Abstract
Anorexia nervosa is a serious psychiatric disorder characterized by restricted eating, a pursuit of thinness, and altered perceptions of body shape and size. Neuroimaging in anorexia nervosa has revealed morphological and functional alterations in the brain. A better understanding of physiological changes in anorexia nervosa could provide a brain-specific health marker relevant to treatment and outcomes. In this study, we applied several advanced magnetic resonance imaging (MRI) techniques to quantify regional and global cerebral blood flow (CBF) in 25 healthy women (HC), 23 patients currently with anorexia (AN-C) and 19 patients in long-term weight recovery following anorexia (AN-WR). Specifically, CBF was measured with pseudo-continuous arterial spin labeling (pCASL) MRI and then verified by a different technique, phase contrast (PC) MRI. Venous T2 values were determined by T2 relaxation under spin tagging (TRUST) MRI, and were used to corroborate the CBF results. These novel techniques were implemented on a standard 3T MRI scanner without any exogenous tracers, and the total scan duration was less than 10 min. Voxel-wise comparison revealed that the AN-WR group showed lower CBF in bilateral temporal and frontal lobes than the AN-C group. Compared with the HC group, the AN-C group also showed higher CBF in the right temporal lobe. Whole-brain-averaged CBF was significantly decreased in the AN-WR group compared with the AN-C group, consistent with the PC-MRI results. Venous T2 values were lower in the AN-WR group than in the AN-C group, consistent with the CBF results. A review of prior work examining CBF in anorexia nervosa is included in the discussion. This study identifies several differences in the cerebral physiological alterations in anorexia nervosa, and finds specific differences relevant to the current state of the disorder.
Keywords: Cerebral blood flow, Arterial spin labeling, Phase contrast, MRI, T2 relaxation under spin tagging (TRUST), Eating disorders
1. Introduction
Anorexia nervosa is a serious psychiatric disorder characterized by calorie restriction leading to significant weight loss, fear of weight gain, and a disturbance in body-image (American Psychiatric Association, 1994). The precise etiology of anorexia nervosa is still unknown, but many factors are thought to contribute to anorexia, including genetic, neural, psychological, and social (Garfinkel and Garner, 1983; Bulik et al., 2008; Kaye et al., 2011; Brown and Keel, 2012; Scott-Van Zeeland et al., 2014). Unfortunately, the success of treatments is very limited, with nearly 5% of patients dying from the disorder, the highest mortality rate for any mental illness (Hoek, 2006; Bulik et al., 2008). A better understanding of the physiological characteristics of brain function in anorexia nervosa may assist in understanding both the causes and consequences of the illness.
Cerebral microvasculature abnormalities may play a significant role in the psychiatric disorders (West, 2007). It is plausible that abnormalities in microvasculature can result in functional deficits because of the coupling between neuronal activity and blood oxygen consumption (Roy and Sherrington, 1890; Kuschinsky, 1991). The most common techniques used to detect this abnormality are to measure brain perfusion and metabolic parameters by nuclear medicine techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT). Techniques based on magnetic resonance imaging (MRI) are more attractive for psychiatric research because they provide both noninvasive and reproducible measures of cerebral microvasculature (Theberge, 2008). Several studies have shown agreement between MRI-based physiological studies and nuclear medicine studies (Liu et al., 2012; Zimny et al., 2015). Arterial spin labeling (ASL) MRI relies on the use of magnetically tagged or labeled blood as an endogenous tracer that does not involve any injection of MRI contrast agent, making it more convenient for subjects. In recent years, it has been used to study several psychiatric diseases, such as schizophrenia (Risterucci et al., 2005; Ota et al., 2014), depression (Doraiswamy et al., 1999; Clark et al., 2001; Clark et al., 2006a; Clark et al., 2006b), dementia (Du et al., 2006; Hayasaka et al., 2006), and addictions (Gazdzinski et al., 2006; Clark et al., 2007).
Anorexia nervosa is associated both with medical complications (Garfinkel et al., 1983; Mitchell and Crow, 2006) and disturbances of brain function (Bailer and Kaye, 2011; Kaye et al., 2011). As such, cerebral vasculature changes may be particularly important in anorexia nervosa, and cerebral blood flow (CBF) might provide a measure of the severity of brain dysfunction occurring in patients with anorexia. Physiological brain differences in anorexia nervosa have been investigated in studies using nuclear medicine-based techniques, with largely heterogeneous results (Gordon et al., 1997; Kuruoglu et al., 1998; Naruo et al., 2001; Råstam et al., 2001; Takano et al., 2001; Chowdhury et al., 2003; Kojima et al., 2005; Lask et al., 2005; Key et al., 2006; Matsumoto et al., 2006; Frank et al., 2007; Yonezawa et al. 2008; Komatsu et al., 2010; Frampton et al., 2011). A review of this literature is included in the discussion and summarized in Table 1.
Table 1.
Prior studies examining regional cerebral blood flow (rCBF) in patients with anorexia nervosa (AN) in chronological order of publication.
| Technique | Experimental Design | Results | |
|---|---|---|---|
| Gordon et al., 1997 |
99mTc HMPAO/ECD SPECT |
rCBF measured at one time point for AN pediatric patients (n, 15, age, 13.1, weight for height, 82%). |
13 or 15 patients with unilateral reduced rCBF in temporal lobe. Follow-up scans with same pattern. |
| Kuruoglu et al., 1998 |
99mTc HMPAO SPECT |
Case-reports of two subjects with AN, aged 16 and 18. Both subjects scanned initially, treated for over a year, rescanned after 3 months of normalized weight. |
Bilateral frontal, parietal, and frontotemporal hypoperfusion at low weight, normal after treatment. |
| Takano et al., 2001 | 123I-IMP SPECT | rCBF compared at one time point for 2 groups: HC (n, 8, age 28.3, BMI, 19.7) and AN (n, 14, age, 21, BMI, 14.0). |
Patients with hypoperfusion in medial prefrontal cortex (MPFC) and anterior cingulate (ACC) and hyperperfusion of thalamus and amygdala- hippocampus. |
| Naruo et al.,2001 | PAO SPECT | rCBF compared at one time point for 3 groups: HC (n, 7, age, 21.9, BMI, 20.0), AN-Ra (n, 7, age, 21.7, BMI, 12.8), and AN-BPb (n, 7, age, 25.6, BMI, 14.5). |
Reduced rCBF in bilateral ACC and frontal regions in AN-R group relative to AN-BP and HC groups. |
| Råstam et al., 2001 |
99mTc HMPAO SPECT |
rCBF compared for AN (n, 21, age 22.1, BMI, 21.2) and non- psychiatric pediatric patients (n, 9, age 9.7, BMI, 16.2). Subjects with AN included both weight- recovered and underweight. |
AN group with hypoperfusion of temporal, parietal, occipital, and orbitofrontal lobes. |
| Chowdhury et al., 2003 |
99mTc ECD SPECT |
rCBF measured at one time point for 15 new-onset pediatric subjects with AN (n, 15, age, 14.9, weight for height, 83%). |
73% of patients with unilateral hypoperfusion of one region (9 with temporal lobe, 5 with parietal lobe, and 3 with frontal lobe and thalamux) |
| Lask et al., 2005 |
99mTc ECD SPECT |
rCBF measured at one time point for pediatric subjects with AN (n, 24, age, 14.4, BMI, not reported) |
66% of patients with unilateral hypoperfusion in different regions (12, temporal; 4 parietal; 5 thalamic) |
| Kojima et al., 2005 |
99mTc HMPAO SPECT |
rCBF measured before and after weight recovery for subjects with AN-R (n, 12, age 18, BMI before treatment, 12.5, BMI after treatment, 15.6, length of treatment, 104 days) and compared to HC measured only once (n, 11, age, 22, BMI, 20.1). |
Before treatment, AN-R group with lower rCBF in ACC, right parietal, insula, occipital lobes. After weight gain, rCBF increased in right parietal with decreased in basal ganglia and cerebellum |
| Key et al., 2006 |
99mTc ECD SPECT |
rCBF compared at one time point for 2 groups of adult women: HC (n, 11, age, 26, BMI, 22), and AN (n, 11, age, 28, BMI, 17). |
8 of 11 patients with hypoperfusion in the anterior temporal lobe and/or caudate nuclei |
| Matsumoro et al., 2006 | 123I-IMP SPECT | rCBF examined before and after weight recovery: AN only (n, 8, age, 18.5, BMI at admission, 12.9, at discharge, 18.8, length of treatment 175 days). |
After treatment, rCBF increased in precuneus, ACC, posterior cingulate gyrus (PCG), right dorsolateral prefrontal (DLPFC), and MPFC. |
| Frank et al., 2007 | [15O] water PET | rCBF compared at one time point for 3 groups: HC (n, 8, age, 26, BMI, 23), weight- recovered AN-R (n, 10, age, 24, BMI, 24), and weight-recovered AN-BP (n, 8, age, 24, BMI, 25). |
rCBF similar across groups. |
| Yonezawa et al., 2008 |
99mTc HMPAO SPECT |
rCBF compared at one time point for 3 groups: HC (n, 10, age, 20.6, BMI, 19.7), AN-R (n, 13, age, 22.2, BMI, 13.8), and AN-BP (n, 13, age, 22.3, BMI, 13.4) |
Both AN-R and AN-BP with decreased perfusion in bilateral subcallosal gyrus, PCG, corpus callosum, midbrain, and pons. |
| Komatsu et al., 2010 | 123I-IMP SPECT | rCBF compared before and after weight recovery for pediatric AN (n = 10, age 13.2 yrs, initial BMI 13.1, post-treatment BMI 16.6, treatment 4 months). |
Following treatment, increased rCBF in bilateral parietal lobe, and right PCG. |
| Frampton et al., 2011 |
99mTc ECD SPECT |
Follow-up of 9 AN participants now in weight-recovery ~4 years after scans for the Chowdhury, 2003 study. |
7 patients with significant and persisting unilateral temporal hypoperfusion. |
AN-R: anorexia nervosa, restricting type
AN-BP: anorexia nervosa, binge-purge type
Here, we applied pseudo-continuous arterial spin labeling (pCASL) as well as other advanced MRI techniques to obtain rapid, non-invasive measures of cerebral physiological parameters, including CBF and venous T2 values, markers of blood oxygenation. Further, we compared these parameters among subjects with a current diagnosis of anorexia nervosa (AN-C), subjects in long-term weight recovery from anorexia nervosa (AN-WR), and healthy women (HC) to determine if cerebral physiological characteristics differed during different stages of the disorder.
