Abstract
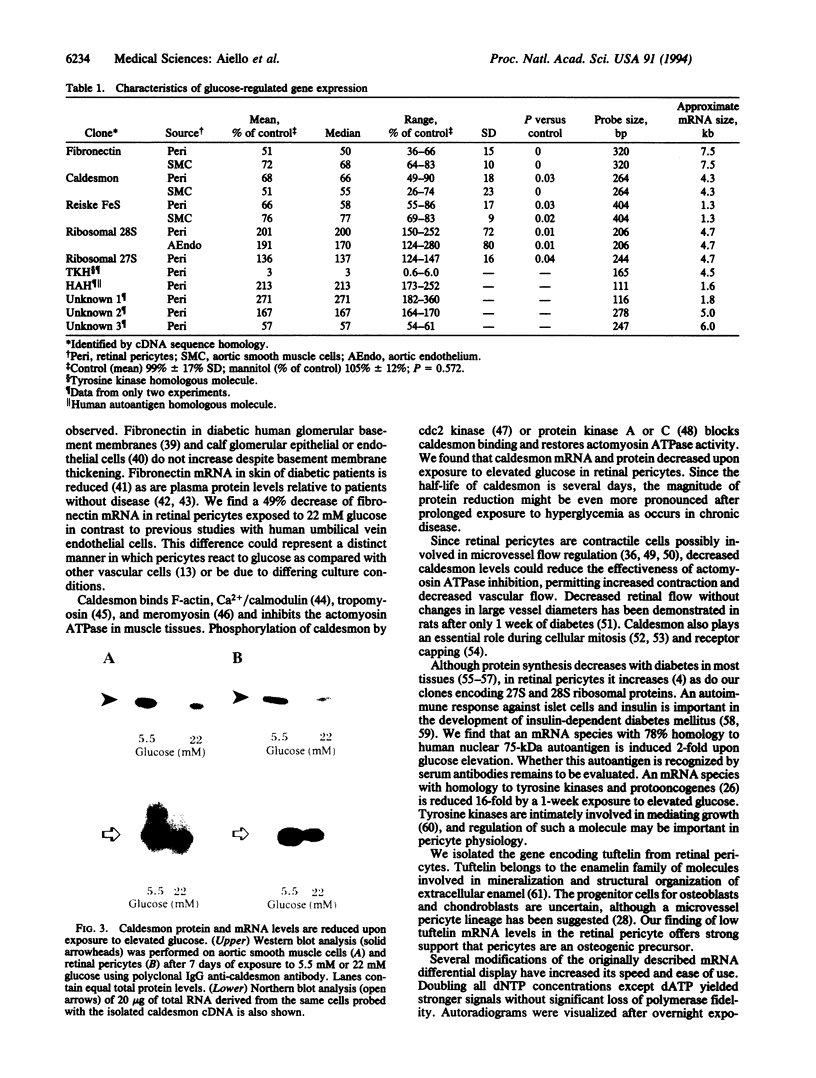
Loss of capillary pericytes, a characteristic finding in diabetic retinopathy, is strongly associated with hyperglycemia. The pathologic aberrations associated with diabetic retinopathy are localized primarily in the retinal capillaries and are only poorly reversed by subsequent euglycemic control. Since hyperglycemia significantly inhibits pericyte growth in culture, we investigated the regulation of gene expression in retinal pericytes exposed to physiologic (5.5mM) and pathologic (20 mM) glucose concentrations. By utilizing modifications of the mRNA differential display technique, over 14,000 mRNA species were screened, and 35 candidate clones were obtained. Partial DNA sequence demonstrated that 25 of these were distinct genes, including 7 known, 16 previously unreported, and 2 sequences with known homologues. Northern blot analysis demonstrated altered gene expression in 10 (40%), undetectable signals in 12 (48%), and nonregulation in 3 (12%). Genes with glucose-regulated expression included those encoding fibronectin (51% +/- 15%, P = 0.003; mean percentage of control +/- SD), caldesmon (68% +/- 18%; P = 0.026), two ribosomal proteins (201% +/- 72%, P = 0.011; 136% +/- 16%, P = 0.036), Rieske FeS reductase (66% +/- 17%; P = 0.029), three previously unreported sequences (57%, 167%, 271%), and molecules homologous to autoantigens (213%) and tyrosine kinases (down 16- to 33-fold). Caldesmon protein concentrations in pericytes and smooth muscle cells demonstrated decreases by Western blot analysis concordant with mRNA levels. These studies identify genes whose expression is significantly altered after 7 days of exposure to elevated glucose levels and provide new targets for understanding the adverse effects of hyperglycemia on vascular cells. In addition, this study provides strong support for the use of differential mRNA display as a method to rapidly isolate differentially expressed genes in metabolic systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderuccio F., Chan E. K., Tan E. M. Molecular characterization of an autoantigen of PM-Scl in the polymyositis/scleroderma overlap syndrome: a unique and complete human cDNA encoding an apparent 75-kD acidic protein of the nucleolar complex. J Exp Med. 1991 Apr 1;173(4):941–952. doi: 10.1084/jem.173.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli-Orlidge A., Saunders K. B., Smith S. R., D'Amore P. A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrieux A., Neeley W. E., Dillmann W. H. Diabetes-induced alterations in the translational activity of specific messenger ribonucleic acids isolated from rat hearts. Circ Res. 1985 Aug;57(2):296–303. doi: 10.1161/01.res.57.2.296. [DOI] [PubMed] [Google Scholar]
- Bond J. F., Farmer S. R. Regulation of tubulin and actin mRNA production in rat brain: expression of a new beta-tubulin mRNA with development. Mol Cell Biol. 1983 Aug;3(8):1333–1342. doi: 10.1128/mcb.3.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryan J., Imai M., Lee R., Moore P., Cook R. G., Lin W. G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989 Aug 15;264(23):13873–13879. [PubMed] [Google Scholar]
- Bursell S. E., Clermont A. C., Shiba T., King G. L. Evaluating retinal circulation using video fluorescein angiography in control and diabetic rats. Curr Eye Res. 1992 Apr;11(4):287–295. doi: 10.3109/02713689209001782. [DOI] [PubMed] [Google Scholar]
