Abstract
BACKGROUND
Despite worldwide consumption of moderate amounts of alcohol, the neural mechanisms that mediate the transition from use to abuse are not fully understood.
METHODS
Here, we conducted a high-through put screen of the amygdala proteome in mice after moderate alcohol drinking (n = 12/group) followed by behavioral studies (n = 6–8/group) to uncover novel molecular mechanisms of the positive reinforcing properties of alcohol that strongly influence the development of addiction.
RESULTS
Two-dimensional difference in-gel electrophoresis with matrix assisted laser desorption ionization tandem time-of-flight identified 29 differentially expressed proteins in the amygdala of nondependent C57BL/6J mice following 24 days of alcohol drinking. Alcohol-sensitive proteins included calcium/calmodulin-dependent protein kinase II alpha (CaMKIIα) and a network of functionally linked proteins that regulate neural plasticity and glutamate-mediated synaptic activity. Accordingly, alcohol drinking increased α-amino-3-hydroxy-5-methyl-4-isooxazole receptor (AMPAR) in central amygdala (CeA) and phosphorylation of AMPAR GluA1 subunit at a CaMKII locus (GluA1-Ser831) in CeA and lateral amygdala. Further, CaMKIIα-Thr286 and GluA1-Ser831 phosphorylation was increased in CeA and lateral amygdala of mice that lever-pressed for alcohol versus the nondrug reinforcer sucrose. Mechanistic studies showed that targeted pharmacologic inhibition of amygdala CaMKII or AMPAR activity specifically inhibited the positive reinforcing properties of alcohol but not sucrose.
CONCLUSIONS
Moderate alcohol drinking increases the activity and function of plasticity-linked protein networks in the amygdala that regulate the positive reinforcing effects of the drug. Given the prominence of positive reinforcement in the etiology of addiction, we propose that alcohol-induced adaptations in CaMKIIα and AMPAR signaling in the amygdala may serve as a molecular gateway from use to abuse.
Keywords: Addiction, Alcohol, AMPA, Amygdala, CaMKII, CaMKII alpha, GluA1, Glutamate, Mice, Proteomics, Reinforcement, Self-administration
Human beings have consumed alcohol for thousands of years; its use is deeply rooted in culture and religion. In developed countries, more than 50% of the adult population consumes alcohol on a regular basis (1) with approximately 9% developing dependence (2). The transition from alcohol use to abuse is a multiphasic process that is characterized initially by repeated intoxication episodes where the powerful positive reinforcing, or rewarding, effects of the drug predominate (3). Thus, defining the neural mechanisms of alcohol reinforcement is critical to a comprehensive understanding of the etiology of this debilitating neuropsychiatric disorder (4).
Although multiple neural systems regulate reinforcement processes, evidence suggests that drugs of abuse gain control over individuals, in part, by usurping glutamate-linked neurotransmission within brain reward circuits (5–7), including the extended amygdala (8). Indeed, chronic alcohol exposure alters glutamate release (9), receptor function (10,11), and glutamate-linked synaptic plasticity (12) in the amygdala. Since glutamate transmission in the amygdala is required for the development of new behavior and retention of actions (13), these neural processes may underlie the development and maintenance of alcohol-seeking behavior (14,15). However, the extent to which initial alcohol drinking alters mechanisms of glutamate-linked transmission in the amygdala and whether these neural targets of alcohol regulate the positive reinforcing effects of the drug remain to be characterized.
To increase understanding of the transition from use to abuse, we first employed an unbiased proteomic strategy to identify molecular changes that are induced in the amygdala by moderate voluntary alcohol drinking in C57BL/6J mice. In four parallel assays, two-dimensional differential in-gel electrophoresis (2D-DIGE) followed by matrix assisted laser desorption ionization tandem time-of-flight (MALDI-TOF/TOF) mass spectrometry showed that alcohol drinking consistently altered expression of 29 amygdala proteins, which included calcium/calmodulin dependent kinase II alpha (CaMKIIα) and other proteins that regulate glutamate neurotransmission. CaMKII is a serine-threonine kinase that plays a prominent role in glutamate neurotransmission and experience-dependent plasticity at the synaptic and behavioral level (16), which makes it a highly significant target in the search for neural mechanisms of how the brain becomes focused on alcohol during the development of addiction (17). Moreover, proteomic studies have found overlapping changes in neural protein expression between rodent models of alcohol drinking (18,19) and postmortem human alcoholic brain (20,21), indicating significant translational value of preclinical approaches (22). However, it remains to be determined if alcohol-sensitive protein networks mechanistically regulate alcohol-seeking behavior in a manner that might influence the development of addiction. Thus, after identifying CaMKIIα as a neural target of moderate drinking, we used operant conditioning techniques to determine if CaMKII signaling in the amygdala mechanistically regulates the reinforcing effects of alcohol in mice.
Glutamatergic α-amino-3-hydroxy-5-methyl-4-isooxazole receptors (AMPARs) play a major role in experience-dependent synaptic and behavioral plasticity (23). CaMKII phosphorylation of AMPARs at the GluA1-Ser831 subunit is required for synaptic plasticity and memory retention (24–26). This makes the CaMKII-AMPAR signaling pathway an ideal candidate for aberrant learning that characterizes addiction (27). Therefore, we also explored the hypothesis that alcohol-induced changes in amygdala CaMKIIα protein expression might be associated with increased glutamate-mediated synaptic activity and mechanistic regulation of alcohol reinforcement via AMPAR function within the amygdala. Collectively, these multidisciplinary experiments identify a novel protein network that includes the CaMKIIα–AMPAR pathway in the amygdala as a target of moderate alcohol drinking that also mechanistically regulates the positive reinforcing effects of the drug, which are critical to the etiology and enduring nature of alcohol use disorders.
METHODS AND MATERIALS
Additional methodological details are in Supplement 1.
Mice
Adult male C57BL/6J mice were singly (voluntary home-cage drinking) or group housed (operant self-administration) and treated in accordance with institutional and National Institutes of Health guidelines.
Voluntary Alcohol Drinking (Home-Cage)
Voluntary home-cage alcohol drinking was measured as described (28). Briefly, mice had home-cage ethanol (10% vol/vol) and water access for 24 days. Control mice had access to two water bottles. Alcohol and water intake were measured at 24-hour intervals. After the final access day, brains were processed for 2D-DIGE analysis, immunoblotting, or immunohistochemistry at the end of the 12-hour light cycle when blood alcohol content (BAC) was zero.
2D-DIGE Protein Expression Profiling of Amygdala
Amygdala punches (1 mm diameter) from alcohol or control mice were pooled (n = 3 mice per condition per sample) to obtain adequate protein for four 2D gels. Samples were sent to Applied Biomics, Inc (Hayward, California) for 2D-DIGE analysis under established protocols (Supplement 1 and www.appliedbiomics.com). Data are presented from protein spots that met a priori criteria: average relative change ≥25%, change present in all four gels, and mean change (alcohol versus water) was statistically significant. Proteins were identified by MALDI-TOF/TOF analysis (Applied Biosystems, Foster City, California).
Immunoblotting
Amygdala punches from alcohol- or water-drinking mice were prepared for evaluation of CaMKIIα, calcium/calmodulin dependent kinase II beta (CaMKIIβ), pCaMKIIThr286, and N-ethylmaleimide sensitive factor (NSF) protein expression via immunoblot as reported (28). Optical density of each band was measured using Scion imaging software (Scion Corporation, Frederick, Maryland). Values were expressed as percent of glyceraldehyde-3-phosphate dehydrogenase.
