Abstract
Objective
Physical activity is critically important for successful aging, but its effect on adiposity markers at older ages is unclear as much of the evidence comes from self-reported data on physical activity. We assessed the associations of questionnaire-assessed and accelerometer-assessed physical activity with adiposity markers in older adults.
Design/Setting/Participants
This was a cross-sectional study on 3940 participants (age range 60-83 years) of the Whitehall II study who completed a 20-item physical activity questionnaire and wore a wrist-mounted accelerometer for 9 days in 2012 and 2013.
Measurements
Total physical activity was estimated using metabolic equivalent hours/week for the questionnaire and mean acceleration for the accelerometer. Time spent in moderate-and-vigorous physical activity (MVPA) was also assessed by questionnaire and accelerometer. Adiposity assessment included body mass index, waist circumference, and fat mass index. Fat mass index was calculated as fat mass/height² (kg/m²), with fat mass estimated using bioimpedance.
Results
Greater total physical activity was associated with lower adiposity for all adiposity markers in a dose-response manner. In men, the strength of this association was 2.4 to 2.8 times stronger with the accelerometer than with questionnaire data. In women, it was 1.9 to 2.3 times stronger. For MVPA, questionnaire data in men suggested no further benefit for adiposity markers past 1 hour/week of activity. This was not the case for accelerometer-assessed MVPA where, for example, compared with men undertaking <1 hour/week of accelerometer-assessed MVPA, waist circumference was 3.06 (95% confidence interval 2.06–4.06) cm lower in those performing MVPA 1–2.5 hours/week, 4.69 (3.47–5.91) cm lower in those undertaking 2.5–4 hours/week, and 7.11 (5.93–8.29) cm lower in those performing ≥4 hours/week.
Conclusions
The association of physical activity with adiposity markers in older adults was stronger when physical activity was assessed by accelerometer compared with questionnaire, suggesting that physical activity might be more important for adiposity than previously estimated.
Keywords: Physical activity, accelerometer, questionnaire, adiposity, body mass index, waist circumference
Increasing life expectancy and the obesity epidemic has led to increase in the number of older adults considered obese.1–3 For instance, the prevalence of obesity [that is, body mass index (BMI) ≥ 30 kg/m²] in the US adults aged 60 years and over is around 35%4 and corresponding figures for European countries vary between 20% and 30%.2,5–7 Obesity has a wide range of adverse health effects, such as reduced mobility, sleep apnea, and higher cardiovascular risk, including diabetes and hypertension,1–3 and recent studies suggest that, contrary to previous beliefs, it might also be a risk factor for fractures.8,9 In addition, although the relative risk of mortality associated with obesity decreases at older ages, the absolute risk continues to increase up to 75 years.1,2
Obesity results from an imbalance between energy intake and energy expenditure. Thus, both diet and physical activity are important to optimize the energy balance for weight control.10 Fat mass increases at older ages while lean body mass (muscle and bone) decreases. As physical activity is known to improve muscular function and bone health,11 its association with adiposity markers in older adults has received particular attention.1–3,12–14 Furthermore, although BMI tends to decrease after age 75, abdominal adiposity, often indicated by waist circumference, continues to increase with age6 and is associated with metabolic risk independently of BMI.15 Multiple adiposity markers must, therefore, be considered in order to better understand adiposity at older ages.
Physical activity in studies is mostly assessed by questionnaire, with significant measurement error resulting in potential biases in the association with adiposity.12,16–20 The correlation between self-reported and objective measures of physical activity is known to be low-to-moderate21,22 and appears to be even lower at older ages.23 Our aim in the present study was to quantify the association between physical activity and adiposity markers, including BMI, waist circumference, and fat mass index (FMI), in older adults using data from a large British population-based study of adults aged 60–83 years with physical activity assessed by both questionnaire and accelerometer.
Methods
Study Population
Data are drawn from the Whitehall II cohort study, established in 1985–1988 on 10,308 individuals (67% men), aged 35–55 years.24 Individuals gave written consent to participate in the study, and the University College London ethics committee approved the study. Since inception, sociodemographic, behavioral, anthropometric, and health-related factors have been assessed approximately every 5 years (1985/1988, 1991/1994, 1997/1999, 2002/2004, 2007/2009, and 2012/2013). Accelerometer measurement was added to the study at the 2012/2013 wave of data collection for participants seen at the central London clinic and for those living in the South-Eastern regions of England who were screened at home. Whitehall II data, protocols, and other metadata are available to the scientific community. Please refer to the Whitehall II data sharing policy at http://www.ucl.ac.uk/whitehallII/data-sharing.
Questionnaire-Based Assessment of Physical Activity
A modified version of the previously validated Minnesota physical activity questionnaire was used.25,26 The instructions were as follows: “We would like to know about your activities at work and in your free time that involve physical activity.” The questionnaire included 20 items on the amount of time spent in the following activities: walking, sport (cycling, soccer, golf, swimming, and 2 open-ended questions on other sports), gardening (weeding, mowing, and 1 open-ended question on other gardening activities), housework (carrying heavy shopping, cooking, hanging out washing, and 2 open-ended questions on other housework), do-it-yourself activity (washing a car, painting, or decorating, and 1 open-ended question on other do-it-yourself activity), and 2 open-ended questions on other activities. For each item, the participants were required to take into account activity patterns over the past 4 weeks to give an indication of usual activity and provide the total number of hours spent in that activity per week (see question 82 of the health survey questionnaire online available at http://www.ucl.ac.uk/whitehallII/pdf/S7_HSQ.pdf).
