Abstract
Patients with classic (type I) Ehlers-Danlos Syndrome (EDS), characterized by heterozygous mutations in the Col5a1 and Col5a2 genes, exhibit connective tissue hyperelasticity and recurrent joint dislocations, indicating a potential regulatory role for collagen V in joint stabilizing soft tissues. This study asked whether the contribution of collagen V to the establishment of mechanical properties is tissue-dependent. We mechanically tested four different tissues from wild type and targeted collagen V-null mice: the flexor digitorum longus tendon (FDL), Achilles tendon (ACH), the anterior cruciate ligament (ACL) and the supraspinatus tendon (SST). Area was significantly reduced in the Col5a1ΔTen/ΔTen group in the FDL, ACH, and SST. Maximum load and stiffness were reduced in the Col5a1ΔTen/ΔTen group for all tissues. However, insertion site and midsubstance modulus were reduced only for the ACL and SST. This study provides evidence that the regulatory role of collagen V in extracellular matrix assembly is tissue-dependent and that joint instability in classic EDS may be caused in part by insufficient mechanical properties of the tendons and ligaments surrounding each joint.
Keywords: collagen V, Ehlers-Danlos Syndrome, mechanical properties, tendon, ligament
1 Introduction
Tendons and ligaments have a complex and unique hierarchical structure that is finely tuned to enable them to withstand high forces when transferring loads between tissues. They are primarily composed of collagen I with collagen molecules assembled as fibrils, which then bundle to form fibers, fascicles and then full tendon proper.1; 2 Surrounding the collagen fibrils is mainly water, proteoglycans with associated glycosaminoglycans (GAGs), as well as other small proteins. Although the mechanical roles of proteoglycans and their GAGs is debated,3-9 it is well established that the collagen fibrils and their structural hierarchy is the primary load-bearing component of tendon and it is able to perform this task by exhibiting many complex dynamic responses to load such as re-alignment, uncrimping, sliding, and deformation of the collagen fibrils and fibers.10-13 This intricate structure is formed during development in a process called fibrillogenesis, in which collagen molecules line up in a staggered pattern to form fibrils and fibrils grow in both length and width; and fibrils are organized to form the higher scale structures.2; 14; 15 This process is highly regulated by a number of molecules, specifically proteoglycans and quantitatively minor collagens, such as collagen V.16-19
Collagen V, although a quantitatively minor component (∼2%) in mature tendon and ligament composition, is a major regulator of fibrillogenesis. Collagen V plays a critical role during the early process of fibril nucleation and reduction of collagen V expression during this process results in fewer collagen I fibrils with increased diameters in tendons, ligaments, dermis and cornea.20-24 In addition, these changes persist past development, resulting in altered biomechanics of mature flexor digitorum longus tendon with reduced area and stiffness.22 Removal of collagen V altogether results in an overall lack of fibril formation in a number of embryonic tissues,23 resulting in embryonic lethality. However, the effects of collagen V in mature tissue can be studied using recently developed conditional mouse model.25 Given the importance of the collagen I structure to tendon function, changes in the structure and/or composition of the matrix via regulation with collagen V may have dramatic effects on tendon/ligament mechanical properties.
A reduction in collagen V is also clinically relevant in classic (type I) Ehlers-Danlos Syndrome (EDS), a rare genetic disease most commonly resulting from a functional loss of one Col5a1 allele.22; 26 Classic EDS patients exhibit connective tissue hyperelasticity and laxity in a variety of tissues, suggesting that collagen V has a role in assembling the hierarchical structure responsible for the adequate function of soft connective tissues. In addition to generalized tissue hyperelasticity, mutations in the collagen V gene have been linked to injury,27 performance deficiencies,28 Achilles tendinopathy,29 and anterior cruciate ligament (ACL) rupture.30 Furthermore, classic EDS also is associated with a prevalence of joint problems, with the highest incidence of joint dislocations and surgeries occurring at the shoulder and knee joints, respectively.31-33 This indicates a potential regulatory role for collagen V in specific tendons and ligaments that directly contribute to joint stability.