Immunohistochemistry
Coronal amygdala sections were prepared from home-cage alcohol- (n = 6) or water-drinking (n = 6) mice for immunohistochemical analysis of pCaMKIIa-Thr286 and pGluA1-Ser831 as previously reported (15).
Electrophysiology
Additional mice underwent the 24-day voluntary home-cage alcohol- or water-drinking procedure for whole-cell voltage clamp recordings from coronal sections of the medial central amygdala (CeA) as detailed in Supplement 1.
Operant Alcohol Self-Administration
Training
Alcohol or sucrose self-administration sessions were conducted in operant conditioning chambers. Lever-press behavior was facilitated using sucrose-fading procedures detailed in Supplement 1. Briefly, responses were trained with sucrose-only reinforcement and then maintained on a fixed-ratio 4 schedule of sweetened alcohol (9% vol/vol ethanol + 2% wt/vol sucrose) reinforcement. Control mice were trained with sucrose (2% wt/vol) reinforcement. After 30 baseline sessions, separate cohorts of mice were used for 1) site-specific microinjections, 2) immunoblotting, or 3) immunohistochemistry.
Microinjections
Following the 30th session, mice were surgically implanted with bilateral injector guides aimed 2 mm above the amygdala (29). After recovery, CaMKII inhibitors KN-93, myristoylated autocamtide-2-related inhibitory peptide (m-AIP), or myristoylated CamKIINtide (m-CAMKIINtide) or the AMPAR antagonist NBQX were infused in the amygdala before alcohol self-administration sessions in separate groups of alcohol or sucrose-control mice to assess regulation of alcohol reinforcement. Potential nonspecific effects were assessed in a locomotor activity assay. Data are presented from mice verified histologically to have injection sites in the amygdala.
RESULTS
Effects of Voluntary Alcohol Drinking on the Amygdala Proteome
Alcohol Drinking
C57BL/6J mice consumed alcohol (10% vol/vol) versus water under home-cage 24-hour two-bottle choice conditions (28) for 24 days (Figure 1A). Total fluid consumption did not differ between groups (Figure 1B, top). Alcohol-exposed mice exhibited preference for alcohol over water (>75%) and showed stable intake at a subdependence dosage (12.8 ± 1.3 g/kg per 24 hours) from days 6 to 24 of access (Figure 1B, bottom). BAC was .0 mg/dL at the onset of the 12-hour dark cycle and 39.1 mg/dL after 6 hours in the dark. Voluntary alcohol drinking produced no major health effects as evidenced by normal growth in alcohol (Δ weight = 1.7 ± .6 g/day) versus water (Δ weight = 2.1 ± .21 g/day) groups. Alcohol-drinking mice exhibited no change in plasma stress hormone level at the time of sacrifice (Figure 1C) or withdrawal symptoms (30), suggesting absence of dependence.
Figure 1.

Effects of voluntary alcohol drinking on the amygdala proteome. (A) Timeline of experimental procedures. (B) (top) Alcohol (EtOH) (n = 12) and water (H2O) (n = 12) exposed mice showed equal daily total fluid intake. (B) (bottom) Alcohol ntake stabilized after 5 days and reached a mean (±SEM) of 12.8 g/kg per day. (C) Alcohol drinking produced no change in levels of the stress hormone corticosterone (CORT) measured at the time of sacrifice. (D) Schematic (left) and photomicrograph (right) of mouse brain section showing location of amygdala tissue punch for two-dimensional differential in-gel electrophoresis (2D-DIGE) and immunoblot studies. (E) Representative 2D-DIGE gel showing differentially expressed proteins (circled) and labeled with spot numbers corresponding to Table 1. Red arrows point to N-ethylmaleimide sensitive factor (NSF) (spot 11) and calcium/calmodulin dependent kinase II alpha (CaMKIIα) (spot 19). (F) Standardized abundance (log) showing significant (p = .006, t test) alcohol-nduced change in CaMKIIα averaged across four replicate 2D gels. (G–I) Representative immunoblots (n = 6/group) and quantitative analysis showing that alcohol drinking significantly increased CaMKIIα and NSF but produced no change in calcium/calmodulin dependent kinase II beta (CaMKIIβ) in the amygdala (CeA) relative to water control mice. Data are relative to glyceraldehyde-3-phos-phate dehydrogenase (GAPDH). (J,K) Representative images and quantitative data showing alcohol drinking-nduced increase in CaMKIIα immunoreactivity (IR) in CeA and lateral amygdala (LA) as compared with H2O control mice. Data are expressed as mean (±SEM). Asterisks indicate significantly different from water (*p < .05; **p < .01), t test. IHC, immunohistochemistry; OD, optical density.
Differentially Expressed Proteins in Amygdala
Amygdala tissue punches (Figure 1D) were taken from alcohol and water-only exposed mice at the onset of the dark cycle on day 25 (Figure 1A). Tissue was pooled (n = 3 per sample per condition) and whole-cell lysates were compared between water and alcohol exposed mice on four replicate gels via 2D-DIGE. Differential in-gel image analysis using DeCyder software (GE Healthcare Bio-Sciences, Pittsburgh, Pennsylvania) identified 30 spots that met the a priori criterion for selection, and 29 were identified using MALDI-TOF/TOF (Figure 1E; Table 1, spots 2–30). Differential in-gel image analysis showed that expression ratios (alcohol/water) ranged from −2.03-fold to 1.72-fold with 14 proteins showing significant decreased expression and 15 showing increased expression (Table 1). Cross-gel normalization was conducted via comparison of an internal standard made up of equal amounts of each sample (alcohol + water) on each gel, which showed that CaMKIIα expression was increased more than five-fold in alcohol-exposed mice (Figure 1F).
Table 1.