For each physical activity, including the open-ended items, we assigned a metabolic equivalent (MET) value using a compendium of energy costs.27 One MET value reflects the intensity of the activity relative to lying quietly. Total physical activity was estimated as MET.hour/week, the sum of the product of the intensity (MET) and weekly duration (hours/week) of all reported activities. In addition, we calculated the total number of hours per week in moderate-and-vigorous physical activity (MVPA) defined as activities with associated MET ≥3 (eg, cycling, weeding, swimming, mowing).27
Accelerometer-Assessed Physical Activity
Participants with no contraindications (that is, allergies to plastic or metal, traveling abroad the following week) were asked to wear a triaxial accelerometer (GeneActiv; Activinsights Ltd, Kimbolton, Cambs, UK, http://www.geneactiv.org/) on their nondominant wrist for 9 consecutive (24-hour) days. The accelerometer was sampled at 85.7 Hz and as in previous studies using new generation accelerometers,28–30 acceleration was expressed relative to gravity (g units; 1 g = 9.81 m.s−²) to reflect the fact that sensors are calibrated relative to gravity. Calibration error was estimated based on static periods in the data and corrected if necessary.31 The Euclidean norm (magnitude) of the 3 raw signals minus 1 g, with negative numbers rounded to zero, was used to quantify the acceleration related to the movement registered and expressed in milligravity (mg, 1 mg = 0.00981 m.s−²).32
Accelerometer data were processed in R (cran.r-project.org/) using the GGIR package and executed on MOVEeCloud (http://movelab.org/research/moveecloud/), a computing facility for physical activity research.33 Data extracted between the first and last midnight were retained for the analysis leading to a maximum of 24-hour measurements for 8 days. Participants were included in the analysis if they had valid data (≥16 hour/day) for at least 2 weekdays and 2 weekend days. Nonwear time was estimated on the basis of the standard deviation (SD) and value range of each accelerometer axis, calculated for moving windows of 60 minutes with 15-minute increments.32 For each 15-minute period of time detected as nonwear time over the valid days, missing data were replaced by the mean value calculated from measurement on other days at the same time of day.30,34
Besides accelerometer-assessed total activity, for each participant duration in moderate-and-vigorous physical activity was also calculated. Because a validated threshold to define MVPA in older adults does not yet exist, we chose the 100 mg threshold based on the fact that walking at 4 km/hour is classified as moderate physical activity27 and is equivalent to an acceleration of 100 mg in a laboratory based study on 30 adults.29 In order to qualify as MVPA, at least 80% of the activity needed to be at 100 mg, for at least a period (bout) of 10 minutes, using moving 10-minute windows. In sensitivity analyses, a more stringent cut-point of 120 mg was chosen to define MVPA.
As the observation period covered 8 days, the data were recoded so that our measure reflected physical activity over 1 week to match the questionnaire-assessed physical activity. If a participant had 3 valid weekend days or 6 weekdays, the wrist acceleration of the first and last full day of measurement (for example, 2 Tuesdays a week apart) were averaged to represent 1 unique day. Then, the mean accelerometer-assessed total physical activity (mg) over a week was calculated as: [(5 × mean daily weekday wrist acceleration + 2 × mean daily week-end wrist acceleration)]/7. The same rescaling was undertaken for time spent in MVPA per week (hour/week).
Adiposity Markers
Adiposity markers were assessed during the clinical examination by a trained nurse. BMI was calculated as weight (kg) divided by height (m) squared. Weight was measured in light clothing, using an electronic Soehnle scale with digital readout (Leifheit AS, Nassau, Germany). Height was measured using a stadiometer with the participant standing completely erect with their head in the Frankfort plane. Waist circumference was taken as the smallest circumference at or below the costal margin. FMI was calculated as the ratio of fat mass (kg) by height (m) squared.35,36 Fat mass was estimated by bioimpedance using the Tanita TBF-300 body composition analyzer (Tanita, Arlington Heights, Ill, www.tanita.com/en/). Bioimpedance data were available on participants seen at the central London clinic (N = 4524) but not at home. As this measure is influenced by the total amount of body fluid,37 those with renal insufficiency (assessed by estimated glomerular filtration rate <15) were excluded from the analysis on FMI (N = 1).
Covariates
Sociodemographic variables included age, sex, ethnicity (White, South Asian, Black, other), marital status (married/cohabiting, single, widowed, divorced/separated), and socioeconomic status, measured by the highest qualification on leaving full-time education (university/higher university degree, higher secondary school, lower secondary school, and lower primary school or below) and occupational position at age 50 years (high, intermediate and low, representing income, and status at work).
Health behaviors were assessed by questionnaire. Smoking status was defined as current, past, and never smokers. Alcohol consumption was assessed using a question on the quantity of alcohol consumed in the previous week and was classified as “no alcohol consumption in the previous week,” “moderate alcohol consumption” (1–14 units/week in women and 1–21 units/week in men), and “heavy drinkers” (15+ units in women and 21+ units in men). The frequency of fruit and vegetable consumption was assessed on a 9-point scale, ranging from “seldom or never” to “4 or more times a day.”
Statistical Analysis
The associations of the physical activity measures (total physical activity and MVPA, both assessed by questionnaire and accelerometer) with adiposity markers were examined using linear regression with adiposity markers as the outcomes. The linearity assumption was tested by comparing the fit of a model including physical activity measures as linear terms to the fit of models including degree 1 and 2 fractional polynomial terms using the deviance differences between the models (fracpoly command in STATA 13′; StataCorp LP, College Station, TX). Subsequently, models were adjusted for sociodemographic and behavioral variables in order to control for potential confounding, including those because of clustering of health behaviors.
We first assessed the association of adiposity markers with total physical activity, assessed using the questionnaire (MET.h/week) and accelerometer (mean acceleration), using quartiles as the metric for these 2 measures was different. Then, total physical activity (continuous) and MVPA (categories) were entered simultaneously into the model, and percentage reduction in the associations was calculated to allow the contribution on MVPA to be estimated. Then, the association of adiposity markers with MVPA was investigated using the following categories for both questionnaire and accelerometer measures: <1.0 hour/week, 1.0–2.5 hours/week, 2.5–4.0 hours/week, and ≥4 hours/week. Analyses were repeated using sex-specific standardized measures of adiposity in order to compare whether the associations differed by adiposity markers. To compare the fit of the models for questionnaire vs accelerometer measures, the Akaike information criterion was used. The interactions of physical activity measures with sex and age (continuous and median-based classes) were also tested. In sensitivity analyses, we repeated the analysis for the association with MVPA using a more stringent (120 mg instead of 100 mg) cut-off for accelerometry. The main analyses were performed using SAS v 9.3 (SAS Institute, Inc, Cary, NC). Analyses based on fractional polynomial models and figures were undertaken with STATA 13 statistical software (StataCorp LP).
