Abstract
In the US, children diagnosed with cancer are living longer, but not without consequences from the same drugs that cured their cancer. In these patients, cardiovascular disease is the leading cause of non-cancer-related morbidity and mortality. Although this review focuses on anthracycline-related cardiomyopathy in childhood cancer survivors, the global lifetime risk of other cardiovascular diseases such as atherosclerosis, arrhythmias and intracardiac conduction abnormalities, hypertension, and stroke also are increased. Besides anthracyclines, newer molecularly targeted agents, such as vascular endothelial growth factor receptor and tyrosine kinase inhibitors, also have been associated with acute hypertension, cardiomyopathy, increased risk of ischemic cardiac events and arrhythmias, and are summarized here. This review also covers other risk factors for chemotherapy-related cardiotoxicity (including both modifiable and non-modifiable factors), monitoring strategies (including both blood and imaging-based biomarkers) during and following cancer treatment, and discusses the management of cardiotoxicity (including prevention strategies such as cardioprotection by use of dexrazoxane).
Introduction
The 5-year survival rate of US childhood cancers has increased from around 60% in the mid-1970s to more than 80% [1]. This increase is attributed, in part, to treatment advancements [1]. Some treatments can cause multi-organ toxicities, including the heart. About 65% of anthracycline-treated childhood cancer survivors have subclinical myocardial dysfunction [2]. Among survivors of childhood cancer, cardiovascular disease is the leading cause of morbidity and mortality after cancer recurrence and secondary malignancies [3, 4]. With increasing numbers of childhood cancer survivors comes a focus on monitoring for treatment-related long-term morbidity and mortality.
We review cardiotoxic effects of anthracyclines and other antineoplastic agents used to treat childhood cancers. We describe the mechanisms by which cardiotoxicity develops, its cardiac risk factors, screening and monitoring techniques, and measures to prevent or reduce cardiotoxicity in these vulnerable patients. We recognize that the increased incidence of global cardiovascular morbidity and mortality in this population has multifactorial mechanisms.
1. Risk Factors
Modifiable risk factors for anthracycline-related cardiotoxicity include drug-related and behavioral factors, such as anthracycline cumulative dose and dose rate, concomitant treatments, physical inactivity, metabolic risk factors, and smoking [5]. Non-modifiable risk factors include age, sex, and genetics [5]. Besides anthracyclines, other potentially cardiotoxic cancer therapies are discussed (Section 4.0).
1.1 Therapy-Related Risk Factors
An established therapy-related risk factor for abnormal left ventricular (LV) function is the cumulative anthracycline dose [6–9]. Since cardiac damage can occur at doses less than 240 mg/m2 [10], there might not be any “safe” dose of anthacyclines. Both cumulative dose and length of follow-up are risk factors for anthracycline cardiotoxicity in survivors. Thus, it is important to understand that a paucity of evidence of cardiotoxicity after relatively short follow-up might not reflect the findings after longer follow-up of the same patient. Much of the cardiotoxicity is progressive in nature.
A long-standing question is whether cardiotoxicity can be attenuated by replacing high peak serum doxorubicin concentrations from bolus infusion with lower peak concentrations associated with continuous infusion [11]. In a multicenter, randomized clinical trial (RCT) of childhood high-risk acute lymphoblastic leukemia (ALL) patients, neither cardioprotection nor event-free survival was better in patients receiving continuous versus bolus infusion doxorubicin [12]. In the absence of cardioprotection, recommendations exist that continuous infusion be discontinued in children because it increases length of hospital stay, costs, and risks of thromboembolic events and mucositis [13].
Mediastinal radiation with or without anthracycline treatment is another risk factor for cardiotoxicity [14, 15]. Low-dose cardiac radiation has been associated with an increased lifetime risk of cardiovascular morbidity and mortality [14, 16]. Among 294 survivors of Hodgkin’s Lymphoma exposed to mediastinal irradiation, 23 had coronary events, including 10 with myocardial infarctions after 6.5 years of follow-up [17]. Cranial irradiation is also a risk factor for cardiovascular disease and abnormalities [18], presumably mediated by growth hormone deficiency and related to lower LV mass [19, 20].
1.2 Behavioral Risk Factors
As in the general population, potentially modifiable cardiovascular risk factors in childhood cancer survivors include inactivity, hypertension, dyslipidemia, endocrinopathies, obesity, diabetes, illicit drug use, alcohol use, caffeine, and smoking, [20, 21, 22–25]. Survivors are more likely to be inactive and less likely to meet physical activity standards than siblings or age- and sex-matched healthy controls [25, 26].
Hypertension incidence in childhood cancer survivors is 7.8% after 15 years of follow-up [27]. Hypertension increases the risk for coronary artery disease (relative risk [RR], 6.1), heart failure (RR, 19.4), valvular disease (RR, 13.6), and arrhythmia (RR, 6.0), and is an independent risk factor for cardiac death (RR, 5.6; 95% CI, 3.2 to 9.7) [28].
Although the incidence of obesity rate among survivors is similar to that of general population [22, 23, 25], certain survivor sub-groups are at increased risk [29]. Those treated with cranial irradiation had lower mean blood insulin growth factor-1 concentrations than unexposed survivors, increasing obesity risk [19]. Obesity increases the risk of insulin resistance and diabetes mellitus [22–24]. Abdominal, brain, and total body irradiation results in an increased incidence of diabetes mellitus in long-term survivors [30]. Smoking prevention and cessation programs may improve long-term outcomes of survivors [31]. Survivors are not more likely than siblings to smoke (15% and 16%, respectively), but may have disproportionately increase risk of adverse health outcomes [32].
1.3 Non-Modifiable Risk Factors
Younger at the time of treatment is associated with anthracycline-related cardiotoxicity [33, 34], including a higher risk of increased LV afterload and decreased LV mass and LV wall thickness [7].
The risk of late-onset depressed LV contractility is higher in girls than in boys, even at the same cumulative doxorubicin dose [7, 33]. Because anthracycline dose calculation is based on body-surface area, and girls have a higher percentage of body fat than boys, and doxorubicin is poorly fat-soluble, the higher risk among girls might be related to lower fat doxorubicin concentrations and higher cardiomyocyte concentrations [7]. Similar sex-related outcome differences after dexrazoxane cardioprotection suggest the possibility of increased free-radical injury susceptibility in girls [35].
Children with pre-existing cardiovascular diseases are at increased risk of anthracycline-related cardiotoxicity, as are children with cancer and pre-treatment elevated cardiac troponin concentrations [36]. Those with family histories of premature cardiovascular disease and genetic susceptibilities to cardiovascular diseases are also at increased risk of cardiotoxicity [37]. Inter-individual variation in risk of anthracycline-related cardiotoxicity at a given dose suggests genetic predisposition [37–41]. Mutations of the hemochromatosis gene (HFE) associated with hereditary hemochromatosis can interfere with iron metabolism and lead to iron overload, increasing susceptibility to anthracycline-related cardiotoxicity [37, 42]. The 10% of children with acute lymphoblastic leukemia (ALL) carrying the HFE C282Y mutation had nine-times the risk of doxorubicin myocardial injury than that of non-carriers [37]. Patients who are homozygous for the G allele of the CBR3 gene, which encodes carbonyl reductase 3, also had an increased risk of cardiomyopathy [39]. These genetic variants may assist in screening, guiding treatment, and in post-chemotherapy monitoring [37]. African-American patients and those with trisomy-21 are also more susceptible to anthracycline-related cardiotoxicity [43].
2. Monitoring Chemotherapy-Induced Cardiotoxicity
Monitoring children treated with chemotherapy might detect early cardiotoxicity even when LV dysfunction is asymptomatic, thus providing opportunities to prevent, attenuate, or reverse pathologic LV remodeling.
2.1 Myocardial Injury and Cardiomyopathy Biomarkers
Cardiac-specific biomarkers detect early anthracycline-induced cardiovascular injury. Cardiac troponins (including serum cardiac troponin T [cTnT] and serum cardiac troponin I [cTnI]) are intra-cardiomyocyte contractile proteins detectable in the blood after active cardiomyocyte injury or necrosis. Highly-sensitive troponin assays predict cardiac structural abnormalities and mortality in the general population [44, 45].
Troponins identify anthracycline-induced cardiomyocyte injury and subsequent cardiomyopathy risk. Cardiac troponin I concentrations can predict the risk of cardiac events in adults receiving high-dose chemotherapy [46]. Adults with persistently elevated cTnI concentrations 1 month after anthracycline therapy had more cardiac events at 3 years (84%) than patients with transient or no cTnI elevations (37% and 1%, respectively) [46]. A randomized clinical trial (RCT) of prophylactic enalapril revealed the short-term benefit of early cardioprotective intervention in high-risk adults with anthracycline-induced elevations in cTnI concentration(s) [47]. One year after anthracycline therapy, LV systolic function was preserved with fewer cardiac events in enalapril-treated patients than controls [47]. This study had a short follow-up but illustrates the potential use of biomarker concentrations to guide cardioprotective therapy for early anthracycline-induced heart disease [48].
Cardiac troponin T concentrations are associated with cardiac outcomes in children receiving moderate-dose anthracyclines for high-risk ALL. Among 134 children, cTnT concentrations were measured before, during, and at the end of anthracycline therapy [36, 49]. More than 15% of children presenting with ALL had elevated cTnT prior to receiving anthracyclines and were at higher risk for anthracycline cardiotoxicity [36]. Concentrations of cTnT were elevated (>0.01 ng/mL) in more than a third of children and, when present during the first 90 days of therapy, were associated with subsequent reduced LV mass, LV thickness-to-dimension ratio (increased pathologic LV remodeling), and LV end-diastolic posterior wall thickness [49].
N-terminal pro-brain natriuretic peptide (NT-proBNP) is an unspecified marker of ventricular wall stress that can be elevated in a number of cardiovascular and non-cardiovascular conditions. For example, elevated NT-proBNP concentrations may indicate cardiomyopathy with increased LV wall stress from pressure or volume overload, and has been shown to predict anthracycline-induced cardiotoxicity in children with high-risk ALL [49]. Concentrations were elevated in more than 90% of 156 children receiving moderate-dose anthracyclines for high-risk ALL and, when detected during the first 90 days of therapy, were associated with an abnormal LV thickness-dimension ratio 4 years later, suggesting pathologic LV remodeling [49]. Other studies have reported acute elevations of NT-proBNP or BNP concentrations during anthracycline therapy; however, they have not always been able to assess associations between biomarker concentrations and LV structural and functional parameters at follow-up [50–52].