Differentially Expressed Proteins Identified by MALDI-TOF/TOF Following 2D-DIGE Analysis of Amygdala from Moderate Alcohol-Drinking Mice Versus Water-Only Control Mice
| Gene ID | Accession # | Protein Name | Spot # | Cell Location | t Test p Value | Fold Change |
|---|---|---|---|---|---|---|
| Decreased Expression | ||||||
| CNRIP1 | 56789033 | Cannabinoid receptor interacting protein 1 | 30 | Cytoplasm | .0012 | −2.03 |
| ENO1 | 54673814 | Enolase 1, alpha non-neuron | 20 | Cytoplasm | .012 | −1.45 |
| HSPA8 | 55250073 | Heat shock protein 8 | 12 | Cytoplasm | .004 | −1.42 |
| FH | 33859554 | Fumarate hydratase 1 | 23 | Cytoplasm | .021 | −1.41 |
| PAFAH1B1 | 2104937 | Platelet-activating factor acetylhydrolase, isoform 1b, subunit 1 | 21 | Cytoplasm | .034 | −1.39 |
| GNB2 | 984551 | G protein beta 2 subunit | 25 | Membrane | .037 | −1.36 |
| ALDOA | 29747888 | Aldolase A, fructose-bisphosphate | 24 | Cytoplasm | .0017 | −1.35 |
| CMPK1 | 23821758 | UMP-CMP kinase (Cytidylate kinase) (Deoxycytidylate kinase) (Cytidine monophosphate kinase) | 29 | Nucleus | .016 | −1.35 |
| DSCAM | 19852058 | Down syndrome cell adhesion molecule-like protein | 15 | Membrane | .014 | −1.33 |
| PGAM1 | 12963669 | Phosphoglycerate mutase 1 | 27 | Cytoplasm | .021 | −1.32 |
| MDH1 | 319837 | Malate dehydrogenase 1 | 26 | Cytoplasm | .0007 | −1.31 |
| DPYSL2 | 40254595 | Dihydropyrimidinase-like 2 | 14 | Cytoplasm | .024 | −1.29 |
| AK1 | 15928666 | Adenylate kinase 1 | 28 | Cytoplasm | .00083 | −1.28 |
| CAP1 | 6671666 | Adenylate cyclase-associated protein 1 | 17 | Membrane | .0096 | −1.27 |
| Increased Expression | ||||||
| ALDH1L1 | 20380027 | Aldehyde dehydrogenase 1 family, member L1 | 7 | Cytoplasm | .005 | 1.26 |
| OLA1 | 21313144 | Obg-like ATPase 1 isoform a | 22 | Cytoplasm | .082 | 1.27 |
| HSP90AA1 | 57242925 | Heat shock protein 1, beta | 9 | Cytoplasm | .0009 | 1.29 |
| ADAM22 | 56790900 | A disintegrin and metalloprotease domain-containing protein 22 | 3 | Membrane | .01 | 1.31 |
| EEF2 | 33859482 | Eukaryotic translation elongation factor 2 | 8 | Cytoplasm | .0009 | 1.31 |
| BASP1 | 45598372 | Brain abundant, membrane attached signal protein 1 | 18 | Nucleus | .0094 | 1.33 |
| CCT7 | 14198388 | Chaperonin subunit 7 (eta) | 16 | Cytoplasm | .003 | 1.34 |
| KIF5C | 45433560 | Kinesin family member 5C | 4 | Cytoplasm | .0048 | 1.35 |
| CAMK2A | 28916677 | Calcium/calmodulin-dependent protein kinase II alpha | 19 | Cytoplasm | .0038 | 1.35 |
| ACLY | 29293809 | ATP citrate lyase | 5 | Cytoplasm | .002 | 1.42 |
| NSF | 1171774 | N-ethylmaleimide sensitive fusion protein | 11 | Cytoplasm | .005 | 1.42 |
| LRPPRC | 37589470 | Leucine-rich PPR motif-containing protein | 2 | Cytoplasm | .044 | 1.43 |
| STXBP1 | 6678179 | Syntaxin binding protein 1 | 13 | Cytoplasm | .002 | 1.44 |
| IMMT | 29427692 | Mitochondrial inner membrane protein | 10 | Cytoplasm | .01 | 1.45 |
| HK1 | 30316354 | Hexokinase, type I | 6 | Cytoplasm | .005 | 1.72 |
Data are sorted in ascending order of fold change (alcohol/water control expression) with positive and negative values indicating an increase or decrease in protein expression, respectively, in the alcohol versus water group. p values represent results of t tests comparing mean protein expression between alcohol and water groups (n = 4 parallel two-dimensional gels).
ATP, adenosine triphosphate; ATPase, adenosine triphosphatase; 2D-DIGE, two-dimensional differential in-gel electrophoresis; MALDI-TOF/TOF, matrix assisted laser desorption ionization tandem time-of-flight.
Bioinformatics
Protein identifiers, fold-change, and p values were submitted to Ingenuity Pathway Analysis (IPA) (Ingenuity Systems, Redwood City, California) for dataset enrichment. Cellular location of proteins was classified as follows: nucleus (2 proteins), cytoplasm (22 proteins), plasma membrane (4 proteins), and other (1 protein) (Table 1). Two networks of known protein-protein interactions related to cell signaling (14 proteins) and cell morphology (8 proteins) were associated with alcohol drinking (Table 2). Protein-protein interactions within the alcohol-sensitive network related to cell signaling and interaction, nervous system development and function, and behavior were visualized using the IPA pathway designer and indicate key interactions, including CaMKIIα modulation of AMPAR GRIA1 (GluA1) subunit (Figure S1 in Supplement 1). Functional clusters were identified by IPA related to known diseases and disorders, molecular and cellular functions, and canonical pathways with potential upstream regulators also suggested (Table 2).
Table 2.
Involvement of Differentially Expressed Proteins in Neural Networks and Functions Identified by Ingenuity Pathway Analysis
| Networks | Focus Proteins | Protein Network | |
|---|---|---|---|
| Cell Signaling and Interaction, Nervous System Development and Function, Behavior | 14 | AK1↓, ALDH1L1↑, ALDOA↓, APP, ATP, BASP1↑, CAMK2A↑, CAP1↓, CAV1, CDK5R1, DSCAM↓, EEF2↑, EGFR, EIF2AK2, ENO1↓, F2R, FABP3, FMR1, GDI1, GRIA1, GRIA2, HK1↑, HSP90AA1↑, HSPA8↓, IFNG, MAP1B, MAP2K1, MAP2K2, MECP2, MMP9, NRGN, NSF↑, PGAM1↓, progesterone, SNAP25 | |
| Cell Morphology, Cell Assembly and Organization, Cell Development | 8 | ADAM22↑, APP, CAMK2A↑, CDK5R1, DAB1, DCX, DLG2, DLG4, DPYSL2↓, GNB2↓, GRIN2A, GRIN2B, HTR2C, INA, KCNA2, KIF5B, KIF5C↑, KLC1, KLC2, LGI1, MAP1B, MBP, MT3, NEFL, NF1, NOS1, NSF↑, PAFAH1B1↓, PAFAH1B2, RAC1, RHOA, RTN4, RTN4R, STXBP1↑, TNIK | |
| Diseases and Disorders | Function | p Value | Proteins |
| Developmental Disorder | Delay of brain development | .002 | PAFAH1B1↓ |
| Neuropsychiatric Disorder | Bipolar disorder | .009 | CAMK2A↑, DPYSL2↓ |
| Schizophrenia | .02 | DPYSL2↓, MDH1↓, PGAM1↓ | |
| Depression | .03 | CAMK2A↑, DPYSL2↓, ENO1↓, HK1↓ | |
| Molecular and Cellular | Function | p Value | Proteins |
| Cellular Development, Function, and Maintenance | Microtubule dynamics Formation of cellular projections | .004 .001 |
AK1↓, ADAM22↑, BASP1↑, CAMK2A↑, DPYSL2↓, DSCAM↓, HSPA8↓, HSP90AA1↑, PAFAH1B1↓, KIF5C↑ |
| Cell-To-Cell Signaling | Synaptic transmission and plasticity, LTP | .004 | CAMK2A↑, DPYSL2↓, EEF2↑, KIF5C↑, NSF↑, PAFAH1B1↓, STXBP1↑ |
| Metabolism | Alcohol catabolic process Glucose catabolic process |
.00001 3.0E-6 |
ALDOA↓, ENO1↓, HK1↑, MDH1↑, PGAM1↓ |
| Top Canonical Pathways | p Value | Proteins | |
| Glucogenesis/Glycolysis | 2.0E-6 | ALDOA↓, ENO1↓, HK1↑, MDH1↓, PGAM1↓ | |
| Krebs Cycle | .002 | FH↓, MDH↓ | |
| Top Upstream Regulators | p Value | Target Proteins in Dataset | |
| MAPT (microtubule-associated protein tau) PSEN1 (presenilin 1) APP (amyloid beta (A4) precursor protein) |
3.9E-12 1.8E-10 1.2E-09 |
ALDOA↓, BASP1↑, CAP1↓, DPYSL2↓, ENO1↓, HK1↑, HSP90AA1↑, HSPA8↓, PGAM1↓, STXBP1↑ | |
| FMR1 (fragile X mental retardation syndrome 1) | 6.2E-09 | ALDOA↓, BASP1↓, CAMK2A↑, EEF2↑, ENO1↓ | |
| HTT (huntingtin) | 1.3E-03 | BASP1↑, CAMK2A↑, HSPA8↓, NSF↑, STXBP1↑ | |
Focus proteins are in bold font. Other proteins (regular font) are included in networks based on known protein interactions in the Ingenuity Pathway Analysis database. Arrows indicate direction of change in protein expression (↓ downregulated, ↑ upregulated) induced by alcohol drinking, as shown in Table 1. Protein clusters with multiple functions are noted by brackets. p values were derived in Ingenuity Pathway Analysis by right-tailed Fisher exact test and indicate relative overrepresentation of proteins in a given function than what is expected by chance.
