Abstract
Tendinopathy is a common musculoskeletal injury whose treatment is limited by ineffective therapeutic interventions. Previously we have shown that tendons ineffectively repair early sub-rupture fatigue damage. In contrast, physiological exercise has been shown to promote remodeling of healthy tendons but its utility as a therapeutic to promote repair of fatigue damaged tendons remains unknown. Therefore, the objective of this study was to assess the utility of exercise initiated 1 and 14 days after onset of fatigue damage to promote structural repair in fatigue damaged tendons. We hypothesized that exercise initiated 14 days after fatigue loading would promote remodeling as indicated by a decrease in area of collagen matrix damage, increased procollagen I and decorin, while decreasing proteins indicative of tendinopathy. Rats engaged in 6-week exercise for 30 min/day or 60 min/day starting 1 or 14 days after fatigue loading. Initiating exercise 1-day after onset of fatigue injury led to exacerbation of matrix damage, particularly at the tendon insertion. Initiating exercise 14 days after onset of fatigue injury led to remodeling of damaged regions in the midsubstance and collagen synthesis at the insertion. Physiological exercise applied after the initial biological response to injury has dampened can potentially promote remodeling of damaged tendons.
Keywords: tendon damage, fatigue, ECM, exercise, tendinopathy
Tendinopathy is a common musculoskeletal injury that affects a wide spectrum of the population. Therapeutic interventions have limited success in treating tendon injury, likely because they are commonly prescribed after development of advanced debilitating symptoms during late stage of the disease.1,2 While the pathogenesis of tendinopathy has not been fully prescribed, degenerative changes in ruptured tendons suggest a process of sub-rupture damage accumulation that ultimately leads to tendon tears in a chronically degenerated environment.3–5 The underlying premise is that the pace at which a tendon repairs the induced damage outpaces effective remodeling.
Accordingly, we have previously established6 an in vivo model of sub-rupture damage accumulation using the rat patellar tendon to investigate mechanisms that underlie early onset of tendinopathy, the time at which therapeutic interventions may be most effective. We found that inducing 1 bout of moderate level fatigue loading results in a 20% stiffness loss that is not recovered out to at least 6 weeks.7 In addition, apoptosis is significantly increased and the expression of genes associated with remodeling is significantly decreased in a manner that is correlated with the amount of structural damage that is induced.8 Taken together, our previous findings suggest that 1 bout of sub-rupture damage leads to altered structure and impaired function that is not repaired due to an environment that promotes excessive cell death and a dampened molecular response.
In contrast, we have previously shown that a low number of applied cycles in vivo, representative of exercise, induces a molecular response that suggests adaptation and remodeling.9 This notion is further corroborated by studies that have shown that prolonged exercise protocols, such as treadmill running, lead to hypertrophy and improved mechanical properties of the tendon.10–12 However, it remains unknown whether exercise can be used as a therapeutic intervention to promote remodeling and repair of fatigue damaged tendons. Therefore, the objective of this study was to assess the utility of exercise to promote structural repair in fatigue damaged tendons. Since the duration and the time of initiation of exercise after the fatigue loading insult is expected to be integral to the resulting response,12 we evaluated the effect of a 6 weeks, 30 or 60 min/day treadmill running protocol that was initiated 1 or 14 days after fatigue loading. The 14-day timepoint was chosen since it coincides with the molecular response and apoptotic activity returning to baseline values after peaking at 7 days, possibly allowing more cells to respond to the exercise.8 We hypothesized that healthy exercise would promote remodeling of fatigue damaged tendons, as indicated by a decrease in area of collagen matrix damage, increased procollagen I13 and decorin,14 while decreasing proteins indicative of a tendinopathic state (such as collagen III and aggrecan).15 We expected that rats engaged in 6-week exercise for 30 min/day would exhibit further improved repair of fatigue damaged tendons than rats that engage in 6 weeks of exercise for 60 min/day. In addition, we expected that rats that initiate exercise immediately after onset of fatigue damage would exhibit inferior repair of fatigue damaged tendons than those that rested for 14 days prior to initiation of the exercise regimen.