These studies substantiate cTnT and NT-proBNP concentrations as markers of early anthracycline-induced cardiotoxicity and validate these during-therapy markers as surrogates of late LV structural status in long-term survivors [36, 49]. For all cardiac biomarkers validated as surrogates for late cardiotoxicity, biomarker-guided cancer chemotherapy should be compared to conventional therapy to determine which has the better oncologic efficacy-to-cardiotoxicity profile [49].
2.2 Other Cardiovascular Biomarkers
Other cardiac biomarkers associated with development or progression of heart failure have not been fully validated in survivors of childhood cancer.
Inflammatory mediators, including interleukin-6, tumor necrosis factor-alpha, and high-sensitivity C-reactive protein, may be useful cardiovascular biomarkers, given the importance of inflammation in the progression of heart failure [53]. These markers can independently predict heart failure and adverse outcomes in non-oncology patients with heart failure [54–56]. High-sensitivity C-reactive protein measured during doxorubicin therapy for childhood ALL increased with higher cumulative doxorubicin doses and cTnT concentrations, but was not predictive of late cardiotoxicity [49]. In a small study of adults with cancer, elevated interleukin-6 concentrations after epirubicin administration correlated with impaired LV contractility even at 18-month follow-up [57].
Cystatin-C, a protease inhibitor from oxidative stress, promotes extracellular cardiac matrix remodeling [58]. Cystatin-C concentrations predict subclinical cardiac structural abnormalities and heart failure in asymptomatic individuals and mortality in patients with heart failure [59, 60]. Plasma cystatin-C concentrations are elevated in early murine doxorubicin-induced cardiomyopathy [58].
Galectin-3 is a β-galactoside-binding lectin that promotes cardiac fibroblast proliferation and collagen synthesis after myocardial injury [61, 62]. Elevated galectin-3 concentrations predict adverse outcomes in heart failure, but have not been evaluated in childhood cancer survivors [63].
MicroRNAs are post-transcriptional gene expression regulators involved in cardiac regeneration, repair, and pathologic remodeling associated with stress and disease [43]. However, this is still an evolving concept. Whether or not circulating myocardial-derived microRNA concentrations are sensitive and specific markers of cardiomyocyte injury and cardiovascular disease is still under debate [65]. MicroRNA dysregulation after anthracycline-induced cardiomyocyte injury was found in mice but not in patients receiving anthracyclines [66, 67].
2.3 Cardiac Imaging during Chemotherapy
Echocardiography is commonly used for monitoring cardiac structure and function. It is non-invasive, painless, and widely available. Early guidelines for monitoring chemotherapy cardiotoxicity involved assessing LV ejection fraction and LV fractional shortening [68]. These guidelines also recommended the frequency of monitoring and the criteria for discontinuing anthracycline therapy; however, these guidelines have been criticized for not being evidence-based [69]. The impact of these guidelines on detecting later cardiac outcomes and of dose-modification on cancer outcomes remains unclear.
LV fractional shortening was evaluated in doxorubicin-treated children with ALL and has not correlated with elevations cTnT. Yet, in that study, cTnT elevations were validated as surrogates of late-occurring abnormalities of LV structure [36, 49]. Thus LV fractional shortening measured during therapy to predict long-term cardiovascular status was not supported by longitudinal studies [36, 49]. Such measurements ascertained during therapy may be abnormal from many causes unrelated to anthracycline-induced myocardial injury. Thus, the fact that these abnormalities do not relate to long-term cardiac status is not surprising [49]. End-therapy measurements of LV structure and function have predictive value for late cardiac status [33]. Both LV ejection fraction and LV fractional shortening are load-dependent, may not be sensitive to detect restrictive anthracycline-related cardiomyopathy, nor reflect changes in LV contractility or mass.
Data on the utility of other echocardiographic techniques for monitoring of children during chemotherapy is limited. The results of 2-dimensional, speckle-tracking-derived longitudinal strain measurements in 19 children at baseline, 4 and 8 months during chemotherapy demonstrate that changes in LV global longitudinal peak systolic strain preceded changes in LV ejection fraction and segmental deformation in mid- and apical-segments and correlate with decreases in LV ejection fraction at later follow-up [70].
Doppler speckle-tracking-derived longitudinal strain echocardiography has been useful in assessing adults for cardiac damage [71]. Comparing the sensitivity of strain-rate imaging using Doppler techniques with conventional echocardiograms in 16 elderly women with breast cancer at baseline and after 3 and 6 cycles of liposomal doxorubicin showed that LV dimensions, ejection fraction, and systolic myocardial velocities were similar between modalities; longitudinal and radial strain and strain-rate were markedly lower, with radial strain showing greater and earlier changes [72].
Similarly, LV 2-dimensional longitudinal and radial systolic strain measured by speckle-tracking were significantly reduced 1 week after chemotherapy in women with breast cancer without LV ejection fraction changes [73]. Measurements of peak systolic myocardial longitudinal, radial and circumferential strain using speckle-tracking in 43 patients with breast cancer at baseline, and at 3 and 6 months during chemotherapy indicated that longitudinal strain-rate predicted the development of lower LV ejection fraction at 6 months [74]. These studies had a short follow-up duration [74].
Evaluation of serum biomarkers and the myocardial performance index (MPI), a global index of LV systolic and diastolic function thought to be shape- and load-independent, at diagnosis and 24 hours after each anthracycline course in 19 children with standard-risk ALL showed that the MPI was increased at higher cumulative anthracycline doses. Similarly, 13 children had increased LV end-diastolic wall thickness, higher MPIs, and reduced LV wall thickening 2 hours after initial anthracycline dosing [75]. Diastolic LV function variables, such as E/A ratio and isovolumic relaxation time, changed markedly, as did longitudinal and radial systolic strain and strain rates [75].
Cardiac MRI may characterize myocardial tissue and assess perfusion abnormalities independent of a good transthoracic window to obtain acceptable echocardiographic images. Of 10 non-Hodgkin's lymphoma patients with cardiac MRI before and 3 months after doxorubicin-based chemotherapy, 5 had a greater than 10% decreases in LV ejection fraction, and three had at least one new or progressive segment of gadolinium-delayed enhancement at follow-up [76]. Global circumferential strain was lower after chemotherapy, suggesting that MRI detected early functional changes [76]. Myocardial contrast enhancement increased by day 3 of anthracyclines, which predicted a decrease in LV ejection fraction at 28 days [77]. The end-systolic LV volume index increased, and LV and RV ejection fraction decreased to below 55% in 15 of 28 young cancer patients during therapy [78].
The optimal timing, modality, measures, and impact of cardiotoxicity monitoring during chemotherapy need to be investigated further.
Radionuclide ventriculography is also used as a modality to detect cardiac dysfunction, which is reliable, reproducible and may be less operator dependent as compared to 2D echocardiography. In patients with limited windows for clear imaging by echocardiography radionuclide ventriculography is a useful option for monitoring ventricular function in this population. For young children, however, the radiation exposure and occasional need for sedation limit the use of radionuclide ventriculography.
2.4 Cardiac Imaging after Chemotherapy
Consensus-based guidelines for cardiotoxicity monitoring following chemotherapy using echocardiography exist [79]. Optimal timing and cost-effectiveness for such monitoring needs further investigation [80]. Left ventricular fractional shortening and ejection fraction are common measures for cardiotoxicity surveillance among children who have received chemotherapy. Six years after chemotherapy, about 30% of 115 children had reduced LV fractional shortening [2]. This has been assessed by others [75, 81]. Both measures are load-dependent, have measurement variability, lack sensitivity for subtle changes in function and have not been tested for long-term clinical outcome correlation. Although measuring LV ejection fraction by 3-dimensional echocardiography or the biplane method may improve reproducibility, and the results may correlate with the LV ejection fraction measured by MRI, there is no “gold-standard” for LV fractional shortening or ejection fraction measurement technique in terms of being a validated surrogate predictive of late clinically significant cardiovascular disease [82, 83]. Other indices, such as LV mass and LV end-diastolic posterior wall thickness, have been greatly reduced at 3, 6, and 8 years of follow-up [12, 33].
Serial myocardial performance, or the Tei index, in 26 children who received chemotherapy showed the index increased before other conventional LV systolic and diastolic functional variables did, even at low doses and as early as a year after chemotherapy [84]. Changes [0.53 (SD 0.08) vs. 0.37 (SD 0.06) in controls] in MPI were noted in as many as 83% of survivors on follow-up, despite normal LV fractional shortening, LV mass, and LV wall thickness [85]. The MPI is affected by LV afterload, preload, contractility, conduction disorders, activation synchrony, and is abnormal whenever any of these factors are abnormal so has not been a good predictor.
Longitudinal LV diastolic function measurements in survivors correlate with anthracycline dose. After chemotherapy, the ratio of early (E) and late (A) mitral inflow velocity (E/A) decreases, isovolumic relaxation time (IVRT) is prolonged, and the pulmonary venous Doppler pattern is abnormal [75, 85]. The ratio of E to the early velocity as measured by tissue Doppler imaging (E') (E/E’) was significantly higher in 32 survivors of childhood cancer than in healthy controls [86]. The clinical importance of the small difference in E/E' between the groups is unclear and the ratio in both groups was normal [86]. The E/A ratio is also load-dependent and only weakly correlated with other diastolic variables and subsequent impaired LV systolic function [87].
Tissue Doppler imaging, in addition to standard echocardiography, was studied in 63 children treated with anthracyclines [88]. Right ventricular wall thickness and LV fractional shortening were lower, and LV diameter was higher than in healthy controls. Tissue Doppler measures of peak late diastolic myocardial velocities and transmyocardial systolic and diastolic velocities were also significantly lower in treated children than in controls. The authors postulated that the transmyocardial velocity differences were secondary to wall stiffness [88]. Twenty-percent of anthracycline-treated children had local paradoxical myocardial movements of the basal and mid LV wall [88].
