Abstract
Expression of GAD1 GABA synthesis enzyme is highly regulated by neuronal activity and reaches mature levels in prefrontal cortex not before adolescence. A significant portion of cases diagnosed with schizophrenia show deficits in GAD1 RNA and protein levels in multiple areas of adult cerebral cortex, possibly reflecting molecular or cellular defects in subtypes of GABAergic interneurons essential for network synchronization and cognition. Here, we review 20 years of progress towards a better understanding of disease-related regulation of GAD1 gene expression. For example, deficits in cortical GAD1 RNA in some cases of schizophrenia are associated with changes in the epigenetic architecture of the promoter, affecting DNA methylation patterns and nucleosomal histone modifications. These localized chromatin defects at the 5′end of GAD1 are superimposed by disordered locus-specific chromosomal conformations, including weakening of long-range promoter-enhancer loopings and physical disconnection of GAD1 core promoter sequences from cis-regulatory elements positioned 50 kilobases further upstream. Studies on the 3-dimensional architecture of the GAD1 locus in neurons, including developmentally regulated higher order chromatin compromised by the disease process, together with exploration of locus-specific epigenetic interventions in animal models, could pave the way for future treatments of psychosis and schizophrenia.
GABAergic Dysfunction in Schizophrenia – A Brief Chronology
Schizophrenia (SCZ)— a major psychiatric disorder with symptoms of delusions, hallucinations disorganized thought and affect, social withdrawal and apathy— lacks unifying neuropathology (Catts et al., 2013; Dorph-Petersen and Lewis, 2011), or narrowly defined genetic risk architectures and disease etiologies (Andreassen et al., 2014; Rodriguez-Murillo et al., 2012). Yet, clinical and translational research conducted over the last 40 years is beginning to identify major building blocks within the complex pathophysiology of SCZ. As highlighted in the various articles in this Special Issue of Schizophrenia Research, one such building block is the inhibitory GABAergic circuitry in the cerebral cortex. While the primary focus of our review will be on the transcriptional dysregulation of the GLUTAMIC ACID DECARBOXYLASE 1 (GAD1) gene, encoding the 67 KDa GABA synthesis enzyme, we will begin with a brief synopsis of past studies in pursuit of the ‘GABAergic hypothesis of SCZ’, which proposes that GABAergic systems could play a key role in the pathophysiology of SCZ. This idea is not new. Thus, 25 years after the first reports described the relatively large amounts of GABA and high levels glutamic acid decarboxylase (GAD) in brain (Roberts and Frankel, 1950, 1951), the role of inhibitory inputs to midbrain dopaminergic neurons was hypothesized to be the key mechanism responsible for excessive and dysregulated dopaminergic activity in psychosis (Smythies et al., 1975; Stevens et al., 1974). While it was quickly recognized that a generalized deficit in GABA signaling is not a characteristic of SCZ—as the symptoms of psychosis remained unresponsive to GABA agonists in early clinical trials (Tamminga et al., 1978)— the idea of region-specific dysfunctions of GABA systems nonetheless continued, up to the present day, to maintain significant traction and in fact, emerged as one of the most popular hypotheses in SCZ research. For example, several studies reported a decrease in GABA levels and GAD activity in the medial temporal lobe, thalamus and ventral striatum of the SCZ postmortem brain (Bird et al., 1977; Perry et al., 1979; Spokes et al., 1980) albeit other investigators reported negative findings (Cross et al., 1979). There were also reports on low GABA levels in the cerebrospinal fluid in at least a subset of patients diagnosed with SCZ (Lichtshtein et al., 1978; van Kammen et al., 1982). Following these early studies on GABA and GAD quantifications in the schizophrenic brain, several papers explored alterations in the inhibitory system in the context of abnormal circuitry. This type of work was mainly focused on the cerebral cortex and hippocampus, starting with Benes’ model proposing excessive excitatory and insufficient inhibitory signaling in the upper layers of the cerebral cortex due to a possible loss of GABAergic neurons and/or supranormal numbers or densities of glutamatergic afferent input into the same cortical layers (Benes et al., 1992a; Benes et al., 1992b). There is also an ongoing discussion if and how decreased expression of GABAergic markers genes in the cerebral cortex could be related to neurodevelopmental alterations (Benes, 2012), including the excessive numbers and densities of subcortical white matter neurons that seem to affect some cases with SCZ (Akbarian et al., 1993a; Akbarian et al., 1995; Akbarian et al., 1993b; Anderson et al., 1996; Benes, 2012; Eastwood and Harrison, 2005; Joshi et al., 2012; Kirkpatrick et al., 1999; Rioux et al., 2003; Yang et al., 2011).
However, the circuitry model with the greatest impact in the field to date was put forward by Lewis and colleagues (Lewis et al., 2005). At the core of this model is a deficit in perisomatic inhibition of cortical pyramidal neurons that involves their axon initiating segments (AIS) as a key control point for the output of cortical information processing (Lewis et al., 2005). The AIS is innervated by a specialized subtype of GABAergic interneuron, the chandelier cell, which is thought to be one of the interneuron types that are dysfunctional in SCZ cerebral cortex as evidenced by molecular alterations both on the pre- and postsynaptic site of the AIS (Pierri et al., 1999; Volk et al., 2002; Woo et al., 1998). Importantly, these postmortem studies, together with related work in preclinical model systems, paved the way for clinical trials and novel treatment approaches aimed at alleviating GABAergic deficits at the AIS and other key nodes of the cortical inhibitory system (Geffen et al., 2012; Lett et al., 2013; Lewis et al., 2008; Radhu et al., 2012; Rowland et al., 2013; Rudolph and Mohler, 2014; Stan and Lewis, 2012). For example to test the concept that reduced GABA signalling from chandelier cells to pyramidal neurons contribute to working memory dysfunction via parvalbumin-positive GABA neurons, a benzodiazepine-like compound, MK-0777, a selective agonist of GABAA α-2 and α-3 subunits, was tested in clinical trials. It was associated with improved performance on cognitive tasks (N-back, AX Continuous Performance Test, and Preparing to Overcome Prepotency tasks) (Lewis et al., 2008). Similarly, a GABA agonist and dopamine antagonist (BL-1020) showed greater improvements on cognitive tasks than risperidone and better than placebo effects on positive and negative symptoms assesses via PANSS and CGI (Geffen et al., 2012). While it is fair to state that none of these studies so far have resulted in a therapeutic breakthrough, they reflect a remarkable advancement for the field. We view them as evidence-based clinical trials in pursuit of drug treatment targets beyond the dopamine receptors that had been the focus of this field for more than half a century (Kapur and Mamo, 2003).