METHODS
Following IACUC approval, forty-eight 9–11 month old female retired breeder Sprague–Dawley rats (Charles River Laboratories International, Inc., Wilmington, MA) were anesthetized (1–5% by volume at flow rate of 4 L/min of isoflurane), and unilaterally fatigue loaded per our established methods.6 Briefly, under aseptic conditions, the left tibia and patella were surgically exposed and clamped, positioning the hind limb at 30 degrees knee flexion. The patella clamp was connected in series to a 50 lb load cell (Transducer Techniques, Temecula, CA), and to an Instron machine (Instron 8841 Materials Testing Solutions, Norwood, MA). Fatigue loading was applied by cycling from 1 to 40 N at 1 Hz for 7,200 cycles. Diagnostic tests were applied before (Diag1) and after (Diag2) fatigue loading by cycling from 1 to 15 N at 1 Hz for 420 and 120 cycles, respectively. As previously described,9,16 change between Diag1 and Diag2 in hysteresis, stiffness of the loading and unloading load-displacement curves, and elongation, was calculated as an indicator of the initial damage induced (damage parameter). This analysis was conducted solely to confirm that all groups were subjected to a similar amount of initial damage. At the completion of loading, skin incisions were sutured using prolene 6-0 sutures (Ethicon, Somerville, NJ). Buprenorphine (0.06 mg/ml/kg) was administered as an analgesic at the end of the surgery and 24 h later, and the rats resumed cage activity.
After fatigue loading, animals were randomly assigned into 1 of 6 groups (n = 6–8/group) as follows: (1) Six weeks of running for 30 min/day, 5 days/week that initiated 1 day after fatigue loading (“immediate 30-min runners”); (2) Six weeks of running for 60 min/day, 5 days/week that initiated 1 day after fatigue loading (“immediate 60-min runners”); (3) Six weeks of cage activity (“6-week Cage–Control”); (4) Six weeks of running for 30 min/day, 5 days/week that initiated 2 weeks after fatigue loading (“delayed 30-min runners”); (5) Six weeks of running for 60 min/day, 5 days/week that initiated 2 weeks after fatigue loading (“delayed 60-min runners”); (6) Six weeks of cage activity (“8-week Cage–Control”). Running was conducted on a customized treadmill (NordicTrack A2350 pro treadmill, Logan, UT) with ten independent lanes. On the first day of running, both the 30 and 60 min groups ran for 15 min at 12 m/min. On the second day of running the 30 min group ran for 25 min at 12 m/min and the 60 min group ran for 25 min at 12 m/min and 15 min at 17 m/min. Both, the 30 and 60 min groups ran their full intended time at 17 m/min starting from the 3rd day of training. Both groups ran 5 days a week with 2 days off for a total of 6 weeks.
Processing of Harvested Tendons
The fatigue loaded quadriceps-patella-patellar tendon-tibia complex was harvested on the day of completion of the running protocol, fixed under 2N of tension in Z-fix (Anatech Ltd, Battle Creek, MI) and decalcified (Decal Chemical Corporation, Tallman, NY). The tendons were separated into medial and lateral halves. The lateral half of the tendon was embedded in methyl methacrylate (MMA) and sectioned into ~130 µm sections for Second Harmonic Generating (SHG) imaging using a 60× oil immersion lens (300× magnification). SHG imaging of thick sections minimizes shearing artifacts from confounding calculation of extracellular matrix (ECM) damage.17,18 Five images were acquired per tendon. ECM damage was identified using established methods.19 The damage area fraction (DAF) was defined as the damaged area relative to total area.19 The medial half of the tendon was embedded in paraffin, sliced into 5 µm sections, and then mounted on paraffin slides. DeCal solution (BioGenex, Inc., San Ramon, CA) was used for antigen retrieval in deparaffinized sections. Endogenous peroxidase activity was quenched using 10%H2O2. Non-specific binding was blocked with Dako Protein block. Immunohistochemical (IHC) staining for collagen III (1:500, Abcam, Cambridge, MA), procollagen I (1:100, Santa Cruz Biotechnology, Inc., Dallas, TX), decorin (1:100, Abcam), or aggrecan (1:2,000, Abcam) primary antibodies was conducted. Incubation in rabbit and goat serum without primary antibody was used as the negative staining control. Sections were then counterstained with toluidine blue. Two sister sections were visually compared to confirm accuracy and repeatability. For each antibody, one blinded section was then used for all analysis.
