Abstract
In today’s cochlear implant (CI) systems, the monopolar (MP) electrode configuration is the most commonly used stimulation mode, requiring only a single current source. However, with an implant that will allow simultaneous activation of multiple independent current sources, it is possible to implement an all-polar (AP) stimulation mode designed to create a focused electrical field. The goal of this experiment was to study the potential benefits of this all-polar mode for reducing uncontrolled electrode interactions compared with the monopolar mode. The five participants who took part in the study were implanted with a research device that was connected via a percutaneous connector to a benchtop stimulator providing 22 independent current sources. The perceptual effects of the AP mode were tested in three experiments. In Experiment 1, the current level difference between loudness-matched sequential and simultaneous stimuli composed of 2 spatially separated pulse trains was measured as function of the electrode separation. Results indicated a strong current-summation interaction for simultaneous stimuli in the MP mode for separations up to at least 4.8 mm. No significant interaction was found in the AP mode beyond a separation of 2.4 mm. In Experiment 2, a forward-masking paradigm was used with fixed equally loud probes in AP and MP modes, and AP maskers presented on different electrode positions. Results indicated a similar spatial masking pattern between modes. In Experiment 3, subjects were asked to discriminate between across-electrode temporal delays. It was hypothesized that discrimination would decrease with electrode separation faster in AP compared to MP modes. However, results showed no difference between the two modes. Overall, the results indicated that the AP mode produced less current spread than MP mode but did not lead to a significant advantage in terms of spread of neuronal excitation at equally loud levels.
Keywords: cochlear implant, psychophysics, stimulation strategies, electrical field
INTRODUCTION
Although the cochlear implant (CI) is rightfully considered as one of the greatest success stories in biomedical science, the device has significant shortcomings. First, the benefit provided by the device varies greatly among patients, with a significant portion (up to 30 %) of the recipients only marginally benefiting for audition-alone speech understanding (Blamey et al. 2013). Secondly, speech in noisy environments and music are still poorly perceived by most recipients (Fu et al. 1998; Friesen et al. 2001; McDermott 2011). It has often been argued that a main limitation of the CI is the spread of current induced by each intra-cochlear electrode: thus, each electrode may activate an inappropriately large range of sensory neurons. Additionally, direct current summation across electrode positions can cause uncontrolled loudness and spectral shape distortions, limiting the ability to simultaneously present spectral or temporal information at more than one cochlear site (Shannon 1983). This study investigated whether a highly focused electrical stimulation mode can reduce unwanted electrode interactions.
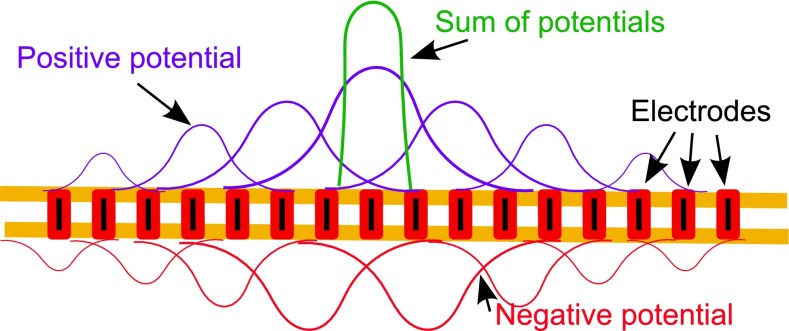
Van den Honert and Kelsall (2007) have demonstrated that if the impedance matrix between all pairs of electrodes can be measured, it is possible to create a potential field localized around one targeted electrode. By selecting simultaneous current levels and phases on each electrode appropriately, the sum of all potentials can result in a gradient confined to a very specific place in the cochlea (schematically shown in Fig. 1), which should consequently activate narrower regions of auditory neurons. This technique is termed all-polar (AP) mode in this paper but has also been described variously as “multipolar” or “phased array.” In this paper, we will designate the electrode around which the controlled current interactions create a focused current field the “AP electrode”. In monopolar (MP) mode, the term “MP electrode” will designate the single intra-cochlear activated electrode.
FIG. 1.
Schematic of the all-polar mode.
The aims of this study were to evaluate if the AP mode would induce percepts that reflect a more focused current field and a narrower region of activated neurons compared to MP stimulation. To produce an AP electrode, it is necessary to simultaneously activate all the individual electrodes using independent current sources. At the time of the study, no commercial device with such a feature was available for research purposes in Australia. Therefore, the experiment was undertaken using an experimental stimulator provided by Cochlear Ltd that was equipped with 22 independent current sources. This stimulator was connected to an implanted intra-cochlear electrode array via a percutaneous connector (van den Honert and Kelsall 2007). A series of experiments was performed to test the spatial specificity of AP stimulation compared to MP stimulation.
The first experiment compared the extent of current summation produced by two simultaneously activated AP and MP electrodes by balancing the loudness of simultaneous versus sequential activation. The second experiment compared the width of spatial forward-masking patterns in the two modes. The third experiment compared the temporal interactions across electrode positions in both modes. Based on behavioral and physiological data and computational models (van den Honert and Kelsall 2007; Bonham and Litvak 2008; Frijns et al. 2011), the AP mode was expected to show (1) less current summation, (2) a narrower masking pattern, and (3) less temporal interaction than the MP mode.
EXPERIMENT 1: CURRENT SUMMATION
Participants and Equipment
Five participants were implanted with the research implant with percutaneous connector. Table 1 lists demographic and hearing-related information for all participants. Recruitment was conducted through the Cochlear Implant Clinic at the Royal Victorian Eye and Ear Hospital and the Hearing CRC. All the participants gave written informed consent and were compensated for their travel expenses. This project conformed to The Code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the Royal Victorian Eye and Ear Hospital Human Research Ethics Committee (Project 11-993H).
TABLE 1.