Speckle-tracking echocardiography can detect subtle changes in cardiac dysfunction, but reproducibility has been a problem. Myocardial strain and strain-rate, as measured by speckle-tracking echocardiography, are dimensionless, angle- and load-independent global measures of LV function. Two-dimensional speckle-tracking echocardiograms in 47 survivors of Hodgkin's lymphoma 22 years after receiving chemotherapy demonstrated global longitudinal and circumferential strain and LV ejection fraction were lower than those of healthy controls [89]. Further, patients treated with anthracyclines had lower global longitudinal strain than that of those treated with radiotherapy. Left ventricular ejection fractions did not differ between groups.
Torsion analysis using speckle-tracking echocardiography in 25 adults before and at 1 and 3 months after treatment showed at 1 month torsion and twisting and untwisting rates had markedly deteriorated, and torsion measures were inversely correlated with cumulative anthracycline dose [90]. At 3 months follow-up, the isovolumic relaxation time was prolonged but all other Doppler indices were unchanged [90]. Another evaluation of torsion variables in 36 children confirmed markedly lower apical twisting and lower peak LV torsion, compared to controls, even in the absence of an abnormal LV ejection fraction [91].
Speckle-strain imaging is time-consuming and usually performed offline. Although many techniques appear sensitive in detecting cardiotoxicity, whether their results correlate with longitudinal cardiac outcomes in children who have received chemotherapy is unknown. The impact and cost of incorporating these techniques into the routine surveillance of children receiving chemotherapy needs study.
Cardiac MRI may provide quality images in echo-poor windows (e.g. in obese patients) to measure LV function; but is time-consuming, may be unavailable, requires trained physician interpretation, and in younger patients, may require sedation. T2-weighted imaging can detect edema and active myocardial inflammation. Gadolinium contrast-infused delayed enhancement can detect patchy sub-endocardial fibrosis in patients after myocardial infarction; it is not as effective for detecting diffuse fibrosis. Because doxorubicin cardiotoxicity may cause myocardial necrosis and fibrosis, cardiac MRI monitoring may be useful. Cardiac MRI found mid-myocardial delayed enhancement in 10 women with breast cancer treated with doxorubicin, all of whom had poor LV function [92].
Myocardial T1 mapping uses the T1 relaxation time to calculate the volume and distribution of gadolinium in the myocardium. Relaxation time is increased in the presence of diffuse myocardial fibrosis [77]. Whether cardiac MRI abnormalities in children can predict cardiac outcomes after chemotherapy remains unknown. Further, experimental imaging techniques using molecular probes designed to image areas of apoptosis are being developed [93].
3 Managing Cardiotoxicity
3.1 Prevention of Cardiotoxicity
There is strong evidence for dexrazoxane as a cardioprotectant. Dexrazoxane is an EDTA-like bis dioxopiperazine that decreases oxygen free radicals through intracellular iron chelation, among other actions, including being a weak topoisomerase inhibitor [94]. In two meta-analyses, dexrazoxane was associated with 60% to 80% fewer clinical and subclinical cardiac events during and after anthracycline-based therapy [95, 96]. Overall, toxicity and measures of tumor response were similar between patients exposed and unexposed to dexrazoxane [95]. Currently, the US FDA approves dexrazoxane for women with metastatic breast cancer who have received 300 mg/m2 of doxorubicin and who may receive additional anthracycline-based therapy. The American Society of Clinical Oncology also recommends considering dexrazoxane for adults with any history of cancer who have already received 300 mg/m2 of doxorubicin-based therapy [97]. Neither of these recommendations to start dexrazoxane after initial anthracycline-induced cardiomyocyte toxicity comports with our current understanding of the mechanism of cardioprotection.
Intermediate or surrogate endpoints in multiple RCTs of children show that dexrazoxane reduces cardiotoxicity [35, 36, 98]. Among 38 children with sarcoma, the rate of subclinical heart failure was lower among children receiving dexrazoxane than in those who did not (22% vs. 67%; P<0.01). Further, the dexrazoxane group received greater cumulative anthracycline doses before cardiotoxicity developed (410 vs. 310 mg/m2, P<0.05) [98].
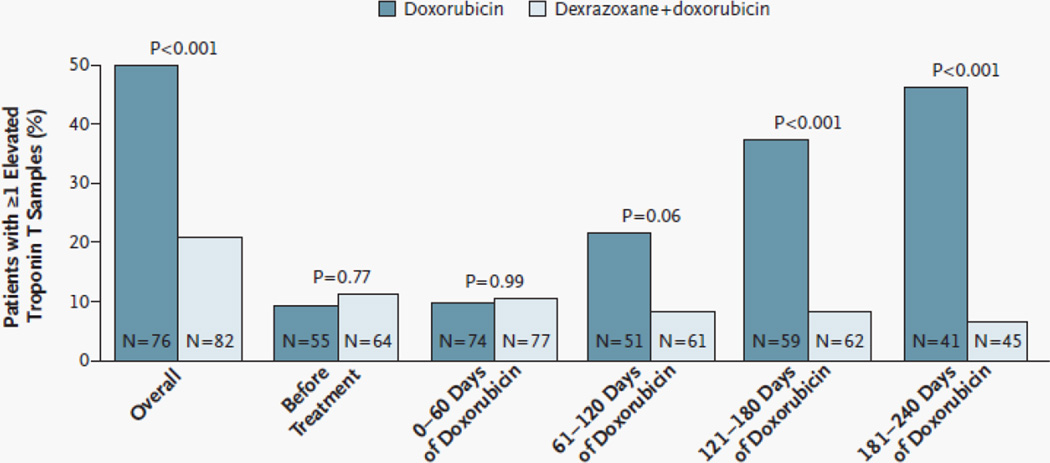
Among 206 children with ALL, those receiving dexrazoxane had fewer episodes of elevated cTnT concentrations after anthracycline treatment (21% vs. 50%, P<0.001; Figure 2) [36]. Longer-term follow-up data from 134 of the 206 children demonstrated various echocardiographic indices of LV structure and function were worse in those not receiving dexrazoxane [35]. Girls benefited from dexrazoxane more than boys, particularly with respect to changes in the LV end-diastolic thickness-to-dimension ratio, a marker of pathologic LV remodeling. Other large studies have found dexrazoxane to be cardioprotective in children treated with doxorubicin for T-cell ALL and lymphoma [99], in children with osteosarcoma [100], whose treatment also included the known cardiotoxic drug traztusamab; and in children with osteosarcoma treated with doxorubicin dose escalations to 600 mg/m2 cumulative dose [101].
Figure 2. Percentage of Patients With at Least One Elevated Serum Cardiac Troponin T Measurement Overall, Before Treatment with Doxorubicin, and During Treatment.
An elevated level of troponin T was defined as one that exceeded 0.01 ng per milliter. The number of patients in whom troponin T was measured at least once during the specified intervals is shown in each bar. (Reprinted with permission from Massachusetts Medical Society [36].)
In all five of these trials, neither recurrence rates nor overall survival differed between treatment groups [98–102]. Moreover, earlier concerns that dexrazoxane may be associated with an increased risk of second cancers in children with Hodgkin’s lymphoma [103] have not been borne out, although such concerns have limited its widespread use among children [104, 105].
The long-term cardiac outcomes of patients treated on 2 Hodgkin’s lymphoma clinical trials remain unknown [103, 106, 107]. An ongoing Children’s Oncology Group study is following these children to determine whether dexrazoxane exposure is associated with longer-term effects on cardiac outcomes and to update the data on second cancers and overall mortality (National Clinical Trial #01790152) [108].
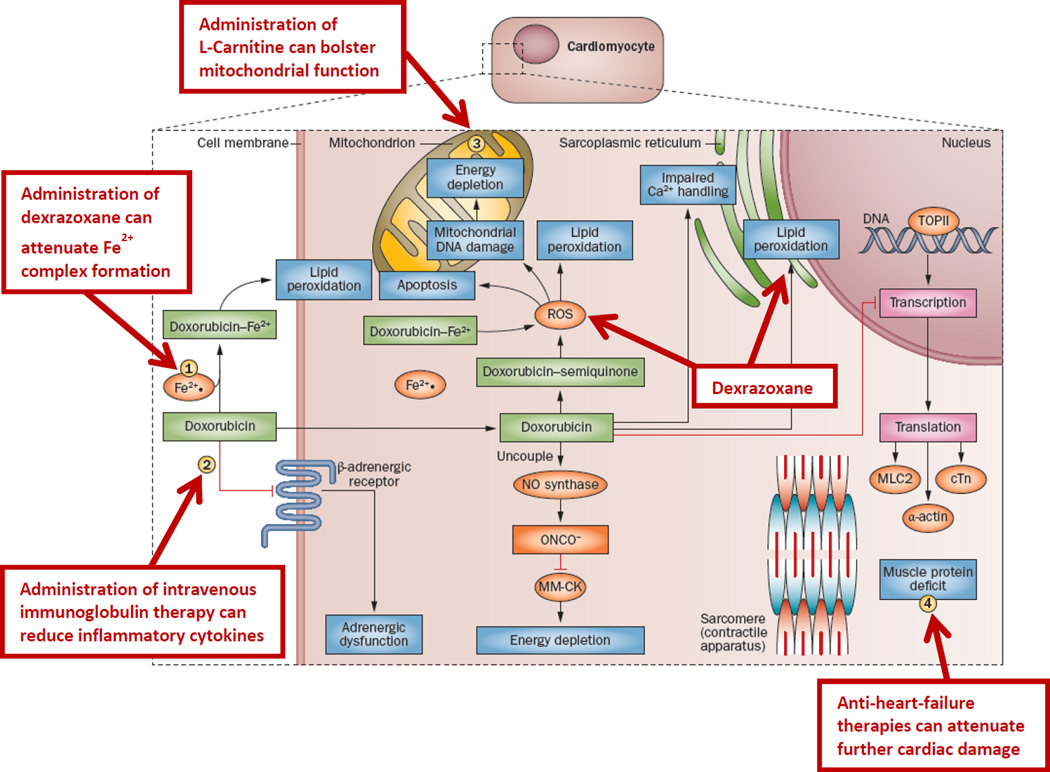
Cardioprotectants tested in RCTs, include amifostine [109], acetylcysteine [110, 111], calcium channel blockers [112, 113], carvedilol [114], coenzyme Q10 [115], and L-carnitine [116] (Figure 1). However, evidence of efficacy from these mostly smaller trials is not compelling. None of these agents is considered standard-of-care [95–97, 117]. Data on the potential cardioprotection associated with liposomal formulations of anthracyclines in children is limited. A Phase 1 study of liposomal daunorubicin in 48 children reported no cardioprotection with substantial cardiotoxicity, although all had been exposed to conventional anthracyclines [118].