Quantification of IHC Staining
Images were acquired under 400× magnification, and were imported to ImageJ software for quantification. Analysis was conducted at the insertion (tibial end) and midsubstance, as previously described.8 Briefly, a region at the insertion was defined for analysis by drawing a trapezoid that outlines a region enclosed by a border between the tendon and fibrocartilage and the surface of the tendon. The tendon midpoint was also defined and imaged throughout the full thickness of the tendon to define the midsubstance. The origin (patellar end) was excluded from this study since cutting the tendons in half did not permit retention of this region in a sufficiently large sample size.
For cellular stains (procollagen I, aggrecan, and decorin), the total number of cells and the number of positively stained cells were counted to yield a positive cell ratio and positive cell density. To quantify the amount of ECM staining (collagen III, aggrecan, and decorin), ImageJ was used to split the color channels yielding a black and white image from the blue channel, wherein the blue counterstain is removed and the remaining black stain in the binary image represents the brown positive ECM staining. The percent of the image or region of interest that is positively stained is then quantified. By using a binary image the intensity of the staining did not influence the quantification percentage of area stained.
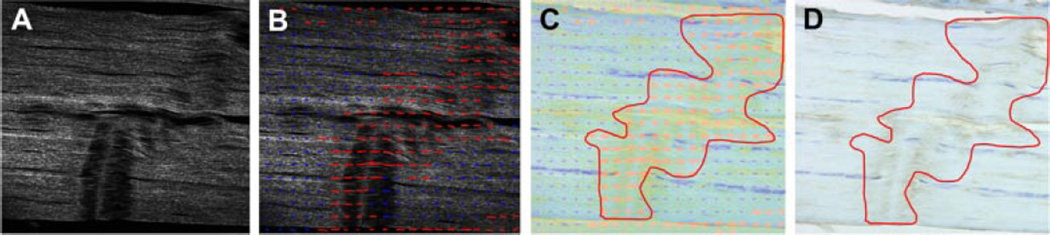
In addition to reporting DAF, and ECM and cell staining for the combined damaged and undamaged regions (bulk tendon) at the insertion and midsubstance, the protein content localized to damaged regions was further analyzed. Briefly, under 200× magnification, SHG and conventional imaging were conducted on each IHC slides for aggrecan, decorin, and collagen III. Identified damage regions were from SHG imaging and were used to mask corresponding regions in IHC images (Fig. 1). This analysis allows us to identify the effect of exercise after fatigue loading on correlated changes between collagen damage and the other evaluated ECM proteins.
Figure 1.
Identification of damaged regions from SHG imaging for assessment of corresponding ECM protein staining. (A) SHG images are acquired; (B) Damaged regions are outlined with red arrows on SHG images; (C) The outlined SHG image is overlayed on the corresponding IHC image (shown for collagen III); (D) The masked IHC stained regions can be analyzed per our described methods.
Statistical Analysis
Non-parametric t tests (Mann–Whitney) and non-parametric ANOVAs (Kruskal–Wallis) were utilized for all two and three group comparisons, respectively. Posthoc Dunn’s was utilized to identify significantly different pairs for ANOVAs. We first assessed the effect of running by comparing running groups (30 and 60 min/day) and their respective cage–control. We then assessed the effect of the time delay until start of exercise after fatigue loading by comparing immediate-runners and delayed-runners that were normalized by the average of their respective controls. Significance was set at p ≤ 0.05 denoted with “*” and a trend20,21 at p ≤ 0.1 denoted with “#”. In all figures, error bars represent the standard deviation.
RESULTS
As expected, there were no differences in initial damage parameters (data not shown) between all groups suggesting that any measured differences at study end reflect the effect of the post fatigue exercise regimen. Contrary to our hypothesis, there were no differences between 30 and 60 min groups at the insertion, midsubstance or in the isolated damaged regions for any of the parameters evaluated leading to subsequent pooling of these groups. Therefore the pooled sets of data will be referred to as “immediate runners” and “delayed runners.”