Participants’ information
| ID | Age (year) | Gender | Duration of severe hearing loss (year) | Etiology of hearing loss | Experience with first implant (year) | CVC word score in MP (%) | Most apical electrode in Experiment I | Probe in Experiment II |
|---|---|---|---|---|---|---|---|---|
| P1 | 54 | F | 9 | Hereditary | 3 | 64 | 20 | 17 |
| P2 | 79 | M | 20 | Unknown | 5 | 30 | 17 | 14 |
| P3 | 77 | M | 22 | Hereditary | 6 | 50 | 17 | 10 |
| P4 | 82 | F | 31 | Antibiotic | 3 | 88 | 18 | |
| P5 | 44 | F | 13 | Hereditary | 11 | 52 | 19 |
Before being implanted with the research device, all the participants had at least 3 years of experience with a first implant on the contralateral side. They agreed to use the research device for 18 months and to participate in research during this time. While not participating in experiments, the participants connected their research implant to a standard sound processor programmed with the ACE strategy (Vandali et al. 2000) using a custom-designed dongle (van den Honert and Kelsall 2007). After the research period, participants were explanted and reimplanted with a standard commercial cochlear implant. The participants did not derive any particular benefit from the research: they were all candidates for a second implant and were financially covered for this by health insurance (although the devices were provided by Cochlear Ltd in this instance). Their motivation was therefore solely altruistic. In order to minimize any risk of infection due to the percutaneous connector, the patients were carefully monitored throughout their time with the experimental processor by medical staff.
Stimuli
AP electrodes were created by first measuring the impedances between all possible pairs of electrodes, which resulted in a weight matrix that defined the relative current amplitudes across the array predicted to produce the focused current field at each electrode position (van den Honert and Kelsall 2007). When adjusting the level of activation for AP electrodes, these weights remained constant.
Stimuli for this experiment were dual-electrode pulse trains in either MP or AP mode. The pulses on the two electrodes were presented either simultaneously or sequentially with an onset to onset delay of 232 μs between electrode positions. The most apical AP electrode position of the electrode pair was fixed and was selected to avoid regions of elevated threshold or non-auditory sensation (see Table 1). The basal electrode position of each electrode pair was separated by 2, 4, 6, or 8 electrode distances or 1.2, 2.4, 3.6, 4.8 mm, respectively, as the electrodes of the participants’ model, the Nucleus® Contour Softip™ perimodiolar electrode array, were separated on average by approximately 0.6 mm (Long et al. 2014). MP dual-electrode stimuli were created using the same electrode positions as the AP dual-electrode stimuli. The duration of the stimuli was 500 ms, within which each period of 10 ms contained two pulses, one on each of two AP or MP electrodes. Each biphasic pulse had a phase width of 100 μs and an interphase gap of 20 μs. The overall levels of the stimuli were set to be comfortable.
Method
Before creating the dual-electrode stimuli, single-electrode threshold levels were measured for all MP and AP electrodes across the array by increasing and decreasing the current in 0.8 dB steps until the participant reported a reliable detection of sound.
All the experimental dual-electrode stimuli were composed of two equally loud single-electrode stimuli presented sequentially or simultaneously. The single-electrode stimuli were loudness balanced to each other using pulse trains of the same overall rate as the dual-electrode stimuli (i.e., double the per-electrode rate of the dual-electrode stimuli) to avoid further current adjustments for overall loudness when the dual-electrode stimuli were created (McKay et al. 2003).
The loudness balancing method replicated the procedure outlined in McKay and McDermott (1999). The most apical electrode of the experiment was selected as reference electrode (see Table 1) and was set to a comfortable level, or at least 1 dB below the maximum level practicable for the device. The stimulus to be balanced and the reference stimulus were presented, separated by a 500-ms silent interval, in random order, in a 2-interval forced-choice task with a 1-up 1-down adaptive rule. The participant was asked to indicate which sound was the louder. If the participant selected the reference, then the level of sound to be balanced was increased or vice versa. The initial step size was 0.6 dB in MP mode and 1.2 dB in AP mode. After two reversals, the step size was halved. The test stopped after a total of 8 reversals. The level of the balanced stimulus was derived as the average of the final 4 reversal points. The difference in step size between MP and AP modes was motivated by pilot experiments that showed that the loudness function in MP mode was approximately twice as steep as in AP mode (i.e., to induce the same loudness change, the physical step in AP mode needs to be twice the size of the step in MP mode). The balancing run was repeated with a different starting level of the stimulus to be balanced: in one trial, it was initiated at 1 dB (in MP mode) or 2 dB (in AP mode) above the reference level, and in the other run, at 1 or 2 dB below the reference level. To counter balance any bias related to the varying stimulus (Marks and Florentine 2011), the role of the reference and the to-be-balanced stimuli were reversed, and two new balancing runs were performed. The difference between the levels of the reference and balanced stimuli in the four trials was averaged and used to set the final balanced level of the stimulus to be balanced. The two balanced levels were then used to form the dual-electrode stimuli.
Sixteen dual-electrode stimuli were created from two stimulation modes (AP and MP), two simultaneities and 4 electrode separations (1.2, 2.4, 3.6, 4.8 mm), and a similar balancing method was used to loudness-balance all the stimuli. When adjusting the levels of the dual-electrode stimuli, the current levels on each electrode were adjusted by equal ratios. First, the MP stimulus with the most apical pair of electrodes in sequential presentation was selected as the reference stimulus. Then, all the other MP stimuli with sequential and simultaneous presentation were loudness-balanced against this reference. Then, an AP reference with the same pair of electrodes as the MP reference was loudness-balanced against the latter. Finally, all the AP stimuli were loudness-balanced against the AP reference.
Results
Threshold Measurements
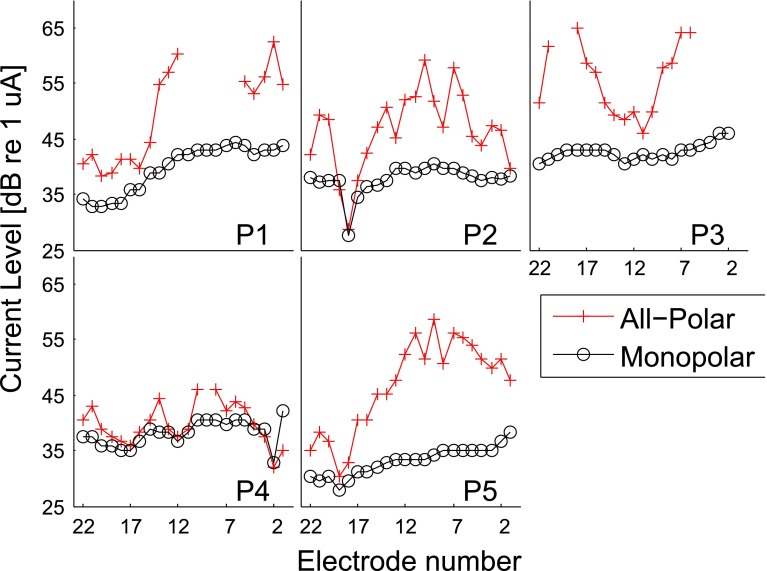
Figure 2 shows all the single-electrode threshold levels in dB re 1 μA. Electrodes are numbered according to the Cochlear device where electrode 22 is the most apical electrode (i.e., inducing the lowest pitch).
