Abstract
Recent investigations involving experiments on intact rabbit renal proximal tubules indicated that organic anion transporter 3 (OAT3) may be involved in the transport of DMPS. Therefore, we have evaluated the interaction of OAT3 with DMPS to determine the impact of OAT3 on basolateral DMPS uptake. We used stably transfected HEK293 cells, expressing human and rabbit orthologs of the exchanger OAT1 and OAT3. Using 6-carboxyfluorescein (6-CF) as a substrate, the IC50 determinations for reduced DMPS (DMPSH) revealed a stronger interaction with OAT1 as compared to OAT3 (rbOat1: 123.3±13.7 µM, hOAT1: 85.1±8.8 µM, rbOat3: 171.7±22.3 µM and hOAT3: 172.2±36.4 µM). However, inhibition of 6-CF uptake by the oxidized form of DMPS (DMPSS), the main form of DMPS in the blood, showed a preference of OAT3 (rbOat1: 237.4±23 µM, hOAT1: 104.6±13.1 µM, rbOat3: 52.4±7.6 µM and hOAT3: 31.6±6.6 µM). In order to determine if DMPSH and DMPSS are substrates for OAT3 we performed efflux studies with [14C]glutarate and inwardly directed gradients of glutarate. The inhibitors trans-stimulated the efflux of [14C]glutarate, suggesting that OAT3 may be able to transport both forms of DMPS. Based on the substantial interaction of OAT3 with DMPSS we conclude that OAT3 represents the dominant basolateral player in renal detoxification processes resulting from use of DMPS.
Keywords: OAT1, mercury, kidney, nephrotoxicity, heavy metal, secretion, detoxification
Introduction
Some heavy metals, including lead, cadmium and mercury, are accumulated during urinary excretion in renal proximal tubule cells, causing chronic renal diseases (Sabolic 2006; Bridges and Zalups 2005b). A common procedure to increase the renal elimination of heavy metals and thus to reduce the burden on the kidneys is the application of the drug “Dimaval”, the active agent of which is 2,3-dimercapto-1-propanesulfonic acid (DMPS). DMPS is negatively charged under physiological conditions and, consequently, belongs to a structural class of compounds collectively referred to as “organic anions” (OAs). Following oral application DMPS is found in the blood in both reduced (DMPSH) and oxidized (DMPSS) forms, with DMPSS representing the predominant species (Hurlbut et al. 1994). DMPS possesses two vicinal, free sulfhydryl groups with a high chelating potency for heavy metals, particularly mercury. Its effectiveness in vivo is due to the high affinity of DMPS for mercury and the ability of DMPS to access the intracellular compartment.
The entry of mercury into renal proximal tubule cells (RPTs) involves organic anion transporters (OATs), especially OAT1 and OAT3 (Lash et al. 2005b). These OATs are well characterized OA/dicarboxylate exchangers located at the basolateral side of proximal tubule cells, that facilitate the uptake of a broad range of organic anions into RPT cells as the first step in renal secretion (Burckhardt and Burckhardt 2003; Wright and Dantzler 2004; Rizwan and Burckhardt 2007). There is some evidence that OAT1 and OAT3 may also play an important role in the detoxification process of heavy metals like mercury, mediating the uptake of DMPS into the proximal tubule cells (Bridges and Zalups 2005a).Human organic anion transporter 1 (hOAT1) is able to translocate both DMPSH (reduced DMPS) and DMPSS (Islinger et al. 2001), and comparison of the uptake characteristics displayed by rabbit OAT1 (expressed heterologously) and the uptake characteristics of the non-perfused rabbit single proximal tubule S2 segments, further supported the idea of an involvement of OAT1 in DMPSH uptake in vivo (Bahn et al. 2002). An extension of these studies was recently published by Lungkaphin and coworkers (Lungkaphin et al. 2004). Based on the fact that rabbit Oat1 (rbOat1) and rabbit Oat3 (rbOat3) can be distinguished by their substrates, p-aminohippurate (PAH) and estrone sulfate (ES) respectively, the authors provided the first evidence that OAT3 may play a role in DMPS, and especially DMPSS, uptake into proximal tubule cells. However, there was no direct proof to support this hypothesis, showing the interaction of OAT3 with DMPS.
