Activated Caspase-9 activates Caspase-3, which then turns on caspase-activated DNase and other pro-apoptotic factors, leading to DNA fragmentation and cell death41, 42.
Hyperglycemia-induced oxidative stress
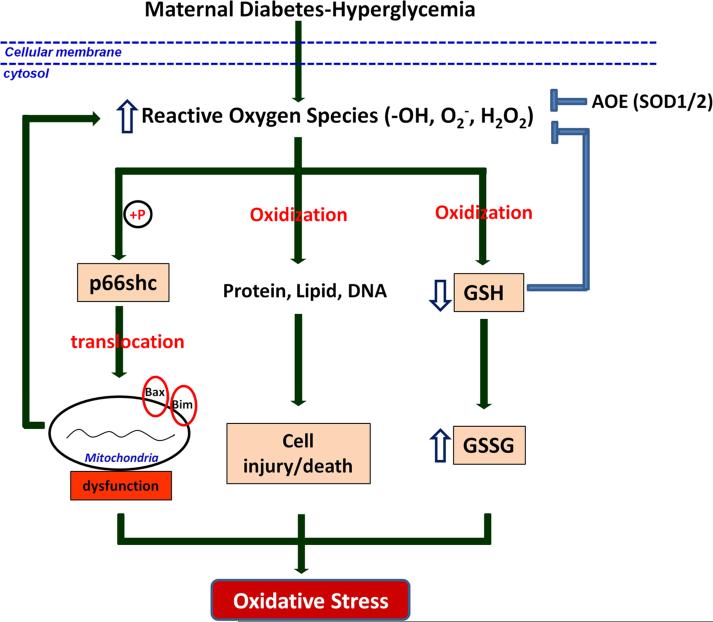
Evidence from clinical and experimental studies demonstrates that diabetes-related hyperglycemia leads to sustained generation of reactive oxygen species (ROS) and depletion of antioxidants, resulting in intracellular oxidative stress from an imbalance in intracellular reduction-oxidation (redox) homeostasis43-48. Under normal physiological conditions, oxygen free radicals, including hydroxyl radicals, superoxide anions, singlet oxygen, and hydrogen peroxide (H2O2), are produced during cellular energy metabolism in mitochondria49-52. Physiologic levels of ROS mediate intracellular signal transduction, which, in turn, regulates a wide range of cellular functions, including proliferation, differentiation, and migration49-51. However, under pathological conditions, excess ROS can oxidize proteins, lipids, and DNA, causing cell injury and cell death53 (Fig. 1).
Figure 1.
Hyperglycemia-induced oxidative stress. Sustained generation of ROS by maternal diabetes-related hyperglycemia activates p66shc by phosphorylation, which further causes mitochondrial dysfunction, aggravating ROS generaction and oxidative stress. Excess ROS, in one hand, causes cell injury and death through oxidizing protein, lipids and DNA; in the other hand, causes oxidization of GSH which results in depletion of antioxidant further augmenting oxidative stress. Generation of AOEs can protect cells by converting free radicals to non-toxic molecules.  : phosphorylation; GSH: glutathione; GSSG: glutathione disulfide; AOE: antioxidative enzyme.
: phosphorylation; GSH: glutathione; GSSG: glutathione disulfide; AOE: antioxidative enzyme.
Intracellular redox homeostasis depends on the relative balance between ROS production and the thiol buffers, glutathione (GSH) and thioredoxin54. Normally, the intracellular environment is maintained in a highly reduced state, which is mediated by high levels of reduced glutathione (GSH) and thioredoxin54. However, ROS produced via various cellular activities converts GSH into oxidized GSSG (glutathione disulfide) (Fig. 1). If ROS production exceeds the cellular thiol-buffering capacity, the oxidizing agents build up in the cell, causing damage and promoting oxidative stress55.
In addition to the GSH antioxidant buffering system, cells also protect themselves by producing antioxidative enzymes (AOEs) that convert damaging radicals to non-toxic molecules56-59 (Fig. 1). Superoxide dismutases (SODs) convert a superoxide anion into hydrogen peroxide, which is then reduced to water by GSH peroxidase (GPx) and catalase (CAT)51, 60. Two types of mammalian intracellular SODs, copper–zinc SOD (CuZn-SOD, or SOD1) and manganese SOD (Mn-SOD, or SOD2), have been extensively studied56, 58; SOD1 is localized in the cytoplasm, SOD2 in the mitochondrial matrix56, 58. It has been shown that SOD1, not only controls the redox state in the cytoplasm, but also regulates mitochondrial homeostasis61, 62. A study using transgenic mouse embryos overexpressing a human SOD1 transgene showed higher SOD activity and lower malformation rate in response to maternal diabetic conditions than wild-type embryos under the same conditions63. These studies strongly suggest that AOEs play an important role in protecting embryos against hyperglycemia-augmented oxidative stress (Fig. 1).
The effects of ROS can be transduced by a number of factors within the cell, including p66Shc, a member of the ShcA family which also includes p46Shc and p52Shc64 (Fig. 1). Unlike other members of this protein family, p66Shc is a specific target of ROS and a critical transducer of oxidative stress signaling leading to apoptosis65, 66. p66Shc is activated via phosphorylation of its serine 36 residue (S36) in the CH2 domain 67, 68. Targeted deletion of the p66Shc gene in the mouse increases cellular resistance to oxidative stress-induced apoptosis69, 70. The apoptotic effect of p66Shc may involve functional activation and/or transcriptional regulation of Bax and Bim 71 (Fig. 1). Recently, reports in animal models have shown that p66Shc plays a critical role in diabetic complications. In our laboratory, p66Shc is also affected in models of diabetic embryopathy. These observations suggest that p66Shc may be an important factor which mediates the effects of oxidative stress in diabetes-induced birth defects.
Oxidative stress-induced pro-apoptotic protein kinase C signaling
Intracellular ROS are generated by a number of mechanisms, including changes in ion homeostasis, membrane lipid metabolism and peroxidation72, 73. In embryos under maternal hyperglycemicconditions, products of arachidonic acid (AA) metabolism (lipoperoxides) have been detected 74, 75. The major pathway of AA metabolism involves cyclooxygenase-2 (COX-2)-catalyzed production of prostaglandin E2 (PGE2)76, 77. Adding PGE2 to the medium of embryos cultured under high glucose conditions prevents malformations, suggesting that PGE2 has a protective effect on embryos exposed to hyperglycemia78.
Other pathways of AA metabolism have also been identified in diabetic patients79, 80. In these alternate pathways, AA is converted into PGE2-like isoprostanes, such as 8-isoprostagladin F2 (8-iso-PGF2) and 8-iso-PGF2α, by non-COX-catalyzed peroxidation involving free radicals79, 80. These PGE2-like isoprostanes have been shown to have damaging effects in animal models and embryos79, 81. In embryos cultured under hyperglycemic conditions, as well as in diabetic patients, the level of 8-iso-PGF2 is dramatically elevated79, 82-84, suggesting a shift in metabolism from AA/PGE2 to AA/isoprostanes (Fig. 2).