Therefore, the purpose of this study was to determine the contribution of collagen V to the establishment of mechanical properties in four different tissues: the flexor digitorum longus tendon (FDL), the Achilles tendon (ACH), the anterior cruciate ligament (ACL) and the supraspinatus tendon (SST). We hypothesized that the absence of collagen V would result in decreased mechanical properties in the the SST and ACL since these tissues contribute directly to joint stability, but not in the ACH or FDL.
2 Methods
2.1 Animal model
A conditional tendon/ligament specific Col5a1ΔTen/ΔTen mouse model was created. Conditional Col5a1flox/flox mice were created as previously described.25 The scleraxis promoter was used to target Cre expression to tendons and ligaments. Scleraxis-Cre (Scx-Cre) transgenic mice were cross-bred with conditional Col5a1flox/flox mice for two generations to create Scx-Cre+/Col5a1flox/flox (Col5a1ΔTen/ΔTen) mice as described.24
2.2 Sample Preparation
This study was approved by the University of Pennsylvania IACUC. Male mice from two genotypes, Col5a1+/+ (Wild Type, n = 7-9) and conditional mouse targeted to tendon/ligament, ScxCre+/Col5a1-/- (Col5a1ΔTen/ΔTen, n = 8-13) were sacrificed at 60 days postnatal. Following euthanasia, the FDL, ACH, ACL, and SST were dissected and prepared for mechanical testing as previously described.17; 34; 35
2.2.1 Flexor Digitorum Longus Tendon (FDL)
FDL Tendons were removed from the ventral aspect of the mouse foot carefully and cleaned free of soft tissue. Verhoeff's stain lines were placed 2.5 mm apart within the mid-substance to track strain optically and the tendon cross-sectional area was then measured using a custom built optical device.36 The tendon was then secured with sandpaper and superglue in custom made grips with a gauge length of approximately 5mm for mechanical testing (Fig. 1A). These specimens also were used in the manuscript initially characterizing the mouse model and limited mechanical parameters were presented.24
Figure 1.
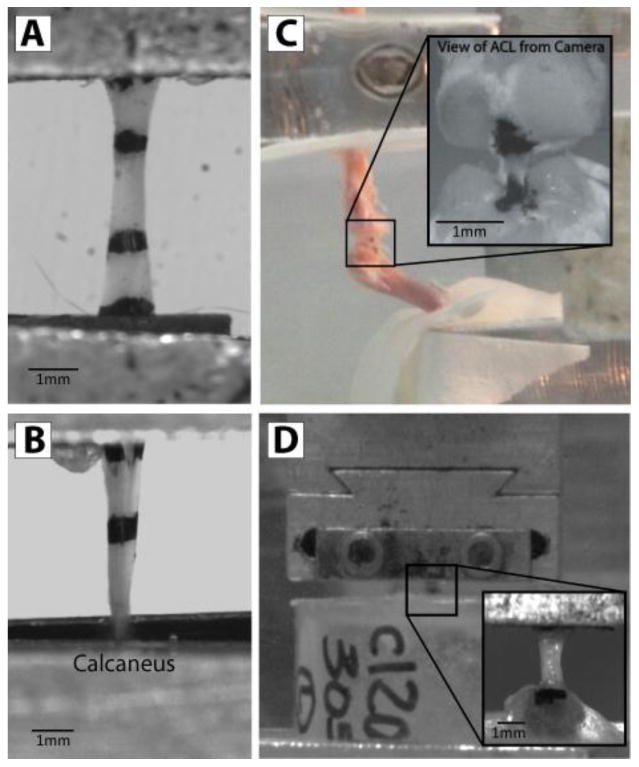
(A) Flexor digitorum longus tendon, (B) Achilles tendon, (C) Anterior cruciate ligament, and (D) Supraspinatus tendon prepared for mechanical testing.