In the present study we explored the interaction of human OAT3 with reduced and oxidized DMPS and compared these data with the characteristics of hOAT1 and of the rabbit orthologs of OAT1 and OAT3 to evaluate the possible contribution of OAT3 to DMPS transport in vivo. Our results illustrate that in both species OAT1 interacts strongly with DMPSH, whereas OAT3 prefers the oxidized form DMPSS. Moreover, trans-stimulation experiments provide the first molecular evidence that OAT3 may translocate both substances, indicating that it may play a substantial role in renal basolateral DMPS uptake.
Materials and Methods
Reagents
Materials used included fetal bovine serum, trypsin, and PBS from Invitrogen (Groningen, The Netherlands). All chemicals were of analytic grade and purchased from Sigma-Aldrich (Deisenhofen, Germany). 6-Carboxyfluorescein (6-CF) was purchased from Molecular Probes (Leiden, The Netherlands). [14C]glutarate (GA), (glutaric acid, [1,5-14C]-, 30.8 mCi/mmol) was obtained from ICN (Costa Mesa, CA).
Handling of DMPSH and preparation of DMPSS
The reduced form of DMPS (DMPSH) in solution is rapidly oxidized (6%/h). For this reason we always used fresh solutions of DMPSH. To obtain DMPSS, DMPSH was oxidized overnight as previously described (Islinger et al. 2001; Lungkaphin et al. 2004).
Stable transfection and cell culture of OATs in HEK293 cells
The recombinant epithelial kidney cell lines T-REx™ HEK293-hOAT1, -hOAT3, -rbOat1 and -rbOat3 were established using the Flp-In™ expression system (Invitrogen, Karlsruhe, Germany) according to the manufacturer’s protocol. Briefly, the cDNAs of hOAT1 (GenBank Acc.-No. AF097490), hOAT3 (BI760120), rbOat1 (AJ242871) and rbOat3 (AF533644 and AJ489526) were cloned into the Flp-In™ expression vector pcDNA5/FRT, containing a Flp recombination target site linked to the hygromycin resistance gene. These constructs were co-transfected with the Flp recombinase expression vector pOG44 into Flp-In™ HEK293 cells. Cells stably expressing hOAT1, hOAT3, rbOat1 or rbOat3 were selected by maintaining cultures in the presence of hygromycin (200 µg/ml). Cells were grown in flasks containing Dulbecco’s modified minimum essential medium (with high glucose) supplemented with 10% fetal bovine serum, penicillin (100 units/ml), streptomycin (100 µg/ml) and blasticidine (5 µg/ml). Cultures were maintained in a humidified atmosphere containing 5% CO2 at 37 °C. Cells were split with a ratio of 1:5 every 3 to 4 days.
Uptake and efflux experiments
The cells were harvested and plated in 24-well plastic dishes (Sarstedt, Nümbrecht, Germany) at a density of 2×105 cells/well. Transport assays were performed 48 h post seeding in mammalian Ringer (MR) solution (in mM): 130 NaCl, 4 KCl, 1 CaCl2, 1 MgSO4, 1 NaH2PO4, 20 HEPES and 18 glucose at a pH of 7.4. For uptake experiments cells were washed twice with 500 µl MR and incubated in buffer containing 5 µM 6-carboxyfluorescein (6-CF) with or without increasing concentrations of DMPSH or DMPSS for 2 min (hOAT1 and rbOat1) and 10 min (hOAT3 and rbOat3) at RT. The incubation was stopped, and the extracellular 6-CF was removed by washing the monolayer 2-3 times with 500 to 1000 µl of ice-cold PBS. Afterwards, the cells were dissolved in 1 ml 0.5 N NaOH and the 6-CF accumulation was determined in a fluorescence spectrophotometer (Hitachi, Tokyo, Japan) at 492/512 nm (excitation/emission). For the determinations of the concentrations of DMPS in its reduced and oxidized forms that blocked 50% of 6-CF uptake (IC50) the following equation (eq. 1) was used and fitted by non-linear regression with SigmaPlot 2001 (SPSS Science, Chicago, IL, USA).
| eq. 1 |
Where ν is the rate of 6-CF uptake in the presence of the inhibitor (DMPS in its respective form), ν0 is the rate of 6-CF uptake in the absence of the inhibitor (in this case set to 100%), I is the inhibitor concentration, and h is the Hill coefficient representing the cooperativity between the tested substances and the transporter.
