Abstract
Observations over the past decade using longitudinal data reveal a gender-specific shift in adrenal steroid production. This shift is represented by an increase in the circulating concentrations of delta 5 steroids in 85% of all women and is initiated only after the menopausal transition has begun. While the associated rise in the major adrenal androgen, dehydroepiandrosterone sulfate (DHEAS), is modest, the parallel rises in dehydroepiandrosteone (DHEA) and androstenediol (Adiol) are much more robust. These increases in circulating steroid concentrations are qualitatively similar on average between ethnicities but quantitatively different between individual women. Both circulating testosterone (T) and androstenedione (Adione) also rise concomitantly but modestly by comparison. This phenomenon presents a new and provocative aspect to the endocrine foundations of the menopausal transition and may provide important clues to understanding the fundamentals of mid-aged women's healthy aging, particularly an explanation for the wide diversity in phenotypes observed during the MT as well as their different responses to hormone replacement therapies. Experimental studies using the nonhuman primate animal model show an acute adrenal response to human chorionic gonadotropin (hCG) challenge as well as the presence of luteinizing hormone receptors (LHR) in their adrenal cortices. These experimental results support the concept that LHRs are recruited to the adrenal cortices of mid-aged women that subsequently function to respond to increasing circulating LH to shunt pregnenolone metabolites towards the delta 5 pathway. Future investigations are required to determine the relationship of these changes in adrenal function to symptoms and health outcomes of mid-aged women.
Keywords: Adrenal glands, Menopause, Gonadal steroid hormones
The endocrine foundation of the menopausal transition (MT) may be more complex than previously considered. Recent observations indicate that the adrenal cortex of mid-aged women may play a significant role in the production of sex steroids. These studies reveal a gender-specific difference in adrenal androgen production during the aging process that is limited to the MT. As a result of these observations, the adrenal cortex is now recognized as a potential important contributor to endocrine foundation of women's healthy aging.1-5 Previous reports had suggested that most of the positive contribution by the adrenals for women was largely through normally secreted weak adrenal androgens such as dehydroepiandrostenone (DHEA) which are subsequently converted peripherally to bioactive estrogens and androgens such as estradiol (E2) and testosterone (T).6, 7 That original concept was acceptable to many because it was supported by independent observations that higher circulating levels of DHEA were associated with the conservation of women's cognitive and administrative function in middle age.8 These reports therefore led logically to intervention experiments with DHEA supplementation 9 but these intervention studies have provided little or no convincing evidence that such treatments have benefit.10 It is still not fully appreciated that increased production of adrenal DHEA, androstenediol (Adiol) and perhaps other compounds can contribute to the different circulating sex steroid profiles in individual women.
There is now direct evidence that the primary peripheral conversion products of DHEA are mainly androgenic not estrogenic,11 suggesting that DHEA is neither directly nor indirectly responsible for preserving the estrogen-dependent integrity of the neural substrate and other estrogen-sensitive tissues. However, because wide, between-women differences in adrenal androgen secretion is now recognized, a plausible and comprehensive role of the adrenal cortex in women's aging 1-5 is emerging. This wide between-woman difference in the apparent production rates of Adiol has generated a new concept to explain the range of phenotypes observed in middle-aged women. We now understand that Adiol, secreted by the adrenal with its inherent dual estrogenic and androgenic bioactivities, is produced in parallel with DHEA, but to a much greater degree than dehydroepiandrosterone sulfate (DHEAS) during the MT Thus these unconjugated delta 5 steroids are likely major contributors to the physiology of mid-aged women.4, 5
Future studies will be needed to determine the relation between these physiologic findings to menopausal symptoms and health issues. Although less potent, the adrenal steroids can reach levels many fold above E2 and T in some women. The possibility that adrenal sex steroids that possess both androgenic and estrogenic bioactivity will provide important clues to explain current conundrums relating to women's healthy aging is quite real. Specifically, individual and ethnic differences in adrenal function must be considered in terms of the difference in symptoms and health trajectories. In addition, the role of multiple estrogen receptors (ERs), their different ratios in estrogen-sensitive organs and changes in circulating ER ligands of different types should be investigated in terms of hormone replacement (HT) strategies. Finally, alterations in changes in the classic hypothalamic-pituitary-adrenal (HPA) must be examined as a possible explanation for the transient changes observed in metabolism and renal function.
Why has this concept been slow to emerge?