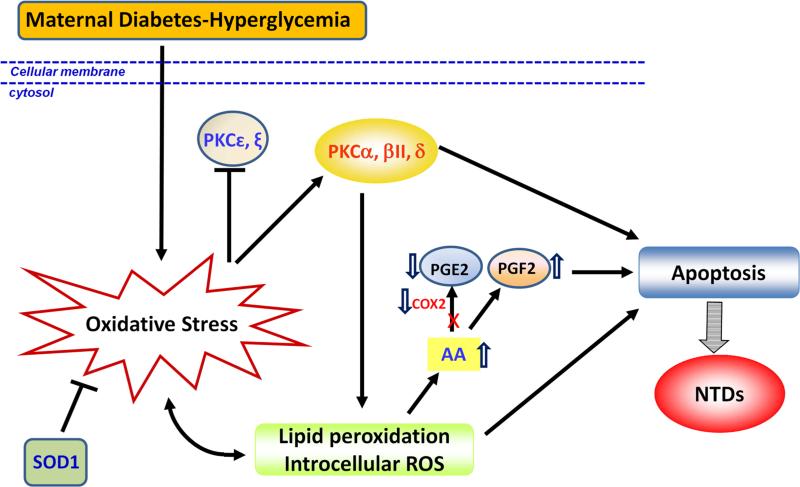
Figure 2.
Oxidative stress-induced pro-apoptotic protein kinase C signaling. Maternal diabetes-induced oxidative stress activates PKCα, βII, δ, while inhibits PKC[.epsilon], ξ. Activated PKCα induces lipid peroxidation, which in turn aggravates oxidative stress, induces apoptosis and diabetic embryopathy. COX2 helps lipid peroxidation product AA convert to PGE2, which has protective effect on embryos from oxidative stress. Hyperglycemia decreases COX2 activity resulting in generation of PGF2 from AA instead of PGE2, finally inducing apoptosis and NTD formation. x: blocked; AA: arachidonic acid. ⇓ downregulated; ⇑ upregulated.
Dietary AA appears to be protective against hyperglycemia-induced damage. We have shown that pregnant diabetic rats supplemented with AA display a reduced incidence of embryonic malformations75, 85, 86. Similar phenomena have also been seen with the addition of AA to embryos cultured in high concentrations of glucose75, 87, 88. Giving AA as a treatment may protect against hyperglycemic insults because exogenous AA may replace the endogenous AA displaced from the cell, thereby repairing and stabilizing cell membrane structure and function.
The PKC family of serine/threonine protein kinases consists of 12 members, which can be divided into the following three groups based on their activation mechanisms:89 1) PKCα, β1, β2, and γ require calcium and diacylglycerol (DAG) for activation; 2) PKCδ, ε, η, ν, and θ require only DAG; 3) PKCμ, ξ, and ι/λ do not require calcium or DAG, but instead require distinct lipid cofactors (e.g., ceramide and phosphatidylinositol-4-phosphate)89, 90 . Substrate specificity of an individual PKC family member involves binding to its specific membrane-bound anchor protein and becoming localized to a particular cellular compartment, such as the plasma membrane, cytoskeleton, mitochondrion, or nucleus89. PKCs are involved in a number of cellular activities, including proliferation, migration, apoptosis, differentiation, and secretion89, 91.
Prolonged activation of PKC by hyperglycemia has been documented in people with diabetes, animal models, and cultured cells92-95. Specific PKC isoforms (α, β2, and δ) are upregulated, while others (ε and ξ) are downregulated in diabetic embryopathy (Fig. 2). Pharmacological inhibition of the activity of PKCα, -β2, and –δ results in significant decreases in NTD rates in embryos cultured under high glucose conditions. Molecular studies have further uncovered a functional role for PKCα activation in diabetic embryopathy96. Deletion of the Prkca gene in PKCα knockout mice significantly blocks caspase activation and apoptosis leading to a reduction in NTD formation rate in diabetic pregnancies96.
Evidence suggests that maternal diabetes-induced oxidative stress is a major contributor to PKC activation. Transgenic overexpression of SOD1, which suppresses oxidative stress and NTD formation63, 97-100 represses maternal diabetes-induced phosphorylation of PKCα/βII and PKCδ97. In addition, PKC activation induces lipid peroxidation97, 101 (Fig. 2), and, thus, may further enhance the degree of oxidative stress seen in embryos subjected to hyperglycemic conditions. Therefore, our experiments have shown that oxidative stress and PKC activation form a positive feedback loop in diabetic embryopathy (Fig. 2).
Hyperglycemia and oxidative stress-altered MAPK signaling
Chronic and excessive oxidative stress results in cell injury and activation of a variety of stress-sensitive signaling pathways that often induce apoptosis73. Members of the mitogen-activated protein kinase (MAPK) family play a large role in programmed cell death and are activated in response to a variety of extracellular stimuli, including ROS102, 103. Activation of these serine/threonine kinases requires phosphorylation103. MAPKs can be grouped into extracellular signal-regulated kinases (ERKs), c-jun N-terminal kinases/stress-activated protein kinases (JNKs/SAPKs), p38, and others102 (Fig. 3). MAPK activity is altered in diabetic patients and in cells cultured in high glucose, suggesting that MAPKs may be involved in hyperglycemia-induced complications93, 104, 105. Although MAPKs are involved in various cellular activities, the ERK pathways primarily mediate cell proliferation, and the JNK and p38 pathways respond to cell stress signals and mediate apoptosis106, 107. Maternal diabetes induces JNK1/2 activation but suppresses ERK phosphorylation associated with increased apoptosis in the developing embryo23, 98, 105, 108, 109 (Fig. 3).
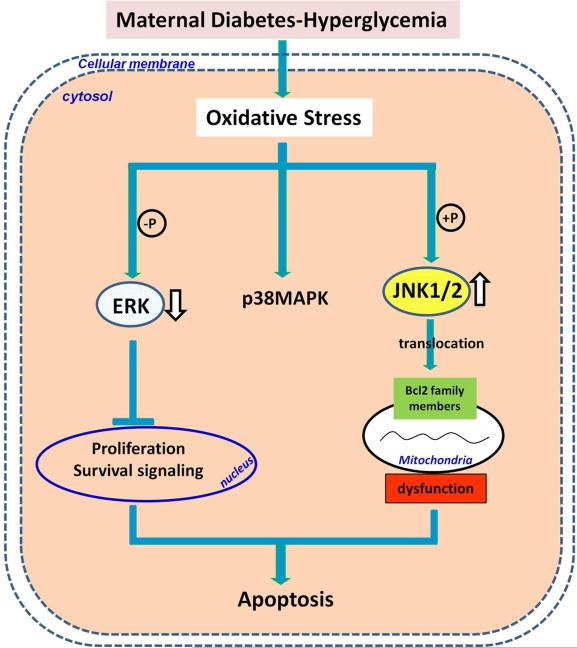
Figure 3.
MAPK signaling in diabetic embryopathy. Among MAPKs, ERK regulates cell proliferation, p38MAPK and JNKs regulate cell apoptosis. Hyperglycemia-induced oxidative stress decreases ERK activity by dephosphorylation and increases JNK1/2 activity by phosphorylation, while has no effect on p38MAPK. Decreased ERK suppresses cell proliferation, blocks cell survival signaling. Activated JNK1/2 induces translocation of Bcl2 family members to mitochondria membrane resulting in mitochondrial dysfunction and apoptosis.  : dephosphorylation;
: dephosphorylation;  : phosphorylation; ⇓ downregulated; ⇑ upregulated.
: phosphorylation; ⇓ downregulated; ⇑ upregulated.