LTP, long-term potentiation.
Independent Confirmation of 2D-DIGE Changes
Immunoblot analysis in separate mice confirmed that alcohol drinking (intake: 12.85 ± .9 g/kg per 24 hours; 69.5 ± 3.0% preference) increased CaMKIIα expression (+60%, t10 = 2.5, p = .015) in amygdala (Figure 1G) with no change in CaMKIIβ (Figure 1H). Additionally, there was a trend for an increase in pCaMKIIα-Thr286 expression after alcohol drinking (water: 100 ± 15.4; alcohol: 132.9 ± 37.8, p = ns) but expression of pCaMKIIa-Thr286 relative to total CaMKIIα was unchanged (water: 66.24% ± 12.04; alcohol: 58.6% ± 22.84, p = ns). NSF, which regulates presynaptic vesicle release and postsynaptic stabilization of AMPARs (31), was increased in the 2D-DIGE experiment (Table 1) and showed an alcohol-induced increase (+40%, t10 = 2.0, p = .042) (Figure 1I) on protein immunoblots.
Prefrontal cortex, nucleus accumbens (NAcb), dorsal striatum, and motor cortex were also assessed for altered CaMKIIα or CaMKIIβ expression. Alcohol drinking increased CaMKIIα expression only in NAcb (p = .026, t test) with no changes in CaMKIIβ (Table S1 in Supplement 1).
Alcohol Drinking Increased CaMKIIα Immunoreactivity in Amygdala Subregions
Immunohistochemistry was used to characterize potential subregional effects of alcohol drinking on CaMKIIα expression. CaMKIIα immunoreactivity (IR) (positive cells per mm2) was increased in the CeA (Figure 1J, t5 = 3.9, p = .01) and lateral amygdala (LA) (Figure 1K, t5 = 3.0, p = .02) but not basolateral amygdala (BLA) of alcohol-drinking mice, demonstrating subregional specificity.
Increased CaMKIIα Phosphorylation by Alcohol Reinforcement
To determine if alcohol’s positive reinforcing effects are associated with altered activation of CaMKIIα, mice were trained to self-administer alcohol (9% vol/vol + sucrose 2% wt/vol) or sucrose only (2% wt/vol) in 1-hour sessions (32). Brains were processed for pCaMKIIα-Thr286 IR immediately after the 30th session (Figure 2A). The two groups showed equivalent lever-pressing behavior (Figure 2B), indicating that differences in pCaMKIIα-Thr286 IR are unrelated to behavioral output or reward exposure. Average alcohol intake was 1.14 ± .1 g/kg per hour (Figure 2C), which resulted in BAC = 61 ± 10 mg/dL per hour (Figure 2D). Under these behavior-matched conditions, alcohol self-administration increased pCaMKIIα-Thr286 IR by fivefold in the CeA (Figure 2E, t12 = 2.2, p = .02) and 1.6-fold in LA (Figure 2F, t12 = 1.9, p = .04) with no change in BLA (data not shown) relative to sucrose control mice, indicating that CaMKIIα activation in the CeA and LA is a target of self-administered alcohol. There was no change in total CaMKIIα IR in any amygdala subregion immediately following operant self-administration (Table S2 in Supplement 1), suggesting alcohol-induced activation of extant CaMKIIα.
Figure 2.
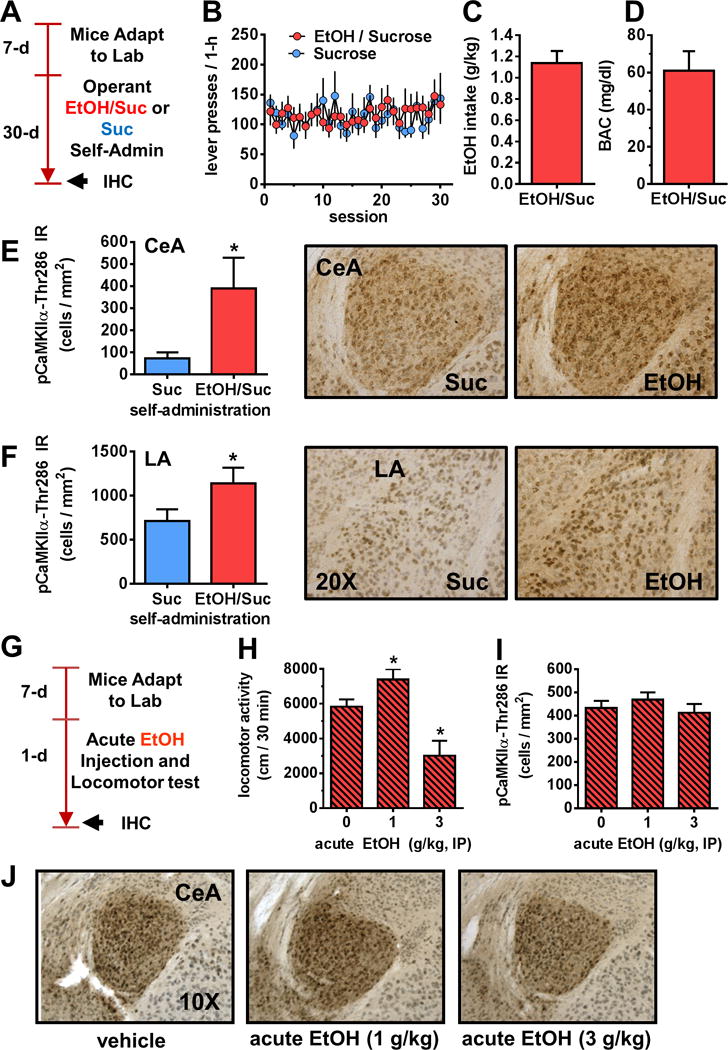
Operant alcohol self-administration, but not acute injection, increases calcium/calmodulin dependent kinase II alpha (CaMKIIα)-Thr286 phosphorylation in the amygdala. (A) Timeline of experimental procedures evaluating CaMKIIα phosphorylation after operant alcohol self-administration (Self-Admin). (B) Total alcohol (EtOH) (n = 7) and sucrose (Suc) (n = 7) reinforced lever-presses as a function of daily 1-hour behavioral test sessions. (C,D) Dosage of EtOH self-administered (g/kg) and resultant blood alcohol concentration (BAC) immediately after the 1-hour session. (E,F) Representative photomicrographs of the cytological pattern of phosphorylated CaMKIIα (pCaMKIIα)-Thr286 immunoreactivity (IR) in the central amygdala (CeA) and lateral amygdala (LA) (right) and analysis (left) showing that operant alcohol self-administration increased pCaMKIIα-Thr286 IR (cells/mm2) in both the CeA and LA. Data represent mean (±SEM) of multiple brain sections from n = 7 mice per condition (*p < .05), t test. (G) Timeline of experiment evaluating effects of acute ethanol injection on locomotor activity and CaMKIIα-Thr286 immunoreactivity. (H–J) Acute EtOH (0–3 g/kg) produced a biphasic effect on locomotor activity (*p < .05 vs. EtOH [0 g/kg] Dunnett’s test) but no effect on CaM-KIIα-Thr286 phosphorylation in the CeA or LA (not shown). IHC, immuno-histochemistry; IP, intraperitoneal.