It has been more than ten years since the first population-based study indicated that a rise of DHEAS and related adrenal steroids is specific to the MT;1 however, the clinical implications of this have not yet been seriously considered. The slow pace of the acceptance and general appreciation of the concept is modest at best even now, when at least one recent descriptive report supports the hypothesis that adrenal components are associated with the MT and their production are associated with circulating levels of luteinizing hormone (LH).12 This report suggests that both a rise in cortisol and a decline in aldosterone are associated with circulating LH levels which are acting on the adrenal. Still, solid and direct evidence for this mechanism is still lacking. It seems likely that these descriptive data will give direction to future studies and will ultimately explain why a change in cortisol production during the MT has been suspected by many, but never clearly confirmed by any for decades. Perhaps more importantly these kinds of observations also support the concept that LH receptors are recruited not only to the zona glumerulosa, but to other cortical zones as well, as described recently in the nonhuman primate (NHP) animal model. Together these observations provide direction for future investigations that reveal mechanisms that explain not only the transient change in adrenal and renal function in mid-aged women, but also why the range of phenotypes expressed by mid-aged women is so great.
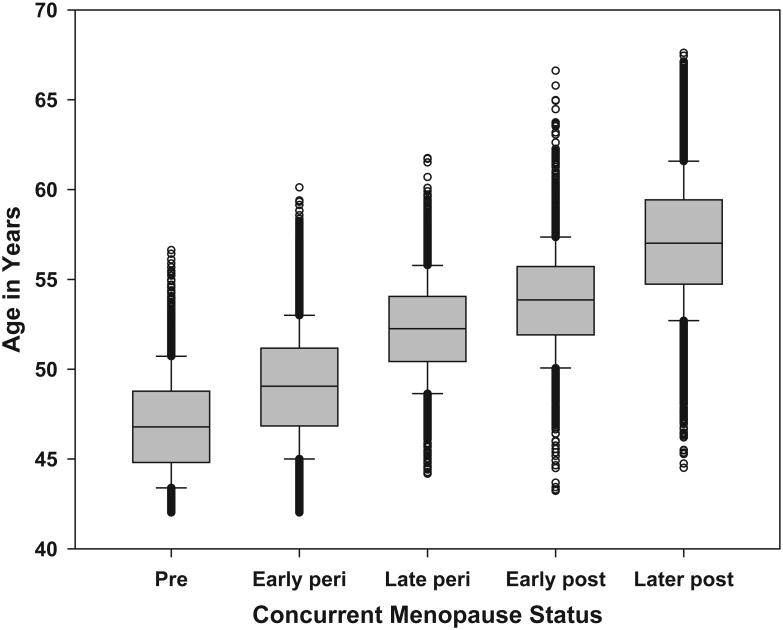
The slow pace of the general appreciation of the concept of an MT-related rise in DHEAS and related adrenal steroids can be attributed to several factors. Evidence of an ovary-adrenal interaction has been accumulating for years and well characterized in rodents. Until now, however, these indications were never coalesced into a coherent concept for higher primate species. While several studies have attempted to demonstrate a change in circulating cortisol during the MT, the results of these investigations have failed to provide conclusive data. Studies of age-related changes are unlikely to identify MT-related changes, given the high degree of overlap in age across menopause stages, as seen in Figure 1; women aged 45 to 55 could be classified in any stage of ovarian function from pre- to later postmenopause. Age-based analyses in this age range therefore can capture all levels of circulating adrenal steroids and produce mean levels that are relatively stable over a wide range of ages.
Figure 1.
Distribution of chronologic age by concurrent menopause status, from the Study of Women's Health Across the Nation (SWAN), baseline through 12th annual follow-up visit. Observations concurrent with pregnancy/breastfeeding, exogenous hormone therapy use, and hysterectomy/bilateral oophorectomy are omitted. Menopause status was categorized as: 1) premenopausal (menstrual period in the past 3 months, no change in regularity over the previous 12 months); 2) early perimenopausal (menstrual period in the past 3 months, increased irregularity over the previous 12 months); 3) late perimenopausal (menstrual bleeding within the previous 12 months but not the previous 3 months); 4) early postmenopausal (0-24 months since the final menstrual period); and 5) later postmenopause (>24 months since the final menstrual period).
