Abstract
Patients with neurogenic orthostatic hypotension (OH) typically have impaired sympathetic nervous system tone and therefore low levels of upright plasma norepinephrine. We report a subset of patients who clinically have typical neurogenic OH but who paradoxically have elevated upright levels of plasma norepinephrine.
We retrospectively studied 83 OH patients evaluated at the Vanderbilt Autonomic Dysfunction Center between August 2007 and May 2013. Based upon standing norepinephrine, patients were dichotomized into a hyperadrenergic orthostatic hypotension group (hyperOH: upright NE ≥3.55 nmol/L [600 pg/mL], n=19) or a non-hyperadrenergic orthostatic hypotension group (nOH: upright NE < 3.55 nmol/L [600 pg/mL], n=64). Medical history and data from autonomic testing, including the Valsalva maneuver (VM), were analyzed. HyperOH patients had profound orthostatic falls in blood pressure, but less severe than in nOH (change in SBP: −53±31 mmHg vs. −68±33 mmHg, P=0.050; change in DBP: −18±23 mmHg vs. −30±17 mmHg, P=0.01). The expected compensatory increase in standing heart rate was similarly blunted in both hyperOH and nOH groups (84±15 bpm vs. 82±14 bpm; P=0.6). HyperOH patients had less severe sympathetic failure as evidenced by smaller falls in DBP during phase 2 of VM, and a shorter VM phase 4 blood pressure recovery time (16.5±8.9 sec vs. 31.6±16.6 sec; P<0.001) than nOH patients.
Neurogenic hyperOH patients have severe neurogenic orthostatic hypotension, but have less severe adrenergic dysfunction than nOH patients. Further work is required to understand if hyperOH patients will progress to nOH or if this represents a different disorder.
Keywords: Hypotension, Orthostatic, Syncope, Autonomic Failure, Pure, Polyneuropathy
INTRODUCTION
Orthostatic hypotension (OH) is defined as a reduction in systolic blood pressure (SBP) >20 mmHg or a reduction in diastolic blood pressure (DBP) >10 mmHg within 3 minutes of standing [1]. It is an increasingly prevalent clinical problem that is responsible for significant morbidity in the elderly [2;3]. OH is associated with a significant economic burden on the health care system with an estimated 160,000 hospitalizations annually in the United States [4]. While ~50% of these hospitalizations are due to acutely reversible conditions such as dehydration or medication effects, the other half represent chronic and longstanding neurogenic OH related to autonomic nervous system failure [4;5]. Therefore, neurogenic OH is associated with reduced upright plasma norepinephrine (NE) levels, a marker of sympathetic nervous system activity [6].
The term “hyperadrenergic orthostatic hypotension” (hyperOH) was first coined by Dr. David Streeten in 1990 when he described a small group of patients with mild OH and normal or elevated levels of upright plasma NE levels [7]. He did not describe patients with severe neurogenic OH who have elevated upright plasma NE levels (≥3.55 nmol/L or 600 pg/mL)[8].
At our tertiary autonomic disorders referral center, we have noted that some patients with neurogenic OH have elevated upright plasma NE. This phenomenon has not been previously recognized in neurogenic OH. Further, the clinical features and characteristics of neurogenic OH patients with paradoxically elevated plasma NE have not been described in the literature. We investigated whether symptomatic patients with neurogenic hyperadrenergic OH (hyperOH) could be distinguished from those patients with non-elevated levels of plasma NE (non-hyperadrenergic OH, nOH) using standard autonomic reflex testing.
METHODS
Patient population
Patients evaluated in the Vanderbilt Autonomic Dysfunction Clinic or admitted to the Vanderbilt Clinical Research Center between August 2007 and May 2013 with a diagnosis of chronic autonomic failure were included in this study if they met the criteria for neurogenic OH (SBP drop ≥20 mmHg (or ≥30 mmHg if the supine SBP was ≥160 mmHg) or DBP drop ≥10 mmHg) [1]and had undergone autonomic function testing which included a digitally acquired Valsalva maneuver (VM) with beat to beat blood pressure (BP) recording. The research has been carried out in accordance with the Declaration of Helsinki (2008) of the World Medical Association. Patients with evidence of a “square-root” wave pattern on VM were excluded as the BP aberrantly stays elevated and constant throughout the strain phases of the VM. This only occurred in 3 patients. All patients gave their written informed and this study was approved by the Vanderbilt University Institutional Review Board. These data have not been previously published.
Demographics and history of symptoms
We reviewed patient charts and abstracted data for age, height, weight, body mass index (BMI), gender, duration of disease at time of presentation, co-morbidities, and medications during presentation. Co-morbid conditions that were noted for analysis included diabetes, obstructive sleep apnea, multiple system atrophy (MSA), and Parkinson’s disease [9]. Medications that were noted for analysis included chronic use of opioid analgesics, benzodiazepines, selective serotonin reuptake inhibitors (SSRI), serotonin norepinephrine reuptake inhibitors (SNRI), fludrocortisone, midodrine, levodopa/carbidopa, beta-blockers, and alpha-1 adrenergic antagonists.
Stand test with catecholamines
The “stand test” involved patients lying down for at least 15 min (to allow for a supine steady state), heart rate (HR), SBP & DBP (Dinamap, Critikon Corp) were measured. The patient then stood for 10 minutes (as tolerated) with HR, SBP, and DBP measured at 1, 3, 5, and 10 min standing. Orthostatic changes were calculated as the difference between supine parameters and measurements obtained at the end of standing. Patients unable to stand for 10 minutes were allowed to sit for the 10 min before blood was sampled. Blood was collected for NE and epinephrine (EPI) in plastic syringes in both supine and upright positions (after at least 10 min of standing or sitting). Samples were then immediately transferred to chilled vacuum tubes with sodium heparin (BD, Franklin Lakes, NJ). Plasma was separated by centrifugation at 4°C and stored at −70°C in collection tubes. Clinical samples were assayed within 4 days. Reduced glutathione 6% (Sigma-Aldrich Inc, St Louis, Mo) was added to the Clinical Research Center samples prior to freezing. Concentrations of NE and EPI were measured by high-performance liquid chromatography with electrochemical detection following batch alumina extraction [10].