FIG. 2.
Threshold levels in monopolar and all-polar mode for the 5 participants.
Some threshold levels could not be reached in AP mode because the system either reached the maximum output limit (18 V) or the safety limit of charge delivered (212 nC per phase, Shannon 1992). Threshold levels were all higher in AP compared to MP mode, except for P4’s electrode 1. Overall, the AP threshold levels were more variable than the MP ones. P2’s thresholds were surprisingly low at electrode 18 in both modes. In order to test whether P2 confused the stimulus with tinnitus, an adaptive three-alternative forced-choice procedure with the stimulus presented in one randomly selected interval was performed at that electrode position. The participant was asked to indicate in which of the 3 intervals he heard a sound. The result from this procedure confirmed the previous measures and indicated that he could indeed perceive the stimuli with such a low level.
Loudness Matching
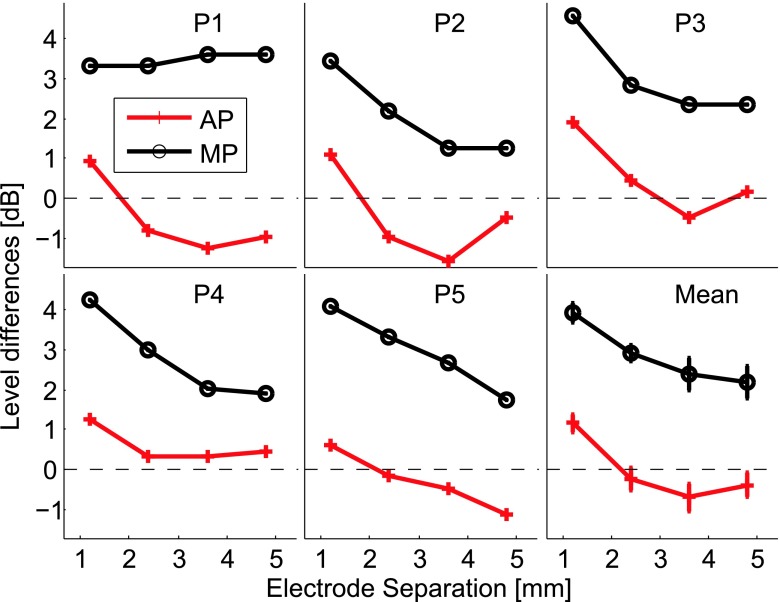
Figure 3 shows the average current level difference between simultaneous and sequential dual-electrode stimuli when adjusted to equal loudness. For MP stimuli, the balanced levels were always higher for sequential compared to simultaneous stimuli (i.e., the simultaneous stimulus had to be reduced in current to achieve the same loudness as the sequential stimulus). On average, when the MP electrodes were separated by only two electrode positions, a difference of 3.9 dB was observed. The current difference between sequential and simultaneous stimuli decreased monotonically with the distance between electrodes. However, when comparing level differences between sequential and simultaneous stimuli in AP mode, a smaller difference of 1.2 dB was observed on average for a separation of 1.2 mm, and between -0.2 and -0.7 dB for greater separations. The level differences were submitted to a 2-way repeated measures ANOVA with stimulation mode (MP vs. AP) and the electrode separation as main factors. The analysis revealed a significant effect of the stimulation mode (F1,4 = 81.9276, P = 0.0008) and the electrode separation (F3,12 = 26.4047, P < 0.00001) but not for the interaction factor (F3,12 = 0.7879, P = 0.5235). Four additional t tests were performed to test whether the AP level differences were significantly different from 0. The critical value was divided by 4 to account for multiple comparisons. The analysis revealed that the level difference was significantly different from 0 only for the stimuli with a separation of 1.2 mm (t4 = 5.58, P = 0.0025).
FIG. 3.
Results of Experiment 1. Average current level difference between simultaneous and sequential stimuli when adjusted to equal loudness in AP mode (red crosses) and MP mode (black circles) for each participant and their average (bottom right panel). The error bars of the last panel show the standard error of the mean.
Discussion
The results of this experiment indicate a significant difference between the AP and MP modes, which might reflect a difference in degree of current spread in each mode. The strong difference of level between equally loud sequential and simultaneous stimuli in MP mode was expected and has been previously reported. Frijns et al. (2009) found a difference of 6 dB between the intensity of equally loud simultaneous and sequential dual-electrode MP stimuli separated by only one electrode (equivalent to 1.1 mm with the Advanced Bionics®HiFocus1J™ electrode array). Landsberger and Galvin (2011) found a slightly smaller difference of about 5.1 dB between similar stimuli also using Advanced Bionics devices. At a similar distance, a difference of approximately 4 dB can be derived from the present experiment. This discrepancy might be caused by differences between subjects, interphase gaps, interpulse gaps, or electrode array geometry in the two studies. It is worth noting that a large current interaction was observed in MP mode even with a separation of 4.8 mm. This distance is often larger than the difference in allocation position of two vowel formants.
In AP mode, the analysis revealed that there was a significant difference in level between equally loud sequential and simultaneous stimuli only when the component AP electrodes were separated by 1.2 mm. This result suggests that the current interactions in AP may be localized to electrode separations less than 2.4 mm. Interestingly, the level differences in AP mode were negative on average for a separation of 2 mm or more (mostly driven by P1 and P2). It might be possible that, due to a non-optimal configuration, the simultaneous presentation of two AP electrodes could interfere in a destructive way. As a result, the overall loudness would be reduced. However, as the average differences for those separations were not significantly different from zero, one can only speculate on these negative differences.
