Abstract
The Escherichia coli SOS system is a well-established model for the cellular response to DNA damage. Control of SOS depends largely on the RecA protein. When RecA is activated by single-stranded DNA in the presence of a nucleotide triphosphate cofactor, it mediates cleavage of the LexA repressor, leading to expression of the 30+-member SOS regulon. RecA activation generally requires the introduction of DNA damage. However, certain recA mutants, like recA730, bypass this requirement and display constitutive SOS expression as well as a spontaneous (SOS) mutator effect. Presently, we investigated the possible interaction between SOS and the cellular deoxynucleoside triphosphate (dNTP) pools. We found that dNTP pool changes caused by deficiencies in the ndk or dcd genes, encoding nucleoside diphosphate kinase and dCTP deaminase, respectively, had a strongly suppressive effect on constitutive SOS expression in recA730 strains. The suppression of the recA730 mutator effect was alleviated in a lexA-deficient background. Overall, the findings suggest a model in which the dNTP alterations in the ndk and dcd strains interfere with the activation of RecA, thereby preventing LexA cleavage and SOS induction.
INTRODUCTION
Exposure of Escherichia coli to agents or conditions that damage DNA or impair DNA replication results in the induction of the SOS response. The expression of the SOS regulon genes is controlled by LexA and RecA proteins (1–3). Binding of the RecA protein to single-stranded DNA (ssDNA) at or near replication blockage sites in the presence of a nucleoside triphosphate causes a conformational change in RecA (active RecA). RecA then promotes cleavage of LexA protein, the repressor of the SOS regulon (4,5). Inactivation of the repressor enables the expression of more than 30 SOS genes (6–8). The early phase of SOS is characterized by generally error-free repair and maintenance processes. However, if the DNA damage level remains too high to be processed by these pathways, error-prone pathways are activated, mediated by error-prone DNA polymerases, causing elevated mutation levels (6).
In E. coli three DNA polymerases are expressed as part of the inducible SOS response: DNA polymerase II, DNA polymerase IV and DNA polymerase V (9). DNA polymerases IV and V are members of the Y family of polymerases. Both lack intrinsic proofreading activity and are considered low-fidelity DNA polymerases. Pol IV is encoded by the dinB gene, and polymerase V is encoded by the umuDC operon. Previous studies (10,11) have indicated that both Pol IV and Pol V have significant access to the replication fork under SOS-induced conditions, although most mutagenesis results from the action of Pol V. Active RecA also promotes autocatalytic cleavage of UmuD protein to UmuD′ and then participates in forming the active form of DNA polymerase V, UmuD′2C-RecA-ATP, also called the mutasome (12–14).
In addition to ssDNA binding, RecA co-protease function also requires binding of a nucleoside triphosphate cofactor (4,15). Various (d)NTP species have been shown to have different efficiencies in promoting RecA activity in vitro, dATP being the most effective, while other nucleotides can inhibit RecA activity (15–18). Interestingly, and possibly related to this, it has also been observed that addition of nucleosides or free bases to the growth medium can affect the level of SOS induction: addition of adenine promotes SOS activity in vivo, while addition of cytidine and guanine is inhibitory (19,20). These data have suggested the possibility that changes in the nucleotide pools may affect the activity of RecA protein (20,21).
In addition, the cellular dNTPs are important both as they serve as DNA precursors and determinants of DNA replication fidelity. Specifically, the relative ratios among the individual dNTPs affect the mispairing rate of DNA polymerases, while the absolute dNTP levels determine the correction of polymerase errors by exonucleolytic proofreading (next-nucleotide effect) (22,23). In this respect, SOS mutagenesis, like normal DNA replication, would be expected to be sensitive to changes in the dNTP levels, as was demonstrated in the case of mutagenesis by ultraviolet (UV) light (24).
In this study, we have investigated how distinct nucleotide pool changes may affect SOS induction and mutagenesis in recA730 strains. In recA730 strains, carrying the RecA E38K mutation (25), RecA protein is constitutively active without the need for the introduction of DNA damage (26,27). As a result, the SOS system is expressed constitutively, resulting in—among others—a spontaneous mutator effect (26,27) due to persistent presence of the PolV mutasome (12–14). In the work described here, dNTP pool alterations were achieved by employing ndk and dcd mutants of E. coli, defective in nucleoside diphosphate kinase and dCTP deaminase, respectively. These mutants have been shown to display altered cellular dNTP pools combined with distinct mutator phenotypes (23,28–31). Our results show, unexpectedly, that the dNTP alterations in these mutants strongly suppress SOS expression and associated mutagenesis.
MATERIALS AND METHODS
Bacterial strains and media
The E. coli strains used in this study are listed in Table 1. Strain MC4118 was described in Maliszewska-Tkaczyk et al. (32) and strain JW2502 in Baba et al. (33). Strain constructions by P1 transduction were done using P1virA. The F′ prolac episomes used in the mutagenesis experiments of Tables 2 and 3 were introduced into the strains of the NR9338 series by conjugation. Strains used in the β-galactosidase assay were derivatives of NR9338 carrying plasmid pSK1002 (34). MC4118 is a lac+ derivative of strain MC4100 created by the method of Diederich et al. (35) as described in Fijalkowska et al. (36). Solid and liquid media were prepared using standard recipes (37). Solid minimal medium (MM) contained 0.5% glucose or 0.4% lactose as a carbon source and 5 μg/ml of thiamine. For experiments with dcd strains the solid media contained additionally 50 μg/ml of thymidine to improve colony growth on the plates (larger colony sizes). Liquid media, used for generation of mutant frequencies and extraction of cellular dNTP pools (see below), did not contain any added thymidine. Antibiotics, when required during strain constructions, were added at 30 μg/ml (kanamycin), 12.5 μg/ml (tetracycline), 50 μg/ml (ampicillin) or 10 μg/ml (chloramphenicol). LB-Rif plates used for the scoring of rifampicin-resistant mutants contained 100-μg/ml rifampicin.
Table 1. Escherichia coli strains used in this work.