Bulk Tendon Response
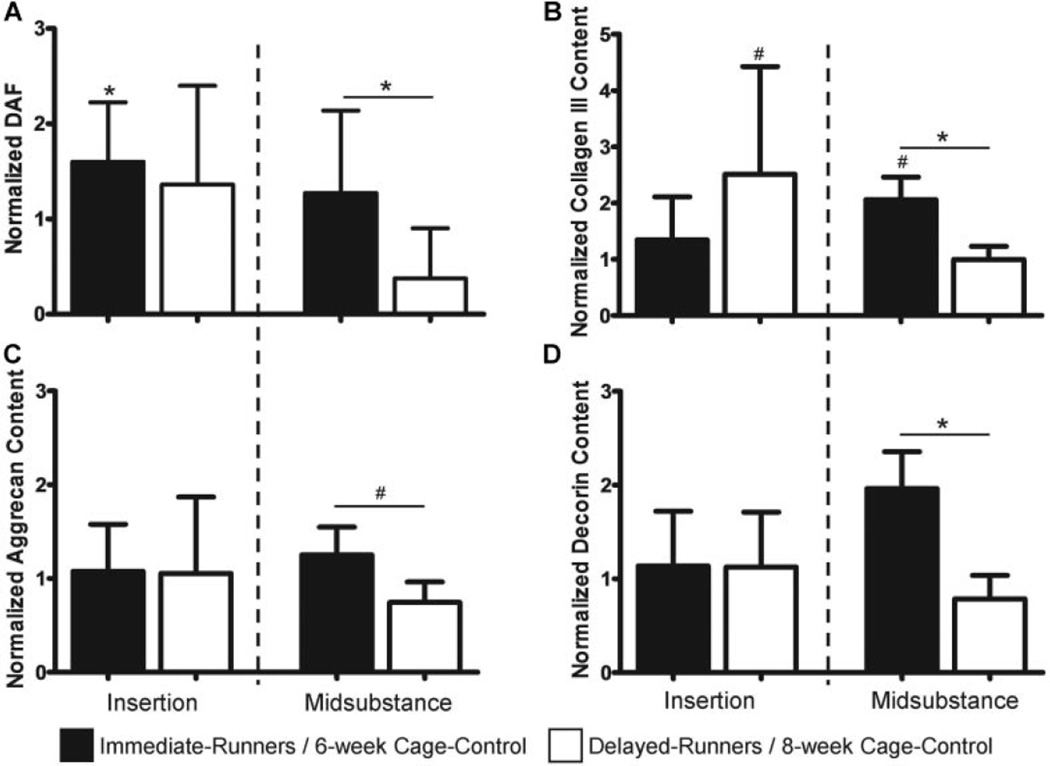
As hypothesized, DAF, indicative of the fraction of the image that exhibits collagen structural damage, was significantly higher than 6-week cage–controls at the insertion in the immediate-runners (p = 0.03) but not the delayed-runners. In support of our hypothesis, DAF was significantly decreased from 8-week cage–control (p = 0.1) and immediate-runners (p = 0.001) in the midsubstance of delayed-runners (Fig. 2A).
Figure 2.
Effect of exercise on ECM collagen damage and ECM protein levels in fatigue damaged tendons. (A) DAF was increased at the insertion of immediate-runners but was decreased in the midsubstance of delayed-runners; (B) Collagen III was increased in the midsubstance on immediate-runners and in the insertion of delayed-runners; (C) Aggrecan and (D) Decorin were not altered in fatigue damaged tendons by exercise.
Exercise resulted in an increase in collagen III content in the midsubstance of immediate-runners relative to 6-week cage–control (p = 0.1) and delayed-runners (p = 0.02), and in the insertion of delayed-runners (p = 0.03) relative to 8-week cage–control (Fig. 2B). Contrary to our hypothesis, aggrecan and decorin ECM staining (%) was not altered by exercise in fatigue damaged tendons in immediate- or delayed-runners (Fig. 2C and D).