EXPERIMENT 2: FORWARD MASKING
Participants
Participants P1, P2, and P3 participated in this experiment.
Stimuli
This experiment tested the masking effect of AP maskers on probe stimuli in AP or MP mode in a forward-masking paradigm. The masker was a 75-ms biphasic pulse train with a pulse rate of 200 pps. The probe was a 20-ms biphasic pulse train with 500-pps rate presented 80 ms after the masker onset (i.e., silent gap of 5 ms). The probe was presented in either AP or MP mode at fixed, equally loud, soft levels. The probe electrode was fixed during the experiment for each participant (electrode 17 for P1, 14 for P2, and 12 for P3) and was selected based on individual thresholds (see Fig. 2) to avoid dead regions or regions with non-auditory sensations. The masker position varied between ±3 electrodes away from the probe position. The masker was always presented in AP mode, at a level that was constrained between masker threshold and either maximum comfortable level or maximum possible level. The masker level was adjusted to just mask the fixed probe to derive a spatial forward-masking function (masker level versus masker position) for each of the AP and MP probes.
Methods
The threshold and maximum comfortable levels were first measured for all masker and probe stimuli. The thresholds were measured using an adaptive 3AFC procedure, with the signal in a randomly selected interval and silence in the other two intervals. The step size was initially 0.6 dB, and after two reversals, was reduced to 0.3 dB. The test stopped after a total of 8 reversals. The threshold level was derived as the average of the last 4 reversal points. The maximum comfortable level was measured to ensure that no uncomfortably loud masking stimuli were presented and to later be able to express the masker level as a function of the electric dynamic range. A loudness category task was used. The level of each stimulus was gradually increased, and the participant reported their sensation of loudness on a scale ranging from: “Not heard,” “Faint,” “Soft,” “Medium,” “Loud,” “Max Comfortable,” “Too Loud”. For some maskers of P3, a loudness category above Medium could not be reached because the current reached the safety limit. The “Max Comfortable” level was then extrapolated from the lower judgments. The AP and MP probes were loudness balanced to each other at a very soft but clearly perceivable level using the same balancing method as outlined in Experiment 1.
The experiment was divided into 12 or 14 blocks. Within each block, the presentation mode of the probe was fixed (half the blocks used a MP probe and the other half an AP probe), as well as the masker position. In each trial of the block, the participants were presented with three intervals each containing a masker and one, randomly selected, also containing the probe. The participant was asked to indicate which interval was different. The level of the masker was varied between its threshold and maximum comfortable level (or maximum safe level) in 7 to 10 equally spaced steps. Overall, at least 30 repetitions of each probe/masker-level combination were collected for each participant. A psychometric function was fitted to the % correct probe identification versus current level for each masker, and the current level for 66 % correct probe identification was interpolated as the masker level just sufficient to mask the probe. Spatial forward-masking functions were then derived by plotting the masker level for probe threshold versus the masker position.
Results
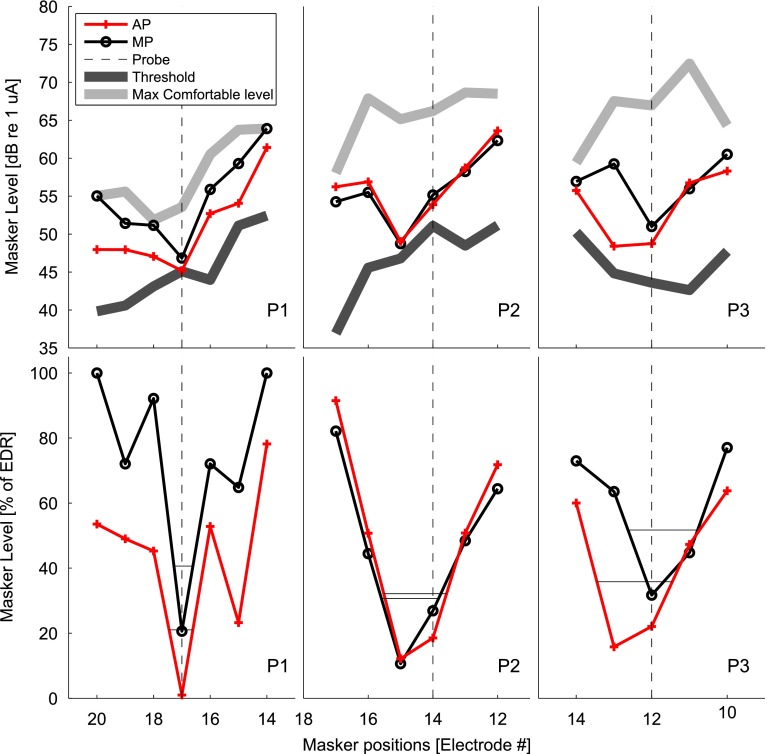
The level of the AP probe was always higher than its loudness-balanced MP probe, as expected, with a difference of 9.85, 15.65, and 8.62 dB for P1, P2, and P3, respectively.
The top panels of Figure 4 show the results for the 3 participants with the masking level denoted in dB re 1 μA. Also shown in these panels are the masker threshold and maximum comfortable levels in the same units. The same masking functions are represented in the bottom panels of Figure 4 with masker level denoted as percentage of the dynamic range (%DR) of each masker. McKay (2012) has argued that representing the forward-masking pattern in %DR and measuring its width at a fixed %DR above the tip is an ecologically valid way to compare the underlying spread of excitation from this type of forward-masking function. The width of the masking patterns at 20 %DR above the tip were calculated (McKay 2012) and reported in Table 2. The results showed different patterns for each participant. For P1, the MP masking pattern had a similar shape to the AP masking pattern but shifted upward by an average of 4 dB. The masking patterns for P2 were almost identical. It is also worth noting that the most efficient masking electrode was one electrode position more basal than the probe. This could indicate a dead region near electrode 14. The widths of the masking patterns of P3 were larger than those of P2 and P1 but showed little difference between the AP and MP modes. The overall masking pattern for P3 was shifted upward in the MP mode compared to AP mode by 3 dB (as for P1) and sideways by one electrode. However, one could argue that the masking patterns were similar, except around the masker electrode 13, which showed a large difference between probe modes. Although there is insufficient data for statistical analysis, it is worth mentioning that the width of the masking pattern for the 3 participants was always wider in AP mode by 0.1 mm compared to the MP mode. This small advantage for MP mode would be further enhanced by P1’s data if the width at a higher %DR above the tip were measured, as the secondary tip was deeper in AP mode. In summary, there was no evidence for these three participants that the spread of neural excitation is reduced in the AP mode compared to the MP mode.