Results
Sample Description
Among the 4880 participants to whom the accelerometer was proposed, 210 had contraindications, 4282 agreed to wear it, and 4040 participants had valid accelerometer data (≥16 hour/day) for at least 2 weekdays and 2 weekend days. Of those, 3940 participants also had data on questionnaire-assessed physical activity, BMI, waist circumference, and all covariates, constituting the main analytic sample of this study. Compared with the 940 participants not included in the analysis, the analytic sample did not differ by age [mean age = 69.3, SD = 5.7; 95% confidence interval (CI) = 69.2, 69.5] vs 69.1 years [SD = 5.7; 95% CI = 68.7, 69.5), P = .20] but was composed of more men (74.2% vs 66.8%, P < .0001) and fewer participants from the lowest occupational position (10.7% vs 13.3%, P = .02). Among these 3940 participants, 3828 (97.1%) had valid data for the 8-day observation period, 76 (1.9%) for 6–7 days, and 36 (0.9%) for 5–4 days. In all, missing data were replaced for 1–2 hours for 26.3% of the participants, >2–5 hours for 1.6% of the participants, >5–10 hours for 1.1% of the participants, and >10–25 hours for 0.4% of the participants. Analyses on FMI were restricted to 3617 participants seen at the clinic. Participants included in analyses on BMI and waist circumference but not on FMI (N = 324) had on average higher BMI (28.5 (95% CI 27.9, 29.1; SD = 5.5) vs 26.4 (95%CI 26.3, 26.6; SD = 4.2) kg/m², P < .0001) and waist circumference [100.0 (95% CI 98.5, 101.6; SD = 14.0) vs 96.0 (95% CI 95.6, 96.4; SD = 12.0) cm, P < .0001]. Table 1 presents the characteristics of the study population. As interactions were found between physical activity measures and sex [all P < .05, except for questionnaire-assessed MVPA with waist circumference (P = .09) and FMI (P = .12)], all analyses were performed separately for men and women. No interactions were found between physical activity and age (P between .18 and .91). The mean total physical activity assessed by questionnaire was 46.7 (95% CI 45.7, 47.7; SD = 27.0) MET.h/week in men and 44.5 (95% CI 42.7, 46.3; SD = 29.0) MET.h/week in women. For accelerometer data, the mean acceleration over a week was 23.4 (95% CI 23.2, 23.7; SD = 6.8) mg in men and 23.1 (95% CI 22.7, 23.5; SD = 6.7) mg in women.
Table 1.
Characteristics of the Study Population (N = 3940)∗
| Characteristics | Men N = 2924 |
Women N = 1016 |
|---|---|---|
| Age (years), mean (SD) | 69.3 (5.7) | 69.4 (5.7) |
| Non-White | 157 (5.4) | 129 (12.7) |
| Married/cohabiting | 2411 (82.5) | 538 (53.0) |
| Education | ||
| Primary school or below | 192 (6.6) | 180 (17.7) |
| Lower secondary school | 902 (30.9) | 347 (34.2) |
| Higher secondary school | 853 (29.2) | 253 (24.9) |
| University degree | 977 (33.4) | 236 (23.2) |
| Occupational position at age 50 years | ||
| Low | 111 (3.8) | 311 (30.6) |
| Intermediate | 1293 (44.2) | 466 (45.9) |
| High | 1520 (52.0) | 239 (23.5) |
| Smoking status | ||
| Current smokers | 90 (3.1) | 39 (3.8) |
| Ex-smokers | 1487 (50.9) | 399 (39.3) |
| Never smokers | 1347 (46.1) | 578 (56.9) |
| Alcohol consumption | ||
| No alcohol in the last week | 442 (15.1) | 336 (33.1) |
| Moderate alcohol consumption | 2002 (68.5) | 542 (53.4) |
| Heavy alcohol consumption | 480 (16.4) | 138 (13.6) |
| Daily consumption of fresh fruit or vegetables | 2292 (78.4) | 839 (82.6) |
Numbers are N (%), otherwise stated.
Associations Between Total Physical Activity and Adiposity Markers
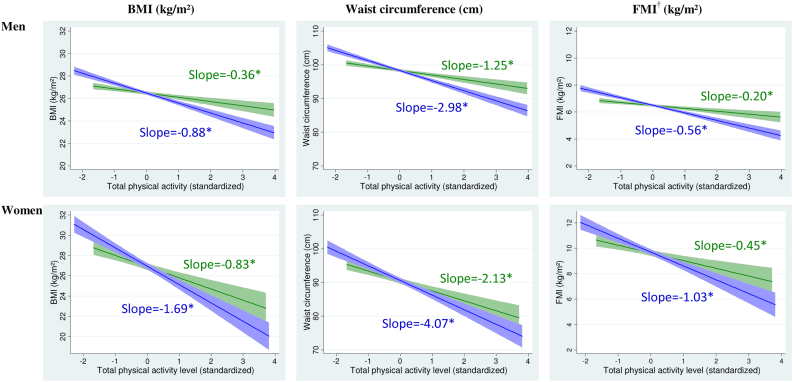
Analyses on the shape of the association between total physical activity and adiposity revealed linear dose-response associations with all adiposity markers (Figure 1). In men, differences in adiposity markers were 2.4–2.8 (1.9–2.3 for women) times higher for an increase of 1SD in the accelerometer measure compared with an increase of 1SD in the questionnaire measure. In models adjusted for sociodemographic and behavioral factors, in men, being in the highest quartile of accelerometer-assessed total physical activity was associated with a 2.68 (95% CI 2.28, 3.08) kg/m² lower BMI compared with being in the lowest quartile (Table 2). The corresponding figure for questionnaire data, at 1.14 (95% CI 0.74, 1.53) kg/m², was 2.4 times smaller. Associations between total physical activity and all adiposity markers were larger in women than in men. For example, the mean difference in BMI between the highest and the lowest quartiles of accelerometer-assessed total physical activity was 4.61 (95% CI 3.65, 5.57) kg/m² in women compared with 2.68 (95% CI 2.28, 3.08) kg/m² in men. After adjustment for MVPA, the associations between total physical activity and adiposity markers were reduced by 7%–29% but remained statistically significant. In addition, analyses using standardized adiposity markers showed that the effect sizes of the associations were similar for all measures of adiposity (Appendix Table A1).
