Abstract
Antiretroviral (ARV) medication diversion to the illicit market has been documented in South Florida, and linked to sub-optimal adherence in people living with HIV. ARV diversion reflects an unmet need for care in vulnerable populations that have difficulty engaging in consistent HIV care due to competing needs and co-morbidities. This study applies the Gelberg-Andersen Behavioral Model of Health Care Utilization for Vulnerable Populations to understand how social vulnerability is linked to ARV diversion and adherence. Cross-sectional data were collected from a targeted sample of vulnerable people living with HIV in South Florida between 2010 and 2012 (n=503). Structured interviews collected quantitative data on ARV diversion, access and utilization of care, and ARV adherence. Logistic regression was used to estimate the goodness-of-fit of additive models that test domain fit. Linear regression was used to estimate the effects of social vulnerability and ARV diversion on ARV adherence. The best fitting model to predict ARV diversion identifies having a low monthly income and unstable HIV care as salient enabling factors that promote ARV diversion. Importantly, health care need factors did not protect against ARV diversion, evidence that immediate competing needs are prioritized even in the face of poor health for this sample. We also find that ARV diversion provides a link between social vulnerability and sub-optimal ARV adherence, with ARV diversion and domains from the Behavioral Model explaining 25% of the variation in ARV adherence. Our analyses reveal great need to improve engagement in HIV care for vulnerable populations by strengthening enabling factors (e.g. patient-provider relationship) to improve retention in HIV care and ARV adherence for vulnerable populations.
INTRODUCTION
Antiretroviral (ARV) medication diversion is the unlawful transmission of ARVs from legal sources (e.g. patients with legitimate prescriptions) to the illicit market (1). Although ARV diversion is a relatively unexplored phenomenon, it has been documented in at least seven U.S. states and may be a widespread practice (2-8). A recent study in South Florida exposed substantial ARV diversion among vulnerable people living with HIV, particularly among those experiencing homelessness, substance dependence, and/or mental illness (9).
ARV diversion represents a serious barrier to medication adherence and has implications for HIV care. Clinical trials benchmark optimal ARV adherence at 95% or better (10) to maximize the long-term success of HIV treatment, viral suppression, and the prevention of onward HIV transmission (11, 12). However, ARV diverters in South Florida have 74% lower odds of optimal adherence to their medications compared to non-diverters (9). Aside from the individual care implications, ARV non-adherence has also been associated with the development and transmission of ARV resistant strains of HIV (13) and can potentially contaminate the medication supply chain with mishandled and tampered medications (8, 14).
Understanding the factors needed for successful engagement in care is central to providing adequate HIV services, especially for vulnerable populations. Successful HIV treatment requires complete and sustained engagement in HIV care and the alignment of numerous factors for optimal adherence (e.g. regimen simplicity, side-effect management, open physician-patient communication, and unhindered access to HIV care) (15); this combined with the long-term nature of ARV therapy makes adherence difficult even for the average person (16), nonetheless for those with competing needs. ARV diversion and non-adherence appear to reflect poor engagement in HIV care and other important wrap-around services (e.g. substance use treatment, mental health, etc.) in populations with competing needs (9, 17); prior research has demonstrated that health-care seeking behavior and adherence is impeded by poor quality of healthcare and lack of access to services to address both competing priorities (e.g. employment services, health coverage, and housing assistance) and co-morbidities (e.g. substance abuse and mental health treatment) (17-20).
Recent research calls to improve the spectrum of engagement in HIV care (21). An estimated 60% of people with HIV in the U.S. do not receive regular HIV care due, in part, to deficits in linkage to and retention in care (21). Prior work has identified ARV non-adherence as indicative of poor HIV care (22). However, no one has considered ARV diversion as an indicator of inadequate HIV care, especially for vulnerable populations. Despite having to make follow-up medical visits every few months for a legitimate ARV prescription, research shows that people divert their ARV medications to meet competing needs (17), indicating that ARV diversion may serve as a proxy for incomplete engagement in care and not being channeled to the proper services to support and ensure adherence. Although South Florida has one of the highest HIV incidence and prevalence rates in the nation (23), the region has also been associated with poor HIV care access, utilization, and engagement (24). This study aims to identify individual-level factors that are associated with ARV diversion and poor adherence in a vulnerable population of people living with HIV.
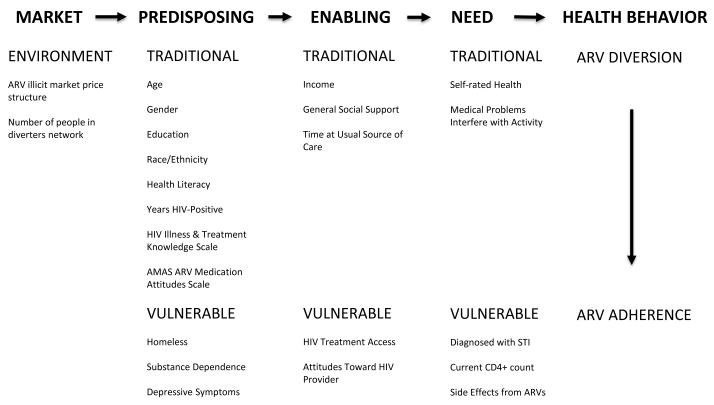
Conceptual Model
The Gelberg-Andersen Behavioral Model of Health Care Utilization for Vulnerable Populations (Behavioral Model) (25) provides a framework for organizing environmental, population, and vulnerability factors into domains associated with health service utilization (or lack thereof). The model posits that there are three domains that influence an individual’s health care utilization – predisposing, enabling, and need factors. Predisposing factors are individual characteristics that are present prior to the onset of the illness and represent a predisposition to use health care services. Traditional predisposing factors include socio-demographic characteristics, health beliefs, knowledge about a medical condition, and attitudes regarding medical care, whereas vulnerable predisposing factors include homelessness, substance abuse, and mental illness. Enabling factors are individual, provider, or community resources that facilitate (or impede) the use of health care services and include personal resources (e.g. income, health insurance, etc.), characteristics of the patient-provider relationship (e.g. perceived quality of engagement), and community resources (e.g. social support). Need factors are an individual’s perceived and evaluated need to receive health care. Examples of perceived need include self-rated health and disease symptoms, whereas examples of evaluated need include CD4+ count and depression diagnosis. The model postulates that, in an equitable healthcare system, individuals with healthcare need should have equal odds of receiving care (or not) (26). In contrast, in an inequitable healthcare system, differences in predisposing and enabling characteristics will better explain healthcare utilization than the presence of healthcare need. Furthermore, the environmental domain is a precursor to individual characteristics and explains contextual factors related to the health care system and other external environmental forces which influence the context in which individual characteristics operate.
The Behavioral Model has been successfully applied to better understand predictors of antiretroviral access and adherence (27-29). This study extends the use of the Behavioral Model to examine predictors of ARV diversion and adherence, which we frame as indicators of inadequate engagement in care. We also consider environmental factors that influence the illicit street market for ARV medications. Utilizing the Behavioral Model, we analyze how model domains and vulnerability affect ARV diversion and adherence.