FIG. 4.
Results of Experiment 2. Top panels: level of an AP masker in dB (re 1 uA) with a probe presented in AP mode (red crosses) and in MP mode (black circles). Also shown are the masker thresholds and maximum comfortable levels (black and gray thick lines). Bottom panels: the same data but with the masker level expressed as % of the dynamic range.
TABLE 2.
The width of the masking pattern at 20 %DR above the tip for MP and AP probes
| MP (mm) | AP (mm) | |
|---|---|---|
| P1 | 0.40 | 0.50 |
| P2 | 1.06 | 1.16 |
| P3 | 1.11 | 1.20 |
Discussions
This experiment was designed to test the hypothesis that AP mode would lead to a more focused masking pattern than MP mode. The results did not support this hypothesis. For one participant, P2, the masking patterns were identical in AP and MP modes. For P1 and P3, the widths of the masking patterns at 20 %DR above the tip were similar between modes, but the shapes were slightly shifted upward, and only for P3, sideward. The upward shift might be explained by a small residual difference in loudness between the loudness-balanced probes. The rightward shift of the masking pattern of P3 could be caused by a dead region located in the basal direction from the probe (Moore and Alcántara 2001).
Overall, the widths of the masking patterns found in this experiment are within the range of monopolar and tripolar masking pattern widths previously reported (Bierer and Faulkner 2010; Landsberger et al. 2012; Fielden et al. 2013).
EXPERIMENT 3: ACROSS-ELECTRODE TEMPORAL DISCRIMINATION
Experiment III measured the ability to discriminate timing differences across electrodes in dual-electrode stimuli. It was hypothesized that if the component electrodes activate an overlapping region of auditory neurons, it will be easier to detect small temporal delays between pulses on the two electrodes. Therefore, it was hypothesized that across-electrode delays in AP dual-electrode stimuli would be more difficult to discriminate than in MP mode, and this ability would reduce faster in AP mode as electrode separation was increased, reflecting a narrower spread of neural activity in AP mode.
Participants
All 5 participants took part in this experiment.
Stimuli
The stimuli were dual-electrode 500-ms pulse trains in AP or MP mode. In each 10-ms period, there were two biphasic pulses separated by a delay of either 1 ms (reference stimulus) or longer (test stimulus). The first pulse of each pair was always presented to a fixed “reference” electrode (see Table 3). The second pulse of each pair was either delivered to the same electrode or one of the 4 adjacent electrodes in the apical direction. For each electrode separation and mode, a reference and a test stimulus were created (differing only in delay between the electrodes). The currents on the component electrodes were adjusted to provide equally loud sensations for single-electrode stimuli as in Experiment 1. These reference and test dual-electrode stimuli were similar to the stimuli used by McKay and McDermott (1999), except in this study, the longer delay of the test stimulus was set to a participant-specific value as described in the Methods section and noted in Table 3. The dual-electrode stimuli were balanced in loudness as follows. First, the MP reference stimulus with both pulses on the reference electrode was set to a comfortable level, and the other MP reference stimuli with non-zero electrode separations were all balanced with it. Next, for each electrode separation, the test MP stimulus (longer delay) was balanced with the corresponding reference stimulus (1 ms delay). The procedure was repeated in AP mode, after balancing the AP and MP reference stimuli with zero electrode separation. Table 3 summarizes the test stimulus delays and reference electrode for each participant.
TABLE 3.
Experiment 3 parameters for each participant
| Participant | Reference electrode | Delay MP (μs) | Delay AP (μs) | Roving level MP (dB) | Roving level AP (dB) |
|---|---|---|---|---|---|
| P1 | 16 | 3608 | 3608 | 0.7 | 2.1 |
| P2 | 12 | 4604 | 3998 | 0.7 | 1.4 |
| P3 | 10 | 5000 | 5000 | 0.88 | 1.9 |
| P4 | 11 | 2800 | 2884 | 0.7 | 1.4 |
| P5 | 14 | 2519 | 2084 | 0.7 | 1.4 |
Methods
For each mode and electrode separation, the discrimination of two dual-electrode stimuli differing in inter-electrode delay was tested in a three-interval forced-choice (3IFC) procedure, in which one random interval in each set of three contained the test stimulus with longer inter-electrode delay. The subject was asked to identify the “different” interval while ignoring loudness variation. To prevent identification based on any small residual loudness difference after loudness balancing, a random level increment was added in each interval. This level was randomly selected within ± an interval set to 4.5 times their individual standard error for loudness balancing (2.3*1.96*SE as discussed in Fraser and McKay 2012). This amount, shown in Table 3, ensured that the variation of loudness was greater than the possible error in loudness matching (Dai and Micheyl 2010).
In order to be able to compare two psychometric functions (correct discrimination versus electrode separation), it is important to ensure that the portion studied outlines the same performance range (Fielden et al. 2014). Therefore, temporal delays for the test stimuli were derived in a preliminary experiment for each participant and mode to reach 80 % accuracy for the easiest condition, in which both pulses in the pulse pair were presented on the same electrode. In this pre-experiment, the test delay was varied between 1 and 5 ms in a similar 3IFC task and the test delay for 80 % accuracy was interpolated (see Table 3).
In the main experiment, the test stimulus delay was set to this individually specified value and discrimination of reference and test stimulus pairs was tested for each electrode separation (including zero separation) in six blocks of trials. Within each block, the mode was kept constant and pairs differing in electrode separation were presented 10 times each in random order. The mode of presentation of the first block was randomly selected then was alternated between blocks. Therefore, a total of 30 trials were collected for each electrode separation and mode.