Altered Expression of GABA Synthesis Enzyme GAD1 is one of the most frequently reported molecular alterations in SCZ brain
The function of multiple subtypes of GABAergic neurons, including the aforementioned Chandelier cells, is thought to be compromised, at least in part, due to altered expression of GAD1 GABA synthesis enzyme (Lewis et al., 2005). GAD1(GAD67) accounts for 80–90% of overall brain GABA, while 10–20% reflects the activity of a related gene, GAD2 (GAD65) (Asada et al., 1997; Condie et al., 1997). To date, there are at least 20 reports in the literature, conducted by multiple groups of investigators on postmortem tissues collected in the U.S., Europe and Australia, reporting downregulated RNA and protein expression specifically of GAD1 in multiple brain regions of SCZ subjects, including prefrontal, medial temporal and occipital cortex and cerebellar cortex and basal ganglia (Akbarian et al., 1995; Benes et al., 2007; Bullock et al., 2008; Curley et al., 2011; Fatemi et al., 2005; Gilabert-Juan et al., 2012; Guidotti et al., 2000; Hashimoto et al., 2008a; Hashimoto et al., 2008b; Hashimoto et al., 2003; Huang et al., 2007; Impagnatiello et al., 1998; Mirnics et al., 2000; Sheng et al., 2012; Thompson Ray et al., 2011; Torrey et al., 2005; Veldic et al., 2005; Veldic et al., 2007; Volk et al., 2000; Volk et al., 2012). Furthermore, in a postmortem cohort comprised of elderly subjects, increased GAD1 expression in SCZ brain has been reported (Dracheva et al., 2004). These studies—each conducted with a sample size typically in the range of 20–120 brains— when taken together, leave little doubt that dysregulated GAD1 expression, primarily manifesting as a decrease in RNA levels, is a type of molecular alteration that is representative for a significant portion of SCZ brains. It has been suggested that approximately one out of three disease cases are affected by a more robust, perhaps more than > 30% downregulation in expression when compared to unaffected controls (Curley et al., 2011; Volk et al., 2012). Interestingly, decreased levels of GAD2, a paralogue of GAD1 resulting from an ancestral gene duplication (Bu and Tobin, 1994), have been reported somewhat less frequently in the SCZ postmortem literature, and could affect the hippocampus of the same cases on the mood or psychosis spectrum (Heckers et al., 2002; Todtenkopf and Benes, 1998).
Given that SCZ is a disorder of complex etiology and substantial subject-to-subject heterogeneities as it pertains to individual genetic risk architectures, environmental exposures and psychiatric manifestations, any type of molecular and cellular abnormality affecting a substantial subset of cases, such as decreased GAD1 expression, is likely to represent adaptive pathophysiology resulting from a fairly broad range of potential factors operating further upstream. Indeed, as outlined in a previous review (Akbarian and Huang, 2006) and in the next chapters, this hypothesis receives strong support from preclinical studies, with a steadily increasing list of animal models and cell culture systems providing insights into the molecular and cellular systems governing neuronal Gad1 expression.
Activity-dependent regulation of GABAergic gene expression in rodent and primate brain
Given that various portions of cerebral cortex show functional hypoactivity during various neuropsychological task performance in many cases with SCZ (Snitz et al., 2005; Streit et al., 2001), it is possible that some of the molecular and cellular changes observed in SCZ brain could reflect secondary changes in response to alterations in neuronal activity. Indeed, a broad range of studies, from in vivo work with non-human primates to ex vivo studies in the cell culture dish, are in support of this hypothesis. For example, 48 hours after monkey visual cortex had been deprived from sensory input in one eye, a marked reduction in GABA and GAD protein levels was found in the ocular dominance columns associated with a deprived eye (Hendry and Jones, 1986), followed by a robust downregulation of Gad1 RNA levels after a period of 15 days (Benson et al., 1994). These and other changes in Gad1 expression in the cerebral cortex of non-human primates are viewed as adaptive responses aimed at restoration of the excitation/inhibition (E/I) balance after afferent activity in the network has changed (Hendry and Jones, 1988; Jones et al., 1994). This hypothesis is also supported by some independent observations in rodents. For example, in rats, kainic acid-induced loss of hippocampal pyramidal neurons leads to a permanent downregulation of Gad1 expression in the same brain region, which could contribute to the rewiring and hyperexcitability of the postlesion hippocampus (Shetty and Turner, 2001). In cultured hippocampal neurons, synaptic inactivity results in reduced GABA levels and Gad1/Gad67 expression, and furthermore, Gad1/Gad67 emerged as a critical regulator for cytosolic levels and vesicular filling of GABA, thereby affecting synaptic homeostasis (Lau and Murthy, 2012). Whether or not such types of activity-dependent mechanisms could have contributed to downregulated cortical GAD1 expression in some of the SCZ cases remains unclear given that the clinical studies are confined to postmortem brain. However, this is a plausible hypothesis given that there is ample evidence for functional hypoactivity affecting multiple cortical areas in SCZ (Cieslik et al., 2013; Diwadkar et al., 2011; Eack et al., 2013; Fujiki et al., 2013; Lee et al., 2014; Ota et al., 2014; Thoresen et al., 2014; Wolwer et al., 2012; Yoon et al., 2013)(and earlier references cited therein).
Prefrontal GAD1 expression steadily increases during childhood and adolescence, and is sensitive to developmental pertubations
Maturation of the cerebral cortex, including its prefrontal areas, extends beyond the second decade of life (Kolb et al., 2012), and such type of prolonged developmental periods may play a key role in the neurobiology of SCZ as a psychiatric disorder with a typical onset of clinical symptoms around adolescence and young adulthood (Weinberger, 1987). Studies monitoring the expression and developmental trajectory of key molecules at inhibitory synapses in primate prefrontal cortex during the transitions from the early postnatal period to adulthood have emphasized the temporal association with a range of environmental risk factors for SCZ, including cannabis abuse (Hoftman and Lewis, 2011). This developmental window of vulnerability is likely to include much earlier time points too, because studies in rodents have linked early postnatal stressors (Zhang et al., 2010), and even prenatal exposure to toxins (Mackowiak et al., 2014) and maternal immune activation (Richetto et al., 2014) to deficits in Gad1/Gad67 expression in adult cortex and hippocampus. Therefore, it is interesting to note that GAD1 expression in human prefrontal cortex is subject to a developmentally regulated steady increase from the late prenatal period to early adolescence and adulthood(Huang et al., 2007), together with a progressive switch from an early GAD1 transcript encoding a 25kDa protein lacking enzymatic activity to full length (67kDa) GAD1(Hyde et al., 2011). Such broad temporal window of vulnerability would make it appear plausible that developmental defects or environmental insults impacting the immature brain indeed play a role for the observed alterations of cortical GAD1 expression later in life.