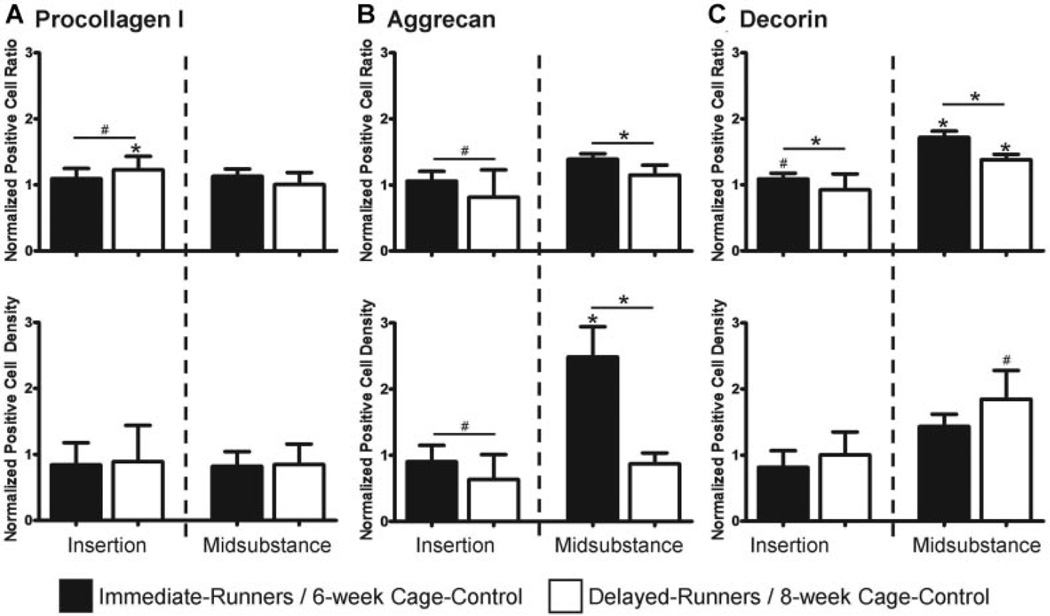
Evaluation of cell staining for procollagen I showed that despite an unaltered positive cell density, there was a higher positive cell ratio at the insertion in delayed-runners compared to 8-week cage–control (p = 0.05) and immediate-runners (p = 0.1) (Fig. 3A). Consistent with our hypothesis, aggrecan cell staining showed decreased positive cell density (p = 0.08) but an unaltered positive cell ratio at the insertion in delayed-runners. Contrary to our hypothesis, there was an increased aggrecan positive cell density (p = 0.04) and positive cell ratio (p = 0.08) in the midsubstance of immediate-runners (Fig. 3B). As expected, immediate-runners exhibited an increased positive cell ratio for decorin at the insertion (p = 0.06) and midsubstance (p < 0.001). As hypothesized, delayed-runners exhibited an increased decorin positive cell density (p = 0.07) and positive cell ratio (p = 0.02) in the midsubstance only (Fig. 3C).
Figure 3.
Effect of exercise on positive cell ratio and density in fatigue damaged tendons. (A) Procollagen I was increased at the insertion of delayed-runners; (B) Aggrecan was increased in the midsubstance of immediate-runners but was decreased at the insertion of delayed-runners; (C) Decorin was increased at the insertion and midsubstance of immediate-runners and in the midsubstance of delayed-runners.
Assessment of Damaged Regions
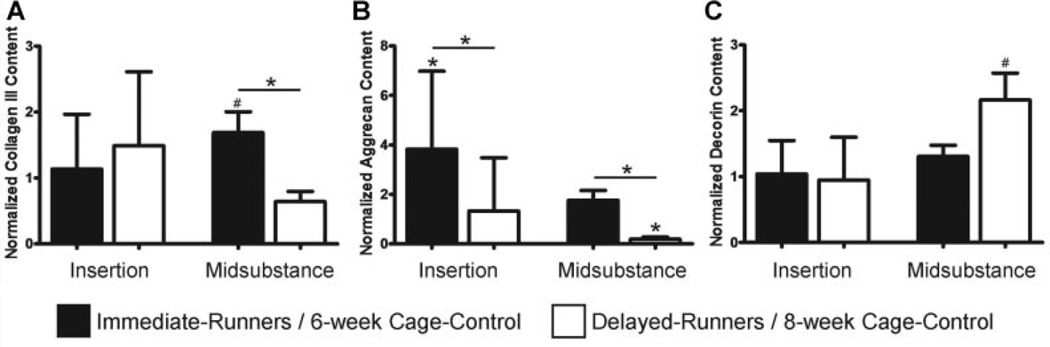
Immediate-runners exhibited an increase in collagen III content in damaged regions in the midsubstance relative to 6-week cage–controls (p = 0.12) and delayed-runners (p = 0.005; Fig. 4A). In support of our hypothesis, immediate-runners exhibited a significant increase in aggrecan content in damaged regions at the insertion compared to 6-week cage–control (p = 0.02) and delayed-runners (p = 0.01). However, delayed-runners exhibited a decrease in aggrecan content in damaged regions in the midsubstance compared to 8-week cage–control (p < 0.01) and immediate-runners (p < 0.01; Fig. 4B). As expected, decorin content was significantly increased (p = 0.04) in the midsubstance of delayed-runners in comparison with 8-week cage–controls (Fig. 4C).
Figure 4.