Fig. 1.
Association of total physical activity with adiposity markers using questionnaire and accelerometer data.  Questionnaire data,
Questionnaire data,  Accelerometer data,
Accelerometer data,  Confidence intervals,
Confidence intervals,  Confidence intervals, Footnotes: Slopes correspond to difference in adiposity markers associated with 1 SD increment in questionnaire (SD = 27.53 MET.h/week) and accelerometer (SD = 6.73 mg) assessed total physical activity. *P < .0001. †Analyses are based on participants seen at the clinic (N = 2725 men and 892 women).
Confidence intervals, Footnotes: Slopes correspond to difference in adiposity markers associated with 1 SD increment in questionnaire (SD = 27.53 MET.h/week) and accelerometer (SD = 6.73 mg) assessed total physical activity. *P < .0001. †Analyses are based on participants seen at the clinic (N = 2725 men and 892 women).
Table 2.
Association of Total Physical Activity Assessed by Questionnaire and Accelerometer With Adiposity Markers
| N (%) | BMI (kg/m²) | Waist Circumference (cm) | FMI∗ (kg/m²) | |
|---|---|---|---|---|
| Men | ||||
| Mean (SD) | 2924 | 26.47 (3.89) | 98.26 (11.24) | 6.47 (2.37) |
| Total physical activity† | Difference‡ (95% CI) | Difference‡ (95% CI) | Difference‡ (95% CI) | |
| Questionnaire measure | ||||
| 1st quartile (least active) | 720 (24.6) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 2nd quartile | 725 (24.8) | −0.79 (−1.18, −0.39) | −2.81 (−3.95, −1.68) | −0.42 (−0.68, −0.17) |
| 3rd quartile | 711 (24.3) | −0.70 (−1.09, −0.30) | −2.92 (−4.07, −1.77) | −0.33 (−0.58, −0.08) |
| 4th quartile (most active) | 768 (26.3) | −1.14 (−1.53, −0.74) | −4.00 (−5.13, −2.87) | −0.66 (−0.91, −0.41) |
| Accelerometer measure | ||||
| 1st quartile (least active) | 733 (25.1) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 2nd quartile | 718 (24.6) | −0.84 (−1.23, −0.45) | −2.36 (−3.47, −1.25) | −0.45 (−0.69, −0.20) |
| 3rd quartile | 730 (25.0) | −1.77 (−2.16, −1.38) | −5.20 (−6.33, −4.08) | −1.05 (−1.30, −0.80) |
| 4th quartile (most active) | 743 (25.4) | −2.68 (−3.08, −2.28) | −8.30 (−9.44, −7.15) | −1.60 (−1.85, −1.35) |
| Women | ||||
| Mean (SD) | 1026 | 27.02 (5.42) | 90.75 (13.18) | 9.66 (3.58) |
| Total physical activity† | Difference‡ (95% CI) | Difference‡ (95% CI) | Difference‡ (95% CI) | |
| Questionnaire measure | ||||
| 1st quartile (least active) | 266 (26.2) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 2nd quartile | 259 (25.5) | −1.37 (−2.29, −0.45) | −2.40 (−4.65, −0.15) | −0.77 (−1.44, −0.10) |
| 3rd quartile | 274 (27.0) | −2.22 (−3.14, −1.30) | −4.89 (−7.13, −2.65) | −1.23 (−1.89, −0.58) |
| 4th quartile (most active) | 217 (21.4) | −2.63 (−3.60, −1.66) | −6.44 (−8.81, −4.06) | −1.61 (−2.30, −0.91) |
| Accelerometer measure | ||||
| 1st quartile (least active) | 252 (24.8) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 2nd quartile | 267 (26.3) | −1.98 (−2.88, −1.08) | −4.26 (−6.47, −2.06) | −0.77 (−1.43, −0.11) |
| 3rd quartile | 255 (25.1) | −2.95 (−3.88, −2.03) | −6.65 (−8.92, −4.38) | −1.59 (−2.26, −0.92) |
| 4th quartile (most active) | 242 (23.8) | −4.61 (−5.57, −3.65) | −10.86 (−13.21, −8.51) | −2.87 (−3.55, −2.19) |
Analyses are based on participants seen at the clinic (N = 2725 men and 892 women).
Total physical activity measures were categorized into quartiles based on the total population (N = 3940).
Models adjusted for age, sex, ethnicity, marital status, education, occupational position at age 50 years, smoking status, alcohol consumption, and fruit and vegetable consumption.