Developmental and disease-associated changes in epigenetic signatures at the GAD1 promoter
The regulatory networks governing the molecular architectures of cortical inhibitory circuitry are exceedingly complex and include a diverse array of transcriptional and post-transcriptional mechanisms. To mention just one recent example from the SCZ literature, prefrontal deficits in the expression of a subset of GABA neuron-specific mRNAs, including Neuropeptide (NPY), were found to be dependent on the regional supply of Brain-derived Neurotrophic Factor (BDNF), which in turn was subject to post-transcriptional control by a microRNA-dependent mechanism (Mellios et al., 2009).
In contrast, as will be further discussed below, defective GAD1 expression in SCZ has been primarily linked to epigenetic abnormalities in chromatin surrounding the GAD1 promoter and transcription start site (note that while the more traditional definition of epi-(greek for ‘over’, ‘above’) genetics is often equated with heritable changes in gene expression and function in the absence of DNA sequence alterations, the term is nowadays much more broadly applied to describe chromatin structure and function in the context of transcription, splicing, genome organization and maintenance and many other mechanisms). It should be mentioned that the process of gene expression and the gene (sequence)-specific occupancies of activated RNA polymerase II complex and subunits associated with transcriptional initiation or elongation cannot be measured easily in postmortem specimens. However, some of the covalent modifications of the nucleosomal core histones that are differentially enriched at sites of actively expressed genes versus repressed and condensed chromatin are sufficiently stable after death. Thus, a number of postmortem brain studies have successfully employed chromatin immunoprecipitation assays, a standard approach to quantify levels of specific histone modifications, at specific genes and loci (Huang et al., 2006; Kurita et al., 2012; Matevossian and Akbarian, 2008; Stadler et al., 2005; Tang et al., 2011), and even embarked on genome-scale epigenome mappings (Cheung et al., 2010; Zhu et al., 2013).
These techniques have now also been applied to GAD1 promoter sequences which are located within a few hundred base pairs from the transcription start site (Chen et al., 2011). Alterations observed include a shift from facilitative towards repressive chromatin-associated histone modifications, and changes in DNA methylation signatures, often in conjunction with altered GAD1 expression in the prefrontal cortex (PFC) of the affected SCZ cases (Grayson and Guidotti, 2012; Huang and Akbarian, 2007; Huang et al., 2007; Tang et al., 2011). It is also noteworthy that common polymorphisms in the proximal GAD1 promoter confer genetic risk for SCZ, impaired working memory performance and accelerated loss of gray matter (Addington et al., 2005; Straub et al., 2007), possibly in conjunction with altered expression of the cation chloride co-transporters NKCC1/KCC2, two key regulators of postsynaptic GABAA receptor-mediated currents (Hyde et al., 2011). These findings, taken together, would suggest that the genetic and epigenetic architecture of the GAD1 promoter is a potential factor for the gene’s dysregulated expression in at least some cases with SCZ. Furthermore, at least one of the histone modifications found at reduced levels at GAD1 regulatory sequences in SCZ, histone H3 trimethylated at lysine 4 (H3K4me3), shows a progressive upregulation at human and mouse GAD1/Gad1 during the extended period of postnatal cortical development until reaching mature levels, in parallel to the corresponding increase of the RNA (Huang et al., 2007). Strikingly, the same epigenetic mark was significantly upregulated in GAD1 chromatin from PFC of SCZ subjects treated with the atypical antipsychotic clozapine before death, and in cerebral cortex of mice subjected to a subchronic (21 days) regimen of daily clozapine injections (Huang et al., 2007). Therefore, the GAD1-bound H3K4me3 could be viewed as a molecular link that interconnects three important factors in the neurobiology of SCZ – GABA neuron dysfunction, developmental mechanisms and the molecular response after exposure to one of the most effective antipsychotic drugs currently available.
Spatial architecture of the GAD1 locus in normal and diseased PFC
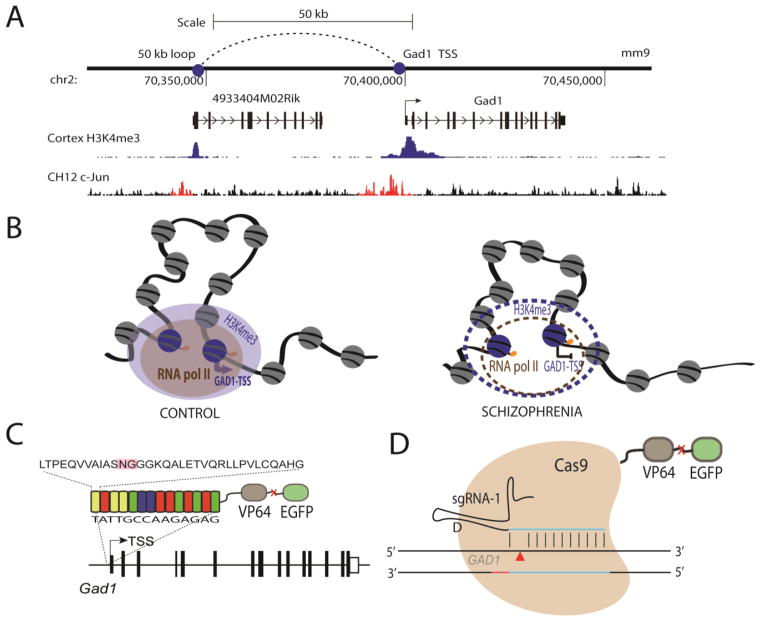
The preceding paragraph summarized evidence for localized epigenetic dysregulation of sequences surrounding the GAD1 transcription start site. However, the regulation of gene expression in a vertebrate cell goes far beyond the genetic and epigenetic architectures of proximal promoters and transcription start sites. Instead, chromosomes and gene expression units inside the cell nucleus are organized as highly complex dynamic 3-dimensional structures that includes chromosomal loopings and physically interactions of enhancer and promoter elements with transcription start sites (Deng and Blobel, 2010; Ribeiro de Almeida et al., 2011; Singh et al., 2012). To date, however, the regulation of supranucleosomal higher order chromatin, beyond the level of DNA methylation and posttranslational modifications of the core histones, largely remains unexplored in normal or diseased human brain. However, this may soon change because it was recently reported that chromosome conformation capture, commonly referred to as 3C (Dekker et al., 2013; van Berkum and Dekker, 2009) and widely viewed as the standard approach to map chromosomal loopings, is applicable to brain tissue collected postmortem (Mitchell et al., 2014). The 3C technique explores physical interactions between DNA fragments separated by Kb or Mb of interspersed sequence; crosslinked chromatin is digested with a specific restriction enzyme, religated and amplified using primer pairs for which forward and reverse primers match to different portions of the genomic locus-of-interest(Mitchell et al., 2014). Chromosomal conformations have been explored at the GAD1 locus(Bharadwaj et al., 2013). One of these loop formations, initially detected in PFC and then confirmed in neuronal cultures derived from pluripotent skin cells, interconnected promoter-proximal sequences, positioned within 1–2kb from the GAD1 transcription start site, with non-coding sequences positioned 50 kb further upstream. These loopings showed a significant weakening in PFC of SCZ subjects affected by decreased GAD1 expression (Bharadwaj et al., 2013). Remarkably, DNA sequences at the GAD1 TSS and 50kb upstream show conservation from rodents to primates, and the GAD1 TSS – 50kb chromatin loop is strikingly similar in both mouse and human brain (Bharadwaj et al., 2013). Furthermore, reported that 3C studies in reporter mice expressing green fluorescent protein in GABAergic neuronal nuclei showed that the Gad1 TSS-50kb loop was much stronger in cortical GABAergic interneurons—which express Gad1—compared to other cortical cells that do not express Gad1. This study (Bharadwaj et al., 2013) therefore could be viewed as ‘proof of principle’, showing that higher order chromatin is specific for cell-type and potentially altered in SCZ, and amenable for follow-up work in animal models and human cell culture systems.