Figure 1. Potential Opportunities for Cardioprotection.
Doxorubicin chemotherapy has a range of effects on cardiomyocytes. It induces lipid peroxidation at the cell and mitochondrial membranes by way of complexing with Fe2+ and induces apoptosis, mitochondrial DNA damage and energy depletion through its production of reactive oxygen species. Furthermore, it impairs Ca2+ processing in the sarcoplasmic reticulum and inhibits the transcription of important muscle elements, weakening the heart muscle. It also downregulates adrenergic receptors and interrupts cell signaling. (1) Administration of dexrazoxane can prevent Fe2+ complex formation. (2) Intravenous immunoglobulin therapy can reduce inflammatory cytokines. (3) L-carnitine can bolster mitochondrial function. (4) Anti-heart-failure therapies, such as angiotensin-converting-enzyme inhibitors and β-blockers, can prevent further damage. Abbreviations: cTn, cardiac troponin; MLC2, myosin light chain 2; MM-CK, myofibrillar isoform of the CK enzyme; ROS, reactive oxygen species; TOPII, topoisomerase 2. (Reprinted with permission from Nature Publishing Group [21].)
3.2 Treatment of Cardiotoxicity
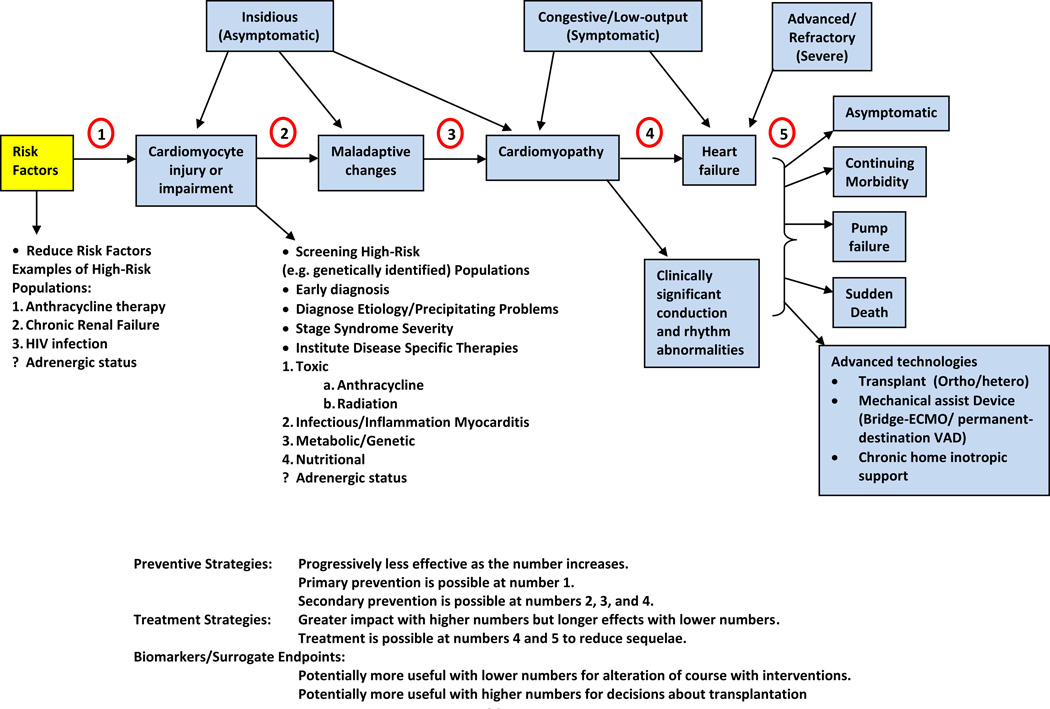
Cardiac dysfunction should be treated as early as possible (Figure 3) [119]. Many patients are treated only when cardiotoxicity becomes symptomatic [120].
Figure 3. Stages in The Course of Pediatric Ventricular Dysfunction.
(1) Primary prevention is possible at this stage by reducing risk factors in high-risk populations (such as those receiving anticancer therapy). (2) Secondary prevention is possible at this stage to reduce the effects of the treatment-induced injury. (3) Secondary prevention is also possible at this stage. (4) Clinically significant conduction and rhythm abnormalities might be observed. (5) Radical therapies might be required at this stage (such as heart transplant) if there is failure of medical management. Preventive strategies are progressively less effective as the toxicity increases. Treatment strategies have a greater impact when used to treat the more-diseased heart, but have longer effects if initiated early. Biomarkers and surrogate end points are potentially useful at early stages to alter the course with interventions, and are potentially useful at later stages to aid decisions about transplantation. (Reprinted with permission from Elsevier [166].)
Chemotherapy-induced cardiotoxicity can be categorized as acute, early-onset, or late-onset [14]. Acute cardiotoxicity is rare, occurring in less than 1% of patients. It is characterized by the onset of heart failure and sinus tachycardia within 1 week of starting anthracycline therapy and usually resolves after stopping anthracycline therapy. Early-onset cardiotoxicity occurs within the first year after completing therapy, and late-onset cardiotoxicity occurs any time thereafter [14]. Early- and late-onset cardiotoxicity may be subclinical, or present as symptomatic restrictive or dilated cardiomyopathy. All manifestations can persist and progress [14, 33].
Cardiac dysfunction in children and adolescents may present as subclinical dilated cardiomyopathy, but later develop into a restrictive cardiomyopathy [33]. Treatments for anthracycline cardiomyopathy depend, in part, on LV preload and LV afterload abnormalities, and the progression of cardiac fibrosis. The goal for treatment at early stages is to prevent pathologic LV remodeling using drugs that target LV preload (diuretics) and LV afterload (angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers; Figure 1). The use of angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers is based on extensive research that goes beyond simply LV afterload reduction. The beneficial effects of angiotensin-converting-enzyme inhibitors for childhood cancer survivors are transient. In a retrospective study of 18 children started on enalapril about 7 years after completing doxorubicin therapy, LV structure and function transiently improved during the first 6 years but this benefit was subsequently lost. For those with heart failure, initial benefits were short-lived. Subsequent management, including heart transplant, failed by 3–5 years in all patients [121]. Effective therapies for anthracycline-associated heart failure have not been tested. Drug regimens might include diuretics (furosemide) for volume overload; inotropes (dobutamine, milrinone, dopamine) to improve contractility and hemodynamics; vasopressors (epinephrine, norepinephrine); vasodilators (sodium nitroprusside); and calcium sensitizers (levosimendan). These are drugs to treat acute, severe heart failure and cardiogenic shock, not just heart failure.
Because anthracycline-related structural changes progress over time from dilated cardiomyopathy to a restrictive cardiomyopathy, it is of critical importance to understand the type of cardiomyopathy causing the heart failure in these patients. Many therapies appropriate for heart failure from dilated cardiomyopathy would be inappropriate for heart failure from restrictive cardiomyopathy. We frequently employ tailored precision therapy based on hemodynamic monitoring of these symptomatic patients.
Beta-adrenergic blocking drugs have been used to treat cardiotoxicity [122]. Carvedilol, a non-selective beta-blocker, has improved LV function and mortality in adults [122–124], but its effects in childhood cancer survivors is unknown.
Clinical heart failure in childhood cancer survivors can rapidly progress to functional impairment that is refractory to drug therapy. In this situation, mechanical support, such as pacemakers, implantable defibrillators, extracorporeal membrane oxygenation, and implantable pulsatile or continuous-flow ventricular assist devices, may be considered. However, evidence for efficacy in children is still limited and is based on studies of general heart failure management. Adverse events, including infection, thrombosis, bleeding, and neurological complications occur in about 40% of patients requiring mechanical assist devices [125].
For those few patients who do not respond well to all other cardiac treatments, heart transplantation is an option for end-stage anthracycline cardiomyopathy [122]. In one multicenter study of 17 children (mean age at cancer diagnosis, 6 years) who underwent heart transplantation at a median of 9.2 years after cancer diagnosis, five underwent heart transplant within 5 years from the end of treatment. There was only one recurrent cancer and the 1-, 2-, and 5-year survival rates were 100%, 92%, and 60%, respectively [126].
Growth hormone therapy has also been used to treat cardiotoxicity. Additionally, LV mass and LV dimension are markedly lower in exposed survivors than in unexposed ones [19]. Together, these findings suggest that growth hormone therapy may help prevent cardiotoxicity, at least among growth-hormone-deficient survivors. In one longitudinal observational study of childhood cancer survivors with somatic growth deficiency, improvements in LV structure and blood pressure occurred with growth hormone therapy, but were lost after therapy was discontinued [127]. Additional studies are still needed to determine the clinical use, timing, dosage, and risks of growth hormone therapy to treat or prevent cardiotoxicity in these survivors.
4 New Chemotherapeutic Agents and Associated Cardiotoxicity
Several molecularly-targeted agents have been developed to improve anti-cancer efficacy and reduce side effects [128]. Although these agents have expanded therapeutic options, some have unanticipated and serious adverse cardiovascular effects [129, 130]. Many are not yet used in children.
A few of the more commonly used molecular agents and their cardiovascular side effects are reviewed below.
4.1 Imatinib
Imatinib, a tyrosine kinase inhibitor, acts against the BCR-ABL fusion gene product found in chronic myeloid leukemia and Philadelphia-positive ALL and is used to treat those two leukemias in both adults and children [131–133]. Imatinib also inhibits other tyrosine kinases, such as KIT and platelet-derived growth factor receptor, which may be mutated in gastrointestinal stromal tumors. Imatinib, FDA-approved since 2001, is rarely associated with congestive heart failure, likely because of the effects of ABL inhibition on cardiomyocytes [134]. With a median follow-up of 4 years, only 22 of 1300 (1.7%) adults enrolled in imatinib clinical trials developed heart failure, and in only 8 cases of the 22 was considered to be related to imatinib [135]. Imatinib cardiotoxicity has not been a concern with children [136, 137].