2. Methods
2.1. Participants
Subjects came to an initial screening appointment to provide written informed consent to participate in this study. The Health Insurance Portability and Accountability Act compliant protocol was approved by the University of Texas Southwestern Institutional Review Board, and written informed consent was obtained from all participants. A total of 67 female subjects, between 18 and 47 years of age were included. All subjects were interviewed using the Structured Clinical Interview for DSM-IV disorders (SCID-RV) to confirm the history of anorexia nervosa in the AN-C (n = 23) and AN-WR (n = 19) groups, and the absence of current or past eating disorders in the HC group (n = 25). All subjects in the AN-C group had met the DSM-IV criteria for anorexia nervosa within the previous 12 months, and were required to be at a stable or increasing weight (no weight loss exceeding 2 kg in preceding 8 weeks). Many of these subjects (16 of 23) had completed an intensive treatment program or partial hospital program for anorexia nervosa within the previous 12 weeks. All subjects in the AN-WR group had met the DSM-IV criteria for AN previously but had maintained a healthy weight, defined as a minimal body-mass index (BMI) greater than or equal to 19.0 kg/m2, for at least 2 years. No participants met criteria for any psychotic disorders, for bipolar disorder, or for a history of a traumatic brain injury. Clinician-administered quantitative assessments of depression (Quick Inventory of Depression, Clinician-Report), and anxiety (Structured Inventory of Generalized Hamilton Anxiety Symptoms, SIGH-A) were obtained. The Eating Attitudes Test-26 was used to assess current disordered eating behaviors in all three groups (Table 2). The participants did not have any safety contraindications for MRI such as metal implants, pacemaker, neurostimulator, body piercings, or claustrophobia.
Table 2.
Participants’ demographic and clinical assessments (mean ± SD)
| HC | AN-C | AN-WR | ANOVA | |
|---|---|---|---|---|
| Average age (years) | 25.5±6.6 | 25.8±8.3 | 30.4±8.4 | 0.09 |
| Intelligence quotient | 120.1 ±11.2 | 120.2±8.3 | 120.9±10.1 | 0.96 |
| Body-mass index (kg/m2) | 22.6±2.7 | 18.2±1.6 | 23.0±3.0 | <0.001a |
| Eating Attitudes Test | 33±3.7 | 36.6±17.8 | 15.2±10.1 | <0.001b |
| Quick Inventory of Depression | 1.5±1.8 | 6.8±5.7 | 4.6±4.1 | <0.001c |
| Hamilton Anxiety | 2.0±2.6 | 9.6±8.6 | 7.3±6.2 | <0.001c |
Abbreviations: HC, healthy controls (n=25); AN-C, currently ill patients (n=23); AN-WR, weight-recovered patients (n=19). ANOVA, analysis of variance. Follow-up Student t-test:
HC vs. AC-C, P<0.001; AN-WR vs. AN-C, P<0.001.
AN-C vs. HC, P<0.001; AN-C vs. AN-WR, P<0.001; AN-WR vs. HC, P=0.005.
AN-C vs. HC, P<0.001; AN-WR vs. HC, P<0.05.
2.2. General MRI procedures
All experiments were conducted on a 3T MR system (Philips Healthcare, Best, The Netherlands). The body coil was used for radiofrequency transmission, and an eight-channel sensitivity encoding (SENSE) head coil was used for receiving (Dai et al., 2008; Aslan and Lu, 2010). A 3D T1-weighted magnetization-prepared-rapid-acquisition-of-gradient-echo (MPRAGE) scan was performed for anatomical reference and the estimation of brain volume. The MPRAGE sequence used the following imaging parameters: repetition time (TR) of 8.1 ms, echo time (TE) of 3.7 ms, flip angle (FA) of 12°, shot interval of 2100 ms, inversion time (TI) of 1100 ms, voxel size of 1 × 1 × 1 mm3, 160 slices with a sagittal slice orientation, and total scan duration of 3 min 57 s.
2.3. Pseudo-continuous arterial spin labeling (pCASL) MRI methods and analysis
The pCASL MRI method was used to obtain regional CBF values and to evaluate regional heterogeneity of CBF change (Aslan and Lu, 2010). Forty pairs of control and labeled images were acquired using a multi-slice echo-planar imaging (EPI) acquisition. Imaging parameters for pCASL experiments were as follows: single-shot gradient-echo EPI, field of view (FOV)=240×240 mm2, matrix=80×80, voxel size=3×3 mm2, 29 slices acquired in ascending order, thickness=5 mm, labeling duration 1650 ms, post-labeling delay 1525 ms, TR/TE =4205/13.81 ms, FA= 90°, and scan duration = 5 min 40 s.
The pCASL control and labeled images were realigned using Statistical Parametric Mapping software (SPM5, Wellcome Department of Imaging Neuroscience, London, UK, www.fil.ion.ucl.ac.uk/spm) running in MATLAB (Mathworks, Natick, MA). The CBF map was calculated using a perfusion kinetic model similar to that described by Thomas et al. (Thomas et al., 2013). For the normalization, MPRAGE images were first segmented to gray matter, white matter and cerebrospinal fluid (CSF) in SPM. Next, the gray matter images were spatially normalized to the gray matter template of the Montreal Neurological Institute (MNI) atlas and applied to the CBF maps. CBF maps were smoothed with a full-width at half-height (FWHH) of 8 mm to reduce noise. A whole brain mask was applied to CBF maps to exclude out-of-brain voxels.
For CBF map analysis, a whole brain voxel-wise analysis of variance (ANOVA) and follow-up post hoc t-tests were conducted in SPM5 to compare data across all three groups. Maps were thresholded using a voxel height of P<0.005 and extent of 256 voxels, which corresponded to a cluster-PFWE-Corrected < 0.001 according to the “3d clustersim” function in AFNI. Region of interest (ROI) analysis on the CBF map was performed using in-house MATLAB scripts. ROIs in different brain lobes were defined from the clusters that showed a significant group difference in the voxel-wise comparison within each lobe. Anatomical masks of major brain lobes were generated by Automated Anatomical Labeling (AAL) software, and were defined in the MNI template space. If an isolated significant cluster was observed in a lobe, the cluster was defined as the functional ROI of this lobe and was applied to the CBF map. If multiple clusters were observed within one lobe, the clusters were combined into one functional ROI of this lobe, and applied to the CBF maps.
