Abstract
Insulin-like growth factor 1 (IGF-1) and interleukin 6 (IL-6) play an important role in the adaptation of both muscle and bone to mechanical stimuli. Here, we provide an overview of the functions of IL-6 and IGF-1 in bone and muscle metabolism, and the intracellular signaling pathways that are well known to mediate these functions. In particular, we discuss the Akt/mammalian target of rapamycin (mTOR) pathway which in skeletal muscle is known for its key role in regulating the rate of mRNA translation (protein synthesis). Since the role of the mTOR pathway in bone is explored to a much lesser extent, we discuss what is known about this pathway in bone and the potential role of this pathway in bone remodeling. We will also discuss the possible ways of influencing IGF-1 or IL-6 signaling by osteocytes and the clinical implications of pharmacological or nutritional modulation of the Akt/mTOR pathway.
Keywords: Osteocyte, Osteoblast, Myoblast, Hypertrophy, mTOR, Mechanical loading, Aging, Osteoporosis
Introduction
Most people know from personal experience that muscles increase in mass when loaded and decrease in mass during a period of physical inactivity. Much less commonly known is that exactly the same principle holds true for bones. This adaptation of bone mass to mechanical demands is facilitated by the activity of osteoblasts and osteoclasts. Osteoclasts remove bone in places of relative unloading, while osteoblasts form bone in places of relative high mechanical loading, leading to a constant adaptation of bone mass and structure [1]. As a result, healthy bones are able to withstand the forces placed upon them, while using a minimum of material. During osteoporosis, this delicate balance between bones’ resistance against mechanical loads and bone mass is clearly disturbed, mostly as a result of enhanced bone loss [2]. Although most studies have focused on the role of osteoblasts and osteoclasts in the emergence of osteoporosis, osteocytes may well be involved in the pathogenesis of this disease. It is well known that osteocytes produce signaling molecules in response to mechanical loading which affect osteoclast and osteoblast recruitment and activity [3, 4]. Our group has shown that nitric oxide and prostaglandin production in response to mechanical loading of cultured osteocytes from osteoporotic individuals differs from that of osteocytes derived from people with a high bone mass [5]. As such, alterations in the osteocyte responses to mechanical loading, in terms of signaling molecule production, may well be involved in the etiology of osteoporosis.
In recent years, a defined picture is emerging of osteocytes as signaling centers, actively communicating with osteoblasts, osteoclasts, marrow cells, cells of the lymphatic system, and the kidney. In addition, potential lines of communication between bone and muscle are currently under much scrutiny [6–9]. The organ most closely connected to the skeleton is arguably the musculature. If bones indeed communicate with muscle, the most likely source of the paracrine and endocrine signal is the osteocyte, which is by far the most abundant cell in adult bones, and osteocyte-derived signals have been shown to reach remote organs [10]. Presuming that osteocyte communication with muscles affects muscle mass, the opposite may also be true. Muscles may communicate to osteocytes, or muscles could communicate directly with osteoblasts and osteoclasts via similar signals that osteocytes employ to dictate osteoclast and osteoblast behavior. Osteocytes and muscle cells show considerable overlap in the molecules they use to regulate their mass in response to mechanical cues. Our group recently showed that differentiated myotubes and osteocytes alike cells produce factors such as nitric oxide (NO), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), and interleukin 6 (IL-6) in response to mechanical loading [11•, 12]. Short-lived molecules are unlikely to do more than autocrine and some paracrine signaling, but elevated levels of muscle-derived IL-6 and IGF-1 are found in the circulation after vigorous exercise [13–15]. We have shown that IL-6 derived from myotubes affects formation of osteoclasts in vitro [12]. This indicates that communication between muscle and bone is theoretically possible. Whether muscle and bone indeed communicate via the production of soluble factors is difficult to establish, but some evidence is available in support of this hypothesis [6, 16]. One complicating factor is that muscles exert mechanical forces in bone, thereby affecting bone mass regardless of the existence of an exchange of biochemical factors. Considering this, it is possible that osteoporosis is simply related to a decrease in the number and/or magnitude of mechanical stimuli in bone, caused by the loss of muscle mass that is common with aging. Maintenance of muscle mass could thus be a therapeutic option in elderly, in order to preserve bone integrity and mass. Even if it turns out that biochemical communication between muscle and bone does not play a significant role in bone homeostasis, it is likely that, considering the similarities in signaling molecules employed by both tissue types, much can be learned from the muscle field in order to generate a better understanding of bone mass regulation and vice versa.
Insulin-Like Growth Factor 1
IGF-1 is a hormone rather similar in molecular structure to insulin. It is produced primarily by the liver under the control of growth hormone, plays an important role in regulating growth in children, and has anabolic effects in adults [17]. By far, the largest amount of IGF-1 in the body is bound to IGF-binding proteins (IGFBPs), which affect the activity of IGF-1. For instance, IGFBP-2 and IGFBP-5 bind IGF-1 at a higher affinity than its receptor, and an increased serum level of these IGFBPs thus result in lower IGF-1 signaling [18]. Several splice variants from the IGF-1 gene have been identified, of which IGF-1Ea and IGF-1Eb/c (also known as mechano growth factor (MGF)) play a role in muscle and bone homeostasis. These two splice variants differ with respect to their nucleotide sequence in exon 5 which encompasses an insert of about 23 amino acids, coding for the E-peptide [19]. The 70 kD IGF-1 domain, coded by exons 3 and 4 of the IGF-1 gene, stimulates osteocyte and osteoblast survival [20, 21], as well as osteoblast differentiation and matrix production [22], while the E peptide in MGF promotes MC3T3-E1 osteoblast proliferation [23]. In addition to these anabolic effects in bone, the 70 kD IGF-1 domain has been shown to promote osteoclastogenesis [24, 25].