2.2.2 Achilles Tendon (ACH)
Achilles tendons were carefully dissected out under a dissection scope and cleaned of excess tissue leaving only the calcaneus and tendon. Verhoeff's stain was applied along the tissue to separate the midsubstance and insertion site for strain analysis. Tendon cross-sectional area was then measured using the custom laser-based device.36 The tendon was secured at a gauge length of approximately 5mm from the insertion site with sandpaper and both the tendon and calcaneus were then placed in custom grips for mechanical testing (Fig. 1B).
2.2.3 Anterior Crucial Ligament (ACL)
Hind limbs were detached from the animals and dissected free of soft tissue leaving only the knee joint intact. The medial and lateral collateral ligaments as well as menisci were then carefully cut away using microscissors to expose the cruciate ligaments. The posterior cruciate ligament was then carefully cut which was confirmed using a posterior drawer test. Soft tissue around the ACL was then carefully trimmed to leave only the femur-ACL-tibia complex intact. Under a dissection microscope, the knee was then imaged in the coronal and sagittal planes to obtain measures for length and width of the ligament. Cross-sectional area was then calculated by assuming an ellipsoidal shape with length and width measured in MIPAV (NIH CIT, Version 5.4.4) from the dissection images. Verhoeff's stain was then applied to the femoral and tibial insertion sites of the ACL for optical strain tracking of the midsubstance of the tissue, leaving a gauge length of approximately 1mm. The femur and tibia were affixed in custom-built testing fixtures using polymethylmethacrylate (PMMA) pots such that the femur was vertical and the tibia was at approximately 60 degrees of flexion (Fig. 1C). Similar to the FDL, these specimens were used in the manuscript initially characterizing the mouse model.24
2.2.4 Supraspinatus Tendon (SST)
All soft tissue was removed from around the tendon, leaving the supraspinatus tendon attached to the humerus. Verhoeff's stain lines were placed on the tendons denoting the insertion site and tendon midsubstance for regions of strain analysis. Tendon cross-sectional area was then measured using a custom laser-based device.36 The humerus was then embedded in a 1-inch diameter acrylic tube piece with the use of PMMA. A second coating of PMMA was applied to prevent failure at the growth plate. The proximal end of the tendon was glued between two pieces of sandpaper with an initial gauge length of approximately 3mm and both the tendon and the acrylic pot were placed in custom grips for tensile testing (Fig. 1D).
2.3 Mechanical Testing and Analysis
Samples were placed in a room temperature phosphate buffered saline bath and loaded in a tensile testing system (model 5543, Instron, Norwood, MA). To determine biomechanical properties, tensile testing along the long axis of the tendon was performed with the following protocol, as previously described:37 preload to 0.02N, followed by preconditioning (10 cycles between 0.02 N and 0.04 N), a 300 second hold followed by stress-relaxation to 5% strain at a rate of 5%/sec for 600 seconds, a return to zero-displacement, 60 second hold, and ramp to failure at 0.1%/second. A 10 N load cell was used for all tests with a resolution of 0.01 N. During testing, images were obtained with a digital camera (Basler, Exton, PA) every 5 seconds for optical strain analysis.
Maximum stress was calculated as the maximum force divided by initial area. A custom Matlab program (Matlab, Natick, MA) was used to optically track strain lines to quantify regional stiffness and modulus in the linear region of the mechanical test.38 The FDL and ACL had only one region of analysis, the midsubstance, while the ACH and SST were analyzed for both insertion site and midsubstance properties. Shapiro-Wilk tests for normality showed some non-normal group distributions. As such, Mann-Whitney tests were used to determine statistical significance between the control and Col5a1ΔTen/ΔTen groups to test our study hypotheses.
3 Results
Structural measurements of gauge length and cross-sectional area as well as sample size are reported in Table 1. Cross-sectional area was significantly reduced in the Col5a1ΔTen/ΔTen FDL, ACH, and SST, but not in the ACL (Fig. 2A). However, percent relaxation was not affected by absence of collagen V in any tissue (Fig. 2B). Maximum load was reduced in the Col5a1ΔTen/ΔTen group in all tissues except the FDL tendon (Fig. 2C). However, maximum stress was severely reduced only in the ACL and SST of the Col5a1ΔTen/ΔTen group (Fig. 2D). There was no difference in maximum stress between groups in the ACH or FDL. Stiffness was significantly reduced in the Col5a1ΔTen/ΔTen group for all tissues and all regions (Fig. 3). Finally, both insertion site and midsubstance modulus were reduced in the Col5a1ΔTen/ΔTen group only for the ACL and SST (Fig. 4).