Data acquisition during autonomic function testing including Valsalva Maneuver
Autonomic function tests were performed while patients were inpatients on the Vanderbilt University Clinical Research Center or in the outpatient setting during their clinic visit. All non-essential vasoactive medications were held on the day of autonomic testing. SBP and DBP were measured continuously by the finger volume clamp method (Nexfin; BMEYE, Amsterdam, The Netherlands) and intermittently with an automated oscillometric brachial cuff (Vital-Guard 450C, Ivy Biomedical Systems). HR was determined by continuous ECG monitoring (Vital-Guard 450C, Ivy Biomedical Systems). ECG and BP data were digitalized with 14-bit resolution at a 500 and 1000 Hz sample frequency using a WINDAQ data acquisition system (DI720; DATAQ, Akron, Ohio, USA) and processed off-line using custom software in PV-Wave language (PV-Wave; Visual Numerics Inc., Houston, Texas, USA) written by one of the authors (AD).
Spectral Analysis (Table 1)
Table 1.
Spectral and Valsalva maneuver parameters
| Abbreviation | Definition | Significance |
|---|---|---|
| RRI-HF | RR interval variability in the high frequency band | Marker of parasympathetic tone |
| RRI-LF | RR interval variability in the low frequency band | Marker of parasympathetic tone |
| SBP-LF | Systolic blood pressure variability in the low frequency band | Marker of sympathetic tone |
| pNN50 | Percentage of consecutive RR intervals that are more than 50 millisecond different from adjacent RR interval | Marker of parasympathetic tone |
| PRT | Pressure recovery time | Marker of sympathetic tone |
| BRS-a | Adrenergic baroreflex sensitivity | Marker of sympathetic tone |
| BRS-v | Cardiovagal baroreflex sensitivity | Marker of parasympathetic tone |
| Phase 2 SBP Decrement | The decrease in systolic blood pressure during the Valsalva maneuver from the start to the lowest point of phase 2 | Marker of sympathetic tone |
| Phase 2 DBP Decrement | The decrease in diastolic blood pressure during the Valsalva maneuver from the start to the lowest point of phase 2 | Marker of sympathetic tone |
Spectral and Valsalva maneuver parameters and their clinical significance.
Data segments of 300 seconds recorded at the beginning of each autonomic function test session during quiet respiratory breathing and stable resting conditions were used for the calculation of heart rate variability parameters, blood pressure variability parameters, and cardiovagal baroreflex sensitivity (BRS-v). A QRS detection algorithm, modified from Pan and Tompkins [11], was used to generate beat-to-beat R–R interval and blood pressure values, and these values were interpolated, low-pass filtered (cutoff 2 Hz), and resampled at 4 Hz. Linear trends were removed and power spectral density was estimated with the Fast Fourier transform-based Welch algorithm using segments of 256 data points. The power in the low frequency range (LF: 0.04 to, 0.15 Hz) and high frequency range (HF: 0.15 to 0.40 Hz) were calculated following the North American Society of Pacing and Electrophysiology Task Force Guidelines[12]. The HF component of the RR intervals (RRI-HF) strongly correlates with parasympathetic heart rate control, assuming that the respiratory rate is between 9 and 24 breaths/min (0.15–0.4 Hz). Similar methods were employed for the continuous systolic blood pressure signal. The low frequency band (SBP-LF) was taken as a measure of sympathetic nervous system tone [13]. In the time-domain, we assessed pNN50, the percentage of consecutive RR intervals that are more than 50 msec different from their neighbor [14], as a marker of parasympathetic tone.
BRS-v was calculated using cross-spectral analysis of the relationships between R–R interval and SBP. Specifically, it was defined as the mean magnitude value of the transfer function in the low-frequency band with negative phase and squared coherence value greater than 0.5 [15].
Valsalva Maneuver
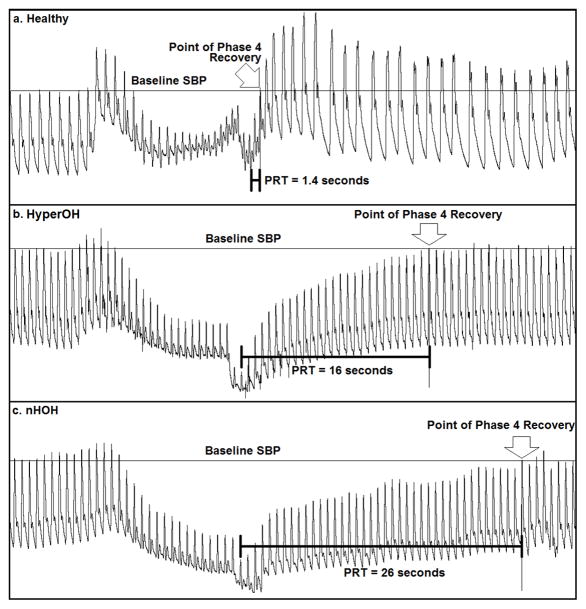
Baseline SBP and DBP were obtained just prior to initiation of the Valsalva maneuver. Patients were asked to maintain an expiratory pressure of at least 30 mmHg for 15 seconds. Typical Valsalva maneuver tracings are presented in Figure 1 for a healthy volunteer, a hyperOH patient and a nOH patient.
Figure 1. Representative Valsalva maneuver traces.
Continuous blood pressure tracings of a healthy subject (Panel a), a patient with hyperadrenergic orthostatic hypotension (hyperOH, Panel b), and a patient with non-hyperadrenergic orthostatic hypotension (nOH, Panel c) are shown. SBP- systolic blood pressure; PRT – pressure recovery time (see text or Figure 2 for details on calculation).
Phase 1 and phase 2 are the “strain phases” of the Valsalva maneuver with a Valsalva induced reduction in cardiac venous return and relative hypotension. Phase 3 and phase 4 are the “recovery phases” of the Valsalva maneuver when cardiac venous return normalizes to baseline levels. Phase 1 includes the period from the onset of the Valsalva maneuver until the SBP peak (Figure 2). Phase 2 lasts from the end of phase 1 until release of the Valsalva maneuver. The lowest BP in phase 2 is the “nadir of phase 2”. If SBP and DBP increased from this nadir during this phase, a “late phase 2” (phase 2L) was present. If no phase 2L was present, the “phase 2 blood pressure decrement” was measured until the end of phase 2. Phase 3 begins after Valsalva release and lasts briefly until BP starts to recover, heralding Phase 4. Phase 4 in normal individuals is notable for a profound recovery in BP, termed “Phase 4 overshoot”, but usually absent in patients with autonomic failure. The DBP was recorded immediately preceding the relevant SBP peak.