Synopsis and implications for future treatments of SCZ
If dysregulated GAD1 expression in PFC and other brain regions is indeed critical in the pathophysiology of at least some cases with SCZ, then one could speculate whether these mechanisms would offer novel therapeutic avenues for a subset of patients carrying this diagnosis. Of note, antipsychotic medications targeting dopaminergic, serotonergic and monoaminergic receptor systems are still the mainstay in SCZ treatment (Kim and Stahl, 2010; Taly, 2013) and broadly applied to a large majority of patients. However, the majority of subjects with SCZ experience an incomplete response to currently prescribed antipsychotic drugs, resulting in significant disability and reduced quality of life (Lieberman et al., 2005; Swartz et al., 2007). Rationale for the development of GAD1 and other GABAergic gene-targeted therapeutic interventions could be built on the following five lines of evidence and starting points: First, prefrontal GAD1 expression is dysregulated in a significant subset, or approximately 30%, of subjects with SCZ (Volk et al., 2012). Second, common polymorphisms in 5′regulatory sequences of GAD1 that affects its expression also confer a statistical risk for childhood-onset SCZ and cortical volume loss (Addington et al., 2005), and are in epistasis, or co-regulated, with other SCZ-relevant genes (Straub et al., 2007; Tao et al., 2012). Third, conditional deletion of one of the two Gad1 alleles in parvalbumin-positive interneurons in the juvenile cortex elicited transient synaptic deficits and increased pyramidal cell excitability (Lazarus et al., 2013). Furthermore, Gad1/Gad67 deficiencies in select interneuron populations of the adolescent cortex result in cell-autonomous defects in connectivity (Chattopadhyaya et al., 2007). Decreased expression of GAD1/GAD67 in conjunction with other SCZ-related deficits and behavioral alterations has also been reported in conditional mutant mice with interneuron-specific ablation of NMDA glutamate receptor subunits (Belforte et al., 2010). These elegant mouse models further emphasize the potential importance of even moderately decreased GAD1 expression in disease states, and warrants additional examination of the homeostatic and compensatory mechanisms that normalized synaptic inhibition in the adult mouse mutant cortex. Fourth, cortical Gad1 expression and levels of transcription-associated histone methylation at the Gad1 promoter showed in some rodent studies a subtle but significant up-regulation after treatment with commonly prescribed antipsychotic drugs (Huang et al., 2007; Lipska et al., 2003). Fifth, bulk GABA tissue levels in the cerebral cortex show robust associations with gamma band oscillations and working memory performance, even under conditions of system and cognitive impairments as seen in SCZ (Chen et al., 2014; Yoon et al., 2010). While cell-type specific prefrontal deficits in GAD1 expression probably are not well represented in measurements of bulk tissue GABA levels, the five independent lines of preclinical and clinical evidence, as summarized above, taken together, provide very reasonable rationale to pursue targeted therapeutic interventions aimed at cortical GAD1 expression in SCZ.
Looking forward: Targeted Epigenetic Interventions at the GAD1 locus for the treatment of SCZ
Genetic engineering-induced GAD1/GAD67 deficiency in cortical interneurons is detrimental for neuronal signaling and cognition and social interactions (Belforte et al., 2010; Chattopadhyaya et al., 2007; Lazarus et al., 2013; Schmidt et al., 2014). Whether or not an experimentally induced increase in Gad1/Gad67 would elicit therapeutic effects in preclinical SCZ models remains to be determined. Among the various options available in the present-day molecular toolbox for altering the expression of a gene-of-interest, we would favor de novo engineered designer transcription factors targeted against specific sequences in the gene-proximal promoter near the TSS, or at distal cis-regulatory elements, including long-range enhancers (Figure 1A). Such type of chromatin-based therapy would bear important advantages over transgene-derived overexpression that relies on some artificial or endogenous promoter that is constitutively active. Instead, promoter- or enhancer-based interventions would modulate, or boost physiologically driven Gad1 expression. For example, a good candidate would be targeting the sequences that engage in a chromosomal loop formation interconnecting the gene-proximal GAD1 promoter with regulatory elements positioned 50kb further upstream to edit looping interactions (Bharadwaj et al., 2013).
Figure 1. GAD1/Gad67 TALE and CRISPR Engineering.
(A) A chromatin loop exists between the Gad1-TSS and a region upstream by 50kb marked by H3K4me3 sites in mouse cortex. It is marked by H3K4me3 sites in cortex and AP1 (c-Jun) sites in CH12 cell lines (ENCODE data) (Bernstein et al., 2012). (B) This 50-kb loop also exists in humans and is characterized by RNA polymerase II binding and H3K4me3 sites. The loop is weakened in the prefrontal cortex (PFC) of subjects with schizophrenia along with decreased GAD1 gene expression, loss of the H3K4me3 mark, and probably also RNA polymerase II binding. (C and D) Designer transcription factors can be targeted to the human or mouse GAD1(Gad1)-TSS or to the upstream 50-kb loop to effectively increase gene expression. (C) We propose to test these mechanisms in mice in vivo, using (C) a TALE or (D) a guide RNA (‘sg-RNA 1’) for the CRISPR-Cas9 protein, each fused to the VP64 enhancer to increase gene expression and EGFP as a fluorescent mark.
‘NG’ (pink) box in (C) marks the hypervariable region of the TALE repeat region.
