Abstract
Background
Impulse control disorder (ICD) and behaviours (ICB) represent a group of behavioural disorders that have become increasingly recognised in Parkinson’s disease (PD) patients who previously used dopaminergic medications, particularly dopamine agonists and levodopa. It has been suggested that these medications can lead to the development of ICB through the abnormal modulation of dopaminergic transmission and signalling in the mesocorticolimbic dopaminergic system. Several studies have reported an association between polymorphisms in the dopamine receptor (DRD) and N-methyl-D-aspartate 2B (GRIN2B) genes with the development of ICB in PD (PD-ICB) patients. Thus, this study aimed to investigate the association of selected polymorphisms within the DRD and GRIN2B genes with the development of ICB among PD patients using high resolution melt (HRM) analysis.
Method
We used high resolution melt (HRM) analysis to genotype 11 polymorphisms in 5 DRD genes [DRD1 (rs4532, rs4867798 and rs265981), DRD2 (ANKK1 rs1800497, rs104894220 and rs144999500), DRD3 (rs3732783 and rs6280), DRD4 (rs1800443), and DRD5 (rs144132215)] and 1 polymorphism in GRIN2B (rs7301328) in PD patients with (cases, n = 52) and without (controls, n = 39) ICB. Cases were obtained from two tertiary movement disorder centres [UKMMC (n = 9) and UMMC (n = 43)]. At both centres, the diagnosis of ICB was made using the QUIP questionnaire. Controls were recruited from PD patients who attended UKMMC and were found to be negative for ICB using the QUIP questionnaire.
Results
The HRM analysis showed that 7 of 11 polymorphisms [DRD1 (rs4532, rs4867798, and rs265981), DRD2 (ANKK1 rs1800497), DRD3 (rs3732783 and rs6280), and GRIN2B (rs7301328)] exhibited a clear distinction between wild-type and variant alleles. Variants of DRD2/ANKK1 rs1800497 (OR = 3.77; 95% CI, 1.38-10.30; p = 0.0044), DRD1 rs4867798 (OR = 24.53; 95% CI, 1.68-357.28; p = 0.0054), DRD1 rs4532 (OR = 21.33; 95% CI, 1.97-230.64; p = 0.0024), and GRIN2B rs7301328 (OR = 25.07; 95% CI, 1.30-483.41; p = 0.0097) were found to be associated with an increased risk of developing ICB among PD patients.
Conclusion
Our findings suggest that polymorphisms in dopamine [DRD1 (rs4532 and rs4867798) and DRD2/ANKK1 rs1800497] and glutamate (GRIN2B rs7301328) receptor genes confer increased risk of ICB development among PD patients.
Electronic supplementary material
The online version of this article (doi:10.1186/s12883-015-0316-2) contains supplementary material, which is available to authorized users.
Keywords: High resolution melt analysis, DNA polymorphism, N-methyl-D-aspartate 2B, Dopamine receptor
Background
Impulse control behaviours/disorders (ICB/ICD) are defined as complex behavioural disorders characterized by the failure to resist the temptation to perform an act that is harmful to the individual or to others [1]. ICB/ICD has been increasingly recognized in PD patients who develop behaviour disorders, such as pathological gambling (5%), hypersexuality (3.6%), compulsive shopping (5.7%), and compulsive eating (4.3%). More than a quarter of PD patients with ICB (PD-ICB) develop two or more behavioural addictions [2]. Patients can also develop other sub-syndromic ICB or ICD, such as compulsive medication use, punding, or hobbyism [3,4]. The primary risk factor for the development of PD-ICB is the use of dopaminergic medications. While some studies have shown that the development of ICB might be dose-dependent, others have suggested that genetic predisposition coupled with inherent ‘subconscious’ personality traits are the root cause of this problem [3,5].
A possible neurobiological explanation for the association of PD with the development of ICB centres on the dopaminergic system, which is involved in reward mechanisms, impulsiveness, and decision-making processes. Dopamine receptors (DRD) play an important role in activating or inhibiting downstream signalling in the dopaminergic pathway. DRD1 and DRD2 are expressed abundantly in the ventral striatum [6] and might mediate the motor effects of dopamine replacement therapies. DRD3 is expressed in the limbic area of the brain, and has been associated with both behavioural addictions [7] and substance use disorders [5]. Both DRD4 and DRD5 have been linked to attention deficit hyperactivity disorder (ADHD) [8].
Polymorphisms in DRD1, especially rs4532, have been studied widely to detect genetic associations with neuropsychiatric disorders [9]. The DRD1 rs4532 polymorphism has been associated with several mental illnesses, including nicotine addiction [10], bipolar disorder [11], and ADHD [12]. Additionally, the T➔C nucleotide substitution located 800 bp upstream of exon 1 (rs265981) in DRD1 has been reported to be associated with ADHD [12-14]. Genetic polymorphisms in DRD3 rs6280 have been reported to be associated with the development of ICB in Korean PD patients [15]. The DRD2/ANKK1 rs1800497 polymorphism is located close to the ankryin repeat and kinase domain containing-1 (ANKK1) gene. The DRD2/ANKK1 rs1800497 variant causes a glutamic acid to lysine substitution in serine/threonine kinase that might affect substrate binding to the D2 receptor [16]. Moreover, the DRD3 rs6280 variant causes a glycine to serine substitution at codon 9 [17], which results in low binding affinity to dopamine [18]. Although DRD4 has not been linked to the development of PD-ICB, this receptor can also be activated by dopamine and has been linked to neurological and psychiatric conditions, such as schizophrenia, bipolar disorder, and addictive behaviour [19]. Additional variants that lead to non-synonymous amino acid substitutions in DRD1–5, such as DRD1 rs4867798, DRD2 rs104894220 and rs144999500, DRD3 rs3732783, DRD4 rs1800443, and DRD5 rs144132215, have not been studied among ICB subjects and, therefore, were included in this study.