Second- and third-generation derivatives of imatinib (e.g., dasatinib, nilotinib, bosutinib) are being used as first-line agents for chronic myeloid leukemia (CML) because of their greater molecular efficacy [138]. Ponatinib, efficacious against CML in patients with the T315I mutation, which renders CML resistant to the other current tyrosine kinase inhibitors was associated with a nearly 12% incidence of serious arterial thrombotic events in adults resulting in withdrawal from the market [139]. The vascular toxicity of nilotinib [140] has raised concerns about the safety of this class of agents, particularly as first-line agents for CML and other cancers [130].
4.2 Trastuzumab
Trastuzumab has cardiovascular side effects. An inhibitor of the human epidermal growth factor receptor (HER2, also known as ErbB2), trastuzumab is primarily used to treat breast cancers with HER2 overexpression [141]. Trastuzumab interferes with cellular repair, leading to increased apoptosis, particularly in the context of concurrent anthracycline therapy, and has been associated with LV systolic dysfunction and heart failure [142]. Anthracycline treatment and chest radiotherapy are risk factors for trastuzumab-associated LV systolic dysfunction [143]. In contrast to anthracycline-associated LV systolic dysfunction, trastuzumab-associated dysfunction is often reversible after discontinuing the drug; patients with LV systolic dysfunction may tolerate trastuzumab re-treatment [144].
Over-expression of HER2 has been found on osteosarcoma cells and is associated with a poorer prognosis. Although a Phase 2 trial of 41 patients with metastatic osteosarcoma found that trastuzumab could be safely given in conjunction with anthracycline-based chemotherapy and the cardioprotectant dexrazoxane (none experienced clinical heart failure and markers of cardiotoxicity were normal), its efficacy remains unclear [110]. Newer anti-HER2 agents, such as lapatinib and pertuzumab, may be less cardiotoxic than trastuzumab [142, 145]. Data with these agents in children are emerging [146, 147].
4.3 Bevacizumab
Antibodies and inhibitors against vascular signaling pathway proteins are also commonly used. Bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor (VEGF) receptors, is approved for treating several metastatic cancers in adults [148, 149]. Heart failure was reported with bevacizumab in patients with metastatic breast cancer who also received anthracyclines or taxanes; bevacizumab is no longer approved for this indication [150].
A meta-analysis associated bevacizumab with a 2-fold higher relative risk of ischemic cardiac events but not of arterial thromboembolic events or stroke [151]. Bevacizumab has been tested in children with high-grade central nervous system tumors [152, 153]. As well is in children with retinopathy of prematurity [154].
4.3 Other Inhibitors of Vascular Endothelial Growth Factor Receptors
Other VEGF receptor and tyrosine kinase inhibitors include sunitinib, sorafenib, axitinib, pazopanib, regorafenib, and vandetanib. These agents affect several signaling pathways and share similar cardiovascular side effects, particularly hypertension and, less commonly, cardiomyopathy. Sunitinib and sorafenib are most used in children, sunitinib in Phase 1 and 2 trials treating solid tumors [155] and sorafenib in Phase 1 trials treating both leukemia and solid tumors [156, 157].
Hypertension caused by VEGF receptor inhibitors is usually acute, occurs within 2 weeks of administration, and may relate to decreased nitrogen oxide concentrations, which increases vascular tone and endothelial cell apoptosis [149]. In adults, the incidence of hypertension has been as high as 40% for sorafenib and 81% for sunitinib [158–161]. In adults with metastatic renal cell carcinoma, hypertension may even increase sunitinib’s anti-cancer efficacy; patients with hypertension have better overall survival than patients without hypertension, regardless of anti-hypertensive therapy [162]. Drug-related hypertension is often controlled with standard anti-hypertensive agents and has not necessitated stopping VEFG receptor inhibitors.
Episodes of cardiomyopathy have been rare, with the incidence of symptomatic heart failure (United States National Cancer Institute Common Terminology Criteria for Adverse Events [CTCAE] grade 3 or higher) ranging from 1.5% to 15% among adults and the incidence of any LV systolic dysfunction ranging from 7% to 28% [158–161, 163]. Left ventricular systolic dysfunction, like that seen with trastuzumab, is often reversible and has not resulted in discontinuations. These agents have been associated with increased ischemic cardiac events and arrhythmias, including QT-interval prolongation [159, 160].
Experience with these agents in children is limited. Among 12 children with solid tumors treated with sunitinib, 2 experienced CTCAE grades 2–3 dose-limiting reductions in LV ejection fraction, leading to exclusion of subsequent patients with a history of anthracycline or cardiac radiation exposure [155]. Among 11 children subsequently enrolled in the same study, none experienced cardiotoxicity, none had remarkable issues with hypertension, and only 2 had grade 1–2 QT-interval prolongation (i.e. a subclinical corrected QT interval >500 milliseconds no more than once) [155]. In another study, among 12 children with relapsed or refractory leukemia treated with sorafenib, none experienced adverse cardiotoxicity, aside from one instance of hypotension [156]. A sorafenib study with 65 children with refractory solid tumors and leukemia reported at least 3 instances of non-dose-limiting hypertension requiring anti-hypertensive medications without sorafenib dose reduction [157].
Given that these agents are usually tested in adults before children, and assuming they are effective, determining their long-term cardiovascular safety in children may require years, if not decades, of close follow-up. An FDA survey of 19 pediatric Phase 1 trials of molecularly-targeted agents approved for adults found cardiotoxicity only with sunitinib [164].
Comparing late-onset cardiotoxicity is difficult because most trials do not typically use cardiovascular measures as primary or secondary endpoints, often exclude patients with a history of cardiovascular diseases or findings, and define cardiotoxicity differently. Even when common definitions are used, such as the CTCAE [165], these definitions, particularly earlier versions, are usually oriented to grading acute toxicities and may not be detailed enough to capture more chronic or late-onset toxicities. Finally, these trials often do not include prospective longer-term monitoring, when important late outcomes may become more detectable [21].
Conclusions
Advancements in treatment have increased survival of children with cancer. These treatments come with a high cost of cardiotoxicity. Research dedicated to preventing, detecting, predicting, and treating cardiotoxicities in survivors of childhood cancer has been informative. Significant gaps in knowledge remain, including the influence of genetic predisposition, the optimal use of both blood- and imaging-based biomarkers both during and following cancer-therapy, the efficacy of potential cardioprotectants, optimal management strategies once subclinical cardiotoxicity has been detected, and the long-term cardiotoxicity of newer chemotherapeutics. Recognizing these opportunities to increase our understanding in these areas should result in continuing progress toward improving life-spanning quality of life by maximizing the efficacy of cancer treatment while minimizing its short- and long-term adverse effects.
Acknowledgements
Eric Chow is supported by grants from the National Cancer Institute (K07 CA151775), the Leukemia and Lymphoma Society, and the St. Baldricks' Foundation.
Footnotes
The authors have no conflicts of interest that are directly relevant to the content of this review.
References
- 1.American Cancer Society. Cancer Facts & Figures 2013. 2013. [Google Scholar]
- 2.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324(12):808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 3.Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, Haddy N, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28(8):1308–1315. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94(4):525–533. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff DD, Layard MW, Basa P, Davis HL, Jr, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 7.Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332(26):1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 8.Orgel E, Zung L, Ji L, Finklestein J, Feusner J, Freyer DR. Early cardiac outcomes following contemporary treatment for childhood acute myeloid leukemia: a North American perspective. Pediatr Blood Cancer. 2013;60(9):1528–1533. doi: 10.1002/pbc.24498. [DOI] [PubMed] [Google Scholar]
- 9.Hudson MM, Rai SN, Nunez C, Merchant TE, Marina NM, Zalamea N, et al. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J Clin Oncol. 2007;25(24):3635–3643. doi: 10.1200/JCO.2006.09.7451. [DOI] [PubMed] [Google Scholar]
- 10.Trachtenberg BH, Landy DC, Franco VI, Henkel JM, Pearson EJ, Miller TL, et al. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol. 2011;32(3):342–353. doi: 10.1007/s00246-010-9878-3. [DOI] [PubMed] [Google Scholar]
- 11.Simbre VC, Duffy SA, Dadlani GH, Miller TL, Lipshultz SE. Cardiotoxicity of cancer chemotherapy: implications for children. Paediatr Drugs. 2005;7(3):187–202. doi: 10.2165/00148581-200507030-00005. [DOI] [PubMed] [Google Scholar]
- 12.Lipshultz SE, Miller TL, Lipsitz SR, Neuberg DS, Dahlberg SE, Colan SD, et al. Continuous versus bolus infusion of doxorubicin in children with ALL: long-term cardiac outcomes. Pediatrics. 2012;130(6):1003–1011. doi: 10.1542/peds.2012-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipshultz SE, Giantris AL, Lipsitz SR, Kimball Dalton V, Asselin BL, Barr RD, et al. Doxorubicin administration by continuous infusion is not cardioprotective: the Dana-Farber 91-01 Acute Lymphoblastic Leukemia protocol. J Clin Oncol. 2002;20(6):1677–1682. doi: 10.1200/JCO.2002.20.6.1677. [DOI] [PubMed] [Google Scholar]
- 14.Adams MJ, Lipshultz SE. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer. 2005;44(7):600–606. doi: 10.1002/pbc.20352. [DOI] [PubMed] [Google Scholar]