A number of different genome editing approaches, including zinc finger nucleases, transcription activator-like effector nucleases (TALEN), clustered regularly interspaced short palindromic repeat(CRISPR)/CAS9 RNA-guided nucleases—all of which were recently introduced to the field (Gaj et al., 2013; Li et al., 2014)—allow for design of sequence-specific DNA binding factors, which when fused to well described transcriptional activation domains such as the VP64 protein, could function as sequence-specific transcription factors (Figure 1C and 1D). Each method targets the sequence differently. Zinc finger proteins are generated in 3 bp selectivity modules. Each modules can then be linked together to target a specific region of interest. TALEs are composed of 33–35 amino acid repeat domains that recognize a single base pair with specificity determined by two hypervariable amino acids. Distinct from zinc finger proteins and TALEs CRISPR/Cas operates via RNA-guides. The CRISPR/Cas system can be guided to specific regions via short guide RNA (sgRNA). These transcription factors and editors can be combined with different effector domains (nucleases, transcriptional activators and repressors, recombinases, transposases, DNA and histone methyltransferases, and histone acetyltransferases) to strengthen the Gad1-TSS/50-kb loop and upregulate Gad1 gene expression in cell culture, mouse GABAergic PFC neurons, and possibly eventually in humans (Cho et al., 2013; Cong et al., 2013). A TALE-based novel engineered transcription factor already has contributed to better understanding of the regulatory mechanisms governing Gad1/Gad67 expression in culture mouse neurons. In cell culture, a TALE-based transcription factor positioned at gene-proximal Gad1 promoter sequences was used with good success to upregulate Gad1 gene expression in cultured neurons (Konermann et al., 2013). It is likely that these approaches will become the essential parts in the molecular toolbox to up-regulate cortical GAD1 expression in the animal model, or at some point in the future, also in human subjects.
Acknowledgments
Funding Source
Work in the authors’ laboratory is supported by funds from the NIH and the Brain & Behavior Research Foundation.
Footnotes
The authors declare no conflicts.
Contributors
Amanda Mitchell and Schahram Akbarian edited and drafted the manuscript. Yan Jiang and Cyril Peter drafted TALE and CRISPR images for the final figure. All authors reviewed the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington AM, Gornick M, Duckworth J, Sporn A, Gogtay N, Bobb A, Greenstein D, Lenane M, Gochman P, Baker N, Balkissoon R, Vakkalanka RK, Weinberger DR, Rapoport JL, Straub RE. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Molecular psychiatry. 2005;10(6):581–588. doi: 10.1038/sj.mp.4001599. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Bunney WE, Jr, Potkin SG, Wigal SB, Hagman JO, Sandman CA, Jones EG. Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Archives of general psychiatry. 1993a;50(3):169–177. doi: 10.1001/archpsyc.1993.01820150007001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain research reviews. 2006;52(2):293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of general psychiatry. 1995;52(4):258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Vinuela A, Kim JJ, Potkin SG, Bunney WE, Jr, Jones EG. Distorted distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Archives of general psychiatry. 1993b;50(3):178–187. doi: 10.1001/archpsyc.1993.01820150016002. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Volk DW, Lewis DA. Increased density of microtubule associated protein 2-immunoreactive neurons in the prefrontal white matter of schizophrenic subjects. Schizophrenia research. 1996;19(2–3):111–119. doi: 10.1016/0920-9964(96)88521-5. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Thompson WK, Dale AM. Boosting the power of schizophrenia genetics by leveraging new statistical tools. Schizophrenia bulletin. 2014;40(1):13–17. doi: 10.1093/schbul/sbt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(12):6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nature neuroscience. 2010;13(1):76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. A new paradigm for understanding gamma-aminobutyric acid cell pathology in schizophrenia? Biological psychiatry. 2012;72(9):712–713. doi: 10.1016/j.biopsych.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(24):10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Sorensen I, Vincent SL, Bird ED, Sathi M. Increased density of glutamate-immunoreactive vertical processes in superficial laminae in cingulate cortex of schizophrenic brain. Cereb Cortex. 1992a;2(6):503–512. doi: 10.1093/cercor/2.6.503. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Alsterberg G, Bird ED, SanGiovanni JP. Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1992b;12(3):924–929. doi: 10.1523/JNEUROSCI.12-03-00924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Huntsman MM, Jones EG. Activity-dependent changes in GAD and preprotachykinin mRNAs in visual cortex of adult monkeys. Cereb Cortex. 1994;4(1):40–51. doi: 10.1093/cercor/4.1.40. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj R, Jiang Y, Mao W, Jakovcevski M, Dincer A, Krueger W, Garbett K, Whittle C, Tushir JS, Liu J, Sequeira A, Vawter MP, Gardner PD, Casaccia P, Rasmussen T, Bunney WE, Jr, Mirnics K, Futai K, Akbarian S. Conserved chromosome 2q31 conformations are associated with transcriptional regulation of GAD1 GABA synthesis enzyme and altered in prefrontal cortex of subjects with schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(29):11839–11851. doi: 10.1523/JNEUROSCI.1252-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird ED, Spokes EG, Barnes J, MacKay AV, Iversen LL, Shepherd M. Increased brain dopamine and reduced glutamic acid decarboxylase and choline acetyl transferase activity in schizophrenia and related psychoses. Lancet. 1977;2(8049):1157–1158. doi: 10.1016/s0140-6736(77)91542-2. [DOI] [PubMed] [Google Scholar]
- Bu DF, Tobin AJ. The exon-intron organization of the genes (GAD1 and GAD2) encoding two human glutamate decarboxylases (GAD67 and GAD65) suggests that they derive from a common ancestral GAD. Genomics. 1994;21(1):222–228. doi: 10.1006/geno.1994.1246. [DOI] [PubMed] [Google Scholar]
- Bullock WM, Cardon K, Bustillo J, Roberts RC, Perrone-Bizzozero NI. Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. The American journal of psychiatry. 2008;165(12):1594–1603. doi: 10.1176/appi.ajp.2008.07121845. [DOI] [PubMed] [Google Scholar]
- Catts VS, Fung SJ, Long LE, Joshi D, Vercammen A, Allen KM, Fillman SG, Rothmond DA, Sinclair D, Tiwari Y, Tsai SY, Weickert TW, Shannon Weickert C. Rethinking schizophrenia in the context of normal neurodevelopment. Frontiers in cellular neuroscience. 2013;7:60. doi: 10.3389/fncel.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54(6):889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Stanford AD, Mao X, Abi-Dargham A, Shungu DC, Lisanby SH, Schroeder CE, Kegeles LS. GABA level, gamma oscillation, and working memory performance in schizophrenia. Neuro Image Clinical. 2014;4:531–539. doi: 10.1016/j.nicl.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dong E, Grayson DR. Analysis of the GAD1 promoter: trans-acting factors and DNA methylation converge on the 5′ untranslated region. Neuropharmacology. 2011;60(7–8):1075–1087. doi: 10.1016/j.neuropharm.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Cheung I, Shulha HP, Jiang Y, Matevossian A, Wang J, Weng Z, Akbarian S. Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(19):8824–8829. doi: 10.1073/pnas.1001702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31(3):230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- Cieslik EC, Muller VI, Kellermann TS, Grefkes C, Halfter S, Eickhoff SB. Shifted neuronal balance during stimulus-response integration in schizophrenia: an fMRI study. Brain structure & function. 2013 doi: 10.1007/s00429-013-0652-1. [DOI] [PubMed] [Google Scholar]