No Effect of Acute Alcohol on CaMKIIα Phosphorylation in the Amygdala
C57BL/6J mice were administered alcohol (0–3 g/kg, intraperitoneal) before a 30-minute locomotor test (Figure 2G). Acute alcohol produced a biphasic effect (F2,29 = 11.5, p = .002) on locomotor activity (Figure 2H) but did not alter pCaMKIIα-Thr286 IR in the CeA (Figure 2I–J) or LA (mean ± SEM cells/mm2: 0 mg/kg = 784.6 ± 56.37; 1 mg/kg = 747.1 ± 86.76; 3 mg/kg = 840.5 ± 173.1) immediately after locomotor sessions.
CaMKII Activity in the Amygdala Is Required for Alcohol Reinforcement
To determine if CaMKII activity in the amygdala regulates the positive reinforcing effects of alcohol, we site-specifically inhibited CaMKII in the amygdala before operant self-administration sessions (33–35) (Figure 3A,B). The cell-permeable CaMKII inhibitor KN-93 (10 μg/.5 μL, n = 8 mice) decreased alcohol-reinforced responding (t7 = 2.5, p = .04) without altering locomotor activity (Figure 3C). Since KN-93 also blocks voltage-gated potassium channels in a CaMKII-independent manner, we examined effects of the selective cell-permeable inhibitor m-AIP, which binds to the calmodulin site and inhibits CaMKII activation (36). Intra-amygdala infusion of m-AIP produced a dose-dependent reduction in alcohol-reinforced responding (F3,6 = 10.4, p = .002) with no effect on sucrose self-administration or locomotor activity (Figure 3D). Infusion of scrambled m-AIP had no effect (Figure 3D, left). We also examined effects of CaMKIIN-tide, an endogenous peptide inhibitor that blocks both CaMKII activation and autophosphorylation (37). Intra-amygdala infusion of CaMKIINtide significantly reduced alcohol-reinforced responding (F3,18 = 5.3, p = .0026) with no effect on sucrose reinforcement or locomotor activity (Figure 4E). Scrambled CaMKIINtide had no effect (Figure 3E, left).
Figure 3.

Calcium/calmodulin dependent kinase II (CaMKII) activity in the amygdala is required for the reinforcing effects of alcohol. (A) Procedural timeline showing number of days during each experimental phase of alcohol (EtOH) and sucrose (Suc) self-administration (Self-Admin). (B) Illustration of drug infusion sites in the amygdala from alcohol/sucrose (red) and sucrose (blue) self-administering mice. (C) Total EtOH reinforced responses (n = 8) during 1-hour sessions plotted as a percentage of artificial cerebrospinal fluid (ACSF) control (left) and locomotor activity plotted as a function of KN-93 dosage (*p < .05 vs. vehicle, t test). (D,E) Effects of CaMKII peptide inhibitors (myristoylated autocamtide-2-related inhibitory peptide [m-AIP] and CaMKII Ntide) on alcohol (m-AIP n = 7; Ntide n = 6) and sucrose (m-AIP n = 7; Ntide n = 5) self-administration and locomotor activity. Figures represent EtOH (left) and sucrose (middle) reinforced responses and locomotor activity (right) during 1-hour sessions plotted as a function of m-AIP (D) or CaMKII Ntide (E) dosage. Scrambled peptide (S) was tested at a single dose for each inhibitor as negative controls to show that a specific sequence rather than the amino acid composition is critical for activity. *p < .05 versus vehicle, Dunnett’s multiple comparison test. [Images in (B) are based on coordinates from Paxinos and Franklin (40)].
Figure 4.

Effect of voluntary alcohol drinking on α-amino-3-hydroxy-5-methyl-4-isooxazole receptor (AMPAR) GluA1-ser831 phosphorylation and glutamate-mediated synaptic activity in the central amygdala (CeA). (A) Timeline of experimental procedures evaluating effects of voluntary home-cage alcohol (EtOH) drinking on GluA1-ser831 immunoreactivity (IR) and glutamate-mediated synaptic activity in the CeA. (B) Confocal microscopy image showing immuno-cytochemical co-expression of calcium/calmodulin dependent kinase II alpha (CaMKIIα) and AMPA GluA1 subunit in CeA of untreated mice. (C,D) Immunohistochemistry (IHC) results showing increased activation (e.g., phosphorylation) of GluA1 at the CaMKIIα recognition site (pGluA1-Ser831) in alcohol-drinking mice in the CeA but no change in the lateral amygdala (LA). (E) Photomicrograph showing the cytological expression of pGluA1-ser831 in subregions of the amygdala. (F) Significant increase in AMPA/N-methyl-D-aspartate (NMDA) ratio and AMPA-mediated spontaneous excitatory postsynaptic current (sEPSC) frequency as a function of alcohol drinking. (G) Tracings show currents from water (H2O)- (n = 8) and alcohol- (n = 8) drinking mice. Asterisks indicate the following: *p < .05; **p < .001, t test. BLA, basolateral amygdala.
Alcohol Drinking Increases AMPAR GluA1 Phosphorylation and Synaptic Activity in CeA
A primary function of CaMKII is to phosphorylate the AMPAR GluA1 subunit at Ser831, which leads to potentiation of AMPAR-mediated synaptic activity and regulation of behavioral processes (38,39) (Figure S1 in Supplement 1). Thus, we sought to determine if voluntary home-cage alcohol dinking increases AMPAR GluA1 phosphorylation and synaptic activity (Figure 4A). Dual-label fluorescent immunohistochemistry with confocal visualization showed that CeA neurons co-express CaMKIIα and GluA1 (Figure 4B). Further, home-cage alcohol drinking (24 days) increased pGluA1-Ser831 IR (CaMKII recognition site) in the CeA (Figure 4C,E; t12 = 2.5, p = .026) but not in the LA (Figure 4D,E; t14 = .5, p = .65). To determine if increased GluA1-Ser831 activation is associated with increased synaptic activity, we examined effects of voluntary alcohol drinking on glutamatergic synaptic activity in the CeA. C57BL/6J mice consumed alcohol (10% vol/vol; mean intake: 13.2l ± l.25 g/kg per day) or water for 24 days under the two-bottle drinking procedure. AMPA/N-methyl-D-aspartate (NMDA) ratio was increased in CeA neurons from alcohol mice (Figure 4F,G; t27 = 3.1, p = .004). Paired-pulse ratio was not different between alcohol (1.29 ± .19) and control mice (1.45 ± .13). However, CeA neurons from alcohol mice showed increased spontaneous excitatory postsynaptic current (sEPSC) frequency (Figure 4F,G; t32 = 2.7, p = .009) with no change in amplitude (alcohol: 21.4 ± 1.2, water: 21.0 ± 1.3).