Table 1.
Gauge length and area measurements for wild type and Col5a1ΔTen/ΔTen groups for all tendons. Data is presented as median ± interquartile range. Boxes indicated by ‘NA’ are not applicable since regional measurements were not made for the FDL and ACL.
| Gauge Length (mm) | Tendon Area (mm2) | Insertion Area (mm2) | Midsubstance Area (mm2) | Sample Size | ||
|---|---|---|---|---|---|---|
| FDL | Col5 WT | 4.9 ± 0.32 | 0.13 ± 0.033 | NA | NA | 8 |
| Col5a1ΔTen/ΔTen | 4.9 ± 0.83 | 0.056 ± 0.016 | NA | NA | 8 | |
| ACL | Col5 WT | 1.1 ± 0.19 | 0.066 ± 0.023 | NA | NA | 7 |
| Col5a1ΔTen/ΔTen | 1.2 ± 0.30 | 0.073 ± 0.035 | NA | NA | 8 | |
| ACH | Col5 WT | 5.1 ± 0.73 | 0.38 ± 0.053 | 0.35 ± 0.093 | 0.34 ± 0.044 | 9 |
| Col5a1ΔTen/ΔTen | 4.8 ± 0.80 | 0.27 ± 0.047 | 0.27 ± 0.061 | 0.25 ± 0.044 | 8 | |
| SST | Col5 WT | 3.1 ± 0.45 | 0.14 ± 0.017 | 0.13 ± 0.0016 | 0.13 ± 0.015 | 7 |
| Col5a1ΔTen/ΔTen | 2.8 ± 0.20 | 0.080 ± 0.057 | 0.074 ± 0.055 | 0.11 ± 0.035 | 9 | |
Figure 2.
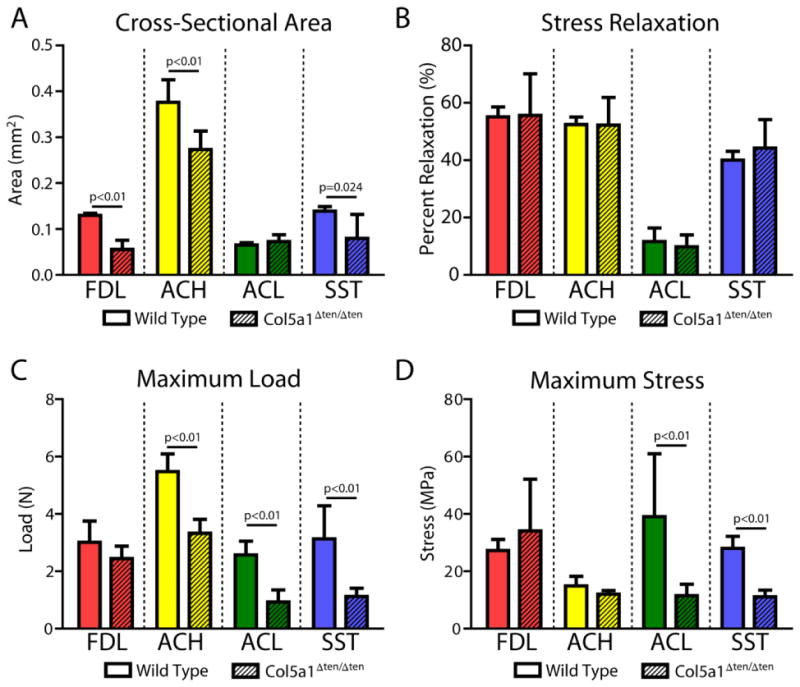
(A) Cross-sectional area was decreased in the Col5a1ΔTen/ΔTen group compared to the wild type group in the FDL, ACH, and SST. (B) Percent relaxation was not altered with collagen V removal in any tissue. (C) Maximum load was decreased in the Col5a1ΔTen/ΔTen group in all tissues except the FDL. (D) Maximum stress was severely reduced in the ACL and SST, but there were no differences between groups in the ACH or FDL. Data presented as median ± interquartile range.