| Strain | Relevant genotype and/or construction | Source or reference |
|---|---|---|
| A. Used for construction | ||
| NR9338 | ara thi Δ(pro-lac) sulA366 | (27) |
| MC4118 | Δ(argF-lac)169 sulA366 | (32) |
| NR11531 | NR9338, but recA730 srl::Tn10 | (27) |
| JW2502 | Δndk::kan | (33) |
| NR11814 | Δndk::cam | (23) |
| BW1040 | dcd-12::Tn10dkan | (40) |
| CC101 | F'CC101 (A·T→C·G) | (39) |
| CC103 | F'CC103 (G·C→C·G) | (39) |
| CC105 | F'CC105 (A·T→T·A) | (39) |
| NR9405 | NR9338, but lexA51 malB::Tn9 | this work |
| B. Used for measurements | ||
| EC9524 | NR9338 [pSK1002] | this work |
| EC9525 | NR11531 (recA730) [pSK1002] | this work |
| EC9529 | NR9338 dcd-12::Tn10dkan [pSK1002] | this work |
| EC9526 | NR9338 Δndk::cam [pSK1002] | this work |
| EC9527 | NR11531 (recA730) dcd-12::Tn10dkan [pSK1002] | this work |
| EC9528 | NR11531 (recA730) Δndk::cam [pSK1002] | this work |
| EC9642 | NR9338, but F'CC101 | this work |
| EC9656 | EC9642 dcd-12::Tn10dkan | this work |
| EC9668 | EC9642 Δndk::kan | this work |
| EC9783 | EC9642 recA730 srl::Tn10 | this work |
| EC9786 | EC9642 recA730 srl::Tn10 dcd-12::Tn10dkan | this work |
| EC9789 | EC9642 recA730 srl::Tn10 Δndk::kan | this work |
| EC9792 | NR9338 lexA51 malB::Tn9 F'CC101 | this work |
| EC9795 | EC9792 dcd-12::Tn10dkan | this work |
| EC9798 | EC9792 Δndk::kan | this work |
| EC9801 | EC9792 recA730 srl::Tn10 | this work |
| EC9804 | EC9792 recA730 srl::Tn10 dcd-12::Tn10dkan | this work |
| EC9807 | EC9792 recA730 srl::Tn10 Δndk::kan | this work |
| EC9644 | NR9338, but F'CC103 | this work |
| EC9658 | EC9644 dcd-12::Tn10dkan | this work |
| EC9670 | EC9644 Δndk::kan | this work |
| EC9784 | EC9644 recA730 srl::Tn10 | this work |
| EC9787 | EC9644 recA730 srl::Tn10 dcd-12::Tn10dkan | this work |
| EC9790 | EC9644 recA730 srl::Tn10 Δndk::kan | this work |
| EC9793 | NR9338 lexA51 malB::Tn9 F'CC103 | this work |
| EC9796 | EC9793 dcd-12::Tn10dkan | this work |
| EC9799 | EC9793 Δndk::kan | this work |
| EC9802 | EC9793 recA730 srl::Tn10 | this work |
| EC9805 | EC9793 recA730 srl::Tn10 dcd-12::Tn10dkan | this work |
| EC9808 | EC9793 recA730 srl::Tn10 Δndk::kan | this work |
| EC9646 | NR9338 F'CC105 | this work |
| EC9660 | EC9646 dcd-12::Tn10dkan | this work |
| EC9672 | EC9646 Δndk::kan | this work |
| EC9785 | EC9646 recA730 srl::Tn10 | this work |
| EC9788 | EC9646 recA730 srl::Tn10 dcd-12::Tn10dkan | this work |
| EC9791 | EC9646 recA730 srl::Tn10 Δndk::kan | this work |
| EC9794 | NR9338 lexA51 malB::Tn9 F'CC105 | this work |
| EC9797 | EC9794 dcd-12::Tn10dkan | this work |
| EC9800 | EC9794 Δndk::kan | this work |
| EC9803 | EC9794 recA730 srl::Tn10 | this work |
| EC9806 | EC9794 recA730 srl::Tn10 dcd-12::Tn10dkan | this work |
| EC9809 | EC9794 recA730 srl::Tn10 Δndk::kan | this work |
| EC9428 | MC4118, but lac+ (AmpR) | this work |
| EC9461 | EC9428 dcd-12::Tn10dkan | this work |
| EC9487 | EC9428 Δndk::cam | this work |
| EC9477 | EC9428 recA730 srl::Tn10 | this work |
| EC9471 | EC9428 recA730 srl::Tn10 dcd-12::Tn10dkan | this work |
| EC9503 | EC9428 recA730 srl::Tn10 Δndk::cam | this work |
| EC9681 | MC4118 but lac+ (AmpR) lexA51 malB::Tn9 | this work |
| EC9684 | EC9681 dcd-12::Tn10dkan | this work |
| EC9682 | EC9681 Δndk::kan | this work |
| EC9685 | EC9681 recA730 srl::Tn10 | this work |
| EC9686 | EC9681 recA730 srl::Tn10 dcd-12::Tn10dkan | this work |
| EC9687 | EC9681 recA730 srl::Tn10 Δndk::kan | this work |
Table 2. Mutant frequencies (lac+ revertants per 108 cells) mediated by indicated base-pair substitutions in recA730- and ndk-related strains.
| Strain | A·T→C·G | G·C→C·G | A·T→T·A | |
|---|---|---|---|---|
| A. | wild-type | 0.31 (0.18–0.82) | 0.16 (0.02–0.34) | 2.4 (1.5–6.9) |
| recA730 | 5.2 (3.4–12.7) | 2.0 (1.1–4.1) | 38 (34–46) | |
| ndk | 0.37 (0.23–0.81) | 0.05 (0.04–0.54) | 22 (15–32) | |
| recA730 ndk | 1.6 (1.0–4.2) | 0.26 (0.19–2.1) | 21 (16–28) | |
| B. | lexA51 | 0.31 (0.07–0.37) | 0.25 (0.11–0.51) | 1.2 (0.53–1.9) |
| lexA51 recA730 | 11 (6.7–15.8) | 2.7 (1.2–4.9) | 36 (31–46) | |
| lexA51 ndk | 0.38 (0.03–0.73) | 0.18 (0.04–0.40) | 17 (11–23) | |
| lexA51 recA730 ndk | 11 (9.2–15) | 2.9 (1.9–4.7) | 69 (56–90) | |
Shown are average values and confidence intervals for 12 independent cultures for each strain. (A) The strains used were the recA730, ndk and recA730 ndk derivatives of EC9642 and (F′CC101), EC9644 (F′CC103) and EC9646 (F′CC105), which revert to lac+ by A·T→C·G, G·C→C·G and A·T→T·A, respectively. (B) The corresponding lexA51 (lexAdef) strains were derivatives of EC9792 (F′CC101), EC9793 (F′CC103) and EC9794 (F′CC105) (see Table 1 and the Materials and Methods section).
Table 3. Mutant frequencies (lac+ revertants per 108 cells) mediated by indicated base-pair substitutions in recA730- and dcd-related strains.
| Strain | A·T→C·G | G·C→C·G | A·T→T·A | |
|---|---|---|---|---|
| A. | wild-type | 0.75 (0.26–1.4) | 0.32 (0.02–0.34) | 2.7 (1.5–6.3) |
| recA730 | 6.9 (6.3–8.7) | 2.5 (0.76–5.6) | 36 (33–41) | |
| dcd | 1.1 (0.47–2.8) | 0.47 (0.16–0.59) | 10 (7.3–13) | |
| recA730 dcd | 4.2 (2.1–10) | 1.3 (0.85–1.9) | 28 (25–34) | |
| B. | lexA51 | 0.36 (0.08–0.49) | 0.16 (0.03–0.35) | 4.8 (3.6–5.8) |
| lexA51 recA730 | 8.4 (4.9–20) | 3.9 (2.0–7.5) | 47 (38–51) | |
| lexA51 dcd | 1.4 (0.85–2.1) | 0.50 (0.12–0.48) | 11 (7.5–16) | |
| lexA51 recA730 dcd | 19 (13–27) | 7.5 (4.7–15) | 48 (37–55) | |
Shown are average values and confidence intervals for 12 independent cultures for each strain. (A) The strains used were the recA730, dcd and recA730 dcd derivatives of EC9642 and (F′CC101), EC9644 (F′CC103) and EC9646 (F′CC105), which revert to lac+ by A·T→C·G, G·C→C·G and A·T→T·A, respectively. (B) The corresponding lexA51 (lexAdef) strains were derivatives of EC9792 (F′CC101), EC9793 (F′CC103) and EC9794 (F′CC105) (see Table 1 and the Materials and Methods section).
Mutant frequency measurements
Mutant frequencies for each strain were determined using a total of 12–20 cultures (1 ml of Luria-Bertani Broth (LB)), started from a single colony (one colony per tube), and growing them to saturation at 37°C. The number of lac revertants was determined by plating 100 μl of undiluted cultures on MM plates containing lactose as carbon source. The number of RifR mutants in each culture was determined by plating 100 μl of undiluted cultures on LB-Rif plates. The viable cell count in the cultures was determined by plating 100 μl of a 10−6 dilution on LB or MM plates containing glucose as carbon source. The plates were incubated for 24–48 h at 37°C. Mutant frequencies were calculated by dividing the number of mutants per plate by the average number of total cells. Sporadic jackpot cultures were removed from the analysis. Statistical analysis was performed using the software program Statistica.
Beta-galactosidase assay
Bacterial cultures containing plasmid pSK1002 (34) were grown at 37°C in LB medium. Overnight cultures were diluted 1:2000 in fresh medium and grown at 37°C with shaking to an OD600 = 0.4. At this point, cells were treated with a DNA-damaging agent (20 Jm−2 of UV, 0.02% of methylmethane sulfonate (MMS) or 0.5 μg/ml of mitomycin C) and grown for additional 2 h. β-galactosidase activity was determined as described (37). The values of β-galactosidase specific activity were calculated as nmoles of o-nitrophenyl-β-D-galactoside hydrolyzed per minute per mg of total protein.
Microarray studies
Bacterial strains were grown in LB. The strains used were of the MC4118 series (see Table 1). Overnight cultures were diluted 1:100 in fresh LB and grown at 37°C with shaking to an OD600 = 0.5. RNA was isolated using the RNeasyBacteria Protect Mini kit (Qiagen). cDNA labeling was performed with the use of FairPlay III Microarray Labeling Kit (Agilent Technologies) and CyDye Cy5 and Cy3 mono-reactive dyes (GE Healthcare). The gene expression levels were determined using the E. coli Gene Expression Microarray system (Agilent Technologies). Two-color hybridizations with dye swap between duplicates were performed according to the Agilent Two-Color Microarray-Based Analysis protocol. Each dye swap combination was repeated three times. For feature extraction, Axon GenePix 4000B scanner and GenePix Pro 6.1 software (Molecular Devices) were used. Statistical analysis using Student's t-test was performed using Acuity 4.0 software (Molecular Devices). Differences in gene expression level were expressed as log2 ratio value in Figure 1. The complete data set was submitted to the GEO repository (series record GSE62898) (http://www.ncbi.nlm.nih.gov/gds).