- Condie BG, Bain G, Gottlieb DI, Capecchi MR. Cleft palate in mice with a targeted mutation in the gamma-aminobutyric acid-producing enzyme glutamic acid decarboxylase 67. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(21):11451–11455. doi: 10.1073/pnas.94.21.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AJ, Crow TJ, Owen F. Gamma-aminobutyric acid in the brain in schizophrenia. Lancet. 1979;1(8115):560–561. doi: 10.1016/s0140-6736(79)90991-7. [DOI] [PubMed] [Google Scholar]
- Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. The American journal of psychiatry. 2011;168(9):921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Marti-Renom MA, Mirny LA. Exploring the three-dimensional organization of genomes: interpreting chromatin interaction data. Nature reviews. Genetics. 2013;14(6):390–403. doi: 10.1038/nrg3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Blobel GA. Do chromatin loops provide epigenetic gene expression states? Current opinion in genetics & development. 2010;20(5):548–554. doi: 10.1016/j.gde.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Pruitt P, Goradia D, Murphy E, Bakshi N, Keshavan MS, Rajan U, Reid A, Zajac-Benitez C. Fronto-parietal hypo-activation during working memory independent of structural abnormalities: conjoint fMRI and sMRI analyses in adolescent offspring of schizophrenia patients. Neuro Image. 2011;58(1):234–241. doi: 10.1016/j.neuroimage.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Lewis DA. Stereological approaches to identifying neuropathology in psychosis. Biological psychiatry. 2011;69(2):113–126. doi: 10.1016/j.biopsych.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracheva S, Elhakem SL, McGurk SR, Davis KL, Haroutunian V. GAD67 and GAD65 mRNA and protein expression in cerebrocortical regions of elderly patients with schizophrenia. Journal of neuroscience research. 2004;76(4):581–592. doi: 10.1002/jnr.20122. [DOI] [PubMed] [Google Scholar]
- Eack SM, Wojtalik JA, Newhill CE, Keshavan MS, Phillips ML. Prefrontal cortical dysfunction during visual perspective-taking in schizophrenia. Schizophrenia research. 2013;150(2–3):491–497. doi: 10.1016/j.schres.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial white matter neuron density in the dorsolateral prefrontal cortex and parahippocampal gyrus in schizophrenia. Schizophrenia research. 2005;79(2–3):181–188. doi: 10.1016/j.schres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophrenia research. 2005;72(2–3):109–122. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Fujiki R, Morita K, Sato M, Kamada Y, Kato Y, Inoue M, Shoji Y, Uchimura N. Reduced prefrontal cortex activation using the Trail Making Test in schizophrenia. Neuropsychiatric disease and treatment. 2013;9:675–685. doi: 10.2147/NDT.S43137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends in biotechnology. 2013;31(7):397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen Y, Keefe R, Rabinowitz J, Anand R, Davidson M. Bl-1020, a new gamma-aminobutyric acid-enhanced antipsychotic: results of 6-week, randomized, double-blind, controlled, efficacy and safety study. The Journal of clinical psychiatry. 2012;73(9):e1168–1174. doi: 10.4088/JCP.12m07642. [DOI] [PubMed] [Google Scholar]
- Gilabert-Juan J, Varea E, Guirado R, Blasco-Ibanez JM, Crespo C, Nacher J. Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neuroscience letters. 2012;530(1):97–102. doi: 10.1016/j.neulet.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Guidotti A. The Dynamics of DNA Methylation in Schizophrenia and Related Psychiatric Disorders. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Archives of general psychiatry. 2000;57(11):1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Molecular psychiatry. 2008a;13(2):147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. The American journal of psychiatry. 2008b;165(4):479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23(15):6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Archives of general psychiatry. 2002;59(6):521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG. Reduction in number of immunostained GABAergic neurones in deprived-eye dominance columns of monkey area 17. Nature. 1986;320(6064):750–753. doi: 10.1038/320750a0. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron. 1988;1(8):701–712. doi: 10.1016/0896-6273(88)90169-9. [DOI] [PubMed] [Google Scholar]
- Hoftman GD, Lewis DA. Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophrenia bulletin. 2011;37(3):493–503. doi: 10.1093/schbul/sbr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Akbarian S. GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PloS one. 2007;2(8):e809. doi: 10.1371/journal.pone.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Matevossian A, Jiang Y, Akbarian S. Chromatin immunoprecipitation in postmortem brain. Journal of neuroscience methods. 2006;156(1–2):284–292. doi: 10.1016/j.jneumeth.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, Akbarian S. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(42):11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, Straub RE, Ye T, Colantuoni C, Herman MM, Bigelow LB, Weinberger DR, Kleinman JE. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(30):11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting P, Sharma RP, Costa E. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(26):15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Huntley GW, Benson DL. Alpha calcium/calmodulin-dependent protein kinase II selectively expressed in a subpopulation of excitatory neurons in monkey sensory-motor cortex: comparison with GAD-67 expression. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14(2):611–629. doi: 10.1523/JNEUROSCI.14-02-00611.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D, Fung SJ, Rothwell A, Weickert CS. Higher gamma-aminobutyric acid neuron density in the white matter of orbital frontal cortex in schizophrenia. Biological psychiatry. 2012;72(9):725–733. doi: 10.1016/j.biopsych.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Progress in neuro-psychopharmacology & biological psychiatry. 2003;27(7):1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kim DH, Stahl SM. Antipsychotic drug development. Current topics in behavioral neurosciences. 2010;4:123–139. doi: 10.1007/7854_2010_47. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Conley RC, Kakoyannis A, Reep RL, Roberts RC. Interstitial cells of the white matter in the inferior parietal cortex in schizophrenia: An unbiased cell-counting study. Synapse. 1999;34(2):95–102. doi: 10.1002/(SICI)1098-2396(199911)34:2<95::AID-SYN2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R. Experience and the developing prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 2):17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Cong L, Platt RJ, Scott DA, Church GM, Zhang F. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500(7463):472–476. doi: 10.1038/nature12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita M, Holloway T, Garcia-Bea A, Kozlenkov A, Friedman AK, Moreno JL, Heshmati M, Golden SA, Kennedy PJ, Takahashi N, Dietz DM, Mocci G, Gabilondo AM, Hanks J, Umali A, Callado LF, Gallitano AL, Neve RL, Shen L, Buxbaum JD, Han MH, Nestler EJ, Meana JJ, Russo SJ, Gonzalez-Maeso J. HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nature neuroscience. 2012;15(9):1245–1254. doi: 10.1038/nn.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Murthy VN. Activity-dependent regulation of inhibition via GAD67. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(25):8521–8531. doi: 10.1523/JNEUROSCI.1245-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus MS, Krishnan K, Huang ZJ. GAD67 Deficiency in Parvalbumin Interneurons Produces Deficits in Inhibitory Transmission and Network Disinhibition in Mouse Prefrontal Cortex. Cereb Cortex. 2013 doi: 10.1093/cercor/bht322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Chun JW, Yoon SY, Park HJ, Kim JJ. Involvement of the mirror neuron system in blunted affect in schizophrenia. Schizophrenia research. 2014;152(1):268–274. doi: 10.1016/j.schres.2013.10.043. [DOI] [PubMed] [Google Scholar]
- Lett TA, Voineskos AN, Kennedy JL, Levine B, Daskalakis ZJ. Treating Working Memory Deficits in Schizophrenia: A Review of the Neurobiology. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. The American journal of psychiatry. 2008;165(12):1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature reviews. Neuroscience. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Li M, Suzuki K, Kim NY, Liu GH, Izpisua Belmonte JC. A cut above the rest: targeted genome editing technologies in human pluripotent stem cells. The Journal of biological chemistry. 2014;289(8):4594–4599. doi: 10.1074/jbc.R113.488247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtshtein D, Dobkin J, Ebstein RP, Biederman J, Rimon R, Belmaker RH. Gamma-aminobutyric acid (GABA) in the CSF of schizophrenic patients before and after neuroleptic treatment. The British journal of psychiatry: the journal of mental science. 1978;132:145–148. doi: 10.1192/bjp.132.2.145. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. The New England journal of medicine. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Lerman DN, Khaing ZZ, Weickert CS, Weinberger DR. Gene expression in dopamine and GABA systems in an animal model of schizophrenia: effects of antipsychotic drugs. The European journal of neuroscience. 2003;18(2):391–402. doi: 10.1046/j.1460-9568.2003.02738.x. [DOI] [PubMed] [Google Scholar]
- Mackowiak M, Bator E, Latusz J, Mordalska P, Wedzony K. Prenatal MAM administration affects histone H3 methylation in postnatal life in the rat medial prefrontal cortex. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2014;24(2):271–289. doi: 10.1016/j.euroneuro.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Matevossian A, Akbarian S. A chromatin assay for human brain tissue. Journal of visualized experiments: JoVE. 2008;(13) doi: 10.3791/717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biological psychiatry. 2009;65(12):1006–1014. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28(1):53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Mitchell AC, Bharadwaj R, Whittle C, Krueger W, Mirnics K, Hurd Y, Rasmussen T, Akbarian S. The Genome in Three Dimensions: A New Frontier in Human Brain Research. Biological psychiatry. 2014;75(12):961–969. doi: 10.1016/j.biopsych.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M, Ishikawa M, Sato N, Okazaki M, Maikusa N, Hori H, Hattori K, Teraishi T, Ito K, Kunugi H. Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophrenia research. 2014;154(1–3):113–118. doi: 10.1016/j.schres.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Perry TL, Kish SJ, Buchanan J, Hansen S. Gamma-aminobutyric-acid deficiency in brain of schizophrenic patients. Lancet. 1979;1(8110):237–239. doi: 10.1016/s0140-6736(79)90767-0. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. The American journal of psychiatry. 1999;156(11):1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Radhu N, Ravindran LN, Levinson AJ, Daskalakis ZJ. Inhibition of the cortex using transcranial magnetic stimulation in psychiatric populations: current and future directions. Journal of psychiatry & neuroscience: JPN. 2012;37(6):369–378. doi: 10.1503/jpn.120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro de Almeida C, Stadhouders R, de Bruijn MJ, Bergen IM, Thongjuea S, Lenhard B, van Ijcken W, Grosveld F, Galjart N, Soler E, Hendriks RW. The DNA-binding protein CTCF limits proximal Vkappa recombination and restricts kappa enhancer interactions to the immunoglobulin kappa light chain locus. Immunity. 2011;35(4):501–513. doi: 10.1016/j.immuni.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Richetto J, Calabrese F, Riva MA, Meyer U. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophrenia bulletin. 2014;40(2):351–361. doi: 10.1093/schbul/sbs195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux L, Nissanov J, Lauber K, Bilker WB, Arnold SE. Distribution of microtubule-associated protein MAP2-immunoreactive interstitial neurons in the parahippocampal white matter in subjects with schizophrenia. The American journal of psychiatry. 2003;160(1):149–155. doi: 10.1176/appi.ajp.160.1.149. [DOI] [PubMed] [Google Scholar]
- Roberts E, Frankel S. gamma-Aminobutyric acid in brain: its formation from glutamic acid. The Journal of biological chemistry. 1950;187(1):55–63. [PubMed] [Google Scholar]
- Roberts E, Frankel S. Glutamic acid decarboxylase in brain. The Journal of biological chemistry. 1951;188(2):789–795. [PubMed] [Google Scholar]
- Rodriguez-Murillo L, Gogos JA, Karayiorgou M. The genetic architecture of schizophrenia: new mutations and emerging paradigms. Annual review of medicine. 2012;63:63–80. doi: 10.1146/annurev-med-072010-091100. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Edden RA, Kontson K, Zhu H, Barker PB, Hong LE. GABA predicts inhibition of frequency-specific oscillations in schizophrenia. The Journal of neuropsychiatry and clinical neurosciences. 2013;25(1):83–87. doi: 10.1176/appi.neuropsych.11120368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. GABAA receptor subtypes: Therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annual review of pharmacology and toxicology. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MJ, Horvath S, Ebert P, Norris JL, Seeley EH, Brown J, Gellert L, Everheart M, Garbett KA, Grice TW, Caprioli RM, Mirnics K. Modulation of behavioral networks by selective interneuronal inactivation. Molecular psychiatry. 2014;19(5):580–587. doi: 10.1038/mp.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng G, Demers M, Subburaju S, Benes FM. Differences in the circuitry-based association of copy numbers and gene expression between the hippocampi of patients with schizophrenia and the hippocampi of patients with bipolar disorder. Archives of general psychiatry. 2012;69(6):550–561. doi: 10.1001/archgenpsychiatry.2011.1882. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Glutamic acid decarboxylase-67-positive hippocampal interneurons undergo a permanent reduction in number following kainic acid-induced degeneration of ca3 pyramidal neurons. Experimental neurology. 2001;169(2):276–297. doi: 10.1006/exnr.2001.7668. [DOI] [PubMed] [Google Scholar]
- Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, Liu EM, Reichel J, Porrati P, Pellegatta S, Qiu K, Gao Z, Ceccarelli M, Riccardi R, Brat DJ, Guha A, Aldape K, Golfinos JG, Zagzag D, Mikkelsen T, Finocchiaro G, Lasorella A, Rabadan R, Iavarone A. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337(6099):1231–1235. doi: 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies JR, Bradley RJ, Linton PH. Meeting report: Biochemical aspects of schizophrenia. Alabama, April 1975. Psychoneuroendocrinology. 1975;1(2):199–201. doi: 10.1016/0306-4530(75)90011-6. [DOI] [PubMed] [Google Scholar]
- Snitz BE, MacDonald A, 3rd, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. The American journal of psychiatry. 2005;162(12):2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Spokes EG, Garrett NJ, Rossor MN, Iversen LL. Distribution of GABA in post-mortem brain tissue from control, psychotic and Huntington’s chorea subjects. Journal of the neurological sciences. 1980;48(3):303–313. doi: 10.1016/0022-510x(80)90103-3. [DOI] [PubMed] [Google Scholar]
- Stadler F, Kolb G, Rubusch L, Baker SP, Jones EG, Akbarian S. Histone methylation at gene promoters is associated with developmental regulation and region-specific expression of ionotropic and metabotropic glutamate receptors in human brain. Journal of neurochemistry. 2005;94(2):324–336. doi: 10.1111/j.1471-4159.2005.03190.x. [DOI] [PubMed] [Google Scholar]
- Stan AD, Lewis DA. Altered cortical GABA neurotransmission in schizophrenia: insights into novel therapeutic strategies. Current pharmaceutical biotechnology. 2012;13(8):1557–1562. doi: 10.2174/138920112800784925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J, Wilson K, Foote W. GABA blockade, dopamine and schizophrenia: experimental studies in the cat. Psychopharmacologia. 1974;39(2):105–119. doi: 10.1007/BF00440842. [DOI] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Molecular psychiatry. 2007;12(9):854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Streit M, Ioannides A, Sinnemann T, Wolwer W, Dammers J, Zilles K, Gaebel W. Disturbed facial affect recognition in patients with schizophrenia associated with hypoactivity in distributed brain regions: a magnetoencephalographic study. The American journal of psychiatry. 2001;158(9):1429–1436. doi: 10.1176/appi.ajp.158.9.1429. [DOI] [PubMed] [Google Scholar]
- Swartz MS, Perkins DO, Stroup TS, Davis SM, Capuano G, Rosenheck RA, Reimherr F, McGee MF, Keefe RS, McEvoy JP, Hsiao JK, Lieberman JA. Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. The American journal of psychiatry. 2007;164(3):428–436. doi: 10.1176/ajp.2007.164.3.428. [DOI] [PubMed] [Google Scholar]
- Taly A. Novel approaches to drug design for the treatment of schizophrenia. Expert opinion on drug discovery. 2013;8(10):1285–1296. doi: 10.1517/17460441.2013.821108. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Crayton JW, Chase TN. Muscimol: GABA agonist therapy in schizophrenia. The American journal of psychiatry. 1978;135(6):746–747. doi: 10.1176/ajp.135.6.746. [DOI] [PubMed] [Google Scholar]
- Tang B, Dean B, Thomas EA. Disease- and age-related changes in histone acetylation at gene promoters in psychiatric disorders. Translational psychiatry. 2011;1:e64. doi: 10.1038/tp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Li C, Newburn EN, Ye T, Lipska BK, Herman MM, Weinberger DR, Kleinman JE, Hyde TM. Transcript-specific associations of SLC12A5 (KCC2) in human prefrontal cortex with development, schizophrenia, and affective disorders. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(15):5216–5222. doi: 10.1523/JNEUROSCI.4626-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. Journal of psychiatry & neuroscience: JPN. 2011;36(3):195–203. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoresen C, Endestad T, Sigvartsen NP, Server A, Bolstad I, Johansson M, Andreassen OA, Jensen J. Frontotemporal hypoactivity during a reality monitoring paradigm is associated with delusions in patients with schizophrenia spectrum disorders. Cognitive neuropsychiatry. 2014;19(2):97–115. doi: 10.1080/13546805.2013.776495. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Benes FM. Distribution of glutamate decarboxylase65 immunoreactive puncta on pyramidal and nonpyramidal neurons in hippocampus of schizophrenic brain. Synapse. 1998;29(4):323–332. doi: 10.1002/(SICI)1098-2396(199808)29:4<323::AID-SYN4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biological psychiatry. 2005;57(3):252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- van Berkum NL, Dekker J. Determining spatial chromatin organization of large genomic regions using 5C technology. Methods Mol Biol. 2009;567:189–213. doi: 10.1007/978-1-60327-414-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kammen DP, Sternberg DE, Hare TA, Waters RN, Bunney WE., Jr CSF levels of gamma-aminobutyric acid in schizophrenia. Low values in recently ill patients. Archives of general psychiatry. 1982;39(1):91–97. doi: 10.1001/archpsyc.1982.04290010065012. [DOI] [PubMed] [Google Scholar]
- Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(6):2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Kadriu B, Maloku E, Agis-Balboa RC, Guidotti A, Davis JM, Costa E. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophrenia research. 2007;91(1–3):51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Archives of general psychiatry. 2000;57(3):237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, Sampson A, Hashimoto T, Lewis DA. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. The American journal of psychiatry. 2012;169(10):1082–1091. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12(10):1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of general psychiatry. 1987;44(7):660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Wolwer W, Stroth S, Brinkmeyer J, Gaebel W. Electrophysiological correlates of planning and monitoring in first episode schizophrenia. Psychiatry research. 2012;203(1):83–88. doi: 10.1016/j.pscychresns.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(9):5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fung SJ, Rothwell A, Tianmei S, Weickert CS. Increased interstitial white matter neuron density in the dorsolateral prefrontal cortex of people with schizophrenia. Biological psychiatry. 2011;69(1):63–70. doi: 10.1016/j.biopsych.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, Carter CS. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(10):3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Raouf S, D’Esposito M, Carter CS. Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biological psychiatry. 2013;74(2):122–129. doi: 10.1016/j.biopsych.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(39):13130–13137. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Adli M, Zou JY, Verstappen G, Coyne M, Zhang X, Durham T, Miri M, Deshpande V, De Jager PL, Bennett DA, Houmard JA, Muoio DM, Onder TT, Camahort R, Cowan CA, Meissner A, Epstein CB, Shoresh N, Bernstein BE. Genome-wide chromatin state transitions associated with developmental and environmental cues. Cell. 2013;152(3):642–654. doi: 10.1016/j.cell.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]