Effect of exercise on ECM protein levels of damaged regions in fatigue damaged tendons. (A) Collagen III was increased in damaged regions of the midsubstance in immediate-runners; (B) Aggrecan was increased in damaged regions at the insertion of immediate-runners but was decreased in damaged regions in the midsubstance of delayed-runners; (C) Decorin was increased in damaged regions in the midsubstance of delayed-runners.
DISCUSSION
In this study, we evaluated the utility of exercise initiated 1 or 14 days after fatigue loading as a therapeutic intervention that can promote remodeling in fatigue damaged tendons. Since it was unknown whether an exercise regimen that imposes repetitive loading on the tendon would be therapeutic or further exacerbate the induced ECM damage, both short (30 min/day) and long (60 min/day) durations of exercise were evaluated. Surprisingly, while exercise altered the repair response of fatigue damaged tendons, there was no difference between the effect of a short and long bout of exercise, suggesting that these protocols were equally beneficial after 6 weeks. This finding has significant clinical implications as it suggests that remodeling can be induced in fatigue damaged tendons even with a daily short bout of exercise.
The effect of initiating exercise after 1 and 14 days from onset of fatigue injury was compared. Our previous finding that showed that the prescribed fatigue loading protocol leads to an immediate stiffness loss that is not recovered out to at least 6-week suggests that the mechanical properties the fatigue damaged tendons were similar at the start of both exercise regimens.7 However, our previous findings showing that onset of fatigue damage results in increased apoptosis and a molecular response that peaks at 7 days but is diminished by 14 days suggests availability of more cells that can respond to exercise that is initiated at 14 days than 1 day after onset of damage.8 These two groups were evaluated to represent two common, clinically relevant patient populations: (1) A patient who engages in one bout of a strenuous damaging activity but continues a regimen of physiological exercise starting the following day; (2) A patient who engages in one bout of a strenuous damaging activity but rests for some time (2 weeks) before initiating exercise.
Interestingly, structural changes, as indicated by damage area fraction of the tendon, suggest that initiating exercise 1 day after onset of fatigue injury leads to exacerbation of ECM damage, particularly at the tendon insertion. Interestingly, the increase in ECM damage at the insertion in this group was not coupled with any changes in ECM protein staining, suggesting generally inadequate remodeling in this region. Further analysis of the isolated damaged regions at the insertion of this group showed an increase in aggrecan content only. Since aggrecan is typically abundant at the insertion of healthy tendons due to the co-existence of tensile and compressive loads in this region,13,14 its increase in damaged regions at the insertion site may suggest progression towards restoration of the loading environment of the cells that may ultimately prompt ECM remodeling. In contrast, analysis of the midsubstance (a tensile load bearing region) suggests further degeneration by early exercise as indicated by unaltered collagen ECM damage, coupled with unaltered procollagen I and increased aggrecan.
Our data suggests that delayed exercise promotes effective remodeling of fatigue damaged tendons. While ECM damage was not altered by delayed exercise at the insertion, increased collagen III and procollagen I that was unaccompanied by similar changes in the isolated damaged regions suggest a compensatory response by the healthy regions. Increased collagen deposition may ultimately lead to decreased relative ECM damage by increasing the number of the collagen fibers available to bear load. In contrast, delayed exercise resulted in decreased amount of ECM damage in the midsubstance that was coupled with decreased aggrecan and increased decorin in the isolated damaged regions, suggesting effective remodeling of the damaged regions. In effect, our data suggests that delayed exercise results in remodeling by repairing damaged regions in the midsubstance but promotes tendon hypertrophy without repair of the ECM damage at the insertion. This data suggests that while physiological exercise may improve tendon functional capacity at the commonly injured tendon insertion, minimal repair of damaged regions suggest a sustained risk of further injury.
