Abstract
The PI3K/Akt signaling pathway is involved in mediating survival of sensory hair cells. Here, we investigated the involvement of PI3K/Akt in noise-induced hearing loss in both temporary and permanent threshold shift noise models. The PI3K regulatory subunit p85α and phosphorylation of Akt on serine 473 (p-Akt S473) are downregulated in sensory hair cells, including both outer and inner hair cells, and supporting cells of the mouse organ of Corti 1 h after exposure to permanent-threshold-shift-inducing noise (PTS noise), but not with temporary-threshold-shift-inducing noise (TTS noise). In contrast, the PI3K catalytic subunit p110α and phosphorylation of Akt on threonine 308 (p-Akt T308) do not change with PTS or TTS noise. Additionally, mice pretreated with p85α small interfering RNA (siRNA) have decreased expression of p-Akt1 (S473) in their sensory hair cells and increased sensitivity to TTS noise-induced hearing loss. Finally, Akt1-knockout mice also have enhanced sensitivity to TTS noise-induced hearing loss. In conclusion, this study suggests that endogenous PI3K/Akt signaling is an intrinsic protective mechanism of the inner ear. Blockade of PI3K/Akt signaling pathways increases sensitivity to TTS noise-induced hearing loss.
Keywords: noise-induced hearing loss, PI3K, p85α siRNA, p-Akt, Akt1-knockout mice
Introduction
Considerable evidence has demonstrated that a decrease in phosphoinositide-3 kinase/protein kinase B (PI3K/Akt) signaling pathways is associated with hearing loss and hair cell death following a variety of insults and stimuli. For example, aminoglycoside treatment results in permanent hearing loss and sensory hair cell loss, and phosphorylation of Akt on serine 473 (p-Akt S473) in sensory hair cells is decreased by such treatment (Jiang et al. 2006). A decrease in p-Akt (S473) in sensory hair cells is also involved in age-related hearing impairment (Sha et al. 2010). On the other hand, Akt has been implicated as an integral part of the protein kinase C (PKC) pathway that promotes spiral ganglion neuron survival (Lallemend et al. 2005). In rat cochlear explants, treatment with an Akt inhibitor promotes gentamicin-induced outer hair cell (OHC) death (Chung et al. 2006) and diminishes the protective function of dexamethasone against TNFα-initiated apoptosis (Haake et al. 2009). Moreover, inhibition of the downstream Akt target NF-κB by aminoglycosides leads to loss of sensory hair cells in explants (Nagy et al. 2005, 2007) and mouse cochleae (Jiang et al. 2006). Taken together, this evidence suggests that increasing PI3K/Akt signaling might protect the inner ear from noxious stimuli.
PI3K plays a key role in various cellular processes, including cell survival, growth, and proliferation. The functions related to PI3K-regulated activation of Akt involve the binding of the pleckstrin homology (PH) domain of Akt to phosphatidylinositol (3,4,5)-triphosphate (PIP3), which is catalyzed by activated PI3K (Hemmings and Restuccia 2012). PI3K family members are lipid enzymes divided into three classes (classes I, II, and III) based on primary structure and regulatory function. PI3K class I is a heterodimer of various catalytic and regulatory subunits, of which the p110 catalytic and p85 regulatory subunits are commonly studied. Akt is one of the major downstream effectors of PI3K (Cantley 2002). The Akt family includes three members (Akt1, Akt2, and Akt3); each exhibits distinct features, although they are structurally homologous. Akt1 and Akt2 are ubiquitously expressed, while Akt3 has confined expression (Agarwal et al. 2013). Phosphorylation of Akt on serine 473 (p-Akt S473) or threonine 308 (p-Akt T308), the two activation sites of Akt, serves as a common hub in many anti-apoptotic pathways (Paez and Sellers 2003).
Although PI3K/Akt signaling pathways are associated with sensory hair cell survival following inner ear insults, the mechanisms through which PI3K/Akt regulate hair cell survival are not fully defined, particularly those induced in response to noise trauma. In this study, we investigate the molecular mechanisms of PI3K/Akt pathways in regulation of hair cell survival under both permanent threshold shift (PTS) and temporary threshold shift (TTS) noise models, in combination with p85α-small interfering RNA (siRNA) techniques using adult CBA/J mice and Akt1-knockout mice to elucidate role of endogenous PI3K/Akt signaling as an intrinsic protective molecule against inner ear insults.
Methods
Animals
Male CBA/J mice at 12 weeks of age (Harlan Sprague Dawley, Indianapolis, IN) had free access to water and a regular mouse diet (Purina 5025, St. Louis, MO) and were kept at 22 ± 1 °C under a standard 12:12 h light-dark cycle to acclimate for 1 week before the experiments. All research protocols were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina (MUSC). Animal care was under the supervision of the Division of Laboratory Animal Resources at MUSC.
C57/B6 Akt1-knockout mice were initially generated and kindly provided by Dr. Birnbaum from the University of Pennsylvania (Cho et al. 2001). After three generations of breeding at the University of Michigan’s Unit for Laboratory Animal Medicine, the fourth generation was used in this study. The animals were kept at 22 ± 1 °C under a 12:12 h light-dark cycle and had free access to water and a regular mouse diet (Purina #5025, St. Louis, MO). At the age of 2 weeks, the genotype of the animal was identified (Cho et al. 2001). The C57/B6 Akt1-knockout mice and their littermates were used for this study at 10 weeks of age in order to avoid the confounding effects of the age-related hearing loss (ahl) allele (Harding et al. 2005). Animal care was under the supervision of the University of Michigan’s Unit for Laboratory Animal Medicine, and all experimental protocols were approved by the University of Michigan Committee on Use and Care of Animals.