Associations Between MVPA and Adiposity Markers
For questionnaire data, differences in adiposity markers were mainly observed between men who reported <1 hour/week of MVPA compared with those reporting more (Appendix Figure A1, Table 3), but no differences in adiposity markers were found for longer durations (all P > .05 for comparison across 1–2.5 hours/week, 2.5–4 hours/week, and ≥4 hours/week duration categories). In contrast, for accelerometer-assessed physical activity, adiposity markers decreased progressively with increasing time spent in MVPA. For example, compared with men performing <1 hour/week of MVPA, waist circumference was 3.06 (95% CI 2.06, 4.06) cm lower in those performing 1–2.5 hours/week, 4.69 (95% CI 3.47, 5.91) cm lower in those performing 2.5–4 hours/week, and 7.11 (95% CI 5.93, 8.29) cm lower in those performing ≥4 hours/week of MVPA. In women, the differences between the association of questionnaire and accelerometer data with adiposity markers were observed but tended to attenuate for longer time spent in MVPA (Appendix Figure A1). Standardized measures of adiposity markers showed that associations between physical activity and all 3 adiposity markers were similar (Appendix Table A2). In both men and women, the fit of the models based on the accelerometer data for total physical activity and MVPA was much better than for the models based on questionnaire data (all Akaike information criteria differences >32, data not tabulated).
Table 3.
Association of Moderate and Vigorous Physical Activity Assessed by Questionnaire and Accelerometer With Adiposity Markers
| Moderate and Vigorous Physical Activity | N (%) | BMI (kg/m²) |
Waist Circumference (cm) |
FMI∗ (kg/m²) |
|---|---|---|---|---|
| Difference† (95% CI) | Difference† (95% CI) | Difference† (95% CI) | ||
| Men | ||||
| Questionnaire measure | ||||
| <1 hour/week | 728 (24.9) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 1–2.5 hours/week | 519 (17.7) | −0.65 (−1.08, −1.22) | −2.61 (−3.85, −1.36) | −0.43 (−0.71, −0.16) |
| 2.5–4 hours/week | 495 (16.9) | −0.88 (−1.32, −0.44) | −3.49 (−4.76, −2.23) | −0.55 (−0.83, −0.28) |
| ≥4 hours/week | 1182 (40.4) | −0.97 (−1.34, −0.61) | −3.73 (−4.78, −2.68) | −0.63 (−0.86, −0.40) |
| Accelerometer measure | ||||
| <1 hour/week | 1303 (44.6) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 1–2.5 hours/week | 727 (24.9) | −0.99 (−1.34, −0.64) | −3.06 (−4.06, −2.06) | −0.54 (−0.76, −0.33) |
| 2.5–4 hours/week | 410 (14.0) | −1.57 (−2.00, −1.14) | −4.69 (−5.91, −3.47) | −0.95 (−1.22, −0.69) |
| ≥4 hours/week | 484 (16.6) | −2.24 (−2.65, −1.83) | −7.11 (−8.29, −5.93) | −1.35 (−1.60, −1.09) |
| Women | ||||
| Questionnaire measure | ||||
| <1 hour/week | 392 (38.6) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 1–2.5 hours/week | 224 (22.0) | −0.99 (−1.89, −0.10) | −2.01 (−4.20, 0.18) | −0.73 (−1.36, −0.09) |
| 2.5–4 hours/week | 182 (17.9) | −1.34 (−2.30, −0.38) | −3.18 (−5.52, −0.83) | −0.78 (−1.46, −0.10) |
| ≥4 hours/week | 218 (21.5) | −2.08 (−2.99, −1.16) | −5.72 (−7.96, −3.48) | −1.34 (−1.99, −0.70) |
| Accelerometer measure | ||||
| <1 hour/week | 586 (57.7) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 1–2.5 hours/week | 228 (22.4) | −2.79 (−3.60, −1.97) | −6.95 (−8.95, −4.94) | −1.74 (−2.30, −1.17) |
| 2.5–4 hours/week | 101 (9.9) | −4.30 (−5.43, −3.17) | −8.95 (−11.72, −6.17) | −2.60 (−3.36, −1.83) |
| ≥4 hours/week | 101 (9.9) | −3.68 (−4.81, −2.54) | −9.37 (−12.15, −6.59) | −2.53 (−3.30, −1.76) |
Analyses are based on participants seen at the clinic (N = 2725 men and 892 women).
Models adjusted for age, sex, ethnicity, marital status, education, occupational position at age 50 years, smoking status, alcohol consumption, and fruit and vegetable consumption.
Sensitivity Analyses
A more stringent cut-off for MVPA (120 mg instead of 100 mg) showed similar results (Appendix Table A3). The associations between adiposity and accelerometer were stronger than that with questionnaire in men than in women leading us to examine whether the correlation between questionnaire and accelerometer measures differed by sex. These results showed it to be similar in men (Spearman correlation = 0.33; 95%CI 0.30–0.36) and women (Spearman correlation = 0.32; 0.27–0.37; P for difference by sex = .70) for total physical activity but stronger for MVPA in women (Spearman correlation = 0.28; 95% CI 0.22–0.33) than in men (Spearman correlation = 0.16; 95% CI 0.12–0.19; P for difference by sex = .0006). On average, men reported spending 1.7 (95% CI1.5, 1.9; SD = 5.4) hours/week more in MVPA than estimated by the accelerometer while women reported 0.9 (95% CI 0.5, 1.3; SD = 6.0) hour/week more (P for difference by sex <.0001).
Discussion
This study of British adults aged 60 to 83 years presents 3 key findings. (1) Total and moderate-and-vigorous physical activity, assessed by questionnaire and accelerometer, were associated with BMI, waist circumference, and FMI, effect sizes being similar across the adiposity markers. (2) Associations between total physical activity and adiposity markers were up to 2.8 times larger for accelerometer- than questionnaire-assessed physical activity. (3) In men, the association of adiposity markers with accelerometer-assessed moderate-and-vigorous physical activity compared with questionnaire data was much stronger, possibly because of greater measurement error in the reporting of time spent in moderate-and-vigorous physical activity by men.