- Cagliero E., Roth T., Roy S., Lorenzi M. Characteristics and mechanisms of high-glucose-induced overexpression of basement membrane components in cultured human endothelial cells. Diabetes. 1991 Jan;40(1):102–110. doi: 10.2337/diab.40.1.102. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Danne T., Spiro M. J., Spiro R. G. Effect of high glucose on type IV collagen production by cultured glomerular epithelial, endothelial, and mesangial cells. Diabetes. 1993 Jan;42(1):170–177. doi: 10.2337/diab.42.1.170. [DOI] [PubMed] [Google Scholar]
- Deutsch D., Palmon A., Fisher L. W., Kolodny N., Termine J. D., Young M. F. Sequencing of bovine enamelin ("tuftelin") a novel acidic enamel protein. J Biol Chem. 1991 Aug 25;266(24):16021–16028. [PubMed] [Google Scholar]
- Diaz-Flores L., Gutierrez R., Lopez-Alonso A., Gonzalez R., Varela H. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res. 1992 Feb;(275):280–286. [PubMed] [Google Scholar]
- Duan R. D., Erlanson-Albertsson C. The effect of pretranslational regulation on synthesis of pancreatic colipase in streptozotocin-induced diabetes in rats. Pancreas. 1992;7(4):465–471. doi: 10.1097/00006676-199207000-00008. [DOI] [PubMed] [Google Scholar]
- Engerman R. L., Kern T. S. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987 Jul;36(7):808–812. doi: 10.2337/diab.36.7.808. [DOI] [PubMed] [Google Scholar]
- Fahmy L. H., Leader D. P. The effect of diabetes and insulin on the polyadenylic acid-containing RNA of rat skeletal muscle. Biochim Biophys Acta. 1980 Jul 29;608(2):344–357. doi: 10.1016/0005-2787(80)90180-x. [DOI] [PubMed] [Google Scholar]
- Heery D. M., Gannon F., Powell R. A simple method for subcloning DNA fragments from gel slices. Trends Genet. 1990 Jun;6(6):173–173. doi: 10.1016/0168-9525(90)90158-3. [DOI] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Characterization of caldesmon binding to myosin. J Biol Chem. 1990 Nov 15;265(32):19672–19678. [PMC free article] [PubMed] [Google Scholar]
- Hettasch J. M., Sellers J. R. Caldesmon phosphorylation in intact human platelets by cAMP-dependent protein kinase and protein kinase C. J Biol Chem. 1991 Jun 25;266(18):11876–11881. [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Jones K. W., Shapero M. H., Chevrette M., Fournier R. E. Subtractive hybridization cloning of a tissue-specific extinguisher: TSE1 encodes a regulatory subunit of protein kinase A. Cell. 1991 Sep 6;66(5):861–872. doi: 10.1016/0092-8674(91)90433-y. [DOI] [PubMed] [Google Scholar]
- KUWABARA T., COGAN D. G. Retinal vascular patterns. VI. Mural cells of the retinal capillaries. Arch Ophthalmol. 1963 Apr;69:492–502. doi: 10.1001/archopht.1963.00960040498013. [DOI] [PubMed] [Google Scholar]
- King G. L., Goodman A. D., Buzney S., Moses A., Kahn C. R. Receptors and growth-promoting effects of insulin and insulinlike growth factors on cells from bovine retinal capillaries and aorta. J Clin Invest. 1985 Mar;75(3):1028–1036. doi: 10.1172/JCI111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Klein B. E., Moss S. E., Davis M. D., DeMets D. L. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984 Apr;102(4):520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- Kolbe M., Kaufman J. L., Friedman J., Dinerstein C., Mackenzie J. W., Boyd C. D. Changes in steady-state levels of mRNAs coding for type IV collagen, laminin and fibronectin following capillary basement membrane thickening in human adult onset diabetes. Connect Tissue Res. 1990;25(1):77–85. doi: 10.3109/03008209009009814. [DOI] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Isolation and characterization of cDNA clones for human and bovine fibronectins. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3218–3222. doi: 10.1073/pnas.80.11.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labat-Robert J., Leutenegger M., Llopis G., Ricard Y., Derouette J. C. Plasma and tissue fibronectin in diabetes. Clin Physiol Biochem. 1984;2(1):39–48. [PubMed] [Google Scholar]
- Labat-Robert J., Robert L. Modifications of fibronectin in age-related diseases: diabetes and cancer. Arch Gerontol Geriatr. 1984 May;3(1):1–10. doi: 10.1016/0167-4943(84)90011-6. [DOI] [PubMed] [Google Scholar]
- Levis R., Penman S. 5'-terminal structures of poly(A)+ cytoplasmic messenger RNA and of poly(A)+ and poly(A)- heterogeneous nuclear RNA of cells of the dipteran Drosophila melanogaster. J Mol Biol. 1978 Apr 25;120(4):487–515. doi: 10.1016/0022-2836(78)90350-9. [DOI] [PubMed] [Google Scholar]
- Li W., Shen S., Khatami M., Rockey J. H. Stimulation of retinal capillary pericyte protein and collagen synthesis in culture by high-glucose concentration. Diabetes. 1984 Aug;33(8):785–789. doi: 10.2337/diab.33.8.785. [DOI] [PubMed] [Google Scholar]
- Liang P., Averboukh L., Keyomarsi K., Sager R., Pardee A. B. Differential display and cloning of messenger RNAs from human breast cancer versus mammary epithelial cells. Cancer Res. 1992 Dec 15;52(24):6966–6968. [PubMed] [Google Scholar]
- Liang P., Averboukh L., Pardee A. B. Distribution and cloning of eukaryotic mRNAs by means of differential display: refinements and optimization. Nucleic Acids Res. 1993 Jul 11;21(14):3269–3275. doi: 10.1093/nar/21.14.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Carpenter M., Smillie L. B., Wang J. H. Phosphorylation of caldesmon by p34cdc2 kinase. Identification of phosphorylation sites. J Biol Chem. 1991 Oct 25;266(30):19971–19975. [PubMed] [Google Scholar]
- Mak A. S., Watson M. H., Litwin C. M., Wang J. H. Phosphorylation of caldesmon by cdc2 kinase. J Biol Chem. 1991 Apr 15;266(11):6678–6681. [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak R. C., Berman A. B., George K. L., Eisenbarth G. S., King G. L. A monoclonal antibody (3G5)-defined ganglioside antigen is expressed on the cell surface of microvascular pericytes. J Exp Med. 1988 Mar 1;167(3):1003–1015. doi: 10.1084/jem.167.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerup J., Lernmark A. Autoimmunity in insulin-dependent diabetes mellitus. Am J Med. 1981 Jan;70(1):135–141. doi: 10.1016/0002-9343(81)90420-4. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Detection of caldesmon in muscle and non-muscle tissues of the chicken using polyclonal antibodies. Biochem Biophys Res Commun. 1985 Mar 15;127(2):533–539. doi: 10.1016/s0006-291x(85)80192-3. [DOI] [PubMed] [Google Scholar]
- Novy R. E., Lin J. L., Lin J. J. Characterization of cDNA clones encoding a human fibroblast caldesmon isoform and analysis of caldesmon expression in normal and transformed cells. J Biol Chem. 1991 Sep 5;266(25):16917–16924. [PubMed] [Google Scholar]
- Orlidge A., D'Amore P. A. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987 Sep;105(3):1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. T., Greene W. C., Reaven G. M. Effect of experimental diabetes mellitus on kidney ribosomal protein synthesis. Diabetes. 1971 Oct;20(10):649–654. doi: 10.2337/diab.20.10.649. [DOI] [PubMed] [Google Scholar]