Figure 3.
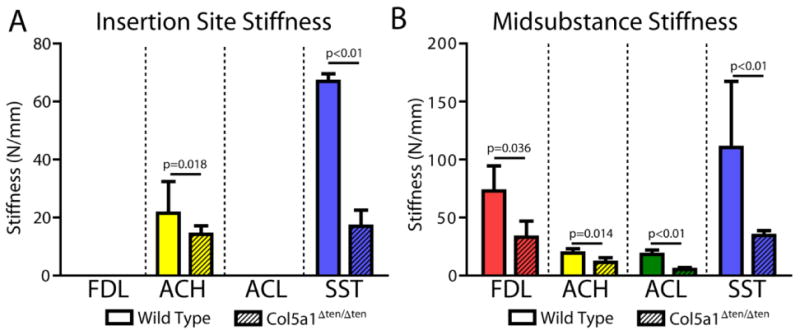
Stiffness was decreased in the Col5a1ΔTen/ΔTen group at the insertion site (A) for the ACH and SST and in the midsubstance (B) for all tissues. Data presented as median ± interquartile range.
Figure 4.
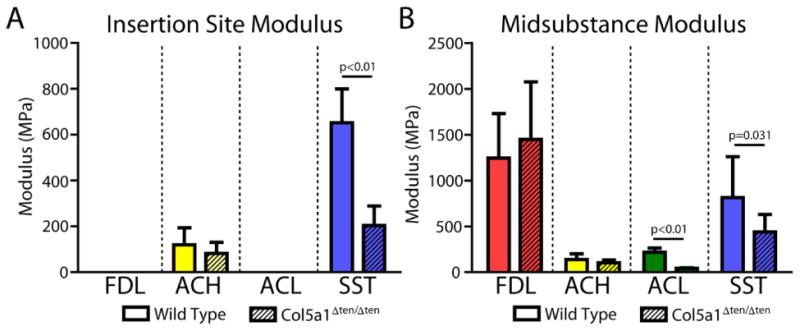
Modulus was decreased in the Col5a1ΔTen/ΔTen group at the insertion site (A) only for the SST and in the midsubstance (B) only for the ACL and the SST. Data presented as median ± interquartile range.
4 Discussion
This study found that Col5a1ΔTen/ΔTen tendons were significantly smaller than their wild type counterparts. As collagen V is a major regulator of fibrillogenesis and specifically of fibril nucleation, this was an expected finding. Removal of fibril nucleation sites results in fewer fibrils being formed in the tissue25 and thus should form smaller tendons/ligaments overall. This finding was not present in the ACLs. However, ligaments have been proposed to be more metabolically active than tendons and contain more cells, which could result in the production of more collagen during development and thus lead to compensation for this change.39 Alternatively, due to the small size of the mouse ACL, it is also possible that small changes may have been present which were not large enough to detect using this method of area measurement.
In coordination with theCol5a1ΔTen/ΔTen group being smaller than the wild type group, the Col5a1ΔTen/ΔTen group also had a reduced maximum load and stiffness than the control group in all tissues. This could be due either to the smaller size of the tissue or to a change in the material quality of the tissue. In the FDL and ACH, there were no differences in modulus at the insertion site (ACH only) or the midsubstance, confirming that the reduced maximum load and stiffness was likely due only to the smaller size of the Col5a1ΔTen/ΔTen tendons. Interestingly, the ACL and SST from the Col5a1ΔTen/ΔTen group did have a significantly decreased modulus at the insertion site (SST only) and the midsubstance, indicating that the quality, in addition to the size, is severely diminished in these tissues.