Moreover, cross-sectional studies – even those using ovarian stage rather than chronologic age – can provide different estimates than longitudinal analyses.13-16 The former infers within-woman change from between-woman comparisons, and these may not be equivalent due to factors such as period or cohort effects.17 In the Study of Women's Health Across the Nation (SWAN), for example, women transitioning to a late stage at different follow-up times differ in many respects, including race/ethnicity, smoking, and socioeconomic status, consistent with cohort effects. A comparison of cross-sectional differences in DHEAS between the early and late transition indicates variability in this difference across annual visits, as well as differences from longitudinal analyses of within-woman change in DHEAS from early to late stage.2 In addition, longitudinal data permit direct calculation of within-woman change, which often provides greater precision;18, 19 in contrast, cross-sectional studies cannot identify participants with large changes who nevertheless appear “normal” cross-sectionally.20
The primary reason that progress has been slow may be because of the wide range of patterns that are observed in the adrenal response to rising LH. Cross-sectional analyses of mid-aged women during the MT tend to meld high, low and non-responders together because individual women have different trajectories in the rise of adrenal steroids.5 Plus, only one laboratory has provided a mechanistic explanation using an appropriate animal model that has the ability to secrete adrenal androgens in a manner similar to humans. The paucity of supportive data is also due largely to the fact that serial blood samples from large longitudinal studies following individual women through the MT have not been widely available for evaluation. In addition, in any ovarian stage group a range of values will be observed because some women have a rapid and robust rise in adrenal steroids while others do not experience any perceptible rise. This between-woman variability – as seen in other reproductive hormones 21 - necessitates a dataset that is large enough to have sufficient representation of smaller subgroups. Even with access to the large SWAN dataset, direct evidence that circulating Adiol reaches effective concentrations currently is limited to less than 200 subjects in pilot analyses,4, 5 still the demonstration of a parallel rise or circulating Adiol with DHEAS in 120 individual subjects over three years essentially insures the generalizability of the concept since the ovarian stage-specific rise in DHEAS has been shown for 85% of over 3000 subjects.2 Perhaps the most limiting factor for broad interest in the general concept has been that experiments with human subjects are extremely challenging and often ethically questionable. Most of the current supportive information is descriptive and few laboratories have access to appropriate nonhuman primate animal models that are required for invasive experimental studies.
From the broadest phylogenetic perspective, gonadal-adrenal relationships have a very long history in the scientific literature 22 but these concepts have only recently been recognized to extend to primates or have clinical significance in humans.23-26 While evidence for luteinizing hormone (LH) receptors has been established in mice,27 their physical presence in human adrenals has been sparingly suggested,28, 29 and these claims have been largely held as contentious. Perhaps the most compelling evidence for a LH/chorionic gonadotropin (CG)-adrenal interaction in humans comes from pregnancy-induced Cushing's disease in women 23, 24 in which increased adrenal androgens are thought to be stimulated by increased circulating CG. Recently the adrenal response to a LH/CG challenge which has long been recognized in rodents has been demonstrated in the nonhuman primate animal model.30, 32 Since it is now assumed that LH receptors are relatively common in the mammalian adrenal cortex, current research now focuses on the mechanism(s) by which these receptors become responsive to circulating LH-like stimulation. Whether or not these mechanisms will be shown to be similar across the wide range of phylogeny that has been better studies remains to be determined. However, studies in non-primate species may not provide direct insight into our understanding of the perimenopausal rise in adrenal steroids since only the higher primates have the ability to secrete large amounts of delta five steroids.
Specifically, there has been some reluctance to accept the data which predict that a modest rise in circulating DHEAS will be paralleled by a much more robust rise in DHEA and Adiol. When plotted schematically (Figure 2) the extreme high levels in circulating steroids demonstrated by a few women indicate that not only the concentration domination of Adiol and DHEA in the late MT, but indicate the wide range of concentration ratios of the different ER ligands both between women and across the MT. The ratio differences of the predominantly alpha contribution of E2 to the predominantly ER beta contribution of Adiol can range from less than 10 fold to over 100 fold in different women at different time points during the MT in the same women. Because the maximal rise in DHEA and Adiol varies broadly among women, it seems possible that the effect of the very high levels may have a different effect than those of the lower levels. This is suggested in the observation that an association of Adiol to vasomotor symptoms (VMS) is found only when both estradiol (E2) and Adiol circulating levels are the highest. Thus the highest Adiol levels may have adverse effects by antagonizing E2 while lower levels may fail to provide a protective effect against E2 stimulation of hyperplastic disease particularly in women in which no rise in DHEAS or its attendant adrenal steroids is observed.1, 2
Figure 2.
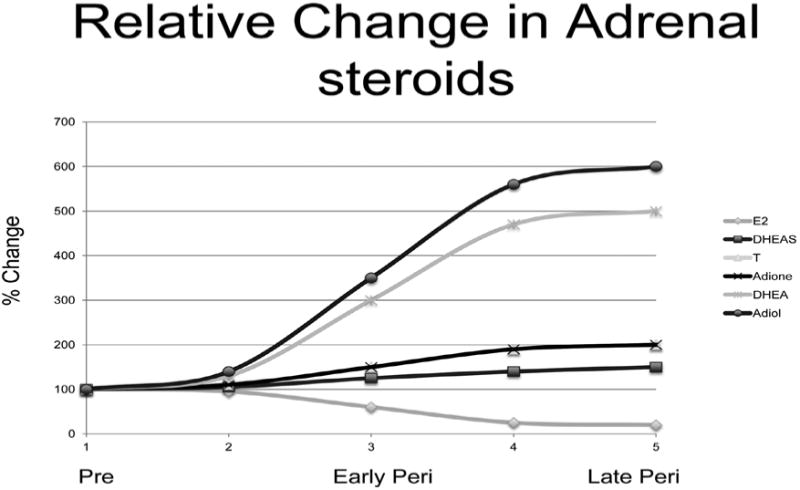
Schematic of maximal circulating levels of the sex steroids across the menopausal transition. During the MT all women experience a modest, similar decline in circulating E2 levels with the steepest decline occurring in the four years surrounding menopause. In contrast approximately 85% of all women experience an increase in circulating androgens throughout the complete menopausal transition. While all five androgens rise, the greatest increase found in Adiol and DHEA which can increase over 500%. Adiol reaches effective biological levels but only in some women This wide range in circulating Adiol concentrations that are associated with the increase in adrenal steroid production during the MT have broad and mixed steroid receptor signaling implications. The ER beta signaling potential of Adiol has particular significance during and after the menopausal transition (MT) since ER beta signaling can impede ER alpha signaling. This suggests that the ER alpha versus ER beta tissue responses to Adiol may be as important, if not more important, than the classic E2/T balance that has previously dominated endocrine studies of the MT. Dehydroepiandrosterone (DHEA) and androstenediol (Adiol) reach the highest circulating levels in some women which can be 5-8-fold above levels observed prior to the menopausal transition. Testosterone (T, colors) and androstenedione (Adione) rise only 2-3 fold during the same interval. Dehydroepiandrosterone sulfate (DHEAS) rises only 15-20%. Estradiol (E2) measured on day 5 of the menstrual cycle falls slightly just prior to and following the last menstrual period. Adapted from McConnell et al.5
What are the implications?