Figure 1.
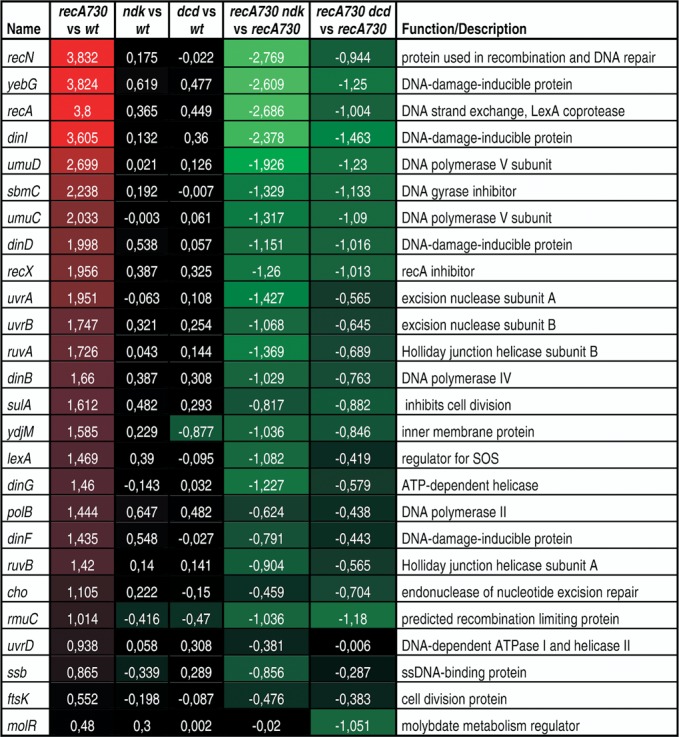
Microarray analysis on recA730 and related strains. Shown are the log2 values for the fold increase (or decrease) in mRNA expression determined using the Agilent E. coli Gene Expression Microarray System for the indicated comparisons. See the Materials and Methods section for details. Red colors indicate increased gene expression, green color reduced expression. Strains used were EC9428 (wt), EC9461 (dcd), EC9487 (ndk), EC9477 (recA730), EC9471 (recA730 dcd) and EC9503 (recA730 ndk) (see Table 1).
dNTP pool measurements
dNTPs were extracted using the procedure described by Diaz et al. (38) with some modifications. Bacterial cultures were grown at 37°C in LB medium. Strains used were of the MC4118 series (see Table 1). Each overnight culture was diluted 1:2000 in fresh medium and grown at 37°C with shaking to an OD600 = 0.3. Cells from 100 ml of culture were collected by filtration on 0.2-μm polycarbonate filters (Sterlitech) and washed with 10 ml of cold saline. Nucleotides were extracted from the filters by incubation in 7 ml of cold 60% methanol for 2 h at −20°C. The lysate was lyophilized and the obtained residue resuspended in 1 ml of water, followed by extraction using 0.5 ml of chloroform. The aqueous phase was collected and re-lyophilized, and the residue resuspended in 0.1-ml water. Determination of dNTPs was done by ion-pairing reverse-phase chromatography, as described in Ahluwalia et al. (22), with minor modifications. Peaks for the individual dNTPs and ATP (ADP) were identified based on retention times of nucleotide standards and confirmed by the UV spectra of the peak. Quantitation of each dNTP was by peak area at 260 nm, corrected for the differential extinction coefficients of the various nucleotides at this wavelength and expressed per OD600 of the corresponding culture at the time of harvest.
RESULTS
The SOS mutator effect of recA730 strains is abrogated by a ndk nucleoside diphosphate kinase deficiency
The studies described in this paper flow from our previous investigations of the mutator effect associated with a defect in nucleoside diphosphate kinase (ndk gene product). Defective ndk strains display distinct changes in their dNTP pool levels (elevation of dCTP and reduction in dATP) and are also mutators (23,28–29). The mutator effect has a defined specificity in terms of the specific base-substitution mutations it promotes, and positive correlations between the dNTP pool changes and the elevated base substitutions have been drawn (23).
As part of the above studies, we were interested in the possible interaction of ndk with the E. coli SOS system. In particular, we focused on the spontaneous SOS mutator effect resulting from constitutive SOS induction in the recA730 mutant (26,27). This recA730 mutator effect, resulting from the error-prone action of the Pol V mutasome on undamaged DNA (12–14), is characterized by its own specificity of mutation, promoting preferentially transversion mutations (27). However, whether any dNTP pool changes may contribute to the spontaneous SOS mutator effect has not been established, and this is an important open question. One recent study on mutagenesis induced by UV light, which is also mediated by SOS induction, revealed dNTP pool elevations in E. coli upon UV irradiation and, importantly, these dNTP changes were shown to contribute to the mutagenic effects of the UV irradiation (24).
Based on these considerations, we undertook a study of a recA730 ndk double mutant strain, focusing on its mutator effects. It was thought that any observed changes in the recA730 mutator effect upon addition of the ndk defect might provide additional insights into the underlying causes of the mutator effect. Unexpectedly, as shown in the data of Table 2A, a negative interaction between the two individual mutators was observed. For these measurements, we used the lacZ reversion system developed by Cupples and Miller (39) which permits scoring of specific base-pair substitution mutations in the lacZ gene. Data for three different lac alleles are shown, reverting by A·T→C·G, G·C→C·G or A·T→T·A transversion mutation, specifically. These alleles were chosen because they have been used productively to demonstrate the magnitude of the recA730 mutator effect (27). In addition, the A·T→T·A transversion is highly diagnostic for the ndk mutator effect (23).
The Table 2A shows that recA730 is a mutator for each of the three lac alleles (17-, 12- and 16-fold enhancement, respectively). The mutator effect of the single ndk strain for the A·T→T·A transversion is also clearly seen (9-fold). Interestingly, in the recA730 ndk double mutant all frequencies are reduced compared to the level of the single recA730 strain: 3-fold for the A·T→C·G, 8-fold for the G·C→C·G and nearly 2-fold for the A·T→T·A transversion. The case of the A·T→T·A transversion is particularly illuminating, as both the recA730 and ndk are strong mutators (16- and 9-fold, respectively) for these events. Nevertheless, when the two are combined, no amplification of the mutant frequency is observed and, in fact, the resulting frequency is even less than a simple additive effect. It appears that the frequency simply returns to that of the single ndk, further implying a loss of the SOS mutator effect.
Effect of the dcd mutator on the SOS mutator effect
E. coli dCTP deaminase, encoded by the dcd gene, catalyzes the formation of dUTP from dCTP, an important step in the ultimate production of dTTP (40). In a dCTP deaminase-deficient strain, dCTP accumulates and the dTTP pool may be reduced (23,30). Like ndk mutants, dcd-defective strains are characterized by a mutator phenotype, which has been correlated with the specific dNTP pool changes occurring in this strain (23,31). When we combined recA730 with the dcd null allele, we likewise observed a negative interaction between the two individual alleles (see Table 3A), although the antimutagenic effect is slightly less than observed for the combination with ndk. Nevertheless, in all cases the mutant frequency for the recA730 dcd double is less than the sum of the two individual mutant frequencies and, in fact, is reduced from the level of the single recA730 strain (1.6-, 1.9- and 1.3-fold, for the three reversions, respectively). For the A·T→T·A transversion, the 13-fold mutator effect for recA730 combined with the 3.7-fold mutator effect for dcd does not lead to any further increase in frequency, but rather a reduction compared to the single recA730 strain. Thus, the dcd defect clearly exerts an antimutagenic effect on the recA730 mutator.