For trans-stimulation experiments, cells were preloaded with [14C]glutarate (1,8 µM) for 1h at 37°C and the efflux was measured for 10 min at 37°C in the presence of glutarate, DMPSH or DMPSS, each in a concentration of 500 µM, and compared to control efflux measured in MR solution without added test agents.
Kinetic and statistical analysis
Unless indicated otherwise, data are the mean (±S.E.M.) of four independent experiments (DMPSH) or three independent experiments (DMPSS) with triplicate determinations each. Statistical analysis was performed using Microsoft Excel (Microsoft, Unterschleißheim, Germany) and SigmaPlot 2001 (SPSS Science, Chicago, IL, USA).
Results
Inhibition of 6-carboxyfluorescein uptake of OAT3 by reduced DMPS (DMPSH)
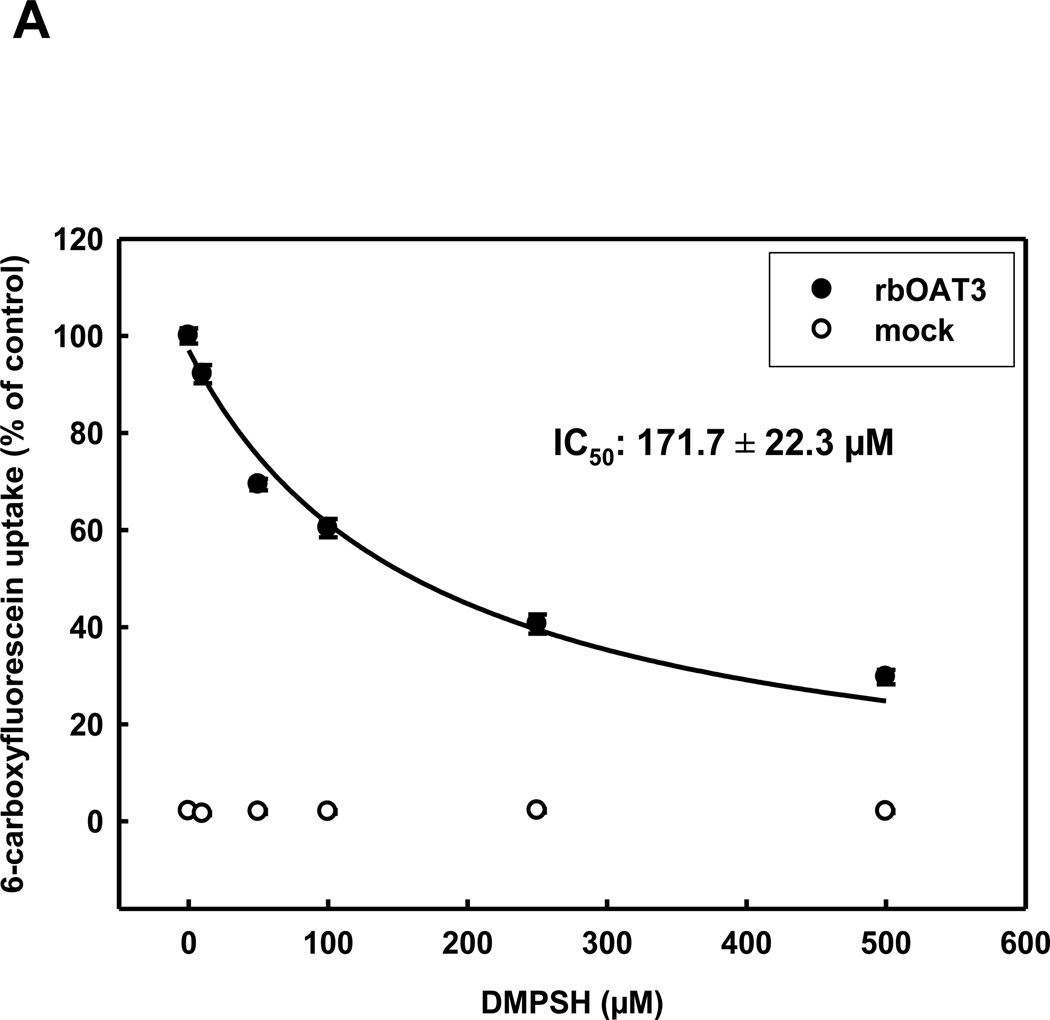