In addition to DRDs, N-methyl-D-aspartate (NMDA) receptors are ionotropic glutamate receptors that are involved in glutamate-mediated neurotransmission in the brain [20]. The NMDA receptor plays a role in PD development because the altered expression of both NMDA receptor subunits and factors that facilitate NMDA receptor activation by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can modulate glutamate release, which leads to the death of nigrostriatal dopamine neurons [21,22]. The NMDA receptor consists of NR1 (GRIN1) and NR2 (GRIN2) subunits. For the NR2 subunit, there are four subtypes—GRIN2A, GRIN2B, GRIN2C, and GRIN2D [23]. GRIN2B acts as the agonist binding site for glutamate and the predominant excitatory neurotransmitter receptor in the mammalian brain [24]. Variants in GRIN2B (c.366C > G, c.2664C > T and c.-200 T > C) are commonly found in Asian populations [25,26]. Other polymorphisms within GRIN2B in the Ashkenazi Jewish population has been reported to be associated with bipolar I disorder, which indicates the involvement of the glutamate signalling pathway [27]. Moreover, the GRIN2B rs7301328 variant has been reported to be associated with the development of ICD in Korean PD patients [15].
Thus, in this study we investigated the associations of selected polymorphisms in DRDs and GRIN2B with the development of ICB among PD patients using high resolution melt (HRM) analysis. We selected 11 polymorphisms within DRD1 (rs4532, rs4867798, and rs265981), DRD2 (ANKK1 rs1800497, rs104894220, and rs144999500), DRD3 (rs3732783 and rs6280), DRD4 (rs1800443), DRD5 (rs144132215), and GRIN2B (rs7301328) for screening. The association of these polymorphisms with ICB development among a cohort of Malaysian PD subjects was then evaluated.
Methods
Subjects and clinical measures
This study involved samples from 91 PD patients obtained from two larger clinical studies on ICB among PD patients, in two tertiary centres in Kuala Lumpur [Universiti Kebangsaan Malaysia Medical Centre (UKMMC) and Universiti Malaya Medical Centre (UMMC)]. In both centres, patients with idiopathic PD (H&Y Stages I-IV), diagnosed by two movement disorder neurologists (NMI and SYL), and on dopaminergic medications were screened for ICB using the QUIP questionnaire. The diagnosis of ICB was based on previously established criteria published elsewhere [28]. The QUIP questionnaire is a validated questionnaire for the detection of ICB in PD. It consists of 3 sections: Section 1 assesses ICDs (sexual, gambling, eating, and buying behaviour), Section 2 assesses other compulsive behaviours, such as compulsive buying, hypersexuality, punding, hobbyism, and walkabout, and Section 3 assesses compulsive medication use [28]. Cases and controls were determined based on QUIP positivity. Fifty-two cases (QUIP positive or PD-ICB) and 39 controls (QUIP negative) were selected randomly for this study. This study was conducted in accordance with the Declaration of Helsinki and was approved by Institutional Ethics Committee from both centres (UKM-DLP-2011-048 for UKMMC and MEC No: 745.81 for UMMC). Written informed consent for participation in the study was obtained from all recruited participants.
DNA samples
Genomic DNA from blood samples was extracted from buffy coat leukocytes using a QiaAmp DNA Blood Mini Kit (Qiagen, Limburg, The Netherlands) according to the manufacturer’s protocol. The concentration and purity (1.7–1.9) of DNA samples were measured using NanoVue™ Plus (GE Healthcare, Berkshire, UK), and the integrity of DNA was analysed using 0.8% agarose gel electrophoresis.
Assay design and polymerase chain reaction optimization
Ten primer sets were designed using Primer3Plus to detect 11 polymorphic loci in DRD1 (rs4532 and rs4867798), DRD2 (ANKK1 rs1800497, rs104894220, and rs144999500), DRD3 (rs3732783 and rs6280), DRD4 rs1800443, DRD5 rs144132215 and GRIN2B rs7301328, which were mined from the National Center for Biotechnology Information (NCBI) database. The primer set for DRD1 rs265981 was synthesized based on a previous study [29]. Secondary structure was predicted using DINAMelts software, which also was used to calculate the melting temperature [30]. All primers were optimized using gradient polymerase chain reaction (PCR) and real-time PCR prior to HRM analysis. Primer sequences and properties are detailed in Table 1.
Table 1.
