Abstract
Temporal modulation detection ability matures over many years after birth and may be particularly sensitive to experience during this period. Profound hearing loss during early childhood might result in greater perceptual deficits than a similar loss beginning in adulthood. We tested this idea by measuring performance in temporal modulation detection in profoundly deaf children and adults fitted with cochlear implants (CIs). At least two independent variables could constrain temporal modulation detection performance in children with CIs: altered encoding of modulation information due to the CI-auditory nerve interface, and atypical development of central processing of sound information provided by CIs. The effect of altered encoding was investigated by testing subjects with one of two different hearing mechanisms (normal hearing vs. CI) and the effect of atypical development was studied by testing two different age groups. All subjects were tested for their ability to detect acoustic temporal modulations of sound amplitude. A comparison of the slope, or cutoff frequency, of the temporal modulation transfer functions (TMTFs) among the four subject groups revealed that temporal resolution was mainly constrained by hearing mechanism: normal-hearing listeners could detect smaller amplitude modulations at high modulation frequencies than CI users. In contrast, a comparison of the height of the TMTFs revealed a significant interaction between hearing mechanism and age group on overall sensitivity to temporal modulation: sensitivity was significantly poorer in children with CIs, relative to the other three groups. Results suggest that there is an age-specific vulnerability of intensity discrimination or non-sensory factors, which subsequently affects sensitivity to temporal modulation in prelingually deaf children who use CIs.
Keywords: cochlear implants, temporal processing, maturational effect
INTRODUCTION
The number of prelingually deaf children who receive a cochlear implant (CI) has been rapidly increasing worldwide. For many of these patients, a CI provides sufficient auditory input to support the development of spoken-language skills as the primary modality of communication (Niparko et al. 2010; Yoshinaga-Itano et al. 2010). However, some children do not achieve this goal (Davidson et al. 2011) and large individual differences in speech perception and language outcomes of implanted children are widely reported (e.g., Geers 2004; Manrique et al. 2004; Niparko et al. 2010). Where an individual patient falls on this outcome spectrum is often not apparent until 2–3 years after CI surgery, when precise clinical measures of spoken-language abilities have been obtained over time (Robbins et al. 2004; Ganek et al. 2012). Although several demographic predictors of CI outcomes have been identified, such as social-family factors (Hallberg et al. 2005; Niparko et al. 2010; Hess et al. 2014), neurocognitive impairments (Waltzman et al. 2000; Holt and Kirk 2005), and etiology (Dahl et al. 2003), unexplained variability in spoken language development in implanted children remains an important barrier to optimizing clinical outcomes in this population.
The fact that age at implantation is the most robust predictor of outcomes in children with prelingual deafness (PLD) who use CIs suggests that altered sensory experience may constrain auditory perceptual development in these children (Geers et al. 2003; Tobey et al. 2003; Nicholas and Geers 2007; Yehudai et al. 2011). Basic psychoacoustic abilities have previously been shown to constrain hearing outcomes of CI users; for example, temporal modulation detection (Cazals et al. 1994; Fu 2002; Won et al. 2011; Gnansia et al. 2014), spectral-ripple discrimination (Henry and Turner 2003; Henry et al. 2005; Won et al. 2007; Won et al. 2010; Anderson et al. 2011), spectral-ripple detection (Litvak et al. 2007; Saoji et al. 2009; Anderson et al. 2012), Schroeder-phase discrimination (Drennan et al. 2008), electrode discrimination (Henry et al. 2000), and place-pitch discrimination (Donaldson and Nelson 2000). It should be noted that most of these previous studies have been conducted with postlingually deaf adults with CIs; thus, little is known about the development of psychoacoustic abilities in children with PLD who use CIs.
There is ample evidence in the literature that early atypical auditory stimulation (including hearing loss) has an impact on development of basic psychoacoustic abilities (e.g., Hall and Grose 1994b; Hall et al. 1995; Wilmington et al. 1994; Kidd et al. 2002; Rance et al. 2004; Halliday and Bishop 2005, 2006). For example, children with corrected or resolved chronic middle ear disease demonstrate atypical binaural processing as evidenced by smaller masking-level differences than normal-hearing (NH) controls (Hall and Grose 1994b; Hall et al. 1995). Similarly, patients with successfully repaired unilateral congenital aural atresia show atypical performance on some binaural processing measures (Wilmington et al. 1994). Children with prelingual sensorineural hearing loss appear to be more susceptible to informational masking than children with NH (Kidd et al. 2002). Children with sensorineural hearing loss also show atypical frequency discrimination and modulation detection compared to NH listeners (Halliday and Bishop 2005, 2006). Thus, early atypical auditory experience may have wide-ranging, long-term effects on basic psychoacoustic abilities.
With regard to CI users, Jung et al. (2012) compared performance in various types of psychoacoustic tasks between children with PLD and postlingually deaf adults who use CIs. Spectral-ripple discrimination was similar in the two groups of subjects, but children with PLD were generally poorer than adult CI users on the tasks where good temporal sensitivities are required such as Schroeder-phase discrimination, melody, and timbre identification. Sanes and Woolley (2011) have suggested that temporal processing, which develops slowly into late adolescence, may be particularly affected by atypical early auditory experience. However, Schroeder-phase discrimination, melody, and timbre identification involve both spectral and temporal sensitivities. Therefore, it is not straightforward to tease apart the contribution of spectral and temporal sensitivities into the differences in performance between the two groups of subjects reported by Jung et al. The current study was designed to specifically examine the temporal processing capabilities in children with PLD.
One issue in studying development of temporal processing is that various non-temporal factors can influence performance. For instance, gap detection is influenced by attention and intensity coding (Irwin et al. 1985; Wightman et al. 1989; Werner et al. 1992; Buss et al. 2012). The current study used the temporal modulation transfer function (TMTF) to separate temporal resolution from non-temporal factors using the method introduced by Viemeister (1979). Amplitude modulation detection thresholds were measured at various modulation frequencies to derive the TMTF. The TMTF can vary independently in both the position of the function along the vertical axis (i.e., height of the TMTF) as well as in the shape of the function (defined by either the cutoff frequency or slope). TMTF height describes the “modulation sensitivity” or “efficiency” of the system to encode temporal modulations and is influenced by temporal resolution, intensity discrimination, attention, and motivation (Buss et al. 2012). In contrast, TMTF shape describes temporal resolution, indicating the time constant that limits the auditory system’s ability to encode temporal information (Hall and Grose 1994a). If the TMTF height, but not the slope of the function, changes during development, it can be inferred that the developmental change reflects processes other than temporal resolution (Hall and Grose 1994a). With respect to implanted children with PLD, both hearing mechanism and developmental change may constrain temporal modulation detection. The goal of the present study was to understand how these factors affect temporal resolution and modulation sensitivity. Thus, TMTFs were investigated in four groups of subjects: school-aged children with NH, early-implanted school-aged children with PLD, NH adults, and postlingually deaf adults with CIs.