2.4. Phase-contrast MRI methods and analysis
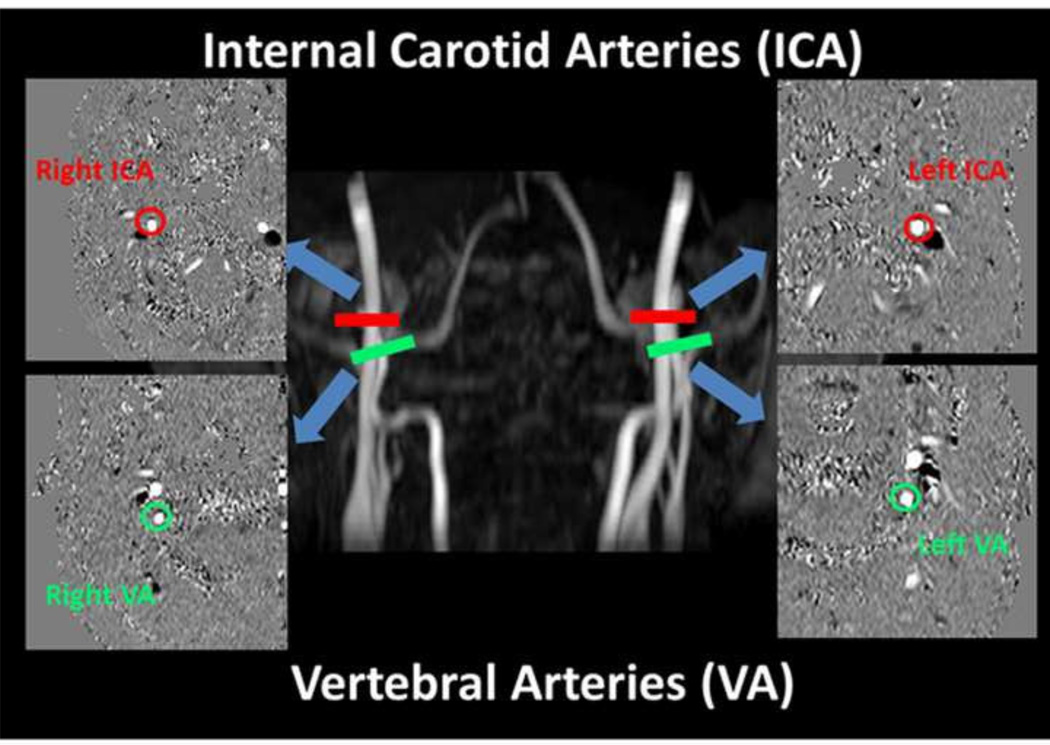
Phase-contrast (PC) flow velocity MRI (Fig. 1) was used to measure the whole-brain, global CBF (Liu et al., 2013). Time-of-flight angiogram was performed before the PC flow measurements to obtain the anatomical information of the feeding arteries of the brain. The slice positioning and imaging parameters followed the optimized protocols established earlier (Liu et al., 2013), as follows: TR/TE/flip angle = 23 ms/3.45 ms/18°, field of view (FOV) =160 × 160 × 70.5 mm3, voxel size = 0.3 × 0.3 × 1.5 mm3, number of slices = 47, one 60-mm saturation slab positioned above the imaging slab, and scan duration = 1.4 min. Since the brain is supplied exclusively by four arteries, left and right internal carotid arteries (ICAs) and left and right vertebral arteries (VAs), we performed four PC-MRI scans, with each scan targeting one specific feeding artery. An automatic algorithm was applied to determine PC-MRI slice positioning of the targeting arteries (Liu et al., 2014). Imaging parameters of PC MRI were as follows: one slice, FOV = 200 × 200 × 5 mm3, voxel size = 0.5 × 0.5 × 5 mm3, 4 averages, maximum velocity encoding = 80 cm/s, and scan duration = 0.5 min for one PC scan. An ROI was then drawn on each of the four arteries based on the magnitude image (Aslan et al., 2010). The ROI mask was applied to the velocity map, and the integration of the velocity within the ROI (i.e., velocity × area) yielded CBF in units of ml/min. To obtain a unit volume CBF value in order to account for brain volume, we use software FSL (FMRIB Software Library, Oxford University, UK) to segment the high-resolution T1 image into gray matter, white matter and cerebrospinal fluid. The brain’s parenchyma volume was given by the sum of gray and white matter volumes, and converted to the weight of the brain by assuming a parenchyma density of 1.06 g/ml (Herscovitch et al., 1985). The CBF (in ml/100 g/min) was normalized to unit volume to account for differences in brain volume across subjects.
Fig. 1.
Slice positions and typical results of phase-contrast (PC) MRI for the quantification of global CBF. Four PC MRI scans, red bars for internal carotid arteries (ICA) and green bars for vertebral arteries (VA) are positioned perpendicular to the respective feeding arteries on an angiogram image (shown in the middle). The corresponding PC MR images for each artery are shown in the side panels, with the blue arrows directing to each artery. The phase images of the target arteries (circled) always appear in the center of the image and are easily identified.
2.5 T2-relaxation under-spin-tagging (TRUST) MRI methods and analysis
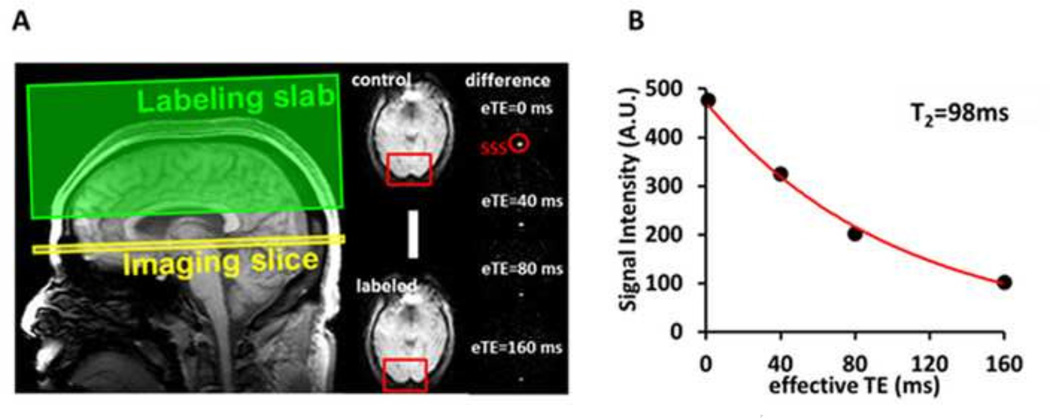
The T2-relaxation under-spin-tagging (TRUST) MRI technique (Fig. 2) provides a measure of whole-brain venous blood T2 values (Lu and Ge, 2008; Ge et al., 2012; Lu et al., 2012). Venous blood T2 is a surrogate marker of blood oxygenation, which is thought to be tightly coupled to blood flow (Liu et al., 2013), and should provide results comparable to both the pCASL and PC-MRI CBF techniques. In the TRUST approach, pure venous blood signal was first isolated from the superior sagittal sinus (SSS) by subtracting the labeled image from the control image (Lu and Ge, 2008) (Fig. 2A). The venous blood signals were then fitted to a monoexponential function to obtain T2 (Fig. 2B). The imaging parameters were as follows: voxel size 3.44 × 3.44 × 5 mm3, TR = 3000 ms, TI = 1022 ms, four effective TEs = 0, 40, 80, and 160 ms, labeling thickness = 100 mm, gap = 22.5 mm, and scan duration = 1.2 min. This procedure did not use any exogenous tracers.
Fig. 2.
Measurement of venous T2 value using T2 relaxation under spin tagging (TRUST) MRI. A. Left figure illustrates imaging slice (yellow) and labeling slab (green) of the TRUST scan. Middle panels show raw images of control and labeled scans. The red boxes illustrate the manually drawn region of interest of the superior sagittal sinus (SSS). Right panels show difference images, i.e. control-labeled. eTE = effective echo time. Red circle highlights the location of the SSS. B. Monoexponential fitting of the signal intensity in SSS as a function of eTE yields blood T2 value.
2.6. Statistical analysis of whole-brain CBF values
A one-way ANOVA was conducted to identify significant differences between the means of three independent groups for the whole-brain CBF values (P<0.05). Post hoc two-tailed Student t-tests (P<0.05) were used to assess specific pairwise differences between the groups.