- 15.Gilladoga AC, Manuel C, Tan CT, Wollner N, Sternberg SS, Murphy ML. The cardiotoxicity of adriamycin and daunomycin in children. Cancer. 1976;37(2 Suppl):1070–1078. doi: 10.1002/1097-0142(197602)37:2+<1070::aid-cncr2820370814>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Little MP, Azizova TV, Bazyka D, Bouffler SD, Cardis E, Chekin S, et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect. 2012;120(11):1503–1511. doi: 10.1289/ehp.1204982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidenreich PA, Schnittger I, Strauss HW, Vagelos RH, Lee BK, Mariscal CS, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. J Clin Oncol. 2007;25(1):43–49. doi: 10.1200/JCO.2006.07.0805. [DOI] [PubMed] [Google Scholar]
- 18.Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22(15):3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 19.Landy DC, Miller TL, Lipsitz SR, Lopez-Mitnik G, Hinkle AS, Constine LS, et al. Cranial irradiation as an additional risk factor for anthracycline cardiotoxicity in childhood cancer survivors: an analysis from the cardiac risk factors in childhood cancer survivors study. Pediatr Cardiol. 2013;34(4):826–834. doi: 10.1007/s00246-012-0539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipshultz SE, Landy DC, Lopez-Mitnik G, Lipsitz SR, Hinkle AS, Constine LS, et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. 2012;30(10):1050–1057. doi: 10.1200/JCO.2010.33.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipshultz SE, Cochran TR, Franco VI, Miller TL. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol. 2013;10:697–710. doi: 10.1038/nrclinonc.2013.195. [DOI] [PubMed] [Google Scholar]
- 22.Landy DC, Lipsitz SR, Kurtz JM, Hinkle AS, Constine LS, Adams MJ, et al. Dietary quality, caloric intake, and adiposity of childhood cancer survivors and their siblings: an analysis from the cardiac risk factors in childhood cancer survivors study. Nutrition and cancer. 2013;65(4):547–555. doi: 10.1080/01635581.2013.770042. [DOI] [PubMed] [Google Scholar]
- 23.Landy DC, Miller TL, Lopez-Mitnik G, Lipsitz SR, Hinkle AS, Constine LS, et al. Aggregating traditional cardiovascular disease risk factors to assess the cardiometabolic health of childhood cancer survivors: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Am Heart J. 2012;163(2):295–301. doi: 10.1016/j.ahj.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller TL, Lipsitz SR, Lopez-Mitnik G, Hinkle AS, Constine LS, Adams MJ, et al. Characteristics and determinants of adiposity in pediatric cancer survivors. Cancer Epidemiol Biomarkers Prev. 2010;19(8):2013–2022. doi: 10.1158/1055-9965.EPI-10-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AM, Lopez-Mitnik G, Somarriba G, Lipsitz SR, Hinkle AS, Constine LS, et al. Exercise capacity in long-term survivors of pediatric cancer: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Pediatr Blood Cancer. 2013;60(4):663–668. doi: 10.1002/pbc.24410. [DOI] [PubMed] [Google Scholar]
- 26.Ness KK, Leisenring WM, Huang S, Hudson MM, Gurney JG, Whelan K, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115(9):1984–1994. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Laar M, Feltbower RG, Gale CP, Bowen DT, Oliver SE, Glaser A. Cardiovascular sequelae in long-term survivors of young peoples' cancer: a linked cohort study. Br J Cancer. 2014 doi: 10.1038/bjc.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messiah SE, Arheart KL, Lopez-Mitnik G, Lipshultz SE, Miller TL. Ethnic group differences in cardiometabolic disease risk factors independent of body mass index among American youth. Obesity (Silver Spring) 2013;21(3):424–428. doi: 10.1002/oby.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meacham LR, Sklar CA, Li S, Liu Q, Gimpel N, Yasui Y, et al. Diabetes mellitus in long-term survivors of childhood cancer Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med. 2009;169(15):1381–1388. doi: 10.1001/archinternmed.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emmons KM, Puleo E, Mertens A, Gritz ER, Diller L, Li FP. Long-term smoking cessation outcomes among childhood cancer survivors in the Partnership for Health Study. J Clin Oncol. 2009;27(1):52–60. doi: 10.1200/JCO.2007.13.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klosky JL, Howell CR, Li Z, Foster RH, Mertens AC, Robison LL, et al. Risky health behavior among adolescents in the childhood cancer survivor study cohort. J Pediatr Psychol. 2012;37(6):634–646. doi: 10.1093/jpepsy/jss046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23(12):2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 34.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 35.Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11(10):950–961. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351(2):145–153. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 37.Lipshultz SE, Lipsitz SR, Kutok JL, Miller TL, Colan SD, Neuberg DS, et al. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer. 2013;119(19):3555–3562. doi: 10.1002/cncr.28256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanco JG, Leisenring WM, Gonzalez-Covarrubias VM, Kawashima TI, Davies SM, Relling MV, et al. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer. 2008;112(12):2789–2795. doi: 10.1002/cncr.23534. [DOI] [PubMed] [Google Scholar]
- 39.Blanco JG, Sun CL, Landier W, Chen L, Esparza-Duran D, Leisenring W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children's Oncology Group. J Clin Oncol. 2012;30(13):1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visscher H, Ross CJ, Rassekh SR, Barhdadi A, Dube MP, Al-Saloos H, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30(13):1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 41.Visscher H, Ross CJ, Rassekh SR, Sandor GS, Caron HN, van Dalen EC, et al. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr Blood Cancer. 2013;60(8):1375–1381. doi: 10.1002/pbc.24505. [DOI] [PubMed] [Google Scholar]
- 42.Miranda CJ, Makui H, Soares RJ, Bilodeau M, Mui J, Vali H, et al. Hfe deficiency increases susceptibility to cardiotoxicity and exacerbates changes in iron metabolism induced by doxorubicin. Blood. 2003;102(7):2574–2580. doi: 10.1182/blood-2003-03-0869. [DOI] [PubMed] [Google Scholar]
- 43.Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15(4):1544–1552. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 44.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304(22):2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356–e375. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- 46.Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109(22):2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 47.Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 48.Colombo A, Meroni CA, Cipolla CM, Cardinale D. Managing cardiotoxicity of chemotherapy. Curr Treat Options Cardiovasc Med. 2013;15(4):410–424. doi: 10.1007/s11936-013-0248-3. [DOI] [PubMed] [Google Scholar]
- 49.Lipshultz SE, Miller TL, Scully RE, Lipsitz SR, Rifai N, Silverman LB, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30(10):1042–1049. doi: 10.1200/JCO.2010.30.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekstein S, Nir A, Rein AJ, Perles Z, Bar-Oz B, Salpeter L, et al. N-terminal-proB-type natriuretic peptide as a marker for acute anthracycline cardiotoxicity in children. J Pediatr Hematol Oncol. 2007;29(7):440–444. doi: 10.1097/MPH.0b013e3180640d42. [DOI] [PubMed] [Google Scholar]
- 51.Erkus B, Demirtas S, Yarpuzlu AA, Can M, Genc Y, Karaca L. Early prediction of anthracycline induced cardiotoxicity. Acta Paediatr. 2007;96(4):506–509. doi: 10.1111/j.1651-2227.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 52.Soker M, Kervancioglu M. Plasma concentrations of NT-pro-BNP and cardiac troponin-I in relation to doxorubicin-induced cardiomyopathy and cardiac function in childhood malignancy. Saudi Med J. 2005;26(8):1197–1202. [PubMed] [Google Scholar]
- 53.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358(20):2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 54.Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, Masson S, et al. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005;112(10):1428–1434. doi: 10.1161/CIRCULATIONAHA.104.508465. [DOI] [PubMed] [Google Scholar]
- 55.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107(11):1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 56.Lee DS, Vasan RS. Novel markers for heart failure diagnosis and prognosis. Curr Opin Cardiol. 2005;20(3):201–210. doi: 10.1097/01.hco.0000161832.04952.6a. [DOI] [PubMed] [Google Scholar]
- 57.Dessi M, Madeddu C, Piras A, Cadeddu C, Antoni G, Mercuro G, et al. Long-term, up to 18 months, protective effects of the angiotensin II receptor blocker telmisartan on Epirubin-induced inflammation and oxidative stress assessed by serial strain rate. Springerplus. 2013;2(1):198. doi: 10.1186/2193-1801-2-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie L, Terrand J, Xu B, Tsaprailis G, Boyer J, Chen QM. Cystatin C increases in cardiac injury: a role in extracellular matrix protein modulation. Cardiovasc Res. 2010;87(4):628–635. doi: 10.1093/cvr/cvq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel PC, Ayers CR, Murphy SA, Peshock R, Khera A, de Lemos JA, et al. Association of cystatin C with left ventricular structure and function: the Dallas Heart Study. Circ Heart Fail. 2009;2(2):98–104. doi: 10.1161/CIRCHEARTFAILURE.108.807271. [DOI] [PubMed] [Google Scholar]
- 60.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115(2):173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta S, Drazner MH, de Lemos JA. Newer biomarkers in heart failure. Heart Fail Clin. 2009;5(4):579–588. doi: 10.1016/j.hfc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 62.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11(9):811–817. doi: 10.1093/eurjhf/hfp097. [DOI] [PubMed] [Google Scholar]
- 63.Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, et al. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol. 2010;99(5):323–328. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov. 2012;11(11):860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fichtlscherer S, Zeiher AM, Dimmeler S. Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol. 2011;31(11):2383–2390. doi: 10.1161/ATVBAHA.111.226696. [DOI] [PubMed] [Google Scholar]
- 66.Horie T, Ono K, Nishi H, Nagao K, Kinoshita M, Watanabe S, et al. Acute doxorubicin cardiotoxicity is associated with miR-146a–induced inhibition of the neuregulin-ErbB pathway. Cardiovasc Res. 2010;87(4):656–664. doi: 10.1093/cvr/cvq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu J, Peng C, Wang W, Jin H, Tang Q, Wei X. Let-7 g is involved in doxorubicin induced myocardial injury. Environ Toxicol Pharmacol. 2012;33(2):312–317. doi: 10.1016/j.etap.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 68.Steinherz LJ, Graham T, Hurwitz R, Sondheimer HM, Schwartz RG, Shaffer EM, et al. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Childrens Cancer Study Group. Pediatrics. 1992;89(5 Pt 1):942–949. [PubMed] [Google Scholar]