MATERIALS AND METHODS
Participants
All experimental procedures relating to the use of human subjects in this study were approved by the Human Subject Institutional Review Boards of the University of Washington and Seattle Children’s Hospital.
CI subjects
Ten children with PLD participated. Their mean age was 11.9 years (range 7–16 years). All ten children had received a CI before 3 years of age. Mean duration of CI use was 9.7 years (range 6–14 years). Table 1 shows demographic information for this subject group. The data for 24 postlingually deafened adults were adopted from a recent study Won et al. (2011). The mean age of the adult CI subjects was 58.8 years (range 25–78 years). The mean duration of hearing loss and CI use of the adult CI subjects were 9.7 and 3.2 years, respectively. Detailed demographic information for 24 adult CI users is provided in Table 2. CI subjects were tested acoustically using their own sound processors set to a comfortable listening level in the sound field. This acoustic approach was selected over direct stimulation to ensure that CI users’ temporal modulation sensitivity was assessed under realistic, every day, listening conditions. Children with PLD in this study all underwent routine clinical mapping by an experienced pediatric CI audiologist. Although mapping procedures for children were quite similar to adults, there was some non-homogeneity in CI devices between and within age groups. In order to ensure that there were no systematic differences between CI maps of adults and children, dynamic ranges were examined for both age groups. Mean dynamic range did not differ significantly between age groups. As with long-term adult CI users, the children in this study had and would be expected to have similarly stable maps (Robinson et al. 2012).
TABLE 1.
Demographics for prelingually deafened children with cochlear implants (CIs)
| Subject | Age (years) | Age at implantation (years) | Duration of CI use (years) | Etiology | Implant type | Strategy |
|---|---|---|---|---|---|---|
| C2 | 12 | 3 | 9 | Connexin 26 | Nucleus 24 | ACE |
| C3 | 10 | 1 | 9 | Unknown | Clarion CII | Fidelity120 |
| C4 | 9 | 1 | 8 | Unknown | Nucleus 24 | ACE |
| C5 | 16 | 3 | 12 | Congenital CMV | Nucleus 22 | ACE |
| C8 | 13 | 2 | 9 | Unknown | Nucleus 24 | ACE |
| C9 | 13 | 2 | 11 | Connexin 26 | Nucleus 24 | ACE |
| C10 | 14 | 3 | 11 | Connexin 26 | Nucleus 24 | ACE |
| C11 | 16 | 2 | 14 | Unknown | Nucleus 24 | SPEAK |
| C12 | 7 | 1 | 6 | Connexin 26 | MedEl Combi40+ | CIS |
| C13 | 9 | 1 | 8 | Unknown | Nucleus 24 | ACE |
CMV cytomegalovirus infection
TABLE 2.
Demographic information for postlingually deafened adults with cochlear implants from Won et al. (2011)
| Subject | Age (years) | Duration of hearing loss (years)a | Duration of implant use (years) | Etiology | Implant type | Strategy |
|---|---|---|---|---|---|---|
| S01 | 61 | 0.3 | 2 | Unknown | Nucleus 24 | ACE |
| S03 | 61 | 5 | 11 | Genetic | Nucleus 22 | SPEAK |
| S04 | 62 | 1 | 3 | Unknown | Nucleus 24 | ACE |
| S12 | 49 | 0 | 2 | Connexin 26 | MED-EL Combi40+ | CIS |
| S34 | 55 | 1.5 | Noise exposure | HiRes90K | HiResolution | |
| S38 | 51 | 9 | 4 | Noise exposure | Nucleus 24 | ACE |
| S40 | 72 | 5 | 6 | Genetic | HiRes90K | HiResolution |
| S41 | 52 | 7 | 5 | Hereditary | HiRes90K | HiResolution |
| S48 | 67 | 10 | 0.5 | Unknown | HiRes90K | HiResolution |
| S49 | 64 | 4 | 0.75 | Hereditary | HiRes90K | Fidelity120 |
| S51 | 56 | 7 | 6 | Hereditary | Clarion CII | HiResolution |
| S52 | 77 | 0 | 0.5 | Noise exposure | HiRes90K | Fidelity120 |
| S53 | 63 | 3 | 7 | Unknown | Clarion CII | Fidelity120 |
| S54 | 25 | 0.5 | 2.5 | Unknown | HiRes90K | HiResolution |
| S55 | 65 | 40 | 1 | Genetic | HiRes90K | Fidelity120 |
| S58 | 64 | 57 | 7 | Noise exposure | Clarion CII | Fidelity120 |
| S59 | 47 | 12 | 2.5 | Noise exposure | HiRes90K | Fidelity120 |
| S61 | 78 | 10 | 1 | Genetic | HiRes90K | Fidelity120 |
| S62 | 32 | 3 | 1 | Unknown | HiRes90K | Fidelity120 |
| S65 | 56 | 2 | 7 | Unknown | Clarion CII | HiResolution |
| S66 | 66 | 3 | 2 | Unknown | HiRes90K | Fidelity120 |
| S69 | 60 | 30 | 2 | Unknown | HiRes90K | Fidelity120 |
| S70 | 59 | 0 | 1 | Genetic | Freedom | ACE |
| S71 | 70 | 15 | 1.5 | Genetic | HiRes90K | Fidelity120 |
aThe duration of their hearing loss before implantation
NH subjects
Seven NH children (mean age 11.1 years, range 8–14 years) were matched to the children with PLD based on chronological age. Seven NH adults (mean age 33.3 years, range 28–39 years) were also tested. All NH subjects had audiometric thresholds of 20 dB HL or less at octave frequencies between 250 and 8,000 Hz. Although ages for the NH and CI adults were not matched, we do not expect that the difference in ages would have significantly affected the modulation sensitivity and the shape of TMTFs.1
Procedure
Temporal modulation detection
All testing was performed in a double-walled, soundproof booth (IAC). Stimuli were presented in free field through a loudspeaker positioned at 0° azimuth and 0° elevation. Subjects sat 1 m from the loudspeaker. Subjects were presented with acoustic stimuli that were 2 s in duration. One of the two 1-s observation intervals consisted of sinusoidally amplitude modulated wide band noise, and the other 1-s observation interval consisted of unmodulated wide band noise. To create the modulated stimuli, the following equation was used:
| 1 |
in which f(t) is the wideband Gaussian noise carrier, mi is the modulation index (i.e., modulation depth), fm is the modulation frequency, and y(t) is a resulting signal. In Eq. 1, “t” indicates time and “×” indicates multiplication. The noise carrier was refreshed for every observation (i.e., created on the fly). Both the modulated and unmodulated signals were gated on and off with 10-ms linear ramps, then concatenated with no gap between the two signals.