Three members of the JNK family, JNK1, −2, and −3, and their splice variants have been characterized110. JNK1 and −2 are ubiquitously expressed, while JNK3 is primarily expressed in the nervous system111. JNKs are activated by phosphorylation by upstream MAPK kinases (MKKs), specifically MKK4 and MKK7. MKKs are, in turn, phosphorylated by other enzymes, one of which is apoptosis signal-regulating kinase (ASK) 1, a kinase which is activated under the influence of ROS112-114 (Fig. 3). JNKs can phosphorylate nuclear proteins, such as c-jun, ATF2, and Elk-1, as well as cytoplasmic proteins, such as Bcl-2 and Bim106, 110. Mice with a null mutation in any individual jnk gene develop normally115, as do double mutants of jnk1/jnk3 or jnk2/jnk3 116. Although jnk1/jnk2 null mutants die in utero due to an abundance of abnormal apoptosis in the brain117, individual jnk gene null mutants are still useful models for delineating apoptotic pathways involving JNKs. It remains unclear how JNK1/2 are activated by maternal diabetes. It is likely that JNK1/2 are activated by oxidative stress because overexpressing the antioxidant enzyme superoxide dismutase 1 (SOD1) in transgenic mice abrogates maternal diabetes-induced JNK1/2 activation98, 109.
Recently, we have revealed a functional role for JNK1/2 activation in diabetic embryopathy. In a cultured embryo system, inhibiting JNK1/ by the pharmacological inhibitor, SP600125, reduced high glucose-induced NTD formation, whereas adding sorbitol, a JNK1/2 activator, induced NTD formation118. Studies using gene knockout mouse models have uncovered a critical role for JNK1/2 activation in maternal diabetes-induced apoptosis and NTD formation98, 118. Deletion of either the Jnk1 gene or the Jnk2 gene abolishes the activation of four transcription factors downstream of JNK1/2, blocks maternal diabetes-induced caspase cascade activation, neural progenitor apoptosis and NTD formation109. These findings support the hypothesis that JNK1 and JNK2 are equally responsible for the induction of diabetic embryopathy, and that the JNK1/2 pathway mediates apoptosis and the teratogenicity of maternal diabetes (Fig. 3).
The reciprocal causation between JNK1/2 activation and ER stress
JNK1/2 activation induces pro-apoptotic cellular events leading to apoptosis. JNK1/2 activation positively modulates the activities and mitochondrial translocation of the proapoptotic Bcl-2 family members119 (Fig. 3). While both JNK1/2 and increased activities of pro-apoptotic Bcl-2 family members have been observed in diabetic embryopathy120, 121, the relationship between these events have not been established in relation to mitochondrial dysfunction, which is manifested in embryos exposed to maternal diabetes122, 123. Recently, endoplasmic reticulum (ER) stress has emerged as a proapoptotic event that is involved in the pathogeneses of diabetic complications124, 125. It is known that one of the unfolded protein response (UPR) sensors, inositol-requiring protein-1α (IRE1α), can activate JNK1/2 under ER stress conditions126 (Fig. 4). In our previous ultracellular study using electronic microscopy, aberrant maturational and cytoarchitectural changes associated with malformations in cultured embryos were observed under high glucose conditions 19, 127. These findings suggest that ER stress may be present in embryos exposed to maternal diabetes, and plays a role in JNK1/2 activation and apoptosis.
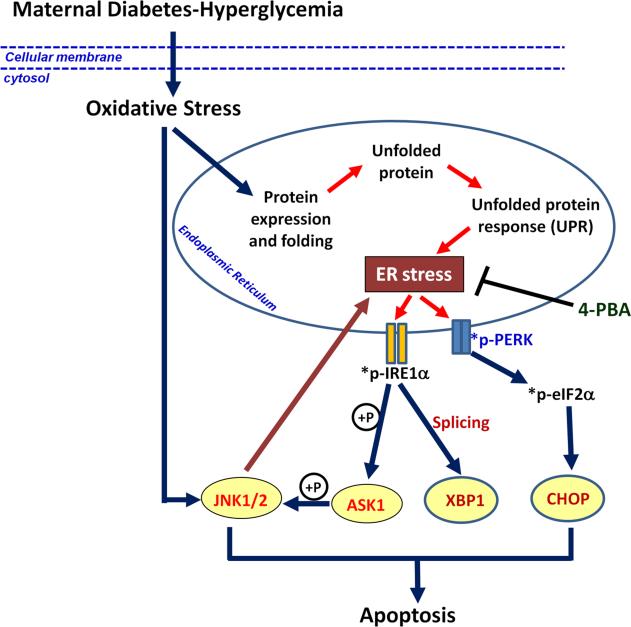
Figure 4.
Oxidative stress-induced JNK1/2 activation and ER stress. Maternal diabetes-induced oxidative stress causes ER stress by aggravating UPR events in ER. ER stress activates UPR sensors IRE1α and PERK by phosphorylation. Activated IRE1α leads to XBP1 splicing and ASK1-JNK1/2 signaling pathway, whereas phospho-PERK activates eIF2α and CHOP, which both finally induce apoptosis and diabetic embryopathy. Activated JNK1/2 can reversely intensify ER stress. 4-PBA blocks ER stress and ER stress-induced apoptosis and NTD formation. *p-: phosphorylated-;  : phosphorylation.
: phosphorylation.
Newly synthesized proteins are folded into their correct three-dimensional structures in the ER. A group of molecular chaperone proteins residing in the ER, such as binding immunoglobulin protein (BiP) and calnexin, is critical for the maintenance of ER luminal homeostasis. Accumulation of misfolded proteins due to ER luminal imbalance triggers ER stress and the induction of apoptosis128, 129. The UPR sensors, particularly IRE1α and protein kinase RNA-like ER kinase (PERK), mediate pro-apoptotic signaling in the ER128, 129 (Fig. 4).
Our recent study demonstrated that neuroepithelial cells in the developing neural tubes of embryos exposed to maternal diabetic conditions possess swollen/stressed ER lumens, and have elevated ER stress markers109. Our work in animal models has shown that maternal diabetes activates IRE1α and PERK109. IRE1α activation leads to splicing of X-box binding protein (XBP1) mRNA and subsequent formation of a transcription activator, whereas PERK activation results in phosphorylation of eukaryotic initiation factor 2α (eIF2α) leading to up-regulation of an apoptotic factor, C/EBP-homologous protein (CHOP)109 (Fig. 4).
Our laboratory has further revealed a causal role for ER stress in maternal diabetes-induced neuroepithelial cell apoptosis and NTD formation by blocking ER stress109. We have shown that the ER stress inhibitor, 4-phenylbutyric acid (4-PBA), diminishes ER stress markers, blocks apoptosis in the developing neural tube and NTD formation in embryos cultured under high glucose conditions109 (Fig. 4). Because it is widely accepted that ER stress activates JNK1/2, we have conducted subsequent studies to show that 4-PBA treatment also can suppress hyperglycemia-induced JNK1/2 activation109. The relationship between JNK1/2 activation and ER stress has been further defined using JNK1 and JNK2 knockout mice. Deletion of either Jnk1 or Jnk2 gene abolishes maternal diabetes-triggered UPR signaling and ER stress, indicating that JNK1/2 activation acts upstream of ER stress (Fig. 4). The observations we have made in our diabetic embryopathy model system agree with findings in another recent study, which showed that inhibiting JNK1/2 prevented ER stress and apoptosis in pancreatic cells130. Therefore, our work has indicated a reciprocal causation between JNK1/2 and ER stress exists in pathogenesis of diabetic embryopathy (Fig. 4).