As both physical activity patterns and body fat distribution change with age, results based on data on younger adults might not apply to older adults. It has been hypothesized that the association between physical activity and adiposity markers reverses at older ages because of the attenuation in age-related weight loss in those engaging in more physical activity.12,13 However, the results from previous studies are inconsistent. Some studies reported greater physical activity to be associated with lower BMI,38–41 waist circumference,38,39 and fat mass,40–42 but not fat-free mass.40 Another study based on individuals aged 70–82 years found that higher energy expenditure was associated with greater total body weight and fat-free mass, but no association was found with fat mass.14 It has also been suggested that the association between moderate physical activity and fat mass would attenuate with age.41 In the present study, we used multiple adiposity markers, the commonly used measures of BMI and waist circumference, but also FMI, which is the ratio of fat mass by height squared. FMI, like BMI, has the advantage of taking into account height differences and facilitates comparison between age and ethnic groups.43 We found that higher physical activity was associated with lower BMI, waist circumference, and FMI, and these associations did not differ by age. In our data, simple measures of adiposity such as BMI or waist circumference had similar results as FMI in their associations with physical activity. There were large differences in adiposity markers between the most and least active individuals according to the accelerometer, up to 2.7 kg/m² for BMI in men and 4.6 kg/m² in women, and up to 8 cm for waist circumference in men and 11 cm in women. In older adults, a difference of 4 to 5 kg/m² in BMI and of 11 to 13 cm in waist circumference is associated with a doubling of the risk of diabetes,44 a 30% increase in risk of heart failure,45 and more than 2-fold greater odds of functional limitations.46
The extent to which there are increasing benefits for weight control at greater physical activity remains unclear. We found a linear association of adiposity markers with total physical activity, which was reduced by less than 30% after taking the effects of moderate-and-vigorous physical activity into account. This suggests benefits for body weight at all levels of physical activity. However, there were large differences in adiposity between those practicing none or little moderate-and-vigorous physical activity compared with the others even though the effects were attenuated at higher levels of activity. In addition, as also shown in some38,42 but not all previous studies,41,47 associations between physical activity and adiposity markers in our study were stronger in women than in men. Potential reasons for these differences include sexual dimorphisms in energy metabolism48 and the type of physical activity undertaken, with women being more likely to choose activities that help maintain their weight.49
At least 2 previous studies have examined associations between physical activity and adiposity markers in older adults using both questionnaire and accelerometer measures.41,50 Our study is based on a much larger sample (previous analyses were on 238 and 636 persons) and shows a consistently stronger association of all adiposity markers with accelerometer rather than questionnaire assessed-physical activity. We used a waterproof accelerometer worn on the wrist instead of the waist or the hip, allowing us to assess physical activity during 8 full days (24 hours). Furthermore, compliance of a wrist-worn accelerometer is better than one worn on the waist or the hip,51 thus, reducing misclassification because of missing data over nonwear periods.
There are several possible explanations for stronger associations with adiposity using accelerometer- rather than questionnaire-based assessments. The questionnaire we used focusses on 20 activities that do not cover all possible activities in a day. Accelerometers were worn over the full 24 hours, over 8 days. In addition, questionnaire-based assessments are likely to have a degree of measurement error52 as the duration of each activity relies on reports from the participant. Although the accelerometer measures the movement of only one body part with inferences that apply to the whole body, it has the advantage of being free of reporting bias. In our data, measurement error of self-reported physical activity appears to be nondifferential with respect to adiposity markers as there was no difference in the correlation between accelerometer and questionnaire assessed physical activity measures across BMI categories (data not tabulated). Thus, the expected bias of the estimate for the physical activity-adiposity association is toward the null, which may explain the weaker association with questionnaire-based assessments.53 Physical activity will continue to be assessed by questionnaire in many large-scale studies because of the ease of administration and lower costs. Increasing recognition of the importance of physical activity for health54 suggests that studies will attempt to measure it better, making accelerometers an important research tool.
Our study has several strengths including the large study sample, analysis of raw rather than “counts” data (metric generated by the monitor based on algorithm specific to each accelerometer brand),32 and high compliance for accelerometer wear among participants. It also has some limitations. First, no causal inferences can be drawn because of the cross-sectional nature of the study. Second, although the sample covered a wide socioeconomic range, data are from an occupational cohort and cannot be assumed to be representative of the general population. In addition, less than 5% of the analytic sample had a BMI greater or equal to 35 kg/m² so the results cannot be extended to the highest levels of adiposity. Third, food habits, associated with both body weight and physical activity, are likely to explain part of the association between physical activity and adiposity markers. In the present analysis, we used “fruit and vegetable consumption” as a marker of healthy diet. It explained between 4% and 9% of the associations with questionnaire-assessed physical activity and around 2% of the associations with accelerometer data. Analyses on a subset of participants for whom we had data on dietary patterns55 showed a reduction of 3% to 15% of the associations with questionnaire data and around 4% of the associations with accelerometer data. As there was no substantial difference in results, we retained the analysis using the measure of fruit and vegetable consumption as it allowed analyses on a larger number of participants. Fourth, although our results are in accordance with previous studies that have used different instruments, such as the Physical Activity Scale for the Elderly questionnaire or other types of accelerometer (eg, Actigraph), they are specific to the instruments used and might not be generalizable across instruments. Further studies are needed to examine the generalizability of our findings across different populations and accelerometer types.
Conclusions
Although the extent to which physical activity is associated with adiposity in older adults continues to be debated, the present study shows strong inverse associations of total and moderate-and-vigorous physical activity, both questionnaire- and accelerometer-assessed, with all adiposity markers. Associations were up to 2.8 times larger using accelerometer- assessed instead of questionnaire-assessed physical activity, suggesting that beneficial effects of physical activity for adiposity at older ages are much greater than previously estimated.
Acknowledgments
The authors thank all of the participating civil service departments and their welfare, personnel, and establishment officers; the British Occupational Health and Safety Agency; the British Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team. The Whitehall II Study team comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants and data entry staff, who make the study possible.
Footnotes
This work was supported by the US National Institutes of Health (R01AG013196; R01AG034454; R01HL036310), the UK Medical Research Council (K013351), the British Heart Foundation (PG/29605), and the Economic and Social Research Council (ES/J023299). The funding organizations had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
The authors declare no conflicts of interest.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jamda.2015.01.086.