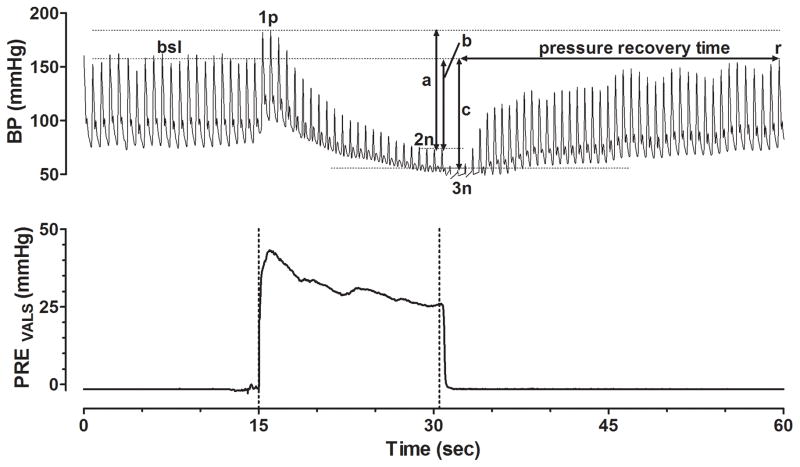
Figure 2. Schematic Parameterization of the Valsalva Maneuver.
Valsalva maneuver from one of our orthostatic hypotension patients is shown with blood pressure displayed on top and expiratory Valsalva pressure on the bottom. Annotations include the baseline (bsl) systolic blood pressure (SBP) prior to the Valsalva maneuver, the peak SBP during Valsalva phase 1 (1p), the nadir of Valsalva phase 2 (2n), and the nadir of phase 3 (3n). Arrow (a) represents the Valsalva phase 2 SBP Decrement. Arrow (b) represents the difference between baseline SBP and the end of Valsalva phase 2n. Arrow (c) represents the difference between baseline SBP and point 3n. Pressure recovery time (PRT) is measured as the time from the nadir SBP of Valsalva phase 3 until SBP recovers back to bsl level at point (r). Adrenergic baroreflex sensitivity index (BRS-a) is equal to the SBP represented by arrow (c) divided by the PRT.
Various Valsalva metrics have been reported to be useful in characterizing the severity of adrenergic dysfunction and were measured in this study (Figure 2). These include the magnitude of phase 2 SBP and DBP decrement relative to the phase 1 peak [16], difference between baseline SBP and the SBP at the end of phase 2 [17], blood pressure recovery time (PRT) [18], and adrenergic baroreflex sensitivity index (BRS-a) [19]. PRT is defined as the amount of time required for the SBP to recover from the nadir of phase 3 to the baseline SBP level [18]. BRS-a is defined as the SBP drop between baseline and the nadir of phase 3 divided by the PRT [19]. The lowest SBP during phase 3 was used to calculate the starting point for the PRT and BRS-a. Other recorded VM parameters included baseline HR, maximum HR during the VM, HR at VM phase 4 recovery, Valsalva ratio (the maximum HR during phase 2 of VM divided by the lowest HR within 30 seconds after the maximum HR), presence/absence of a VM late phase 2, and presence/absence of a VM phase 4 SBP overshoot.
Statistical Analyses
Independent 2-tailed Student’s t-tests were used to compare the continuous variables between the hyperOH and nOH groups, and in the case of PRT, between these 2 parameters and published control data [18]. Fisher’s exact tests were used to analyze categorical data. Spearman’s correlation was used to calculate correlation coefficient between standing plasma NE levels against PRT and BRS-a. Data are presented as mean±standard deviation. Probability values ≤0.05 were considered statistically significant. Statistical analyses were performed using SPSS for Windows version 19 (IBM Corp., Armonk, NY) and GraphPad Prism version 5.02 (GraphPad Software Inc., La Jolla, CA). GraphPad Prism was used to create the figures.
RESULTS
Demographics (Table 2)
Table 2.
Demographics and Posture Study
| Hyperadrenergic Orthostatic Hypotension | Non-hyperadrenergic Orthostatic Hypotension | P-value | |
|---|---|---|---|
| Total Subjects (n) | 19 | 64 | -- |
| Male Gender (%) | 11/20 (52%) | 37/65 (56%) | 0.879 |
| Age (years) | 69±8 | 65±11 | 0.128 |
| Weight (kilogram) | 74±15 | 81±18 | 0.135 |
| Height (cm) | 174±12 | 173±10 | 0.590 |
| BMI (kg/m2) | 24±4 | 27±5 | 0.026* |
| Years of disease at presentation (years) | 5.1±3.5 | 7.1±5.4 | 0.132 |
| Supine | |||
| Heart Rate (beats/min) | 67±10 | 71±13 | 0.217 |
| Systolic Blood Pressure (mmHg) | 155±32 | 158±31 | 0.776 |
| Diastolic Blood Pressure (mmHg) | 83±14 | 88±14 | 0.257 |
| Norepinephrine (nmol/L)[pg/mL] | 2.81±2.03 [475±344] | 0.86±0.76 [145±129] | 0.001* |
| Epinephrine (nmol/L)[pg/mL] | 0.110±0.07 [20±13] | 0.09±0.10 [17±19] | 0.608 |
| Standing | |||
| Heart Rate (beats/min) | 84±15 | 82±14 | 0.625 |
| Systolic Blood Pressure (mmHg) | 103±38 | 90±23 | 0.166 |
| Diastolic Blood Pressure (mmHg) | 66±21 | 58±11 | 0.145 |
| Norepinephrine (nmol/L) [pg/mL] | 6.12±1.99 [1035±336] | 1.34±0.85 [226±144] | <0.001* |
| Epinephrine (nmol/L) [pg/mL] | 0.20±0.25 [37±46] | 0.18±0.21[33±39] | 0.730 |
| Change from Supine to Standing | |||
| Heart Rate (beats/min) | 16±10 | 10±14 | 0.073 |
| Systolic Blood Pressure (mmHg) | −53±31 | −68±33 | 0.050* |
| Diastolic Blood Pressure (mmHg) | −18±23 | −30±17 | 0.011* |
| Norepinephrine (nmol/L) [pg/mL] | 3.30±1.48 [559±251] | 0.70±0.48 [81±119] | <0.001* |
| Epinephrine (nmol/L) [pg/mL] | 0.09±0.21 [17±38] | 0.08±0.17 [16±32] | 0.891 |
Data presented as mean±standard deviation. Continuous data were analyzed using Student’s t-test comparing hyperadrenergic vs. nonhyperadrenergic orthostatic hypotension groups, and categorical data were analyzed using a Fisher’s Exact Test.