The JNK1/2 upstream kinase, ASK1, initiates a key molecular signaling pathway
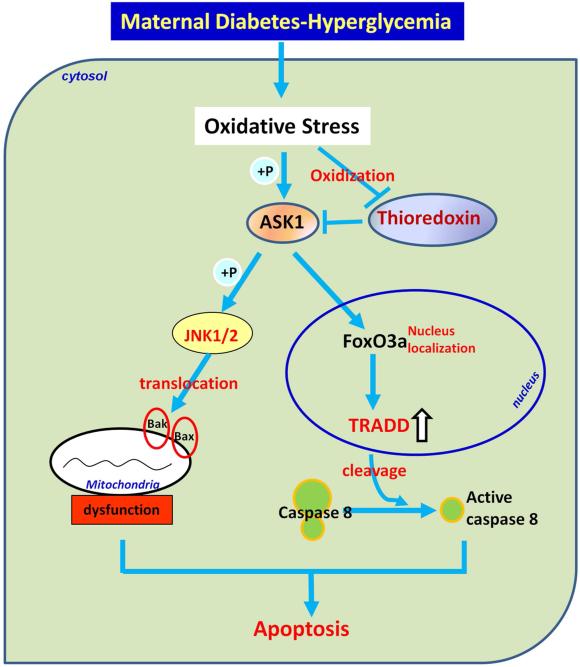
The upstream kinase of JNK1/2 has been identified as ASK1, which is activated by oxidative stress98 (Fig. 5). Under nondiabetic conditions, the ASK1 endogenous inhibitor, thioredoxin, is tightly associated with ASK1. Under diabetic conditions, the interaction of thioredoxin and ASK1 is disrupted and ASK1 is autophosphorylated and activated (Fig. 5). ASK1 induces the activation of Forkhead transcription factor 3a (FoxO3a), which in turn up-regulates the expression of a pro-apoptotic factor, tumor necrosis factor receptor type 1-associated DEATH domain protein (TRADD)131 (Fig. 5). TRADD up-regulation results in caspase 8 cleavage and neuroepithelial cell apoptosis (Fig. 5). Deletion of the Ask1 gene, the FoxO3a gene, or thioredoxin treatment ameliorates maternal diabetes-induced apoptosis and NTD formation. Our work has revealed a comprehensive pathway, the ASK1-JNK1/2-FoxO3a-TRADD-caspase 8 pathway131 (Fig. 5), which provides important insights into our understanding of the mechanisms underlying the teratologenicity of diabetes.
Figure 5.
Oxidative stress activates ASK1 signaling pathway. Oxidative stress activates ASK1 by disrupting its interaction with oxidized-thioredoxin. Free ASK1 quickly autophosphorylates and further phosphorylates JNK1/2, induces translocation of Bcl2 family members to mitochondria and apoptosis. Phospho-ASK1 can also increase nucleus transpotation of transcription factor FoxO3a, which induces TRADD expression. Up-regulation of TRADD leads to caspase 8 cleavege and apoptosis. 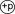 : phosphorylation; ⇑ upregulated.
: phosphorylation; ⇑ upregulated.
Future perspectives and clinical relevance
The prevalence rate of diabetes in women of childbearing age is rising all over the world, turning this chronic condition into a global pandemic that is associated with significant adverse maternal, fetal and neonatal outcomes132-137. Hyperglycemia during the periconceptional period and later in gestation is a major teratogenic factor10, 138-141 causing a range of adverse outcomes from fetal death, to congenital anomalies, to accelerated fetal growth and delivery complications, to higher rates of metabolic syndrome in adults due to altered in utero programming142, 143.
Clinical interventions are intended to help patients achieve and maintain euglycemia138, 144. Glycemic control is managed by measuring levels of a patient's glycohemoglobin (HgA1c), self-monitoring or continuous glucose monitoring. However, many controversies still exist in the medical community regarding the best glucose monitoring methods, desired glucose target values and treatment options.
When measured by a healthcare practitioner, a patient's HgA1c reading reflects her mean concentration of blood glucose levels in the prior 4-6 weeks. In addition, although self-monitored blood glucose readings or continuous glucose monitoring add information on the fluctuations of a patient's glucose levels, and may reveal short-term hypo- or hyperglycemia, the exact combination of monitoring techniques, and how frequently finger-stick blood glucose and HgA1c measurements should be performed is still undetermined. The debate also continues regarding which glucose values are associated with adverse outcomes, and which glucose values should be attained to improve pregnancy outcomes. Even if an ideal glucose level is determined, the available treatment options to achieve euglycemia vary greatly: from diet and exercise alone; to oral hypoglycemic drugs, combined with short, intermediate and/or long acting, self-injectable agents; to insulin pumps. Although maternal diabetes-associated adverse pregnancy outcomes may be reduced by normalizing a woman's blood glucose levels, her risk of complications remains higher than the risk in healthy women. As the number of people with diagnosed and undiagnosed diabetes continues to rise145, and considering that many pregnancies are unplanned, achieving euglycemia at the periconceptional period is almost an unreachable goal. Therefore, there is a significant need to develop new and improved strategies to prevent diabetes-associated birth defects and pregnancy complications.
Work in animal models, as well as translational research, has opened up a new era of intervention for diabetic pregnant women. Studies that have focused on the mechanism of diabetes-induced congenital anomalies have revealed that enhanced production of ROS, impaired antioxidant capability, high oxidative stress and increased abnormal apoptosis are some of the major underlying causes for hyperglycemia-induced adverse events in animal models of diabetic pregnancies98-100, 131, 146-149. Targeted interventions aimed at blocking the events causing altered cell function and excess cell death may offer other strategies for reducing diabetes associated mal effects. Antioxidants, inhibitors of PKC specific isoforms, thioredoxin, 4-PBA and caspase inhibitors are good candidates for therapeutic intervention131, 149, 150. However, further research is needed to prove safety and efficacy of any of these candidates in women with diabetes.
Condensation.
Pro-apoptotic kinase signaling mediates the effect of oxidative stress in diabetic embryopathy
Acknowledgments
The studies are supported by NIH R01DK083243, R01DK101972, R56 DK095380 (P. Y), R01DK103024 (to P. Y and E. A. R) and the Basic Science Award, American Diabetes Association (to P. Y). We thank the support from the Office of Dietary Supplements, National Institute of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None of the authors have a conflict of interest.