Effect of ndk and dcd on rifampicin-resistant mutations
In addition to the lac reversion system described above, we also investigated combined mutational effects using the rpoB forward assay. In this assay, rifampicin-resistant mutants are scored at a large number of sites in the rpoB gene (41). The data in Table 4A show that, for rifampicin-resistant mutants, recA730 is a strong mutator (18-fold). The ndk and dcd single-mutant strains also display a mutator phenotype for this target, 6.5-fold for ndk and 2.5-fold for dcd. Importantly, when combining recA730 with ndk a 2-fold reduction was observed compared to the single recA730 mutant, and a 1.5-fold reduction was seen for the recA730 dcd combination. Thus, the data of Tables 2–4 clearly indicate a negative effect of the ndk and dcd defects on the SOS mutator effect.
Table 4. Mutability of recA730- and ndk- or dcd-related strains as measured in the rpoB forward target (frequency of rifampicin-resistant mutants).
| Strain | RifR per 108 cells | |
|---|---|---|
| A. | wild-type | 3.5 (2.4–4.6) |
| recA730 | 63 (51–73) | |
| ndk | 23 (19–33) | |
| recA730 ndk | 30 (24–37) | |
| dcd | 8.7 (5.0–11.5) | |
| recA730 dcd | 41 (33–51) | |
| B. | lexA51 | 5.7 (4.4–7.2) |
| lexA51 recA730 | 79 (65–95) | |
| lexA51 ndk | 42 (32–52) | |
| lexA51 recA730 ndk | 115 (103–163) | |
| lexA51 dcd | 9.3 (6.4–12.5) | |
| lexA51 recA730 dcd | 88 (58–100) | |
Shown are average values and confidence intervals for 12 independent cultures for each strain. (A) Strains used were EC9428 (wt), EC9477 (recA730), EC9487 (ndk), EC9503 (recA730 ndk), EC9461 (dcd), EC9471 (recA730 dcd) and (B) the lexAdef derivatives EC9681 (lexA51), EC9685 (lexA51 recA730), EC9682 (lexA51 ndk), EC9687 (lexA51 recA730 ndk), EC9684 (lexA51 dcd) and EC9686 (lexA51 recA730 dcd) (see Table 1 and the Materials and Methods section).
Effect of ndk and dcd on umuDC expression
As the recA730 SOS mutator effect is strictly dependent on the mutagenic action of Pol V, the product of the umuDC operon, one simple possibility for the observed antimutator effect would be reduced activity or expression of Pol V in the double mutant strains. One way to test this is by using the umuC::lacZ gene fusion, which has been used previously as a direct indicator for Pol V expression and, indirectly, as an indicator for overall SOS expression (34). The data in Table 5 clearly show that umuC expression, as measured by units of β-galactosidase, while significantly elevated in the recA730 strain, is strongly reduced from that level in the double mutants. This reduction provides a ready explanation for the observed reduction in mutator activity. Importantly, the data also show that the ndk and dcd deficiencies interfere with umuC expression in a wild-type (rec+) background when the cells are treated by the DNA damaging agents mitomycin C, MMS or UV light, which otherwise effectively induce the SOS response.
Table 5. UmuC expression levels in recA730 strains or after treatment with DNA-damaging agents.
| Treatment | Strain | β-galactosidase units |
|---|---|---|
| None | wild-type | 16 |
| recA730 | 206 | |
| recA730 ndk | 34 | |
| recA730 dcd | 90 | |
| Mitomycin C | wild-type | 3629 |
| ndk | 1556 | |
| dcd | 1659 | |
| MMS | wild-type | 999 |
| ndk | 297 | |
| dcd | 754 | |
| UV | wild-type | 1322 |
| ndk | 969 | |
| dcd | 483 |
β-galactosidase level as determined using the umuC::lacZ gene fusion of plasmid pSK1002 as described in the Materials and Methods section. The strains used were EC9524 (wt), EC9525 (recA730), EC9528 (recA730 ndk), EC9527 (recA730 dcd), EC9526 (ndk) and EC9529 (dcd) (see Table 1).
Expression of the entire SOS regulon is diminished in ndk and dcd backgrounds
To investigate whether the observed reduction in Pol V expression is unique to the umuDC operon or may reflect an general shutdown of the SOS system (lexA regulon), we performed microarray analysis of gene expression profiles in each of the single ndk, dcd and recA730 strains, as well as the recA730 ndk and recA730 dcd doubles. The complete results are available at GEO repository (series record GSE62898). In Figure 1 we display a subset of the data, focusing on a set of established members of the SOS regulon (7).
The data for recA730 in comparison to the wild-type clearly show the induction of the SOS regulon. For example, the recN, yebG and recA expression levels are induced by around 14-fold, dinI by 12-fold and umuD and umuC by 6.5- and 4-fold, respectively. In contrast, the dcd and ndk defects do not cause any SOS induction. The ndk and dcd defects, however, do cause a significant reduction of SOS gene expression in the recA730 background. The ndk deficiency has the strongest effect, reducing recA expression by as much as 6.5-fold, dinI by 5.2-fold and umuD and umuC by 3.8- and 2.5-fold, respectively, in comparison to the single recA730. The dcd deficiency reduces recA expression by 2-fold, dinI by 2.75 fold and umuD and umuC expression by 2.3- and 2.1-fold, respectively. Based on these data, we conclude that the ndk or dcd-mediated impairment of the SOS mutator effect is due to a general inhibition of the SOS regulon. Therefore, the source of the inhibitory effect likely resides at the level of the RecA–LexA interaction, which controls the expression of the SOS system through the RecA-facilitated cleavage of LexA.
Effect of ndk and dcd on the SOS mutator in a lexA(Def) background
We also investigated the ability of the ndk and dcd deficiencies to inhibit the recA730 mutator effect in a lexA-deficient background (lexA51). The results are shown in Tables 2B, 3B and 4B. They indicate that when the LexA repressor is absent, the inhibitory effect of the ndk and dcd deficiencies is diminished or no longer observed. It appears that in several cases the mutator effects in the double mutants can be described as a simple additive effect of the individual mutator effects. These results are consistent with the RecA-mediated cleavage of LexA as the critical step affected by the ndk and dcd deficiencies. We have also monitored this issue by quantitative polymerase chain reaction, analyzing the expression of three individual SOS-inducible genes (yebG, recN and recA) (see Supplementary Figure S1). The results confirm the inhibition of each of these genes in the corresponding ndk and dcd backgrounds, as well as their recovery in their lexA51 derivatives.
The SOS mutator effect depends on cleavage of both LexA, determining the expression level of the umuDC operon, and UmuD. The latter cleavage yields UmuD′, which is an essential component of the Pol V mutasome (14,42–43). Thus, in principle, the inhibitory effect exerted by the ndk and dcd deficiencies in the lex+ background could be due to an inhibition of both cleavages. Nevertheless, the observed recovery of the mutator effect in the lexA(Def) background indicates that the cleavage of UmuD must not be a rate-limiting factor under these conditions and that sufficient UmuD′ is being produced. Differential cleavage of LexA and UmuD under conditions of RecA protein alterations has been reported (44,45), and this may be part of the explanation. Also, production of Pol V (UmuCD’2) is subject to several regulatory steps (42), and the rate-limiting step may not simply reside at the RecA level. Note that, as expected, mutagenesis in the recA730 ndk lexA51 mutants is still fully umuDC dependent (results not shown).
Measurement of dNTP pool changes
The active form of RecA responsible for the autocleavage of LexA has been determined to be a RecA homopolymer bound to ssDNA as an extended and dynamic nucleofilament (46,47). The active state of this nucleofilament also requires the presence of ATP (or dATP) nucleotide cofactor bound at the RecA monomer–monomer interfaces (47). As the ndk and dcd deficiencies are characterized by substantial changes in the levels of individual nucleotides, notably the dNTPs (23,28), it is reasonable to propose that such changes in the nucleotide levels may affect the activation state of RecA (and RecA730).
In Figure 2 we show the results of our dNTP pools measurements. The first important finding is that the constitutive induction of the SOS response in the recA730 strain is not associated with any significant pool alteration compared to the wild-type strain. Second, distinct dNTP pool changes are seen in the ndk and dcd strains, consistent with previous reports (23). For both ndk and dcd, there was a large increase in the level of dCTP (12- and 10-fold enhancements, respectively). In the ndk strain, there was also a small increase in the dTTP pool (1.5-fold) and a decrease in dATP pool (2-fold). In the dcd strain, there also was a 3-fold reduction in the dTTP pool. Finally, and importantly, the ndk- and dcd-mediated dNTP pool alterations remain essentially unaffected by the recA730 allele.
Figure 2.