Loci of selected DRD and GRIN2B SNPs and the corresponding primer pairs used for high resolution melt analysis
| SNP | Chromosome | Location | Allele changes | Amino acid changes | Forward primer (5′➔3′) | Reverse primer (5′➔3′) | Amplicon size (bp) |
|---|---|---|---|---|---|---|---|
| DRD1 rs4532 | chr 5 (q35.2) | 19681423 | C > T | Nil | GAACAGAGAAGTCCCTCTCCAC | CTGGAAATCTGACTGACCCCTA | 147 |
| DRD1 rs4867798 | chr 5 (q35.2) | 19679572 | T > C | Nil | GGGCTCTTCTTAAGTTGGCTTT | GGACACAGATAAATGCAAGGTG | 189 |
| DRD1 rs265981 | chr 5 (q35.2) | 174870940 | T > C | Nil | GCTCTCTCCCAAGGAAGCTC | GTGCGTTTGGGGAAAGGATC | 141 |
| DRD2/ANKK1 rs1800497 | chr 11 (q23.2) | 16833244 | C > T | Glu > Lys | CTCTAGGAAGGACATGATGCCC | GCAACACAGCCATCCTCAAAG | 128 |
| DRD2 rs104894220 | chr 11 (q23.2) | 16850073 | G > A | Val > Ile | CATGCCCATGCTGTACAATACG | GTACCTGCGTTATTGAGTCCGA | 126 |
| DRD2 rs144999500 | chr 11 (q23.2) | 16845792 | G > A | Pro > Leu | GAGCATCTGAGTGGCTTTCTTCTC | GAGAAGAATGGGCATGCCAAAG | 150 |
| DRD3 rs3732783 | chr 3 (q13.31) | 20385935 | T > C | Ala > Ala | AGTAGGAGAGGGCATAGTAGGC | CTGGGCTATGGCATCTCTGAG | 116 |
| DRD3 rs6280 | chr 3 (q13.31) | 20385961 | C > T | Gly > Ser | AGTAGGAGAGGGCATAGTAGGC | CTGGGCTATGGCATCTCTGAG | 116 |
| DRD4 rs1800443 | chr 11 (p15.5) | 579830 | T > G | Val > Gly | TACTGTGCGGCCTCAACGAC | GGGTAGGAAGAAGGAGCACAC | 104 |
| DRD5 rs144132215 | chr 4 (p16.1) | 966018 | G > T | Gly > Trp | GTCCATCCTCATCTCCTTCATTCC | CTGGAGTCACAGTTCTCTGCAT | 159 |
| GRIN2B rs7301328 | chr 12 (p13.1) | 6778901 | G > C | Pro > Pro | CTCCCTGCAGCCCCTTTTTA | CGCCCAGATCCTCGATTTCA | 109 |
High resolution melting-Polymerase chain reaction
The HRM analysis was carried out in 10 μl reaction volumes containing 30 ng genomic DNA, 0.7 μM primers, and 1× Type-It HRM-PCR master mix (HotStarTaq Plus DNA Polymerase, EvaGreen Dye, and an optimized concentration of Q-solution, dNTPs, and MgCl2; Qiagen). The primer pairs used for HRM analysis are summarised in Table 1. Reactions were run on a 5-plex HRM real-time PCR machine (Rotor-Gene™ 6000, Qiagen) consisting of 95°C for 5 min for initial denaturation, 45 cycles of 95°C for 10 sec and 60°C for 30 sec followed by a high resolution melting phase (65-90°C for 2 sec). Data were analysed using Rotor-Gene 6000 Series Software 1.7. To ensure accuracy, raw data were selected according to the following criteria.
-
i.
The Ct value and amplification rate of the samples based on comparative quantitative analysis must be less than 30 cycles and more than 1.4 cycles, respectively.
-
ii.
Samples must show a single peak in a derivative melt-curve analysis ranging from 75-85°C.
-
iii.
The confidence value for each genotype prediction must be greater than 90% when compared to the genotype based on reference samples and confirmed by DNA sequencing analysis.
Sequencing
A total of 17% of samples were selected for DNA sequencing analysis to confirm the HRM genotyping results. Samples selected from each variant cluster were purified using a Fragment DNA purification kit (Intron Biotechnology, South Korea) followed by Sanger sequencing analysis to confirm the nucleotide polymorphisms. Sequences were analysed and alignments were performed using DNA Baser v3.5.4 software [31]. A Phred score of 20 or greater was used to indicate high quality sequencing results.
Data and statistical analysis
SNPStats [32] was used to assess genotypic frequencies, Hardy–Weinberg equilibrium (HWE) and linkage disequilibrium (LD) among PD patients with or without ICB. The HWE test was performed to examine the genotypic distributions of polymorphisms in PD-ICB patients. The HWE deviation of allele and genotype frequencies was assessed by Fisher’s exact analysis. The logistic regression was used to estimate the odds ratio (OR) and 95% confidence interval (CI) for associations between each locus and the presence of PD-ICB. The analysis was used to estimate the regression coefficient and the association in the log odds of the subject with a polymorphism. The association with disease is modelled depending on the response variable. The variable response was defined as a binary response (categorical variable) and logistic regression was used to assess the proportion of variation in the response between the polymorphisms and other factors such as gender, age, ethnicity, dosage of medication and duration of PD. The association was based on co-dominant, dominant, recessive, overdominant, and log-additive models between each polymorphism in PD-ICB patients. The right model was selected based on the significant p value and the lowest Bayesian Information Criterion (BIC) score. Pair-wise LD statistics (D’ and r2) were used to determine the robustness of LD between polymorphisms. In all statistical analyses, comparisons were considered to be significant when p < 0.0167 after Bonferroni correction for multiple comparisons [33].
Results
Demographic analysis of subjects
A total of 91 PD patients with (QUIP positive or cases; n = 52) and without ICB (QUIP negative or controls; n = 39) were recruited from a total of 280 PD patients from two medical centres and were analysed. Comparison between the cases and controls in terms of age, sex and racial distribution is presented in Table 2. The mean disease duration for the cases and controls were 8.2 ± 0.7 and 5.8 ± 0.7 years, respectively. Most PD-ICB patients received a combination therapy consisting of levodopa and dopamine agonist (54%) followed by levodopa monotherapy (29%). In the control group, 39% of patients received levodopa alone whereas 36% of patient received a combination of levodopa and dopamine agonist. The other control (15%) and PD-ICB (13%) patients received dopamine agonist alone. The mean daily levadopa dose and dopamine agonist dose were higher in the cases than the controls (Table 2).
Table 2.