References
- 1.Reece EA, Homko C, Miodovnik M, Langer O. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Arkansas, May 2002. J Matern Fetal Neonatal Med. 2002;12:362–4. doi: 10.1080/jmf.12.6.362.364. [DOI] [PubMed] [Google Scholar]
- 2.Sever L, Lynberg MC, Edmonds LD. The impact of congenital malformations on public health. Teratology. 1993;48:547–49. doi: 10.1002/tera.1420480603. [DOI] [PubMed] [Google Scholar]
- 3.Feig DS, Palda VA. Type 2 diabetes in pregnancy: a growing concern. Lancet. 2002;359:1690–92. doi: 10.1016/S0140-6736(02)08599-9. [DOI] [PubMed] [Google Scholar]
- 4.Molsted-Pedersen L, Tygstrup I, Pedersen J. Congenital malformations in newborn infants of diabetic mothers. Lancet. 1964:i, 1124–26. doi: 10.1016/s0140-6736(64)91805-7. [DOI] [PubMed] [Google Scholar]
- 5.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 6.Greene MF, Hare JW, Cloherty JP, Benacerraf BR, Soeldner JS. First-trimester hemoglobin A1 and risk for major malformation and spontaneous abortion in diabetic pregnancy. Teratology. 1989;39:225–31. doi: 10.1002/tera.1420390303. [DOI] [PubMed] [Google Scholar]
- 7.Rose BI, Graff S, Spencer R, Hensleigh P, Fainstat T. Major congenital anomalies in infants and glycosylated hemoglobin levels in insulin-requiring diabetic mothers. J Perinatol. 1988;8:309–11. [PubMed] [Google Scholar]
- 8.Lucas MJ, Leveno KJ, Williams ML, Raskin P, Whalley PJ. Early pregnancy glycosylated hemoglobin, severity of diabetes, and fetal malformations. Am J Obstet Gynecol. 1989;161:426–31. doi: 10.1016/0002-9378(89)90536-x. [DOI] [PubMed] [Google Scholar]
- 9.Key TC, Giuffrida R, Moore TR. Predictive value of early pregnancy glycohemoglobin in the insulin- treated diabetic patient. Am J Obstet Gynecol. 1987;156:1096–100. doi: 10.1016/0002-9378(87)90117-7. [DOI] [PubMed] [Google Scholar]
- 10.Miller E, Hare JW, Cloherty JP, et al. Elevated maternal hemoglobin A1c in early pregnancy and major congenital anomalies in infants of diabetic mothers. The New England journal of medicine. 1981;304:1331–34. doi: 10.1056/NEJM198105283042204. [DOI] [PubMed] [Google Scholar]
- 11.Ylinen K, Aula P, Stenman UH, Kesaniemi-Kuokkanen T, Teramo K. Risk Of Minor And Major Fetal Malformations In Diabetics With High Haemoglobin A1C Values In Early Pregnancy. Brmed J (Clin Resed) 1984;289:345–46. doi: 10.1136/bmj.289.6441.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson UJ, Cederberg J, Wentzel P. Congenital malformations in offspring of diabetic mothers--animal and human studies. Rev Endocr Metab Disord. 2003;4:79–93. doi: 10.1023/a:1021879504372. [DOI] [PubMed] [Google Scholar]
- 13.Persson B. Prevention of fetal malformation with antioxidants in diabetic pregnancy. Pediatr Res. 2001;49:742–43. doi: 10.1203/00006450-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Fuhrmann K, Reiher H, Semmler K, Glockner E. The effect of intensified conventional insulin therapy before and during pregnancy on the malformation rate in offspring of diabetic mothers. Exp Clin Endocrinol. 1984;83:173–77. doi: 10.1055/s-0029-1210327. [DOI] [PubMed] [Google Scholar]
- 15.Kitzmiller JL, Gavin LA, Gin GD, Jovanovic-Peterson L, Main EK, Zigrang WD. Preconception care of diabetes. Glycemic control prevents congenital anomalies. JAMA : the journal of the American Medical Association. 1991;265:731–36. [PubMed] [Google Scholar]
- 16.Holing EV, Beyer CS, Brown ZA, Connell FA. Why don't women with diabetes plan their pregnancies? Diabetes Care. 1998;21:889–95. doi: 10.2337/diacare.21.6.889. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson UJ, Borg LA, Cederberg J, et al. Pathogenesis of diabetes-induced congenital malformations. Ups J Med Sci. 2000;105:53–84. doi: 10.1517/03009734000000055. [DOI] [PubMed] [Google Scholar]
- 18.Reece EA, Pinter E, Homko C, Wu YK, Naftolin F. The yolk sac theory: closing the circle on why diabetes-associated malformations occur. J Soc Gynecol Investig. 1994;1:3–13. [PubMed] [Google Scholar]
- 19.Pinter E, Reece EA, Leranth CZ, et al. Yolk sac failure in embryopathy due to hyperglycemia: ultrastructural analysis of yolk sac differentiation associated with embryopathy in rat conceptuses under hyperglycemic conditions. Teratology. 1986;33:73–84. doi: 10.1002/tera.1420330110. [DOI] [PubMed] [Google Scholar]
- 20.Wentzel P, Wentzel CR, Gareskog MB, Eriksson UJ. Induction of embryonic dysmorphogenesis by high glucose concentration, disturbed inositol metabolism, and inhibited protein kinase C activity. Teratology. 2001;63:193–201. doi: 10.1002/tera.1034. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson UJ, Bone AJ, Turnbull DM, Baird JD. Timed interruption of insulin therapy in diabetic BB/E rat pregnancy: effect on maternal metabolism and fetal outcome. Acta Endocrinol (Copenh) 1989;120:800–10. doi: 10.1530/acta.0.1200800. [DOI] [PubMed] [Google Scholar]
- 22.Reece EA, Wiznitzer A, Homko CJ, Hagay Z, Wu YK. Synchronization of the factors critical for diabetic teratogenesis: an in vitro model. American journal of obstetrics and gynecology. 1996;174:1284–8. doi: 10.1016/s0002-9378(96)70672-5. [DOI] [PubMed] [Google Scholar]
- 23.Reece EA, Eriksson UJ. The pathogenesis of diabetes-associated congenital malformations. Obstet Gynecol Clin North Am. 1996;23:29–45. doi: 10.1016/s0889-8545(05)70243-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Z, Reece EA. Experimental mechanisms of diabetic embryopathy and strategies for developing therapeutic interventions. J Soc Gynecol Investig. 2005;12:549–57. doi: 10.1016/j.jsgi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Northrup H, Volcik KA. Spina bifida and other neural tube defects. Curr Probl Pediatr. 2000;30:313–32. doi: 10.1067/mpp.2000.112052. [DOI] [PubMed] [Google Scholar]
- 26.Moley KH. Hyperglycemia and apoptosis: mechanisms for congenital malformations and pregnancy loss in diabetic women. Trends Endocrinol Metab. 2001;12:78–82. doi: 10.1016/s1043-2760(00)00341-6. [DOI] [PubMed] [Google Scholar]
- 27.Fine EL, Horal M, Chang TI, Fortin G, Loeken MR. Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes. 1999;48:2454–62. doi: 10.2337/diabetes.48.12.2454. [DOI] [PubMed] [Google Scholar]
- 28.Forsberg H, Eriksson UJ, Welsh N. Apoptosis in embryos of diabetic rats. Pharmacol Toxicol. 1998;83:104–11. doi: 10.1111/j.1600-0773.1998.tb01452.x. [DOI] [PubMed] [Google Scholar]
- 29.Sun F, Kawasaki E, Akazawa S, et al. Apoptosis and its pathway in early post-implantation embryos of diabetic rats. Diabetes Res Clin Pract. 2005;67:110–8. doi: 10.1016/j.diabres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Evan GI, Brown L, Whyte M, Harrington E. Apoptosis and the cell cycle. [Review] [84 refs]. Current Opinion in Cell Biology. 1995;7:825–34. doi: 10.1016/0955-0674(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 31.Buja LM, Eigenbrodt ML, Eigenbrodt EH. Apoptosis and necrosis. Basic types and mechanisms of cell death. [Review] [81 refs]. Archives of Pathology & Laboratory Medicine. 1993;117:1208–14. [PubMed] [Google Scholar]
- 32.Davies AM. Regulation of neuronal survival and death by extracellular signals during development. Embo J. 2003;22:2537–45. doi: 10.1093/emboj/cdg254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vila M, Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:365–75. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- 34.Farber E. Programmed cell death: necrosis versus apoptosis. [Review] [37 refs]. Modern Pathology. 1994;7:605–09. [PubMed] [Google Scholar]
- 35.Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003;40:401–13. doi: 10.1016/s0896-6273(03)00601-9. [DOI] [PubMed] [Google Scholar]