A two-interval, two-alternative adaptive forced-choice procedure was used to measure the modulation detection thresholds (MDTs) for six modulation frequencies including 10, 50, 100, 150, 200, and 300 Hz. Stimuli were presented at 65 dBA. Subjects were instructed to choose the interval that contained the modulated noise. The correct answer was provided visually after each sound presentation. A two-down, one-up adaptive procedure was used to measure the modulation depth (mi) threshold, converging on 70.7 %. The tracking history started with a modulation depth of 100 % (i.e., mi = 1) and decreasing in steps of 4 dB from the first to the fourth reversal, and 2 dB for the next 10 reversals. For each tracking history, the final 10 reversals were averaged to obtain the MDT for that tracking history. In most cases, about 50 trials were needed for a single tracking history. In this paper, MDTs are reported in dB relative to 100 % modulation (i.e., 20log10(mi)). Subjects completed all six modulation frequencies in random order, and then the subjects repeated a new set of six modulation frequencies with newly created random order. The sequence of testing was randomized within and across subjects. A third adaptive track was obtained if the difference between the first two tracks exceeded 3 dB for a given modulation frequency. The final threshold for each modulation frequency was the mean of these two (or three) adaptive tracks. This threshold estimation method has previously been shown to be efficient in reducing the duration of testing while limiting within-subject performance variability (e.g., Hall and Grose 1994a; and see Figure 9 in Won et al. 2011). In most children, however, the difference in MDTs between the first and second tracks did not exceed 3 dB. Before actual testing, experimenters played example stimuli for subjects until they became familiar with the stimuli and task.
The TMTFs for each subject group were characterized using two metrics: the shape (slope or cutoff frequency) and y-intercept of the functions. To estimate these metrics, MDTs at six modulation frequencies were fit with two different functions. The two fitting functions are presented in Eqs. (2) and (3) in the “Results.” Because a single subject was tested on the temporal modulation detection test for six different modulation frequencies, some correlations would exist among MDTs at six modulation frequencies for a given subject. Therefore, a nonlinear mixed effects model (Lindstrom and Bates 1990) was used to assess the effects of two metrics (the shape and y-intercept of the TMTFs) among the four groups while controlling for the correlations across MDTs within subjects. Statistical analyses were performed using IBM SPSS statistics version 20 (IBM Inc., USA) and R version 2.15.2 (http://www.r-project.org) and p values less than 0.05 were considered statistically significant.
Speech perception tests
Speech reception thresholds in steady-state noise were measured for implanted children. Twelve equally difficult spondee words were presented in the presence of speech-shaped, steady-state noise (Turner et al. 2004; Won et al. 2007). After hearing a spondee word that was selected in random, subjects were instructed to identify the word by clicking on the virtual button labeled with the spondee word. The same testing materials and procedure were used for both children and adults. The spondees, two-syllable words with equal emphasis on each syllable (e.g., “birthday,” “padlock,” “sidewalk”), were recorded by a female talker (F0 range 212–250 Hz). Duration of the steady-state noise was 2 s and the onset of the spondees was 500 ms after the onset of the noise. A closed-set, 12-alternative forced-choice task with an one-up, one-down adaptive tracking procedure was used to determine thresholds converging on 50 % correct (Levitt 1971). The level of the target speech was fixed at 65 dBA. The noise level was varied with a step size of 2-dB. Feedback was not provided. For all subjects, the adaptive track started with +10 dB signal-to-noise ratio condition. The threshold for a single adaptive track was estimated by averaging the signal-to-noise ratios for the final 10 of 14 reversals. Three adaptive tracks were repeated to determine the average thresholds. Speech identification in quiet for PLD with CIs was also evaluated using 50 simple monosyllabic words (consonant-nucleus vowel-consonant word). A word list was randomly chosen out of ten lists for each subject (Peterson and Lehiste 1962) and stimuli were presented in quiet at 65 dBA. Subjects were instructed to repeat the word that they heard. A total percent correct score was calculated after 50 presentations as the percent of words correctly repeated. Speech perception data were not collected for all children with PLD due to constraints on participation schedules.
RESULTS
Characteristics of TMTFs for four subject groups
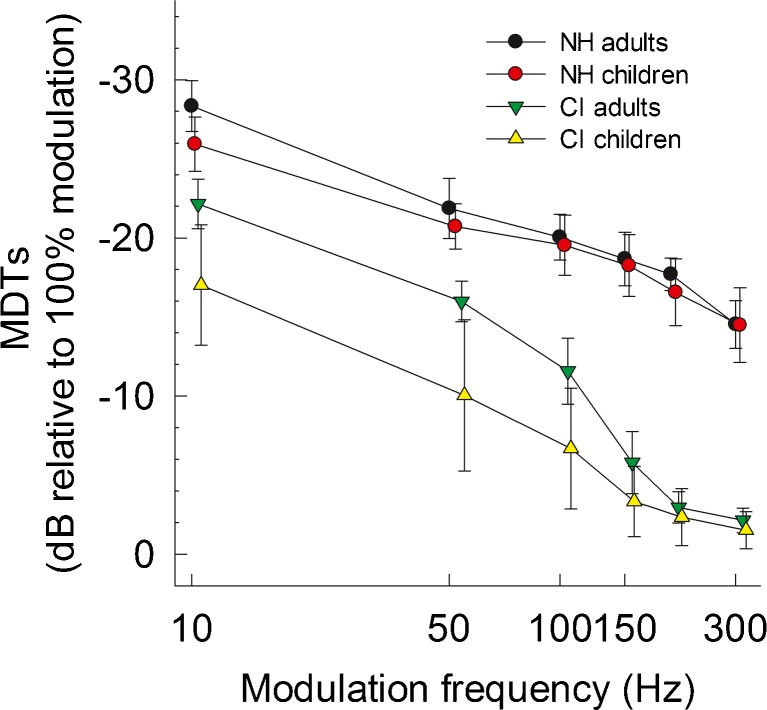
Figure 1 shows the TMTFs for four subject groups, plotting mean MDT as a function of modulation frequency. For all four subject groups, modulation detection sensitivity decreased as the modulation frequency was increased. The low-pass filter characteristic of TMTFs is typical for acoustic hearing either with normal sensitivity or hearing loss (Viemeister 1979; Bacon and Viemeister 1985) and for electric hearing either with direct stimulation on a single electrode or acoustic stimulation using the sound processor (Shannon 1992; Won et al. 2011). When comparing hearing mechanisms (normal vs. CI), mean MDTs were poorer in the CI groups relative to the NH groups across the range of modulation frequencies. When comparing age groups within the same hearing mechanism, children showed poorer MDTs across modulation frequencies than did the adult groups. This difference between age groups appeared larger in the CI group than in the NH group, particularly for low modulation frequencies.