Noise Exposure
CBA/J mice at 12 weeks of age (one mouse per stainless steel wire cage approximately 9 × 9 × 9 cm) were exposed to a broadband noise (BBN) with a frequency spectrum from 2 to 20 kHz at 106 dB SPL for 2 h to induce permanent threshold shifts (PTS) or 96 dB SPL to induce temporary threshold shifts (TTS). C57/B6 Akt1-knockout mice and their littermates at the age of 10 weeks were exposed to BBN at 96 dB SPL to induce TTS. The sound intensity of the environment surrounding the cages was 65 dB as measured with a sound level meter (model 1200; Quest Technologies, Oconomovoc, WI). The sound exposure chamber is fitted with a loudspeaker (model 2450H; JBL, Northridge, CA) driven by a power amplifier (model XLS 202D; Crown Audio, Elkhart, IN) fed from a CD player (model CD-200; Tascam TEAC American, Montebello, CA). Audio CD sound files were created and equalized with audio editing software (Audition 3; Adobe System, Inc., San Jose, CA). Sound levels were calibrated with the sound level meter at multiple locations within the sound chamber to ensure uniformity of the sound field and were measured before and after exposure to ensure stability. Control mice were kept in silence (without use of the loudspeaker) within the same chamber for 2 h.
Auditory Brainstem Responses (ABRs)
Mice were anesthetized with an intra-peritoneal (IP) injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and then placed in a sound-isolated and electrically shielded booth (Acoustic Systems, Austin, TX). Body temperature was monitored and maintained near 37 °C with a heating pad. Acoustic stimuli were delivered monaurally to a Beyer earphone attached to a customized plastic speculum inserted into the ear canal. Sub-dermal electrodes were inserted at the vertex of the skull, under the left ear, and under the right ear (ground). ABRs were measured at 8, 16, and 32 kHz in adult CBA/J mice. Akt1-knockout mice and their littermates were measured at 6, 12, and 24 kHz due to the limitation of the ABR equipment at that time when the experiments were conducted. We have compared ABR scores at 24 and 32 kHz in CBA/J mice under both our TTS and PTS noise conditions and did not observe differences in auditory thresholds between 24 and 32 kHz. Tucker Davis Technology (TDT) System III hardware and SigGen/Biosig software (TDT, Alachua, FL) were used to present the stimuli (15 ms duration tone bursts with 1 ms rise-fall time) and record the response. Up to 1024 responses were averaged for each stimulus level. Thresholds were determined for each frequency by reducing the intensity in 10 dB increments and then in 5 dB steps near threshold until no organized responses were detected. Thresholds were estimated between the lowest stimulus level where a response was observed and the highest level without response. All ABR measurements were conducted by the same experimenter. The ABR scores were assigned by an expert who was blinded to the treatment conditions.
Intra-Tympanic Delivery of siRNA
The methods for siRNA delivery into the adult mouse inner ear in vivo were modified from a recent study (Oishi et al. 2013). Briefly, after anesthesia, a retroauricular incision was made to approach the temporal bone. The otic bulla was identified ventral to the facial nerve, and a shallow hole was made in the thin part of the otic bulla with a 30-G needle and enlarged with a dental drill to a diameter of 2 mm in order to visualize the round window. A customized sterile micro medical tube was inserted into the hole just above the round window niche to slowly deliver 0.6 μg (15 μL) of pre-designed p85α siRNA (Life Technologies #S71608, Carlsbad, CA) to cover the round window niche. After the siRNA was delivered, the hole was covered with surrounding muscle and glued with tissue adhesive. Finally, the skin incision was closed with tissue adhesive and the mouse was kept in the surgical position for about 1 h. Seventy-two hours after siRNA delivery, the animals were exposed to BBN or kept in silence in the noise chamber for 2 h. Since a hole of 2-mm diameter was made for visualization of the round window niche, we used 15 μL of the siRNA solution for delivery, although the volume of the mouse middle ear is 5–8 μL, to allow for expected leakage.
Immunocytochemistry for Cochlear Surface Preparations and Cyosections
The temporal bones were removed immediately after decapitation and perfused through the scala with a solution of 4 % paraformaldehyde in PBS, pH 7.4, and kept in this fixative overnight at 4 °C. The cochleae were then rinsed in PBS. Before decalcification of each cochlea in a 4 % solution of sodium EDTA for 72 h at 4 °C (adjusted with HCl to pH 7.4), the apical otic capsule was removed. The EDTA solution was changed daily for 3 days and maintained at 4 °C. Following decalcification, each cochlea for immunocytochemistry was dissected under a microscope by removing the softened otic capsule, stria vascularis, Reissner’s membrane, and tectorial membrane. The remaining tissue, including the modiolus and cochlear sensory epithelium, was permeabilized in 3 % Triton X-100 solution for 30 min at room temperature. For preparations of cryostat sections, cochleae were embedded following decalcification with 4 % EDTA. Sections of 8-μm thickness were incubated in 0.3 % Triton X-100 in phosphate-buffered saline (PBS) for 30 min at room temperature. The specimens were washed three times (10 min each) with PBS and blocked with 10 % goat serum for 30 min at room temperature, followed by incubation with monoclonal rabbit anti-PI3K p85α (Millipore #04-403, 1:50), monoclonal rabbit anti-PI3K p110α antibody (Cell Signaling Technology #4249, 1:50), monoclonal rabbit anti-p-Akt (S473) (Cell Signaling Technology #4060, 1:50), monoclonal rabbit anti-p-Akt (T308) (Cell Signaling Technology #2965, 1:50), in darkness at 4 °C for 72 h. After washing three times, the tissues were then incubated with the Alexa Fluor 594-conjugated secondary antibody at a concentration of 1:200 at 4 °C overnight in darkness. For the p85α antibody only, biotinylated goat anti-rabbit IgG secondary antibody (Vector Laboratories Inc. #PK-4001, 1:100) was used instead. Following three washes, the specimens were incubated with Alexa Fluor 594 streptavidin (Invitrogen #S-32356, 1:100) at 4 °C overnight. Following three more washes, the specimens were incubated with Alexa Fluor 488 Phalloidin (Life Technologies #12379, 1:100) at room temperature for 60 min. Finally, after washing at least three times, the specimens were dissected in PBS by removing the modiolus under micro-dissection and divided into three segments (apex, middle, and base). Each segment was mounted on slides with anti-fade mounting media. Control incubations were routinely processed without primary antibody treatments. Immunolabeled images were taken using a Zeiss laser confocal microscope (Zeiss LSM 510).