METHODS
Data were drawn from a larger mixed-methods study that examined ARV diversion in indigent, substance abusing people living with HIV in South Florida. Quantitative data were collected from a targeted sample of vulnerable people living with HIV in South Florida between 2010 and 2012 (n=503). Recruitment was guided by targeted sampling – a method used to recruit hard-to-reach populations – in neighborhoods with high prevalence of HIV and concentrated poverty (30). Field staff used direct outreach to recruit participants through the systematic distribution of study cards and flyers in street venues and HIV service organizations within the target areas. Participants were eligible for the study if they were at least 18 years old, reported recent substance use, were confirmed HIV-positive, and were prescribed ARV treatment. Participants were considered to be diverters if they reported selling their ARVs at least once in the prior three months. Nearly equal numbers diverters (n=251) and non-diverters (n=252) were enrolled. All project staff completed certification for protection of human subjects and study protocols were approved by the Institutional Review Boards of University of Delaware (predecessor institution) and Nova Southeastern University. An NIH Certificate of Confidentiality was also obtained and a copy offered to participants. After attaining informed consent, trained project staff administered face-to-face structured interviews.
The study questionnaire collected quantitative data on individual, provider, and environmental factors related to ARV diversion, access and utilization of care, and ARV adherence. Participants received a $30 stipend upon completion of the interview, which averaged one hour in duration.
Measures
Figure 1 shows the Behavioral Model as operationalized in this study. This analysis examined two outcome measures. First, current ARV diversion stems from the question, “When was the last time, if ever, that you sold or traded any of your HIV medications to another person for any reason?” Current ARV diversion was then re-coded into ARV diversion in the past three months or not (yes/no). Second, percent ARV adherence was measured using the ACTG instrument (31), which assessed self-reported adherence in the past seven days. Percent ARV adherence was calculated by taking the total weekly ARV doses taken and dividing this number by the total ARV doses prescribed (and multiplying by 100 to attain a percentage from 0 to 100).
Figure 1.
Gelberg-Andersen Behavioral Model of Health Care Utilization for Vulnerable Populations
ARV illicit market factors measure the external environment and are hypothesized to influence ARV diversion. Knowledge of the market price structure measured whether or not the participant was familiar with the trends of the illicit market prices of ARVs. Embeddedness in the illicit ARV market was measured by the number of people the participant personally knew that were involved in selling/trading their ARVs (e.g. number of people in their diverter network).
Predisposing factors included basic socio-demographic characteristics and variables that measure vulnerability. The Global Appraisal of Individual Needs (GAIN) instrument (32) was used to assess a range of socio-demographics, social support, substance dependence (DSM-IV R), and mental health. Traditional predisposing factors included age, gender, education, race/ethnicity, and years HIV-positive. In addition, we included: a 7-item HIV treatment knowledge scale (with questions such as “Skipping a dose of HIV medications is fine; you can catch up at your next dose” and “Taking a drug holiday is good”); with possible scores ranging from 0 (no knowledge) to 7 (very knowledgeable) (α=0.43) (33), an ARV medication attitude scale – a 10-item summative scale with possible scores ranging from 0 (negative attitude) to 10 (positive attitude) (α=0.61) (34), and a health literacy scale – a 3-item summative scale with possible scores ranging from 0 (poor literacy) to 12 (excellent literacy) (α=0.77) (35). Vulnerable predisposing measures included being homeless in the last 30 days, substance dependence in the last 30 days – a 7-item summative scale with possible scores ranging from 0 to 7 (a score of 3 is considered dependent; α=0.89), and depressive symptoms in the last 12 months scale – a 9-item summative scale with scores ranging from 0 (no/minimal depression) to 9 (severe depression) (a score from 2 to 5 is considered moderate depression; α=0.87).
Traditional enabling factors included monthly income, length of time (months) with their usual source of HIV care, and a general social support scale – a 3-item summative scale adapted from the HIV Health Care Cost and Services Utilization Study (HCSUS) (36) (with questions such as someone to give you money if you really needed it; someone to help with chores, errands, or appointments if you were sick) with scores ranging from 3 (low levels of support) to 15 (high levels of support) (α=0.76). Vulnerable enabling factors included an HIV treatment access scale – which was calculated using the general access to care questions from the HCSUS (36) and includes a 7-item summative scale (with questions such as “Places where I can get medical care are very conveniently located” and “I have easy access to medical specialists I need”) with possible scores ranging from 7 (poor treatment access) to 28 (excellent treatment access) (α=0.81) (36), and an attitudes toward HIV provider scale – a 12-item summative scale with scores ranging from 12 (negative attitudes) to 48 (positive attitudes) (α=0.87) (37).
Need factors measured general health need and HIV-related and sexual health need. Traditional need factors included self-rated health on a Likert scale and the number of days in which medical problems interfered with daily activity in the past 90 days. Vulnerable need factors measured HIV-related health need with current CD4+ count and a score that measures the number of side effects reported from ARVs and the severity of each side effect. Sexual health need was measured by having been diagnosed with an STI in the last 12 months.
Data Analysis
Data analysis began with descriptive statistics of the sample and bivariate logistic regression was conducted to document the direction, magnitude, and significance of the statistical associations between Behavioral Model domains and ARV diversion. All the assumptions of logistic regression were tested for and met prior to analyses and listwise deletion resulted in an analytic sample size of n=477.
To test the Behavioral Model, multiple logistic regression was then conducted in two steps. First, the effect of each domain of the Behavioral Model was estimated separately and model fit was compared between individual domains and the full model using the likelihood ratio test and the Bayesian Information Criterion (BIC), two goodness-of-fit measures appropriate for the comparison of nested models (not reported). Second, based on the results from the first step, the best-fitting domain was added first followed by other domains in order by their rank in model fit. Finally, unadjusted and adjusted linear regression was conducted to estimate the effect of ARV diversion on ARV adherence.
RESULTS
Table 1 describes our sample (n=477) characteristics using dimensions from the Behavioral Model. About half of the participants recently diverted their ARVs (48%), reporting diverting an average of 2.3 times (data not shown). Participants self-reported an average of 79% adherence to their ARVs. Of environmental factors, more than half of participants (53%) had some familiarity with the price structure of the ARV illicit market and were, on average, greatly embedded in the market with five to six people in their diverter network.
Table 1.