Besides being an endocrine molecule, IGF-1 also exerts effects in a paracrine/autocrine manner in many tissues. IGF-1 is produced locally in bone and is able to exert effects in bone in an autocrine manner [26]. Since then, the question “how much of the anabolic effect of IGF-1 in bone is autocrine in nature?” has remained unsolved until Elis et al. showed that, in the absence of tissue-specific IGF-1 gene expression, maintaining long-term elevated IGF-1 levels in serum by IGF-1 gene overexpression in the liver was sufficient to restore skeletal architecture and mechanical function [27•]. In addition, conditional disruption of the IGF-1 gene in osteocytes under the control of the DMP-1 promoter slightly reduces bone mineral content, but not mineral density, and IGF-1 knockout in osteocytes does not alter plasma levels of IGF-1 [28]. This suggests that IGF-1 produced locally in bone has a minor role in bone development compared to IGF-1 produced in the liver. However, the importance of local production of IGF-1 in bone in response to mechanical loading may be a different story altogether. Mechanical loading is anabolic for bone, and osteocytes have been shown to produce IGF-1 in response to mechanical loading in vitro and in vivo [29•, 30]. In addition, mechanical loading increases bone formation in wild-type mice but not in mice with osteocytes deficient in IGF-1 [31••]. From the above, it was concluded that IGF-1 plays a pivotal role in the response of bone to mechanical forces.
In skeletal muscle, the application of mechanical loading, either applied passively by stretching or actively by neuronal-initiated contractile activity, stimulates the expression of IGF-1 Ea and MGF [32]. IGF-1 Ea acts on the muscle stem cells (i.e., satellite cells (MuSC) located between the sarcolemma and the basal lamina (Fig. 1) by stimulation of their differentiation into myotubes [32, 33]. In contrast, MGF E peptide has been shown to stimulate proliferation and migration of myoblasts [33, 34]. For an injured muscle fiber, MGF will enhance the capacity for regeneration while for intact mechanically overloaded muscle fibers this will lead to an expansion of the pool of myonuclei within the muscle fiber and as such an increase in the amount of DNA serving as template for transcription. Besides the effects of IGF-1 splice variants on MuSCs, IGF-1 signaling also occurs in the mature muscle fiber. Like insulin, the 70 kD IGF-1 domain stimulates muscle fiber hypertrophy (i.e., increases muscle fiber diameter) [35, 36] by concurrently stimulating the rate of protein synthesis and inhibiting the rate of protein degradation [37]. This dual role makes IGF-1 in muscle an autocrine and paracrine growth factor with a strong potency to induce muscle hypertrophy.
Fig. 1.
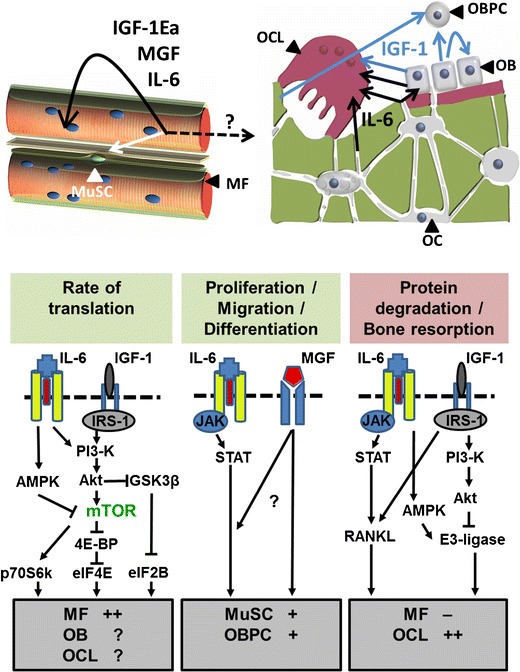
Insulin-like 1 (IGF-1) and interleukin-6 (IL-6) signaling in the regulation of skeletal muscle adaptation and bone metabolism. a In skeletal muscle, the machinery for protein synthesis and degradation resides within the muscle fibers, which are multinucleated cells. The muscle satellite cells (MuSCs) reside between the sarcolemma (i.e., plasma membrane) and the basal lamina. b In bone, osteoid is synthesized by osteoblasts (OB) which are derived from osteogenic progenitor cells and differentiate into osteocytes (OC) when buried in their own matrix. Osteocytes have long extensions embedded within the canaliculi and are highly sensitive to mechanical loading. In response to mechanical stimuli, myofibers (MF) produce paracrine/endocrine factors such as IL-6, and both splice variants of IGF-1, i.e., mechano growth factor (MGF) and IGF-1Ea. These factors are able to affect myofibers, MuSCs, and cells in other organs such as the liver and potentially bone. IL-6 slows down the rate of translation in myofibers while enhancing the rate of protein breakdown via 5′ adenosine monophosphate-activated protein kinase (AMPK), resulting in a net catabolic effect. On the other hand, IL-6 stimulates the proliferation and differentiation of satellite cells via the Janus kinase/Signal Transducer and Activator of Transcription (JAK/STAT) pathway, which would be an anabolic response. IGF-1 stimulates the rate of protein translation in myofibers via the phosphatidylinositol 3 kinase (PI3-K)/Akt/mammalian target of rapamycin (mTOR) pathway while inhibiting the expression of catabolic ubiquitin E3 ligases resulting in an increase in muscle mass. MGF stimulates satellite cell proliferation and differentiation, but it is so far unclear via which pathway this occurs. Mechanically-loaded osteocytes and osteoblasts produce IGF-1 (likely both splice variants), and IGF-1 is released from the matrix during osteoclastic (OCL) bone resorption. Mechanically-loaded osteocytes and osteoblasts also produce IL-6, as do apoptotic osteocytes. IGF-1 enhances differentiation of osteoblast precursors cells (OBPC) via the PI3-K/AktT/mTOR pathway (not shown) and activity and formation of osteoclasts via stimulation of RANKL expression in osteoblasts and osteocytes. IL-6 also stimulates osteoblast precursor differentiation and osteoclast formation, via an increase in RANKL expression by osteoblasts. Whether IL-6 and IGF-1 affect the rate of protein translation in osteoblasts or osteoclasts is currently unknown. IRS-1 insulin receptor substrate-1; eIF4E eukaryotic initiation factor 4E; 4E-BP eIF4E-binding protein; p70S6K p70S6 kinase; eIF2B eukaryotic initiation factor 2B. GSK3β glycogen synthase kinase 3β
Interleukin 6
IL-6 is a pleiotropic cytokine which is produced by a variety of cell types such as T-cells and macrophages, but IL-6 is also readily produced by other cell types, such as smooth muscle cells, fibroblasts, skeletal muscle fibers, osteoblasts, and osteocytes [38••, 3]. IL-6 requires gp130 (ubiquitously expressed) and IL-6 receptor (IL-6R) for signaling. IL-6R is expressed by a limited population of cells, amongst which osteoblasts and osteoclasts as well as muscle cells [39]. Human osteocytes express high amounts of IL-6R at week 8 and 14 during gestation, and MLO-Y4 osteocytes have been shown to express IL-6R mRNA [40]. Even if a cell does not express IL-6R, it can respond to IL-6 because IL-6R exists in a soluble form in the serum where it acts as an agonist. IL-6R can end up in the serum because it is shed from cell membranes through cleaving by ADAM17 or ADAM10. Interestingly, osteocytes express ADAM10, making osteocytes possible modulators of IL-6 signaling [41].