3. Results
3.1. Demographic and clinical comparisons
No significant differences in age or intelligence quotient were observed across the AN-C, AN-WR and HC groups (Table 2). As expected, the mean BMI value of the AN-C group was significantly lower than those of the other two groups. Similarly, the three groups differed in the amount of reported eating disorder symptoms, with the AN-C group showing significantly more symptoms than both the AN-WR and HC groups. The AN-WR group continued to report more eating disorder symptoms than the HC group. Although both the AN-C and AN-WR groups showed significantly more anxiety and depression symptoms than the HC group, they did not differ from each other in these symptoms, which are associated with other psychiatric illnesses that are commonly comorbid with eating disorders (Dellava et al., 2011; Touchette et al., 2011).
3.2. Whole-brain cerebral blood flow
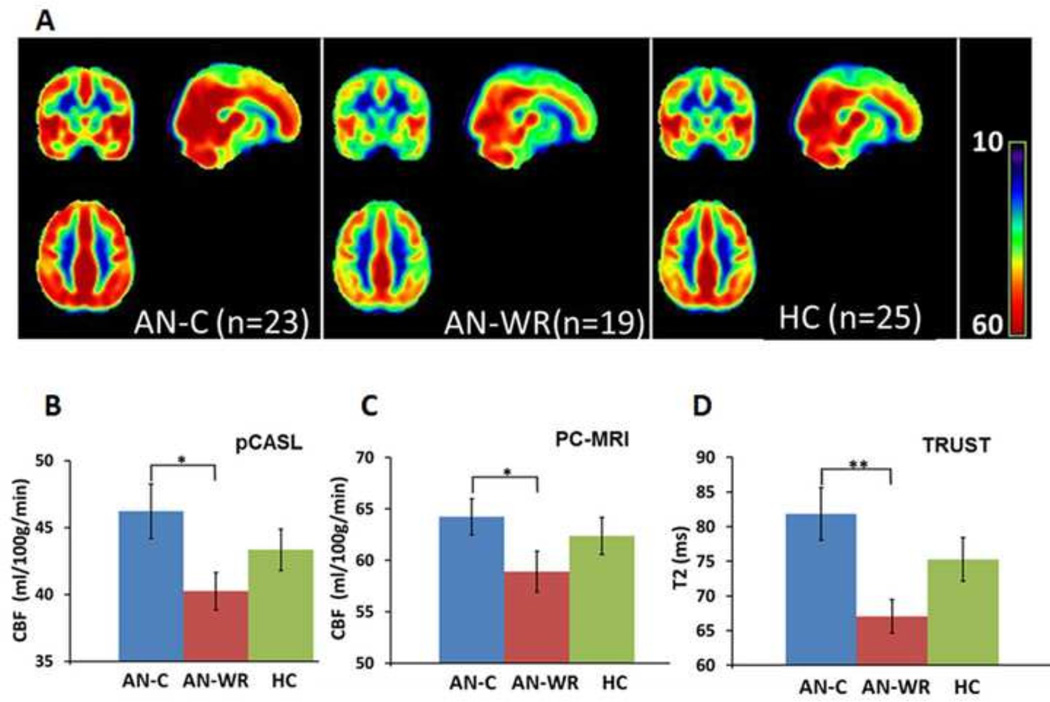
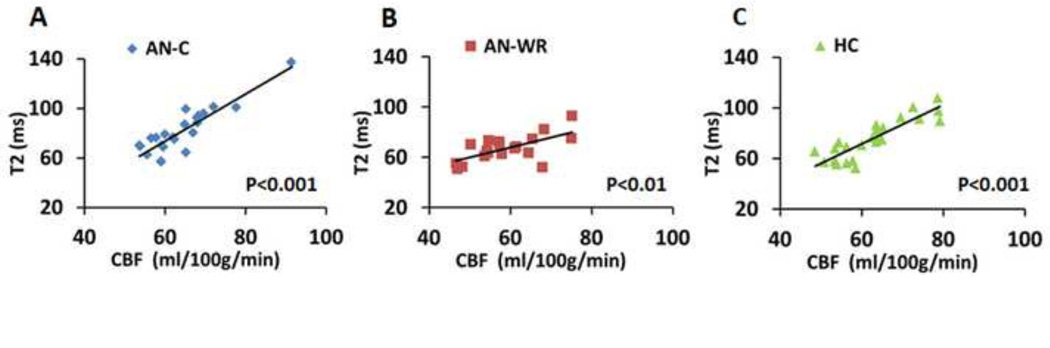
Three different techniques were included to assess whole-brain CBF. First, using pCASL MRI, we found that group-averaged CBF maps showed differences among the AN-C, AN-WR and HC groups (Fig. 3A). Whole-brain-averaged CBF values were extracted from the pCASL CBF maps (Fig 3B, mean CBF±SEM in ml/100g/min, AN-C 46.2±2.0, AN-WR 40.2±1.4, HC 43.3±1.5), with a post hoc t-test showing a significant difference between the AN-C and AN-WR groups (t=2.4, P=0.02). Second, using PC-MRI (Methods in Fig.1), we also found that the AN-C group had significantly higher CBF values (CBF±SEM in ml/100g/min, AN-C 64.2±1.8, AN-WR 58.9±2.0, HC 62.4±1.8, t-test, t=2.0, P=0.05) than the AN-WR group (Fig. 3C). Finally, TRUST-MRI (Methods in Fig. 2) provided a noninvasive assessment of cerebral blood oxygenation. Group differences in the T2 were seen (mean±SEM, AN-C 81.8±3.8 ms, AN-WR 67.0±2.4 ms, HC 75.3±3.2 ms, ANOVA, F=3.9, P=0.03), with the post hoc pair-wise comparisons also demonstrating that the group difference was based on elevated T2 in the AN-C group relative to the AN-WR group (t-test, t=3.0, P=0.005) (Fig. 3D). For all three groups, venous T2 was highly correlated with CBF (Fig. 4), confirming its utility as an indicator of CBF (AN-C: R=0.9, P<0.001, AN-WR: R=0.6, P=0.003, HC: R=0.9, P<0.001).
Fig. 3.
Whole-brain cerebral blood flow. A. Group-averaged CBF maps for AN-C, AN-WR, and HC groups. CBF differences are visually apparent in bilateral frontal and temporal lobes. B. Mean CBF of each group using pCASL technique. C. Mean CBF of each group using PC-MRI technique. D. Mean T2 values for each group using TRUST technique. For BCD, the AN-C mean is in blue, the AN-WR mean in red, and the HC mean in green. The error bar is the standard error of the mean. One asterisk corresponds to P ≤0.05; two asterisks indicate P<0.01.
Fig. 4.
Scatter plots correlating the T2 values and the CBF obtained using PC-MRI for individual subjects. A. AN-C subjects. B. AN-WR subjects. C. HC subjects. There are strong correlations for all three groups.
3.3. Regional differences in cerebral blood flow
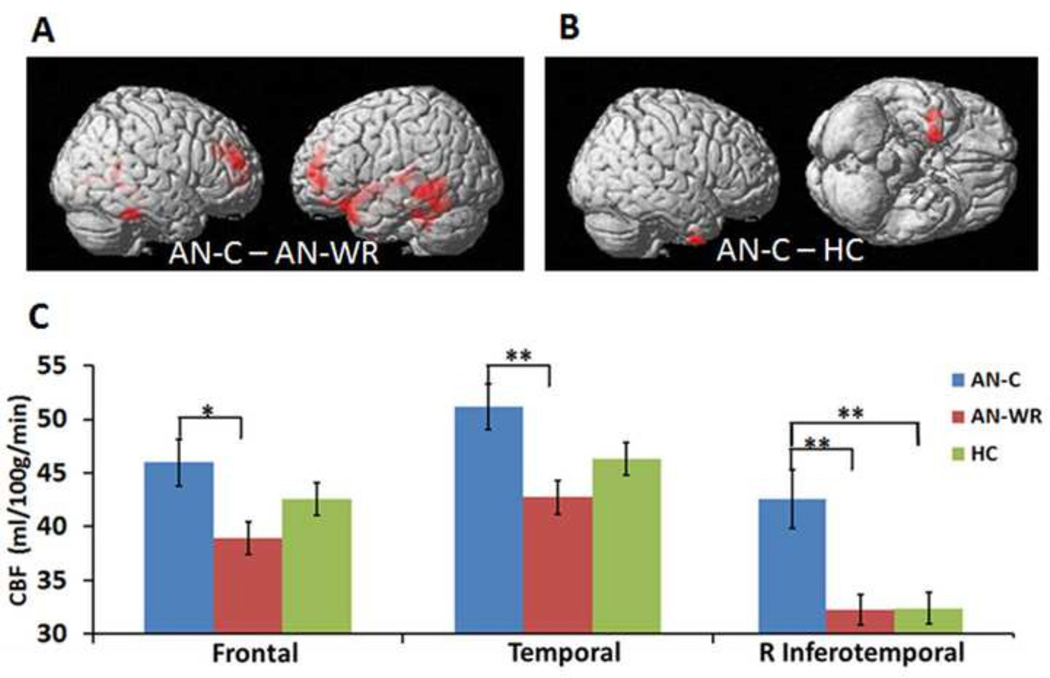
The pCASL MRI technique allows examination of regional differences in CBF. On visual inspection of the whole-brain pCASL maps (Fig. 3A), it is apparent that differences are present mainly in the frontal and temporal lobes. These regional differences in the CBF map were further characterized in two ways. First, whole-brain voxel-wise t-tests were used to compare the CBF maps. Significant clusters resulted from two comparisons as follows: the AN-C group relative to the AN-WR group (Table 3, Fig. 5A) and the AN-C group relative to the HC group (Table 3, Fig. 5B). There were no significant differences in CBF in either direction in the AN-WR and HC comparisons.