The primary implication of the observation of an ovarian stage-specific shift in adrenal androgen production during the MT in a small cohort is that it likely occurs to some degree in most women. Even when a modest rise of adrenal androgens occurs as it does in approximately 15% of all women, it could represent a general physiological aspect of women's endocrine aging by its absence. If this can be confirmed by direct longitudinal evaluation of a larger number of mid-aged women, then this would represent a direct challenge to the long-held dogma that it is the decline in ovarian function that is the unique endocrine hallmark of the MT. Importantly this paradigm shift, in turn, may then challenge the rationale for providing similar estrogen-based interventions to women without information regarding their adrenal status. Already personalized therapies are becoming the norm and incorporating an adrenal screen would seem to be a likely adjunct. If circulating Adiol is proven to be a primary ER beta ligand that reaches effective circulating concentrations in some women, then future HT therapies may need to consider the circulating ER-alpha/ER-beta balance in directing interventions in the future. Furthermore, if the smaller rise in DHEAS that is observed in many women is proven to be the “tip of the iceberg” with circulating Adiol rising many-fold more in most women, then the concept of intracrinology, which implies that peripheral conversion DHEAS/DHEA to more bioactive metabolites is responsible for the benefits of higher DHEAS, will also need to be reconsidered in terms of women's healthy aging. Essentially, most of what we think we know and how we address what we do not know will be addressed in evaluating how important the adrenal contributions are shown to be.
Concerns were raised by three recent large women's health studies 33-35 that failed to demonstrate a protective effect of some hormone replacement therapies on preventing coronary heart disease and cognitive decline. These studies have led to a rethinking of the basic endocrinology of middle-aged women. In an attempt to explain the results of large, controlled population-based studies. As a result a few new hypotheses have been put forward. The most popular of these is the “timing hypothesis” 36 which posits that the same hormone interventions can have different effects and possibly different risks for women of different ages or stages of ovarian function. This and other widely disseminated theories have confused patients, perplexed physicians and outraged some research scientists. However, these all seem to foreshadow the recognition that the endocrine characteristics of mid-aged women are not singularly defined by ovarian function.
Most recently and adding to the complexity, a clinical trial of a million Chinese women concluded that E+P intervention in postmenopausal women does not increase the risk for CHD in Chinese women but may increase the risk for breast cancer.37 These findings may indicate that endocrine differences in ethnicity should be added to the science of women's healthy aging. Even more doubt is cast on the conventional wisdom of HT by the results of experimental studies using the nonhuman primate (NHP) animal model.30-32 In contrast to the benefits of estrogen alone in stimulating dendritic spine growth in the dorsal lateral prefrontal cortex (dlPFC) that is associated with preserving cognitive performance in aged non-human primates,38 the combined treatment of estrogen with a progestin, which is the conventional therapy, has no beneficial effect on spinogenesis.39 Taken together these new but incomplete data that are used to ascribe risks and benefits of HT, have resulted in a near 30% reduction in women's desire to use conventional HT and placed their physicians in the awkward position of trying to explain the unexplainable. The emerging concept is now that HT carries more benefit than risks and should be considered adequate but not optimal. Recent literature in the lay press indicates the controversy continues.40
The mechanistic basis for a large portion of the changes that occur during the MT is incomplete and the pharmacologic basis for treating its symptoms is currently based on this incomplete information. However, progress is being made as three previous conundrums have been addressed, at least in theory. First, it is now apparent that between-woman and ethnic differences in the events associated with the MT can be explained by differences in circulating steroid hormones if the adrenal component is taken into consideration.1-5 Second, the apparent benefits of pharmacologic estrogen replacement, regardless of endogenous estrogen production, can be explained by the antagonistic actions of high circulating Adiol levels.4, 5 Third, the different responses of individual women to the same HT replacement therapy will likely be explained by the observation that adrenal steroid production is accelerated to different degrees in individual women 5 and different in response to different HT regimens.22
What is the experimental evidence?