- 69.Lipshultz SE, Sanders SP, Goorin AM, Krischer JP, Sallan SE, Colan SD. Monitoring for anthracycline cardiotoxicity. Pediatrics. 1994;93(3):433–437. [PubMed] [Google Scholar]
- 70.Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25(7):733–740. doi: 10.1016/j.echo.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 71.Ruggiero A, De Rosa G, Rizzo D, Leo A, Maurizi P, De Nisco A, et al. Myocardial performance index and biochemical markers for early detection of doxorubicin-induced cardiotoxicity in children with acute lymphoblastic leukaemia. Int J Clin Oncol. 2013;18(5):927–933. doi: 10.1007/s10147-012-0458-9. [DOI] [PubMed] [Google Scholar]
- 72.Jurcut R, Wildiers H, Ganame J, D'Hooge J, De Backer J, Denys H, et al. Strain rate imaging detects early cardiac effects of pegylated liposomal Doxorubicin as adjuvant therapy in elderly patients with breast cancer. J Am Soc Echocardiogr. 2008;21(12):1283–1289. doi: 10.1016/j.echo.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 73.Stoodley PW, Richards DA, Hui R, Boyd A, Harnett PR, Meikle SR, et al. Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. Eur J Echocardiogr. 2011;12(12):945–952. doi: 10.1093/ejechocard/jer187. [DOI] [PubMed] [Google Scholar]
- 74.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107(9):1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ganame J, Claus P, Eyskens B, Uyttebroeck A, Renard M, D'Hooge J, et al. Acute cardiac functional and morphological changes after Anthracycline infusions in children. Am J Cardiol. 2007;99(7):974–977. doi: 10.1016/j.amjcard.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 76.Lunning MA, Kutty S, Rome ET, Li L, Padiyath A, Loberiza F, et al. Cardiac Magnetic Resonance Imaging for the Assessment of the Myocardium After Doxorubicin-based Chemotherapy. Am J Clin Oncol. 2013;21(12):1283–1289. doi: 10.1097/COC.0b013e31829e19be. [DOI] [PubMed] [Google Scholar]
- 77.Wassmuth R, Lentzsch S, Erdbruegger U, Schulz-Menger J, Doerken B, Dietz R, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging-a pilot study. Am Heart J. 2001;141(6):1007–1013. doi: 10.1067/mhj.2001.115436. [DOI] [PubMed] [Google Scholar]
- 78.Oberholzer K, Kunz RP, Dittrich M, Thelen M. [Anthracycline-induced cardiotoxicity: cardiac MRI after treatment for childhood cancer] Rofo. 2004;176(9):1245–1250. doi: 10.1055/s-2004-813416. [DOI] [PubMed] [Google Scholar]
- 79.Shankar SM, Marina N, Hudson MM, Hodgson DC, Adams MJ, Landier W, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121(2):e387–e396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 80.Wong FL, Bhatia S, Landier W, Francisco L, Leisenring W, Hudson MM, et al. Efficacy and cost-effectiveness of the Children's Oncology Group long-term follow-Up guidelines for early detection of treatment-related cardiac compromise in childhood cancer survivors. Ann Intern Med. 2014 doi: 10.7326/M13-2498. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stapleton GE, Stapleton SL, Martinez A, Ayres NA, Kovalchin JP, Bezold LI, et al. Evaluation of longitudinal ventricular function with tissue Doppler echocardiography in children treated with anthracyclines. J Am Soc Echocardiogr. 2007;20(5):492–497. doi: 10.1016/j.echo.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 82.Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21(16):1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 83.Jenkins C, Bricknell K, Hanekom L, Marwick TH. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol. 2004;44(4):878–886. doi: 10.1016/j.jacc.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 84.Eidem BW, Sapp BG, Suarez CR, Cetta F. Usefulness of the myocardial performance index for early detection of anthracycline-induced cardiotoxicity in children. Am J Cardiol. 2001;87(9):1120–1122. doi: 10.1016/s0002-9149(01)01476-x. A9. [DOI] [PubMed] [Google Scholar]
- 85.Iarussi D, Indolfi P, Casale F, Martino V, Di Tullio MT, Calabro R. Anthracycline-induced cardiotoxicity in children with cancer: strategies for prevention and management. Paediatr Drugs. 2005;7(2):67–76. doi: 10.2165/00148581-200507020-00001. [DOI] [PubMed] [Google Scholar]
- 86.Karakurt C, Kocak G, Ozgen U. Evaluation of the left ventricular function with tissue tracking and tissue Doppler echocardiography in pediatric malignancy survivors after anthracycline therapy. Echocardiography. 2008;25(8):880–887. doi: 10.1111/j.1540-8175.2008.00695.x. [DOI] [PubMed] [Google Scholar]
- 87.Dorup I, Levitt G, Sullivan I, Sorensen K. Prospective longitudinal assessment of late anthracycline cardiotoxicity after childhood cancer: the role of diastolic function. Heart. 2004;90(10):1214–1216. doi: 10.1136/hrt.2003.027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kapusta L, Thijssen JM, Groot-Loonen J, Antonius T, Mulder J, Daniels O. Tissue Doppler imaging in detection of myocardial dysfunction in survivors of childhood cancer treated with anthracyclines. Ultrasound Med Biol. 2000;26(7):1099–1108. doi: 10.1016/s0301-5629(00)00252-0. [DOI] [PubMed] [Google Scholar]
- 89.Tsai HR, Gjesdal O, Wethal T, Haugaa KH, Fossa A, Fossa SD, et al. Left ventricular function assessed by two-dimensional speckle tracking echocardiography in long-term survivors of Hodgkin's lymphoma treated by mediastinal radiotherapy with or without anthracycline therapy. Am J Cardiol. 2011;107(3):472–477. doi: 10.1016/j.amjcard.2010.09.048. [DOI] [PubMed] [Google Scholar]
- 90.Motoki H, Koyama J, Nakazawa H, Aizawa K, Kasai H, Izawa A, et al. Torsion analysis in the early detection of anthracycline-mediated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2012;13(1):95–103. doi: 10.1093/ejechocard/jer172. [DOI] [PubMed] [Google Scholar]
- 91.Cheung YF, Li SN, Chan GC, Wong SJ, Ha SY. Left ventricular twisting and untwisting motion in childhood cancer survivors. Echocardiography. 2011;28(7):738–745. doi: 10.1111/j.1540-8175.2011.01429.x. [DOI] [PubMed] [Google Scholar]
- 92.Fallah-Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J Cardiovasc Magn Reson. 2008;10:5. doi: 10.1186/1532-429X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dash R, Chung J, Chan T, Yamada M, Barral J, Nishimura D, et al. A molecular MRI probe to detect treatment of cardiac apoptosis in vivo. Magn Reson Med. 2011;66(4):1152–1162. doi: 10.1002/mrm.22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Minotti G, Recalcati S, Menna P, Salvatorelli E, Corna G, Cairo G. Doxorubicin cardiotoxicity and the control of iron metabolism: quinone-dependent and independent mechanisms. Methods Enzymol. 2004;378:340–361. doi: 10.1016/S0076-6879(04)78025-8. [DOI] [PubMed] [Google Scholar]
- 95.Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27(1):127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 96.Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer. 2013;49(13):2900–2909. doi: 10.1016/j.ejca.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 97.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011;(6):CD003917. doi: 10.1002/14651858.CD003917.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wexler LH, Andrich MP, Venzon D, Berg SL, Weaver-McClure L, Chen CC, et al. Randomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicin. J Clin Oncol. 1996;14(2):362–372. doi: 10.1200/JCO.1996.14.2.362. [DOI] [PubMed] [Google Scholar]
- 99.Asselin B, Devidas M, Zhou T, Camitta BM, Lipshultz SE. Cardioprotection and safety of dexrazoxane (DRZ) in children treated for newly diagnosed T-cell acute lymphoblastic leukemia (T-ALL) or advanced stage lymphoblastic leukemia (T-LL) J Clin Oncol. 2012:9504. doi: 10.1200/JCO.2015.60.8851. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ebb D, Meyers P, Grier H, Bernstein M, Gorlick R, Lipshultz SE, et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the children's oncology group. J Clin Oncol. 2012;30(20):2545–2551. doi: 10.1200/JCO.2011.37.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kopp LM, Bernstein ML, Schwartz CL, Ebb D, Krailo MD, Grier HE, et al. The effects of dexrazoxane on cardiac status and second malignant neoplasms (SMN) in doxorubicin-treated patients with osteosarcoma (OS) J Clin Oncol. 2012:9503. (abstract) [Google Scholar]
- 102.Vrooman LM, Neuberg DS, Stevenson KE, Asselin BL, Athale UH, Clavell L, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer. 2011;47(9):1373–1379. doi: 10.1016/j.ejca.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol. 2007;25(5):493–500. doi: 10.1200/JCO.2005.02.3879. [DOI] [PubMed] [Google Scholar]
- 104.Walker DM, Fisher BT, Seif AE, Huang YS, Torp K, Li Y, et al. Dexrazoxane use in pediatric patients with acute lymphoblastic or myeloid leukemia from 1999 and 2009: analysis of a national cohort of patients in the Pediatric Health Information Systems database. Pediatr Blood Cancer. 2013;60(4):616–620. doi: 10.1002/pbc.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seif CE, Walker DM, Li Y, Huang YS, Kavcic M, Torp K, et al. Dexrazoxane exposure and risk of secondary acute myeloid leukemia in pediatric cancer patients. Pediatr Blood Cancer. 2014 Mar 26; doi: 10.1002/pbc.25043. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tebbi CK, Mendenhall NP, London WB, Williams JL, Hutchison RE, Fitzgerald TJ, et al. Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2012;59(7):1259–1265. doi: 10.1002/pbc.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schwartz CL, Constine LS, Villaluna D, London WB, Hutchison RE, Sposto R, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood. 2009;114(10):2051–2059. doi: 10.1182/blood-2008-10-184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chow EJ, Asselin BL, Schwartz CL, Doody DR, Leisenring WM, Aggarwal S, et al. Late mortality and relapse after dexrazoxane (DRZ) treatment: an update from the Children’s Oncology Group (COG) J Clin Oncol. 2014:10024. doi: 10.1200/JCO.2014.59.4473. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gallegos-Castorena S, Martinez-Avalos A, Mohar-Betancourt A, Guerrero-Avendano G, Zapata-Tarres M, Medina-Sanson A. Toxicity prevention with amifostine in pediatric osteosarcoma patients treated with cisplatin and doxorubicin. Pediatr Hematol Oncol. 2007;24(6):403–408. doi: 10.1080/08880010701451244. [DOI] [PubMed] [Google Scholar]
- 110.Myers DF, O'Connell JB, Subramanian R. Myocarditis resolving after discontinuation of procainamide. Int J Cardiol. 1983;4(3):322–324. doi: 10.1016/0167-5273(83)90091-8. [DOI] [PubMed] [Google Scholar]