The demographic and clinical characteristics of ICB in PD patients
| Variables | Control (n = 39) | Case (n = 52) |
|---|---|---|
| Age | ||
| Mean ± SE | 63.46 ± 1.344 | 62.42 ± 1.087 |
| Median | 63.00 | 63.00 |
| Range | 42 - 85 | 42 - 77 |
| Duration of PD | ||
| Mean ± SE | 5.77 ± 0.745 | 8.17 ± 0.725 |
| Median | 4.00 | 8.00 |
| Range | 1.0 - 20.0 | 0.0 - 23.0 |
| Gender, n (%) | ||
| Male | 27 (69) | 38 (73) |
| Female | 12 (31) | 14 (27) |
| Ethnicity, n (%) | ||
| Malay | 13 (33) | 8 (15) |
| Chinese | 25 (64) | 34 (66) |
| Indian | 1 (3) | 8 (15) |
| Others | 0 (0) | 2 (4) |
| Drug Medication, n (%) | ||
| No Medication | 4 (10) | 2 (4) |
| Levadopa only | 15 (39) | 15 (29) |
| Dopamine agonist only | 6 (15) | 7 (13) |
| Levadopa + Dopamine agonist | 14 (36) | 28 (54) |
| Dosage of Levadopa | ||
| Mean ± SE | 172.79 ± 26.89 | 345.98 ± 41.53 |
| Median | 150.00 | 150.00 |
| Range | 0.00 - 675.00 | 0.00 - 675.00 |
| Dosage of DA | ||
| Mean ± SE | 1.05 ± 0.19 | 82.79 ± 12.16 |
| Median | 1.00 | 80.00 |
| Range | 0.00 - 5.00 | 0.00 - 360.00 |
| Diagnosis, n (%) | ||
| One repetitive behaviour | 0 (0) | 30 (57) |
| >1 repetitive behaviour | 1 (3) | 15 (29) |
| Compulsive medication | 1 (3) | 2 (4) |
| >1 repetitive behaviour + Compulsive medication | 0 (0) | 5 (10) |
| No repetitive behaviour + Compulsive medication | 37 (94) | 0 (0) |
High resolution melt analyses
In this study, 11 polymorphisms were analysed, but only 7 polymorphisms showed allelic variation [DRD1 (rs4532, rs4867798, rs265981), DRD2/ANKK1 rs1800497, DRD3 (rs3732783 and rs6280), and GRIN2B rs7301328] (Figure 1). The other 4 polymorphisms [DRD2 (rs104894220 and rs144999500), DRD4 rs1800443, and DRD5 rs144132215] screened were found to be monomorphic (Additional file 1: Figure S1). We have successfully established 11 HRM assays for the detection of polymorphisms in DRD1-5 and GRIN2B. The HRM assays were very efficient, sensitive, and specific in identifying nucleotide transitions (C > T or T > C) and transversions (G > C) in our samples. Approximately 17% of the total numbers of HRM reactions were sequenced and the results were 100% in agreement with the HRM profiles. The differential melting curves for the 7 corresponding polymorphisms (C > T, T > C, or G > C) clearly distinguished the wild-type from the variant genotypes. The amplicons derived from the DRD1 rs4532, DRD2/ANKK1 rs1800497, and DRD3 rs6280 mutant alleles showed a single nucleotide change from C to T. Polymorphisms in DRD1 rs4867798, DRD1 rs265981, and DRD3 rs3732783 showed a single nucleotide change from T to C. Analysis of the amplicons derived from GRIN2B rs7301328 showed a single nucleotide change from G to C. All analysed SNPs were deposited in dbSNP build B145 (http://www.ncbi.nlm.nih.gov/SNP/) with the following accession numbers; ss1713988434 (rs265981), ss1713988435 (rs4532), ss1713988436 (rs4867798), ss1713988437 (rs104894220), ss1713988438 (rs144999500), ss1713988439 (rs1800497), ss1713988440 (rs3732783), ss1713988441 (rs6280), ss1713988442 (rs1800443), ss1713988443 (rs144132215) and ss1713988444 (rs7301328).
Figure 1.
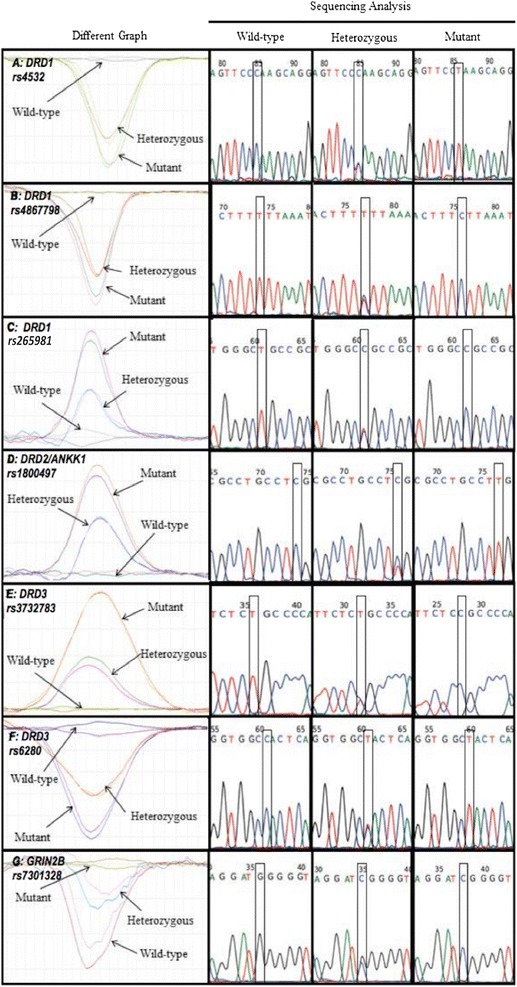
The HRM profiles and sequence analyses of (a) DRD1 rs4532, (b) DRD1 rs4867798, (c) DRD1 rs265981, (d) DRD2/ANKK1 rs1800497, (e) DRD3 rs3732783, (f) DRD3 rs6280, and (g) GRIN2B rs7301328, which are presented along with their respective sequencing results.
Genotype distribution in Parkinson’s disease–impulse control disorder patients
No significant deviation was observed in the distribution of genotype and allele frequencies for all polymorphisms (except for DRD3 rs3732783) based on HWE analysis between the case and control groups (Table 3). The frequency of the C allele for DRD3 rs3732783 was significantly higher (p < 0.01) in the PD population. Moreover, pair-wise LD measurements between polymorphisms in DRD1 based on the Dʹ statistic showed no difference in LD among the polymorphisms (rs4532, rs4867798, and rs265981). By contrast, as expected, DRD3 showed strong LD (D′ > 0.99) between polymorphisms (rs3732783 and rs6280) that could be observed using multiple-SNP analysis (Table 4). In multiple haplotype analysis of DRD1 and DRD3, we detected low frequencies for all alleles (Additional file 2: Table S1).
Table 3.