P-value <0.05
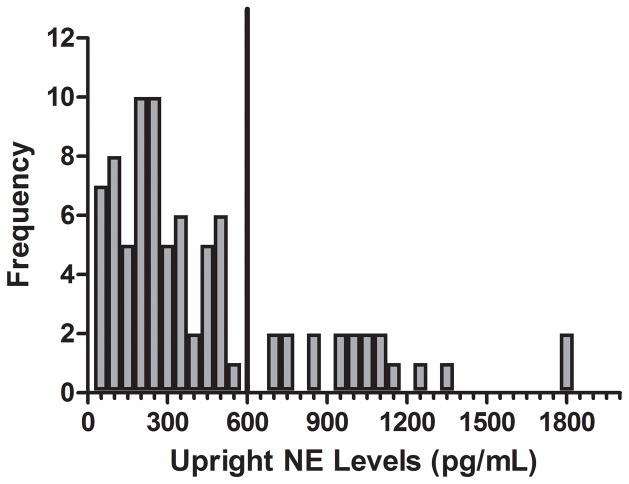
A total of 83 patients met the inclusion criteria. Upright plasma NE levels ranged between 0.14 to 10.87 nmol/L (23 to 1839 pg/mL; Figure 3). Nineteen patients (55% male) had a plasma upright NE level ≥ 3.55 nmol/L (hyperOH). The nOH group consisted of the other 64 patients (57% male). There was no significant difference in age between hyperOH and nOH patients (69±8 years vs. 65±11 years, P=0.1). The BMI was lower in the hyperOH patients than the nOH patients (24.3±3.9 kg/m2 vs. 27.9±4.7 kg/m2, P=0.03), although height (P=0.6) and weight (P=0.1) were not different between groups. Onset of disease symptoms was non-significantly more recent in the hyperOH patients compared to the nOH patients (5.1±3.5 years vs. 7.1±5.4 years, P=0.1).
Figure 3. The distribution of upright plasma norepinephrine levels.
The distribution of upright plasma norepinephrine into intervals of 0.30 nmol/L (50 pg/mL) is shown for our entire study population (n=83). A line has been drawn at 600 pg/mL to dichotomize our study population into a hyperadrenergic orthostatic hypotension group (hyperOH) to the left of the line, and a non-hyperadrenergic orthostatic hypotension group (nOH) to the right of the line. There is a normal distribution to the left of the line, with outliers to the right of this line.
Stand Test with Catecholamines (Table 2)
The supine HR (P=0.2), SBP (P=0.8), and DBP (P=0.3) were not different between groups. Maximum standing times were not different between the hyperOH and nOH groups (875±585 sec vs. 728±639 sec, P=0.375). Standing HR was similar for hyperOH patients and nOH patients (84±15 bpm vs. 82±14 bpm, P=0.6), as were the standing SBP (P=0.2) and DBP (P=0.1). The orthostatic increase in HR was similar between hyperOH and nOH patients (16±10 bpm vs. 10±14 bpm, P=0.07). Both groups experienced large drops in blood pressure with standing, but the drop in SBP (−53±31 mmHg vs. −68±33 mmHg, P=0.05) and DBP (−18±23 mmHg vs. −30±17 mmHg, P=0.01) were smaller in hyperOH patients than nOH patients.
Supine plasma NE was much greater in the hyperOH group than the nOH group (2.81 ± 2.03 nmol/L [475±344 pg/mL] vs. 0.86±0.76 nmol/L [145±129 pg/mL], P=0.001). On standing, the plasma norepinephrine was considerably higher in the hyperOH group (6.12±1.99 nmol/L [1035±336 pg/mL] vs. 1.34±0.85 nmol/L [226±144 pg/mL], P<0.001).
Co-morbidities and Medication Use (Table 3)
Table 3.
Comorbidities and medication use.
| Hyperadrenergic Orthostatic Hypotension | Non-hyperadrenergic Orthostatic Hypotension | P-value | |
|---|---|---|---|
| Total Subjects (n) | 19 | 64 | -- |
| Comorbidities | |||
| Parkinson’s Disease | 3 (16%) | 5 (8%) | 0.376 |
| Diabetes | 2 (11%) | 3 (5%) | 0.322 |
| Multiple System Atrophy | 0 (0%) | 13 (20%) | 0.033* |
| Obstructive Sleep Apnea | 2 (11%) | 12 (19%) | 0.506 |
| Medication Usage | |||
| Opioid Analgesic Use | 3 (16%) | 4 (6%) | 0.193 |
| Benzodiazepine Use | 9 (47%) | 5 (8%) | <0.001* |
| Tricyclic Antidepressant Use | 0 (0%) | 1 (2%) | 1.000 |
| Stimulant Use | 0 (0%) | 2 (3%) | 1.000 |
| Levodopa Use | 3 (16%) | 5 (8%) | 0.376 |
| Midodrine Use | 5 (26%) | 31 (48%) | 0.116 |
| Fludrocortisone Use | 1 (5%) | 19 (30%) | 0.033* |
| Selective Serotonin Reuptake Inhibitor Use | 7 (37%) | 14 (22%) | 0.232 |
| Serotonin-Norepinephrine Reuptake Inhibitor Use | 2 (11%) | 3 (5%) | 0.322 |
| Alpha Blocker Use | 0 (0%) | 3 (5%) | 1.000 |
| Beta Blocker Use | 6 (32%) | 6 (9%) | 0.026* |
Data presented as incidence of disease or medication use in each study group. Reported P values are for Chi-square tests using Fisher’s Exact Method.
P-values <0.05
The prevalence of Parkinson’s disease (P=0.4), diabetes mellitus (P=0.3) and obstructive sleep apnea (P=0.5) were not different between the groups. MSA was exclusively seen in the nOH group with a prevalence of 20%.