Three relevant experiments using the nonhuman primate animal model have been recently reported.21-23 In the first experiment, mature macaque females were either ovariectomized (OVX) or chemically castrated prior to a challenge with human chorionic gonadotropin (hCG) which has luteinizing hormone (LH) bioactivity. In all animals a brisk rise of DHEAS was observed with no change in ovarian steroid production indicating the ability of the adrenal cortex to respond to bioactive LH. In the second study, OVX mature female macaques were treated with either estrogen alone (E) or estrogen plus progesterone (E+P) to simulate conventional HT. The results of this study demonstrate that the effect of the same HT intervention is distinctly different between younger and older animals and the width of the adrenal cortices changed in parallel with the circulating levels of DHEAS. Finally, in the third report, the adrenal cortices from the animals in the second study were stained to reveal the presence of LH receptors (LHR), thus explaining how the adrenal increases steroid production when ovarian entrainment of LH declines. This third study confirmed the presence of LH receptors in the adrenal cortex of higher primates similarly to what has been reported in rodents. Perhaps it is the validation of an animal model that will likely provide the experimental avenue for understanding the underlying mechanism(s) is the most important aspect of these studies.
Collectively, these and future data can lead to a comprehensive explanation and the construction of an endocrine foundation for the events and progression of the MT. This construction will need to be consistent with and predictive of the unexpected results of recent population-based studies relating to mid-aged women. The higher circulating concentrations of Adiol compared to the circulating concentrations of E2 found in some mid-aged women are consistent with concentrations that have been shown to stimulate protein expression and cell proliferation in human, estrogen-sensitive cells in vitro, 41, 42 sufficient to elicit an estrogenic response in vivo in the immature female rat 43 and capable of contributing to circulating estrogenicity in middle-aged women. However, not all women share the most robust rise in Adiol as approximately 15% remain at or near pre-menopausal levels and most women experience levels between these two extremes. This wide range of circulating Adiol (1-8 nM), therefore, very likely explains the wide range of estrogen-related phenotypes and symptoms that are observed in perimenopausal women which has been difficult to reconcile with the relatively narrow range of circulating E2 levels during this same time period.44 The initial rise of circulating follicle stimulating hormone, the increase in the metabolic syndrome and other menopausal symptoms occur during the early peri-menopause, while the first detectable decline in circulating E2 does not occur until two years prior to the final menstrual period,44 or two to three years later than the onset of symptoms. This confined and narrow range in the decline in circulating E2 concentrations occurs when DHEAS, DHEA and Adiol are accelerating at their highest rates.2 Furthermore, the inter-women differences in DHEAS, DHEA and Adiol are the greatest of any unconjugated steroid hormone at that time 1-5 and the one hundred-fold higher circulating concentrations of Adiol in some women, compared to the levels of circulating estradiol, and its lesser affinity to sex hormone binding protein compensates for its lower estrogenic bioactivity compared to E2.
Several compelling relationships between ovarian function, adrenal steroid secretion and health outcomes are found in recent observations using the NHP animal model that compares interventions of E-alone intervention (which does not increase adrenal steroid production) and E+P (which induces increases adrenal cortex width and delta 5 steroid production). E-alone intervention has generally been shown to have superior benefits to mid-aged women lacking a uterus compared to E+P. In addition E-alone is associated with increased dendritic spinogenisis in the frontal cortex 45 and cognition 38 in experiments using the NHP. In contrast E+P has been linked with several adverse conditions in postmenopausal women and does not preserve dendritic spines or brain function in the NHP animal model.29 E+P intervention has been associated with increased breast cancer in perimenopausal Chinese women compared to E-alone 37 and E+P has been associated with an increase in cardiovascular disease in Caucasian women who initiate E+P HT more than six years post menopause.40 These observations suggest three interpretations. First, E-alone may be more protective of brain structure-function than E+P which more closely simulates the normal pattern of ovarian steroid production in younger, reproductive aged women. Second, E+P intervention has ethnic- and age-specific risks. Third, the benefit of E-alone may be associated with its ability to decrease adrenal steroid production in mid-aged women. Together these interpretations support the hypothesis that health benefits and risks are related to the relative circulating levels of individual sex steroids which may be predominantly emanating from the adrenal cortex as women age.
What is still needed?