Supplementary Data
References
- 1.Chapman I.M. Obesity in old age. Front Horm Res. 2008;36:97–106. doi: 10.1159/000115358. [DOI] [PubMed] [Google Scholar]
- 2.Mathus–Vliegen E.M. Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: A guideline. Obes Facts. 2012;5:460–483. doi: 10.1159/000341193. [DOI] [PubMed] [Google Scholar]
- 3.Michalakis K., Goulis D.G., Vazaiou A. Obesity in the ageing man. Metabolism. 2013;62:1341–1349. doi: 10.1016/j.metabol.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. 2013:1–8. [PubMed] [Google Scholar]
- 5.Gutierrez–Fisac J.L., Leon–Munoz L.M., Regidor E. Trends in obesity and abdominal obesity in the older adult population of Spain (2000–2010) Obes Facts. 2013;6:1–8. doi: 10.1159/000348493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moody A. Health Survey of England 2012: Adult anthropometric measures, overweight, and obesity. Available at: http://www.hscic.gov.uk/catalogue/PUB13218/HSE2012–Ch10–Adult–BMI.pdf [serial online] 2014;1.
- 7.von R.A., Steffen A., Floegel A. Trend in obesity prevalence in European adult cohort populations during follow–up since 1996 and their predictions to 2015. PLoS One. 2011;6:e27455. doi: 10.1371/journal.pone.0027455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonnelli S., Caffarelli C., Nuti R. Obesity and fracture risk. Clin Cases Miner Bone Metab. 2014;11:9–14. doi: 10.11138/ccmbm/2014.11.1.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Premaor M.O., Comim F.V., Compston J.E. Obesity and fractures. Arq Bras Endocrinol Metabol. 2014;58:470–477. doi: 10.1590/0004-2730000003274. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Obesity and overweight. Last updated 08/2014. Last accessed Janaury 19, 2015.
- 11.World Health Organization. Physical activity. Fact sheet N°385. Available at: http://www.who.int/mediacentre/factsheets/fs385/en/ [serial online] 2014; Accessed July, 2014.
- 12.Dziura J., Mendes de L.C., Kasl S., DiPietro L. Can physical activity attenuate aging–related weight loss in older people? The Yale Health and Aging Study, 1982–1994. Am J Epidemiol. 2004;159:759–767. doi: 10.1093/aje/kwh105. [DOI] [PubMed] [Google Scholar]
- 13.Ekelund U., Brage S., Franks P.W. Physical activity energy expenditure predicts changes in body composition in middle–aged healthy whites: Effect modification by age. Am J Clin Nutr. 2005;81:964–969. doi: 10.1093/ajcn/81.5.964. [DOI] [PubMed] [Google Scholar]
- 14.Manini T.M., Everhart J.E., Anton S.D. Activity energy expenditure and change in body composition in late life. Am J Clin Nutr. 2009;90:1336–1342. doi: 10.3945/ajcn.2009.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen I., Katzmarzyk P.T., Ross R. Waist circumference and not body mass index explains obesity–related health risk. Am J Clin Nutr. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 16.Genton L., Karsegard V.L., Chevalley T. Body composition changes over 9 years in healthy elderly subjects and impact of physical activity. Clin Nutr. 2011;30:436–442. doi: 10.1016/j.clnu.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Shiroma E.J., Sesso H.D., Lee I.M. Physical activity and weight gain prevention in older men. Int J Obes (Lond) 2012;36:1165–1169. doi: 10.1038/ijo.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gortmaker S.L., Swinburn B.A., Levy D. Changing the future of obesity: Science, policy, and action. Lancet. 2011;378:838–847. doi: 10.1016/S0140-6736(11)60815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strasser B. Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2013;1281:141–159. doi: 10.1111/j.1749-6632.2012.06785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Hees V.T. The challenge of assessing physical activity in populations. Lancet. 2012;380:1555–1556. doi: 10.1016/S0140-6736(12)61876-5. [DOI] [PubMed] [Google Scholar]
- 21.Helmerhorst H.J., Brage S., Warren J. A systematic review of reliability and objective criterion–related validity of physical activity questionnaires. Int J Behav Nutr Phys Act. 2012;9:103. doi: 10.1186/1479-5868-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prince S.A., Adamo K.B., Hamel M.E. A comparison of direct versus self–report measures for assessing physical activity in adults: A systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyrstad S.M., Hansen B.H., Holme I.M., Anderssen S.A. Comparison of self–reported versus accelerometer–measured physical activity. Med Sci Sports Exerc. 2014;46:99–106. doi: 10.1249/MSS.0b013e3182a0595f. [DOI] [PubMed] [Google Scholar]
- 24.Marmot M.G., Smith G.D., Stansfeld S. Health inequalities among British civil servants: The Whitehall II study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs D.R., Jr., Ainsworth B.E., Hartman T.J., Leon A.S. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Richardson M.T., Leon A.S., Jacobs D.R., Jr. Comprehensive evaluation of the Minnesota Leisure Time Physical Activity Questionnaire. J Clin Epidemiol. 1994;47:271–281. doi: 10.1016/0895-4356(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 27.Ainsworth B.E., Haskell W.L., Herrmann S.D. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 28.da Silva I.C., van Hees V.T., Ramires V.V. Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int J Epidemiol. 2014;43:1959–1968. doi: 10.1093/ije/dyu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hildebrand M., van Hees V.T., Hansen B.H., Ekelund U. Age–group comparability of raw accelerometer output from wrist– and hip–worn monitors. Med Sci Sports Exerc. 2014;46:1816–1824. doi: 10.1249/MSS.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 30.Sabia S., van Hees V.T., Shipley M.J. Association between questionnaire– and accelerometer–assessed physical activity: The role of sociodemographic factors. Am J Epidemiol. 2014;179:781–790. doi: 10.1093/aje/kwt330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Hees V.T., Fang Z., Langford J. Auto–calibration of accelerometer data for free–living physical activity assessment using local gravity and temperature: An evaluation on four continents. J Appl Physiol (1985) 2014;117:738–744. doi: 10.1152/japplphysiol.00421.