The distribution of polymorphisms in the case and control groups
| SNP | Allele frequency | Genotype frequency | p -value | ||||
|---|---|---|---|---|---|---|---|
| Wild type | Heterozygote | Mutant | |||||
| DRD1 rs4532 | Control | C (0.06) | T (0.94) | CC (0.02) | TC (0.07) | TT (0.90) | 0.26 |
| Case | C (0.24) | T (0.76) | CC (0.06) | TC (0.37) | TT (0.57) | ||
| DRD1 rs4867798 | Control | T (0.68) | C (0.32) | TT (0.42) | TC (0.52) | CC (0.06) | 0.35 |
| Case | T (0.54) | C (0.46) | TT (0.36) | TC (0.36) | CC (0.28) | ||
| DRD1 rs265981 | Control | T (0.18) | C (0.82) | TT (0.03) | TC (0.32) | CC (0.66) | 0.51 |
| Case | T (0.21) | C (0.79) | TT (0.02) | TC (0.36) | CC (0.66) | ||
| DRD2/ANKK1 rs1800497 | Control | C (0.48) | T (0.52) | TT (0.30) | TC (0.44) | CC (0.26) | 0.27 |
| Case | C (0.71) | T (0.29) | TT (0.09) | TC (0.40) | CC (0.51) | ||
| DRD3 rs3732783 | Control | T (1.00) | C (0.00) | TT (1.00) | TC (0.00) | CC (0.00) | 0.0014** |
| Case | T (0.95) | C (0.05) | TT (0.94) | TC (0.02) | CC (0.04) | ||
| DRD3 rs6280 | Control | C (0.24) | T (0.76) | CC (0.05) | TC (0.50) | TT (0.57) | 0.61 |
| Case | C (0.33) | T (0.76) | CC (0.08) | TC (0.38) | TT (0.42) | ||
| GRIN2B rs7301328 | Control | G (0.50) | C (0.50) | GG (0.57) | CG (0.38) | CC (0.05) | 0.47 |
| Case | G (0.28) | C (0.72) | GG (0.42) | CG (0.50) | CC (0.08) | ||
Data were analysed using Fisher’s exact test. ** , p < 0.01, which indicates a statistically significant difference.
Table 4.
Pairwise linkage disequilibrium among DRD1 and DRD3 variants by D’ statistic
| DRD1 | rs4532 | rs4867798 | rs265981 |
|---|---|---|---|
| rs4532 | - | 0.250 | 0.789 |
| rs4867798 | - | - | 0.011 |
| rs265981 | - | - | - |
| DRD3 | rs3732783 | rs6280 | |
| rs3732783 | - | 0.996 | |
| rs6280 | - | - |
Association of DRDs and GRIN2B polymorphisms with Parkinson disease–Impulse control disorder
We evaluated the associations between polymorphisms in DRD and GRIN2B with ICB risk among Malaysian PD patients (Table 5). Among the 7 polymorphisms examined, only 4 were associated with the development of ICB among PD patients. Logistic regression analysis (adjusted for gender, age, ethnicity, dosage of medication and duration of PD) showed that there was a significantly higher risk of ICB that was associated with the DRD1 rs4867798 C allele (OR = 24.53; 95% CI, 1.68-357.28; p = 0.0054) and the GRIN2B rs7301328 C allele (OR = 25.07; 95% CI, 1.30-483.41; p = 0.0097). Similarly, the DRD1 rs4532 T allele (OR = 21.33; 95% CI, 1.97-230.64; p = 0.0024) and T allele in DRD2/ANKK1 rs1800497 (OR = 3.77; 95% CI, 1.38–10.30; p = 0.0044) were significantly associated with a higher risk of developing ICB in PD patients. The DRD1 rs4867798 and GRIN2B rs7301328 polymorphisms showed a recessive mode of inheritance, suggesting that the dominant allele has an advantage over the recessive allele. Notably, DRD1 rs4532 showed an overdominant mode of inheritance in which heterozygous patients (TC) have an increased risk of ICB compared to homozygous patients (CC or TT). By contrast, the T allele in DRD2/ANKK1 rs1800497 was shown to have an additive genetic effect, whereby the risk of a patient with 2 copies of the T allele doubles compared to a heterozygous patient. No association was detected between the other polymorphisms and ICB in PD patients.
Table 5.
Association between PD-ICB and polymorphisms in genotypes among cases and controls
| Model | Genotype | Control (%) | Case (%) | OR (95% CI) |
|---|---|---|---|---|
| DRD1 rs4532 (C > T) | ||||
| Overdominant | CC-TT | 35 (94.6) | 32 (61.5) | 1.00 |
| TC | 2 (5.4) | 20 (38.5) | 21.33 (1.97-230.64)** | |
| DRD1 rs4867798 (T > C) | ||||
| Recessive | TT-TC | 29 (96.7) | 32 (71.1) | 1.00 |
| CC | 1 (3.3) | 13 (28.9) | 24.53 (1.68-357.28)** | |
| DRD2/ANKK1 rs1800497 (C > T) | ||||
| Log-additive | T- | - | - | 3.77 (1.38-10.30) ** |
| GRIN2B rs7301328 (G > C) | ||||
| Recessive | GG-CG | 24 (82.8) | 20 (47.6) | 1.00 |
| CC | 5 (17.2) | 22 (52.4) | 25.07 (1.30-483.41) ** | |
** , p < 0.01, which indicates a statistically significant difference.
Discussion
This study showed that the rs4532 and rs4867798 variants in DRD1 were associated with ICB in PD patients. DRD1 encodes one of the major receptors in the brain that mediate the actions of the neurotransmitter dopamine in various psychomotor functions [10]; data suggest that polymorphisms in the promoter region of DRD1 may play a role in the neurobiology of ICB [29]. Previous studies have shown that the rs4532 polymorphism in the 5′-UTR is significantly associated with compulsive addictive behaviour [11,34,35]. These studies have shown that the T allele of rs4532 imparts a higher risk of developing compulsive addictive behaviour in healthy subjects. Similarly, our study showed that this allele was significantly associated with an increased risk of developing ICB in PD patients. To date, there has been no report that the rs4867798 polymorphism in the 3′-UTR of DRD1 was associated with ICB. However, we found that the C allele of rs4867798 was significantly associated with a greater risk of developing ICB in PD patients. In our haplotype-based analysis, we detected no LD between rs4532 and rs4867798 in DRD1. Therefore, the rs4532 and rs4867798 variants were independently associated with the development of ICB among PD patients.