The use of SNRI medications (P=0.3), SSRI medications (P=0.2), opioid analgesics (P=0.2), levodopa/carbidopa (P=0.4), and alpha-adrenergic antagonists (P=1.000) were similar between the groups. Beta-blockers were used by more hyperOH patients than nOH patients (32% vs. 7%, P=0.03), as were benzodiazepines (47% vs. 9%, P<0.001). In contrast, fludrocortisone use was less prevalent in the hyperOH patients than the nOH patients (5% vs. 30%, P=0.03), while midodrine use was similar between both groups (26% vs. 48%, P=0.1).
Spectral Analyses (Table 4)
Table 4.
Cardiovagal Baroreflex sensitivity, Heart Rate Variability, and Blood Pressure Variability
| Hyperadrenergic Orthostatic Hypotension | Non-hyperadren ergic Orthostatic Hypotension | P-Values | Healthy Subjects | |
|---|---|---|---|---|
| Heart Rate Variability | ||||
| pNN50% (%) | 10.5±24.5 | 6.5±16.3 | 0.403 | 36.5±25.5 |
| LF-RRI (ms2) | 364±719 | 220±610 | 0.398 | 1144±1093 |
| HF-RRI (ms2) | 297±678 | 165±517 | 0.375 | 2147±3688 |
| Blood Pressure Variability | ||||
| LF-SYS (mmHg2) | 9.0±13.0 | 5.0±6.0 | 0.229 | 6.7±5.4 |
| Cardiovagal Baroreflex Sensitivity (ms/mmHg) | 6.0±6.2 | 4.1±3.5 | 0.113 | 12.5±7.6 |
Data presented as mean±standard deviation. Reported P values are for t-tests comparing hyperadrenergic vs. non-hyperadrenergic orthostatic hypotension groups. ms, milliseconds; mmHg, millimeters of mercury; pNN50%, percent of adjacent R-R intervals (RRI) greater than 50ms; LF-RRI, RRI power in the low frequency range; HF-RRI, RRI power in the high frequency range. The data for healthy subjects that are presented for comparison are from reference 20.
In the time-domain, pNN50 was lower in both hyperOH patients (10.5±24.5%) and nOH patients (6.5±16.3%) than we have previously found in healthy control subjects (36.5±25.5%) [20]. pNN50 did not differ between hyperOH and nOH patients (P=0.4).
RRI-HF was not different between hyperOH patients (297±678 msec2) and nOH patients (164±517 msec2; P=0.4).
SBP-LF was not different between hyperOH patients (9.0±13.0 mmHg2) and nOH patients (5.0±6.0 mmHg2; P=0.2).
Mean cardiovagal baroreflex sensitivity gain did not differ between hyperOH patients and nOH patients, although there was a non-significant trend toward higher BRS-v in hyperOH patients (6.0±6.2 mmHg/ms vs. 4.1±3.5 mmHg/ms; P=0.1).
Valsalva Maneuver (Table 5)
Table 5.
Valsalva Maneuver Metrics
| Hyperadrenergic Orthostatic Hypotension (n=19) | Nonhyperadrenergic Orthostatic Hypotension (n=64) | P-value | |
|---|---|---|---|
| Pressure Recovery Time (sec) | 16.5±8.9 | 31.6±16.6 | <0.001* |
| Adrenergic baroreflex sensitivity (mmHg/sec) | 5.8±4.0 | 3.9±4.3 | 0.093 |
| Phase 2 SBP Decrement (mmHg) | 77±38 | 78±25 | 0.895 |
| Phase 2 DBP Decrement (mmHg) | 16±10 | 25±13 | 0.008* |
| Difference between baseline SBP and end of phase 2 SBP (mmHg) | 52±32 | 63±27 | 0.140 |
| Difference between baseline DBP and end of phase 2 DBP (mmHg) | 9±10 | 13±14 | 0.310 |
| Valsalva Ratio | 1.18±0.15 | 1.12±0.12 | 0.081 |
| Baseline heart rate (beats/min) | 73±14 | 73±11 | 0.840 |
| Maximum heart rate (beats/min) | 85±15 | 84±13 | 0.825 |
| Heart rate at conclusion of Valsalva maneuver (beats/min) | 78±13 | 74±12 | 0.278 |
| Presence of phase 2 late (%) | 4 (21%) | 8 (13%) | 0.464 |
| Presence of phase 4 overshoot (%) | 2 (11%) | 2 (3%) | 0.223 |
Data presented as mean±standard deviation. Reported P values are for t-test comparing hyperadrenergic vs. Non-hyperadrenergic orthostatic hypotension groups unless otherwise noted for continuous data and Fisher’s Exact test for categorical data. sec, seconds; mmHg, millimeters of mercury.
P-value <0.05
During the Valsalva maneuver, baseline SBP (143±24 mmHg vs. 146±28 mmHg, P=0.6), baseline HR (73±14 vs. 73±11, P=0.8), maximum HR (85±15 vs. 84±13. P=0.8), HR during phase 4 recovery (77±13 vs. 74±12, P=0.278), and the Valsalva Ratio (1.18±0.14 vs. 1.12±0.12, P=0.08) did not differ between hyperOH and nOH groups.
Sympathetically Mediated Vasoconstriction
Valsalva phase 2L was present in 4 of 19 hyperOH patients (21%) vs. 8 of 64 (13%) nOH patients (P=0.5). Valsalva phase 4 overshoot occurred in 2 of 19 hyperOH patients (10%) vs. 2 of 64 (5%) nOH patients (P=0.2). No individuals from either group had both a phase 2L and a phase 4 overshoot during their Valsalva maneuver.