Descriptive characteristics and bivariate odds ratio (OR) of ARV diversion in adults living with HIV in South Florida (N=477)
| Variables | n‡ | % | OR | 95% CI |
|---|---|---|---|---|
| HEALTH BEHAVIOR | ||||
| Diverted ARVs in past 90 days Percent Adherence to ARVs |
230 79.1 (31.7) |
48 0 to 100 |
||
| ENVIRONMENT FACTORS | ||||
| ARV illicit market price structure | ||||
| Unfamiliar | 225 | 47 | 1.00 | |
| Familiar | 252 | 53 | 8.75 | (5.77, 13.27)*** |
| Number of people in diverters network | 5.8 (11.2) | 0 to 96 | 1.04 | (1.02, 1.06)*** |
| PREDISPOSING FACTORS | ||||
| Traditional Domain | ||||
| Age | 46.2 (7.8) | 19 to 71 | 1.00 | (0.98, 1.02) |
| Gender | ||||
| Female | 189 | 40 | 1.00 | |
| Male | 288 | 60 | 1.98 | (1.36, 2.87)*** |
| Education | ||||
| High School or more | 265 | 56 | 1.00 | |
| Less than High School | 212 | 44 | 1.44 | (1.00, 2.08)* |
| Race/Ethnicity | ||||
| Black/African-American | 323 | 68 | 1.00 | |
| Non-Hispanic White | 67 | 14 | 0.9 | (0.56, 1.59) |
| Hispanic/Latino | 87 | 18 | 0.59 | (0.36, 0.96)* |
| Health Literacy Scale | 9.1 (3.3) | 0 to 12 | 0.90 | (0.85, 0.95)*** |
| Years HIV-positive | 13.0 (7.3) | 0 to 30 | 1.00 | (0.98, 1.02) |
| HIV Illness & Treatment Knowledge Scale | 5.8 (1.2) | 1 to 7 | 0.70 | 0.60, 0.82)*** |
| AMAS ARV Medication Attitudes Scale | 8.3 (1.7) | 1 to 10 | 0.81 | (0.72, 0.91)*** |
| Vulnerable Domain | ||||
| Homeless (last 30 days) | 146 | 31 | 2.29 | (1.53, 3.41)*** |
| Substance Dependence Scale (last 30 days ) | 3.4 (2.5) | 0 to 7 | 1.22 | (1.14, 1.32)*** |
| Depressive Symptom Scale (last 12 months) | 5.3 (3.0) | 0 to 9 | 1.10 | (1.03, 1.17)** |
| ENABLING FACTORS | ||||
| Traditional Domain | ||||
| Income (dollars/month) | ||||
| 501 or more | 324 | 68 | 1.00 | |
| 0 to 500 | 153 | 32 | 2.29 | (1.55, 3.40)*** |
| General Social Support Scale | 10.2 (3.2) | 3 to 15 | 0.97 | (0.92, 1.03) |
| Time with Usual Source of Care (months) | ||||
| More than 3 years | 234 | 49 | 1.00 | |
| 1-3 years | 140 | 29 | 1.38 | (0.91, 2.10) |
| Less than 12 months | 103 | 22 | 1.80 | (1.12, 2.87)* |
| Vulnerable Domain | ||||
| HIV Treatment Access | 23.8 (4.3) | 9 to 28 | 0.95 | (0.91, 0.99)* |
| Attitudes Toward HIV Provider | 43.8 (5.4) | 12 to 48 | 0.97 | (0.94, 1.00) |
| NEED FACTORS | ||||
| Traditional Domain | ||||
| Self-rated health | ||||
| Excellent/Very Good | 92 | 19 | 1.00 | |
| Good | 142 | 30 | 1.44 | (0.85, 2.44) |
| Fair | 189 | 40 | 1.21 | (0.73, 2.00) |
| Poor | 54 | 11 | 1.58 | (0.80, 3.10) |
| Medical Problems Interfere with Activity (last 90 days) | ||||
| 0 days | 196 | 41 | 1.00 | |
| Less than 31 days | 162 | 34 | 1.52 | (1.00, 2.31) |
| More than 31 days | 119 | 25 | 2.04 | (1.29, 3.25)** |
| Vulnerable Domain | ||||
| Diagnosed with STI in last 12 months | 137 | 29 | 1.04 | (0.70, 1.55) |
| Current CD4+ count | ||||
| ≥200 | 362 | 76 | 1.00 | |
| <200 | 80 | 17 | 1.08 | (0.67, 1.75) |
| Missing | 35 | 7 | 0.91 | (0.45, 1.83) |
| Side effects from ARVs | 6.5 (13.7) | 0 to 71 | 0.99 | (0.98, 1.01) |
Source: ARV Diversion Study 2010-2012; Exponentiated coefficients; 95% confidence intervals in brackets
mean(standard deviation) and range for continuous variables
Of the predisposing factors, participants averaged 46.2 years old, were male (62%), had a high school or more education (56%), and were Black/African-American (67%). Participants were also fairly health literate scoring an average of 9.1 (out of 12) on the health literacy scale. In terms of their HIV disease, participants averaged living with HIV for 13.0 years (with a range from 0 to 30 years), scored well (5.8 average out of 7) on the HIV treatment knowledge scale, and reported positive attitudes toward their ARV medication, scoring an average of 8.3 (out of 10) on the ARV medication attitudes scale. Many participants demonstrated vulnerability with 31% reporting homelessness in the last 30 days and the average participant evaluated as substance dependent and moderately depressed.
Of enabling factors, most participants report an income of $500 or more a month (68%). Participants reported moderate social support, averaging 10.2 on the general social support scale (out of 15). Almost half of participants (49%) had been at their usual source of care for more than three years whereas 22% reported being with their usual source of care for less than 12 months. Participants scored well on the HIV treatment access scale (averaging 23.8 out of 28) and generally reported positive attitudes toward their HIV care provider (averaging 43.8 out of 48).
Of need factors, only 19% of participants rated their health as either excellent or very good. Most participants reported that medical problems had interfered with their daily activity at least one day in the past 90 days (59%). Almost a third of participants (29%) had been diagnosed with an STI in the last 12 months. The majority of participants (76%) report having a current CD4+ count of 200 or more. Participants scored an average of 6.5 in side effects and severity of side effects from their ARV medications (ranging from 0 to 71).
Based on the bivariate logistic regression, market factors, predisposing factors, and some enabling factors stand out as significant predictors of current ARV diversion (see Table 1). Of the market factors, those familiar with the price structure of the ARV illicit market had almost nine times the odds of diverting their ARVs than those who were unfamiliar with the market. Embeddedness in the ARV illicit market was also a significant predictor of current ARV diversion. For every additional person known that diverted their ARVs, there was a 4% increase in odds of ARV diversion.
Of the predisposing factors, males had over twice the odds of females of diverting their personal ARV medications. As expected, higher scores on the health literacy scale, the HIV illness and treatment knowledge scale, and the ARV medication attitudes scale were associated with significantly lower odds of ARV diversion. In contrast, recent homelessness, greater substance dependence, and more depressive symptoms were associated with significantly greater odds of ARV diversion.