Table 3.
Significant CBF differences of cluster level statistics across 3 groups
| Comparison | Number of voxels |
Z scores | Coordinates |
Brain regions |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| AN-C>AN- WR |
617 | 4.29 | 42 | 52 | 22 | Frontal |
| 3.76 | 52 | 34 | 28 | Frontal | ||
| 3.67 | 50 | 52 | 2 | Frontal | ||
| 3596 | 3.94 | −46 | 52 | 14 | Frontal | |
| 3.71 | −38 | −38 | 2 | Temporal | ||
| 3.64 | −26 | 20 | −32 | Temporal | ||
| 553 | 3.86 | 62 | −40 | −28 | Temporal | |
| 3.02 | 64 | −50 | 14 | Temporal | ||
| 2.99 | 54 | −34 | −6 | Temporal | ||
| 1570 | 3.82 | −66 | −42 | −6 | Temporal | |
| 3.66 | −42 | −34 | −32 | |||
| 3.57 | −60 | −52 | −20 | Temporal | ||
| 451 | 3.10 | 42 | −76 | 4 | Occipital | |
| 3.01 | 18 | −66 | 4 | Limbic | ||
| 2.89 | 34 | −64 | 4 | Temporal | ||
| AN-C>HC | 320 | 3.68 | 24 | 2 | −44 | Temporal |
| 3.56 | 42 | 8 | −48 | |||
| 3.45 | 42 | 0 | −48 | |||
Abbreviations: HC, healthy controls (n=25); AN-C, currently ill patients (n=23); AN-WR, weight-recovered patients (n=19). The x, y and z coordinates are Montreal Neurological Institute (MNI) atlas designations of peak from SPM5; brain regions are defined using PickAtlas software; Z-scores are at the peak MNI coordinate of the cluster. Threshold for significance: cluster PFWE < 0.001.
Fig. 5.
Regional cerebral blood flow. A. Whole-brain voxel-wise t-test for the AN-C and AN-WR groups. Red regions are those with significantly higher CBF in the AN-C group relative to the AN-WR; differences are primarily in the bilateral temporal and frontal lobes. B. Whole-brain voxel-wise t-test for the AN-C and HC groups. The red region in the inferotemporal cortex has significantly higher CBF in the AN-C group relative to the HC group. For both A and B, significance threshold for differences was set at cluster PFWE < 0.001. C. ROI analysis for frontal lobe, temporal lobe and right inferior temporal gyrus. The AN-C group mean is in blue, the AN-WR group mean in red, and the HC group mean in green. Error bar is the standard error of the mean. One asterisk corresponds to P ≤0.05; two asterisks indicate P<0.01.
Second, follow-up ROI analysis was performed to obtain regional CBF values within these regions, using masks of the frontal and temporal lobes and the single cluster in the right inferior temporal gyrus (RITG) as ROIs. One-way ANOVA showed main effects of group on all ROIs (frontal F=5.5, P=0.006; temporal F=5.0, P=0.009; RITG F=8.7, P=0.0004). The mean CBF values in the frontal lobe (mean±SEM, AN-C 51.6±2.5, AN-WR 41.6±1.8, HC 47.0±1.8 unit in ml/100 g/min), the temporal lobe (mean ±SEM, AN-C 50.8±2.5, AN-WR 41.1±2.0, HC 44.8±1.7 unit in ml/100 g/min) and the right inferotemporal gyrus (mean ±SEM, AN-C 42.4±2.7, AN-WR 32.3±1.4, HC 32.3±1.5 unit in ml/100 g/min) are shown in Fig. 5C. The significant differences in the frontal and temporal clusters resulted from the AN-C and AN-WR comparison (frontal lobe AN-C/AN-WR comparison, t=3.3, corrected P=0.004; temporal lobe AN-C/AN-WR comparison, t=3.0, corrected P=0.01). In the RITG, the AN-C showed significantly higher CBF than both the AN-WR (t=3.3, P=0.002) and HC (t=33, P=0.002) groups.
4. Discussion
Physiological changes in the brain may be important in both illness and recovery in anorexia nervosa. Here, we applied three different techniques to examine CBF and venous oxygenation in healthy women (HC), women currently experiencing anorexia (AN-C), and women in long-term recovery from anorexia (AN-WR). This experimental design allowed us to extract information about both acute and chronic changes in brain physiology in anorexia nervosa. We measured CBF and venous T2 values quantitatively, rapidly (<10 min) and non-invasively. Interestingly, we found that the AN-WR group had significantly lower CBF than the AN-C group based on both pCASL, and PC-MRI techniques. Voxel-wise comparisons using the pCASL data showed that these differences were most pronounced in the bilateral temporal and frontal cortex. The TRUST MRI technique was used to confirm that there were lower venous blood T2 values in AN-WR group compared with the AN-C group.
4.1. Clinical considerations
Although there is no previous literature on quantitative global CBF measurement in anorexia nervosa, some studies have investigated regional CBF (rCBF) by PET-CT or SPECT. In a reviewing this literature, we identified 14 studies examining rCBF in anorexia nervosa (Table 1). Six studies measured rCBF in subjects when they were underweight (Gordon et al., 1997; Naruo et al., 2001; Takano et al., 2001; Chowdhury et al., 2003; Key et al., 2006; Yonezawa et al., 2008). Six studies focused on pediatric patients (Gordon et al., 1997; Kuruoglu et al., 1998; Chowdhury et al., 2003; Lask et al., 2005; Komatsu et al., 2010; Frampton et al., 2011), and five on adult patients (Takano et al., 2001; Kojima et al., 2005; Key et al., 2006; Matsumoto et al., 2006; Frank et al., 2007; Yonezawa et al., 2008). Five studies compared rCBF before and after weight recovery, three with primarily pediatric subjects (Kuruoglu et al., 1998; Komatsu et al., 2010; Frampton et al., 2011), and two in adults (Kojima et al., 2005; Matsumoto et al., 2006). There are four studies examining rCBF in currently-ill adult women, but the BMI for subjects in three of these studies (Naruo et al., 2001; Takano et al., 2001; Yonezawa et al., 2008) was substantially lower than in our study, leaving only one study of 11 subjects by Key et al. (Key et al., 2006) containing subjects comparable to our AN-C group based on BMI and age. Similarly, there is one study by Frank et al. (Frank et al., 2007), using PET to assess rCBF in 18 long-term weight recovered subjects comparable to our AN-WR group.
Here, we observed elevated rCBF both in the frontal and temporal cortices and in whole-brain measures for the AN-C group, a result that differs from hypoperfusion reported previously. However, there are differences in the clinical populations and comparisons performed. The AN-C group examined here primarily consisted outpatients who were compared with long-term weight-recovered subjects as well as healthy subjects. In contrast, the previous adult studies (Naruo et al., 2001; Takano et al., 2001; Key et al., 2006; Yonezawa et al., 2008) examined patients with anorexia soon after initial admission for treatment, a time when symptom severity is typically highest and when both acute and chronic effects of starvation are likely, and compared these subjects with healthy women.