AMPAR Activity in the CeA Regulates the Reinforcing Effects of Alcohol
To determine if AMPAR activity in the amygdala is required for alcohol reinforcement, we investigated whether operant self-administration of alcohol is associated with increased GluA1 activation. Mice were trained to lever-press with sweetened alcohol or sucrose reinforcement. The two groups were behavior-matched and earned an equal number of reinforcers, and alcohol intake was 1.3 ± .1 g/kg per hour. Immediately after the 30th self-administration session, brains were processed for pGluA1-Ser831 IR (Figure 5A). Operant alcohol self-administration increased pGluA1-Ser831 IR by twofold in CeA (Figure 5B,D; t14 = 2.2, p = .04) and LA (Figure 5C,D; t14 = 2.1, p = .03) relative to sucrose, indicating that GluA1-containing AMPARs in the CeA are a target of self-administered alcohol. Separate mice were then surgically implanted with guide cannulae and the competitive AMPAR antagonist NBQX (0–3 μg/.5 μL, n = 10) was infused in CeA before operant self-administration sessions (Figure 5A,E). NBQX infusion in CeA significantly reduced sweetened alcohol-self-administration (Figure 5F; t9 = 5.8, p = .0003) but had no effect on sucrose-reinforced responding or open-field locomotor activity in separate tests (data not shown).
Figure 5.
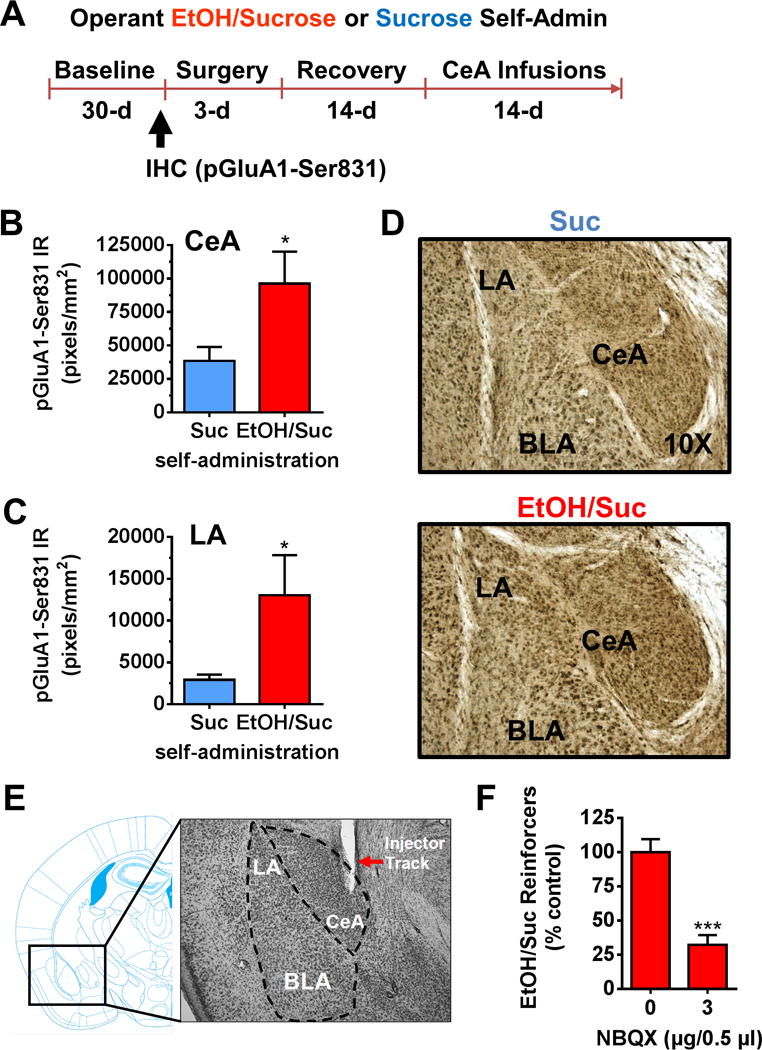
Operant alcohol self-administration increases α-amino-3-hydroxy-5-methyl-4-isooxazole receptor (AMPAR) GluA1-ser831 phosphorylation in amygdala subregions and AMPAR activity is required for the reinforcing effects of alcohol (EtOH). (A) Procedural timeline showing number of days during each experimental phase of alcohol and sucrose (Suc) self-administration (Self-Admin). (B,C) Operant alcohol self-administration (n = 7) increased AMPAR pGluA1-Ser831 immunoreactivity (IR) in the central amygdala (CeA) and lateral amygdala (LA) as compared with sucrose control mice (n = 7). (D) Representative images of coronal sections of the amygdala showing subregional specificity of alcohol-nduced increase in pGluA1-Ser831 from sucrose and EtOH + sucrose (EtOH/Suc) self-administering mice. (E) Representative photomicrograph of coronal brain section showing injector track in CeA juxtaposed with mouse brain atlas. (F) Intra-amygdala infusion of the AMPAR antagonist NBQX significantly reduced alcohol self-administration (n = 10) during 1-hour session. Asterisks indicate the following: *p < .05; ***p < .001, t test. BLA, basolateral amygdala; IHC, immunohistochemistry.
DISCUSSION
Effects of Voluntary Alcohol Drinking on the Amygdala Proteome
This study was designed to increase understanding of the etiology of alcohol addiction by identifying protein networks that are targeted by voluntary alcohol drinking in the amygdala, a key set of nuclei that mediate drug reward and reinforcement (8). A high throughput proteomic screen identified 29 individual proteins and two networks that were differentially expressed after 24 days of alcohol drinking in nondependent mice. IPA identified significant overexpression of alcohol-sensitive proteins in networks that regulate bipolar disorder, schizophrenia, and depression, suggesting novel pathways by which alcohol drinking may alter the predisposition or course of neuropsychiatric disorders (41). Moderate alcohol drinking also altered proteins associated with cell morphology, energy balance, and metabolism, which are critical to brain function. Potential upstream regulators were linked to Alzheimer’s disease, fragile X syndrome, and Huntington’s disease indicating that moderate alcohol use may interact with a variety of conditions across the life span (41).
These findings support prior proteomic and genomic work. For instance, alcohol drinking reduced HSPA8 in the amygdala of alcohol-preferring rats (18). DPYSL2 (CRMP-2), ENO1, HK1, HSP90AA1, MDH1, PGAM1, and STXBP1 were altered in cortex and midbrain of alcohol-dependent, nondependent, and control C57BL/6J mice (19). ENO1, HSPA8, PGAM1, and STXBP1 were reported in at least four alcohol-related proteomic studies [reviewed by (22)]. Genomic studies of alcohol response identified 13 of the 29 alcohol-sensitive targets detected in this study, including Kif5c, NSF, PAFAH1B1, and STXBP1, in rodents and humans (Table S3 in Supplement 1). These findings suggest that effects of alcohol drinking we observed in nondependent mice may translate to later stages of alcohol addiction as modeled in dependent rodents and directly assessed in postmortem human alcoholic studies.
Importantly, protein networks that regulate cell signaling and plasticity were altered by alcohol drinking. Prominent in this cluster was CaMKIIα, which is a principal mechanism of synaptic function including driving AMPARs to the synapse during long-term potentiation (42,43). Proteins linked to CaM-KII and AMPAR function were also increased, including KiF5C, a molecule that shuttles CaMKIIα and AMPAR GluA2 subunits to the postsynaptic density (44); EEF2, an elongation factor that modulates CaMKIIα messenger RNA translation (45); and NSF, an adenosine triphosphatase that stabilizes synaptic AMPARs (31). Additionally, NSF and STXBP-1 play significant roles in calcium (Ca2+)-dependent synaptic vesicle exocytosis during neurotransmitter release (46) and alcohol drinking (47). Collectively, these proteins represent potential cellular machinery for increasing CaMKII expression or activity and upregulation of AMPAR synaptic activity, which suggests enhanced glutamate transmission in the early stages of alcohol addiction.