Valsalva Markers of Adrenergic Function
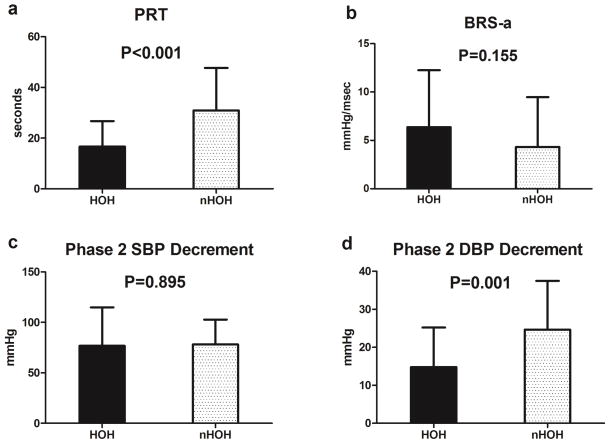
PRT was shorter in the hyperOH than nOH patients (16.5±8.9 sec vs. 31.6±16.6 sec, P<0.001, Figure 4a). PRT for both hyperOH (P<0.001) and nOH (P<0.001) were longer than published data for PRT in healthy control subjects (2.00±1.98 sec) [18]. There was a non-significant trend toward greater BRS-a in hyperOH patients than nOH patients (5.8±4.0 mmHg/sec vs. 3.9±4.3 mmHg/seconds, P=0.093, Figure 4b). Phase 2 SBP decrement from phase 1 was not different between the hyperOH and the nOH patients (77±38 mmHg vs. 78±25 mmHg; P=0.9; Figure 4c), while Phase 2 DBP decrement was considerably smaller in hyperOH patients than nOH patients (16±10 mmHg vs. 25±13, P=0.008, Figure 4d). PRT was negatively and significantly correlated with standing plasma NE (ρ = −0.459, P<0.001) and BRS-a was positively and significantly correlated with standing plasma NE (ρ = 0.364, P=0.001)
Figure 4. Summary Valsalva Metrics between the patient groups.
Summary Valsalva data are presented for hyperadrenergic orthostatic hypotension (hyperOH) and non-hyperadrenergic orthostatic hypotension (nOH) patients. The panels show Pressure Recovery Time (PRT; panel a), adrenergic baroreflex sensitivity (BRS-a; panel b), the fall in systolic blood pressure (SBP; panel c) and the fall in diastolic blood pressure (DBP; panel d) during phase 2 of the Valsalva maneuver. Data are presented as mean±standard error of the mean. Student’s t-tests were used to generate P values.
There were non-significant trends to smaller drops in blood pressure with Valsalva in hyperOH patients than nOH patients. These include the difference between baseline SBP and the SBP at the end of phase 2 (52±31 mmHg vs. 63±27 mmHg, P=0.1), difference between baseline DBP and the DBP at the end of phase 2 (9±10 mmHg vs. 13±14 mmHg, P=0.3), and the difference between baseline SBP and the SBP at the nadir of phase 3 (73±33 mmHg vs. 87±25 mmHg, P=0.1).
Discussion
We define “hyperadrenergic orthostatic hypotension” as the presence of paradoxically high upright plasma NE level ≥ 3.55 nmol/L (600 pg/mL) in neurogenic orthostatic hypotension patients and extensively describe this novel group of patients with severe neurogenic OH for the first time. Compared to classical OH patients with low plasma NE, these patients have less severe adrenergic impairment.
Nomenclature - Hyperadrenergic Orthostatic Hypotension
Dr. David Streeten first coined the term “hyperadrenergic orthostatic hypotension” in 1990 to describe mild OH in the presence of normal to elevated levels of upright plasma NE in 8 young patients with large orthostatic increases in HR [7]. In contrast to the patients reported in that study, the patients in our hyperOH group had large orthostatic drops in blood pressure with only a modest increase in HR (Table 1). This hemodynamic pattern is typical of neurogenic OH, and not consistent with a transient cause of OH such as hypovolemia [21;22]. More recently “hyperadrenergic” has been used to describe patients with upright plasma NE >3.55 nmol/L (600 pg/mL), a threshold met by only 2 of the 8 patients in Streeten’s study [8]. Moreover, those two patients were younger adults (age 49 and 37) [7]. The hyperOH patients in this study represent older adults with orthostatic hypotension in the presence of truly elevated upright plasma NE ≥ 3.55 nmol/L (600 pg/mL) and represent a different population than those patients described by Dr. Streeten.
Hyperadrenergic OH Patients Have Less Severe Adrenergic Dysfunction
Despite fairly significant OH in both groups, patients with hyperOH had less severe sympathetic noradrenergic nerve dysfunction than nOH patients.
The PRT is a function of sympathetic nervous system mediated vasoconstriction. BP will recover quickly with normal vasoconstrictive function. With worsening adrenergic failure, the BP recovery time (as measured by PRT) will be longer. The PRT is a validated, reliable and convenient metric that can be used even in cases when late phase 2 is absent, as is common in patients with neurogenic OH [17–19]. In addition, PRT correlates well with muscle sympathetic nerve activity (MSNA), the gold standard for evaluating sympathetic nerve function [19;23].
While a normal PRT in healthy individuals is 2.00±1.98 sec [18], the PRT for the hyperOH and the nOH patients were significantly longer (Figure 4a). A previous study has reported a PRT of 30.0 sec in a group with orthostatic hypotension, which is almost identical to the PRT in our nOH patients [18]. Vogel et al. reported that a “borderline orthostatic hypotension” group (defined as orthostatic SBP drop >10mmHg but <30 mmHg), had a PRT of 6.6 sec [18]. Our hyperOH patients had a PRT of 16 sec, indicating that the severity of their adrenergic dysfunction lies somewhere in the continuum between moderate and severe adrenergic failure.
The BRS-a correlates well with adrenergic function, and this has been validated with MSNA in a previous study [19]. The BRS-a was severely blunted in both OH groups compared to healthy individuals (24.5±19.3 mmHg/s) [19]. The BRS-a has been shown to be less sensitive than PRT in discriminating those individuals with moderate OH from severe OH, which is consistent with the patients in our study [17].
Hyperadrenergic OH: Clinical Characteristics
Underlying Diagnoses
MSA is a rapidly progressive neurodegenerative disorder associated with severe orthostatic hypotension and has a mean survival of less than 9 years [24]. The absence of MSA in the hyperOH group suggests that a hyperOH state may not be a clinical feature of MSA.
Chronic orthostatic hypotension associated with many conditions, such as diabetes, Parkinson’s, and MSA, is progressive [5], and increases with severity over time [25;26]. The nOH group in our study also had evidence of more severe disease compared to the hyperOH group. This suggests that the hyperOH phenotype may be a manifestation of a milder and less well developed stage of neurogenic orthostatic hypotension. The hyperOH group presented with a trend toward a shorter duration of disease, which is consistent with this theory.