IL-6 was first discovered to mediate bone loss associated with estrogen-withdrawal in mice [42]. More recently, it has been described that increased circulating IL-6 levels in patients with Duchenne muscular dystrophy seem responsible for increased osteoclastic bone resorption [43]. IL-6 most likely stimulates osteoclastogenesis indirectly, by increasing Rankl gene expression by osteoblasts [44, 39, 45]. Elevated IL-6 levels could thus explain why the low grade systemic inflammation as occurs after the menopause enhances osteoclastogenesis and reduces bone mass [46]. On the other end of the spectrum, mice lacking IL-6 also have a low bone mass [47]. Furthermore, IL-6 null mice show reduced osteoblast numbers and delayed fracture healing [47]. Indeed, IL-6-type cytokines promote differentiation of committed osteoblastic cells toward a more mature phenotype [48], and IL-6 has been shown to enhance osteoblast differentiation in stem cells [49]. This double edged role of IL-6 in bone, i.e., both stimulatory for osteoclasts and osteoblasts, is also reflected in the production of IL-6 by osteocytes. Cultured MLO-Y4 osteocytes produce high amounts of IL-6 [29•]. Fluid shear stress at low physiological levels stimulates IL-6 production in osteocytes in vitro [38••]. On the other hand, IL-6 has also been shown to be expressed by osteocytes when they undergo apoptosis in response to bone unloading [3]. Overall, these data indicate that in bone IL-6 has a key role in bone metabolism.
In skeletal muscle, multiple roles have been identified for IL-6 [50]. Contractile activity stimulates IL-6 expression in humans and rodent skeletal muscle [51, 52], which is paralleled by elevated serum levels in humans [14, 53]. IL-6 produced by muscle is generally known for its role in glycogen metabolism and insulin signaling [54], but IL-6 may also be involved in the regulation of muscle fibers size. Direct chronic IL-6 administration to skeletal muscle induces muscle fiber atrophy [55]. However, recovery of gastrocnemius muscle from disuse atrophy is attenuated in IL-6 knockout mice [56], and overload-induced muscle hypertrophy is blunted in IL-6 deficient mice [57]. These reports suggest opposite roles of IL-6 in the regulation of muscle fiber size and regeneration, similarly to bone.
Intracellular Signaling Pathways Activated BY IGF-1 AND IL-6
In skeletal muscle, IGF-1 stimulates the rate of protein synthesis via two ways: (1) IGF-1 increases the rate of mRNA transcription of myofilaments such as α-skeletal actin, a major constituent of the muscle contractile apparatus [36, 58], and (2) IGF-1 stimulates the phosphatidylinositol 3 kinase (PI3-K)/Akt (also known as protein kinase B) pathway which activates the mammalian target of rapamycin (mTOR). Downstream targets of mTOR are p70S6 kinase (p70S6k) and heat- and acid-stable protein 1 (PHAS-1, also referred to as 4E-BP) [59]. Activated mTOR phosphorylates P70S6K, thereby activating the ribosomal protein S6, which is involved in the translation of mRNAs [59] (Fig. 1). In addition, mTOR inhibits 4EBP, which is a negative regulator of the translation initiation factor eIF4E, thereby further enhancing the rate of translation [59, 60]. Akt also inactivates glycogen synthase kinase 3 β (GSK3β), thereby preventing the inhibitory effect of GSK3β on eukaryotic initiation factor 2B (eIF2B), which results in an enhanced rate of mRNA translation [61] (Fig. 1). Apart from its potency to enhance the rate of protein synthesis, IGF-1 also attenuates the rate of protein degradation via PI3-K/Akt/FOXO pathway [62]. Activated Akt phosphorylates the transcription factor FOXO, causing its cytoplasmic localization, which reduces the rate of transcription of muscle-specific ubiquitin E3 ligases (Fig. 1). Muscle E3 ligases will tack contractile proteins with ubiquitin, which marks them for degradation within the 26S-proteasome system. Taken together, IGF-1 is a highly potent growth factor resulting in muscle hypertrophy by reducing protein degradation and stimulating the rate of protein synthesis through a combination of increasing mRNA content of contractile proteins and increasing the rate of translation per mRNA.
Although the role of the mTOR pathway in IGF-1-induced muscle hypertrophy has been extensively studied, this pathway attracted little attention in the bone world, until it has been shown recently that IGF-1 may have an anabolic effect on bone via stimulation of the PI3-K/Akt/mTOR pathway [63••]. IGF-1 released from the bone matrix during bone remodeling stimulates osteoblastic differentiation of recruited mesenchymal stem cells (MSCs) by activation of Akt/mTOR [63••]. The observation that mTOR mediates osteoblast differentiation has also been shown in experiments where rapamycin, a potent mTOR inhibitor, suppressed WNT7B-induced osteoblast differentiation in ST2 mouse bone marrow derived cells, as determined by assessing alkaline phosphatase activity and von Kossa staining [64]. As mentioned before, activated Akt not only affects mTOR, but also inhibits GSK3β. Thereby, Akt activates cellular β-catenin signaling in osteocytes [65••], explaining why conditional disruption of IGF-1 in osteocytes abolishes the loading-induced increase in β-catenin protein levels in osteocytes [31••]. The activation of β-catenin by mechanical loading in osteocytes inhibits osteocyte apoptosis [66, 67].