The severity of the result of collagen V deficiency across the tissues was varied (Table 2). In these bitransgenic models with Cre expression beginning during embryonic development it is unlikely that this could be due to inefficient removal of the collagen V gene. Previous studies have shown that this animal model results in the absence of collagen V expression in the FDL and ACL, as well as in cornea.24; 25 Mechanical changes in this study were larger in the ACL and the SST (Table 2), which contribute to joint stability as the knee and shoulder joints are primarily stabilized by soft tissues. This could imply that the response to load in joint stabilizing tendons and ligaments is fundamentally different from other tissues or that collagen V plays a larger regulatory role in joint stabilizing tendons and ligaments. Further supporting this concept, these animals exhibit increased joint flexibility as well as abnormal gait.24 While collagen V is known to mainly affect fibril structure, it is possible that the non-fibrillar extracellular matrix in these joint stabilizing tendons/ligaments is altered by the absence of collagen V as well, thus producing lower quality tissues unable to compensate for the structural changes.
Table 2.
Percent change between the wild type and Col5a1ΔTen/ΔTen groups for each comparison. Significant differences are represented in bold text and shaded boxes. Boxes indicated by ‘NA’ are not applicable since insertion site properties were not measured for the FDL and ACL.
| Tendon Area | Max Load | Max Stress | Insertion Stiffness | Midsubstance Stiffness | Insertion Modulus | Midsubstance Modulus | |
|---|---|---|---|---|---|---|---|
| FDL | -57% | -19% | 25% | NA | -55% | NA | 16% |
| ACL | 12% | -64% | -70% | NA | -71% | NA | -80% |
| ACH | -27% | -39% | -19% | -34% | -41% | -31% | -26% |
| SST | -42% | -64% | -60% | -75% | -69% | -69% | -46% |
This study is not without limitations. While this study utilized a conditional mouse model with a targeted deletion of collagen V in tendons and ligaments, classic EDS is associated with heterozygous mutations in collagen V. However, recent studies have shown similar mechanical changes in our lab in the heterozygous collagen V mouse, implying a dose-dependent response, and future studies will employ this model to confirm these results in the other tissues.22 Additionally, this study investigated only the tendons and ligaments that stabilize joints, but did not measure the properties of any other stabilizing tissue, such as joint capsule or cartilage. Although the tissues investigated here stabilize joints primarily surrounded by other tendons/ligaments (rotator cuff in the shoulder and other cruciate ligaments in the knee), detrimental changes in joint capsule or cartilage could also contribute significantly to joint instability. Finally, this study only measured the response to load at the macroscale, but there could be changes in the response to load at the fiber or fibril level that could lead to the macroscale mechanical deficiencies in the joint stabilizing tendons and ligaments. Future studies will examine the static and dynamic response to load at multiple hierarchical levels.
Despite these limitations, this study supports the conclusion that the effect of collagen V deficiency is tissue-specific. Subsequently, it is possible that insufficient mechanical properties of the tendons and ligaments surrounding joints contribute in part to joint instability in classic EDS patients, although the mechanism is still unknown. Furthermore, improving the mechanical integrity of surrounding tendons/ligaments of joints, such as the shoulder and knee, could be a potential therapeutic target for improving the quality of life in this patient population.
Acknowledgments
This study was supported by NIH/NIAMS (T32-AR007132, AR044745, AR065995), the NSF GRFP and the Penn Center for Musculoskeletal Disorders (NIH, P30 AR050950).
Footnotes
Author Contributions Statement: BK Connizzo has contributed to all aspects of this study, including research design, data acquisition, interpretation/analysis of data, and drafting/revision of the manuscript. BR Freedman, JH Fried, and M Sun were significantly involved in data acquisition and analysis/interpretation of the data. DE Birk and LJ Soslowsky have contributed significantly in research design, interpretation/analysis of data and drafting/revision of the manuscript. All authors have read and approved the final submitted manuscript.