Effect of Alcohol Drinking and Operant Self-Administration on Amygdala CaMKIIα Expression and Activation
CaMKII is a family of Ca2+-activated Ser/Thr protein kinases that mediate many intracellular responses in the brain including regulation of ion channels, neurotransmitter synthesis and release, and synaptic plasticity (16). Here, we sought to determine if alcohol drinking alters CaMKII protein expression and activation in brain regions known to regulate behavioral plasticity and drug self-administration. Results showed that moderate drinking increased CaMKIIα expression in mouse amygdala and NAcb, brain regions that regulate alcohol reinforcement (48,49). No change occurred in CaMKIIβ or in CaMKII (α or β) expression in other brain regions examined. Further, immunohistochemistry results showed that alcohol drinking-induced increases in CaMKIIα expression in the amygdala were localized to the CeA and LA, indicating subregional specificity. Since CaMKII expression characterizes amygdala projection neurons (50), these results may indicate alcohol-enhanced neuronal output from LA and CeA to target regions, such as the NAcb.
CaMKIIα is activated when neuronal depolarization leads to Ca2+ entry in the cell. Calmodulin then binds calcium and the Ca2+/calmodulin complex leads to CaMKIIα phosphorylation at Thr286 (pCaMKIIa-Thr286). CaMKIIα activation in the amygdala is required for certain types of behavioral plasticity (51). In our experiments, lever-press responding maintained by alcohol increased pCaMKIIα-Thr286 IR in the CeA and LA as compared with behavior-matched sucrose control mice, indicating that differential CaMKIIα phosphorylation is alcohol-specific and not associated with general learning or context effects. Moreover, acute alcohol injection had no effect, suggesting that CaMKIIα phosphorylation in the amygdala may represent an adaptive response to alcohol self-administration or repeated exposure. Given the critical role of CaMKIIα in associative learning and memory (52), these findings suggest that alcohol drinking-induced upregulation of CaMKIIα expression and activation may reflect increased learning about the drug and associated stimuli.
CaMKII Activation in the Amygdala: A Novel Mechanism of Alcohol’s Reinforcing Effects
CaMKII is an ideal molecular candidate for initiating and sustaining maladaptive plasticity in reinforcement processes in the early stages of alcohol addiction due to its prominent role in normal, or adaptive, cellular and behavioral plasticity (53). However, the extent to which CaMKIIα regulates the positive reinforcing effects of alcohol was unknown. To address this gap in knowledge, we assessed the effects of site-specific pharmacologic inhibition of CaMKII activity in the amygdala of separate groups of behavior-matched mice trained to lever-press for sweetened alcohol or sucrose only reinforcement. Results showed that bilateral microinjection of the CaMKII inhibitor KN-93 in the amygdala significantly decreased alcohol-reinforced lever presses in the absence of nonspecific motor effects. To further delineate the mechanistic role of CaMKII in alcohol reinforcement, we evaluated effects of the highly selective CaMKII inhibitor m-AIP, which binds directly to the calmodulin site and prevents initial CaMKII activation (e.g., phosphorylation) (35). m-AIP produced a dose-dependent reduction in alcohol-reinforced responses without effect on behavior-matched sucrose control mice or locomotor activity. No effects of scrambled m-AIP were observed on alcohol reinforcement. Second, behavioral tests were conducted following amygdala infusion of m-CaMKIIN-tide, a cell-permeable peptide inhibitor that blocks both Ca2+/calmodulin phosphorylation and Ca2+-independent autophos-phorylation of CaMKII (36). m-CaMKIINtide produced a dose-dependent and selective reduction in alcohol-reinforced responding with similar potency and efficacy of m-AIP. These data indicate that the positive reinforcing effects of alcohol require initial Ca2+-calmodulin activation of CaMKII in the amygdala and that Ca2+-independent autophosphorylation may not regulate alcohol reinforcement.
Alcohol Drinking Increases AMPAR-Mediated Synaptic Activity in the CeA
Following activation, cytoplasmic CaMKII can translocate to the cell membrane where it potentiates AMPAR-mediated activity via phosphorylation of GluA1-Ser831 (26,37). Results of this study show that neurons in the CeA co-express CaMKIIα and GluA1 and that alcohol drinking increases pGluA1-Ser831 IR in the CeA, suggesting an anatomical basis by which alcohol drinking might increase AMPAR activity.
To address potential changes in AMPAR function, we measured AMPA/NMDA ratio as an index of synaptic strength (54,55). Alcohol-drinking mice showed increased AMPA/NMDA ratios, suggesting that voluntary alcohol intake increases synaptic drive in the CeA. Since exposure to chronic ethanol vapor increases ethanol-induced glutamate levels in CeA (9), we measured response to paired pulses as an index of probability of transmitter release (56). Paired-pulse ratio was not different between alcohol and water control mice. Spontaneous excitatory postsynaptic currents were also examined to characterize potential synaptic sites of action. Alcohol-drinking mice showed an increase in sEPSC frequency but no change in amplitude. An increase in the frequency of sEPSCs in the absence of change in paired-pulse ratio may indicate an increase in the number of functional AMPARs at previously silent synapses (55,57). A potential mechanism for increased AMPA-mediated synaptic activity is that alcohol increased expression of NSF in the amygdala (Tables 1 and 2; Figure 1), which promotes surface expression and increased function of AMPARs (31,58). This is complementary to evidence showing that alcohol dependence is associated with increased excitatory postsynaptic current amplitude, GluA1 surface expression, GluA1-Ser831 phosphorylation, and CaMKIIα-Thr286 phosphorylation in rat BLA (11). Together, these findings suggest that voluntary alcohol drinking during the nondependent state initiates molecular adaptations in amygdala CaMKIIα and AMPAR signaling that subsequently characterize dependence.
AMPARs in the Amygdala Regulate the Reinforcing Effects of Alcohol
To determine if AMPAR activity in the amygdala regulates the positive reinforcing effects of alcohol, we first examined pGluA1-Ser831 IR in the amygdala immediately after an operant alcohol self-administration session. pGluA1-Ser831 IR was increased in the CeA and LA by twofold as compared with sucrose control mice, indicating a specific effect of alcohol on AMPAR GluA1 activation that is independent of motor activity or general reinforcement processes. Next, we infused the AMPAR antagonist NBQX (0 or 3 μg/μL) in the amygdala before self-administration sessions. NBQX significantly reduced operant alcohol self-administration but had no effect in parallel behavior-matched sucrose-only control mice or in locomotor activity tests. These results indicate that operant alcohol self-administration rapidly increases AMPAR phosphorylation at a CaMKII substrate (GluA1-Ser831) and that AMPAR activity in the amygdala is required for alcohol’s positive reinforcing effects. Given the prepotency of positive reinforcement during the initial stages of addiction, this suggests that AMPAR activity in the amygdala may play a critical role in the etiology of alcoholism. It will be important to extend the current work to determine if identified mechanisms of positive reinforcement regulate aspects of pathological drinking in rodent models that characterize abuse and dependence (59).
Conclusion
Alcohol addiction is a pathological progression that ranges from initial drug use to abuse. In this study, we sought to shed new light on the neurobiological changes that occur in the brain during the initial use stage of alcohol addiction. Taking an unbiased proteomic approach, we found that a relatively short duration (24 days) of voluntary alcohol drinking altered expression of key plasticity-linked protein networks in mouse amygdala that, in turn, regulate the positive reinforcing properties of alcohol. Functional proteomic studies that identify the pleiotropic neural targets of initial alcohol use and validate their mechanistic significance in animal behavioral models are essential for a complete understanding of the etiology of alcohol addiction.