IL-6 signaling occurs through binding of IL-6 with the IL-6 receptors (IL-6R), which subsequently bind to gp130 receptors [68]. In skeletal muscle, both MuScs and myofibers express IL-6, IL-6R, and gp130 receptor. In MuSCs, IL-6-induced proliferation and migration occurs via the JAK/STAT pathway, which stimulates the expression of proliferation-associated transcription factors cyclin D1 and c-myc [57, 69]. Regarding the effects of IL-6 on the myofiber (MF), several studies have shown that IL-6 modulates both the rate of protein synthesis and that of protein breakdown [70–73]. Primary human MuSCs differentiated into myotubes showed increased phosphorylation of Akt after exposure to IL-6 [70, 71] suggesting an enhancement of the mTOR signaling. However, the opposite has been reported in mice overexpressing IL-6 in quadriceps muscle. IL-6 attenuated the activity of mTOR via its stimulatory effect on 5′-adenosine monophosphate-activated protein kinase (AMPK) [72•]. This enzyme is phosphorylated and activated by a low energy status (i.e., increased ratio AMP/ATP), but is also a downstream target of IL-6 [73]. Activated AMPK has a multitude of regulatory functions in skeletal muscle: (1) stimulation of biosynthesis of mitochondria and fatty acid oxidation and glucose uptake [74], (2) inhibition of mTOR [75], and (3) stimulation of expression of muscle-specific E3 ligases [76, 77] which is linearly related to increased muscle protein degradation. The actual effects of IL-6 on the PI3-K/Akt/mTOR pathway within muscle fibers remain to be determined. Note that there may also an indirect way by which IL-6 affect the Akt/mTOR signaling within muscle. Transgenic overexpression of IL-6 or its exogenous injection in mice is associated with reduced circulating IGF-1 levels, likely due to increased proteolysis of the IGF-1 binding protein 3 (IGFBP3) and enhanced clearance of IGF-1 [78].
In bone, IL-6 signals via gp130/IL-6R thereby activating the JAK/STAT pathway, which leads to activation of the transcription factor “nuclear factor kappa-light-chain-enhancer of activated B cells” [79]. IL-6 also activates the Ras/Raf pathway leading to the activation of the transcription factor C/EBPβ, also known as “nuclear factor for IL-6” [80]. The latter binds promoter sequences of amongst others iNOS and COX2 genes and stimulates IGF-1 expression, which all play a role in the regulation of bone metabolism [81]. The C/EBPβ gene gives rise to three proteins: LAP*, LAP, and LIP. LAP mediates osteoblast maturation and osteoblast-stimulated osteoclastogenesis while LIP inhibits osteoblast-stimulated osteoclastogenesis [82]. In osteoclasts, the balance between LAP and LIP expression is determined by mTOR activity, i.e., low mTOR activity favoring LAP, while high mTOR activity favors LIP [83]. Whether IL-6 affects mTOR activity in osteoblasts and/or osteocytes is currently unknown.
Role for the mTOR Pathway in Bone
From the foregoing, it is clear that there are similarities and dissimilarities in the way IGF-1 and IL-6 are employed by bone and muscle to achieve changes in tissue mass, and that much can be learned from one field in order to generate a better understanding of regulation of tissue mass in the other field. A similarity between muscle and bone is that the balance between protein formation and degradation determines tissue mass. In order for protein formation to increase in muscle, the muscle increases the amount of nuclei (DNA) per fiber, the amount of RNA per available myonucleus (rate of transcription), as well as the rate of translation per mRNA molecule. In bone, the protein matrix is deposited outside of the cells rather than intracellular, and bone formation and degradation occur by two separate cell types, but otherwise it makes sense that in order for bone matrix production to increase, the same principles apply as for muscle. Indeed, an increase in proliferation and differentiation of osteoblast precursors increases the number of osteoblast nuclei (DNA) per volume bone. For example, MGF enhances the number of osteogenic cells in bone through stimulation of proliferation [23]. One might then question: what is next? In order to increase bone formation rate in an efficient manner, the activity per osteoblast needs to be enhanced as well. The master switch in the rate of protein translation in muscle, as in many other cell types, is the PI3-K/Akt/mTOR pathway, as discussed above [84]. It is possible that mTOR plays a very analogous role in osteoblasts, although the little evidence that is currently available for such a role is ambiguous. On the one hand, genetic disruption of mTOR signaling by deleting Raptor for 3 weeks in the osteoblast lineage in 1-month-old mice did not affect bone mass or strength, suggesting that mTOR signaling in osteoblasts may not be essential for maintaining bone homeostasis on the short term [64]. On the other hand, targeted induction of the WNT7B gene in osteoblasts dramatically enhanced bone mass due to increased osteoblast number, activity, and significantly stimulated bone formation, but not when the cofactor for mTOR signaling Raptor was absent [64]. Thus, Wnt-7B requires mTOR to promote bone formation [64]. In addition, the importance of mTOR signaling for osteoblasts is exemplified by the experiments in which PI3K/mTOR inhibitors, designed as oncostatic drugs, were tested in vivo. Male mice received amongst others the PI3-K/mTOR inhibitors EZ235 and PI103, which reduced BV/TV by 36 and 37 %, respectively [85••]. Of course, this could still be through an effect of mTOR on osteoblast differentiation rather than on the rate of translation.
Whether the mTOR pathway is important for osteocytes is unknown, but it stands to reason that this pathway affects osteocyte biology. When oxygen and nutrients are not abundant, for instance in osteocytes that are not in direct contact with the vasculature, it makes sense that these cells slow down their metabolism and recycle cell components as much as possible through autophagy. Since mTOR activation enhances the energy consuming process of protein translation and inhibits autophagy [86•], one could deduce that mTOR activity needs to stay low in unstimulated osteocytes [87]. Alterations in mTOR activity could thus have profound effects on osteocyte survival and bone metabolism.
Conclusion
Taken together, IL-6 and IGF-1 are extremely important regulators of bone and muscle metabolism, which makes them interesting targets for the treatment of osteopenia or sarcopenia. However, IGF-1 and IL-6 are ubiquitously expressed and signal in many cell types, which makes it difficult to target these molecules only in bone and muscle but not in other tissues. The same holds for the signaling pathway that IGF-1 and IL-6 have in common, i.e., the mTOR pathway. As the mTOR pathway is ubiquitously involved in many cells types, activation of this pathway specifically in bone will be a challenge, although targeted delivery of chemicals using bisphosphonates may be an option. However, even if bone can be reached as a single target, IGF-1, IL-6, and mTOR signaling seem to be a two-edged sword: All seem to stimulate osteogenic differentiation and might be essential in bone development and healing. Knockout of IL-6 in otherwise healthy animals and inhibition of mTOR with compounds such as rapamycin certainly have deleterious effects in bone mass. However, both IGF-1 and IL-6 may also stimulate bone resorption, and mTOR activation in osteoclast precursors stimulates osteoclast formation [83]. IL-6 inhibitors are available in the clinic for suppression of inflammation in rheumatoid arthritis, where they seem to be osteoanabolic, rather than catabolic [88]. Pharmacological intervention in these pathways will thus require fine tuning of dose and kinetics (e.g., continuous or intermitted) in order to achieve an adequate anabolic effect.