Results
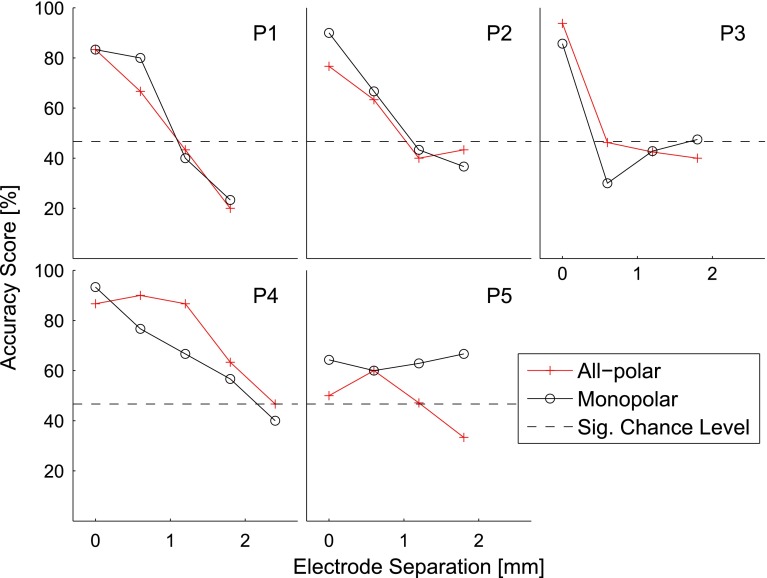
Figure 5 shows the results for the 5 participants. All except P5 showed an accuracy score around 80 % for both modes with zero electrode separation (confirming the accuracy of the preliminary experiment to select the pulse delays). Results from P5 for zero separation were highly inconsistent with the preliminary tests and variable between repeated blocks, and therefore were not considered in the statistical analyses. Overall, Figure 5 shows a very similar pattern of results for the two modes. When a score fell below the significantly above-chance level (dashed line in the figure and defined as the maximum number of correct responses that would occur by chance 5 % of the time), it indicated that the participant could not reliably discriminate the two stimuli. This performance level was reached at one electrode separation for P3, two electrode separation for P1 and P2, and four electrode separation for P4. A repeated measures analysis of variance (ANOVA) with main factors of electrode separation (df = 3) and mode (df = 1) indicated a significant effect of electrode separation (p = 0.0164) but no effect of mode or its interaction with electrode separation.
FIG. 5.
Results of Experiment 3. Accuracy scores as function of electrode separation (in mm).
Discussion
McKay and McDermott (1999) found that participants reached chance performance with electrode separations ranging from 1 to 6 mm for the condition where the pulse width was 100 μs or smaller. The analogous results from this study (in MP mode) is within the lower end of that range, which might be expected as the delay differences between reference and test stimuli were lesser in the current study compared to that of McKay and McDermott. No significant difference between the AP and MP modes was observed in this experiment. This result was not consistent with the hypothesis, as the discrimination score for the AP condition was hypothesized to drop faster with increasing electrode separation compared to the MP condition.
Recently, Fielden et al (2014) performed a very similar experiment with 9 participants comparing tripolar mode to monopolar mode. Consistent with the current results, their average results showed an almost identical pattern on average for both modes, indicating no benefit on average from the tripolar mode. All their participants’ results reached or approached the level of not significantly different from chance after an electrode separation of 1.1 to 5.5 mm. Only one participant retained a score of 70 % after 6.6 mm separation in MP mode.
GENERAL DISCUSSION
This paper describes three experiments designed to assess the capability of the AP stimulation mode to induce less perceptual interaction between spatially separated stimuli than the MP mode. Threshold measurements (Fig. 2) showed that threshold levels were all higher and more variable in AP than MP mode. This variability has already been observed for threshold levels in tripolar mode (Bierer 2007; Landsberger et al. 2012). It has been argued that a more focused stimulation strategy should reflect the inhomogeneity of the auditory nerves in an impaired auditory system (Long et al. 2014) and, therefore, AP or tripolar threshold levels should be more variable than MP thresholds. Interestingly, Long et al. (2014) have shown a significant correlation between the variability of the AP thresholds across the array and speech understanding with ten participants using a similar device to the one used in this study (r2 = 0.82, df = 9, p = 0.0003). This correlation was replicated with the 5 participants of this study. Speech understanding was estimated with CVC scores available from previous clinical assessments (see Table 1). The threshold variability was evaluated as the standard deviation of the first derivative of the threshold levels, ignoring thresholds too high to be determined. Measures of threshold variability and CVC scores were strongly negatively correlated (r2 = 0.86, df = 4, p = 0.02).
Results from Experiment 1 showed large level differences of equally loud simultaneous compared to non-simultaneous presentation of dual-electrode stimuli when presented in MP mode. On the other hand, in AP mode, the level differences were small and limited to small electrode separations. This result can be interpreted as evidence of less current summation between the two AP electrodes than that between two MP electrodes. Thus, Experiment 1 shows that the AP mode can create a current field that is more focused than MP mode.
The results from Experiment 2 indicated similar spatial forward-masking patterns in the two modes. Similarly, the results from Experiment 3 showed no difference between the two modes in across-electrode temporal discrimination ability. These results were surprising considering the results from Experiment 1 and suggest that the spread of neural activation from equally loud AP and MP electrodes is similar in spite of the AP current field being more focused.
Several hypotheses can be proposed to explain this discrepancy. First, the three experiments measured processes at different levels of the auditory pathway. For example, it is possible that, although the AP mode produces a more focused neural excitation pattern, a higher-level process might integrate temporal information across independent regions, explaining the similar pattern of AP and MP modes in Experiment 3.