- 36.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 37.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–25. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol. 2006;18:685–9. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–13. doi: 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 40.van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16:203–13. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- 41.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–67. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 42.Ferraro E, Corvaro M, Cecconi F. Physiological and pathological roles of Apaf1 and the apoptosome. J Cell Mol Med. 2003;7:21–34. doi: 10.1111/j.1582-4934.2003.tb00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dincer Y, Akcay T, Alademir Z, Ilkova H. Assessment of DNA base oxidation and glutathione level in patients with type 2 diabetes. Mutat Res. 2002;505:75–81. doi: 10.1016/s0027-5107(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 44.Sakamaki H, Akazawa S, Ishibashi M, et al. Significance of glutathione-dependent antioxidant system in diabetes- induced embryonic malformations. Diabetes. 1999;48:1138–44. doi: 10.2337/diabetes.48.5.1138. [DOI] [PubMed] [Google Scholar]
- 45.Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull. 1993;49:642–52. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- 46.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 47.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 48.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 49.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–8. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 50.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO reports. 2002;3:420–5. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raha S, Robinson BH. Mitochondria, oxygen free radicals, and apoptosis. Am J Med Genet. 2001;106:62–70. doi: 10.1002/ajmg.1398. [DOI] [PubMed] [Google Scholar]
- 52.Bauer G. Reactive oxygen and nitrogen species: efficient, selective, and interactive signals during intercellular induction of apoptosis. Anticancer Res. 2000;20:4115–39. [PubMed] [Google Scholar]
- 53.Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221–31. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- 54.Sen CK. Cellular thiols and redox-regulated signal transduction. Curr Top Cell Regul. 2000;36:1–30. doi: 10.1016/s0070-2137(01)80001-7. [DOI] [PubMed] [Google Scholar]
- 55.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 56.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 57.Mates JM, Sanchez-Jimenez F. Antioxidant enzymes and their implications in pathophysiologic processes. Front Biosci. 1999;4:D339–45. doi: 10.2741/mates. [DOI] [PubMed] [Google Scholar]
- 58.Kahl R, Kampkotter A, Watjen W, Chovolou Y. Antioxidant enzymes and apoptosis. Drug Metab Rev. 2004;36:747–62. doi: 10.1081/dmr-200033488. [DOI] [PubMed] [Google Scholar]
- 59.Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145–69. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 60.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aquilano K, Vigilanza P, Rotilio G, Ciriolo MR. Mitochondrial damage due to SOD1 deficiency in SH-SY5Y neuroblastoma cells: a rationale for the redundancy of SOD1. Faseb J. 2006;20:1683–5. doi: 10.1096/fj.05-5225fje. [DOI] [PubMed] [Google Scholar]
- 62.O'Brien KM, Dirmeier R, Engle M, Poyton RO. Mitochondrial protein oxidation in yeast mutants lacking manganese-(MnSOD) or copper- and zinc-containing superoxide dismutase (CuZnSOD): evidence that MnSOD and CuZnSOD have both unique and overlapping functions in protecting mitochondrial proteins from oxidative damage. J Biol Chem. 2004;279:51817–27. doi: 10.1074/jbc.M405958200. [DOI] [PubMed] [Google Scholar]
- 63.Hagay ZJ, Weiss Y, Zusman I, et al. Prevention of diabetes-associated embryopathy by overexpression of the free radical scavenger copper zinc superoxide dismutase in transgenic mouse embryos. American journal of obstetrics and gynecology. 1995;173:1036–41. doi: 10.1016/0002-9378(95)91323-8. [DOI] [PubMed] [Google Scholar]
- 64.Pellegrini M, Pacini S, Baldari CT. p66SHC: the apoptotic side of Shc proteins. Apoptosis. 2005;10:13–8. doi: 10.1007/s10495-005-6057-8. [DOI] [PubMed] [Google Scholar]
- 65.Graiani G, Lagrasta C, Migliaccio E, et al. Genetic deletion of the p66Shc adaptor protein protects from angiotensin II-induced myocardial damage. Hypertension. 2005;46:433–40. doi: 10.1161/01.HYP.0000174986.73346.ba. [DOI] [PubMed] [Google Scholar]
- 66.Ravichandran KS. Signaling via Shc family adapter proteins. Oncogene. 2001;20:6322–30. doi: 10.1038/sj.onc.1204776. [DOI] [PubMed] [Google Scholar]
- 67.Trinei M, Giorgio M, Cicalese A, et al. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21:3872–8. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 68.Obreztchikova M, Elouardighi H, Ho M, Wilson BA, Gertsberg Z, Steinberg SF. Distinct signaling functions for Shc isoforms in the heart. J Biol Chem. 2006;281:20197–204. doi: 10.1074/jbc.M601859200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Migliaccio E, Giorgio M, Mele S, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 70.Giorgio M, Migliaccio E, Orsini F, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–33. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 71.Pacini S, Pellegrini M, Migliaccio E, et al. p66SHC promotes apoptosis and antagonizes mitogenic signaling in T cells. Mol Cell Biol. 2004;24:1747–57. doi: 10.1128/MCB.24.4.1747-1757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29:323–33. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 73.Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxid Redox Signal. 2002;4:405–14. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 74.Goldman AS, Baker L, Piddington R, Marx B, Herold R, Egler J. Hyperglycemia-induced teratogenesis is mediated by a functional deficiency of arachidonic acid. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:8227–31. doi: 10.1073/pnas.82.23.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinter E, Reece EA, Leranth CZ, et al. Arachidonic Acid Prevents Hyperglycemia-Associated Yolk Sac damage and embryopathy. Am J Obstet Gynecol. 1986;155:691–702. doi: 10.1016/s0002-9378(86)80001-1. [DOI] [PubMed] [Google Scholar]
- 76.Kudo I, Murakami M. Regulatory functions of prostaglandin E2 synthases. Adv Exp Med Biol. 2003;525:103–6. doi: 10.1007/978-1-4419-9194-2_20. [DOI] [PubMed] [Google Scholar]
- 77.Claria J. Cyclooxygenase-2 biology. Curr Pharm Des. 2003;9:2177–90. doi: 10.2174/1381612033454054. [DOI] [PubMed] [Google Scholar]
- 78.Goto MP, Goldman AS, Uhing MR. PGE2 prevents anomalies induced by hyperglycemia or diabetic serum in mouse embryos. Diabetes. 1992;41:1644–50. doi: 10.2337/diab.41.12.1644. [DOI] [PubMed] [Google Scholar]
- 79.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc NatlAcadSci USA. 1990;87:9383–87. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morrow JD. The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab Rev. 2000;32:377–85. doi: 10.1081/dmr-100102340. [DOI] [PubMed] [Google Scholar]
- 81.Wentzel P, Eriksson UJ. 8-Iso-PGF(2alpha) administration generates dysmorphogenesis and increased lipid peroxidation in rat embryos in vitro. Teratology. 2002;66:164–68. doi: 10.1002/tera.10068. [DOI] [PubMed] [Google Scholar]
- 82.Gopaul NK, Anggard EE, Mallet AI, Betteridge DJ, Wolff SP, Nouroozzadeh J. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. 1995;368:225–29. doi: 10.1016/0014-5793(95)00649-t. [DOI] [PubMed] [Google Scholar]
- 83.Decsi T, Minda H, Hermann R, et al. Polyunsaturated fatty acids in plasma and erythrocyte membrane lipids of diabetic children. Prostaglandins Leukot Essent Fatty Acids. 2002;67:203–10. doi: 10.1054/plef.2002.0420. [DOI] [PubMed] [Google Scholar]