Quantification of the Immunofluorescence Signals from Basal Turn Surface Preparations
Immunofluorescence (red) of p85α, p110α, and p-Akt (S473) in OHCs on surface preparations was quantified from original confocal images, each taken with a ×63 magnification lens under identical conditions and equal parameter settings for laser gains and photomultiplier tube gains, using ImageJ software (National Institutes of Health, Bethesda, MD). All the surface preparations were counter-labeled with Alexa Fluor 488 phalloidin (green) for labeling cell structure in order to identify the comparable parts of the OHCs for capture of confocal images. The borders of each individual OHC were outlined based on the phalloidin labeling. The immunofluorescence of p85α, p110α, and p-Akt (S473) in OHCs was measured in the basal turn of surface preparations in 0.12-mm segments, each containing about 60 OHCs. The intensity of the background fluorescence was subtracted, and the average fluorescence per cell was then calculated. The relative fluorescence was quantified by normalizing the ratio of the average fluorescence in OHCs of treated groups to that of control groups from the same set of experiments handled in parallel with identical procedures.
Extraction of Total Protein
For each sample, cochleae from both ears of one mouse were rapidly removed and dissected in ice-cold PBS containing complete™ mini EDTA-free protease inhibitor cocktail tablets (Roche Diagnostic, Indianapolis, IN) at pH 7.4. To extract total protein, tissues from the cochleae of one mouse were homogenized in ice-cold RIPA lysis buffer containing RIPA lysis buffer base (50 mM Tris-HCl, 1 % IGEPAL, 0.25 % Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM NaF) plus Phosphatase Inhibitor Cocktails II and III (Sigma-Aldrich, St. Louis, MO) and Roche protease inhibitor by using a glass/glass micro tissue grind pestle and tube for 30 s. After 30 min on ice, tissue debris were removed by centrifugation at 10,000×g at 4 °C for 10 min, and the supernatants were retained as the total protein fractions. Protein concentrations were determined using the Bio-Rad Protein Assay dye reagent (Bio-Rad, Hercules, CA) with bovine serum albumin as a protein standard. Two cochleae from the same mouse were pooled for each sample.
Western Blot Analysis
Protein samples (50 μg each) were separated by SDS-PAGE. After electrophoresis, the proteins were transferred onto a nitrocellulose membrane (Pierce, Rockford, IL) and blocked with 5 % nonfat dry milk in PBS-0.1 % Tween 20 (PBS-T). The membranes were incubated with anti-p85α (1:1000), anti-p110α (1:1000), anti-p-Akt (S473) (1:1000), anti-total Akt1/2 (Cell Signaling Technology #9272, 1:1000), or anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Millipore #ABS16, 1:10,000) at 4 °C overnight, and then washed three times (10 min each) with PBS-T buffer. Membranes were incubated with an appropriate secondary antibody at a concentration of 1:2500 for 1 h. Following extensive washing of the membranes, the immunoreactive bands were visualized by SuperSignal West Dura Extended Duration Substrate or Pierce ECL Western Blotting Substrate (Thermo Scientific, Waltham, MA). The membranes were then stripped and re-labeled for GAPDH at a concentration of 1:20,000 as a control for sample loading.
X-ray films of Western blots were scanned and analyzed using ImageJ software. The band densities were first normalized to the background. Next, the probing protein/GAPDH ratio was calculated from the band densities run on the same gel. Finally, the difference in the ratio of the control and experimental bands was analyzed for statistical significance. If three independent trials showed nearly identical band densities, we concluded there was no change in the variable. If the band densities were different between treatment and control groups in three independent trials, we increased n to five or six in order to assess statistical significance.
Statistical Analysis
Data were analyzed using IBM SPSS Statistics Premium V21 and GraphPad software for Windows. The group size (n) in vivo was determined by the variability of measurements and the magnitude of the differences between groups. Based on our previous and current preliminary studies, we determined that five to eight animals per group generally provides sufficient statistical power. Statistical methods used included one-way analysis of variance (ANOVA), Tukey’s multiple comparisons, unpaired t tests, and one-sample t tests. All tests were two-tailed and a p value <0.05 was considered statistically significant. Data are presented as means and SD.