Of the enabling factors, those with a monthly income of $500 or less had more than twice the odds of ARV diversion than those with a greater monthly income. Participants with greater HIV treatment access had significantly lower odds of ARV diversion. Those with less than a year with their usual source of care had significantly greater odds of ARV diversion than those at their usual source of care for more than three years. Of need factors, reporting that medical problems interfered with daily activity for at least one day in the last 90 days was associated with significantly greater odds of ARV diversion.
Table 2 reports odds ratios and model fit statistics for four multiple logistic regression models that predict ARV diversion using domains from the Behavioral Model. Model 1 estimates the effect of market factors on ARV diversion. Familiarity with the price structure of the ARV illicit market increases the odds of diversion by more than eight-fold, net of the number of people in their diverter network. Model 2 adds predisposing factors to examine ARV diversion. Controlling for market and other predisposing factors, being male, recently homeless, and substance dependent are each associated with significantly increased odds of ARV diversion, whereas HIV illness and treatment knowledge is associated with significantly decreased odds of ARV diversion. Model 3 adds enabling factors and, net of other factors, monthly income below $500 and less time with usual source of HIV care remain significantly associated with increased odds of ARV diversion. Model 4 adds need factors and, net of all other dimensions, reporting that medical problems interfered with daily activity is significantly associated with greater odds of ARV diversion in a graduated manner, increasing in magnitude with increasing functional interference. Contrary to what we hypothesized, for every additional side effect from ARVs there was a significant decrease in odds of ARV diversion. Overall, the best model fit is model 3 (compared to model 4) which includes all domains except for need factors.
Table 2.
Odds Ratios of ARV Diversion using the Health Care Utilization Model (N=477)
| Model 1: Environment Factors |
Model 2: + Predisposing Factors |
Model 3: + Enabling Factors |
Model 4: + Need Factors |
|
|---|---|---|---|---|
| ENVIRONMENT FACTORS | ||||
| ARV illicit market price structure | ||||
| Unfamiliar | 1.00 | 1.00 | 1.00 | 1.00 |
| Familiar | 8.41*** (5.46,12.95) |
8.47*** (5.22,13.75) |
10.05*** (6.00,16.85) |
10.63*** (6.24,18.11) |
| Number of people in diverters network | 1.01 (0.99,1.03) |
1.00 (0.98,1.02) |
1.00 (0.98,1.02) |
1.01 (0.98,1.03) |
| PREDISPOSING FACTORS | ||||
| Traditional Domain | ||||
| Age | 1.01 (0.98,1.04) |
1.02 (0.99,1.05) |
1.01 (0.98,1.05) |
|
| Gender | ||||
| Female | 1.00 | 1.00 | 1.00 | |
| Male | 2.27*** (1.40,3.67) |
2.12** (1.28,3.51) |
2.28** (1.35,3.85) |
|
| Education | ||||
| High School Education or more | 1.00 | 1.00 | 1.00 | |
| Less than High School Education | 1.40 (0.86,2.26) |
1.30 (0.80,2.13) |
1.34 (0.80,2.25) |
|
| Race/Ethnicity | ||||
| Black/African-American | 1.00 | 1.00 | 1.00 | |
| Non-Hispanic White | 1.13 (0.57,2.26) |
1.25 (0.62,2.53) |
1.28 (0.61,2.66) |
|
| Hispanic/Latino | 0.56 (0.30,1.03) |
0.62 (0.33,1.17) |
0.65 (0.34,1.26) |
|
| Health Literacy | 0.95 (0.89,1.03) |
0.96 (0.89,1.03) |
0.95 (0.88,1.03) |
|
| Years HIV Positive | 0.99 (0.95,1.02) |
1.00 (0.96,1.03) |
0.99 (0.96,1.03) |
|
| HIV Illness & Treatment Knowledge Scale | 0.74** (0.60,0.91) |
0.75** (0.60,0.92) |
0.74** (0.59,0.92) |
|
| AMAS ARV Medication Attitudes Scale | 0.96 (0.83,1.12) |
0.99 (0.84,1.16) |
0.97 (0.81,1.15) |
|
| Vulnerable Domain | ||||
| Homeless (within 30 days) | ||||
| No | 1.00 | 1.00 | 1.00 | |
| Yes | 1.97** (1.19,3.25) |
1.66 (0.97,2.83) |
1.56 (0.89,2.72) |
|
| Substance Dependence (last 30 days ) | 1.14** (1.04,1.25) |
1.16** (1.05,1.28) |
1.15** (1.04,1.28) |
|
| Depressive Symptom (last year) | 0.99 (0.91,1.08) |
1.00 (0.91,1.08) |
0.99 (0.89,1.09) |
|
| ENABLING FACTORS | ||||
| Traditional Domain | ||||
| Income ($/month) | ||||
| More than $500 | 1.00 | 1.00 | ||
| $0 to 500/month income | 2.28** (1.35,3.85) |
2.25** (1.31,3.87) |
||
| General Social Support | 1.05 (0.98,1.14) |
1.05 (0.97,1.14) |
||
| Time at Usual Source of Care | ||||
| More than 3 years | 1.00 | 1.00 | ||
| 1-3 years | 1.19 (0.68,2.07) |
1.16 (0.66,2.06) |
||
| Less than 12 months | 1.92* (1.04,3.54) |
1.74 (0.92,3.29) |
||
| Vulnerable Domain | ||||
| HIV Treatment Access | 0.99 (0.92,1.06) |
0.98 (0.91,1.05) |
||
| Attitudes Toward HIV Provider | 1.02 (0.96,1.07) |
1.01 (0.96,1.07) |
||
| NEED FACTORS | ||||
| Traditional Domain | ||||
| Self-rated health | ||||
| Excellent/Very Good | 1.00 | |||
| Good | 1.26 (0.62,2.53) |
|||
| Fair | 0.69 (0.34,1.39) |
|||
| Poor | 1.00 (0.37,2.65) |
|||
| Medical Problems Interfere with Activity | ||||
| 0 days | 1.00 | |||
| Less than 31 days | 1.88* (1.05,3.35) |
|||
| More than 31 days | 2.054* (1.03,4.08) |
|||
| Vulnerable Domain | ||||
| Diagnosed with STI (last year) | ||||
| No | 1.00 | |||
| Yes | 1.22 (0.70,2.13) |
|||
| Current CD4+ count | ||||
| ≥200 | 1.00 | |||
| <200 | 0.79 (0.41,1.52) |
|||
| Missing | 0.59 (0.24,1.48) |
|||
| Side effects from ARVs | 0.98* (0.96,1.00) |
|||
| BIC | 557.9 | 572.9 | 591.5 | 631.3 |
| Wald test (Chi-square) | 82.61 | 36.45 | 15.18 | 14.72 |
| p-value | 0.00 | 0.00 | 0.02 | 0.10 |
| Log Likelihood Ratio Test vs. Full Model 4 | 93.22 | 34.14 | 15.68 | - |
| p-value (Log Likelihood Ratio Test) | 0.000 | 0.003 | 0.074 | - |
Source: ARV Diversion Study 2010-2012; Exponentiated coefficients; 95% confidence intervals in brackets
Logistic Regression using Block Addition of Dimensions from Health Care Utilization Model
p < 0.05,
p < 0.01,
p < 0.001
Table 3 reports the unadjusted and adjusted coefficients of models that estimate how well ARV diversion predicts percent adherence to ARV medications. Current ARV diverters have a 23% drop in percent ARV adherence compared to non-diverters, which remains at a 19% drop in percent ARV adherence when controlling for all other dimensions of the health care utilization model. The unadjusted model explains 14% of the variation in ARV adherence, whereas the adjusted model is a better fitting model and explains 25% of the variation in adherence. Upon further examination of the adjusted model, ARV diversion is the strongest predictor of ARV adherence, which corresponds to the largest beta coefficient (in absolute values) in the model (β=−0.30 for ARV diversion, compared to β=0.14 for ARV medication attitudes and β=−0.13 for substance dependence, results not shown).