Alternatively, one could try to specifically target IL-6 and IGF-1 signaling in muscle by pharmacological means, in order to increase both muscle and bone mass. Indeed, overexpression of IGF-1 in the musculature of mice enhanced bone mass as well [89]. Increased muscle mass could be anabolic for bone since muscles exert mechanical forces in bone. Exercise, which stimulates IL-6 and IGF-1 production in bone and muscle, as well as the production of a large quantity of other growth factors and signaling molecules, may be a safe and efficient way to enhance both bone and muscle mass.
In muscle, the mTOR pathway is activated via the food supplement leucine, which transiently stimulated the rate of translation in muscle cells in vitro [90, 91]. Whether supplements can be applied to affect the proliferation of osteoblasts and the rate of translation in these cells is yet unknown and remains to be determined. However, until very fine tuned and tissue-specific pharmacological interventions are available, a combination of sufficient exercise and a balanced diet containing sufficient amounts of for example leucine are a safe and cheap way to help maintain muscle and bone mass.
Acknowledgments
We would like to thank Guus Baan for his excellent assistance in the graphic design of the figures.
Compliance wit Ethics Guidelines
ᅟ
Conflict of Interest
AD Bakker and RT Jaspers both declare no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies by AD Bakker and RT Jaspers involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Footnotes
This article is part of the Topical Collection on Osteocytes
Astrid D. Bakker and Richard T. Jaspers contributed equally to this work.
Contributor Information
Astrid D. Bakker, Phone: +31 20 5980224, Email: a.bakker@acta.nl
Richard T. Jaspers, Phone: +31 20 5988463, Email: r.t.jaspers@vu.nl
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Goodship AE, Lanyon LE, McFie H. Functional adaptation of bone to increased stress. An experimental study. J Bone Joint Surg Am. 1979;61(4):539–46. [PubMed] [Google Scholar]
- 2.Tanck E, Bakker AD, Kregting S, Cornelissen B, Klein-Nulend J, Van Rietbergen B. Predictive value of femoral head heterogeneity for fracture risk. Bone. 2009;44(4):590–5. doi: 10.1016/j.bone.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Cheung WY, Simmons CA, You L. Osteocyte apoptosis regulates osteoclast precursor adhesion via osteocytic IL-6 secretion and endothelial ICAM-1 expression. Bone. 2012;50(1):104–10. doi: 10.1016/j.bone.2011.09.052. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AF, Saunders MM, Shingle DL, Cimbala JM, Zhou Z, Donahue HJ. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol. 2007;292(1):C545–52. doi: 10.1152/ajpcell.00611.2005. [DOI] [PubMed] [Google Scholar]
- 5.Bakker AD, Klein-Nulend J, Tanck E, Heyligers IC, Albers GH, Lips P, Burger EH. Different responsiveness to mechanical stress of bone cells from osteoporotic versus osteoarthritic donors. Osteoporos Int. 2006;17(6):827–33. doi: 10.1007/s00198-006-0072-7. [DOI] [PubMed] [Google Scholar]
- 6.Jaspers RT, Bravenboer N. Biochemical interaction between muscle and bone: a physiological reality? Clin Rev Bone Miner Metab. 2014;12:27–43. doi: 10.1007/s12018-014-9156-7. [DOI] [Google Scholar]
- 7.Shen H, Grimston S, Civitelli R, Thomopoulos S. Deletion of connexin43 in osteoblasts/osteocytes leads to impaired muscle formation in mice. J Bone Miner Res. 2014. doi:10.1002/jbmr.2389. [DOI] [PMC free article] [PubMed]
- 8.Hamrick MW, Shi X, Zhang W, Pennington C, Thakore H, Haque M, Kang B, Isales CM, Fulzele S, Wenger KH. Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone. 2007;40(6):1544–53. doi: 10.1016/j.bone.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kellum E, Starr H, Arounleut P, Immel D, Fulzele S, Wenger K, Hamrick MW. Myostatin (GDF-8) deficiency increases fracture callus size, Sox-5 expression, and callus bone volume. Bone. 2009;44(1):17–23. doi: 10.1016/j.bone.2008.08.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vervloet MG, Massy ZA, Brandenburg VM, Mazzaferro S, Cozzolino M, Urena-Torres P, Bover J, Goldsmith D. Bone: a new endocrine organ at the heart of chronic kidney disease and mineral and bone disorders. Lancet Diabetes Endocrinol. 2014;2(5):427–36. doi: 10.1016/S2213-8587(14)70059-2. [DOI] [PubMed] [Google Scholar]
- 11.•.Juffer P, Bakker AD, Klein-Nulend J, Jaspers RT. Mechanical loading by fluid shear stress of myotube glycocalyx stimulates growth factor expression and nitric oxide production. Cell Biochem Biophys. 2014;69(3):411–9. doi: 10.1007/s12013-013-9812-4. [DOI] [PubMed] [Google Scholar]
- 12.Juffer P, Jaspers RT, Klein-Nulend J, Bakker AD. Mechanically loaded myotubes affect osteoclast formation. Calcif Tissue Int. 2014;94(3):319–26. doi: 10.1007/s00223-013-9813-8. [DOI] [PubMed] [Google Scholar]
- 13.Ardawi MS, Rouzi AA, Qari MH. Physical activity in relation to serum sclerostin, insulin-like growth factor-1, and bone turnover markers in healthy premenopausal women: a cross-sectional and a longitudinal study. J Clin Endocrinol Metab. 2012;97(10):3691–9. doi: 10.1210/jc.2011-3361. [DOI] [PubMed] [Google Scholar]
- 14.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. Faseb J. 2002;16(11):1335–47. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 15.Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund PB. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529(Pt 1):237–42. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;10(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- 17.Schoenle E, Zapf J, Humbel RE, Froesch ER. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982;296(5854):252–3. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- 18.Stewart CE, Rotwein P. Growth, differentiation, and survival: Multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76(4):1005–26. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- 19.Goldspink G. Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology. 2005;20:232–8. doi: 10.1152/physiol.00004.2005. [DOI] [PubMed] [Google Scholar]