Results
PTS Noise Decreases PI3K Regulatory Subunit p85α, but not Catalytic Subunit p110α, in OHCs
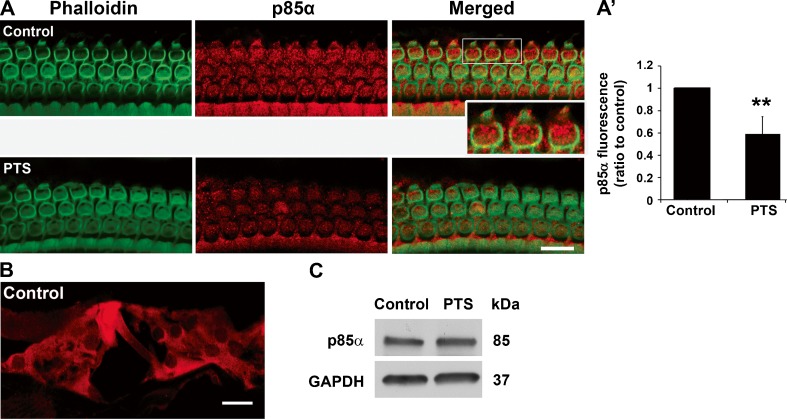
A CBA/J mouse model has been characterized in our laboratory to study noise-induced temporary threshold shifts (TTS) and permanent threshold shifts (PTS). Exposure of 3-month-old male CBA/J mice to 92 or 96 dB SPL at 2–20 kHz broadband noise (BBN) for 2 h results in TTS, while exposure to either 98 or 106 dB SPL results in PTS (Yuan et al. 2014, submitted). To examine whether noise exposure alters the levels of PI3K in sensory hair cells, we first assessed the levels of PI3K regulatory subunit p85α and catalytic subunit p110α on surface preparations of CBA/J mice 1 h after PTS or TTS noise exposure. Both p85α and p110α showed punctuate immunolabeling in OHCs. The punctuate immunolabeling of p85α in OHCs was weaker after PTS noise compared to controls without noise exposure (Fig. 1A, red, inset enlarged OHCs show punctuate labeling) but did not change after TTS noise compared to the controls (data not shown). Quantitative analysis of the p85α immunolabeling in OHCs from original confocal images revealed that PTS noise significantly diminished the levels of p85α. The ratio of immunofluorescence in controls without noise exposure to those with PTS noise exposure was 1:0.6 (Fig. 1(A’), t3 = 5.167, p = 0.014). Next, cochlear cryosections of mice without noise exposure were immunolabeled with p85α antibody in order to illustrate the localization of p85α in the organ of Corti. The immunolabeled p85α was observed in all types of cells in the organ of Corti, including OHCs and IHCs and Deiters cells (Fig. 1B). In addition, the levels of p85α in cochlear homogenates were detected by Western blot and showed a single band with no difference between samples from control mice without noise and mice 1 h after PTS noise (Fig. 1C). In contrast, punctuate immunolabeling of p110α in OHCs underwent no obvious change even after PTS noise exposure (t3 = 0.2828, p = 0.80, data not shown). In addition, the levels of p110α in cochlear homogenates detected by Western blots showed a single band with no difference between samples from control mice without noise and mice 1 h after PTS noise (data not shown).
Fig. 1.
PTS noise decreases p85α levels in OHCs. A PTS noise diminishes the immunolabeling of p85α (red) in OHCs of surface preparations compared to control samples without noise exposure. The enlarged inset image of the three OHCs circumscribed by the white rectangle demonstrates punctuate labeling of p85α. Images were taken from cochlear basal turns and each figure is representative of four individual mice for each condition. Green: phalloidin labeling of sensory hair cells. Scale bar = 10 μm. A’ Quantification of p85α-associated fluorescence in OHCs confirms a significant reduction. Data are presented as mean + SD; n = 4 for each group; **p < 0.01. B A cryosection of the normal adult CBA/J mouse inner ear shows p85α immunolabeling (red) in the organ of Corti, including in OHCs, IHCs, and supporting cells. The image was taken from the cochlear basal turn and is representative of three individual mice. Scale bar = 10 μm. C Western blot analysis of total cochlear homogenates revealed no difference in the levels of p85α between control and PTS noise-exposed mice. GAPDH serves as sample loading control; n = 4.
PTS Noise Decreases p-Akt S473 but Not p-Akt T308 in OHCs and Increases p-Akt S473 in Total Cochlear Homogenates
Next, we examined the levels of Akt phosphorylated at either activation site on surface preparations of CBA/J mice 1 h after exposure to TTS or PTS noise. p-Akt (S473) had punctuate-appearing expression (Fig. 2A, inset enlarged OHCs, red) in OHCs that decreased 1 h after PTS noise, but expression was unchanged following TTS noise compared to controls (Fig. 2A). Quantification of the immunolabeling of p-Akt (S473) in OHCs confirmed a significant decrease after PTS noise (t5 = 7.147, p < 0.001) but not after TTS noise (t2 = 0.231, p = 0.84) compared to control cochleae without noise treatment (Fig. 2(A’)). Additionally, the immunolabeling of p-Akt (T308) in OHCs did not change after PTS noise (t2 = 0.2937, p = 0.80, data not shown). Furthermore, p-Akt (S473) was localized in all types of cochlear cells, including OHCs, IHCs, and Deiters cells, by immunolabeling p-Akt (S473) antibody on cochlear cryosections of CBA/J mice without noise exposure (Fig. 2B, green). Additionally, total Akt1/2 in cochlear homogenates of control animals and those 1 h post-PTS noise showed single bands on Western blots with no difference between these two groups (Fig. 2C). However, the levels of p-Akt (S473) increased 2-fold 1 h after PTS noise exposure by Western blot analysis of total cochlear homogenates (Fig. 2D, t5 = 2.735, p = 0.041).
Fig. 2.