Table 3.
Simple and Multiple Linear Regression to Predict How ARV Diversion Affects Percent of ARV Adherence (n=477)‡
| Unadjusted Coefficient |
Adjusted Coefficient‡ |
||
|---|---|---|---|
| Diverted ARVs (90 days) | −23.49 (−28.89, −18.08) |
−18.97 (−25.57, −12.38) |
|
| p-value | < 0.001 | < 0.001 | |
|
|
|
||
| R-square | 0.14 | 0.25 | |
| BIC | 4591.40 | 4705.96 | |
| RMSE | 29.46 | 28.42 | |
Source: ARV Diversion Study 2010-2012
Adjusted models include all market, predisposing, enabling, and need factors
DISCUSSION
This is the first study to apply the Gelberg-Andersen Behavioral Model of Health Care Utilization for Vulnerable Populations to better understand ARV diversion as a personal health behavior, which is directly related to HIV care service utilization. Our analysis identifies ARV diversion as a possible link between social vulnerability and adherence. We also reveal a great need to improve the spectrum of engagement in HIV care for vulnerable populations. Lastly, we find environmental variables that measured the ARV illicit market greatly improved model fit.
Our findings indicate that the diversion of ARVs to the black market is a proxy for vulnerability that captures competing needs and reflects a need for intervention. The role of vulnerability in enabling ARV diversion becomes apparent with a number of significant factors emerging from the predisposing vulnerable domain (see Table 1). Although we recruited a high needs sample, our analysis demonstrates that having less than a high school education, experiencing recent homelessness, being substance dependent, and having depressive symptoms are significant predisposing vulnerable correlates of ARV diversion. Compared to the national HIV patient population, our sample suffered significant disparities in terms of income, substance dependence, and psychological distress (38). These factors of vulnerability are also consistent correlates of patient ARV adherence with previous studies associating psychosocial factors such as depression (39-43), active drug use (39-41, 43, 44), alcohol use (41, 44), and the lack of social stability with suboptimal levels of ARV adherence (45-47). This study highlights ARV diversion as a potential link between social vulnerability and suboptimal ARV adherence.
We identify that the best fit model to predict ARV diversion (see Model 3 of Table 2) included all domains except for ‘need’; a finding that may indicate an inadequate engagement in HIV care among our vulnerable sample. Our study finds that ARV diversion occurred despite low CD4 counts and poor self-rated health, even though all study participants were at least minimally engaged in HIV care to obtain a legitimate ARV prescription (required for study eligibility). This finding provides evidence that immediate competing needs are prioritized even in the face of poor health for this sample; an observation that may reflect a relative inaccessibility or under-utilization of more intensive HIV-related services that may be required to avoid diversion behavior (e.g. treatment for substance dependence and mental illness, intensive case management, support for adherence and side-effects, and financial and subsistence support).
The best fitting model to predict ARV diversion identifies having a low monthly income ($500 or less) and unstable HIV care (less than 12 months) as salient enabling factors that promote ARV diversion, net of other factors. There is limited understanding regarding the extent to which financial barriers impede access to and quality of HIV care in the U.S. (21), especially among populations with extreme health care need. Programs like Ryan White and the AIDS Drug Assistance Program (ADAP) provide financial support for low-income individuals, and although almost all of the participants in the current study had health insurance, over half of respondents from a qualitative study on ARV diversion report selling their medication for money to meet their subsistence needs (e.g. food, housing rent, utility or transportation bills, etc.) with many noting that poor health precluded them from working for a steady income (17). Future studies should assess the extent to which financial constraints other than HIV-specific health costs (e.g. low personal income, perceived financial barriers, etc.) may impact complete engagement in care (21). Furthermore, additional research on how social vulnerability and comorbidities affect ARV adherence are needed as ARV diversion is found to occur in the context of homelessness, substance dependence and mental health problems (9, 17). Interestingly, although housing instability was significantly associated with ARV diversion in Model 2, the addition of enabling factors to the model (see Table 2) diminished this association. We speculate that the economic precariousness associated with homelessness that drives ARV diversion is mitigated by enabling resources such as income, social support, and access to and quality of care, which were accounted for separately in Model 3. Access to such resources and services is critical for unstably housed individuals, and may enable them to improve their ARV adherence.
Our study findings call to enhance the patient-provider relationship to improve engagement in HIV care (48, 49). We find that individuals who stayed with the same HIV care provider for one year or longer had significantly lower odds of ARV diversion than those with less than one year with their provider. Enhancing the patient-physician relationship plays an important role in appointment adherence (50-52) and long-term engagement in HIV care (53). Additionally, patients’ perceptions of provider engagement and satisfaction with their provider have been associated with HIV medication adherence (54-56). An improved patient-physician relationship allows communication to address problems with adherence, adverse medication side-effects, and to link vulnerable patients to other critical services that could reduce diversion behavior (21, 57, 58). Furthermore, improved engagement in HIV care is linked to increased utilization of case management services, mental health services, substance abuse treatment, transportation assistance, and housing assistance (21, 59). Future HIV intervention research to enhance the patient-provider relationship in vulnerable populations could test interventions that clinically implement: 1) efforts to foster supportive clinical environments for patients, 2) greater time investment for the provider per patient visit (60), 3) training for providers to facilitate patient-centered care (61) and collaborative negotiation with patients in setting treatment goals and strategies (e.g. for stress management) (49), 4) patient navigation and intensive case management to improve linkage to care, 5) text-message reminders (e.g. of doses, appointments, etc.) to improve adherence (21), and 6) intervention to decrease internalized HIV-stigma to improve utilization of HIV care and adherence (62).
This study also found that the inclusion of external environmental factors (e.g. ARV illicit market factors) in the Behavioral Model significantly improved model fit to predict ARV diversion. Being familiar with the price structure and embeddedness in illicit ARV networks were salient predictors of ARV diversion. Research on ARV diversion in South Florida indicates that pill brokers target indigent people living with HIV to divert their medication for money due to financial hardship, to support their substance use, or because of adherence problems due to side effects or regimen complexity (9, 14). Findings from this study highlight the need for structural interventions that utilize existing diverter networks to intervene on barriers to complete engagement in HIV care and ARV adherence.