- 111.Wagdi P, Rouvinez G, Fluri M, Aeschbacher B, Thoni A, Schefer H, et al. [Cardioprotection in chemo- and radiotherapy for malignant diseases--an echocardiographic pilot study] Praxis (Bern 1994) 1995;84(43):1220–1223. [PubMed] [Google Scholar]
- 112.Kraft J, Grille W, Appelt M, Hossfeld DK, Eichelbaum M, Koslowski B, et al. Effects of verapamil on anthracycline-induced cardiomyopathy: preliminary results of a prospective multicenter trial. Haematol Blood Transfus. 1990;33:566–570. doi: 10.1007/978-3-642-74643-7_103. [DOI] [PubMed] [Google Scholar]
- 113.Milei J, Marantz A, Ale J, Vazquez A, Buceta JE. Prevention of adriamycin-induced cardiotoxicity by prenylamine: a pilot double blind study. Cancer Drug Deliv. 1987;4(2):129–136. doi: 10.1089/cdd.1987.4.129. [DOI] [PubMed] [Google Scholar]
- 114.Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48(11):2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 115.Iarussi D, Auricchio U, Agretto A, Murano A, Giuliano M, Casale F, et al. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Mol Aspects Med. 1994;(15 Suppl):s207–s212. doi: 10.1016/0098-2997(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 116.Waldner R, Laschan C, Lohninger A, Gessner M, Tuchler H, Huemer M, et al. Effects of doxorubicin-containing chemotherapy and a combination with L-carnitine on oxidative metabolism in patients with non-Hodgkin lymphoma. J Cancer Res Clin Oncol. 2006;132(2):121–128. doi: 10.1007/s00432-005-0054-8. [DOI] [PubMed] [Google Scholar]
- 117.Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lowis S, Lewis I, Elsworth A, Weston C, Doz F, Vassal G, et al. A phase I study of intravenous liposomal daunorubicin (DaunoXome) in paediatric patients with relapsed or resistant solid tumours. Br J Cancer. 2006;95(5):571–580. doi: 10.1038/sj.bjc.6603288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zerra P, Cochran TR, Franco VI, Lipshultz SE. An expert opinion on pharmacologic approaches to reducing the cardiotoxicity of childhood acute lymphoblastic leukemia therapies. Expert Opin Pharmacother. 2013;14(11):1497–1513. doi: 10.1517/14656566.2013.804911. [DOI] [PubMed] [Google Scholar]
- 120.Cardinale D, Bacchiani G, Beggiato M, Colombo A, Cipolla CM. Strategies to prevent and treat cardiovascular risk in cancer patients. Semin Oncol. 2013;40(2):186–198. doi: 10.1053/j.seminoncol.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 121.Lipshultz SE, Lipsitz SR, Sallan SE, Simbre VC, 2nd, Shaikh SL, Mone SM, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol. 2002;20(23):4517–4522. doi: 10.1200/JCO.2002.12.102. [DOI] [PubMed] [Google Scholar]
- 122.Ewer MS, Yeh ET. Cancer and the heart. Hamilton Lewiston, NY: BC Decker Inc; 2006. Distributed by BC Decker. [Google Scholar]
- 123.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial The SAVE Investigators. N Engl J Med. 1992;327(10):669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 124.Sliwa K, Norton GR, Kone N, Candy G, Kachope J, Woodiwiss AJ, et al. Impact of initiating carvedilol before angiotensin-converting enzyme inhibitor therapy on cardiac function in newly diagnosed heart failure. J Am Coll Cardiol. 2004;44(9):1825–1830. doi: 10.1016/j.jacc.2004.05.087. [DOI] [PubMed] [Google Scholar]
- 125.Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128(17):1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 126.Ward KM, Binns H, Chin C, Webber SA, Canter CE, Pahl E. Pediatric heart transplantation for anthracycline cardiomyopathy: cancer recurrence is rare. J Heart Lung Transplant. 2004;23(9):1040–1045. doi: 10.1016/j.healun.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 127.Lipshultz SE, Vlach SA, Lipsitz SR, Sallan SE, Schwartz ML, Colan SD. Cardiac changes associated with growth hormone therapy among children treated with anthracyclines. Pediatrics. 2005;115(6):1613–1622. doi: 10.1542/peds.2004-1004. [DOI] [PubMed] [Google Scholar]
- 128.Targeted Cancer Therapies. [Accessed November 26, 2013];National Cancer Institute. http://www.cancer.gov/cancertopics/factsheet/Therapy/targeted.
- 129.Mann DL. Targeted cancer therapeutics: the heartbreak of success. Nat Med. 2006;12(8):881–882. doi: 10.1038/nm0806-881. [DOI] [PubMed] [Google Scholar]
- 130.Groarke JD, Cheng S, Moslehi J. Cancer-drug discovery and cardiovascular surveillance. N Engl J Med. 2013;369(19):1779–1781. doi: 10.1056/NEJMp1313140. [DOI] [PubMed] [Google Scholar]
- 131.Suttorp M, Millot F. Treatment of pediatric chronic myeloid leukemia in the year 2010: use of tyrosine kinase inhibitors and stem-cell transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:368–376. doi: 10.1182/asheducation-2010.1.368. [DOI] [PubMed] [Google Scholar]
- 132.Oliansky DM, Camitta B, Gaynon P, Nieder ML, Parsons SK, Pulsipher MA, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review ASBMT Position Statement. Biol Blood Marrow Transplant. 2012;18(7):979–981. doi: 10.1016/j.bbmt.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 133.Oliansky DM, Larson RA, Weisdorf D, Dillon H, Ratko TA, Wall D, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of adult acute lymphoblastic leukemia: update of the 2006 evidence-based review. Biol Blood Marrow Transplant. 2012;18(1):16–17. doi: 10.1016/j.bbmt.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 134.Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12(8):908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 135.Atallah E, Durand JB, Kantarjian H, Cortes J. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood. 2007;110(4):1233–1237. doi: 10.1182/blood-2007-01-070144. [DOI] [PubMed] [Google Scholar]
- 136.Bond M, Bernstein ML, Pappo A, Schultz KR, Krailo M, Blaney SM, et al. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: a Children's Oncology Group study. Pediatr Blood Cancer. 2008;50(2):254–258. doi: 10.1002/pbc.21132. [DOI] [PubMed] [Google Scholar]
- 137.Schultz KR, Bowman WP, Aledo A, Slayton WB, Sather H, Devidas M, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children's oncology group study. J Clin Oncol. 2009;27(31):5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pavey T, Hoyle M, Ciani O, Crathorne L, Jones-Hughes T, Cooper C, et al. Dasatinib, nilotinib and standard-dose imatinib for the first-line treatment of chronic myeloid leukaemia: systematic reviews and economic analyses. Health Technol Assess. 2012;16(42):1–277. doi: 10.3310/hta16420. ii-iv. [DOI] [PubMed] [Google Scholar]
- 139.FDA Drug Safety Communication. FDA asks manufacturer of the leukemia drug Iclusig (ponatinib) to suspend marketing and sales. [Accessed November, 26, 2013];U.S. Food and Drug Administration. 2013 http://www.fda.gov/Drugs/DrugSafety/ucm373040.htm.
- 140.Tefferi A. Nilotinib treatment-associated accelerated atherosclerosis: when is the risk justified? Leukemia. 2013;27(9):1939–1940. doi: 10.1038/leu.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 142.De Keulenaer GW, Doggen K, Lemmens K. The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res. 2010;106(1):35–46. doi: 10.1161/CIRCRESAHA.109.205906. [DOI] [PubMed] [Google Scholar]
- 143.Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104(17):1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23(31):7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 145.Perez EA, Koehler M, Byrne J, Preston AJ, Rappold E, Ewer MS. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc. 2008;83(6):679–686. doi: 10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- 146.Albanell J, Montagut C, Jones ET, Pronk L, Mellado B, Beech J, et al. A phase I study of the safety and pharmacokinetics of the combination of pertuzumab (rhuMab 2C4) and capecitabine in patients with advanced solid tumors. Clin Cancer Res. 2008;14(9):2726–2731. doi: 10.1158/1078-0432.CCR-07-1980. [DOI] [PubMed] [Google Scholar]
- 147.Fouladi M, Stewart CF, Blaney SM, Onar-Thomas A, Schaiquevich P, Packer RJ, et al. Phase I trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28(27):4221–4227. doi: 10.1200/JCO.2010.28.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 149.Bair SM, Choueiri TK, Moslehi J. Cardiovascular complications associated with novel angiogenesis inhibitors: emerging evidence and evolving perspectives. Trends Cardiovasc Med. 2013;23(4):104–113. doi: 10.1016/j.tcm.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sekeres MA. The avastin story. N Engl J Med. 2011;365(15):1454–1455. doi: 10.1056/NEJMc1109550. [DOI] [PubMed] [Google Scholar]
- 151.Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol. 2010;49(3):287–297. doi: 10.3109/02841860903524396. [DOI] [PubMed] [Google Scholar]
- 152.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 153.MacDonald TJ, Aguilera D, Kramm CM. Treatment of high-grade glioma in children and adolescents. Neuro Oncol. 2011;13(10):1049–1058. doi: 10.1093/neuonc/nor092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Darlow BA, Ells AL, Gilbert CE, Gole GA, Quinn GE. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed. 2013;98(2):F170–F174. doi: 10.1136/archdischild-2011-301148. [DOI] [PubMed] [Google Scholar]
- 155.Dubois SG, Shusterman S, Ingle AM, Ahern CH, Reid JM, Wu B, et al. Phase I and pharmacokinetic study of sunitinib in pediatric patients with refractory solid tumors: a children's oncology group study. Clin Cancer Res. 2011;17(15):5113–5122. doi: 10.1158/1078-0432.CCR-11-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol. 2011;29(24):3293–3300. doi: 10.1200/JCO.2011.34.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Widemann BC, Kim A, Fox E, Baruchel S, Adamson PC, Ingle AM, et al. A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a Children's Oncology Group Phase I Consortium report. Clin Cancer Res. 2012;18(21):6011–6022. doi: 10.1158/1078-0432.CCR-11-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 160.Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 161.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008;19(9):1613–1618. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- 162.Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763–773. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 164.Paoletti X, Geoerger B, Doz F, Baruchel A, Lokiec F, Le Tourneau C. A comparative analysis of paediatric dose-finding trials of molecularly targeted agent with adults' trials. Eur J Cancer. 2013;49(10):2392–2402. doi: 10.1016/j.ejca.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 165.Adverse Events/CTCAE. [Accessed November 26, 2013];National Cancer Institute. 2013 http://ctep.cancer.gov/protocolDevelopment/adverse_effects.htm.
- 166.Lipshultz SE. Ventricular dysfunction clinical research in infants, children and adolescents. Prog Pediatr Cardiol. 2000;12(1):1–28. doi: 10.1016/s1058-9813(00)00076-x. [DOI] [PubMed] [Google Scholar]