There is some urgency in the need to initiate investigations to gain a deeper understanding of the endocrine foundations of the MT. Until recently HT interventions to ameliorate symptoms and adverse health outcomes have been considered adequate but not optimal. Overall the benefits were perceived to outweigh the risks and most women follow their physician's advice. However, recent evidence indicates that many women have disregarded this judgment in order to draw their own conclusion and make the decision to forego conventional HT in order to avoid possible health risks. In doing so it is now claimed in the lay press that they are putting themselves at risk for early mortality.
At least three avenues of research should be considered immediately. First, an additional broad epidemiological study is needed to characterize the relationship of adrenal function to symptoms and health outcomes of mid-aged women. These studies need to consider the possibility that circulating C-19 diols which have been largely ignored in the past, may contribute to the phenotypic differences that are observed during and after the MT. Second, clinical trials should be planned to evaluate the potential impact of increased adrenal delta five steroids and the possible benefits of intervening to control their levels. In addition simple and safe studies with gondadotropin releasing hormone agonists (GnRHas) should be conducted to determine if regulating circulating LH may be used as a means of regulating adrenal steroidogenesis. Third, experiments should be conducted in appropriate animal models to define and understand the mechanism(s) involved in the causal pathways that regulate adrenal steroidogenesis during the MT. Does the shift in adrenal steroid production act to shunt the flow of pregnenolone from progesterone and ultimately cortisol to the delta five pathway to permit the five-ten-fold rise in DHEA and Adiol? The much greater increase in circulating DHEA and Adiol indicate a shift in the steroidogenesis in the adrenal cortex. Depending on what level in the overall steroidogenic pathways this occurs, the result could be quite different in individual women. An increase above pregnenolone formation could result in a general increase in both delta 4 and delta 5 steroids and this would be consistent with increases in circulating cortisol suggested by some observation. However, a change in at the level of P450 c17 in the delta 5 pathway could lead to a shunting of pregnenolone products to delta 5 at the expense of the delta 4 pathway and result in a decrease in aldosterone and a compensatory rise in ACTH to maintain normal circulating cortisol levels. More importantly the degree in which the delta 5 pathway is affected would result in quite different steroid production profiles in individual women. While these scenarios are only speculative at this time, they seem to be consistent with the wide range of symptoms and different results that have been reported for women during the MT (Figure 3). Such a shift could have the opposite effect in humans compared to what is observed in rodent models. Instead of increasing cortisol, the drain on the precursor pregnenolone could result in less progesterone available for the production of cortisol.
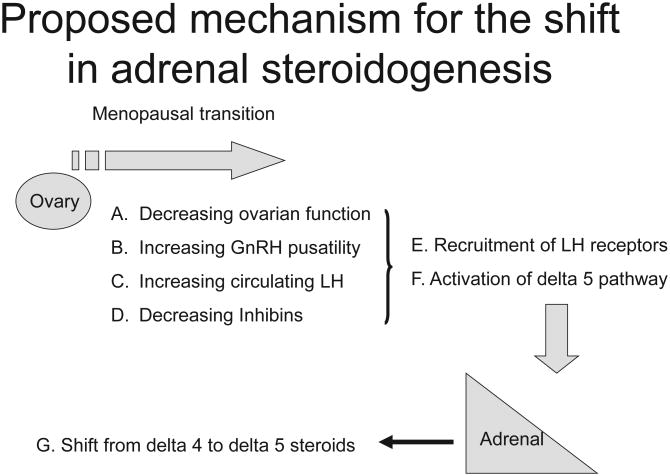
Figure 3.
A proposed mechanistic sequence for the shift in adrenal steroidogenesis during the menopausal transition. The progression of steps involves a decline in ovarian function (A) which leads to increased GNRH pulsatility (B), increasing luteinizing hormone (C) and decreased inhibins (D). In combination these events there is a recruitment of luteinizing hormone receptors in the adrenal cortex (E) and activation of the delta 5 steroid pathway (F). The chronic elevation of circulating luteinizing hormone acting on the activated luteinizing hormone receptors result in a shift from the delta 4 to delta 5 pathway.
Acknowledgments
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG0 12531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). In addition support from the CNPRC Base Grant (P51 RR00169 and the Morrison PPG (P01 AG01675) are gratefully acknowledged. The content of this articleis solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán-Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999;Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009;University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010– 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 –2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services)
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI2001 – 2012; New England Research Institutes, Water-town, MA - Sonja McKinlay, PI1995 – 2001.