Finally, to better understand the extent to which ARV diversion is linked to adherence, we ran linear regression models to predict percent ARV adherence using ARV diversion (Table 3). ARV diversion was the strongest predictor of percent of ARV adherence, explaining 14% of the variation in percent ARV adherence. According to the R-square and RMSE, the model which adjusted for all domains in the Behavioral Model provided a better fit than the unadjusted model and explained 25% of the variation in percent ARV adherence. ARV diversion is just one factor of many that influences adherence, but it also captures the socioeconomic marginalization and social instability present in the lives of our vulnerable sample.
Study findings should be considered in light of several limitations. First, this is a cross-sectional study therefore limiting any assertions of causality between ARV diversion and ARV adherence, health care utilization, and other health problems or needs affecting our sample. Second, our study sample is a highly specific group of vulnerable people living with HIV in South Florida, therefore also limiting the generalizability of findings to other groups of people or regions in the U.S. Third, our measures of ARV illicit market factors were limited to two variables, although the variables did provide a novel addition to the Behavioral Model. Nevertheless, these variables appeared to sufficiently capture dynamics of the ARV illicit market and improved model fit. We adapted external environmental measures to best suit our study, but future studies should include other external environment measures such as changes in insurance coverage and economic factors. Lastly, our measure of ARV adherence is based on self-reported data, and social desirability bias may have prompted participants to over-estimate their levels of ARV adherence. Even so, a recent review of ARV adherence finds that the majority of studies use self-reported ARV adherence as the main measure of treatment adherence (40-43, 63-69).
This paper finds that ARV drug diversion links social vulnerability to poor medication adherence in vulnerable, substance abusing people living with HIV in South Florida. ARV adherence is a complex and dynamic process that not only influences the patient’s health, but also determines overall treatment success and HIV transmissibility (70, 71). Our study highlights the importance of investing resources to improve enabling factors such as: 1) targeted financial and housing support provided by state- and federal-assistance programs, and, 2) clinical intervention research to enhance patient-provider relationships in vulnerable populations. Perhaps most critical is the need to improve screening, referral and linkage to substance use and psychological treatment among vulnerable HIV-positive patients – as these are clearly linked to ARV diversion behavior; addressing these recommendations could improve engagement in HIV care and medication adherence of high needs populations.
Acknowledgments
This research is supported by PHS Grant Number R01DA023157 from the National Institute on Drug Abuse. NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of this report; or in the decision to submit the paper for publication.
REFERENCES
- 1.Inciardi JA, Surratt HL, Kurtz SP, Burke JJ. The diversion of prescription drugs by health care workers in Cincinnati, Ohio. Substance use & misuse. 2006;41(2):255–64. doi: 10.1080/10826080500391829. [DOI] [PubMed] [Google Scholar]
- 2.Associated Press . AIDS drugs surface on the black market. New York Times; 1995. [Google Scholar]
- 3.Dorschner J. Activists: HIV care scam uses homeless. Miami Herald. 2005 Jun 30; 2005. [Google Scholar]
- 4.Flaherty M, Gaul G. Florida Medicaid fraud costs millions, report says. Washington Post: Dec 19, 2003. 2003. [Google Scholar]
- 5.Glasgow K. Their product? The AIDS medications intended to cure them. Miami New Times; 1999. The New Dealers: They’re poor, black, and HIV-positive. [Google Scholar]
- 6.Kurtz SP, Buttram ME, Surratt HL. Vulnerable infected populations and street markets for ARVs: Potential implications for PrEP rollout in the USA. AIDS care. 2013:1–5. doi: 10.1080/09540121.2013.837139. (ahead-of-print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Surratt HL, Kurtz SP. A national perspective on the abuse and diversion of prescription drugs. Nova Southeastern University Faculty Symposium; Nova Southeastern University; 2013. [Google Scholar]
- 8.Bharara P, Manhattan US. Attorney Announces Charges Against 48 Individuals in Massive Medicaid Fraud Scheme Involving the Diversion and Trafficking of Prescription Drugs. Scheme Caused Losses Estimated at More than Half-a-Billion Dollars to Medicaid and is Believed to Be the Largest Single Prescription Drug Diversion Scheme Ever Charged at One Time. 2012.
- 9.Surratt HL, Kurtz SP, Cicero TJ, O’Grady C, Levi-Minzi MA. Antiretroviral Medication Diversion Among HIV-Positive Substance Abusers in South Florida. American journal of public health. 2013;103(6):1026–8. doi: 10.2105/AJPH.2012.301092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher CV, Testa MA, Brundage RC, Chesney MA, Haubrich R, Acosta EP, et al. Four Measures of Antiretroviral Medication Adherence and Virologic Response in AIDS Clinical Trials Group Study 359. Journal of Acquired Immune Deficiency Syndromes. 2005;40(1):301–16. doi: 10.1097/01.qai.0000180078.53321.6a. [DOI] [PubMed] [Google Scholar]
- 11.Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. Aids. 2001;15(9):1181–3. doi: 10.1097/00002030-200106150-00015. Epub 2001/06/21. [DOI] [PubMed] [Google Scholar]
- 12.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of internal medicine. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lucas GM. Antiretroviral adherence, drug resistance, viral fitness and HIV disease progression: a tangled web is woven. Journal of Antimicrobial Chemotherapy. 2005;55(4):413–6. doi: 10.1093/jac/dki042. [DOI] [PubMed] [Google Scholar]
- 14.Inciardi JA, Surratt HL, Kurtz SP, Cicero TJ. Mechanisms of prescription drug diversion among drug-involved club-and street-based populations. Pain Medicine. 2007;8(2):171–83. doi: 10.1111/j.1526-4637.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uldall KK, Palmer B, Whetten K, Mellins C. Adherence in people living with HIV/AIDS, mental illness, and chemical dependency: A review of the literature. AIDS care. 2004;16(Suppl.1):S71–S96. doi: 10.1080/09540120412331315277. [DOI] [PubMed] [Google Scholar]
- 16.Weaver KE, Llabre MM, Durán RE, Antoni MH, Ironson G, Penedo FJ, et al. A Stress and Coping Model of Medication Adherence and Viral Load in HIV-Positive Men and Women on Highly Active Antiretroviral Therapy (HAART) Health Psychology. 2005;34(4):385–92. doi: 10.1037/0278-6133.24.4.385. [DOI] [PubMed] [Google Scholar]
- 17.Tsuyuki K, Surratt HL, Levi-Minzi MA, O’Grady CL, Kurtz SP. The Demand for Antiretroviral Drugs in the Illicit Marketplace: Implications for HIV Disease Management Among Vulnerable Populations. AIDS and Behavior. 2014:1–12. doi: 10.1007/s10461-014-0856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. Aids. 2004;18(Suppl 1(Suppl.1)):S19–25. doi: 10.1097/00002030-200418001-00004. Epub 2004/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingersoll KS, Heckman CJ. Patient-clinician relationships and treatment system effects on HIV medication adherence. AIDS and behavior. 2005;9(1):89–101. doi: 10.1007/s10461-005-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharpe TT, Lee LM, Nakashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected black women. Journal of Community Health. 2004;29(2):117–27. doi: 10.1023/b:johe.0000016716.99847.9b. [DOI] [PubMed] [Google Scholar]
- 21.Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical infectious diseases. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tucker JS, Edelen MO, Burnam MA, Sherbourne CD, Kung F-Y, Gifford A. Psychosocial Mediators of Antiretroviral Nonadherence in HIV-positive Adults with Substance Use and Mental Health Problems. Health Psychology. 2004;23(4):363–70. doi: 10.1037/0278-6133.23.4.363. [DOI] [PubMed] [Google Scholar]
- 23.CDC HIV Surveillance Report. Feb, 2013. 2011. Report No.