- 84.Wentzel P, Welsh N, Eriksson UJ. Developmental damage, increased lipid peroxidation, diminished cyclooxygenase-2 gene expression, and lowered prostaglandin E2 levels in rat embryos exposed to a diabetic environment. Diabetes. 1999;48:813–20. doi: 10.2337/diabetes.48.4.813. [DOI] [PubMed] [Google Scholar]
- 85.Reece EA, Wu YK, Wiznitzer A, et al. Dietary polyunsaturated fatty acid prevents malformations in offspring of diabetic rats. American journal of obstetrics and gynecology. 1996;175:818–23. doi: 10.1016/s0002-9378(96)80005-6. [DOI] [PubMed] [Google Scholar]
- 86.Reece EA, Wu YK, Zhao Z, Dhanasekaran D. Dietary vitamin and lipid therapy rescues aberrant signaling and apoptosis and prevents hyperglycemia-induced diabetic embryopathy in rats. American journal of obstetrics and gynecology. 2006;194:580–5. doi: 10.1016/j.ajog.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 87.Dhanasekaran N, Wu YK, Reece EA. Signaling pathways and diabetic embryopathy. Semin Reprod Endocrinol. 1999;17:167–74. doi: 10.1055/s-2007-1016223. [DOI] [PubMed] [Google Scholar]
- 88.Engstrom E, Haglund A, Eriksson UJ. Effects of maternal diabetes or in vitro hyperglycemia on uptake of palmitic and arachidonic acid by rat embryos. Pediatr Res. 1991;30:150–3. doi: 10.1203/00006450-199108000-00005. [DOI] [PubMed] [Google Scholar]
- 89.Dempsey EC, Newton AC, Mochly-Rosen D, et al. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–38. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 90.Shirai Y, Saito N. Activation mechanisms of protein kinase C: maturation, catalytic activation, and targeting. J Biochem (Tokyo) 2002;132:663–8. doi: 10.1093/oxfordjournals.jbchem.a003271. [DOI] [PubMed] [Google Scholar]
- 91.Wright MM, Mcmaster CR. Phospholipid synthesis, diacylglycerol compartmentation, and apoptosis. Biol Res. 2002;35:223–9. doi: 10.4067/s0716-97602002000200014. [DOI] [PubMed] [Google Scholar]
- 92.Curtis TM, Scholfield CN. The role of lipids and protein kinase Cs in the pathogenesis of diabetic retinopathy. Diabetes Metab Res Rev. 2004;20:28–43. doi: 10.1002/dmrr.431. [DOI] [PubMed] [Google Scholar]
- 93.Srivastava AK. High glucose-induced activation of protein kinase signaling pathways in vascular smooth muscle cells: a potential role in the pathogenesis of vascular dysfunction in diabetes (review). Int J Mol Med. 2002;9:85–9. [PubMed] [Google Scholar]
- 94.Way KJ, Katai N, King GL. Protein kinase C and the development of diabetic vascular complications. Diabet Med. 2001;18:945–59. doi: 10.1046/j.0742-3071.2001.00638.x. [DOI] [PubMed] [Google Scholar]
- 95.Park JY, Takahara N, Gabriele A, et al. Induction of endothelin-1 expression by glucose: an effect of protein kinase C activation. Diabetes. 2000;49:1239–48. doi: 10.2337/diabetes.49.7.1239. [DOI] [PubMed] [Google Scholar]
- 96.Cao Y, Zhao Z, Eckert RL, Reece EA. Protein kinase Cbeta2 inhibition reduces hyperglycemia-induced neural tube defects through suppression of a caspase 8-triggered apoptotic pathway. American journal of obstetrics and gynecology. 2011;204:226, e1–5. doi: 10.1016/j.ajog.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X, Weng H, Reece EA, Yang P. SOD1 overexpression in vivo blocks hyperglycemia-induced specific PKC isoforms: substrate activation and consequent lipid peroxidation in diabetic embryopathy. American journal of obstetrics and gynecology. 2011;205:84, e1–6. doi: 10.1016/j.ajog.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X, Weng H, Xu C, Reece EA, Yang P. Oxidative stress-induced JNK1/2 activation triggers proapoptotic signaling and apoptosis that leads to diabetic embryopathy. Diabetes. 2012;61:2084–92. doi: 10.2337/db11-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang F, Reece EA, Yang P. Superoxide dismutase 1 overexpression in mice abolishes maternal diabetes-induced endoplasmic reticulum stress in diabetic embryopathy. American journal of obstetrics and gynecology. 2013;209:345, e1–7. doi: 10.1016/j.ajog.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weng H, Li X, Reece EA, Yang P. SOD1 suppresses maternal hyperglycemia-increased iNOS expression and consequent nitrosative stress in diabetic embryopathy. American journal of obstetrics and gynecology. 2012;206:448, e1–7. doi: 10.1016/j.ajog.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.von Ruecker AA, Han-Jeon BG, Wild M, Bidlingmaier F. Protein kinase C involvement in lipid peroxidation and cell membrane damage induced by oxygen-based radicals in hepatocytes. Biochemical and biophysical research communications. 1989;163:836–42. doi: 10.1016/0006-291x(89)92298-5. [DOI] [PubMed] [Google Scholar]
- 102.Kyosseva SV. Mitogen-activated protein kinase signaling. Int Rev Neurobiol. 2004;59:201–20. doi: 10.1016/S0074-7742(04)59008-6. [DOI] [PubMed] [Google Scholar]
- 103.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17:287–96. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 104.Kikkawa R, Koya D, Haneda M. Progression of diabetic nephropathy. Am J Kidney Dis. 2003;41:S19–21. doi: 10.1053/ajkd.2003.50077. [DOI] [PubMed] [Google Scholar]
- 105.Reece EA, Ma XD, Wu YK, Dhanasekaran D. Aberrant patterns of cellular communication in diabetes-induced embryopathy. I. Membrane signalling. J Matern Fetal Neonatal Med. 2002;11:249–53. doi: 10.1080/jmf.11.4.249.253. [DOI] [PubMed] [Google Scholar]
- 106.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–49. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 107.Lin A, Dibling B. The true face of JNK activation in apoptosis. Aging Cell. 2002;1:112–6. doi: 10.1046/j.1474-9728.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 108.Yang P, Zhao Z, Reece EA. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. American journal of obstetrics and gynecology. 2008;198:130, e1–7. doi: 10.1016/j.ajog.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 109.Li X, Xu C, Yang P. c-Jun NH2-terminal kinase 1/2 and endoplasmic reticulum stress as interdependent and reciprocal causation in diabetic embryopathy. Diabetes. 2013;62:599–608. doi: 10.2337/db12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin A. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays. 2003;25:17–24. doi: 10.1002/bies.10204. [DOI] [PubMed] [Google Scholar]
- 111.Martin JH, Mohit AA, Miller CA. Developmental expression in the mouse nervous system of the p493F12 SAP kinase. Brain Res Mol Brain Res. 1996;35:47–57. doi: 10.1016/0169-328x(95)00181-q. [DOI] [PubMed] [Google Scholar]
- 112.Ichijo H, Nishida E, Irie K, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–4. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 113.Cross JV, Templeton DJ. Oxidative stress inhibits MEKK1 by site-specific glutathionylation in the ATP-binding domain. Biochem J. 2004;381:675–83. doi: 10.1042/BJ20040591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. Embo J. 1998;17:2596–606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.She QB, Chen N, Bode AM, Flavell RA, Dong Z. Deficiency of c-Jun-NH(2)-terminal kinase-1 in mice enhances skin tumor development by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 2002;62:1343–8. [PubMed] [Google Scholar]
- 116.Dong C, Yang DD, Tournier C, et al. JNK is required for effector T-cell function but not for T-cell activation. Nature. 2000;405:91–4. doi: 10.1038/35011091. [DOI] [PubMed] [Google Scholar]