Levels of p-Akt (S473) decrease in OHCs but increase in total cochlear homogenates after PTS noise exposure. A Surface preparations show weak immunolabeling of p-Akt (S473) in OHCs 1 h after PTS noise, but not after TTS noise exposure. The enlarged inset images, each of three OHCs, demonstrate the punctuate labeling of p-Akt (S473). Images were taken from cochlear basal turns and each figure is representative of three individual mice for each condition. Green: phalloidin labeling of OHCs; red: p-Akt. Scale bar = 10 μm. A’ Quantification of p-Akt-associated fluorescence in OHCs confirms a significant decrease under PTS but not under TTS conditions. Data are presented as means + SD; n = 6 for PTS or control, n = 3 for TTS; **p < 0.01. B Immunolabeled p-Akt (S473) (green) in a cryosection from a normal adult CBA/J mouse shows localization in the organ of Corti, including in OHCs, IHCs, and Deiters cells. Red: PI-labeled nuclei. Scale bar = 10 μm. C There were no changes in total Akt1/2 levels 1 h after PTS noise in total cochlear tissue homogenates by Western blots. GAPDH serves as the loading control; n = 3. D The levels of p-Akt (S473) increased in total cochlear homogenates 1 h after PTS noise exposure. Data are presented as means + SD; *p < 0.05; n = 6.
p-Akt (S473) Is a Downstream Effector of p85α
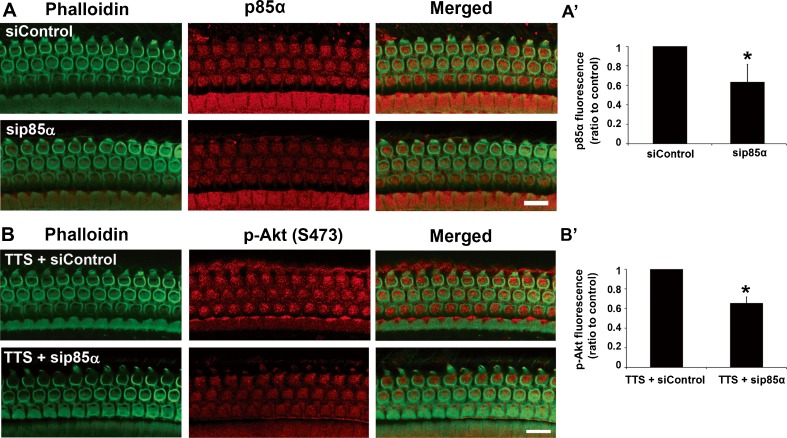
PTS noise reduces both p85α and p-Akt (S473) levels in OHCs. In order to confirm that p-Akt (S473) is a downstream effector protein of PI3K in OHCs, we silenced p85α in CBA/J mice. First, local delivery of p85α siRNA led to diminished expression of p85α protein in OHCs 72 h after siRNA treatment (Fig. 3A). Quantitative analysis of the silencing efficiency showed that the p85α siRNA reduced expression by 30 % in OHCs compared to control cochleae that were treated with scrambled control siRNA (Fig. 3(A’), t4 = 4.489, p = 0.011). Since there were no observable changes in the levels of p-Akt (S473) under TTS noise conditions, these conditions were exploited to examine if p-Akt (S473) is a downstream effector protein of p85α in OHCs. CBA/J mice were exposed to TTS noise 72 h after pretreatment with p85α siRNA. One hour after the TTS noise exposure, surface preparations were immunolabeled with an anti-p-Akt (S473) antibody. The immunolabeling of p-Akt (S473) was relatively weak after pretreatment with p85α siRNA compared to those treated with control siRNA (Fig. 3B). Quantification of p-Akt (S473) immunolabeling in OHCs verifies a significant reduction in p85α-siRNA-treated cochleae (Fig. 3(B’), t2 = 9.4, p = 0.011).
Fig. 3.
Silencing p85α reduces the expression of p-Akt in OHCs. A Local delivery of p85α-siRNA (sip85α) diminishes the expression of p85α in OHCs. Images were taken from the basal turn and each figure is representative of five individual mice for each condition. Scale bar = 10 μm. Green: phalloidin labeling of cell structure; red: p85α. A’ Quantification of p85α-associated fluorescence in OHCs reveals a significant decrease in the expression of p85α in OHCs treated with p85α siRNA. Data are presented as the mean + SD; n = 5 for each group; *p < 0.05. B Under TTS noise conditions, treatment with p85α siRNA reduces the expression of p-Akt (S473) in OHCs. Images are taken from the basal turn and each figure is representative of three individual mice for each condition. Scale bar = 10 μm. Green: phalloidin labeling of sensory hair cell structure; red: p-Akt. B’ Quantification of p-Akt-associated fluorescence in OHCs shows a significant decrease with p85α siRNA treatment 1 h after TTS noise. Data are presented as the mean + SD; n = 3 for each group; *p < 0.05.