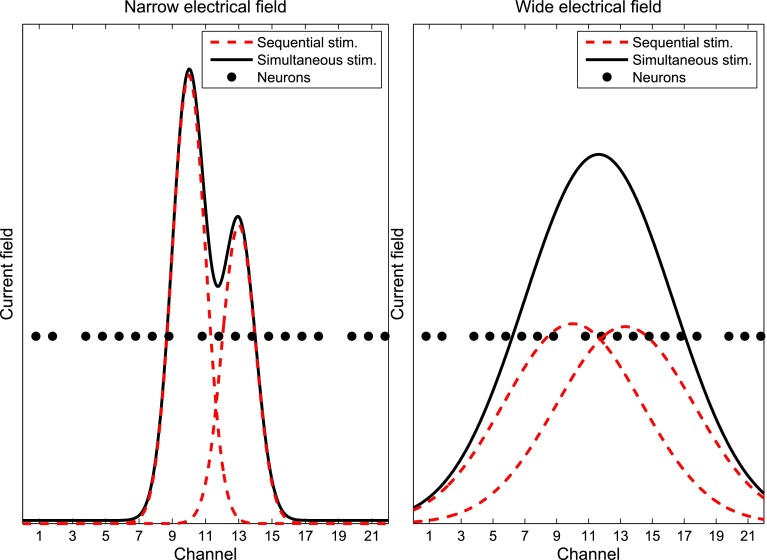
Results may also be explained by making the distinction between the width of the electric field and the width of the neural region excited. Models have demonstrated that AP mode should produce a narrower electrical field than MP mode at similar current amplitudes. A narrower field might be perceived as “sharper” but also softer as less neurons are excited. Therefore, to reach the same loudness in AP as in MP mode, the overall current needs to be increased and this can lead to a wider region of neurons excited. Figure 6 illustrates that hypothesis by showing two possible electrical fields activated simultaneously (black lines) or sequentially (red dashed lines). The left and right panels illustrate a scenario with two narrow or wide electrical fields centered on electrodes 10 and 13, respectively. Each electrical field on its own (red) will excite the same number of neurons (2 in this example) and thus will be perceived with the same loudness. For the sake of the demonstration, it is assumed that the sensation of loudness is only related to the number of neurons excited and, therefore, each single-electrode stimulus can be considered as equally loud. Thus, adjusting the stimuli to produce the same loudness has led to the same spatial spread of neural activity. When the two stimuli are presented sequentially on two different electrodes, a total of four neurons are activated in each case. When the stimuli are presented simultaneously (black line), current summation will occur and some additional neurons will be activated. In the narrow current field in the left panel, the overall loudness will increase marginally, as only one additional neuron will be activated. However, with the less-focused electrical field, the current summation is much greater and a larger number of additional neurons will be activated.
FIG. 6.
Model showing two hypothetical electrical fields activated simultaneously (black lines) or sequentially (red dashed lines). The left panel illustrates a scenario with two narrow electrical fields centered on electrodes 10 and 13. The right panel illustrates a scenario with two wide electrical fields.
The simple cartoon in Figure 6 represents a special case in which there is very sparse neural survival, so that, in order to recruit neurons to achieve a specific loudness, it is necessary to recruit them along the cochlear length rather than recruiting more neurons in the area close to the electrode. If, however, there was good neural survival near the target electrodes, it would be possible to recruit neurons in the same location but with different thresholds as the stimulus increases, leading to a narrower spread of excitation for the same loudness in the focused stimulation case compared to the less-focused case (as observed in Bierer and Faulkner 2010).
Thus, the difference in results between Experiment 1 and Experiments 2 and 3 may be due to the participants in these experiments having insufficient spiral ganglion cell density to result in a narrower spread of neural activity from focused current fields. In order to be recruited for this experiment, the participants needed to already have one cochlear implant and also to have no residual hearing in the contralateral ear that received the research device. It is therefore possible that our 5 participants had a low density of residual spiral ganglion cells in the research ear. Participants with shorter durations of deafness and more residual hearing might have produced a different result. For example, Smith et al (2013) demonstrated improved spectral resolution using a similar stimulation mode to AP using the same Nucleus research implant.
Finally, it might be possible that the lack of differences seen in Experiments 2 and 3 could be attributed simply to a non-optimal configuration of the AP electrodes. The weight matrices were created following the procedure outlined by van den Honert and Kelsall (2007). Although this procedure seemed appropriate at the time of the experiments, it is unclear whether it is optimal. For example, the method to set the weight on the diagonal is still debatable. As it is not possible to measure the cochlear tissue potential using the electrode that is delivering current, the weight on each point of the diagonal was extrapolated from its neighboring electrodes. Other methods have been proposed such as Optimized Multipolar Stimulation (Smith 2009; Smith et al. 2013) where the value of diagonal is enhanced, half way between the MP and AP mode; or through a computational model (Frijns et al 2011). Although our method may not have been optimal, the results of Experiment 1 do suggest that the AP mode did succeed in creating a more focused electric field. Furthermore, Experiment 1 demonstrated that the AP mode allowed simultaneous activation of two AP electrodes with greatly reduced current summation compared to MP mode. Thus, one of the main advantages of AP mode may be the ability to activate different cochlear places with simultaneous pulses, or pulses overlapping in time, leading to the possibility of fine timing control of activity across cochlear place. Additionally, from the practical point of view, simultaneous electrode activation would allow higher rates and consequently lower currents to be used. Some commercial strategies (Advanced Bionics HiRes-P, HiRes-P with Fidelity 120, and Optima-P “parallel stimulation” options) use simultaneous pulses on pairs of channels in MP mode to increase the pulse rate; perhaps these strategies would achieve improved speech perception outcomes if current focusing were also used, as has been recently shown in tripolar mode (Srinivasan et al. 2013).
CONCLUSIONS
The AP stimulation mode resulted in less current summation for simultaneously activated AP electrodes compared to MP electrodes, suggesting benefits of AP mode for new strategies that provide fine across-electrode timing information with simultaneous or overlapping pulses, but did not lead to differences in the width of neural activity for equally loud single-electrode stimuli for these participants.
Acknowledgments
The authors would like to thank The Hearing CRC and Robert Briggs, Bev Sheridan, Michelle Knight, Kerrie Plant, Nik Kydas, and John Heasman for their help and support in the clinical management of the participants, Michelle Moran and Arielle Umansky for supplying the clinic-tested speech scores, and finally the editor, Ruth Litovsky, and two anonymous reviewers for their helpful and insightful comments. This project was funded by The Garnett Passe and Rodney Williams Memorial Foundation and supported by Cochlear Ltd. The Bionics Institute acknowledges the support it receives from the Victorian Government through its Operational Infrastructure Support Program.
Contributor Information
Jeremy Marozeau, Phone: +45 4525 4790, Email: jemaroz@elektro.dtu.dk.