- 24.Mizuno Y, Wilkinson J, Santibanez S, Rose CD, Knowlton A, Handley K, et al. Correlates of health care utilization among HIV-seropositive injection drug users. AIDS care. 2006;18(5):417–25. doi: 10.1080/09540120500162247. [DOI] [PubMed] [Google Scholar]
- 25.Gelberg L, Andersen R, Leake B. The Behavioral Model for Vulnerable Populations: Application to Medical Care Use and Outcomes for Homeless People. Health Services Research. 2000;34(6):1273–302. [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? Journal of Health and Social Behavior. 1995 Mar;36:1–10. [PubMed] [Google Scholar]
- 27.Andersen R, Bozzette S, Shapiro M, St. Clair P, Morton S, Crystal S, et al. Access of vulnerable groups to antiretroviral. therapy among persons in care for HIV disease in the United States. HCSUS Consortium. HIV Cost and Services Utilization Study. Health Services Research. 2000;35(2):389–416. [PMC free article] [PubMed] [Google Scholar]
- 28.Katz MH, Cunningham WE, Fleishman JA, Andersen RM, Kellogg T, Bozzette SA, et al. Effect of Case Management on Unmet Needs and Utilization of Medical Care and Medications among HIV-Infected Persons. Annals of internal medicine. 2001;135(8):557–65. doi: 10.7326/0003-4819-135-8_part_1-200110160-00006. [DOI] [PubMed] [Google Scholar]
- 29.Smith SR, Kirking DM. Access and Use of Medications in HIV Disease. Health Services Research. 1999;34(1 Pt 1):123–44. [PMC free article] [PubMed] [Google Scholar]
- 30.Miami Dade County Department of Health [cited 2013 August 29];Miami-Dade County Reported HIV (Not AIDS) Cases Through 2012. 2012 Available from: http://www.dadehealth.org/downloads/2012%20HIV%20Reported.pdf.
- 31.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. AIDS care. 2000;12(3):255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 32.Dennis ML, Titus JC, White MK, Unsicker JI, Hodgkins D. Global Appraisal of Individual Needs - Initial (GAIN-I) Chestnut Health Systems; Bloomington, IL: 2002. [Google Scholar]
- 33.Van Servellen G, Carpio F, Lopez M, Garcia-Teague L, Herrera G, Monterrosa F, et al. Program to enhance health literacy and treatment adherence in low-income HIV-infected Latino men and women. Aids patient care and STDs. 2003;17(11):581–94. doi: 10.1089/108729103322555971. [DOI] [PubMed] [Google Scholar]
- 34.Viswanathan H, Anderson R, Thomas J., III Evaluation of an antiretroviral medication attitude scale and relationships between medication attitudes and medication nonadherence. Aids patient care and STDs. 2005;19(5):306–16. doi: 10.1089/apc.2005.19.306. [DOI] [PubMed] [Google Scholar]
- 35.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Family Medicine. 2004;36(8):588–94. [PubMed] [Google Scholar]
- 36.RAND Corporation [cited 2013 August 29];HIV Cost and Services Utilization Study Survey 1998. Available from: http://www.rand.org/content/dam/rand/www/external/health/surveys_tools/hcsus/hcsussurvey.pdf.
- 37.Bodenlos JS, Grothe KB, Kendra K, Whitehead D, Copeland AL, Brantley PJ. Attitudes toward HIV health care providers scale: Development and validation. Aids patient care and STDs. 2004;18(12):714–20. doi: 10.1089/apc.2004.18.714. [DOI] [PubMed] [Google Scholar]
- 38.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus–infected adults in the United States. Archives of General Psychiatry. 2001;58(8):721–8. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 39.Singh N, Squier C, Sivek C, Wagener M, Nguyen MH, Yu V. Determinants of compliance with antiretroviral therapy in patients with human immunodeficiency virus: prospective assessment with implications for enhancing compliance. AIDS care. 1996;8(3):261–70. doi: 10.1080/09540129650125696. [DOI] [PubMed] [Google Scholar]
- 40.Kleeberger CA, Buechner J, Palella F, Detels R, Riddler S, Godfrey R, et al. Changes in adherence to highly active antiretroviral therapy medications in the Multicenter AIDS Cohort Study*. AIDS (London, England) 2004;18(4):683–8. doi: 10.1097/00002030-200403050-00013. [DOI] [PubMed] [Google Scholar]
- 41.Mohammed H, Kieltyka L, Richardson-Alston G, Magnus M, Fawal H, Vermund SH, et al. Adherence to HAART among HIV-infected persons in rural Louisiana. Aids patient care and STDs. 2004;18(5):289–96. doi: 10.1089/108729104323076025. [DOI] [PubMed] [Google Scholar]
- 42.Peretti-Watel P, Spire B, Schiltz M-A, Bouhnik A-D, Heard I, Lert F, et al. Vulnerability, unsafe sex and non-adherence to HAART: evidence from a large sample of French HIV/AIDS outpatients. Social science & medicine. 2006;62(10):2420–33. doi: 10.1016/j.socscimed.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Gebo KA, Keruly J, Moore RD. Association of social stress, illicit drug use, and health beliefs with nonadherence to antiretroviral therapy. Journal of general internal medicine. 2003;18(2):104–11. doi: 10.1046/j.1525-1497.2003.10801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golin CE, Liu H, Hays RD, Miller LG, Beck CK, Ickovics J, et al. A prospective study of predictors of adherence to combination antiretroviral medication. Journal of general internal medicine. 2002;17(10):756–65. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh N, Berman SM, Swindells S, Justis JC, Mohr JA, Squier C, et al. Adherence of Human Immunodeficiency Virus—Infected Patients to Antiretroviral Therapy. Clinical infectious diseases. 1999;29(4):824–30. doi: 10.1086/520443. [DOI] [PubMed] [Google Scholar]
- 46.Friedland GH, Williams A. Attaining higher goals in HIV treatment: the central importance of adherence. Aids. 1999;13(1):S61–S72. [PubMed] [Google Scholar]
- 47.Ammassari A, Trotta MP, Murri R, Castelli F, Narciso P, Noto P, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published literature. J Acquir Immune Defic Syndr. 2002;31:S123–7. doi: 10.1097/00126334-200212153-00007. [DOI] [PubMed] [Google Scholar]