Inhibition of PI3K/Akt Signaling Increases Sensitivity to Noise Damage
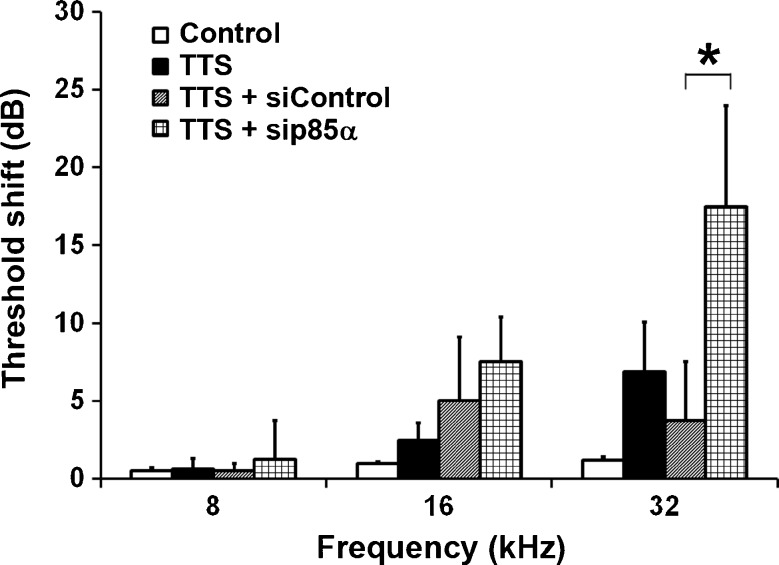
Since the levels of p85α and p-Akt (S473) in OHCs were unchanged by TTS noise treatment, we silenced p85α in CBA/J mice to examine whether blockade of PI3K increases the sensitivity to noise damage, as assessed by ABR measurements. Under TTS noise conditions, auditory thresholds at all three measured frequencies (8, 16, and 32 kHz) recovered completely to baseline by 7 days after the exposure and only had non-significant elevations above baseline at 3 days post-exposure. However, pretreatment with p85α siRNA resulted in a significant increase in threshold shifts at 32 kHz, but not at 8 and 16 kHz, 3 days after TTS noise exposure compared to the other three groups (control without TTS noise, TTS noise only, and control siRNA treatment plus TTS noise) (Fig. 4, F3,15 = 5.943, p = 0.0072). Further statistical analysis using Tukey’s multiple comparisons test showed that the auditory threshold shifts at 32 kHz in p85α-silenced mice were significantly higher than those of siRNA control mice (p = 0.0087). Finally, auditory thresholds of p85α-silenced mice were not statistically different from control siRNA-treated mice 7 days post-exposure, and both groups returned to baseline auditory function by 14 days. In addition, mice pretreated with p85α siRNA or scrambled siRNA had comparable auditory threshold shifts 14 days after PTS noise exposure.
Fig. 4.
Silencing p85α increases sensitivity to noise-induced hearing loss. Under TTS noise conditions, auditory threshold shifts recovered to baseline 3 days after the exposure in 3-month-old CBA/J mice, while after pretreatment with p85α-siRNA the auditory thresholds remain significantly elevated at 32 kHz compared to control siRNA-treated mice. Data are presented as means + SD; n = 8 in the TTS noise group, n = 4 in all other groups; *p < 0.05.
Next, Akt1-knockout mice were used to examine whether knockdown of Akt1 increases the sensitivity to noise under TTS noise conditions. First, we detected the knockdown of Akt1 protein in whole cochlear homogenates of Akt1-knockout mice compared to their wild-type littermates. Probing the cochlear homogenates with total Akt (1/2) antibody showed that the band density was significantly reduced in Akt1-knockout mice compared to their wild-type littermates (Fig. 5A, t2 = 43.755, p < 0.001). There was no significant difference between wild-type and Akt1-heterozygous mice (t2 = 1.239, p = 0.34), but there were nearly significant differences between Akt1-knockout and heterozygous mice (t4 = 2.701, p = 0.054). Finally, Akt1-knockout mice and their littermates (wild types and heterozygotes) were exposed to TTS noise. The auditory threshold shifts of the three groups at 24 kHz were significantly different 3 days after the exposure (Fig. 5B, F2,29 = 4.351, p = 0.023), but not at 6 and 12 kHz (data not shown). Further analysis using Tukey’s multiple comparisons test revealed that the elevation of auditory thresholds induced by TTS noise were significantly higher in Akt1-knockout mice than their wild-type littermates (p = 0.018). Finally, the auditory thresholds of wild-type and heterozygous mice completely recovered to baseline 7 days after TTS noise, while Akt1-knockout mice took 14 days to recover completely. In addition, Akt1-knockout mice and their littermates had similar auditory threshold shifts 14 days after PTS noise exposure.
Fig. 5.
Akt1-knockout mice have increased sensitivity to noise-induced hearing loss. A Western blot analysis of total cochlear homogenates shows a significant decrease in the expression of total Akt1/2 in Akt1-knockout mice. Data are presented as means + SD; n = 3 in each group; **p < 0.001. B Under TTS noise conditions, auditory thresholds recovered to baseline 3 days after the exposure in wild-type mice, while those of Akt1-knockout mice remained significantly elevated at 24 kHz. Data are shown as means + SD; Akt+/+: n = 8, Akt+/−: n = 16, Akt−/−: n = 6; *p < 0.05.
Discussion
The salient finding of this study is that PTS noise diminishes the levels of p85α and p-Akt (S473) signaling molecules in OHCs, while TTS noise does not induce any alteration in p85α or p-Akt (S473). While both C57/B6 Akt1-knockout mice and adult CBA/J mice treated with p85α siRNA have increased sensitivity to TTS noise, neither has increased sensitivity to PTS noise-induced hearing loss nor OHC loss, providing further confirmation that the endogenous PI3K/Akt signaling pathway plays a role in survival against inner ear stress.
The PI3K/Akt signaling cascade serves as a common hub for anti-apoptotic pathways. The decrease in the levels of the regulatory subunit of PI3K, p85α, in OHCs after PTS noise might relate to insulin-like growth factor 1 (IGF-1) signaling pathways as IGF-1 binds to its receptor, IGF-1 tyrosine kinase receptor (IGF-1R), to activate PI3K/Akt pathways (Varela-Nieto et al. 2003). In fact, activation of IGF-1 has been reported to cause an increase in the PI3K/Akt signaling pathway and block apoptosis in sensory hair cells in organ cultures treated with neomycin (Hayashi et al. 2013). Additionally, treatment with IGF-1 prevents hair cell damage from ischemic injury (Fujiwara et al. 2008). The fact that PTS noise decreases p85α in OHCs provides further evidence that p85α may be a potential target for prevention of noise-induced hearing loss (NIHL).