Steering Committee: Susan Johnson, Current Chair and Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Conflicts of interest.—The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Lasley BL, Santoro N, Gold EB, Sowers MF, Crawford S, Weiss G, et al. The relationship of circulating DHEAS, testosterone and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab. 2002;87:3760–7. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- 2.Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, et al. Circulating Dehydroepiandrosterone Sulfate Concentrations during the Menopausal Transition. J Clin Endocrinol Metab. 2009;94:2945–51. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lasley B, Crawford S, Laughlin G, Santoro N, McConnell D, Crandall C, et al. Circulating Dehydroepiandrosterone Levels in Women with Bilateral Salpingo-Oophorectomy during the Menopausal Transition. Human Reproduction. 2010 doi: 10.1097/gme.0b013e3181fb53fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lasley BL, Stanczyk FX, Gee NA, Chen J, El Khoudary, Crawford S, et al. Androstenediol complements estradiol during the menopausal transition. Menopause. 2012;19:657–63. doi: 10.1097/gme.0b013e31823df577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McConnell DS, Santoro N, Randolf JR, Stanczyk FZ, Lasley BL. Menopausal transition stage-specific changes in circulating adrenal androgens. Menopause. 2012;19:650–7. doi: 10.1097/gme.0b013e31823fe274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labrie F, Cusan L, Gomez JL, Martel C, Bérubé R, Bélanger P, et al. Changes in serum DHEA and eleven of its metabolites during 12-month percutaneous administration of DHEA. J Steroid Biochem Mol Biol. 2008;110:1–9. doi: 10.1016/j.jsbmb.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Labrie F, Archer D, Bouchard C, Fortier M, Cusan L, Gomez JL, et al. Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause. 2009;16:907. doi: 10.1097/gme.0b013e31819e8e2d. [DOI] [PubMed] [Google Scholar]
- 8.Davis SR, Shah SM, McKenzie DP, Kulkarni J, Davison SL, Bell RJ. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab. 2008;93:801–8. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- 9.Mamas L, Mamas E. Premature ovarian failure and dehydroepiandrosterone. Fertility and Sterility. 2009;91:644–6. doi: 10.1016/j.fertnstert.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 10.Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab. 2011;96:1642–53. doi: 10.1210/jc.2010-2888. [DOI] [PubMed] [Google Scholar]
- 11.Bird CE, Murphy J, Boroomand K, Finnis W, Dressel D, Clark AF. Dehydroepiandrosterone: kinetics of metabolism in normal men and women. J Clin Endocrinol Metab. 1978;47:818–22. doi: 10.1210/jcem-47-4-818. [DOI] [PubMed] [Google Scholar]
- 12.Saxena AR, Seely EW. Luteinizing hormone correlates with adrenal function in postmenopausal women. Menopause. 2012;19:1280–3. doi: 10.1097/gme.0b013e31825540c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melton LJ, III, Khosla S, Atkinson EJ, O'Connor MK, O'Fallon WM, Riggs BL. Cross-sectional versus longitudinal evaluation of bone loss in men and women. Osteop Int. 2000;11:592–9. doi: 10.1007/s001980070080. [DOI] [PubMed] [Google Scholar]
- 14.Pattishall EN, Helms RW, Strope GL. Noncomparability of cross-sectional and longitudinal estimates of lung growth in children. Pediatr Pulmonol. 1989;7:22–8. doi: 10.1002/ppul.1950070107. [DOI] [PubMed] [Google Scholar]
- 15.Hendrick DJ, Becklake M, Hanley JA. Discordance between cross-sectional and longitudinal studies for the effect of dust on COPD: Why? COPD. 2005;2:395–404. doi: 10.1080/15412550500346436. [DOI] [PubMed] [Google Scholar]
- 16.Rönnlund M, Lövdén M, Nilsson LG. Cross-sectional versus longitudinal age gradients of tower of Hanoi performance: the role of practice effects and cohort differences in education. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15:40–67. doi: 10.1080/13825580701533751. [DOI] [PubMed] [Google Scholar]
- 17.Louis TA, Robins J, Dockery DW, Spiro A, III, Ware JH. Explaining discrepancies between longitudinal and cross-sectional models. J Chron Dis. 1986;39:831–9. doi: 10.1016/0021-9681(86)90085-8. [DOI] [PubMed] [Google Scholar]
- 18.Givens ML, Chensheng L, Bartell SM, Pearson MA. Estimating dietary consumption patterns among children: A comparison between cross-sectional and longitudinal study designs. Environ Res. 2007;103:325–30. doi: 10.1016/j.envres.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of longitudinal data. 2nd. Oxford: Oxford University Press; 2002. [Google Scholar]
- 20.Vollmer VM. Reconciling cross-sectional with longitudinal observations on annual decline. Occup Med. 1993;8:339–51. [PubMed] [Google Scholar]
- 21.Tepper PG, Randolph JF, Jr, McConnell DS, Crawford SL, El Khoudary SR, Joffe H, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women's Health Across the Nation (SWAN) J Clin Endocrinol Metab. 2012;97:2872–80. doi: 10.1210/jc.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkes AS. The adrenal-gonadal relationship. Physiological Reviews. 1945;25:203–54. [Google Scholar]