References
- 1.Birk DE, Southern JF, Zycband EI, et al. Collagen fibril bundles: a branching assembly unit in tendon morphogenesis. Development. 1989;107:437–443. doi: 10.1242/dev.107.3.437. [DOI] [PubMed] [Google Scholar]
- 2.Birk DE, Trelstad RL. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fessel G, Snedeker JG. Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol. 2009;28:503–510. doi: 10.1016/j.matbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Fessel G, Snedeker JG. Equivalent stiffness after glycosaminoglycan depletion in tendon--an ultra-structural finite element model and corresponding experiments. J Theor Biol. 2011;268:77–83. doi: 10.1016/j.jtbi.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Dunkman AA, Buckley MR, Mienaltowski MJ, et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013;32:3–13. doi: 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matuszewski PE, Chen YL, Szczesny SE, et al. Regional Variation in Human Supraspinatus Tendon Proteoglycans: Decorin, Biglycan, and Aggrecan. Connect Tissue Res. 2012 doi: 10.3109/03008207.2012.654866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmadzadeh H, Connizzo BK, Freedman BR, et al. Determining the contribution of glycosaminoglycans to tendon mechanical properties with a modified shear-lag model. J Biomech. 2013 doi: 10.1016/j.jbiomech.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley MR, Huffman GR, Iozzo RV, et al. The location-specific role of proteoglycans in the flexor carpi ulnaris tendon. Connect Tissue Res. 2013;54:367–373. doi: 10.3109/03008207.2013.832232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redaelli A, Vesentini S, Soncini M, et al. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons--a computational study from molecular to microstructural level. J Biomech. 2003;36:1555–1569. doi: 10.1016/s0021-9290(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 10.Connizzo BK, Yannascoli SM, Soslowsky LJ. Structure-function relationships of postnatal tendon development: A parallel to healing. Matrix Biol. 2013;32:106–116. doi: 10.1016/j.matbio.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller KS, Connizzo BK, Feeney E, et al. Characterizing local collagen fiber re-alignment and crimp behavior throughout mechanical testing in a mature mouse supraspinatus tendon model. J Biomech Eng. 2012;45:2061–2065. doi: 10.1016/j.jbiomech.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Screen HR, Lee DA, Bader DL, et al. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc Inst Mech Eng H. 2004;218:109–119. doi: 10.1243/095441104322984004. [DOI] [PubMed] [Google Scholar]
- 13.Screen HR, Toorani S, Shelton JC. Microstructural stress relaxation mechanics in functionally different tendons. Med Eng Phys. 2013;35:96–102. doi: 10.1016/j.medengphy.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev Dyn. 1995;202:229–243. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- 15.Birk DE, Zycband EI, Woodruff S, et al. Collagen fibrillogenesis in situ: fibril segments become long fibrils as the developing tendon matures. Dev Dyn. 1997;208:291–298. doi: 10.1002/(SICI)1097-0177(199703)208:3<291::AID-AJA1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 16.Ansorge HL, Meng X, Zhang G, et al. Type XIV Collagen Regulates Fibrillogenesis: Premature Collagen Fibril Growth and Tissue Dysfunction in Null Mice. J Biol Chem. 2009;284:8427–8438. doi: 10.1074/jbc.M805582200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang G, Ezura Y, Chervoneva I, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. Journal of Cellular Biochemistry. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G, Chen S, Goldoni S, et al. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 20.Segev F, Heon E, Cole WG, et al. Structural abnormalities of the cornea and lid resulting from collagen V mutations. Invest Ophthalmol Vis Sci. 2006;47:565–573. doi: 10.1167/iovs.05-0771. [DOI] [PubMed] [Google Scholar]