The decreased levels of p-Akt (S473) in OHCs after exposure to PTS noise are in accordance with early reports that p-Akt (S473) levels decrease after aminoglycoside treatment or with aging (Jiang et al. 2006; Chung et al. 2006; Haake et al. 2009; Sha et al. 2010). However, the levels of p-Akt (S473) increase in whole cochlear homogenates according to Western blot analysis, implicating another cochlear cell type in response to PTS noise stress. Such an increase may protect other cochlear cell types against the insults, as we do not observe damage to supporting cells or SGCs under the noise conditions (Zheng et al. 2014). This discrepancy between the levels of p-Akt (S473) in OHCs and whole cochlear tissue preparations may explain, at least to some extent, the common finding that sensory hair cells are particularly vulnerable to inner ear insults.
Activation of Akt1 depends on distinct enzymes to phosphorylate two residues (T308 and S473) (Alessi et al. 1996). Phosphorylation of S473 decreases in sensory hair cells under the PTS noise, but not under TTS noise conditions, while phosphorylation of the T308 residue is unchanged under either PTS or TTS noise. This may agree with the notion that Akt is one of the downstream signaling targets of PI3K since pretreatment with p85α siRNA decreased expression of p-Akt (S473) in OHCs after TTS noise exposure. However, activation of p-Akt via mTOR2 signaling cascades is also involved in regulation of p-Akt (S473). Additionally, inhibition of mTOR1 by rapamycin derivatives increases mTOR2, resulting in activation of p-Akt (S473) (Sarbassov et al. 2005; McDonald et al. 2008; Breuleux et al. 2009; Vadlakonda et al. 2013). In line with this notion, attenuation of PTS noise-induced hearing loss with rapamycin may also be due in part to the activation of p-Akt (S473) (Yuan et al. 2014, submitted).
The most significant findings of this study are that reduction of PI3K/Akt signals in adult CBA/J mice by pretreatment with p85α siRNA or knockdown of Akt1 in C57/B6 mice increases sensitivity to TTS noise but does not alter PTS or OHC survival, indicating that PI3K/Akt signals apparently take part in stress-induced protective pathways that work toward restoring homeostasis in the cell and thereby decrease sensitivity to TTS noise. Thus, PI3K/Akt pathways do afford some protection against stresses that are sub-lethal to the sensory hair cells, as in TTS noise. However, such protective capacity cannot combat a major onslaught as with the severity of the PTS noise stress. PI3K/Akt and their restorative capacity may not be sufficient to protect from OHC death, so silencing p85α or knockout of Akt1 would not affect cell fate. Furthermore, PTS noise induces multiple cell death pathways in which cell death signaling overcomes that of endogenous protective molecules that may circumvent PI3K/Akt (Zheng et al. 2014). Additionally, cellular ATP depletion occurs after PTS noise, not TTS noise, resulting in activation of AMPK in OHCs (Chen et al. 2012). Activation of AMPK regulates pro-apoptotic proteins such as c-Jun N-terminal kinase (JNK) (Concannon et al. 2010; Nagashima et al. 2011). Treatment with a JNK inhibitor attenuates NIHL (Wang et al. 2003). There could also be some overlap in the functions of Akt1 and Akt2, which have 82 % amino acid homology (Laine et al. 2002). Both Akt1 and Akt2 are involved in cancer pathogenesis by blocking apoptotic pathways and the isoforms are assumed to have identical or similar substrate specificity (Walker et al. 1998). In summary, this study reinforces the notion that endogenous PI3K/Akt signaling pathways are protective against inner ear insults to some extent and suggests that the PI3K/Akt pathway is only one of multiple molecular signaling pathways involved in noise-induced hearing loss.
Acknowledgments
The research project described was supported by grant R01 DC009222 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health. This work was conducted in part in the WR Building at MUSC in renovated space supported by grant C06 RR014516. Animals were housed in MUSC CRI animal facilities supported by grant C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. We thank Dr. Jochen Schacht for his valuable comments on the manuscript.
Author Disclosure Statement
The data contained in the manuscript have not been previously published and have not been submitted elsewhere and will not be submitted elsewhere while under review. All research protocols were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina (MUSC) or by the University of Michigan (UM) Committee on Use and Care of Animals. Animal care was under the supervision of Division of Laboratory Animal Resources at MUSC or of Unit for Laboratory Animal Medicine at UM. All authors have reviewed the contents of the manuscript, approve of its contents, and validate the accuracy of the data.
Conflict of Interest
There are no conflicts of interest for any of the authors.
Contributor Information
Jun Chen, Email: edondongcccj@aliyun.com.
Hu Yuan, Email: yuanhud@163.com.
Andra E. Talaska, Email: atalaska@umich.edu
Su-Hua Sha, Phone: 843-792-8324, Email: shasu@musc.edu.