FIG. 1.
Comparison of the average temporal modulation transfer functions measured in four subject groups. Error bars indicate 95 % confidence intervals across the subjects for modulation detection thresholds at each modulation frequency.
TMTFs for the four subject groups were analyzed using two different fitting functions, which have previously been used to characterize the TMTF (Hall and Grose 1994a; Won et al. 2011). Each equation includes a coefficient corresponding to TMTF height as well as TMTF shape. Equation 2 shows an exponential function with two fitting parameters:
| 2 |
in which fm is the modulation frequency, |y(fm)| is the absolute value of the MDT, and × indicates multiplication. Coefficients A and b are two fitting parameters. The coefficient b determines the slope of the function, which describes how MDTs change with increasing modulation frequency. TMTF shape, characterized in Eq. 2 by slope of the function, ultimately indicates the time constant that limits the ability of the system to encode temporal envelope modulations. TMTF height, characterized by the coefficient A, represents the y-intercept of the function and describes “efficiency” or “sensitivity” of the system to encode temporal modulations.
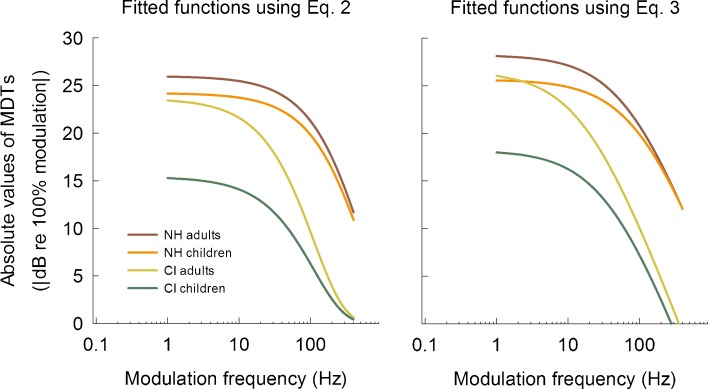
The left panel of Figure 2 shows fitted functions on averaged MDTs using Eq. 2 for the four different subject groups. The effects of hearing mechanism and age groups on the coefficients b and A were examined using a nonlinear mixed effects model. Table 3 shows the results from the nonlinear mixed effects model for all subject groups. The coefficient b was the same for NH adults and children, and it was similar for CI adults and children. However, there was a significant difference between the hearing mechanisms [F(1,47) = 82.2, p < 0.001], but there was not a significant effect of age group within each hearing mechanism [F(1,47) = 0.4, p = 0.512 for NH group; F(1, 47) = 2.5, p = 0.122 for CI group], indicating that hearing mechanism was the main determinant for the slope of the fitted TMTF. A different pattern of results was observed for the coefficient A. As shown in Table 3, NH adults, NH children, and CI adults showed a very similar value of the coefficient A, and there were no statistically significant differences among three groups [F(2,47) = 1.0, p = 0.346]. On the other hand, implanted children showed an apparently lower value of the coefficient A than the other three groups [F(1,47) = 1759.6, p < 0.001].
FIG. 2.
Fitted functions for the four subject groups using Eqs. 2 and 3.
Table 3.
Results across the four subject groups from the nonlinear mixed effects model using the exponential fit (Eq. 2)
| |f(x)| = A exp bx | b estimate (standard error) | A estimate (standard error) |
|---|---|---|
| NH adults | −0.002 (0.000) * | 26.56 (1.18) * |
| NH children | −0.002 (0.000) * | 24.60 (1.18) * |
| CI adults | −0.009 (0.001) * | 24.67 (0.70) * |
| CI children | −0.011 (0.002) * | 18.41 (1.12) * |
Significance of each parameter estimate for each subject group is indicated by the asterisk
*p < 0.001
Due to the modest sample sizes of children in the present study, they were within the range expected to provide reasonable power in a linear regression model (Cohen 1992). Determining sample sizes required for the current nonlinear model is less straightforward due to the large number of possible nonlinear functions. Therefore, we conducted a power analysis based on the actual sample size. Coefficients A and b in NH children were significantly different from those in CI children (p = 0.0004 and p < 0.0001, respectively), and the powers associated with these differences were quite high (96.27 and 99.99 %, respectively).
Equation 3 shows the second fitting function that describes the low-pass filter characteristic of the TMTFs:
| 3 |
in which fm is the modulation frequency, |(y(fm)| is the absolute value of the MDT, coefficients k and α are the fitting parameters that describe the function (Formby and Muir 1988; Hall and Grose 1994a). The shape of the function was defined by computing the time constant: π = 1000/(2πfc) ms. Here, fc indicates low-pass cutoff frequency (i.e., 0.414 × α), the coefficient for fitted TMTF shape in Eq. 3. As for Eq. 2, TMTF height was taken as the y-intercept which was derived by computing 20 × log10(k/α). Fitted functions using Eq. 3 are shown in the right panel of Figure 2. When this logarithmic function was used, the results were similar to the results obtained with the exponential function (Eq. 2): the TMTF slope was determined by hearing mechanism and the values of y-intercept for the implanted children were significantly lower than those for the three other groups.
Relationship between TMTF and speech intelligibility
Previous studies have demonstrated a significant relationship between temporal modulation detection and speech perception in CI users (Cazals et al. 1994; Fu 2002; Won et al. 2011; Gnansia et al. 2014). Using the exponential function (Eq. 2), significant correlations were found between the coefficient b and consonant-nucleus vowel-consonant monosyllabic word recognition (r = 0.53, p = 0.008, N = 24) and speech reception thresholds in steady noise (r = −0.58, p = 0.003, N = 24) for postlingually deafened adults with CIs Won et al. (2011). However, for the same group of subjects, the coefficient A was not significantly correlated with either speech measure. The same analyses were performed for children with PLD. Neither TMTF coefficient was significantly correlated with any speech tasks in this group of participants.
DISCUSSION
To our knowledge, the present study is the first to examine development of temporal processing ability in children with PLD who use CIs. The goal of this initial investigation was to understand how early atypical sensory experience with a CI affects temporal processing skills. To this end, the TMTFs for amplitude modulation detection were obtained and examined as a function of hearing group (CI or NH) and age (adult, school age). Differences in TMTF shape were interpreted as reflecting temporal resolution variability whereas differences in TMTF height were interpreted as reflecting modulation sensitivity. While both factors are involved in temporal processing, studies of normal-hearing subjects suggest different developmental time courses for these factors (Hall and Grose 1994a).