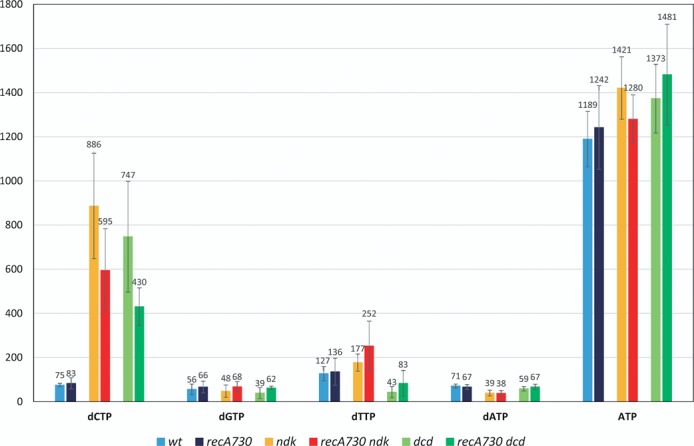
dNTP levels in recA730 and related strains. dNTP levels were determined by HPLC analysis as described in the Materials and Methods section. The numbers represent the mean of three independent experiments and are expressed on the Y-axis as milliabsorbance units (A260) measured by the HPLC instrument per OD600 of the bacterial cultures at harvest. Strains used were EC9428 (wt), EC9461 (dcd), EC9487 (ndk), EC9477 (recA730), EC9471 (recA730 dcd) and EC9503 (recA730 ndk) (see Table 1).
As ATP is generally considered the main in vivo activator for RecA protein (46), we also monitored the ATP content of the cells. Both our HPLC analysis used for the dNTP analysis described above and a series of separate ATP assays using the BacTiter-Glo™ (Promega) reagent (see Supplementary Figure S2) revealed no significant changes in ATP level or the ATP/ADP ratios for any of the strains.
DISCUSSION
Suppression of SOS in ndk and dcd defective backgrounds
In the present work we describe several important observations regarding the activity of the E. coli SOS system under conditions of altered dNTP pool levels. Specifically, we show that SOS expression is inhibited in the ndk and dcd backgrounds characterized by dNTP pool changes. The findings relate primarily to the spontaneous SOS induction in the constitutive recA730 mutant, but may also apply to other instances where the SOS system is induced, for example by DNA damaging agents in the wild-type strain (Table 5). The observation that the ndk or dcd deficiencies suppress the recA730-mediated SOS mutator effect was surprising when first made. As these deficiencies themselves produce distinct mutator effects (23,26,28–29,31), our experiments revealed a case where combined mutator effects lead to a reduction rather than an enhancement of effects. Previous studies on ndk and dcd strains have revealed their mutator effects to result from dNTP pool alterations by at least two mechanisms: (i) enhanced production of mispairing errors (due to the dNTP imbalances) and (ii) reduced exonucleolytic proofreading of any mispairings due to elevation of certain dNTPs (22–23,48). As the SOS mutator activity results from the mutagenic action of Pol V, either by producing increased levels of base–base mismatches during any of its DNA synthesis or, alternatively, by error-prone extension of mismatches created by Pol III holoenzyme (49), the SOS mutator effect would likewise be expected to be sensitive to the dNTP pool changes. Clearly, exactly the opposite effect was observed, and subsequent experiments showed that the negative effect of ndk and dcd results from a suppression of the entire SOS regulon.
How can the ndk and dcd deficiencies cause suppression of the SOS system? Our data show that the suppression results from a reduced ability of RecA730 to mediate LexA cleavage. While other explanations must also be considered (see below), the most direct hypothesis may be that the altered dNTP levels negatively impact the RecA filament. The precise structure and dynamics of the active RecA filament are still being investigated, but it is clear that it consists of an extended polymeric structure of possibly hundreds of RecA monomers long wound around ssDNA and, importantly, containing a nucleoside triphosphate cofactor, such as ATP (46,47), which is essential for its active status. Active filaments are dynamic, generally growing at the 3′ end and undergoing disassembly at the 5′ end. The filament also possesses a robust ATPase activity (16,50) that is critical for certain RecA functions, like homologous recombination, but which is not required for others, such as LexA cleavage and SOS induction (51). Upon ATP hydrolysis, the ADP-containing filaments become inactive and tend to dissociate from the DNA. Generally, filament formation depends on a slow, rate-limiting nucleation step on available ssDNA, followed by a more rapid extension step (46). The nucleation step is rate-limiting, as the availability of ssDNA is controlled effectively by the E. coli single-stranded binding (SSB) protein, which keeps RecA away from the (limited) amount available under normal circumstances. Even after DNA-damaging treatments, such as UV-light irradiation, when increased amounts of ssDNA favor formation of the filament, nucleation still requires assistance of the RecFOR RecA-loading system (46).
The SOS constitutive nature of the recA730 mutant (E38K) has been reported to result from an increased association rate of RecA730 protein with ssDNA, resulting in an increased ability to displace SSB protein (51,52) and permitting filament nucleation in the absence of DNA damage and without requiring assistance of the RecFOR RecA loading complex (52,53). However, importantly, filament formation by RecA730 is still fully dependent on the nucleotide cofactor (51). The (d)NTP cofactor requirement for the activation of RecA and RecA730 provides an attractive starting point for interpreting the inhibitory effects of the ndk and dcd deficiencies. Both deficiencies promote significant changes in the cellular dNTP levels, which may interfere with the ongoing activation of the filament. The primary in vivo cofactor for RecA is believed to be ATP. This is based on its effectiveness in activating RecA in vitro and, further, its significant overabundance compared to all other cellular (d)NTPs in vivo. Nevertheless, it is interesting to note that under several in vitro conditions dATP is slightly more effective than ATP in promoting several RecA activities (15,17,51,54–55). Our studies revealed no significant changes in the level of ATP (or that of the other rNTPs) in the ndk- or dcd-deficient strains, consistent with an earlier study on the ndk deficiency (28). Furthermore, the dcd-encoded dCTP deaminase is highly specific for the dCTP substrate (56) and is not likely to play a role in rNTP metabolism. Thus, we may assume that the inhibitory effects are mediated specifically by the observed changes in the level of the dNTPs. Both strains display a strong increase in the dCTP level (10- to 12-fold). In addition, the ndk strain showed a small increase in the dTTP level (1.5-fold) and a decrease in the dATP level (2-fold), while the dcd strain had a lowered dTTP pool (3-fold). Similar changes, both qualitatively and quantitatively, were noted in previous studies on these deficiencies in slightly different strain backgrounds (23).
Little in vivo information is available about the possible role of the cellular dNTPs in RecA activation, although a number of studies have addressed the role of alternative cofactors using in vitro experiments (15,17–18,57–59). These studies revealed that nucleotides other than ATP or dATP are able to function at least in some of the RecA-mediated reactions, however with reduced efficiency. For example, in early studies on in vitro cleavage of lambda repressor, which may resemble in part LexA cleavage (17), UTP and dUTP were able to stimulate cleavage of the phage repressor to some extent, while (d)GTP, (d)CTP and dTTP were ineffective. In competition with ATP at equimolar concentrations, (d)UTP, (d)GTP and (d)CTP behaved as competitive inhibitors for the ATP-mediated reaction, while dTTP was a very potent inhibitor. Similar results were obtained by Phyzicky and Roberts (15) in a study of lambda repressor cleavage by the conditionally constitutive RecA441 protein. In vitro studies (18,58–59) have suggested that certain alternative (d)NTP cofactors, such as UTP or (d)CTP can, like ATP, bind to RecA to yield a high-affinity DNA binding state, but they can only do so with reduced efficiency. Others, like dGTP or dTTP, were not able to generate such a state. It was suggested that a main difference between ATP-containing active complexes and those containing alternative (d)NTPs reflects their effect on the RecA-DNA binding: whereas ATP promotes strong RecA-DNA binding, other (d)NTPs promote only weak binding, leading to enhanced dissociation of the filament (58).