- Roy S., Sala R., Cagliero E., Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A. 1990 Jan;87(1):404–408. doi: 10.1073/pnas.87.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. W., Warren R. W. Quantitation of microgram amounts of protein in SDS-mercaptoethanol-tris electrophoresis sample buffer. Anal Biochem. 1977 Dec;83(2):773–777. doi: 10.1016/0003-2697(77)90084-7. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura H., Spiro R. G. Studies on macromolecular components of human glomerular basement membrane and alterations in diabetes. Decreased levels of heparan sulfate proteoglycan and laminin. Diabetes. 1987 Mar;36(3):374–381. doi: 10.2337/diab.36.3.374. [DOI] [PubMed] [Google Scholar]
- Shirinsky V. P., Birukov K. G., Koteliansky V. E., Glukhova M. A., Spanidis E., Rogers J. D., Campbell J. H., Campbell G. R. Density-related expression of caldesmon and vinculin in cultured rabbit aortic smooth muscle cells. Exp Cell Res. 1991 Jun;194(2):186–189. doi: 10.1016/0014-4827(91)90352-u. [DOI] [PubMed] [Google Scholar]
- Siperstein M. D. Diabetic microangiopathy, genetics, environment, and treatment. Am J Med. 1988 Nov 28;85(5A):119–130. doi: 10.1016/0002-9343(88)90404-4. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Srikanta S., Ricker A. T., McCulloch D. K., Soeldner J. S., Eisenbarth G. S., Palmer J. P. Autoimmunity to insulin, beta cell dysfunction, and development of insulin-dependent diabetes mellitus. Diabetes. 1986 Feb;35(2):139–142. doi: 10.2337/diab.35.2.139. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Cooper G. M. ret transforming gene encodes a fusion protein homologous to tyrosine kinases. Mol Cell Biol. 1987 Apr;7(4):1378–1385. doi: 10.1128/mcb.7.4.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. S., Dahl H. H., Mercer J. F., Green A. K., Fisher M. J. The effect of streptozotocin-induced diabetes on phenylalanine hydroxylase expression in rat liver. Biochem J. 1989 Nov 15;264(1):185–190. doi: 10.1042/bj2640185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G. Actin filaments in the acrosomal reaction of Limulus sperm. Motion generated by alterations in the packing of the filaments. J Cell Biol. 1975 Feb;64(2):289–310. doi: 10.1083/jcb.64.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton R. G., Kilo C., Williamson J. R. Pericyte-endothelial relationships in cardiac and skeletal muscle capillaries. Microvasc Res. 1979 Nov;18(3):325–335. doi: 10.1016/0026-2862(79)90041-4. [DOI] [PubMed] [Google Scholar]
- Usui S., Yu L., Yu C. A. Cloning and sequencing of a cDNA encoding the Rieske iron-sulfur protein of bovine heart mitochondrial ubiquinol-cytochrome c reductase. Biochem Biophys Res Commun. 1990 Mar 16;167(2):575–579. doi: 10.1016/0006-291x(90)92063-6. [DOI] [PubMed] [Google Scholar]
- Walker G., Kerrick W. G., Bourguignon L. Y. The role of caldesmon in the regulation of receptor capping in mouse T-lymphoma cell. J Biol Chem. 1989 Jan 5;264(1):496–500. [PubMed] [Google Scholar]
- Watson M. H., Kuhn A. E., Novy R. E., Lin J. J., Mak A. S. Caldesmon-binding sites on tropomyosin. J Biol Chem. 1990 Nov 5;265(31):18860–18866. [PubMed] [Google Scholar]
- Yamakita Y., Yamashiro S., Matsumura F. Characterization of mitotically phosphorylated caldesmon. J Biol Chem. 1992 Jun 15;267(17):12022–12029. [PubMed] [Google Scholar]