Temporal Resolution
CI listeners demonstrated steeper TMTF slopes and lower cutoff frequencies, suggesting that temporal resolution was worse for CI listeners relative to NH listeners. This finding should not be taken as contradictory to the earlier work by Shannon (1992), where normal or even higher TMTF cutoff frequencies for CI listeners were shown, due to the methodological differences between the two studies. Shannon utilized direct electrode stimulation whereas the present study used acoustic stimuli presented via the clinical CI processor. Although the present study tested modulation frequencies below 300 Hz, which is lower than the typical envelope cutoff frequency of the CI speech processors (Zeng et al. 2008), signal processing through the speech processor is the most likely candidate to explain the differences in TMTF cutoff frequency between stimulus presentation methods (acoustic vs. direct stimulation for CI users).
More importantly to the purposes of the present study, the limitations of the speech processor should have been similar in both age groups of CI listeners. The shape of the TMTF was adult-like in both groups of school-aged children compared to adults with the same hearing mechanism. Thus, experience with a CI from an early age does not appear to impede development of temporal resolution at least by school-age. This does not rule out the possibility that temporal resolution develops more slowly in children with PLD who use CIs than in children with NH. The age at which TMTF cutoff frequency is adult-like in NH children, at least 4 years old, is well below the mean age of the children tested in the present study (Hall and Grose 1994a). Without testing implanted children with different lengths of CI experience or testing the same cohort longitudinally, the precise time-course for maturation of temporal resolution in CI users cannot be determined from the present study. This is an important area for future research.
Previous work has shown that amplitude modulation detection of acoustic stimuli presented via the sound processor is significantly correlated with speech perception in adult CI listeners (Won et al. 2011; Gnansia et al. 2014). The present study is consistent with these earlier findings. The fact that coefficient b was significantly correlated with speech perception suggests that temporal resolution for acoustic stimuli is a significant factor for speech understanding in adult CI listeners. The lack of a significant correlation for coefficient A suggests that sensitivity to amplitude modulation (discussed below) is less of a factor. Although similar correlations did not reach significance for children who used CIs, this should be interpreted cautiously given the small sample size in this study. Future research into the relationship between temporal resolution, modulation sensitivity, and speech perception in children who use CIs is warranted.
Temporal Modulation Sensitivity
In contrast to TMTF shape and temporal resolution, results from the present study indicate that temporal modulation sensitivity is delayed in school-aged CI users relative to NH counterparts. That is, TMTF height is lower in school-aged CI users relative to adult CI users whereas TMTF heights of school-aged NH children and NH adults are similar. Given that implanted children and adults were both long-term users, underwent similar psychophysical testing procedures, and did not differ in map dynamic range, this result is unlikely to be attributable to systematic biases in length of CI experience, mapping or loudness scaling between age groups. Moreover, there were no systematic differences in CI devices or coding strategies between children with PLD and postlingually deafened adults (Tables 1 and 2). Therefore, this finding suggests that the developmental time course for temporal modulation sensitivity may be prolonged in implanted children relative to NH children. In terms of degree, the difference between mean TMTF height in school-age CI users and adults (6.3 dB) is comparable to the approximately 6 dB immaturity reported in 4 year old NH children (Hall and Grose 1994a). In other words, implanted children in this group demonstrated average TMTF height immaturity similar to 4 year old NH children. The mean duration of CI use for the child CI group was 9.7 years with a range between 6 and 14 years. Thus, even considering the “hearing ages” of the CI listeners, they would be expected to have developed adult-like modulation sensitivity by this time if the rate of development was similar to NH children.
As previously discussed, temporal modulation sensitivity reflects multiple underlying factors including temporal resolution, intensity resolution, and non-sensory factors such as attention (Sanes and Woolley 2011). Thus, the atypical development of modulation sensitivity in children with CIs could have been explained by any of these factors. Unfortunately, little is known about how these factors are affected by early atypical auditory experience with a CI. In adult listeners, intensity discrimination is an important factor in temporal modulation detection performance at low modulation frequencies for both CI users (Donaldson and Viemeister 2000) and NH listeners (Wojtczak and Viemeister 1999). In NH children, intensity resolution is markedly immature at birth, and does not become adult-like until 4–6 years old (Olsho et al. 1987; Werner and Gillenwater 1990; Trehub et al. 1991; Tharpe and Ashmead 2001). Development of intensity representation reflects maturation of external and middle ear structures as well as cochlear and auditory nerve development (Abdala and Keefe 2012). It is possible that atypical auditory nerve stimulation during early auditory development has an impact on the development of intensity coding in children with PLD who use CIs.
Non-sensory factors such as attention could also explain atypical modulation sensitivity development in CI listeners. For instance, higher incidences of inattentiveness and behavioral control are reported in pediatric CI users (Knutson et al. 2000; Beer et al. 2014; Kronenberger et al. 2013). In addition, auditory deprivation has been implicated in reorganization of visual attention processes to favor a wider spatial distribution of selective attention (Bavelier et al. 2000, 2001; Proksch and Bavelier 2002; Rothpletz et al. 2003). Furthermore, the development of sustained attention in children with PLD may be positively influenced by the degree of early auditory access (Quittner et al. 1994; Smith et al. 1998; Horn et al. 2005). Obviously, children with better behavioral control, selective and sustained attention could be expected to be better at any psychoacoustic task including amplitude modulation detection. Without testing attention, and controlling for differences statistically, it is not feasible to determine whether this was a factor in the present results.
Characterization of auditory development in children with PLD who use CIs has most extensively involved electrophysiologic studies of young CI users. Based on electrically evoked auditory electrophysiologic data, it has been shown that latencies of potentials at the level of the auditory nerve, brainstem, and midbrain mature at similar rates in young NH and CI listeners (Gordon et al. 2006). Similar maturation rates are seen even after relatively long periods of deprivation, suggesting that peripheral auditory plasticity is maintained beyond the critical period typically reported for maximal speech perception and language development (Thai-Van et al. 2007). In contrast, longer latency cortical evoked potentials appear to mature more slowly, or in some cases they do not develop at all, in CI listeners relative to NH listeners (Ponton and Eggermont 2001). Therefore, these electrophysiologic data suggest that there are differential effects of early atypical auditory experience with a CI on auditory development based on a peripheral-central gradient (Eggermont and Moore 2012). While the present study cannot implicate particular structures along the auditory pathway, it would seem that the present finding that modulation sensitivity develops atypically in pediatric CI listeners is most likely to be mediated by cortical mechanisms of intensity coding or attention.