Another reason for this discrepancy may be differences in data processing. Some studies (Naruo et al., 2001; Kojima et al., 2005) used normalized CBF maps scaled to an overall mean CBF of 50 ml/100 g/min instead of the original CBF maps used here. Further, although hypoperfusion has generally been reported, each study has found different regions. Using SPECT, Chowdhury et al. (2003) observed hypoperfusion differences in the temporal and frontal lobes, whereas Naruo et al. (2001) and Takano et al. (2001) both found hypoperfusion of the anterior cingulate cortex (ACC). Similarly, using PET to examine glucose metabolism in the brain, Delvenne et al. (1995) found hypometabolism of glucose in the frontal lobe, but Miller et al. (2004) observed hypometabolism of glucose in the temporal lobes. In sum, all of these data, as well as our data, suggest that CBF may be most sensitive to altered metabolic needs present in the temporal and frontal regions in anorexia nervosa.
Another major difference in this study is its cross-sectional design and inclusion of both current-patients as well as long-term weight-recovered patients with a history of anorexia. Only one study, Frank et al. (2007), compared long-term weight-recovered adult patients with healthy subjects and reported no differences in rCBF. Consistent with that study, we did not observe differences in rCBF in the comparison of the AN-WR group with the HC group. However, we found differences in the comparisons of the AN-WR group with the AN-C group. These differences may reflect changes related to the process of recovery from anorexia. Most previous studies examining the effects of weight recovery on cerebral physiology have focused on short-term weight changes (Gordon et al., 1997; Kuruoglu et al., 1998; Komatsu et al., 2010), with only one examining long-term changes after sustained recovery (Frampton et al., 2011). Both Gordon et al. (1997) and Frampton et al. (2011) similarly found temporal lobe hypoperfusion after weight restoration, but Kuruoglu et al. (1998) and Komatsu et al. (2010) reported hypoperfusion before treatment and normal brain perfusion after acute weight gain. In sum, all of these studies suggest perfusion differences are present in anorexia and may be altered by weight gain.
If the CBF alteration reflects physiological change in anorexia nervosa, what are some possible mechanisms? CBF is regulated under the influence of neural, chemical, metabolic and physical factors, and mechanisms of this regulation in the brain are not completely understood. Studies have reported a significant correlation between hematocrit and BMI in anorexia nervosa (Nova et al., 2008), and CBF also appears to vary inversely with hematocrit (Ainslie and Ogoh, 2010). One theory is that CBF changes with arterial O2 content to maintain a steady level of cerebral oxygen transportation (Brown et al., 1985). Because the AN-C cohort has a lower BMI, this group might have a lower hematocrit value than the AN-WR cohort, and thus the CBF differences observed in the AN-C and AN-WR groups may result from compensations designed to maintain normal cerebral oxygen transportation in spite of fluctuations in hematocrit. Alternatively, during illness, AN-C patients may adapt to a lower oxygen delivery to the brain that persists after weight recovery.
4.2. Technical considerations
The earlier nuclear medicine studies required the injection of an exogenous tracer which exposes patients to radiation, whereas the pCASL, PC-MRI, and TRUST techniques are noninvasive, fast and reliable (Luh et al., 1999; Wong, 2007; Dai et al., 2008; Aslan et al., 2010). Because of the increased efficiency and reduced risk of both pCASL and PC-MRI compared with SPECT in examining CBF, future work using this technique may provide more detailed understanding of the cerebral physiological changes occurring in AN. Specifically, this technique can easily be coupled with other MRI paradigms. Ideally, longitudinal changes related to both acute and sustained weight recovery in anorexia nervosa could be examined. Larger cohorts of subjects at different stages of the disease would provide an understanding of how cerebral physiological differences may contribute to both illness and recovery.
PC-MRI was used to evaluate CBF in addition to pCASL because quantification of the pCASL signal involves more complex modeling and parameter assumptions than PC-MRI. Specifically, the longitudinal relaxation time of blood (T1) is an important parameter for quantification and optimization of blood flow measurement based on the ASL technique (Detre et al., 1992). T1 values vary with field strength (Stanisz et al., 2005) but also depend on temperature and the hematocrit fraction (Hct) (Bryant et al., 1990; Silvennoinen et al., 2003). Lu et al. (2004) demonstrated that blood samples with a lower Hct tend to have a longer T1. Approximately one third of patients currently with anorexia show anemia, due to iron and vitamin deficiencies, and might have a lower than normal hematocrit (Hutter et al., 2009; Cleary et al., 2010). Therefore, variations in hematocrit could be a confounding factor for the pCASL technique for this study. Because PC-MRI is not dependent on the Hct value, we capitalized on this technique to support the CBF findings obtained from the pCASL technique. PC-MRI is widely used to quantify whole-brain CBF, by measuring and combining flow flux at the main feeding arteries of the brain. By adding an algorithm that provides precise and accurate scanning guidance to help automate position planning of the majority of arteries, PC is also capable of measuring CBF quantitatively, rapidly (less than 2 min), and non-invasively (Liu et al., 2014). The PC-MRI measure of CBF showed similar results as the pCASL measure (Fig. 3).
Our observation of lower CBF in the AN-WR group relative to the AN-C group was also corroborated by the venous T2 data. For the TRUST-MRI data, the venous T2 value, a marker of blood oxygenation, was found to be decreased in the AN-WR group. From extensive physiology literature, it is known that blood oxygenation is tightly coupled to CBF, which forms the basis of the blood-oxygen-level-dependent (BOLD) functional MRI brain-mapping technique. Thus, the finding of a lower venous oxygenation in the AN-WR group is consistent with and provides further support to the notion of a lower CBF in AN-WR.
4.3. Limitations
There are several limitations to these studies. First, this is a cross-sectional study comparing three groups: women that have recently had anorexia, women that had anorexia but are in long-term weight recovery, and healthy women. Based on the disease process and recovery rates following adult anorexia nervosa, a group that successfully maintains weight recovery from the illness may differ from a group make up of those that are unable to recover. Thus, the CBF differences observed in the recovered group may reflect either pre-existing differences in those patients or changes resulting from recovery. Another limitation relates to comorbid psychiatric illnesses. Both the AN-C and AN-WR groups showed significantly more anxiety and depression symptoms than the HC group, but did not differ from each other in those measures. Two depression studies have measured CBF with similar techniques: Clark et al. (2006a) found elevated CBF in bilateral amygdala, and Doraiswamy et al. (1999) found lower CBF in the left periventricular and parietal region. We found differences in other regions, consistent with prior work in anorexia, supporting that the differences observed here are specific to anorexia nervosa. Another limitation of the studies is related to the possibility that group differences in hematocrit affect the calculation of CBF. Both anemia and bone marrow changes are common in anorexia, but resolve with weight gain (Hutter et al., 2009; Cleary et al., 2010). In future studies, measurement of hematocrit in concert with the CBF and venous oxygenation would provide a more detailed mechanistic understanding of brain oxygenation and CBF. The ideal study would include a large cohort of patients and controls, with longitudinal measures of BMI, CBF and hematocrit in patients during the course of treatment: initial presentation, at discharge following intensive treatment, and years after initial treatment. In this way, the progress of the disorder and its effects on cerebrovasculature could be better understood. This study design is possible using the non-invasive techniques described here.
5. Conclusions
The present study used two different non-invasive techniques to assess CBF. Findings suggest patients in long-term recovery from anorexia, compared with currently ill patients, have lower CBF values. These differences in CBF occurred primarily in temporal and frontal lobes in the AN-C and AN-WR comparison. Additionally, TRUST MRI revealed lower venous T2 values in AN-WR patients, further supporting the CBF findings. The AN-C cohort also showed elevated CBF compared with the HC cohort in the right temporal lobe. These data support the idea that alterations in the cerebral physiology are present in anorexia, and further suggest that localized differences may be present in the temporal and frontal lobes. Future studies should focus on development of a mechanistic and functional understanding of the consequences of these CBF alterations.
Highlights.
Regional and global cerebral blood flow (CBF) is examined in anorexia nervosa with MRI techniques.
We compare healthy women (HC), women currently with anorexia (AN-C), and women in weight-recovery following anorexia (AN-WR).
Whole-brain CBF was less in the AN-WR group than in the AN-C group.
Venous T2 relaxation values were less in the AN-WR group than in the AN-C group.
Regional differences were primarily in the frontal and temporal lobes.