Both the rs4532 and rs4867798 polymorphisms are located outside of the coding region of DRD1. However, to date, no polymorphism has been found to alter the amino acid sequence in the DRD1 coding region [36]. Thus, these polymorphisms located in the 3′- and 5′-UTRs of DRD1 are likely to affect mRNA stability, and to subsequently affect DRD1 expression [10]. It is possible that these two polymorphisms, rs4532 and rs4867798, interfere with mRNA stability, which could affect the binding site of microRNA (miRNA) or change the secondary structure of mRNA. However, further experiments will be needed to test these possibilities.
Among all polymorphisms in DRD2/ANKK1, rs1800497 has been the most frequently implicated in addiction disorders [37]. This polymorphism was previously reported to be associated with cocaine addiction and pathological gambling in the general population [38,39]. In our study, the DRD2/ANKK1 rs1800497 variant was shown to be associated with PD-ICB, which was consistent with a previous study of PD-ICD subjects [40]. By contrast, Lee et al. [15] and Vallelunga et al. [41] showed that there were no associations of DRD2/ANKK1 rs1800497 variants with ICB/ICD among PD subjects. DRD2 is involved in the mesocorticolimbic pathway, which is mainly distributed in the striatum [16] and also affects motor control [15]. The rs1800497 variant in DRD2/ANKK1 has been associated with decreased receptor density in the striatum [42]. In agreement with these speculative points [16,17,41], the rs1800497 variant in DRD2/ANKK1 changes the glutamic acid to lysine (from an amino acid group with a negatively charged side chain to positively charged residue) that might result in a significant protein structure modification that leads to reduced expression of the receptor and the development of neuropsychiatric disorders among PD patients. Therefore, molecular studies of the effect of DRD2/ANKK1 rs1800497 variants should be explored.
In GRIN2B, the rs7301328 variant was previously found to be associated with PD under a dominant model [40]. In our study, we found that the same polymorphism exhibited an increased risk of developing ICB among PD subjects and was in accord with a study conducted by Lee et al. [15]. The rs7301328 variant causes a synonymous single nucleotide substitution. It alters the DNA sequence, but does not change the encoded amino acid sequence. This polymorphism has been found to be associated with alcohol dependence [43] and schizophrenia [26,44]. The association between the NMDA receptor subunits based on polymorphisms in GRIN1 and GRIN2B and PD-ICB has not been thoroughly explored. Thus, screening for polymorphisms in the GRIN1 subunit and subtypes of GRIN2 genes could provide important insights into the understanding of gene-to-gene interactions that influence ICB among PD subjects.
Conclusion
In summary, we have shown that variants in DRD1 rs4867798, DRD1 rs4532, DRD2/ANKK1 rs1800497 and GRIN2B rs7301328 are associated with an increased ICB risk among PD patients. Future studies of gene-to-gene interactions and also identifying the miRNA binding site domains could yield an improved understanding of how synonymous polymorphisms can lead to ICB development. Furthermore, the combinatorial effects of individual polymorphisms in genes that participate in the dopaminergic and glutamategic pathways should be examined. Additional assessment by psychiatrists will relate the association of these ICB susceptible polymorphisms to ICD.
Availability of supporting data
Two additional files were provided as supporting data. Additional file 1: Figure S1 contains a supplementary figure that depicts high resolution melting normalised curves and DNA sequencing results for selected DRD2, DRD4 and DRD5 SNPs. Additional file 2: Table S1 contains a supplementary table for haplotype analysis of selected DRD1 and DRD3 SNPs.
Acknowledgements
This work was supported by grant DIP-2012-026 (awarded to N.M.I.), a Universiti Putra Malaysia Putra Grant (UPM/700/2/1/GP-IPS/2013/9399833 to K.-H.L.), the Malaysian Ministry of Education Exploratory Research Grant Scheme (ERGS/1/11/SKK/UPM/03/ to P.-S.C.), and the Malaysian Ministry of Education Fundamental Research Grant Scheme (04-01-12-1126FR to K.H.L.). S.Z.A is supported by the Malaysian Ministry of Science, Technology and Innovation ScienceFund (02-01-04-SF1306 awarded to P.-S.C.). We would like to thank Professor Dato’ Dr. Lye Munn Sann, Department of Community Health, Universiti Putra Malaysia, for his advice and review on statistical analyses.
Additional files
A High Resolution Melting normalised curve for (A) DRD2 rs104894220, (B) DRD2 rs144999500, (C) DRD4 rs1800443, and (D) DRD5 rs144132215, which are presented along with their respective sequencing results.
Haplotype analysis of selected DRD1 and DRD3 SNPs.
Footnotes
Shahidee Zainal Abidin, Eng Liang Tan, King-Hwa Ling and Norlinah Mohamed Ibrahim contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SZA, ELT, AJ, AXL, MHNAH, NFPR and SA carried out the data collection, gDNA extraction, quality control screening and high-resolution melting analysis. NAAM, AAA, SYL, PSC, KHL and NMI coordinated the recruitment of subjects, processing of samples and overall progression of the study. All subjects were recruited and examined by ELT, SA, SYL and NMI. SZA performed and SCC supervised as well as validated the statistical analysis. SZA, KHL, PSC, SCC, AAA and NMI drafted the manuscript. KHL and NMI conceived of the study and participated in its design. All authors read and approved the final manuscript.
Contributor Information
Shahidee Zainal Abidin, Email: shah.68000@gmail.com.
Eng Liang Tan, Email: drtanengliang@gmail.com.
Soon-Choy Chan, Email: soonchoyc@yahoo.com.