With regard to our present in vivo study, a more complicated situation is at hand, as one needs to consider the in vivo competition between ATP and any of the alternative (d)NTPs. ATP can be estimated to be present at a level ∼20- to 100-fold higher than of the individual dNTPs (Figure 2; see also (28)). Despite this, the extended nature of the nucleofilaments, which may be many hundreds of RecA monomers in length (46), may make the occasional insertion of alternative cofactors hard to avoid. If such insertions were to lead to premature dissociation or were to interfere otherwise with the functioning or the growth dynamics of the filament, they could become a rate-limiting factor for overall RecA activity. If correct, it would not be surprising if the currently observed dNTP pool changes, including the strong elevation of the dCTP pool (10-fold or greater), would cause a significant restriction on the activity of the SOS system. This is an attractive hypothesis that is open to further investigation in view of the increased availability of ways to manipulate the in vivo dNTP pools (e.g. (22,48,60)). The issue is also open to in vitro studies using (d)NTP mixtures mimicking in vivo concentrations. As ndk and dcd have in common the strong elevation of the dCTP concentration, one may surmise that the concentration of this particular nucleotide is most relevant for the observed SOS shutdown. This possibility finds further support in the earlier studies with the recA441 allele (formerly tif-1). This allele contains two mutations, E38K (as in recA730) and I298V (61,62). The I298V substitution acts as a temperature-dependent suppressor of the E38K-mediated SOS induction, such that the constitutive SOS induction is only seen at higher temperature (e.g. 42°C). Importantly, this conditional phenotype is also influenced by the composition of the growth medium: the presence of adenine stimulates SOS induction, while addition of guanine and cytidine inhibits induction (19,63). Thus, not only do these observations point to the likely importance of the composition of the intracellular nucleotide pools, they also suggest that in particular cytosine (and guanine) nucleotides would be the stronger inhibitors of SOS induction. Careful measurements of the (d)NTP pools under these conditions may provide more insight into these issues. In related results (not presented here), we have observed that the recA730 mutator phenotype is likewise sensitive to the medium composition (adenine stimulates while guanine and cytosine diminish its mutator phenotype) (see also (21)).
In addition to the direct effect of the dNTPs as components of the RecA filament, as proposed above, more indirect explanations for their effect should be considered. Overall, the dynamics of the RecA filament, encompassing its formation, growth and breakdown are complex, and are under the control of large number of gene products identified so far, such as RecX, DinI, RecBCD, RecFOR, RadA, XthA and others, which can work in positive or negative ways (46,64–67). Thus, it is formally possible that the dNTP alterations of the ndk and dcd mutants cause changes in the expression or activity of any of these proteins, and may negatively affect the stability of the filament in this manner. For example, increased activity of the UvrD helicase, one of whose activities is to remove RecA from DNA (68), was shown to suppress SOS expression (69). Also, the availability of ssDNA substrates may be altered in dNTP pool bias mutants, for instance by their effect on replication rate or fork progress, such that SOS induction might be affected. Finally, it is to be noted that analysis of two different constitutive recA mutants has shown their precise modes of loading onto the DNA to be distinct (66). Thus, depending on the precise rate-limiting steps for SOS induction in each case, the effect of dNTP alterations could be more or less pronounced and, hence, some caution is required when extrapolating from the recA730 results to the general case of SOS induction.
Lack of dNTP pool changes in constitutively induced SOS cells
A second main result from our study is the observation that SOS induction per se is not associated with major changes in the dNTP pool levels. As dNTP pool alterations are contributing factors to mutagenesis, the lack of such changes may simplify further analysis of the SOS mutator effect. For example, in a previous study (27) we argued that the SOS mutator effect results primarily from the error-prone extension by Pol V of replication errors created by Pol III holoenzyme (HE) (rather than from errors made by Pol V itself). Inherent in this model was the assumption that the pattern of DNA replication errors in normal and SOS-induced cells is the same and that the preferential induction of transversion mutations in recA730 strains simply reflects the preferential access of Pol V to transversion mismatches, which are the more likely impediments for continued DNA synthesis by Pol III holoenzyme (HE) (27). The lack of observed dNTP pool changes in the recA730 strain is fully consistent with this aspect of the model.
In contrast, Gon et al. (24) reported dNTP elevations of several-fold for each of the four dNTPs after treatment of E. coli with UV light, which also effectively induces the SOS system. This dNTP increase was correlated with an increase in expression of the nrdAB genes encoding the ribonucleotide reductase (RNR), the main enzyme involved in dNTP synthesis (70), and a corresponding increase in the RNR protein level. This induction of RNR in response to UV light irradiation is consistent with previous reports on the inducibility of the nrdAB operon by DNA damage (71–73). As the nrdAB operon is not part of the SOS regulon, its induction by DNA-damaging treatments presumably reflects a LexA-independent pathway of gene expression control (73). Our microarray measurements likewise did not reveal any alterations in the nrdAB operon expression in the recA730 strain. Further consistent with this, our microarray experiments with the recA730 strain did not reveal altered expression among the large group of genes involved in nucleotide metabolism, although there were two exceptions, a 2-fold increase in add (adenosine deaminase) and a 4-fold increase in gpt (guanine phosphoribosyltransferase) (for complete results see GEO repository, series record GSE62898). Finally, the measured dNTP pools in the recA730 ndk and recA730 dcd double mutants were essentially unchanged from those in the single ndk and dcd mutants, further corroborating the lack of control of the SOS system on the dNTP pools.
The SOS response is an important system, which is regulated at a large number of different levels (6,43,46,74–75). These levels of regulation ensure the timely induction, progression and termination of the response. The current work suggests that the cellular (d)NTP concentrations are yet one more determining factor for the expression and control of this system.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
Acknowledgments
We thank Drs M. Frazier and M. Longley of the National Institute of Environmental Health Sciences for their careful reading of the manuscript for this paper. We also thank Dr Marek Skoneczny of the Institute of Biochemistry and Biophysics for his assistance with the microarray analysis.
FUNDING
The Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences [project number Z01 ES065086]; International PhD Projects Program of the Foundation for Polish Science [project number MPD/2009-3/2] "Studies of Nucleic Acids and Proteins - from Basic to Applied Research" and European Union Regional Development Fund. Funding for the open access charge: the National Institute of Environmental Health Sciences.
Conflict of interest statement. None declared.
REFERENCES
- 1.Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. In: Hanawalt P, Setlow R, editors. Molecular Mechanisms for Repair of DNA. Part A. Vol. 5. NY: Springer; 1975. pp. 355–367. [DOI] [PubMed] [Google Scholar]
- 2.Little J., Edmiston S., Pacelli L., Mount D. Cleavage of the Escherichia coli LexA protein by the RecA protease. Proc. Natl. Acad. Sci. U.S.A. 1980;77:3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janion C. Inducible SOS response system of DNA repair and mutagenesis in Escherichia coli. Int. J. Biol. Sci. 2008;4:338–344. doi: 10.7150/ijbs.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig N.L., Roberts J. Function of nucleoside triphosphate and polynucleotide in Escherichia coli RecA protein-directed cleavage of phage lambda repressor. J. Biol. Chem. 1981;256:8039–8044. [PubMed] [Google Scholar]