- 23.Lacroix A, Ndiaye N, Tremblay J, Hamet P. Ectopic and abnormal hormone receptors in adrenal Cushing's syndrome. Endocr Rev. 2001;22:75–110. doi: 10.1210/edrv.22.1.0420. [DOI] [PubMed] [Google Scholar]
- 24.Christopoulos S, Bourdeau I, Lacroix A. Aberrant expression of hormone receptors in adrenal Cushing's syndrome. Pituitary. 2004;7:225–35. doi: 10.1007/s11102-005-1083-7. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay JR, Nieman LK. Adrenal disorders in pregnancy. Endocrinol Metab Clin North Am. 2006;35:1–20. doi: 10.1016/j.ecl.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Carlson HE. Human adrenal cortex hyperfunction due to LH/hCG. Mol Cell Endocrinol. 2007;269:46–50. doi: 10.1016/j.mce.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Bernichtein S, Peltoketo H, Huhtaniemi I. Adrenal hyperplasia and tumours in mice in connection with aberrant pituitary-gonadal function. Mol Cell Endocrinol. 2009;300:164–8. doi: 10.1016/j.mce.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Pabon JE, Li X, Lei ZM, Sanfilippo JS, Yussman MA, Rao CV. Novel presence of luteinizing hormone/chorionic gonadotropin receptors in human adrenal glands. J Clin Endocrinol Metab. 1996;81:2397–400. doi: 10.1210/jcem.81.6.8964884. [DOI] [PubMed] [Google Scholar]
- 29.Nishii, Nomura M, Sekine Y, Koike H, Matsui H, Shibata Y, et al. Luteinizing hormone releasing hormone agonist reduces serum adrenal androgen levels in prostate cancer patients: Implications for the effect of LH on the adrenal glands. J Androl. 2012;33:1233–8. doi: 10.2164/jandrol.112.016493. [DOI] [PubMed] [Google Scholar]
- 30.Moran FM, Chen J, Gee NA, Lohstroh PN, Lasley BL. Dehydroepiandrosterone sulfate levels reflect endogenous luteinizing hormone production and response to human chorionic gonadotropin challenge in older female macaque (Macaca fascicularis) Menopause. 2013;20:329–35. doi: 10.1097/GME.0b013e3182698f80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conley AJ, Morrison JH, Stanczyk FX, Gee NA, Lasley BL, Borowics P, et al. Modulation of adrenal androgen production with estrogen versus estrogen plus progesterone. Menopause. 2013 doi: 10.1097/GME.0b013e318273a070. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lasley BL, Conley AJ, Hyde DM, Ventimiglia FF, Gee NA, Morrison RH, et al. Luteinizing hormone receptors in the adrenal cortex of female higher primates. Presented at the Annual Meeting of the Endocrine Society. 2013 [Google Scholar]
- 33.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. A randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 34.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. HERS Research Group Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. Erratum in: JAMA 2002;288:1064. [DOI] [PubMed] [Google Scholar]
- 35.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 36.Hotis HN, Mack WJ. Hormone replacement therapy and the association with coronary heart disease and overall mortality: Clinical application of the timing hypothesis. J Steroid Biochem. 2013 doi: 10.1016/j.jsbmb.2013.06.011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Su IH, Chen YC, Hwang WT, Liu Z, Su TP, Chen TJ, et al. Risks and benefits of menopausal hormone therapy in postmenopausal Chinese women. Menopause. 2012;19:931–41. doi: 10.1097/gme.0b013e31824362ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–14. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohm DT, Bloss EB, Janssen WG, Dietz KC, Wadsworth S, Gee NA, et al. Clinically relevant hormone treatment regimens fail to induce spinogenesis in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2012;32:11700–5. doi: 10.1523/JNEUROSCI.1881-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sifferlin A. Hormone-replacement therapy: could estrogen have saved 50,000 lives? Time Magazine. 2013 [Google Scholar]
- 41.Poulan R, Labrie F. Stimulation of cell growth by C-19 steroids of adrenal origin in the ZR-75-1 human breast cancer cell line. Cancer Research. 1985;46:4933–7. [PubMed] [Google Scholar]
- 42.Seymour-Munn K, Adams JB. Estrogenic effects of 5-androstene-3beta, 17beta diol at physiological concentrations and its possible implications in the etiology of breast cancer. Endocrinol. 1981;112:486–91. doi: 10.1210/endo-112-2-486. [DOI] [PubMed] [Google Scholar]
- 43.Van Doorn LG, Berenschot-Roozendaal J, Poortman J, Thijssen JH, Schwarz F. Binding characteristics of 5-androstene-3 beta, 17 beta-diol and estradiol-17 beta to the cytoplasmic estrogen receptor of the immature rat uterus. J Steroid Biochem. 1982;16:661–71. doi: 10.1016/0022-4731(82)90103-0. [DOI] [PubMed] [Google Scholar]
- 44.Randolph JF, Jr, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88:1516–22. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 45.Hao J, Rapp PR, Janssen WGM, Lou W, Lasley BL, Hof PR, et al. Interactive effects of age and estrogen on cognition and pyramidal neuron in monkey prefrontal cortex. PNAS. 2007;104:11465–7. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]