- 48.Flickinger TE, Saha S, Moore RD, Beach MC. Higher quality communication and relationships are associated with improved patient engagement in HIV care. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2013;63(3):362–6. doi: 10.1097/QAI.0b013e318295b86a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson MO, Chesney MA, Goldstein RB, Remien RH, Catz S, Gore-Felton C, et al. Positive provider interactions, adherence self-efficacy, and adherence to antiretroviral medications among HIV-infected adults: A mediation model. AIDS Patient Care & Stds. 2006;20(4):258–68. doi: 10.1089/apc.2006.20.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bodenlos JS, Grothe KB, Whitehead D, Konkle-Parker DJ, Jones GN, Brantley PJ. Attitudes toward health care providers and appointment attendance in HIV/AIDS patients. Journal of the Association of Nurses in AIDS Care. 2007;18(3):65–73. doi: 10.1016/j.jana.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Kempf M-C, McLeod J, Boehme AK, Walcott MW, Wright L, Seal P, et al. A qualitative study of the barriers and facilitators to retention-in-care among HIV-positive women in the rural southeastern United States: implications for targeted interventions. Aids patient care and STDs. 2010;24(8):515–20. doi: 10.1089/apc.2010.0065. [DOI] [PubMed] [Google Scholar]
- 52.Johnson MO, Sevelius JM, Dilworth SE, Saberi P, Neilands TB. Preliminary support for the construct of health care empowerment in the context of treatment for human immunodeficiency virus. Patient Prefer Adherence. 2012;6(1):395–404. doi: 10.2147/PPA.S30040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mallinson RK, Rajabiun S, Coleman S. The provider role in client engagement in HIV care. Aids patient care and STDs. 2007;21(S1):S-77–S-84. doi: 10.1089/apc.2007.9984. [DOI] [PubMed] [Google Scholar]
- 54.Bakken S, Holzemer WL, Brown M-A, Powell-Cope GM, Turner JG, Inouye J, et al. Relationships between perception of engagement with health care provider and demographic characteristics, health status, and adherence to therapeutic regimen in persons with HIV/AIDS. Aids patient care and STDs. 2000;14(4):189–97. doi: 10.1089/108729100317795. [DOI] [PubMed] [Google Scholar]
- 55.Demmer C. Relationship with health care provider and adherence to HIV medications. Psychological reports. 2003;93(2):494–6. doi: 10.2466/pr0.2003.93.2.494. [DOI] [PubMed] [Google Scholar]
- 56.Roberts KJ. Physician-patient relationships, patient satisfaction, and antiretroviral medication adherence among HIV-infected adults attending a public health clinic. Aids patient care and STDs. 2002;16(1):43–50. doi: 10.1089/108729102753429398. [DOI] [PubMed] [Google Scholar]
- 57.Hart E, Curtis H, Wilkins E, Johnson M. National review of first treatment change after starting highly active antiretroviral therapy in antiretroviral-naïve patients. HIV medicine. 2007;8(3):186–91. doi: 10.1111/j.1468-1293.2007.00451.x. [DOI] [PubMed] [Google Scholar]
- 58.Yuan Y, L’italien G, Mukherjee J, Iloeje U. Determinants of discontinuation of initial highly active antiretroviral therapy regimens in a US HIV-infected patient cohort*. HIV medicine. 2006;7(3):156–62. doi: 10.1111/j.1468-1293.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 59.Conviser R, Pounds M. The role of ancillary services in client-centred systems of care. AIDS care. 2002;14(S1):119–31. doi: 10.1080/09540120220150018. [DOI] [PubMed] [Google Scholar]
- 60.Wilson IB, Kaplan S. Physician-patient communication in HIV disease: the importance of patient, physician, and visit characteristics. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2000;25(5):417–25. doi: 10.1097/00042560-200012150-00006. [DOI] [PubMed] [Google Scholar]
- 61.Epstein RM, Franks P, Fiscella K, Shields CG, Meldrum SC, Kravitz RL, et al. Measuring patient-centered communication in patient–physician consultations: theoretical and practical issues. Social science & medicine. 2005;61(7):1516–28. doi: 10.1016/j.socscimed.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Levi-Minzi MA, Surratt HL. HIV stigma among substance abusing people living with HIV/AIDS: Implications for HIV treatment. AIDS Patient Care & Stds. 2014;28(8):1–10. doi: 10.1089/apc.2014.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fong O, Ho C, Fung L, Lee F, Tse W, Yuen C, et al. Determinants of adherence to highly active antiretroviral therapy (HAART) in Chinese HIV/AIDS patients. HIV medicine. 2003;4(2):133–8. doi: 10.1046/j.1468-1293.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- 64.Goldman DP, Smith JP. Can patient self-management help explain the SES health gradient? Proceedings of the National Academy of Sciences. 2002;99(16):10929–34. doi: 10.1073/pnas.162086599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleeberger CA, Phair JP, Strathdee SA, Detels R, Kingsley L, Jacobson LP. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2001;26(1):82–92. doi: 10.1097/00126334-200101010-00012. [DOI] [PubMed] [Google Scholar]
- 66.Eldred LJ, Wu AW, Chaisson RE, Moore RD. Adherence to antiretroviral and pneumocystis prophylaxis in HIV disease. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1998;18(2):117–25. doi: 10.1097/00042560-199806010-00003. [DOI] [PubMed] [Google Scholar]
- 67.Kalichman SC, Ramachandran B, Catz S. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. Journal of general internal medicine. 1999;14(5):267–73. doi: 10.1046/j.1525-1497.1999.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiser S, Wolfe W, Bangsberg D, Thior I, Gilbert P, Makhema J, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. JAIDS. 2003;34(3):281–8. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- 69.Lanièce I, Ciss M, Desclaux A, Diop K, Mbodj F, Ndiaye B, et al. Adherence to HAART and its principal determinants in a cohort of Senegalese adults. Aids. 2003;17:S103–S8. doi: 10.1097/00002030-200317003-00014. [DOI] [PubMed] [Google Scholar]
- 70.Ickovics JR, Meade C. Adherence to HAART among patients with HIV: breakthroughs and barriers. AIDS care. 2002;14(3):309–18. doi: 10.1080/09540120220123685. [DOI] [PubMed] [Google Scholar]
- 71.Moatti J-P, Souteyrand Y. Editorial: HIV/AIDS social and behavioural research: past advances and thoughts about the future. Social science & medicine. 2000;50(11):1519–32. doi: 10.1016/s0277-9536(99)00462-1. [DOI] [PubMed] [Google Scholar]