Acknowledgments
Funding for this research was provided by K23 MH093684 and a 2012 NARSAD Young Investigator Award to Dr. McAdams, and NIH R01 MH084021, NIH R01 NS067015, and NIH R01 AG042753 grants to Dr. Lu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainslie PN, Ogoh S. Regulation of cerebral blood flow in mammals during chronic hypoxia: a matter of balance. Experimental Physiology. 2010;95:251–262. doi: 10.1113/expphysiol.2008.045575. [DOI] [PubMed] [Google Scholar]
- Aslan S, Lu H. On the sensitivity of ASL MRI in detecting regional differences in cerebral blood flow. Magnetic Resonance Imaging. 2010;28:928–935. doi: 10.1016/j.mri.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan S, Xu F, Wang PL, Uh J, Yezhuvath US, van Osch M, Lu H. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magnetic Resonance in Medicine. 2010;63:765–771. doi: 10.1002/mrm.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Bailer UF, Kaye WH. Serotonin: imaging findings in eating disorders. Current topics in Behavioral Neuroscience. 2011;6:59–79. doi: 10.1007/7854_2010_78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MM, Wade JP, Marshall J. Fundamental importance of arterial oxygen content in the regulation of cerebral blood flow in man. Brain. 1985;108(Pt 1):81–93. doi: 10.1093/brain/108.1.81. [DOI] [PubMed] [Google Scholar]
- Brown TA, Keel PK. Current and emerging directions in the treatment of eating disorders. Substance Abuse: Research and Treatment. 2012;6:33–61. doi: 10.4137/SART.S7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RG, Marill K, Blackmore C, Francis C. Magnetic relaxation in blood and blood clots. Magnetic Resonance in Medicine. 1990;13:133–144. doi: 10.1002/mrm.1910130112. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Thornton L, Pinheiro AP, Plotnicov K, Klump KL, Brandt H, Crawford S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, Mitchell J, Nutzinger D, Strober M, Treasure J, Woodside DB, Berrettini WH, Kaye WH. Suicide attempts in anorexia nervosa. Psychosomatic Medicine. 2008;70:378–383. doi: 10.1097/PSY.0b013e3181646765. [DOI] [PubMed] [Google Scholar]
- Chowdhury U, Gordon I, Lask B, Watkins B, Watt H, Christie D. Early-onset anorexia nervosa: is there evidence of limbic system imbalance? The International Journal of Eating Disorders. 2003;33:388–396. doi: 10.1002/eat.10155. [DOI] [PubMed] [Google Scholar]
- Clark CP, Brown GG, Archibald SL, Fennema-Notestine C, Braun DR, Thomas LS, Sutherland AN, Gillin JC. Does amygdalar perfusion correlate with antidepressant response to partial sleep deprivation in major depression? Psychiatry Research: Neuroimaging. 2006a;146:43–51. doi: 10.1016/j.pscychresns.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CP, Brown GG, Eyler LT, Drummond SP, Braun DR, Tapert SF. Decreased perfusion in young alcohol-dependent women as compared with age-matched controls. The American Journal of Drug and Alcohol Abuse. 2007;33:13–19. doi: 10.1080/00952990601082605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CP, Brown GG, Frank L, Thomas L, Sutherland AN, Gillin JC. Improved anatomic delineation of the antidepressant response to partial sleep deprivation in medial frontal cortex using perfusion-weighted functional MRI. Psychiatry Research: Neuroimaging. 2006b;146:213–222. doi: 10.1016/j.pscychresns.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CP, Frank LR, Brown GG. Sleep deprivation, EEG, and functional MRI in depression: preliminary results. Neuropsychopharmacology. 2001;25:S79–S84. doi: 10.1016/S0893-133X(01)00324-4. [DOI] [PubMed] [Google Scholar]
- Cleary BS, Gaudiani JL, Mehler PS. Interpreting the complete blood count in anorexia nervosa. Eating Disorders. 2010;18:132–139. doi: 10.1080/10640260903585540. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magnetic Resonance in Medicine. 2008;60:1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellava JE, Kendler KS, Neale MC. Generalized anxiety disorder and anorexia nervosa: evidence of shared genetic variation. Depression and Anxiety. 2011;28:728–733. doi: 10.1002/da.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvenne V, Lotstra F, Goldman S, Biver F, De Maertelaer V, Appelboom-Fondu J, Schoutens A, Bidaut LM, Luxen A, Mendelwicz J. Brain hypometabolism of glucose in anorexia nervosa: a PET scan study. Biological Psychiatry. 1995;37:161–169. doi: 10.1016/0006-3223(94)00189-A. [DOI] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magnetic Resonance in Medicine. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, MacFall J, Krishnan KR, O'Connor C, Wan X, Benaur M, Lewandowski M, Fortner M. Magnetic resonance assessment of cerebral perfusion in depressed cardiac patients: preliminary findings. The American Journal of Psychiatry. 1999;156:1641–1643. doi: 10.1176/ajp.156.10.1641. [DOI] [PubMed] [Google Scholar]
- Du AT, Jahng GH, Hayasaka S, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin KP, Miller BL, Weiner MW, Schuff N. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology. 2006;67:1215–1220. doi: 10.1212/01.wnl.0000238163.71349.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton I, Watkins B, Gordon I, Lask B. Do abnormalities in regional cerebral blood flow in anorexia nervosa resolve after weight restoration? European Eating Disorders Review. 2011;19:55–58. doi: 10.1002/erv.1047. [DOI] [PubMed] [Google Scholar]
- Frank GK, Bailer UF, Meltzer CC, Price JC, Mathis CA, Wagner A, Becker C, Kaye WH. Regional cerebral blood flow after recovery from anorexia or bulimia nervosa. The International Journal of Eating Disorders. 2007;40:488–492. doi: 10.1002/eat.20395. [DOI] [PubMed] [Google Scholar]
- Garfinkel PE, Garner DM. The multidimensional nature of anorexia nervosa. In: Garfinkel PE, editor. Anorexia Nervosa: Recent Developments in Research. New York: Alan R Liss, Inc.; 1983. pp. 3–14. [Google Scholar]
- Gazdzinski S, Durazzo T, Jahng GH, Ezekiel F, Banys P, Meyerhoff D. Effects of chronic alcohol dependence and chronic cigarette smoking on cerebral perfusion: a preliminary magnetic resonance study. Alcoholism, Clinical and Experimental Research. 2006;30:947–958. doi: 10.1111/j.1530-0277.2006.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Zhang Z, Lu H, Tang L, Jaggi H, Herbert J, Babb JS, Rusinek H, Grossman RI. Characterizing brain oxygen metabolism in patients with multiple sclerosis with T2-relaxation-under-spin-tagging MRI. Journal of Cerebral Blood Flow and Metabolism. 2012;32:403–412. doi: 10.1038/jcbfm.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Lask B, Bryant-Waugh R, Christie D, Timimi S. Childhood-onset anorexia nervosa: towards identifying a biological substrate. The International Journal of Eating Disorders. 1997;22:159–165. doi: 10.1002/(sici)1098-108x(199709)22:2<159::aid-eat7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Du AT, Duarte A, Kornak J, Jahng GH, Weiner MW, Schuff N. A non-parametric approach for co-analysis of multi-modal brain imaging data: application to Alzheimer's disease. NeuroImage. 2006;30:768–779. doi: 10.1016/j.neuroimage.2005.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscovitch P, Mintun MA, Raichle ME. Brain oxygen utilization measured with oxygen-15 radiotracers and positron emission tomography: generation of metabolic images. Journal of Nuclear Medicine. 1985;26:416–417. [PubMed] [Google Scholar]
- Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Current Opinion in Psychiatry. 2006;19:389–394. doi: 10.1097/01.yco.0000228759.95237.78. [DOI] [PubMed] [Google Scholar]
- Hutter G, Ganepola S, Hofmann WK. The hematology of anorexia nervosa. The International Journal of Eating Disorders. 2009;42:293–300. doi: 10.1002/eat.20610. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wagner A, Fudge JL, Paulus M. Neurocircuity of eating disorders. Current Topics in Behavioral Neurosciences. 2011;6:37–57. doi: 10.1007/7854_2010_85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key A, O’Brien A, Gordon I, Christie D, Lask B. Assessment of neurobiology in adults with anorexia nervosa. European Eating Disorders Review. 2006;14:308–314. [Google Scholar]