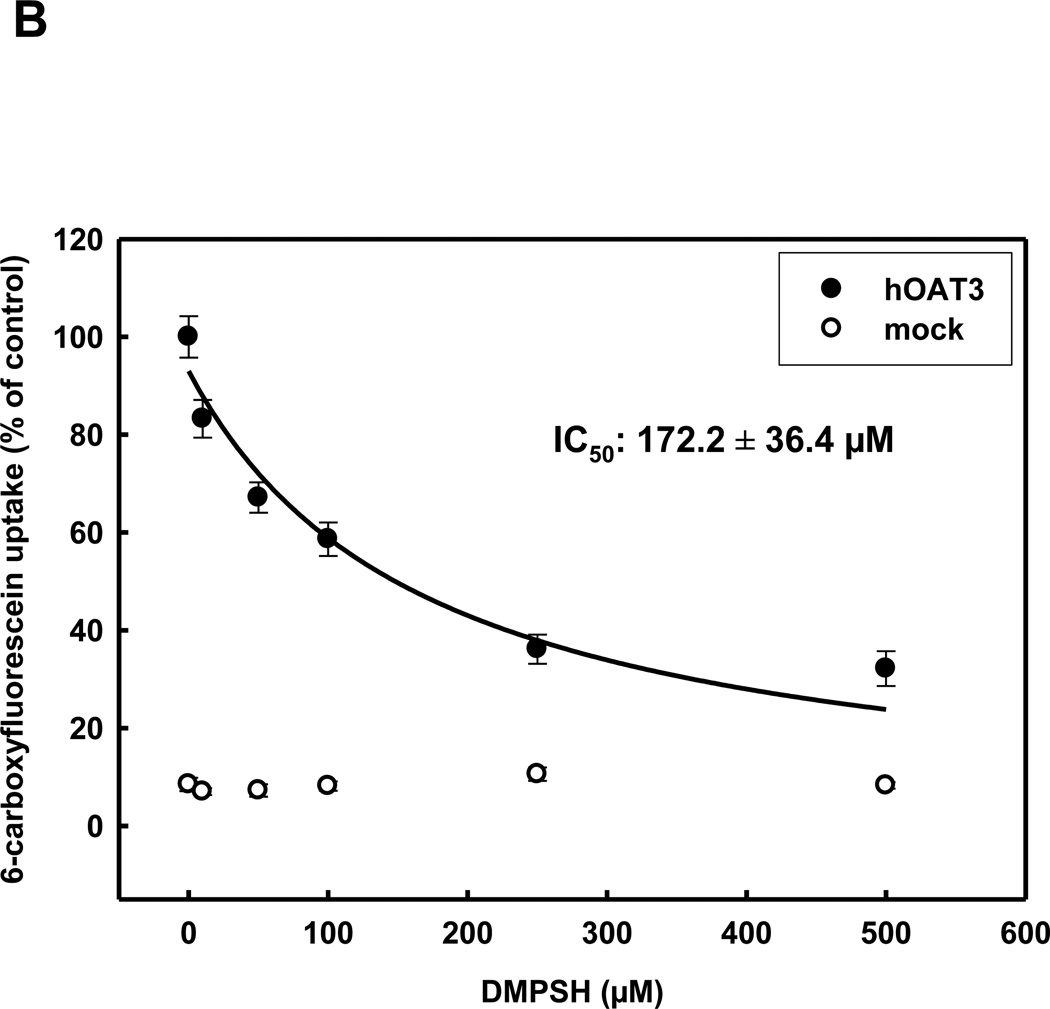
In order to determine the interaction of OAT3 with the reduced form of 2,3-dimercapto-1-propanesulfonate (DMPSH) and to match this data to OAT1 transport characteristics, we measured the uptake of 6-carboxyfluorescein uptake by stably transfected HEK293 cells, expressing rbOat1, hOAT1, rbOat3 or hOAT3, or by non-transfected cells - (generally referred as mock cells), in the presence of increasing concentrations of DMPSH. These experiments resulted in IC50-values of 85.1±8.8 µM for hOAT1 and 123.3±13.7 µM for rbOat1 (see table 1). Human and rabbit OAT3 displayed a lower (compared to OAT1), but species independent interaction with DMPSH, with IC50-values of 172.2±36.4 µM (hOAT3) and 171.7±22.3 µM (rbOat3)(Fig. 1A+B).