Other studies have investigated the potential use of exercise as a therapeutic for injured tendons. For instance, Godbout et al.22 investigated the effect of exercise to treat experimentally induced tendinopathy. However, in contrast to our study, Godbout et al. used collagenase to induce tendinopathy leading to increased inflammation and cellularity, and ultimately a biological environment that starkly differs from the degenerated environment that is prompted by our subrupture fatigue model which lacks inflammation and is characterized by increased apoptosis.8 Interestingly, despite substantial differences in these models, both Godbout et al.22 and our study concluded that delayed exercise was more beneficial to the injured tendon compared to immediate exercise. In contrast, in an Achilles tendon transection model, Eliasson et al.23 found that exercise was beneficial in both immediate and delayed starting points in comparison to sustained immobilization. While exercise seems to serve as a therapeutic for these three distinctly different tendon injuries (sub-rupture mechanical injury, collagenase-induced tendinopathy and laceration), we expect that a different underlying mechanism, namely modulation of inflammation,24 is the likely mechanism by which exercise modulates injury in the collagenase-induced tendinopathy and laceration. In contrast, the absence of inflammation in our sub-rupture damaged tendons suggests that an entirely different mechanism, likely one that restores the microenvironment of the cells, is prompted by exercise and will be the focus of future investigation.
The greatest limitation associated with this study is that only one section was analyzed for each immunohistochemical stain. Although the sections were chosen randomly by a blinded user, it is likely that a larger sampling would reduce the variability within each group and allow detection of more subtle differences between groups.
Given that only one half of the tendon was available for IHC and the high number of markers evaluated (in conjunction with their associated negative control slides), it was not possible to evaluate a larger number of slides for each marker. Another limitation is that the SHG mask used to analyze IHC ECM staining in damaged regions was derived from imaging of each thin paraffin section. In contrast, the bulk DAF analysis that was reported was conducted on thick MMA sections so as to minimize any confounding effect of sectioning artifacts on DAF quantification. However, since IHC staining is most effective on thin sections, regional correlation between damage and ECM IHC staining necessitating SHG imaging of thin sections to generate sample specific damage masks. We expect that artifacts from thin sectioning have the same effect on all groups and do not affect the overall conclusion that exercise results in remodeling of the damaged regions. Also, damage regions were identified but were not further classified by severity. We suspect that remodeling from exercise may lead to not only a decrease in damage area fraction, but also a decreased severity of damage in damaged regions. Future studies will further striate the ECM damage by severity. Lastly, while a strength of this study is that it utilizes our established animal model to investigate the utility of therapeutic interventions during early tendinopathy, inherent differences between animals and humans limit direct translation of our findings for clinical therapeutics. Despite this limitation, we expect that findings from this study, along with future investigation of the associated underlying mechanisms, provide a guide for development of clinical interventions.
In conclusion, we have shown that physiological exercise has the potential to exacerbate or promote remodeling of induced sub-rupture damage. Interestingly, initiating exercise after the initial biological response of the tendon to injury has diminished is integral to a resulting therapeutic outcome. One possible explanation for the difference in effect of immediate and delayed exercise is the potential availability of a larger cell population that can respond to the exercise after a brief period of time (2 weeks) from onset of injury. Another possible explanation is that the environment of the living cells is altered between initial onset of injury and 2 weeks later, changing the sensitivity of the cells to mechanical loading by exercise. Future studies will assess alterations by exercise in apoptotic activity, cell morphology and ECM interactions in damaged and undamaged regions.
ACKNOWLEDGEMENTS
The authors acknowledge Matthew Anderson and Damien Laudier for their contributions. Multiphoton microscopy was performed in the microscopy shared resource facility supported by 1S10RR026639-01 from NIH. This study was funded by AR52743 (N.A.P.) from NIAMS/NIH.
Footnotes
AUTHORS’ CONTRIBUTIONS
R.B. contributed to the data collection, data analysis, data interpretation and manuscript preparation. M.B. contributed to the study design, data collection and manuscript preparation. N.G. contributed to the data collection and data analysis. E.L.F. contributed to the study design and data interpretation. N.A.P. contributed to study design, data collection, data analysis, data interpretation, and manuscript preparation. All authors have read and approved the final submitted version of the document.