Concomitant Medications
There were several medications that were disproportionately used more often in one group than the other. Overall, this discrepant pattern of medication use is most consistent with less severe disease in the hyperOH group. Midodrine and fludrocortisone are commonly prescribed in the treatment of severe neurogenic orthostatic hypotension [21]. Significantly fewer patients with hyperOH were on fludrocortisone than nOH patients, and there was a similar trend to less frequent midodrine use in hyperOH patient. Benzodiazepine use was also considerably greater in the hyperOH population. Physicians may be discouraged from prescribing benzodiazepines to nOH patients due to their greater symptoms and higher potential for falls [27]. Both acutely and chronically administered benzodiazepines decrease levels of norepinephrine at baseline and during times of stress [28;29], making it unlikely to be a cause of the higher NE seen in the hyperOH group.
Finally, the greater use of beta-blockers in the hyperOH population reflects less severe disease in this group. The use of beta-blockers has acutely been shown to increase levels of norepinephrine due to impaired clearance [30;31], but this effect on the study is likely minimal given only a minority of patients in the hyperOH group were taking a beta-blocker (6 of 19). The more prevalent use of beta-blockers in this population is better explained by a pattern of pharmaceutical prescribing more appropriate for a less severe disease phenotype.
Possible Mechanism
The underlying mechanism behind hyperOH may be a partial autonomic neuropathy of the sympathetic nerves innervating the lower extremities, as proposed by Dr. Streeten in his seminal paper. In his study, the few patients with an elevated upright plasma NE level >3.55 nmol/L (600 pg/mL exhibited signs of greater responsiveness in the lower versus upper extremity to norepinephrine infusion, indicating autonomic sympathetic denervation preferentially in the lower limbs [7]. Selective impairment of these nerves results in excessive pooling of blood and causes orthostatic hypotension. It is unclear why these nerves in the lower extremity are affected preferentially but may be related to their longer length, which are affected first in certain types of neuropathy such as diabetic length-dependent distal symmetric polyneuropathy [32]. HyperOH may then be an earlier form of typical nOH when the upper extremities, which have not yet been affected, perceive this orthostatic hypotension and via the baroreflex, compensate for autonomic sympathetic insufficiency in the lower extremities by producing excessive amounts of norepinephrine.
Another possibility could be decreased clearance of norepinephrine coupled with impaired norepinephrine sensitivity resulting in orthostatic hypotension. Norepinephrine has been shown to be elevated in some elderly individuals due to decreased renal clearance [33]. Though norepinephrine sensitivity was not measured, endocrinopathies such as type 2 diabetes have a similar pathogenesis. Type 2 diabetics are well-documented to have excessive insulin secretion in an effort to stem growing insulin resistance to maintain normal blood glucose, followed by frank diabetes when insulin secretion drops below even normal levels [34–36]. Neurogenic hyperOH patients share, perhaps, a similar fate when they arrive at this “burn-out phase”, akin to the old cliché of a candle burning brightest just before it goes out.
Limitations
The main limitation to this study is the relatively modest sample size for the hyperOH group, although this is currently the largest study examining this hyperOH phenomenon. It is possible that a larger study sample size would allow more complete clinical characterization of this population, with further discriminating features achieving statistical significance. Another limitation is that we were unable to control for baseline hypovolemia. Hypovolemia has previously been shown to significantly impact the SBP changes during VM phase 2 [37].
Conclusion
In conclusion, “neurogenic hyperadrenergic orthostatic hypotension” describes the condition of neurogenic orthostatic hypotension in the presence of elevated levels of plasma norepinephrine. Our data suggests that these individuals have severe orthostatic hypotension, but less severe adrenergic impairment compared to typical neurogenic orthostatic hypotension patients. These patients comprise a substantial minority of patients with neurogenic orthostatic hypotension. From this limited study population we postulate that hyperOH may be an earlier manifestation of typical neurogenic OH, and these patients may develop typical nOH over time. Further studies will be required to elucidate the natural history of this disorder.
Clinical Perspectives.
We have defined “neurogenic hyperadrenergic orthostatic hypotension” as neurogenic orthostatic hypotension in the presence of an upright plasma norepinephrine level > 3.55 nmol/L (600 pg/mL).
Neurogenic hyperadrenergic orthostatic hypotension occurs in a substantial minority of patients with neurogenic orthostatic hypotension and have less severe sympathetic nervous system dysfunction compared to typical orthostatic hypotension patients
Hyperadrenergic orthostatic hypotension may reflect an earlier stage of classic neurogenic orthostatic hypotension, although further studies are required to elucidate the natural history of this disorder.
Summary Statement.
“Neurogenic hyperadrenergic orthostatic hypotension” is neurogenic orthostatic hypotension that is associated with paradoxically elevated levels of norepinephrine. This condition has not been extensively studied. Our study finds this population has less severe adrenergic dysfunction compared to classic orthostatic hypotension populations.
Acknowledgments
We would like to thank our patients who participated in this project and to recognize the highly professional care provided by the staff of the Elliot V. Newman Clinical Research Center.
Funding: Supported in part by NIH grants R01 HL102387, R01 HL071784, P01 HL56693, U54 NS065736, and the Clinical and Translational Science Award UL1 TR000445.
Footnotes
Author Contributions:
Philip Mar, Cyndya Shibao, and Satish Raj participated in the development of study design and drafting of the manuscript for content. Philip Mar, Satish Raj, Italo Biaggioni, André Diedrich, and David Robertson participated in the analysis and interpretation of data. Emily Garland and Bonnie Black participated in the conduction of the study and collection of data. All authors were involved with subsequent revisions of the manuscript prior to its final form.