Ameerah Jaafar, Email: ameerah.jaafar@gmail.com.
Alex Xuen Lee, Email: libk201@hotmail.com.
Mohd Hamdi Noor Abd Hamid, Email: medinoor89@gmail.com.
Nor Azian Abdul Murad, Email: nor_azian@ppukm.ukm.edu.my.
Nur Fadlina Pakarul Razy, Email: nurmp89@yahoo.com.
Shahrul Azmin, Email: shahrulazmin@hotmail.com.
Azlina Ahmad Annuar, Email: azlina_aa@um.edu.my.
Shen Yang Lim, Email: limshenyang@ymail.com.
Pike-See Cheah, Email: cheahpikesee@upm.edu.my.
King-Hwa Ling, Email: lkh@upm.edu.my.
Norlinah Mohamed Ibrahim, Email: norlinah@ppukm.ukm.edu.my.
References
- 1.Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S80–4. doi: 10.1016/S1353-8020(11)70026-8. [DOI] [PubMed] [Google Scholar]
- 2.Weintraub D, Potenza MN. Impulse control disorders in Parkinson’s disease. Curr Neurol Neurosci Rep. 2006;6:302–6. doi: 10.1007/s11910-006-0022-y. [DOI] [PubMed] [Google Scholar]
- 3.Voon V, Fernagut P-O, Wickens J, Baunez C, Rodriguez M, Pavon N, et al. Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol. 2009;8:1140–9. doi: 10.1016/S1474-4422(09)70287-X. [DOI] [PubMed] [Google Scholar]
- 4.Lim S-Y, O’Sullivan SS, Kotschet K, Gallagher DA, Lacey C, Lawrence AD, et al. Dopamine dysregulation syndrome, impulse control disorders and punding after deep brain stimulation surgery for Parkinson’s disease. J Clin Neurosci. 2009;16:1148–52. doi: 10.1016/j.jocn.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol. 2008;75:63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 7.Holden C. “Behavioral” addictions: do they exist? Science. 2001;294:980–2. doi: 10.1126/science.294.5544.980. [DOI] [PubMed] [Google Scholar]
- 8.Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 9.Wong AH, Buckle CE, Van Tol HH. Polymorphisms in dopamine receptors: what do they tell us? Eur J Pharmacol. 2000;410:183–203. doi: 10.1016/S0014-2999(00)00815-3. [DOI] [PubMed] [Google Scholar]
- 10.Huang W, Ma JZ, Payne TJ, Beuten J, Dupont RT, Li MD. Significant association of DRD1 with nicotine dependence. Hum Genet. 2008;123:133–40. doi: 10.1007/s00439-007-0453-9. [DOI] [PubMed] [Google Scholar]
- 11.Kim D-J, Park BL, Yoon S, Lee H-K, Joe K-H, Cheon Y-H, et al. 5’ UTR polymorphism of dopamine receptor D1 (DRD1) associated with severity and temperament of alcoholism. Biochem Biophys Res Commun. 2007;357:1135–41. doi: 10.1016/j.bbrc.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 12.Misener VL, Luca P, Azeke O, Crosbie J, Waldman I, Tannock R, et al. Linkage of the dopamine receptor D1 gene to attention-deficit/hyperactivity disorder. Mol Psychiatry. 2004;9:500–9. doi: 10.1038/sj.mp.4001440. [DOI] [PubMed] [Google Scholar]
- 13.Bobb AJ, Addington AM, Sidransky E, Gornick MC, Lerch JP, Greenstein DK, et al. Support for association between ADHD and two candidate genes: NET1 and DRD1. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:67–72. doi: 10.1002/ajmg.b.30142. [DOI] [PubMed] [Google Scholar]
- 14.Minowa MT, Minowa T, Monsma FJ, Sibley DR, Mouradian MM. Characterization of the 5’ flanking region of the human D1A dopamine receptor gene. Proc Natl Acad Sci U S A. 1992;89:3045–9. doi: 10.1073/pnas.89.7.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J-Y, Lee EK, Park SS, Lim J-Y, Kim HJ, Kim JS, et al. Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson’s disease. Mov Disord. 2009;24:1803–10. doi: 10.1002/mds.22678. [DOI] [PubMed] [Google Scholar]
- 16.Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23:540–5. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Fan H, Xu Y, Zhang K, Huang X, Zhu Y, et al. Converging evidence implicates the dopamine D3 receptor gene in vulnerability to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:613–9. doi: 10.1002/ajmg.b.31203. [DOI] [PubMed] [Google Scholar]
- 18.Lundstrom K, Turpin MP. Proposed schizophrenia-related gene polymorphism: expression of the Ser9Gly mutant human dopamine D3 receptor with the Semliki Forest virus system. Biochem Biophys Res Commun. 1996;225:1068–72. doi: 10.1006/bbrc.1996.1296. [DOI] [PubMed] [Google Scholar]
- 19.Gervasini G, Gordillo I, García-Herráiz A, Flores I, Jiménez M, Monge M, et al. Influence of dopamine polymorphisms on the risk for anorexia nervosa and associated psychopathological features. J Clin Psychopharmacol. 2013;33:551–5. doi: 10.1097/JCP.0b013e3182970469. [DOI] [PubMed] [Google Scholar]
- 20.Hess S, Daggett L, Crona J, Deal C, Lu C, Urrutia A, et al. Cloning and functional characterization of human heteromeric N-methyl-D- aspartate receptors. J Pharmacol Exp Ther. 1996;278:808–16. [PubMed] [Google Scholar]
- 21.Mandel S, Grünblatt E, Maor G, Youdim MBH. Early and late gene changes in MPTP mice model of Parkinson’s disease employing cDNA microarray. Neurochem Res. 2002;27:1231–43. doi: 10.1023/A:1020989812576. [DOI] [PubMed] [Google Scholar]