- 20.Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141(7):2674–82. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- 21.Hill PA, Tumber A, Meikle MC. Multiple extracellular signals promote osteoblast survival and apoptosis. Endocrinology. 1997;138(9):3849–58. doi: 10.1210/endo.138.9.5370. [DOI] [PubMed] [Google Scholar]
- 22.Hock JM, Centrella M, Canalis E. Insulin-like growth factor I has independent effects on bone matrix formation and cell replication. Endocrinology. 1988;122(1):254–60. doi: 10.1210/endo-122-1-254. [DOI] [PubMed] [Google Scholar]
- 23.Deng M, Zhang B, Wang K, Liu F, Xiao H, Zhao J, Liu P, Li Y, Lin F, Wang Y. Mechano growth factor E peptide promotes osteoblasts proliferation and bone-defect healing in rabbits. Int Orthop. 2011;35(7):1099–106. doi: 10.1007/s00264-010-1141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill PA, Reynolds JJ, Meikle MC. Osteoblasts mediate insulin-like growth factor-I and -II stimulation of osteoclast formation and function. Endocrinology. 1995;136(1):124–31. doi: 10.1210/endo.136.1.7828521. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Nishida S, Sakata T, Elalieh HZ, Chang W, Halloran BP, Doty SB, Bikle DD. Insulin-like growth factor-I is essential for embryonic bone development. Endocrinology. 2006;147(10):4753–61. doi: 10.1210/en.2006-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canalis E, McCarthy T, Centrella M. Isolation and characterization of insulin-like growth factor I (somatomedin-C) from cultures of fetal rat calvariae. Endocrinology. 1988;122(1):22–7. doi: 10.1210/endo-122-1-22. [DOI] [PubMed] [Google Scholar]
- 27.•.Elis S, Courtland HW, Wu Y, Rosen CJ, Sun H, Jepsen KJ, et al. Elevated serum levels of IGF-1 are sufficient to establish normal body size and skeletal properties even in the absence of tissue IGF-1. J Bone Miner Res. 2010;25(6):1257–66. This study demonstrates that overexpression of IGF-1 in livers of IGF-1 null mice elevated serum IGF-1. This did not overcome skeletal deficiencies during neonatal and early postnatal growth, but did fully compensated for the absence of locally produced IGF-1 between 4 and 16 weeks of age. [DOI] [PMC free article] [PubMed]
- 28.Sheng MH, Zhou XD, Bonewald LF, Baylink DJ, Lau KH. Disruption of the insulin-like growth factor-1 gene in osteocytes impairs developmental bone growth in mice. Bone. 2013;52(1):133–44. doi: 10.1016/j.bone.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 29.•.Juffer P, Jaspers RT, Lips P, Bakker AD, Klein-Nulend J. Expression of muscle anabolic and metabolic factors in mechanically loaded MLO-Y4 osteocytes. Am J Physiol Endocrinol Metab. 2012;302(4):E389–95. This study shows osteocytes subjected to a pulsating fluid shear stress produce muscle anaboic growth factors IGF-1, MGF, VEGF, HGF but not myostatin. This indicates a high similarity in mechanotransduction between muscle fiber and osteocytes. [DOI] [PubMed]
- 30.Reijnders CM, Bravenboer N, Tromp AM, Blankenstein MA, Lips P. Effect of mechanical loading on insulin-like growth factor-I gene expression in rat tibia. J Endocrinol. 2007;192(1):131–40. doi: 10.1677/joe.1.06880. [DOI] [PubMed] [Google Scholar]
- 31.••.Lau KH, Baylink DJ, Zhou XD, Rodriguez D, Bonewald LF, Li Z, Ruffoni D, Muller R, Kesavan C, Sheng MH. Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am J Physiol Endocrinol Metab. 2013;305(2):E271–81. doi: 10.1152/ajpendo.00092.2013. [DOI] [PubMed] [Google Scholar]
- 32.Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol. 2007;102(2):573–81. doi: 10.1152/japplphysiol.00866.2006. [DOI] [PubMed] [Google Scholar]
- 33.Mills P, Dominique JC, Lafreniere JF, Bouchentouf M, Tremblay JP. A synthetic mechano growth factor E Peptide enhances myogenic precursor cell transplantation success. Am J Transplant. 2007;7(10):2247–59. doi: 10.1111/j.1600-6143.2007.01927.x. [DOI] [PubMed] [Google Scholar]
- 34.Yang SY, Goldspink G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett. 2002;522(1–3):156–60. doi: 10.1016/S0014-5793(02)02918-6. [DOI] [PubMed] [Google Scholar]
- 35.Jaspers RT, van Beek-Harmsen BJ, Blankenstein MA, Goldspink G, Huijing PA, van der Laarse WJ. Hypertrophy of mature Xenopus muscle fibres in culture induced by synergy of albumin and insulin. Pflugers Arch. 2008;457(1):161–70. doi: 10.1007/s00424-008-0499-0. [DOI] [PubMed] [Google Scholar]
- 36.Semsarian C, Wu MJ, Ju YK, Marciniec T, Yeoh T, Allen DG, Harvey RP, Graham RM. Skeletal muscle hypertrophy is mediated by a Ca2 + -dependent calcineurin signalling pathway. Nature. 1999;400(6744):576–81. doi: 10.1038/23054. [DOI] [PubMed] [Google Scholar]
- 37.Glass DJ. A signaling role for dystrophin: inhibiting skeletal muscle atrophy pathways. Cancer Cell. 2005;8(5):351–2. doi: 10.1016/j.ccr.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 38.••.Bakker AD, Kulkarni RN, Klein-Nulend J, Lems WF. IL-6 alters osteocyte signaling toward osteoblasts but not osteoclasts. J Dent Res. 2014;93(4):394–9. doi: 10.1177/0022034514522485. [DOI] [PubMed] [Google Scholar]
- 39.Taguchi Y, Yamamoto M, Yamate T, Lin SC, Mocharla H, DeTogni P, Nakayama N, Boyce BF, Abe E, Manolagas SC. Interleukin-6-type cytokines stimulate mesenchymal progenitor differentiation toward the osteoblastic lineage. Proc Assoc Am Physicians. 1998;110(6):559–74. [PubMed] [Google Scholar]