REFERENCES
- 1.Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486–490. [PubMed] [Google Scholar]
- 2.Rowe V, Hemmings S, Barton C, et al. Conservative management of midportion achilles tendinopathy: a mixed methods study, integrating systematic review and clinical reasoning. Sports Med. 2012;42:941–967. doi: 10.1007/BF03262305. [DOI] [PubMed] [Google Scholar]
- 3.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- 4.Tallon C, Maffulli N, Ewen SW. Ruptured achilles tendons are significantly more degenerated than tendinopathic tendons. Med Sci Sports Exerc. 2001;33:1983–1990. doi: 10.1097/00005768-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11:533–578. [PubMed] [Google Scholar]
- 6.Fung DT, Wang VM, Andarawis-Puri N, et al. Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech. 2010;43:274–279. doi: 10.1016/j.jbiomech.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andarawis-Puri N, Flatow EL. Tendon fatigue in response to mechanical loading. J Musculoskelet Neuronal Interact. 2011;11:106–114. [PMC free article] [PubMed] [Google Scholar]
- 8.Andarawis-Puri N, Philip A, Laudier D, et al. Temporal effect of in vivo tendon fatigue loading on the apoptotic response explained in the context of number of fatigue loading cycles and initial damage parameters. J Orthop Res. 2014;32:1097–1103. doi: 10.1002/jor.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andarawis-Puri N, Sereysky JB, Sun HB, et al. Molecular response of the patellar tendon to fatigue loading explained in the context of the initial induced damage and number of fatigue loading cycles. J Orthop Res. 2012;30:1327–1334. doi: 10.1002/jor.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrissey D, Roskilly A, Twycross-Lewis R, et al. The effect of eccentric and concentric calf muscle training on achilles tendon stiffness. Clin Rehabil. 2011;25:238–247. doi: 10.1177/0269215510382600. [DOI] [PubMed] [Google Scholar]
- 11.Birch HL, McLaughlin L, Smith RK, et al. Treadmill exercise-induced tendon hypertrophy: assessment of tendons with different mechanical functions. Equine Vet J Suppl. 1999;30:222–226. doi: 10.1111/j.2042-3306.1999.tb05222.x. [DOI] [PubMed] [Google Scholar]
- 12.Bohm S, Mersmann F, Tettke M, et al. Human achilles tendon plasticity in response to cyclic strain: effect of rate and duration. J Exp Biol. 2014;217:4010–4017. doi: 10.1242/jeb.112268. [DOI] [PubMed] [Google Scholar]
- 13.Chuen FS, Chuk CY, Ping WY, et al. Immunohistochemical characterization of cells in adult human patellar tendons. J Histochem Cytochem. 2004;52:1151–1157. doi: 10.1369/jhc.3A6232.2004. [DOI] [PubMed] [Google Scholar]
- 14.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- 15.Samiric T, Parkinson J, Ilic MZ, et al. Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol. 2009;28:230–236. doi: 10.1016/j.matbio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Andarawis-Puri N, Sereysky JB, Jepsen KJ, et al. The relationships between cyclic fatigue loading, changes in initial mechanical properties, and the in vivo temporal mechanical response of the rat patellar tendon. J Biomech. 2012;45:59–65. doi: 10.1016/j.jbiomech.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fung DT, Sereysky JB, Basta-Pljakic J, et al. Second harmonic generation imaging and Fourier transform spectral analysis reveal damage in fatigue-loaded tendons. Ann Biomed Eng. 2010;38:1741–1751. doi: 10.1007/s10439-010-9976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fung DT, Wang VM, Laudier DM, et al. Subrupture tendon fatigue damage. J Orthop Res. 2009;27:264–273. doi: 10.1002/jor.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sereysky JB, Andarawis-Puri N, Ros SJ, et al. Automated image analysis method for quantifying damage accumulation in tendon. J Biomech. 2010;43:2641–2644. doi: 10.1016/j.jbiomech.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desbiens NA. A novel use for the word “trend” in the clinical trial literature. Am J Med Sci. 2003;326:61–65. doi: 10.1097/00000441-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Fitts DA. Ethics and animal numbers: informal analyses, uncertain sample sizes, inefficient replications, and type I errors. J Am Assoc Lab Anim Sci. 2011;50:445–453. [PMC free article] [PubMed] [Google Scholar]
- 22.Godbout C, Ang O, Frenette J. Early voluntary exercise does not promote healing in a rat model of achilles tendon injury. J Appl Physiol (1985) 2006;101:1720–1726. doi: 10.1152/japplphysiol.00301.2006. [DOI] [PubMed] [Google Scholar]
- 23.Eliasson P, Andersson T, Aspenberg P. Achilles tendon healing in rats is improved by intermittent mechanical loading during the inflammatory phase. J Orthop Res. 2012;30:274–279. doi: 10.1002/jor.21511. [DOI] [PubMed] [Google Scholar]
- 24.Yang JH, Sakamoto H, Xu EC, et al. Biomechanical regulation of human monocyte/macrophage molecular function. Am J Pathol. 2000;156:1797–1804. doi: 10.1016/S0002-9440(10)65051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]