- 22.Marti M, Mela F, Bianchi C, Beani L, Morari M. Striatal dopamine-NMDA receptor interactions in the modulation of glutamate release in the substantia nigra pars reticulata in vivo: opposite role for D1 and D2 receptors. J Neurochem. 2002;83:635–44. doi: 10.1046/j.1471-4159.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 23.Wu S-L, Wang W-F, Shyu H-Y, Ho Y-J, Shieh J-C, Fu Y-P, et al. Association analysis of GRIN1 and GRIN2B polymorphisms and Parkinson’s disease in a hospital-based case–control study. Neurosci Lett. 2010;478:61–5. doi: 10.1016/j.neulet.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Q, Che R, Zhang Z, Wang P, Li J, Li Y, et al. Positive association between GRIN2B gene and bipolar disorder in the Chinese Han Population. Psychiatry Res. 2011;185:290–2. doi: 10.1016/j.psychres.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Liou Y-J, Wang Y-C, Chen J-Y, Bai Y-M, Lin C-C, Liao D-L, et al. Association analysis of polymorphisms in the N-methyl-D-aspartate (NMDA) receptor subunit 2B (GRIN2B) gene and tardive dyskinesia in schizophrenia. Psychiatry Res. 2007;153:271–5. doi: 10.1016/j.psychres.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Ohtsuki T, Sakurai K, Dou H, Toru M, Yamakawa-Kobayashi K, Arinami T. Mutation analysis of the NMDAR2B (GRIN2B) gene in schizophrenia. Mol Psychiatry. 2001;6:211–6. doi: 10.1038/sj.mp.4000808. [DOI] [PubMed] [Google Scholar]
- 27.Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA, et al. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77:918–36. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weintraub D, Stewart S, Shea JA, Lyons KE, Pahwa R, Driver-dunckley ED, et al. Impulse control disorder in Parkinson Disease. NIH Public Access. 2010;24:1461–1467. [Google Scholar]
- 29.Da Silva Lobo DS, Vallada HP, Knight J, Martins SS, Tavares H, Gentil V, et al. Dopamine genes and pathological gambling in discordant sib-pairs. J Gambl Stud. 2007;23:421–33. doi: 10.1007/s10899-007-9060-x. [DOI] [PubMed] [Google Scholar]
- 30.Markham NR, Zuker M. DINAMelt web server for nucleic acid melting prediction. Nucleic Acids Res. 2005;33(Web Server issue):W577–81. doi: 10.1093/nar/gki591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esteves ARF, Domingues AF, Ferreira IL, Januário C, Swerdlow RH, Oliveira CR, et al. Mitochondrial function in Parkinson’s disease cybrids containing an nt2 neuron-like nuclear background. Mitochondrion. 2008;8:219–28. doi: 10.1016/j.mito.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Solé X, Guinó E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22:1928–9. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- 33.Miller RG. Simultaneous Statistical Inference. New York, NY: Springer New York; 1981. [Google Scholar]
- 34.Limosin F, Loze J-Y, Rouillon F, Adès J, Gorwood P. Association between dopamine receptor D1 gene DdeI polymorphism and sensation seeking in alcohol-dependent men. Alcohol Clin Exp Res. 2003;27:1226–8. doi: 10.1097/01.ALC.0000081624.57507.87. [DOI] [PubMed] [Google Scholar]
- 35.Comings DE, Gade R, Wu S, Chiu C, Dietz G, Muhleman D, et al. Studies of the potential role of the dopamine D1 receptor gene in addictive behaviors. Mol Psychiatry. 1997;2:44–56. doi: 10.1038/sj.mp.4000207. [DOI] [PubMed] [Google Scholar]
- 36.Komarova AV, Brocard M, Kean KM. The case for mRNA 5’ and 3’ end cross talk during translation in a eukaryotic cell. Prog Nucleic Acid Res Mol Biol. 2006;81:331–67. doi: 10.1016/S0079-6603(06)81009-3. [DOI] [PubMed] [Google Scholar]
- 37.Noble EP. Addiction and its reward process through polymorphisms of the D2 dopamine receptor gene: a review. Eur Psychiatry. 2000;15:79–89. doi: 10.1016/S0924-9338(00)00208-X. [DOI] [PubMed] [Google Scholar]
- 38.Haile CN, Kosten TR, Kosten TA. Genetics of dopamine and its contribution to cocaine addiction. Behav Genet. 2007;37:119–45. doi: 10.1007/s10519-006-9115-2. [DOI] [PubMed] [Google Scholar]
- 39.Comings DE, Rosenthal RJ, Lesieur HR, Rugle LJ, Muhleman D, Chiu C, et al. A study of the dopamine D2 receptor gene in pathological gambling. Pharmacogenetics. 1996;6:223–34. doi: 10.1097/00008571-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Hassan A, Okun M, Serie D, Heckman M, Ahlskog J, Ross O. Comparison of DRD2, DRD3 and NMDA receptor genotype polymorphisms in Parkinson’s patients and controls. Neurology. 2013;80:P05. doi: 10.1212/WNL.0b013e31827f0f91. [DOI] [Google Scholar]
- 41.Vallelunga A, Flaibani R, Formento-Dojot P, Biundo R, Facchini S, Antonini A. Role of genetic polymorphisms of the dopaminergic system in Parkinson’s disease patients with impulse control disorders. Parkinsonism Relat Disord. 2012;18:397–9. doi: 10.1016/j.parkreldis.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Pohjalainen T, Rinne JO, Någren K, Lehikoinen P, Anttila K, Syvälahti EK, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–60. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 43.Kim JH, Park M, Yang SY, Jeong BS, Yoo HJ, Kim J-W, et al. Association study of polymorphisms in N-methyl-D-aspartate receptor 2B subunits (GRIN2B) gene with Korean alcoholism. Neurosci Res. 2006;56:220–3. doi: 10.1016/j.neures.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Li D, He L. Association study between the NMDA receptor 2B subunit gene (GRIN2B) and schizophrenia: A HuGE review and meta-analysis. Genet Med. 2007;9:4–8. doi: 10.1097/01.gim.0000250507.96760.4b. [DOI] [PubMed] [Google Scholar]