- 40.Dame JB, Juul SE. The distribution of receptors for the pro-inflammatory cytokines interleukin (IL)-6 and IL-8 in the developing human fetus. Early Hum Dev. 2000;58(1):25–39. doi: 10.1016/S0378-3782(00)00064-5. [DOI] [PubMed] [Google Scholar]
- 41.Dallas DJ, Genever PG, Patton AJ, Millichip MI, McKie N, Skerry TM. Localization of ADAM10 and Notch receptors in bone. Bone. 1999;25(1):9–15. doi: 10.1016/S8756-3282(99)00099-X. [DOI] [PubMed] [Google Scholar]
- 42.Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, Ciliberto G, Rodan GA, Costantini F. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994;13(5):1189–96. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rufo A, Del Fattore A, Capulli M, Carvello F, De Pasquale L, Ferrari S, Pierroz D, Morandi L, De Simone M, Rucci N, Bertini E, Bianchi ML, De Benedetti F, Teti A. Mechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humans. J Bone Miner Res. 2011;26(8):1891–903. doi: 10.1002/jbmr.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duplomb L, Baud’huin M, Charrier C, Berreur M, Trichet V, Blanchard F, Heymann D. Interleukin-6 inhibits receptor activator of nuclear factor kappaB ligand-induced osteoclastogenesis by diverting cells into the macrophage lineage: Key role of Serine727 phosphorylation of signal transducer and activator of transcription 3. Endocrinology. 2008;149(7):3688–97. doi: 10.1210/en.2007-1719. [DOI] [PubMed] [Google Scholar]
- 45.Udagawa N, Takahashi N, Katagiri T, Tamura T, Wada S, Findlay DM, Martin TJ, Hirota H, Taga T, Kishimoto T, Suda T. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J Exp Med. 1995;182(5):1461–8. doi: 10.1084/jem.182.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abu-Taha M, Rius C, Hermenegildo C, Noguera I, Cerda-Nicolas JM, Issekutz AC, Jose PJ, Cortijo J, Morcillo EJ, Sanz MJ. Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol. 2009;183(2):1393–402. doi: 10.4049/jimmunol.0803157. [DOI] [PubMed] [Google Scholar]
- 47.Yang X, Ricciardi BF, Hernandez-Soria A, Shi Y, Pleshko Camacho N, Bostrom MP. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41(6):928–36. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellido T, Borba VZ, Roberson P, Manolagas SC. Activation of the Janus kinase/STAT (signal transducer and activator of transcription) signal transduction pathway by interleukin-6-type cytokines promotes osteoblast differentiation. Endocrinology. 1997;138(9):3666–76. doi: 10.1210/endo.138.9.5364. [DOI] [PubMed] [Google Scholar]
- 49.Erices A, Conget P, Rojas C, Minguell JJ. Gp130 activation by soluble interleukin-6 receptor/interleukin-6 enhances osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. Exp Cell Res. 2002;280(1):24–32. doi: 10.1006/excr.2002.5627. [DOI] [PubMed] [Google Scholar]
- 50.Munoz-Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280(17):4131–48. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol. 2000;528(Pt 1):157–63. doi: 10.1111/j.1469-7793.2000.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Croisier JL, Camus G, Venneman I, Deby-Dupont G, Juchmes-Ferir A, Lamy M, Crielaard JM, Deby C, Duchateau J. Effects of training on exercise-induced muscle damage and interleukin 6 production. Muscle Nerve. 1999;22(2):208–12. doi: 10.1002/(SICI)1097-4598(199902)22:2<208::AID-MUS8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 53.Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283(6):E1272–8. doi: 10.1152/ajpendo.00255.2002. [DOI] [PubMed] [Google Scholar]
- 54.Pedersen BK, Edward F. Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol. 2009;107(4):1006–14. doi: 10.1152/japplphysiol.00734.2009. [DOI] [PubMed] [Google Scholar]
- 55.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98(3):911–7. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 56.Washington TA, White JP, Davis JM, Wilson LB, Lowe LL, Sato S, Carson JA. Skeletal muscle mass recovery from atrophy in IL-6 knockout mice. Acta Physiol (Oxf) 2011;202(4):657–69. doi: 10.1111/j.1748-1716.2011.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7(1):33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Spangenburg EE, Bowles DK, Booth FW. Insulin-like growth factor-induced transcriptional activity of the skeletal alpha-actin gene is regulated by signaling mechanisms linked to voltage-gated calcium channels during myoblast differentiation. Endocrinology. 2004;145(4):2054–63. doi: 10.1210/en.2003-1476. [DOI] [PubMed] [Google Scholar]
- 59.Bolster DR, Kimball SR, Jefferson LS. Translational control mechanisms modulate skeletal muscle gene expression during hypertrophy. Exerc Sport Sci Rev. 2003;31(3):111–6. doi: 10.1097/00003677-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16(12):1472–87. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welsh GI, Miller CM, Loughlin AJ, Price NT, Proud CG. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett. 1998;421(2):125–30. doi: 10.1016/S0014-5793(97)01548-2. [DOI] [PubMed] [Google Scholar]
- 62.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14(3):395–403. doi: 10.1016/S1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 63.••.Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, Crane J, Frassica F, Zhang L, Rodriguez JP, Xiaofeng J, Shoshana Y, Shouhong X, Argiris E, Mei W, Xu C. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18(7):1095–101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J, Tu X, Esen E, Joeng KS, Lin C, Arbeit JM, Ruegg MA, Hall MN, Ma L, Long F. WNT7B promotes bone formation in part through mTORC1. PLoS Genet. 2014;10(1):e1004145. doi: 10.1371/journal.pgen.1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.••.Santos A, Bakker AD, Zandieh-Doulabi B, de Blieck-Hogervorst JM, Klein-Nulend J. Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem Biophys Res Commun. 2010;391(1):364–9. doi: 10.1016/j.bbrc.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z, Guo J. Mechanical induction of BMP-7 in osteocyte blocks glucocorticoid-induced apoptosis through PI3K/AKT/GSK3beta pathway. Cell Biochem Biophys. 2013;67(2):567–74. doi: 10.1007/s12013-013-9543-6. [DOI] [PubMed] [Google Scholar]