- 5.Mount D. A mutant of Escherichia coli showing constitutive expression of the lysogenic induction and error-prone DNA repair pathways. Proc. Natl. Acad. Sci. U.S.A. 1977;74:300–304. doi: 10.1073/pnas.74.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker G. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol. Rev. 1984;48:60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández De Henestrosa A.R., Ogi T., Aoyagi S., Chafin D., Hayes J.J., Ohmori H., Woodgate R. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol. Microbiol. 2000;35:1560–1572. doi: 10.1046/j.1365-2958.2000.01826.x. [DOI] [PubMed] [Google Scholar]
- 8.Lewis L.K., Harlow G.R., Gregg-Jolly L.A., Mount D.W. Identification of high affinity binding sites for LexA which define new DNA damage-inducible genes in Escherichia coli. J. Mol. Biol. 1994;241:507–523. doi: 10.1006/jmbi.1994.1528. [DOI] [PubMed] [Google Scholar]
- 9.Napolitano R., Janel-Bintz R., Wagner J., Fuchs R.P. All three SOS-inducible DNA polymerases (Pol II, Pol IV and Pol V) are involved in induced mutagenesis. EMBO J. 2000;19:6259–6265. doi: 10.1093/emboj/19.22.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuban W., Banach-Orlowska M., Schaaper R.M., Jonczyk P., Fijalkowska I.J. Role of DNA polymerase IV in Escherichia coli SOS mutator activity. J. Bacteriol. 2006;188:7977–7980. doi: 10.1128/JB.01088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang M., Pham P., Shen X., Taylor J.S., O'Donnell M., Woodgate R., Goodman M.F. Roles of E. coli DNA polymerases IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature. 2000;404:1014–1018. doi: 10.1038/35010020. [DOI] [PubMed] [Google Scholar]
- 12.Tang M., Shen X., Frank E.G., O'Donnell M., Woodgate R., Goodman M.F. UmuD’(2)C is an error-prone DNA polymerase, Escherichia coli pol V. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8919–8924. doi: 10.1073/pnas.96.16.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlacher K., Leslie K., Wyman C., Woodgate R., Cox M.M., Goodman M.F. DNA polymerase V and RecA protein, a minimal mutasome. Mol. Cell. 2005;17:561–572. doi: 10.1016/j.molcel.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Q., Karata K., Woodgate R., Cox M.M., Goodman M.F. The active form of DNA polymerase V is UmuD’(2)C-RecA-ATP. Nature. 2009;460:359–363. doi: 10.1038/nature08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phizicky E.M., Roberts J.W. Induction of SOS functions: regulation of proteolytic activity of E. coli RecA protein by interaction with DNA and nucleoside triphosphate. Cell. 1981;25:259–267. doi: 10.1016/0092-8674(81)90251-8. [DOI] [PubMed] [Google Scholar]
- 16.Weinstock G.M., McEntee K., Lehman I. Hydrolysis of nucleoside triphosphates catalyzed by the RecA protein of Escherichia coli. J. Biol. Chem. 1981;256:8829–8834. [PubMed] [Google Scholar]
- 17.Weinstock G.M., McEntee K. RecA protein-dependent proteolysis of bacteriophage lambda repressor Characterization of the reaction and stimulation by DNA-binding proteins. J. Biol. Chem. 1981;256:10883–10888. [PubMed] [Google Scholar]
- 18.Menetski J., Varghese A., Kowalczykowski S.C. Properties of the high-affinity single-stranded DNA binding state of the Escherichia coli RecA protein. Biochemistry. 1988;27:1205–1212. doi: 10.1021/bi00404a021. [DOI] [PubMed] [Google Scholar]
- 19.Kirby E., Jacob F., Goldthwait D. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 1967;58:1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llagostera M., Guerrero R., Villaverde A, Barbé J. Effect of adenine, cytidine and guanosine on the expression of the SOS system in Escherichia coli. J. Gen. Microbiol. 1985;131:113–118. doi: 10.1099/00221287-131-1-113. [DOI] [PubMed] [Google Scholar]
- 21.Tessman E., Peterson P. Plaque color method for rapid isolation of novel recA mutants of Escherichia coli K-12: new classes of protease-constitutive recA mutants. J. Bacteriol. 1985;163:677–687. doi: 10.1128/jb.163.2.677-687.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahluwalia D., Bienstock R.J., Schaaper R.M. Novel mutator mutants of E. coli NrdAB ribonucleotide reductase: insight into allosteric regulation and control of mutation rates. DNA Repair (Amst). 2012;11:480–487. doi: 10.1016/j.dnarep.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaaper R.M., Mathews C.K. Mutational consequences of dNTP pool imbalances in E. coli. DNA Repair (Amst). 2013;12:73–79. doi: 10.1016/j.dnarep.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gon S., Napolitano R., Rocha W., Coulon S., Fuchs R.P. Increase in dNTP pool size during the DNA damage response plays a key role in spontaneous and induced-mutagenesis in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2011;108:19311–19316. doi: 10.1073/pnas.1113664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ennis D.G., Levine A.S., Koch W.H., Woodgate R. Analysis of recA mutants with altered SOS functions. Mutat. Res. 1995;336:39–48. doi: 10.1016/0921-8777(94)00045-8. [DOI] [PubMed] [Google Scholar]
- 26.Witkin E.M., McCall J.O., Volkert M.R., Wermundsen I.E. Constitutive expression of SOS functions and modulation of mutagenesis resulting from resolution of genetic instability at or near the recA locus of Escherichia coli. Mol. Gen. Genet. 1982;185:43–50. doi: 10.1007/BF00333788. [DOI] [PubMed] [Google Scholar]
- 27.Fijalkowska I.J., Dunn R.L., Schaaper R.M. Genetic requirements and mutational specificity of the Escherichia coli SOS mutator activity. J. Bacteriol. 1997;179:7435–7445. doi: 10.1128/jb.179.23.7435-7445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Q., Zhang X., Almaula N., Mathews C.K., Inouye M. The gene for nucleoside diphosphate kinase functions as a mutator gene in Escherichia coli. J. Mol. Biol. 1995;254:337–341. doi: 10.1006/jmbi.1995.0620. [DOI] [PubMed] [Google Scholar]
- 29.Miller J., Funchain P., Clendenin W., Huang T., Nguyen A. Escherichia coli strains (ndk) lacking nucleoside diphosphate kinase are powerful mutators for base substitutions and frameshifts in mismatch-repair-deficient strains. Genetics. 2002;13:5–13. doi: 10.1093/genetics/162.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuhard J., Thomassen E. Altered deoxyribonucleotide pools in P2 eductants of Escherichia coli K-12 due to deletion of the dcd gene. J. Bacteriol. 1976;126:999–1001. doi: 10.1128/jb.126.2.999-1001.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gawel D., Fijalkowska I.J., Jonczyk P., Schaaper R.M. Effect of dNTP pool alterations on fidelity of leading and lagging strand DNA replication in E. coli. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2014;759:22–28. doi: 10.1016/j.mrfmmm.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maliszewska-Tkaczyk M., Jonczyk P., Bialoskorska M., Schaaper R.M., Fijalkowska I.J. SOS mutator activity: unequal mutagenesis on leading and lagging strands. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12678–12683. doi: 10.1073/pnas.220424697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K.A., Tomita M., Wanner B.L., Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006;2 doi: 10.1038/msb4100050. 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oda Y., Nakamura S., Oki I., Kato T., Shinagawa H. Evaluation of the new system (umu-test) for the detection of environmental mutagens and carcinogens. Mutat. Res. 1985;147:219–229. doi: 10.1016/0165-1161(85)90062-7. [DOI] [PubMed] [Google Scholar]
- 35.Diederich L., Rasmussen L.J., Messer W. New cloning vectors for integration in the lambda attachment site attB of the Escherichia coli chromosome. Plasmid. 1992;28:14–24. doi: 10.1016/0147-619x(92)90032-6. [DOI] [PubMed] [Google Scholar]
- 36.Fijalkowska I.J., Jonczyk P., Tkaczyk M.M., Bialoskorska M., Schaaper R.M. Unequal fidelity of leading strand and lagging strand DNA replication on the Escherichia coli chromosome. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10020–10025. doi: 10.1073/pnas.95.17.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller J.H. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 38.Diaz M., Ray M., Wheeler L.J., Verkoczy L.K., Mathews C.K. Mutagenesis by AID, a molecule critical to immunoglobulin hypermutation, is not caused by an alteration of the precursor nucleotide pool. Mol. Immunol. 2003;40:261–268. doi: 10.1016/s0161-5890(03)00113-5. [DOI] [PubMed] [Google Scholar]