- 21.Wenstrup RJ, Florer JB, Davidson JM, et al. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem. 2006;281:12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- 22.Wenstrup RJ, Smith SM, Florer JB, et al. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. J Biol Chem. 2011;286:20455–20465. doi: 10.1074/jbc.M111.223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenstrup RJ, Florer JB, Brunskill EW, et al. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 24.Sun M, Connizzo BK, Adams SM, et al. Targeted deletion of collagen V in tendons and ligaments results in a classic Ehlers-Danlos Syndrome joint phenotype. Am J Pathol. 2015 doi: 10.1016/j.ajpath.2015.01.031. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun M, Chen S, Adams SM, et al. Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model. J Cell Sci. 2011;124:4096–4105. doi: 10.1242/jcs.091363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malfait F, Coucke P, Symoens S, et al. The molecular basis of classic Ehlers-Danlos syndrome: a comprehensive study of biochemical and molecular findings in 48 unrelated patients. Hum Mutat. 2005;25:28–37. doi: 10.1002/humu.20107. [DOI] [PubMed] [Google Scholar]
- 27.Laguette MJ, Abrahams Y, Prince S, et al. Sequence variants within the 3′-UTR of the COL5A1 gene alters mRNA stability: implications for musculoskeletal soft tissue injuries. Matrix Biol. 2011;30:338–345. doi: 10.1016/j.matbio.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Collins M, Posthumus M. Type V collagen genotype and exercise-related phenotype relationships: a novel hypothesis. Exerc Sport Sci Rev. 2011;39:191–198. doi: 10.1097/JES.0b013e318224e853. [DOI] [PubMed] [Google Scholar]
- 29.September AV, Cook J, Handley CJ, et al. Variants within the COL5A1 gene are associated with Achilles tendinopathy in two populations. Br J Sports Med. 2009;43:357–365. doi: 10.1136/bjsm.2008.048793. [DOI] [PubMed] [Google Scholar]
- 30.Posthumus M, September AV, O'Cuinneagain D, et al. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. Am J Sports Med. 2009;37:2234–2240. doi: 10.1177/0363546509338266. [DOI] [PubMed] [Google Scholar]
- 31.Stanitski DF, Nadjarian R, Stanitski CL, et al. Orthopaedic manifestations of Ehlers-Danlos syndrome. Clin Orthop Relat Res. 2000:213–221. doi: 10.1097/00003086-200007000-00029. [DOI] [PubMed] [Google Scholar]
- 32.Sacheti A, Szemere J, Bernstein B, et al. Chronic pain is a manifestation of the Ehlers-Danlos syndrome. J Pain Symptom Manage. 1997;14:88–93. doi: 10.1016/s0885-3924(97)00007-9. [DOI] [PubMed] [Google Scholar]
- 33.Ainsworth SR, Aulicino PL. A survey of patients with Ehlers-Danlos syndrome. Clin Orthop Relat Res. 1993:250–256. [PubMed] [Google Scholar]
- 34.Ansorge H, Adams S, Birk D, et al. Mechanical, Compositional, and Structural Properties of the Post-natal Mouse Achilles Tendon. Annals of Biomedical Engineering. 2011;39:1904–1913. doi: 10.1007/s10439-011-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connizzo BK, Sarver JJ, Iozzo RV, et al. Effect of age and proteoglycan deficiency on collagen fiber re-alignment and mechanical properties in mouse supraspinatus tendon. J Biomech Eng. 2013;135:021019. doi: 10.1115/1.4023234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peltz CD, Perry SM, Getz CL, et al. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J Orthop Res. 2009;27:416–420. doi: 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson PS, Lin TW, Jawad AF, et al. Investigating tendon fascicle structure-function relationships in a transgenic-age mouse model using multiple regression models. Ann Biomed Eng. 2004;32:924–931. doi: 10.1023/b:abme.0000032455.78459.56. [DOI] [PubMed] [Google Scholar]
- 38.Derwin KA, Soslowsky LJ, Green WD, et al. A new optical system for the determination of deformations and strains: calibration characteristics and experimental results. J Biomech. 1994;27:1277–1285. doi: 10.1016/0021-9290(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 39.Amiel D, Frank C, Harwood F, et al. Tendons and ligaments: a morphological and biochemical comparison. J Orthop Res. 1984;1:257–265. doi: 10.1002/jor.1100010305. [DOI] [PubMed] [Google Scholar]