- 67.Kitase Y, Lee S, Gluhak-Heinrich J, Johnson ML, Harris SE, Bonewald LF. CCL7 is a protective factor secreted by mechanically loaded osteocytes. J Dent Res. 2014;93(11):1108–15. doi: 10.1177/0022034514553008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122(4):143–59. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 69.Kurosaka M, Machida S. Interleukin-6-induced satellite cell proliferation is regulated by induction of the JAK2/STAT3 signalling pathway through cyclin D1 targeting. Cell Prolif. 2013;46(4):365–73. doi: 10.1111/cpr.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol. 2006;20(12):3364–75. doi: 10.1210/me.2005-0490. [DOI] [PubMed] [Google Scholar]
- 71.Weigert C, Hennige AM, Brodbeck K, Haring HU, Schleicher ED. Interleukin-6 acts as insulin sensitizer on glycogen synthesis in human skeletal muscle cells by phosphorylation of Ser473 of Akt. Am J Physiol Endocrinol Metab. 2005;289(2):E251–7. doi: 10.1152/ajpendo.00448.2004. [DOI] [PubMed] [Google Scholar]
- 72.•.White JP, Puppa MJ, Gao S, Sato S, Welle SL, Carson JA. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metab. 2013;304(10):E1042–52. doi: 10.1152/ajpendo.00410.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carson JA, Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev. 2010;38(4):168–76. doi: 10.1097/JES.0b013e3181f44f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hardie DG. Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism. Proc Nutr Soc. 2011;70(1):92–9. doi: 10.1017/S0029665110003915. [DOI] [PubMed] [Google Scholar]
- 75.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277(27):23977–80. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 76.Tong JF, Yan X, Zhu MJ, Du M. AMP-activated protein kinase enhances the expression of muscle-specific ubiquitin ligases despite its activation of IGF-1/Akt signaling in C2C12 myotubes. J Cell Biochem. 2009;108(2):458–68. doi: 10.1002/jcb.22272. [DOI] [PubMed] [Google Scholar]
- 77.Krawiec BJ, Nystrom GJ, Frost RA, Jefferson LS, Lang CH. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab. 2007;292(6):E1555–67. doi: 10.1152/ajpendo.00622.2006. [DOI] [PubMed] [Google Scholar]
- 78.De Benedetti F, Meazza C, Oliveri M, Pignatti P, Vivarelli M, Alonzi T, Fattori E, Garrone S, Barreca A, Martini A. Effect of IL-6 on IGF binding protein-3: a study in IL-6 transgenic mice and in patients with systemic juvenile idiopathic arthritis. Endocrinology. 2001;142(11):4818–26. doi: 10.1210/endo.142.11.8511. [DOI] [PubMed] [Google Scholar]
- 79.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tominaga H, Maeda S, Hayashi M, Takeda S, Akira S, Komiya S, Nakamura T, Akiyama H, Imamura T. CCAAT/enhancer-binding protein beta promotes osteoblast differentiation by enhancing Runx2 activity with ATF4. Mol Biol Cell. 2008;19(12):5373–86. doi: 10.1091/mbc.E08-03-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Franchimont N, Gangji V, Durant D, Canalis E. Interleukin-6 with its soluble receptor enhances the expression of insulin-like growth factor-I in osteoblasts. Endocrinology. 1997;138(12):5248–55. doi: 10.1210/endo.138.12.5559. [DOI] [PubMed] [Google Scholar]
- 82.Iyer VV, Kadakia TB, McCabe LR, Schwartz RC. CCAAT/enhancer-binding protein-beta has a role in osteoblast proliferation and differentiation. Exp Cell Res. 2004;295(1):128–37. doi: 10.1016/j.yexcr.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 83.Smink JJ, Tunn PU, Leutz A. Rapamycin inhibits osteoclast formation in giant cell tumor of bone through the C/EBPbeta - MafB axis. J Mol Med (Berl) 2012;90(1):25–30. doi: 10.1007/s00109-011-0823-6. [DOI] [PubMed] [Google Scholar]
- 84.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293(2):E453–9. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 85.••.Smith GC, Ong WK, Costa JL, Watson M, Cornish J, Grey A, Gamble GD, Dickinson M, Leung S, Rewcastle GW, Han W, Shepherd PR. Extended treatment with selective phosphatidylinositol 3-kinase and mTOR inhibitors has effects on metabolism, growth, behaviour and bone strength. FEBS J. 2013;280(21):5337–49. doi: 10.1111/febs.12428. [DOI] [PubMed] [Google Scholar]
- 86.•.Pantovic A, Krstic A, Janjetovic K, Kocic J, Harhaji-Trajkovic L, Bugarski D, Trajkovic V. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone. 2013;52(1):524–31. doi: 10.1016/j.bone.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 87.Srinivas V, Bohensky J, Zahm AM, Shapiro IM. Autophagy in mineralizing tissues: Microenvironmental perspectives. Cell Cycle. 2009;8(3):391–3. doi: 10.4161/cc.8.3.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kume K, Amano K, Yamada S, Kanazawa T, Ohta H, Hatta K, Amano K, Kuwaba N. The effect of tocilizumab on bone mineral density in patients with methotrexate-resistant active rheumatoid arthritis. Rheumatology (Oxford, England) 2014;53(5):900–3. doi: 10.1093/rheumatology/ket468. [DOI] [PubMed] [Google Scholar]
- 89.Alzghoul MB, Gerrard D, Watkins BA, Hannon K. Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. Faseb J. 2004;18(1):221–3. doi: 10.1096/fj.03-0293fje. [DOI] [PubMed] [Google Scholar]
- 90.Deldicque L, Sanchez Canedo C, Horman S, De Potter I, Bertrand L, Hue L, Francaux M. Antagonistic effects of leucine and glutamine on the mTOR pathway in myogenic C2C12 cells. Amino Acids. 2008;35(1):147–55. doi: 10.1007/s00726-007-0607-z. [DOI] [PubMed] [Google Scholar]
- 91.Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab. 2008;295(4):E868–75. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