- 39.Cupples C.G., Miller J.H. A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc. Natl. Acad. Sci. U.S.A. 1989;86:5345–5349. doi: 10.1073/pnas.86.14.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L., Weiss B. dcd (dCTP deaminase) gene of Escherichia coli: mapping, cloning, sequencing, and identification as a locus of suppressors of lethal dut (dUTPase) mutations. J. Bacteriol. 1992;174:5647–5653. doi: 10.1128/jb.174.17.5647-5653.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garibyan L., Huang T., Kim M., Wolff E., Nguyen A., Nguyen T., Diep A., Hu K., Iverson A., Yang H., et al. Use of the rpoB gene to determine the specificity of base substitution mutations on the Escherichia coli chromosome. DNA Repair (Amst). 2003;2:593–608. doi: 10.1016/s1568-7864(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 42.Ollivierre J.N., Sikora J.L., Beuning P.J. Dimer exchange and cleavage specificity of the DNA damage response protein UmuD. Biochim. Biophys. Acta—Proteins Proteomics. 2013;1834:611–620. doi: 10.1016/j.bbapap.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez M., Woodgate R. The ‘tale’ of umuD and its role in SOS mutagenesis. BioEssays. 2002;24:141–148. doi: 10.1002/bies.10040. [DOI] [PubMed] [Google Scholar]
- 44.Konola J., Guzzo A., Gow J.B., Walker G.C., Knight K.L. Differential cleavage of LexA and UmuD mediated by recA Pro67 mutants: implications for common LexA and UmuD binding sites on RecA. J. Mol. Biol. 1998;276:405–415. doi: 10.1006/jmbi.1997.1531. [DOI] [PubMed] [Google Scholar]
- 45.Mustard J.A., Little J.W. Analysis of Escherichia coli RecA interactions with LexA, λ CI, and UmuD by site-directed mutagenesis of recA. J. Bacteriol. 2000;182:1659–1670. doi: 10.1128/jb.182.6.1659-1670.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox M.M. Regulation of bacterial RecA protein function. Crit. Rev. Biochem. Mol. Biol. 2007;42:41–63. doi: 10.1080/10409230701260258. [DOI] [PubMed] [Google Scholar]
- 47.Chen Z., Yang H., Pavletich N.P. Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures. Nature. 2008;453:489–484. doi: 10.1038/nature06971. [DOI] [PubMed] [Google Scholar]
- 48.Ahluwalia D., Schaaper R.M. Hypermutability and error catastrophe due to defects in ribonucleotide reductase. Proc. Natl. Acad. Sci. U.S.A. 2013;110:18596–18601. doi: 10.1073/pnas.1310849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fijalkowska I.J., Schaaper R.M., Jonczyk P. DNA replication fidelity in Escherichia coli: a multi-DNA polymerase affair. FEMS Microbiol. Rev. 2012;36:1105–1121. doi: 10.1111/j.1574-6976.2012.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell C.E. Structure and mechanism of Escherichia coli RecA ATPase. Mol. Microbiol. 2005;58:358–366. doi: 10.1111/j.1365-2958.2005.04876.x. [DOI] [PubMed] [Google Scholar]
- 51.Gruenig M.C., Renzette N., Long E., Chitteni-pattu S., Ross B., Cox M.M., Sandler S.J. RecA-mediated SOS induction requires an extended filament conformation but no ATP hydrolysis. Mol. Microbiol. 2009;69:1165–1179. doi: 10.1111/j.1365-2958.2008.06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lavery P.E., Kowalczykowski S.C. Biochemical basis of the constitutive repressor cleavage activity of RecA730 protein: a comparison to RecA441 and RecA803 proteins. J. Biol. Chem. 1992;267:20648–20658. [PubMed] [Google Scholar]
- 53.Wang T.C.V., Chang H.Y., Hung J.L. Cosuppression of recF, recR and recO mutations by mutant recA alleles in Escherichia coli cells. Mutat. Res. 1993;294:157–166. doi: 10.1016/0921-8777(93)90024-b. [DOI] [PubMed] [Google Scholar]
- 54.Menetski J.P., Kowalczykowski S.C. Enhancement of Escherichia coli RecA protein enzymatic function by dATP. Biochemistry. 1989;28:5871–5881. doi: 10.1021/bi00440a025. [DOI] [PubMed] [Google Scholar]
- 55.Robu M.E., Inman R.B., Cox M.M. RecA protein promotes the regression of stalled replication forks in vitro. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8211–8218. doi: 10.1073/pnas.131022698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beck C.F., Eisenhardt A.R., Neuhard J. Deoxycytidine triphosphate deaminase of Salmonella typhimurium. Purification and characterization. J. Biol. Chem. 1975;250:609–616. [PubMed] [Google Scholar]
- 57.Wang W.B., Sassanfar M., Tessman I., Roberts J.W., Tessman E.S. Activation of protease-constitutive RecA proteins of Escherichia coli by all of the common nucleoside triphosphates. J. Bacteriol. 1988;170:4816–4822. doi: 10.1128/jb.170.10.4816-4822.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ellouze C., Selmane T., Kim H.K., Tuite E., Nordén B., Mortensen K., Takahashi M. Difference between active and inactive nucleotide cofactors in the effect on the DNA binding and the helical structure of RecA filament. Dissociation of RecA–DNA complex by inactive nucleotides. Eur. J. Biochem. 1999;262:88–94. doi: 10.1046/j.1432-1327.1999.00357.x. [DOI] [PubMed] [Google Scholar]
- 59.Wigle T., Lee A., Singleton S. Conformationally selective binding of nucleotide analogues to Escherichia coli RecA: a ligand-based analysis of the RecA ATP binding site. Biochemistry. 2006;45:4502–4513. doi: 10.1021/bi052298h. [DOI] [PubMed] [Google Scholar]
- 60.Gawel D., Hamilton M.D., Schaaper R.M. A novel mutator of Escherichia coli carrying a defect in the dgt gene, encoding a dGTP triphosphohydrolase. J. Bacteriol. 2008;190:6931–6939. doi: 10.1128/JB.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knight K.L., Aoki H., Ujita L., McEntee K. Identification of the amino acid substitutions in two mutant forms of the RecA protein from Escherichia coli: RecA441 and RecA629. J. Biol. Chem. 1984;259:11279–11283. [PubMed] [Google Scholar]
- 62.Kawashima H., Horii T., Ogawa T., Ogawa H. Functional domains of Escherichia coli RecA protein deduced from the mutational sites in the gene. Mol. Gen. Genet. 1984;193:288–292. doi: 10.1007/BF00330682. [DOI] [PubMed] [Google Scholar]
- 63.Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol. Gen. Genet. 1972;119:139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- 64.Long J.E., Massoni S.C., Sandler S.J. RecA4142 causes SOS constitutive expression by loading onto reversed replication forks in Escherichia coli K-12. J. Bacteriol. 2010;192:2575–2582. doi: 10.1128/JB.01623-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Massoni S.C., Leeson M.C., Long J.E., Gemme K., Mui A., Sandler S.J. Factors limiting SOS expression in log-phase cells of Escherichia coli. J. Bacteriol. 2012;194:5325–5333. doi: 10.1128/JB.00674-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Long J.E., Renzette N., Centore R.C., Sandler S.J. Differential requirements of two recA mutants for constitutive SOS expression in Escherichia coli K-12. PLoS One. 2008;3:e4100. doi: 10.1371/journal.pone.0004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Centore R.C., Lestini R., Sandler S.J. XthA (Exonuclease III) regulates loading of RecA onto DNA substrates in log phase Escherichia coli cells. Mol. Microbiol. 2008;67:88–101. doi: 10.1111/j.1365-2958.2007.06026.x. [DOI] [PubMed] [Google Scholar]
- 68.Veaute X., Delmas S., Selva M., Jeusset J., Le Cam E., Matic I., Fabre F., Petit M.-A. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli. EMBO J. 2005;24:180–189. doi: 10.1038/sj.emboj.7600485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centore R.C., Leeson M.C., Sandler S.J. UvrD303, a hyperhelicase mutant that antagonizes RecA-dependent SOS expression by a mechanism that depends on its C-terminus. J. Bacteriol. 2009;191:1429–1438. doi: 10.1128/JB.01415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reichard P. Ribonucleotide reductases: substrate specificity by allostery. Biochem. Biophys. Res. Commun. 2010;396:19–23. doi: 10.1016/j.bbrc.2010.02.108. [DOI] [PubMed] [Google Scholar]
- 71.Filpula D., Fuchs J.A. Regulation of ribonucleoside diphosphate reductase synthesis in Escherichia coli: increased enzyme synthesis as a result of inhibition of deoxyribonucleic acid synthesis. J. Bacteriol. 1977;130:107–113. doi: 10.1128/jb.130.1.107-113.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibert I., Calero S., Barbé J. Measurement of in vivo expression of nrdA and nrdB genes of Escherichia coli by using lacZ gene fusions. Mol. Gen. Genet. 1990;220:400–408. doi: 10.1007/BF00391745. [DOI] [PubMed] [Google Scholar]
- 73.Courcelle J., Khodursky A., Peter B., Brown P.O., Hanawalt P.C. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics. 2001;158:41–64. doi: 10.1093/genetics/158.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel M., Jiang Q., Woodgate R., Cox M.M., Goodman M.F. A new model for SOS-induced mutagenesis: how RecA protein activates DNA polymerase V. Crit. Rev. Biochem. Mol. Biol. 2010;45:171–184. doi: 10.3109/10409238.2010.480968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goodman M.F. The discovery of error-prone DNA Polymerase V and its unique regulation by RecA and ATP. J. Biol. Chem. 2014;289:26772–26782. doi: 10.1074/jbc.X114.607374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