Supplementary Material
Acknowledgments
This work was supported by Grants R37 AA014983 (CWH), F31 AA022028 (MCS) and P60 AA11605 (CWH and TLK) from the National Institute on Alcohol Abuse and Alcoholism and by the Bowles Center for Alcohol Studies at the University of North Carolina at Chapel Hill.
The authors would like to thank Drs. David F. Werner and Michael Chua for expert technical assistance.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2014.10.020.
References
- 1.Lowenfels AB. Epidemiologic studies of alcohol-related disease in the 20th century. J Epidemiol Biostat. 2000;5:61–66. [PubMed] [Google Scholar]
- 2.Grant BF. Alcohol consumption, alcohol abuse and alcohol dependence. The United States as an example. Addiction. 1994;89:1357–1365. doi: 10.1111/j.1360-0443.1994.tb03730.x. [DOI] [PubMed] [Google Scholar]
- 3.Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2013;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, et al. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. J Neurosci. 2012;32:15124–15132. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 7.Winder DG, Egli RE, Schramm NL, Matthews RT. Synaptic plasticity in drug reward circuitry. Curr Mol Med. 2002;2:667–676. doi: 10.2174/1566524023361961. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF. Neuroadaptive mechanisms of addiction: Studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: An in vitro and in vivo analysis. J Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology. 2006;31:988–996. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- 11.Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology. 2012;62:2430–2439. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2011;61:1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Gass JT, Sinclair CM, Cleva RM, Widholm JJ, Olive MF. Alcohol-seeking behavior is associated with increased glutamate transmission n basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol. 2011;16:215–228. doi: 10.1111/j.1369-1600.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: Blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 17.Chandler LJ. Ethanol and brain plasticity: Receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther. 2003;99:311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 18.Bell RL, Kimpel MW, Rodd ZA, Strother WN, Bai F, Peper CL, et al. Protein expression changes in the nucleus accumbens and amygdala of inbred alcohol-preferring rats given either continuous or scheduled access to ethanol. Alcohol. 2006;40:3–17. doi: 10.1016/j.alcohol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Gorini G, Roberts AJ, Mayfield RD. Neurobiological signatures of alcohol dependence revealed by protein profiling. PLoS One. 2013;8:e82656. doi: 10.1371/journal.pone.0082656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander-Kaufman K, Cordwell S, Harper C, Matsumoto I. A proteome analysis of the dorsolateral prefrontal cortex in human alcoholic patients. Proteomics Clin Appl. 2007;1:62–72. doi: 10.1002/prca.200600417. [DOI] [PubMed] [Google Scholar]
- 21.Lewohl JM, Van Dyk DD, Craft GE, Innes DJ, Mayfield RD, Cobon G, et al. The application of proteomics to the human alcoholic brain. Ann N Y Acad Sci. 2004;1025:14–26. doi: 10.1196/annals.1316.002. [DOI] [PubMed] [Google Scholar]
- 22.Gorini G, Bell RL, Mayfield RD. Molecular targets of alcohol action: Translational research for pharmacotherapy development and screening. Prog Mol Biol Transl Sci. 2011;98:293–347. doi: 10.1016/B978-0-12-385506-0.00007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 24.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 25.Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 26.Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the α-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 27.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 28.Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, et al. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- 29.Hodge CW, Haraguchi M, Erickson H, Samson HH. Ventral tegmental microinjections of quinpirole decrease ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1993;17:370–375. doi: 10.1111/j.1530-0277.1993.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 30.Besheer J, Lepoutre V, Hodge CW. Preclinical evaluation of riluzole: Assessments of ethanol self-administration and ethanol withdrawal symptoms. Alcohol Clin Exp Res. 2009;33:1460–1468. doi: 10.1111/j.1530-0277.2009.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, et al. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- 32.Faccidomo S, Besheer J, Stanford PC, Hodge CW. Increased operant responding for ethanol in male C57BL/6J mice: Specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology (Berl) 2009;204:135–147. doi: 10.1007/s00213-008-1444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2010;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodge CW, Chappelle AM, Samson HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1996;20:1631–1638. doi: 10.1111/j.1530-0277.1996.tb01709.x. [DOI] [PubMed] [Google Scholar]
- 35.Sansom AJ, Smith PF, Darlington CL, Laverty R. The effects of protein kinase C and calmodulin kinase II inhibitors on vestibular compensation in the guinea pig. Brain Res. 2000;882:45–54. doi: 10.1016/s0006-8993(00)02786-4. [DOI] [PubMed] [Google Scholar]
- 36.Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci U S A. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Mueller TI, Lavori PW, Keller MB, Swartz A, Warshaw M, Hasin D, et al. Prognostic effect of the variable course of alcoholism on the 10-year course of depression. Am J Psychiatry. 1994;151:701–706. doi: 10.1176/ajp.151.5.701. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Franklin KBJ. The Mouse Brain in Sterotaxic Coordinates. 2. New York: Academic Press; 2001. [Google Scholar]
- 41.Quaid KA, Dinwiddie H, Conneally PM, Nurnberger JI., Jr Issues n genetic testing for susceptibility to alcoholism: Lessons from Alzheimer’s disease and Huntington’s disease. Alcohol Clin Exp Res. 1996;20:1430–1437. doi: 10.1111/j.1530-0277.1996.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: Requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 43.Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 44.Hirokawa N. mRNA transport in dendrites: RNA granules, motors, and tracks. J Neurosci. 2006;26:7139–7142. doi: 10.1523/JNEUROSCI.1821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheetz AJ, Nairn AC, Constantine-Paton M. NMDA receptor-mediated control of protein synthesis at developing synapses. Nat Neurosci. 2000;3:211–216. doi: 10.1038/72915. [DOI] [PubMed] [Google Scholar]
- 46.Rizo J, Sudhof TC. Snares and Munc18 in synaptic vesicle fusion. Nat Rev Neurosci. 2002;3:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- 47.Fehr C, Shirley RL, Crabbe JC, Belknap JK, Buck KJ, Phillips TJ. The syntaxin binding protein 1 gene (Stxbp1) is a candidate for an ethanol preference drinking locus on mouse chromosome 2. Alcohol Clin Exp Res. 2005;29:708–720. doi: 10.1097/01.alc.0000164366.18376.ef. [DOI] [PubMed] [Google Scholar]
- 48.Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19:1486–1493. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 49.Schroeder JP, Olive F, Koenig H, Hodge CW. Intra-amygdala infusion of the NPY Y1 receptor antagonist BIBP 3226 attenuates operant ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1884–1891. doi: 10.1097/01.ALC.0000098875.95923.69. [DOI] [PubMed] [Google Scholar]
- 50.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjo-prajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodrigues SM, Farb CR, Bauer EP, LeDoux JE, Schafe GE. Pavlovian fear conditioning regulates Thr286 autophosphorylation of Ca2+/calmodulin-dependent protein kinase II at lateral amygdala synapses. J Neurosci. 2004;24:3281–3288. doi: 10.1523/JNEUROSCI.5303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 53.Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Perkel DJ, Nicoll RA. Evidence for all-or-none regulation of neurotransmitter release: Implications for long-term potentiation. J Physiol. 1993;471:481–500. doi: 10.1113/jphysiol.1993.sp019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 56.Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 57.Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: Implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 58.Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- 59.Hopf FW, Lesscher HM. Rodent models for compulsive alcohol intake. Alcohol. 2014;48:253–264. doi: 10.1016/j.alcohol.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