- 117.Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–76. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 118.Yang P, Zhao Z, Reece EA. Involvement of c-Jun N-terminal kinases activation in diabetic embryopathy. Biochemical and biophysical research communications. 2007;357:749–54. doi: 10.1016/j.bbrc.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 119.Prakasam A, Ghose S, Oleinik NV, et al. JNK1/2 regulate Bid by direct phosphorylation at Thr59 in response to ALDH1L1. Cell death & disease. 2014;5:e1358. doi: 10.1038/cddis.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang P, Zhao Z, Reece EA. Blockade of c-Jun N-terminal kinase activation abrogates hyperglycemia-induced yolk sac vasculopathy in vitro. American journal of obstetrics and gynecology. 2008;198:321, e1–7. doi: 10.1016/j.ajog.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 121.Zabihi S, Eriksson UJ, Wentzel P. Folic acid supplementation affects ROS scavenging enzymes, enhances Vegf-A, and diminishes apoptotic state in yolk sacs of embryos of diabetic rats. Reproductive toxicology. 2007;23:486–98. doi: 10.1016/j.reprotox.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 122.Xu C, Li X, Wang F, Weng H, Yang P. Trehalose prevents neural tube defects by correcting maternal diabetes-suppressed autophagy and neurogenesis. American journal of physiology Endocrinology and metabolism. 2013;305:E667–78. doi: 10.1152/ajpendo.00185.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang X, Borg LA, Eriksson UJ. Altered mitochondrial morphology of rat embryos in diabetic pregnancy. The Anatomical record. 1995;241:255–67. doi: 10.1002/ar.1092410212. [DOI] [PubMed] [Google Scholar]
- 124.Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS letters. 2009;583:1521–7. doi: 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lupachyk S, Watcho P, Stavniichuk R, Shevalye H, Obrosova IG. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes. 2013;62:944–52. doi: 10.2337/db12-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 127.Reece EA, Pinter E, Leranth CZ, et al. Ultrastructural analysis of malformations of the embryonic neural axis induced by in vitro hyperglycemic conditions. Teratology. 1985;32:363–73. doi: 10.1002/tera.1420320306. [DOI] [PubMed] [Google Scholar]
- 128.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO reports. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes & development. 1998;12:982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Verma G, Bhatia H, Datta M. JNK1/2 regulates ER-mitochondrial Ca2+ crosstalk during IL-1beta-mediated cell death in RINm5F and human primary beta-cells. Molecular biology of the cell. 2013;24:2058–71. doi: 10.1091/mbc.E12-12-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang P, Li X, Xu C, et al. Maternal hyperglycemia activates an ASK1-FoxO3acaspase 8 pathway that leads to embryonic neural tube defects. Science signaling. 2013;6:ra74. doi: 10.1126/scisignal.2004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Casson IF, Clarke CA, Howard CV, et al. Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. Bmj. 1997;315:275–8. doi: 10.1136/bmj.315.7103.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Galindo A, Burguillo AG, Azriel S, Fuente Pde L. Outcome of fetuses in women with pregestational diabetes mellitus. Journal of perinatal medicine. 2006;34:323–31. doi: 10.1515/JPM.2006.062. [DOI] [PubMed] [Google Scholar]
- 134.Hawthorne G, Robson S, Ryall EA, Sen D, Roberts SH, Ward Platt MP. Prospective population based survey of outcome of pregnancy in diabetic women: results of the Northern Diabetic Pregnancy Audit, 1994. Bmj. 1997;315:279–81. doi: 10.1136/bmj.315.7103.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Inkster ME, Fahey TP, Donnan PT, Leese GP, Mires GJ, Murphy DJ. Poor glycated haemoglobin control and adverse pregnancy outcomes in type 1 and type 2 diabetes mellitus: systematic review of observational studies. BMC pregnancy and childbirth. 2006;6:30. doi: 10.1186/1471-2393-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klemetti M, Nuutila M, Tikkanen M, Kari MA, Hiilesmaa V, Teramo K. Trends in maternal BMI, glycaemic control and perinatal outcome among type 1 diabetic pregnant women in 1989-2008. Diabetologia. 2012;55:2327–34. doi: 10.1007/s00125-012-2627-9. [DOI] [PubMed] [Google Scholar]
- 137.Stuebe AM, Landon MB, Lai Y, et al. Maternal BMI, glucose tolerance, and adverse pregnancy outcomes. American journal of obstetrics and gynecology. 2012;207:62, e1–7. doi: 10.1016/j.ajog.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fuhrmann K, Reiher H, Semmler K, Glockner E. The effect of intensified conventional insulin therapy before and during pregnancy on the malformation rate in offspring of diabetic mothers. Experimental and clinical endocrinology. 1984;83:173–7. doi: 10.1055/s-0029-1210327. [DOI] [PubMed] [Google Scholar]
- 139.Greene MF, Hare JW, Cloherty JP, Benacerraf BR, Soeldner JS. First-trimester hemoglobin A1 and risk for major malformation and spontaneous abortion in diabetic pregnancy. Teratology. 1989;39:225–31. doi: 10.1002/tera.1420390303. [DOI] [PubMed] [Google Scholar]
- 140.Karlsson K, Kjellmer I. The outcome of diabetic pregnancies in relation to the mother's blood sugar level. American journal of obstetrics and gynecology. 1972;112:213–20. doi: 10.1016/0002-9378(72)90118-4. [DOI] [PubMed] [Google Scholar]
- 141.Widness JA, Goldman AS, Susa JB, Oh W, Schwartz R. Impermeability of the rat placenta to insulin during organogenesis. Teratology. 1983;28:327–32. doi: 10.1002/tera.1420280304. [DOI] [PubMed] [Google Scholar]
- 142.Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. International journal of obesity. 2008;32(Suppl 7):S62–71. doi: 10.1038/ijo.2008.240. [DOI] [PubMed] [Google Scholar]
- 143.Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA : the journal of the American Medical Association. 2001;286:2516–8. doi: 10.1001/jama.286.20.2516. [DOI] [PubMed] [Google Scholar]
- 144.Yee LM, Cheng YW, Inturrisi M, Caughey AB. Effect of gestational weight gain on perinatal outcomes in women with type 2 diabetes mellitus using the 2009 Institute of Medicine guidelines. American journal of obstetrics and gynecology. 2011;205:257, e1–6. doi: 10.1016/j.ajog.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oakley GP., JR Failing to prevent birth defects caused by maternal diabetes mellitus. American journal of obstetrics and gynecology. 2012;206:179–80. doi: 10.1016/j.ajog.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 146.C RC, Horvat D, Leonard D, et al. Hyperglycemia impairs cytotrophoblast function via stress signaling. American journal of obstetrics and gynecology. 2014 doi: 10.1016/j.ajog.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 147.Yang P, Cao Y, Li H. Hyperglycemia induces inducible nitric oxide synthase gene expression and consequent nitrosative stress via c-Jun N-terminal kinase activation. American journal of obstetrics and gynecology. 2010;203:185, e5–11. doi: 10.1016/j.ajog.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang P, Li H. Epigallocatechin-3-gallate ameliorates hyperglycemia-induced embryonic vasculopathy and malformation by inhibition of Foxo3a activation. American journal of obstetrics and gynecology. 2010;203:75, e1–6. doi: 10.1016/j.ajog.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang P, Reece EA. Role of HIF-1alpha in maternal hyperglycemia-induced embryonic vasculopathy. American journal of obstetrics and gynecology. 2011;204:332, e1–7. doi: 10.1016/j.ajog.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Correa A, Gilboa SM, Botto LD, et al. Lack of periconceptional vitamins or supplements that contain folic acid and diabetes mellitus-associated birth defects. American journal of obstetrics and gynecology. 2012;206:218, e1–13. doi: 10.1016/j.ajog.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]