Reference List
- 1.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 2.Low PA. Prevalence of orthostatic hypotension. Clin Auton Res. 2008;18(Suppl 1):8–13. doi: 10.1007/s10286-007-1001-3. [DOI] [PubMed] [Google Scholar]
- 3.Poon IO, Braun U. High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther. 2005;30:173–178. doi: 10.1111/j.1365-2710.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 4.Shibao C, Grijalva CG, Raj SR, Biaggioni I, Griffin MR. Orthostatic hypotension-related hospitalizations in the United States. Am J Med. 2007;120:975–980. doi: 10.1016/j.amjmed.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Robertson D, Robertson RM. Causes of chronic orthostatic hypotension. Arch Intern Med. 1994;154:1620–1624. [PubMed] [Google Scholar]
- 6.Ziegler MG, Lake CR, Kopin IJ. The sympathetic-nervous-system defect in primary orthostatic hypotension. N Engl J Med. 1977;296:293–297. doi: 10.1056/NEJM197702102960601. [DOI] [PubMed] [Google Scholar]
- 7.Streeten DH. Pathogenesis of hyperadrenergic orthostatic hypotension. Evidence of disordered venous innervation exclusively in the lower limbs. J Clin Invest. 1990;86:1582–1588. doi: 10.1172/JCI114878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM, Robertson D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. J Appl Physiol (1985) 1998;84:914–921. doi: 10.1152/jappl.1998.84.3.914. [DOI] [PubMed] [Google Scholar]
- 9.Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RM. Prevalence of orthostatic hypotension in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2011;17:724–729. doi: 10.1016/j.parkreldis.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacob G, Shannon JR, Black B, et al. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation. 1997;96:575–580. doi: 10.1161/01.cir.96.2.575. [DOI] [PubMed] [Google Scholar]
- 11.Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE Trans Biomed Eng. 1985;32:230–236. doi: 10.1109/TBME.1985.325532. [DOI] [PubMed] [Google Scholar]
- 12.Malik M, Bigger T, Malliani A, Moss A, Schwartz P. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 13.Diedrich A, Jordan J, Tank J, et al. The sympathetic nervous system in hypertension: assessment by blood pressure variability and ganglionic blockade. J Hypertens. 2003;21:1677–1686. doi: 10.1097/00004872-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 15.Persson PB, DiRienzo M, Castiglioni P, et al. Time versus frequency domain techniques for assessing baroreflex sensitivity. J Hypertens. 2001;19:1699–1705. doi: 10.1097/00004872-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Sandroni P, Benarroch EE, Low PA. Pharmacological dissection of components of the Valsalva maneuver in adrenergic failure. J Appl Physiol (1985 ) 1991;71:1563–1567. doi: 10.1152/jappl.1991.71.4.1563. [DOI] [PubMed] [Google Scholar]
- 17.Novak P. Assessment of sympathetic index from the Valsalva maneuver. Neurology. 2011;76:2010–2016. doi: 10.1212/WNL.0b013e31821e5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology. 2005;65:1533–1537. doi: 10.1212/01.wnl.0000184504.13173.ef. [DOI] [PubMed] [Google Scholar]
- 19.Schrezenmaier C, Singer W, Swift NM, Sletten D, Tanabe J, Low PA. Adrenergic and vagal baroreflex sensitivity in autonomic failure. Arch Neurol. 2007;64:381–386. doi: 10.1001/archneur.64.3.381. [DOI] [PubMed] [Google Scholar]
- 20.Nwazue VC, Paranjape SY, Black BK, et al. Postural tachycardia syndrome and inappropriate sinus tachycardia: role of autonomic modulation and sinus node automaticity. J Am Heart Assoc. 2014;3:e000700. doi: 10.1161/JAHA.113.000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman R. Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med. 2008;358:615–624. doi: 10.1056/NEJMcp074189. [DOI] [PubMed] [Google Scholar]
- 22.Tuboly G, Rudas L, Csillik A, et al. Haemodynamic parameters and cognitive function during modeled acute volume loss. Acta Physiol Hung. 2012;99:118–125. doi: 10.1556/APhysiol.99.2012.2.4. [DOI] [PubMed] [Google Scholar]
- 23.Rudas L, Crossman AA, Morillo CA, et al. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol. 1999;276:H1691–H1698. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- 24.Schrag A, Wenning GK, Quinn N, Ben-Shlomo Y. Survival in multiple system atrophy. Mov Disord. 2008;23:294–296. doi: 10.1002/mds.21839. [DOI] [PubMed] [Google Scholar]
- 25.Stefanova N, Bucke P, Duerr S, Wenning GK. Multiple system atrophy: an update. Lancet Neurol. 2009;8:1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- 26.Mabuchi N, Hirayama M, Koike Y, et al. Progression and prognosis in pure autonomic failure (PAF): comparison with multiple system atrophy. J Neurol Neurosurg Psychiatry. 2005;76:947–952. doi: 10.1136/jnnp.2004.049023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999;47:30–39. doi: 10.1111/j.1532-5415.1999.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 28.Stratton JR, Halter JB. Effect of a benzodiazepine (alprazolam) on plasma epinephrine and norepinephrine levels during exercise stress. Am J Cardiol. 1985;56:136–139. doi: 10.1016/0002-9149(85)90582-x. [DOI] [PubMed] [Google Scholar]
- 29.Hossmann V, Maling TJ, Hamilton CA, Reid JL, Dollery CT. Sedative and cardiovascular effects of clonidine and nitrazepam. Clin Pharmacol Ther. 1980;28:167–176. doi: 10.1038/clpt.1980.146. [DOI] [PubMed] [Google Scholar]
- 30.Best JD, Halter JB. Blood pressure and norepinephrine spillover during propranolol infusion in humans. Am J Physiol. 1985;248:R400–R406. doi: 10.1152/ajpregu.1985.248.4.R400. [DOI] [PubMed] [Google Scholar]
- 31.Rosen SG, Supiano MA, Perry TJ, et al. Beta-adrenergic blockade decreases norepinephrine release in humans. Am J Physiol. 1990;258:E999–1005. doi: 10.1152/ajpendo.1990.258.6.E999. [DOI] [PubMed] [Google Scholar]
- 32.Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. 2014;14:473. doi: 10.1007/s11910-014-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esler M, Skews H, Leonard P, Jackman G, Bobik A, Korner P. Age-dependence of noradrenaline kinetics in normal subjects. Clin Sci (Lond) 1981;60:217–219. doi: 10.1042/cs0600217. [DOI] [PubMed] [Google Scholar]
- 34.Leahy JL. Pathogenesis of type 2 diabetes mellitus. Arch Med Res. 2005;36:197–209. doi: 10.1016/j.arcmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukushima M, Usami M, Ikeda M, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross-sectional study of Japanese type 2 diabetes. Metabolism. 2004;53:831–835. doi: 10.1016/j.metabol.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 37.van Kraaij DJ, Jansen RW, Hoefnagels WH. Monitoring hypovolemia in healthy elderly subjects by measuring blood pressure response to Valsalva’s maneuver. Geriatr Nephrol Urol. 1999;9:73–79. doi: 10.1023/a:1008331930548. [DOI] [PubMed] [Google Scholar]