Hugh J. McDermott, Email: hmcdermott@bionicsinstitute.org
Brett A. Swanson, Email: bswanson@cochlear.com
Colette M. McKay, Email: cmckay@bionicsinstitute.org
References
- Bierer JA. Threshold and channel interaction in cochlear implant users: evaluation of the tripolar electrode configuration. J Acoust Soc Am. 2007;121:1642–1653. doi: 10.1121/1.2436712. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Faulkner KF. Identifying cochlear implant channels with poor electrode-neuron interface: partial tripolar, single-channel thresholds and psychophysical tuning curves. Ear Hear. 2010;31:247–258. doi: 10.1097/AUD.0b013e3181c7daf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey P, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: an update with 2251 patients. Audiol Neurootol. 2013;18:36–47. doi: 10.1159/000343189. [DOI] [PubMed] [Google Scholar]
- Bonham BH, Litvak LM. Current focusing and steering: modeling, physiology, and psychophysics. Hear Res. 2008;242:141–153. doi: 10.1016/j.heares.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Micheyl C. On the choice of adequate randomization ranges for limiting the use of unwanted cues in same-different, dual-pair, and oddity tasks. Atten Percept Psychophys. 2010;72:538–547. doi: 10.3758/APP.72.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielden CA, Kluk K, McKay CM. Interpulse interval discrimination within and across channels: comparison of monopolar and tripolar mode of stimulation. J Acoust Soc Am. 2014;135:2913–2922. doi: 10.1121/1.4869687. [DOI] [PubMed] [Google Scholar]
- Fielden CA, Kluk K, McKay CM. Place specificity of monopolar and tripolar stimuli in cochlear implants: the influence of residual masking. J Acoust Soc Am. 2013;133:4109–4123. doi: 10.1121/1.4803909. [DOI] [PubMed] [Google Scholar]
- Fraser M, McKay CM. Temporal modulation transfer functions in cochlear implantees using a method that limits overall loudness cues. Hear Res. 2012;283:59–69. doi: 10.1016/j.heares.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110:1150–1163. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Frijns JH, Dekker DM, Briaire JJ. Neural excitation patterns induced by phased-array stimulation in the implanted human cochlea. Acta Otolaryngol. 2011;131:362–370. doi: 10.3109/00016489.2010.541939. [DOI] [PubMed] [Google Scholar]
- Frijns JH, Kalkman RK, Vanpoucke FJ, Bongers JS, Briaire JJ. Simultaneous and non-simultaneous dual electrode stimulation in cochlear implants: evidence for two neural response modalities. Acta Otolaryngol. 2009;129:433–439. doi: 10.1080/00016480802610218. [DOI] [PubMed] [Google Scholar]
- Fu Q-J, Shannon RV, Wang X. Effects of noise and spectral resolution on vowel and consonant recognition: acoustic and electric hearing. J Acoust Soc Am. 1998;104:3586–3596. doi: 10.1121/1.423941. [DOI] [PubMed] [Google Scholar]
- Landsberger DM, Galvin JJ. Discrimination between sequential and simultaneous virtual channels with electrical hearing. J Acoust Soc Am. 2011;130:1559–1566. doi: 10.1121/1.3613938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger DM, Padilla M, Srinivasan AG. Reducing current spread using current focusing in cochlear implant users. Hear Res. 2012;284:16–24. doi: 10.1016/j.heares.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CJ, Holden TA, McClelland GH, Parkinson WS, Shelton C, Kelsall DC, Smith ZM. Examining the electro-neural interface of cochlear implant users using psychophysics, CT scans, and speech understanding. J Assoc Res Otolaryngol. 2014;15:293–304. doi: 10.1007/s10162-013-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks LE, Florentine M. Measurement of loudness, part I: methods, problems and pitfalls. In: Florentine M, Popper AN, Fay RR, editors. Springer handbook of auditory research: loudness. New-York: Springer; 2011. pp. 17–56. [Google Scholar]
- McDermott H. Music perception. In: Zeng FG, Popper AN, Fay RR, editors. Auditory prostheses: new horizons. New York: Springer; 2011. pp. 305–339. [Google Scholar]
- McKay CM. Forward masking as a method of measuring place specificity of neural excitation in cochlear implants: a review of methods and interpretation. J Acoust Soc Am. 2012;131:2209–2224. doi: 10.1121/1.3683248. [DOI] [PubMed] [Google Scholar]
- McKay CM, McDermott HJ. The perceptual effects of current pulse duration in electrical stimulation of the auditory nerve. J Acoust Soc Am. 1999;106:998–1009. doi: 10.1121/1.428052. [DOI] [PubMed] [Google Scholar]
- McKay CM, Henshall KR, Farrell RJ, McDermott HJ. A practical method of predicting the loudness of complex electrical stimuli. J Acoust Soc Am. 2003;113:2054–2063. doi: 10.1121/1.1558378. [DOI] [PubMed] [Google Scholar]
- Moore BC, Alcántara JI. The use of psychophysical tuning curves to explore dead regions in the cochlea. Ear Hear. 2001;22:268–278. doi: 10.1097/00003446-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Multichannel electrical stimulation of the auditory nerve in man. II. Channel interaction. Hear Res. 1983;12:1–16. doi: 10.1016/0378-5955(83)90115-6. [DOI] [PubMed] [Google Scholar]
- Shannon RV. A model of safe levels for electrical stimulation. IEEE Trans Biomed Eng. 1992;39:424–426. doi: 10.1109/10.126616. [DOI] [PubMed] [Google Scholar]
- Smith ZM (2009) Multi-electrode channel configurations. In: Patent Application Publication (CN102361666A, ed). US
- Smith ZM, Parkinson WS, Long CJ (2013) Multipolar current focusing increases spectral resolution in cochlear implants. In: Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual International Conference of the IEEE, pp 2796-2799: IEEE [DOI] [PubMed]
- Srinivasan AG, Padilla M, Shannon RV, Landsberger DM. Improving speech perception in noise with current focusing in cochlear implant users. Hear Res. 2013;299:29–36. doi: 10.1016/j.heares.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Honert C, Kelsall DC. Focused intracochlear electric stimulation with phased array channels. J Acoust Soc Am. 2007;121:3703–3716. doi: 10.1121/1.2722047. [DOI] [PubMed] [Google Scholar]
- Vandali AE, Whitford LA, Plant KL, Clark GM. Speech perception as a function of electrical stimulation rate: using the Nucleus 24 cochlear implant system. Ear Hear. 2000;21:608–624. doi: 10.1097/00003446-200012000-00008. [DOI] [PubMed] [Google Scholar]