- Kojima S, Nagai N, Nakabeppu Y, Muranaga T, Deguchi D, Nakajo M, Masuda A, Nozoe S, Naruo T. Comparison of regional cerebral blood flow in patients with anorexia nervosa before and after weight gain. Psychiatry Research: Neuroimaging. 2005;140:251–258. doi: 10.1016/j.pscychresns.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Nagamitsu S, Ozono S, Yamashita Y, Ishibashi M, Matsuishi T. Regional cerebral blood flow changes in early-onset anorexia nervosa before and after weight gain. Brain & Development. 2010;32:625–630. doi: 10.1016/j.braindev.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Kuruoglu AC, Kapucu O, Atasever T, Arikan Z, Isik E, Unlu M. Technetium-99m–HMPAO brain SPECT in anorexia nervosa. Journal of Nuclear Medicine. 1998;39:304–306. [PubMed] [Google Scholar]
- Kuschinsky W. Coupling of function, metabolism, and blood flow in the brain. Neurosurgical Review. 1991;14:163–168. doi: 10.1007/BF00310651. [DOI] [PubMed] [Google Scholar]
- Lask B, Gordon I, Christie D, Frampton I, Chowdhury U, Watkins B. Functional neuroimaging in early-onset anorexia nervosa. The International Journal of Eating Disorders. 2005;(37 Suppl):S49–S51. doi: 10.1002/eat.20117. discussion S87-49. [DOI] [PubMed] [Google Scholar]
- Liu P, Lu H, Filbey FM, Pinkham AE, McAdams CJ, Adinoff B, Daliparthi V, Cao Y. Automatic and reproducible positioning of phase-contrast MRI for the quantification of global cerebral blood flow. PloS One. 2014;9:e95721. doi: 10.1371/journal.pone.0095721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Uh J, Devous MD, Adinoff B, Lu H. Comparison of relative cerebral blood flow maps using pseudo-continuous arterial spin labeling and single photon emission computed tomography. NMR in Biomedicine. 2012;25:779–786. doi: 10.1002/nbm.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Xu F, Lu H. Test-retest reproducibility of a rapid method to measure brain oxygen metabolism. Magnetic Resonance in Medicine. 2013;69:675–681. doi: 10.1002/mrm.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magnetic Resonanc e in Medicine. 2004;52:679–682. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magnetic Resonance in Medicine. 2008;60:357–363. doi: 10.1002/mrm.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Grgac K, Liu P, Qin Q, van Zijl P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magnetic Resonance in Medicine. 2012;67:42–49. doi: 10.1002/mrm.22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magnetic Resonance in Medicine. 1999;41:1246–1254. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Kitabayashi Y, Narumoto J, Wada Y, Okamoto A, Ushijima Y, Yokoyama C, Yamashita T, Takahashi H, Yasuno F, Suhara T, Fukui K. Regional cerebral blood flow changes associated with interoceptive awareness in the recovery process of anorexia nervosa. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2006;30:1265–1270. doi: 10.1016/j.pnpbp.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Miller KK, Deckersbach T, Rauch SL, Fischman AJ, Grieco KA, Herzog DB, Klibanski A. Testosterone administration attenuates regional brain hypometabolism in women with anorexia nervosa. Psychiatry Research: Neuroimaging. 2004;132:197–207. doi: 10.1016/j.pscychresns.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Michell JE, Crow S. Medical complications of anorexia nervosa and bulimia nervosa. Current Opinions in Psychiatry. 2006;19:438–443. doi: 10.1097/01.yco.0000228768.79097.3e. [DOI] [PubMed] [Google Scholar]
- Naruo T, Nakabeppu Y, Deguchi D, Nagai N, Tsutsui J, Nakajo M, Nozoe S. Decreases in blood perfusion of the anterior cingulate gyri in anorexia nervosa restricters assessed by SPECT image analysis. BMC Psychiatry. 2001;1:2. doi: 10.1186/1471-244X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nova E, Lopez-Vidriero I, Varela P, Casas J, Marcos A. Evolution of serum biochemical indicators in anorexia nervosa patients: a 1-year follow-up study. Journal of Human Nutrition and Dietetics. 2008;21:23–30. doi: 10.1111/j.1365-277X.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- Ota M, Ishikawa M, Sato N, Okazaki M, Maikusa N, Hori H, Hattori K, Teraishi T, Ito K, Kunugi H. Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophrenia Research. 2014;154:113–118. doi: 10.1016/j.schres.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Råstam M, Bjure J, Vestergren E, Uvebrant P, Gillberg IC, Wentz E, Gillberg C. Regional cerebral blood flow in weight-restored anorexia nervosa: a preliminary study. Developmental Medicine & Child Neurology. 2001;43:239–242. doi: 10.1017/s0012162201000457. [DOI] [PubMed] [Google Scholar]
- Risterucci C, Jeanneau K, Schoppenthau S, Bielser T, Kunnecke B, von Kienlin M, Moreau JL. Functional magnetic resonance imaging reveals similar brain activity changes in two different animal models of schizophrenia. Psychopharmacology. 2005;180:724–734. doi: 10.1007/s00213-005-2204-8. [DOI] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. The Journal of Physiology. 1890;11(1–2):85–158. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Bloss CS, Tewhey R, Bansal V, Torkamani A, Libiger O, Duvvuri V, Wineinger N, Galvez L, Darst BF, Smith EN, Carson A, Pham P, Phillips T, Villarasa N, Tisch R, Zhang G, Levy S, Murray S, Chen W, Srinivasan S, Berenson G, Brandt H, Crawford S, Crow S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, La Via M, Mitchell JE, Strober M, Rotondo A, Treasure J, Woodside DB, Bulik CM, Keel P, Klump KL, Lilenfeld L, Plotnicov K, Topol EJ, Shih PB, Magistretti P, Bergen AW, Berrettini W, Kaye W, Schork NJ. Evidence for the role of EPHX2 gene variants in anorexia nervosa. Molecular Psychiatry. 2014;19:724–732. doi: 10.1038/mp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvennoinen MJ, Kettunen MI, Kauppinen RA. Effects of hematocrit and oxygen saturation level on blood spin-lattice relaxation. Magnetic Resonance in Medicine. 2003;49:568–571. doi: 10.1002/mrm.10370. [DOI] [PubMed] [Google Scholar]
- Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magnetic Resonance in Medicine. 2005;54:507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- Takano A, Shiga T, Kitagawa N, Koyama T, Katoh C, Tsukamoto E, Tamaki N. Abnormal neuronal network in anorexia nervosa studied with I-123-IMP SPECT. Psychiatry Research: Neuroimaging. 2001;107:45–50. doi: 10.1016/s0925-4927(01)00093-2. [DOI] [PubMed] [Google Scholar]
- Theberge J. Perfusion magnetic resonance imaging in psychiatry. Topics in Magnetic Resonance Imaging. 2008;19:111–130. doi: 10.1097/RMR.0b013e3181808140. [DOI] [PubMed] [Google Scholar]
- Thomas B, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, Lu H. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. Journal of Magnetic Resonance Imaging. 2013;38:1177–1183. doi: 10.1002/jmri.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchette E, Henegar A, Godart NT, Pryor L, Falissard B, Tremblay RE, Cote SM. Subclinical eating disorders and their comorbidity with mood and anxiety disorders in adolescent girls. Psychiatry Research. 2011;185:185–192. doi: 10.1016/j.psychres.2010.04.005. [DOI] [PubMed] [Google Scholar]
- West JB. Pulmonary Physiology and Pathophysiology: An Integrated, Case-Based Approach. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2007. [Google Scholar]
- Wong EC. Vessel-encoded arterial spin-labeling using pseudocontinuous tagging. Magnetic Resonance in Medicine. 2007;58:1086–1091. doi: 10.1002/mrm.21293. [DOI] [PubMed] [Google Scholar]
- Yonezawa H, Otagaki Y, Miyake Y, Okamoto Y, Yamawaki S. No differences are seen in the regional cerebral blood flow in the restricting type of anorexia nervosa compared with the binge eating/purging type. Psychiatry and Clinical Neurosciences. 2008;62:26–33. doi: 10.1111/j.1440-1819.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- Zimny A, Bladowska J, Macioszek A, Szewczyk P, Trypka E, Wojtynska R, Noga L, Leszek J, Sasiadek M. Evaluation of the posterior cingulate region with FDG-PET and advanced MR techniques in patients with amnestic mild cognitive impairment: comparison of the methods. Journal of Alzheimer’s Disease. 2015;44(1):329–338. doi: 10.3233/JAD-132138. [DOI] [PubMed] [Google Scholar]