CONCLUSIONS
The present study demonstrated that temporal resolution for acoustically presented amplitude modulation is constrained by hearing mechanism but is adult-like in school-age children regardless of hearing mechanism. In contrast, sensitivity to amplitude modulation appears to develop atypically in children with PLD who use CIs. Given the fact that implanted children show, on average, speech perception abilities similar to those observed in implanted adults (Dowell et al. 2002; Jung et al. 2012), this limitation may not affect performance on some basic clinical speech perception measures. However, given that slow modulation sensitivity is critical for speech perception (Drullman et al. 1994), further research is needed to elucidate underlying factors responsible for maturation of modulation sensitivity in pediatric CI users. Investigations into development of cortical representation of intensity in the auditory systems of children with PLD who use CIs should be encouraged. When possible, developmental psychoacoustic studies of pediatric CI users should attempt to control for, or take into account, differences in attention between age or hearing mechanism groups.
Acknowledgments
We appreciate the dedicated efforts of our subjects. This study was supported by NIH grants R01-DC007525 (JTR), P30-DC04661, F31-DC009755 (JHW), K23DC013055 (DLH), and an AOS Clinician-Scientist Award (DLH) and an educational fellowship from Advanced Bionics Corporation. We thank Dr. Sohee Oh for statistical assistance.
References
- Abdala C, Keefe DH. Morphological and functional ear development. Springer Handb Audit. 2012;42:19–59. doi: 10.1007/978-1-4614-1421-6_2. [DOI] [Google Scholar]
- Anderson ES, Nelson DA, Kreft H, Nelson PB, Oxenham AJ. Comparing spatial tuning curves, spectral ripple resolution, and speech perception in cochlear implant users. J Acoust Soc Am. 2011;130:364–375. doi: 10.1121/1.3589255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ES, Oxenham AJ, Nelson PB, Nelson DA. Assessing the role of spectral and intensity cues in spectral ripple detection and discrimination in cochlear-implant users. J Acoust Soc Am. 2012;132:3925–3934. doi: 10.1121/1.4763999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon SP, Viemeister NF. Temporal modulation transfer functions in normal-hearing and hearing-impaired listeners. Audiology. 1985;24:117–134. doi: 10.3109/00206098509081545. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Tomann A, Hutton C, Mitchell T, Corina D, Liu G, Neville H. Visual attention to the periphery is enhanced in congenitally deaf individuals. J Neurosci. 2000;20:RC93. doi: 10.1523/JNEUROSCI.20-17-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Brozinsky C, Tomann A, Mitchell T, Neville H, Liu G. Impact of early deafness and early exposure to sign language on the cerebral organization for motion processing. J Neurosci. 2001;21:8931–8942. doi: 10.1523/JNEUROSCI.21-22-08931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer J, Kronenberger WG, Castellanos I, Colson BG, Henning SC, Pisoni DB (2014) Executive functioning skills in preschool-age children with cochlear implants. Journal of speech, language, and hearing research [DOI] [PMC free article] [PubMed]
- Buss E, Hall JW, 3rd, Grose JH. Development of auditory coding as reflected in psychophysical performance. In: Werner LA, editor. Human Auditory Development, Springer Handbook of Auditory Research. LLC: Springer Science Business Media; 2012. pp. 107–136. [Google Scholar]
- Cazals Y, Pelizzone M, Saudan O, Boex C. Low-pass filtering in amplitude-modulation detection associated with vowel and consonant identification in subjects with cochlear implants. J Acoust Soc Am. 1994;96:2048–2054. doi: 10.1121/1.410146. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis. Curr Dir Psychol Sci. 1992;1(3):98–101. doi: 10.1111/1467-8721.ep10768783. [DOI] [Google Scholar]
- Dahl HH, Wake M, Sarant J, Poulakis Z, Siemering K, Blamey P. Language and speech perception outcomes in hearing-impaired children with and without connexin 26 mutations. Audiol Neurootol. 2003;8:263–268. doi: 10.1159/000071998. [DOI] [PubMed] [Google Scholar]
- Davidson LS, Geers AE, Blamey PJ, Tobey EA, Brenner CA. Factors contributing to speech perception scores in long-term pediatric cochlear implant users. Ear Hear. 2011;32:19S–26S. doi: 10.1097/AUD.0b013e3181ffdb8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GS, Nelson DA. Place-pitch sensitivity and its relation to consonant recognition by cochlear implant listeners using the MPEAK and SPEAK speech processing strategies. J Acoust Soc Am. 2000;107:1645–1658. doi: 10.1121/1.428449. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Viemeister NF. Intensity discrimination and detection of amplitude modulation in electric hearing. J Acoust Soc Am. 2000;108:760–763. doi: 10.1121/1.429609. [DOI] [PubMed] [Google Scholar]
- Dowell RC, Dettman SJ, Blamey PJ, Barker EJ, Clark GM. Speech perception in children using cochlear implants: prediction of long-term outcomes. Cochlear Implants Int. 2002;3:1–18. doi: 10.1179/cim.2002.3.1.1. [DOI] [PubMed] [Google Scholar]
- Drennan WR, Longnion JK, Ruffin C, Rubinstein JT. Discrimination of Schroeder-phase harmonic complexes by normal-hearing and cochlear-implant listeners. J Assoc Res Otolaryngol: JARO. 2008;9:138–149. doi: 10.1007/s10162-007-0107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drullman R, Festen JM, Plomp R. Effect of reducing slow temporal modulations on speech reception. J Acoust Soc Am. 1994;95:2670–2680. doi: 10.1121/1.409836. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Moore JK. Morphological and functional development of the auditory nervous system. Springer Handb Audit. 2012;42:61–105. doi: 10.1007/978-1-4614-1421-6_3. [DOI] [Google Scholar]
- Formby C, Muir K. Modulation and gap detection for broadband and filtered noise signals. J Acoust Soc Am. 1988;84:545–550. doi: 10.1121/1.396831. [DOI] [PubMed] [Google Scholar]
- Fu QJ. Temporal processing and speech recognition in cochlear implant users. Neuroreport. 2002;13:1635–1639. doi: 10.1097/00001756-200209160-00013. [DOI] [PubMed] [Google Scholar]
- Ganek H, Robbins AM, Niparko JK. Language outcomes after cochlear implantation. Otolaryng Clin N Am. 2012;45:173−+. doi: 10.1016/j.otc.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Geers AE. Speech, language, and reading skills after early cochlear implantation. Arch. Otolaryngol--Head Neck Surg. 2004;130:634–638. doi: 10.1001/archotol.130.5.634. [DOI] [PubMed] [Google Scholar]