References
- Agarwal E, Brattain MG, Chowdhury S. Cell survival and metastasis regulation by Akt signaling in colorectal cancer. Cell Signal. 2013;25:1711–1719. doi: 10.1016/j.cellsig.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Breuleux M, Klopfenstein M, Stephan C, Doughty CA, Barys L, Maira SM, Kwiatkowski D, Lane HA. Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Mol Cancer Ther. 2009;8:742–753. doi: 10.1158/1535-7163.MCT-08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Chen FQ, Zheng HW, Hill K, Sha SH. Traumatic Noise Activates Rho-Family GTPases through Transient Cellular Energy Depletion. J Neurosci. 2012;32:12421–12430. doi: 10.1523/JNEUROSCI.6381-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Chung WH, Pak K, Lin B, Webster N, Ryan AF. A PI3K pathway mediates hair cell survival and opposes gentamicin toxicity in neonatal rat organ of Corti. J Assoc Res Otolaryngol. 2006;7:373–382. doi: 10.1007/s10162-006-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon CG, Tuffy LP, Weisova P, Bonner HP, Davila D, Bonner C, Devocelle MC, Strasser A, Ward MW, Prehn JH. AMP kinase-mediated activation of the BH3-only protein Bim couples energy depletion to stress-induced apoptosis. J Cell Biol. 2010;189:83–94. doi: 10.1083/jcb.200909166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Hato N, Nakagawa T, Tabata Y, Yoshida T, Komobuchi H, Takeda S, Hyodo J, Hakuba N, Gyo K. Insulin-like growth factor 1 treatment via hydrogels rescues cochlear hair cells from ischemic injury. Neuroreport. 2008;19:1585–1588. doi: 10.1097/WNR.0b013e328311ca4b. [DOI] [PubMed] [Google Scholar]
- Haake SM, Dinh CT, Chen S, Eshraghi AA, Van De Water TR. Dexamethasone protects auditory hair cells against TNFalpha-initiated apoptosis via activation of PI3K/Akt and NFkappaB signaling. Hear Res. 2009;255:22–32. doi: 10.1016/j.heares.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA, Vos JD. The effect of an age-related hearing loss gene (Ahl) on noise-induced hearing loss and cochlear damage from low-frequency noise. Hear Res. 2005;204:90–100. doi: 10.1016/j.heares.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Yamamoto N, Nakagawa T, Ito J. Insulin-like growth factor 1 inhibits hair cell apoptosis and promotes the cell cycle of supporting cells by activating different downstream cascades after pharmacological hair cell injury in neonatal mice. Mol Cell Neurosci. 2013;56C:29–38. doi: 10.1016/j.mcn.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Schacht J. Kanamycin alters cytoplasmic and nuclear phosphoinositide signaling in the organ of Corti in vivo. J Neurochem. 2006;99:269–276. doi: 10.1111/j.1471-4159.2006.04117.x. [DOI] [PubMed] [Google Scholar]
- Laine J, Kunstle G, Obata T, Noguchi M. Differential regulation of Akt kinase isoforms by the members of the TCL1 oncogene family. J Biol Chem. 2002;277:3743–3751. doi: 10.1074/jbc.M107069200. [DOI] [PubMed] [Google Scholar]
- Lallemend F, Hadjab S, Hans G, Moonen G, Lefebvre PP, Malgrange B. Activation of protein kinase CbetaI constitutes a new neurotrophic pathway for deafferented spiral ganglion neurons. J Cell Sci. 2005;118:4511–4525. doi: 10.1242/jcs.02572. [DOI] [PubMed] [Google Scholar]
- McDonald PC, Oloumi A, Mills J, Dobreva I, Maidan M, Gray V, Wederell ED, Bally MB, Foster LJ, Dedhar S. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res. 2008;68:1618–1624. doi: 10.1158/0008-5472.CAN-07-5869. [DOI] [PubMed] [Google Scholar]
- Nagashima R, Yamaguchi T, Kuramoto N, Ogita K. Acoustic overstimulation activates 5′-AMP-activated protein kinase through a temporary decrease in ATP level in the cochlear spiral ligament prior to permanent hearing loss in mice. Neurochem Int. 2011;59:812–820. doi: 10.1016/j.neuint.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Nagy I, Monge A, Albinger-Hegyi A, Schmid S, Bodmer D. NF-kappaB is required for survival of immature auditory hair cells in vitro. JARO. 2005;6:260–268. doi: 10.1007/s10162-005-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy I, Caelers A, Monge A, Bonabi S, Huber AM, Bodmer D. NF-kappaB-dependent apoptotic hair cell death in the auditory system. Audiol Neurootol. 2007;12:209–220. doi: 10.1159/000101328. [DOI] [PubMed] [Google Scholar]
- Oishi N, Chen FQ, Zheng HW, Sha SH. Intra-tympanic delivery of short interfering RNA into the adult mouse cochlea. Hearing Res. 2013;296:36–41. doi: 10.1016/j.heares.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez J, Sellers WR. PI3K/PTEN/AKT pathway. A critical mediator of oncogenic signaling. Cancer Treat Res. 2003;115:145–167. doi: 10.1007/0-306-48158-8_6. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sha SH, Chen FQ, Schacht J. PTEN attenuates PIP3/Akt signaling in the cochlea of the aging CBA/J mouse. Hear Res. 2010;264:86–92. doi: 10.1016/j.heares.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlakonda L, Dash A, Pasupuleti M, Anil Kumar K, Reddanna P. The Paradox of Akt-mTOR Interactions. Front Oncol. 2013;3:165. doi: 10.3389/fonc.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Nieto I, de la Rosa EJ, Valenciano AI, Leon Y. Cell death in the nervous system: lessons from insulin and insulin-like growth factors. Mol Neurobiol. 2003;28:23–50. doi: 10.1385/MN:28:1:23. [DOI] [PubMed] [Google Scholar]
- Walker KS, Deak M, Paterson A, Hudson K, Cohen P, Alessi DR. Activation of protein kinase B beta and gamma isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B alpha. Biochem J. 1998;331(Pt 1):299–308. doi: 10.1042/bj3310299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23:8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Chen J, Hill K, Lemasters J, Yang SM, Sha SH (2014) Autophay attenuates noise-induced hearing loss by reducing oxidative stress. ARS, manuscript submitted [DOI] [PMC free article] [PubMed]
- Zheng HW, Chen J, Sha SH. Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death Dis. 2014;5:e1262. doi: 10.1038/cddis.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]