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hees V.T., Gorzelniak L., Dean Leon E.C. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLoS One. 2013;8:e61691. doi: 10.1371/journal.pone.0061691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiden H., Woodman S., Watson P., Cala J. Developing cloud applications using the e–Science Central platform. Philos Trans A Math Phys Eng Sci. 2013;371:20120085. doi: 10.1098/rsta.2012.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catellier D.J., Hannan P.J., Murray D.M. Imputation of missing data when measuring physical activity by accelerometry. Med Sci Sports Exerc. 2005;37:S555–S562. doi: 10.1249/01.mss.0000185651.59486.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dulloo A.G., Jacquet J., Solinas G. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes (Lond) 2010;34:S4–S17. doi: 10.1038/ijo.2010.234. [DOI] [PubMed] [Google Scholar]
- 36.VanItallie T.B., Yang M.U., Heymsfield S.B. Height–normalized indices of the body's fat–free mass and fat mass: Potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 37.Elia M. Body composition by whole–body bioelectrical impedance and prediction of clinically relevant outcomes: Overvalued or underused? Eur J Clin Nutr. 2013;67:S60–S70. doi: 10.1038/ejcn.2012.166. [DOI] [PubMed] [Google Scholar]
- 38.Ewald B., McEvoy M., Attia J. Pedometer counts superior to physical activity scale for identifying health markers in older adults. Br J Sports Med. 2010;44:756–761. doi: 10.1136/bjsm.2008.048827. [DOI] [PubMed] [Google Scholar]
- 39.Stamatakis E., Davis M., Stathi A., Hamer M. Associations between multiple indicators of objectively–measured and self–reported sedentary behaviour and cardiometabolic risk in older adults. Prev Med. 2012;54:82–87. doi: 10.1016/j.ypmed.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Pelclova J., Gaba A., Tlucakova L., Pospiech D. Association between physical activity (PA) guidelines and body composition variables in middle–aged and older women. Arch Gerontol Geriatr. 2012;55:e14–e20. doi: 10.1016/j.archger.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Foong Y.C., Aitken D., Winzenberg T. The association between physical activity and reduced body fat lessens with age—Results from a cross–sectional study in community–dwelling older adults. Exp Gerontol. 2014;55:107–112. doi: 10.1016/j.exger.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Bann D., Kuh D., Wills A.K. Physical activity across adulthood in relation to fat and lean body mass in early old age: Findings from the Medical Research Council National Survey of Health and Development, 1946–2010. Am J Epidemiol. 2014;179:1197–1207. doi: 10.1093/aje/kwu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kyle U.G., Genton L., Pichard C. Body composition: What's new? Curr Opin Clin Nutr Metab Care. 2002;5:427–433. doi: 10.1097/00075197-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Vazquez G., Duval S., Jacobs D.R., Jr., Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: A meta–analysis. Epidemiol Rev. 2007;29:115–128. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- 45.Djousse L., Bartz T.M., Ix J.H. Adiposity and incident heart failure in older adults: The cardiovascular health study. Obesity (Silver Spring) 2012;20:1936–1941. doi: 10.1038/oby.2011.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houston D.K., Stevens J., Cai J. Abdominal fat distribution and functional limitations and disability in a biracial cohort: The Atherosclerosis Risk in Communities Study. Int J Obes (Lond) 2005;29:1457–1463. doi: 10.1038/sj.ijo.0803043. [DOI] [PubMed] [Google Scholar]
- 47.Carson V., Wong S.L., Winkler E. Patterns of sedentary time and cardiometabolic risk among Canadian adults. Prev Med. 2014;65C:23–27. doi: 10.1016/j.ypmed.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Wu B.N., O'Sullivan A.J. Sex differences in energy metabolism need to be considered with lifestyle modifications in humans. J Nutr Metab. 2011;2011:391809. doi: 10.1155/2011/391809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jonason P.K. An evolutionary psychology perspective on sex differences in exercise behaviors and motivations. J Soc Psychol. 2007;147:5–14. doi: 10.3200/SOCP.147.1.5-14. [DOI] [PubMed] [Google Scholar]
- 50.Harris T.J., Owen C.G., Victor C.R. A comparison of questionnaire, accelerometer, and pedometer: Measures in older people. Med Sci Sports Exerc. 2009;41:1392–1402. doi: 10.1249/MSS.0b013e31819b3533. [DOI] [PubMed] [Google Scholar]
- 51.Freedson P.S., John D. Comment on “estimating activity and sedentary behavior from an accelerometer on the hip and wrist.”. Med Sci Sports Exerc. 2013;45:962–963. doi: 10.1249/MSS.0b013e31827f024d. [DOI] [PubMed] [Google Scholar]
- 52.Ferrari P., Friedenreich C., Matthews C.E. The role of measurement error in estimating levels of physical activity. Am J Epidemiol. 2007;166:832–840. doi: 10.1093/aje/kwm148. [DOI] [PubMed] [Google Scholar]
- 53.de Klerk N.H., English D.R., Armstrong B.K. A review of the effects of random measurement error on relative risk estimates in epidemiological studies. Int J Epidemiol. 1989;18:705–712. doi: 10.1093/ije/18.3.705. [DOI] [PubMed] [Google Scholar]
- 54.Ekelund U., Ward H.A., Norat T. Physical activity and all–cause mortality across levels of overall and abdominal adiposity in European men and women: The European Prospective Investigation into Cancer and Nutrition Study (EPIC) Am J Clin Nutr. 2015 doi: 10.3945/ajcn.114.100065. Jan 14. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akbaraly T.N., Singh–Manoux A., Marmot M.G., Brunner E.J. Education attenuates the association between dietary patterns and cognition. Dement Geriatr Cogn Disord. 2009;27:147–154. doi: 10.1159/000199235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.