- Geers A, Brenner C, Davidson L. Factors associated with development of speech perception skills in children implanted by age five. Ear Hear. 2003;24:24S–35S. doi: 10.1097/01.AUD.0000051687.99218.0F. [DOI] [PubMed] [Google Scholar]
- Gnansia D, Lazard DS, Leger AC, Fugain C, Lancelin D, Meyer B, Lorenzi C. Role of slow temporal modulations in speech identification for cochlear implant users. Int J Audiol. 2014;53:48–54. doi: 10.3109/14992027.2013.844367. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Papsin BC, Harrison RV. An evoked potential study of the developmental time course of the auditory nerve and brainstem in children using cochlear implants. Audiol Neurootol. 2006;11:7–23. doi: 10.1159/000088851. [DOI] [PubMed] [Google Scholar]
- Hall JW, 3rd, Grose JH. Development of temporal resolution in children as measured by the temporal modulation transfer function. J Acoust Soc Am. 1994;96:150–154. doi: 10.1121/1.410474. [DOI] [PubMed] [Google Scholar]
- Hall JW, 3rd, Grose JH. The effect of conductive hearing loss on the masking-level difference: insert versus standard earphones. J Acoust Soc Am. 1994;95:2652–2657. doi: 10.1121/1.409834. [DOI] [PubMed] [Google Scholar]
- Hall JW, 3rd, Grose JH, Pillsbury HC (1995) Long-term effects of chronic otitis media on binaural hearing in children. Archives of otolaryngology--head & neck surgery 121:847–852 [DOI] [PubMed]
- Hallberg LR, Ringdahl A, Holmes A, Carver C. Psychological general well-being (quality of life) in patients with cochlear implants: importance of social environment and age. Int J Audiol. 2005;44:706–711. doi: 10.1080/14992020500266852. [DOI] [PubMed] [Google Scholar]
- Halliday LF, Bishop DV. Frequency discrimination and literacy skills in children with mild to moderate sensorineural hearing loss. J Speech Lang Hear Res. 2005;48:1187–1203. doi: 10.1044/1092-4388(2005/083). [DOI] [PubMed] [Google Scholar]
- Halliday LF, Bishop DV. Is poor frequency modulation detection linked to literacy problems? A comparison of specific reading disability and mild to moderate sensorineural hearing loss. Brain Lang. 2006;97:200–213. doi: 10.1016/j.bandl.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW. The resolution of complex spectral patterns by cochlear implant and normal-hearing listeners. J Acoust Soc Am. 2003;113:2861–2873. doi: 10.1121/1.1561900. [DOI] [PubMed] [Google Scholar]
- Henry BA, McKay CM, McDermott HJ, Clark GM. The relationship between speech perception and electrode discrimination in cochlear implantees. J Acoust Soc Am. 2000;108:1269–1280. doi: 10.1121/1.1287711. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW, Behrens A. Spectral peak resolution and speech recognition in quiet: normal hearing, hearing impaired, and cochlear implant listeners. J Acoust Soc Am. 2005;118:1111–1121. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- Hess C, Zettler-Greeley C, Godar SP, Ellis-Weismer S, Litovsky RY. The effect of differential listening experience on the development of expressive and receptive language in children with bilateral cochlear implants. Ear: Hear. E-pub ahead of print; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RF, Kirk KI. Speech and language development in cognitively delayed children with cochlear implants. Ear Hear. 2005;26:132–148. doi: 10.1097/00003446-200504000-00003. [DOI] [PubMed] [Google Scholar]
- Horn DL, Davis RAO, Pisoni DB, Miyamoto RT. Development of visual attention skills in prelingually deaf children who use cochlear implants. Ear Hear. 2005;26:389–408. doi: 10.1097/00003446-200508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RJ, Ball AK, Kay N, Stillman JA, Rosser J. The development of auditory temporal acuity in children. Child Dev. 1985;56:614–620. doi: 10.2307/1129751. [DOI] [PubMed] [Google Scholar]
- Jung KH, Won JH, Drennan WR, Jameyson E, Miyasaki G, Norton SJ, Rubinstein JT. Psychoacoustic performance and music and speech perception in prelingually deafened children with cochlear implants. Audiol Neurootol. 2012;17:189–197. doi: 10.1159/000336407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd G, Jr, Arbogast TL, Mason CR, Walsh M. Informational masking in listeners with sensorineural hearing loss. J Assoc Res Otolaryngol: JARO. 2002;3:107–119. doi: 10.1007/s101620010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson JF, Wald RL, Ehlers SL, Tyler RS. Psychological consequences of pediatric cochlear implant use. Ann Oto Rhinol Laryngol Suppl. 2000;185:109–111. doi: 10.1177/0003489400109s1247. [DOI] [PubMed] [Google Scholar]
- Kronenberger WG, Pisoni DB, Henning SC, Colson BG. Executive functioning skills in long-term users of cochlear implants: a case control study. J Pediatr Psychol. 2013;38:902–914. doi: 10.1093/jpepsy/jst034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H (1971) Transformed up-down methods in psychoacoustics. The Journal of the Acoustical Society of America 49:Suppl 2:467 + [PubMed]
- Lindstrom MJ, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990;46:673–687. doi: 10.2307/2532087. [DOI] [PubMed] [Google Scholar]
- Litvak LM, Spahr AJ, Saoji AA, Fridman GY. Relationship between perception of spectral ripple and speech recognition in cochlear implant and vocoder listeners. J Acoust Soc Am. 2007;122:982–991. doi: 10.1121/1.2749413. [DOI] [PubMed] [Google Scholar]
- Manrique M, Cervera-Paz FJ, Huarte A, Molina M. Advantages of cochlear implantation in prelingual deaf children before 2 years of age when compared with later implantation. Laryngoscope. 2004;114:1462–1469. doi: 10.1097/00005537-200408000-00027. [DOI] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE. Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss. J Speech Lang Hear Res: JSLHR. 2007;50:1048–1062. doi: 10.1044/1092-4388(2007/073). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang NY, Quittner AL, Fink NE, Team CDI. Spoken language development in children following cochlear implantation. JAMA. 2010;303:1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsho LW, Koch EG, Halpin CF, Carter EA. An observer-based psychoacoustic procedure for use with young infants. Dev Psychol. 1987;23:627–640. doi: 10.1037/0012-1649.23.5.627. [DOI] [Google Scholar]
- Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ. Of kittens and kids: altered cortical maturation following profound deafness and cochlear implant use. Audiol Neurootol. 2001;6:363–380. doi: 10.1159/000046846. [DOI] [PubMed] [Google Scholar]
- Proksch J, Bavelier D. Changes in the spatial distribution of visual attention after early deafness. J Cogn Neurosci. 2002;14:687–701. doi: 10.1162/08989290260138591. [DOI] [PubMed] [Google Scholar]
- Quittner AL, Smith LB, Osberger MJ, Mitchell TV, Katz DB. The impact of audition on the development of visual-attention. Psychol Sci. 1994;5:347–353. doi: 10.1111/j.1467-9280.1994.tb00284.x. [DOI] [Google Scholar]
- Rance G, McKay C, Grayden D. Perceptual characterization of children with auditory neuropathy. Ear Hear. 2004;25:34–46. doi: 10.1097/01.AUD.0000111259.59690.B8. [DOI] [PubMed] [Google Scholar]
- Robbins AM, Koch DB, Osberger MJ, Zimmerman-Phillips S, Kishon-Rabin L. Effect of age at cochlear implantation on auditory skill development in infants and toddlers. Arch Otolaryngol. 2004;130:570–574. doi: 10.1001/archotol.130.5.570. [DOI] [PubMed] [Google Scholar]
- Robinson EJ, Davidson LS, Uchanski RM, Brenner CM, Geers AE. A longitudinal study of speech perception skills and device characteristics of adolescent cochlear implant users. J Am Acad Audiol. 2012;23:341–349. doi: 10.3766/jaaa.23.5.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothpletz AM, Ashmead DH, Thorpe AM. Responses to targets in the visual periphery in deaf and normal-hearing adults. J Speech Lang Hear Res: JSLHR. 2003;46:1378–1386. doi: 10.1044/1092-4388(2003/107). [DOI] [PubMed] [Google Scholar]
- Sanes DH, Woolley SM. A behavioral framework to guide research on central auditory development and plasticity. Neuron. 2011;72:912–929. doi: 10.1016/j.neuron.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoji AA, Litvak L, Spahr AJ, Eddins DA. Spectral modulation detection and vowel and consonant identifications in cochlear implant listeners. J Acoust Soc Am. 2009;126:955–958. doi: 10.1121/1.3179670. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Temporal modulation transfer functions in patients with cochlear implants. J Acoust Soc Am. 1992;91:2156–2164. doi: 10.1121/1.403807. [DOI] [PubMed] [Google Scholar]
- Smith LB, Quittner AL, Osberger MJ, Miyamoto R. Audition and visual attention: the developmental trajectory in deaf and hearing populations. Dev Psychol. 1998;34:840–850. doi: 10.1037/0012-1649.34.5.840. [DOI] [PubMed] [Google Scholar]
- Thai-Van H, Cozma S, Boutitie F, Disant F, Truy E, Collet L. The pattern of auditory brainstem response wave V maturation in cochlear-implanted children. Clin Neurophysiol. 2007;118:676–689. doi: 10.1016/j.clinph.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Tharpe AM, Ashmead DH. A longitudinal investigation of infant auditory sensitivity. Am J Audiol. 2001;10:104–112. doi: 10.1044/1059-0889(2001/011). [DOI] [PubMed] [Google Scholar]
- Tobey EA, Geers AE, Brenner C, Altuna D, Gabbert G. Factors associated with development of speech production skills in children implanted by age five. Ear Hear. 2003;24:36S–45S. doi: 10.1097/01.AUD.0000051688.48224.A6. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Schneider BA, Thorpe LA, Judge P. Observational measures of auditory-sensitivity in early infancy. Dev Psychol. 1991;27:40–49. doi: 10.1037/0012-1649.27.1.40. [DOI] [Google Scholar]
- Turner CW, Gantz BJ, Vidal C, Behrens A, Henry BA. Speech recognition in noise for cochlear implant listeners: benefits of residual acoustic hearing. J Acoust Soc Am. 2004;115:1729–1735. doi: 10.1121/1.1687425. [DOI] [PubMed] [Google Scholar]
- Viemeister NF. Temporal modulation transfer functions based upon modulation thresholds. J Acoust Soc Am. 1979;66:1364–1380. doi: 10.1121/1.383531. [DOI] [PubMed] [Google Scholar]
- Waltzman SB, Scalchunes V, Cohen NL. Performance of multiply handicapped children using cochlear implants. Am. J. Otol. 2000;21:329–335. doi: 10.1016/S0196-0709(00)80040-X. [DOI] [PubMed] [Google Scholar]
- Werner LA, Gillenwater JM. Pure-tone sensitivity of 2-week-old to 5-week-old infants. Infant Behav Dev. 1990;13:355–375. doi: 10.1016/0163-6383(90)90040-F. [DOI] [Google Scholar]
- Werner LA, Marean GC, Halpin CF, Spetner NB, Gillenwater JM. Infant auditory temporal acuity: gap detection. Child Dev. 1992;63:260–272. doi: 10.2307/1131477. [DOI] [PubMed] [Google Scholar]
- Wightman F, Allen P, Dolan T, Kistler D, Jamieson D. Temporal resolution in children. Child Dev. 1989;60:611–624. doi: 10.2307/1130727. [DOI] [PubMed] [Google Scholar]
- Wilmington D, Gray L, Jahrsdoerfer R. Binaural processing after corrected congenital unilateral conductive hearing loss. Hear Res. 1994;74:99–114. doi: 10.1016/0378-5955(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Wojtczak M, Viemeister NF. Intensity discrimination and detection of amplitude modulation. J Acoust Soc Am. 1999;106:1917–1924. doi: 10.1121/1.427940. [DOI] [PubMed] [Google Scholar]
- Won JH, Drennan WR, Rubinstein JT. Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users. J Assoc Res Otolaryngol: JARO. 2007;8:384–392. doi: 10.1007/s10162-007-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JH, Drennan WR, Kang RS, Rubinstein JT. Psychoacoustic abilities associated with music perception in cochlear implant users. Ear Hear. 2010;31:796–805. doi: 10.1097/AUD.0b013e3181e8b7bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JH, Drennan WR, Nie K, Jameyson EM, Rubinstein JT. Acoustic temporal modulation detection and speech perception in cochlear implant listeners. J Acoust Soc Am. 2011;130:376–388. doi: 10.1121/1.3592521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehudai N, Tzach N, Shpak T, Most T, Luntz M. Demographic factors influencing educational placement of the hearing-impaired child with a cochlear implant. Otol Neurotol. 2011;32:943–947. doi: 10.1097/MAO.0b013e31821a8407. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Baca RL, Sedey AL (2010) Describing the trajectory of language development in the presence of severe-to-profound hearing loss: a closer look at children with cochlear implants versus hearing aids. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 31:1268–1274 [DOI] [PMC free article] [PubMed]
- Zeng FG, Rebscher S, Harrison W, Sun X, Feng H. Cochlear implants: system design, integration, and evaluation. IEEE Rev Biomed Eng. 2008;1:115–142. doi: 10.1109/RBME.2008.2008250. [DOI] [PMC free article] [PubMed] [Google Scholar]