Table 1.
| OAT1 | OAT3 | |||
|---|---|---|---|---|
| rabbit | human | rabbit | human | |
| DMPSH | 123.3 ± 13.7 | 85.1 ± 8.8 | 171.7 ± 22.3 | 172.2 ± 36.4 |
| DMPSS | 237.4 ± 23 | 104.6 ± 13.1 | 52.4 ± 7.6 | 31.6 ± 6.6 |
Fig. 1.
Concentration dependence of rbOAT3 (A) and hOAT3 (B) mediated uptake of 5 µM 6-CF into HEK293-OAT cells using various concentrations of DMPSH for 10 min at RT. Each point represents the mean of triplicate measurements from 4 separate experiments.
Inhibition of 6-carboxyfluorescein uptake of OAT3 by oxidized DMPS (DMPSS)
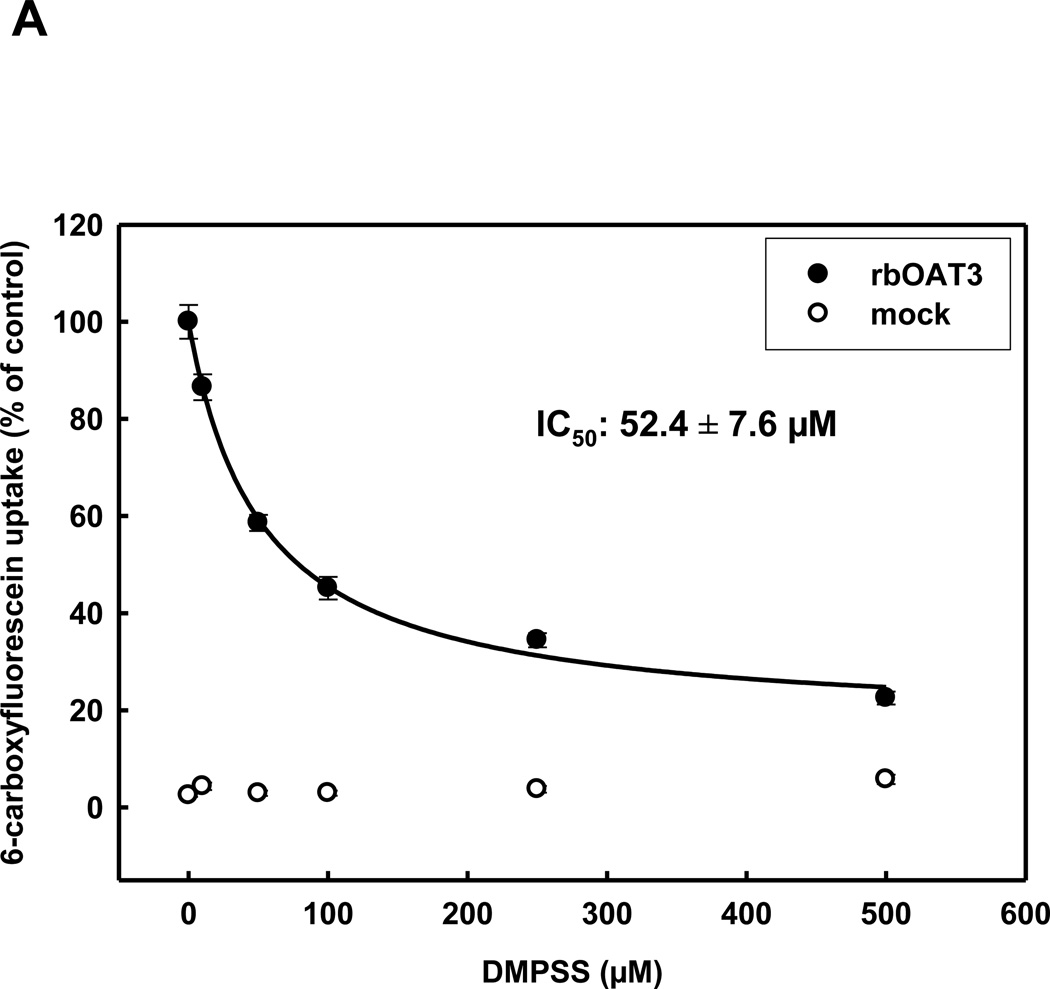
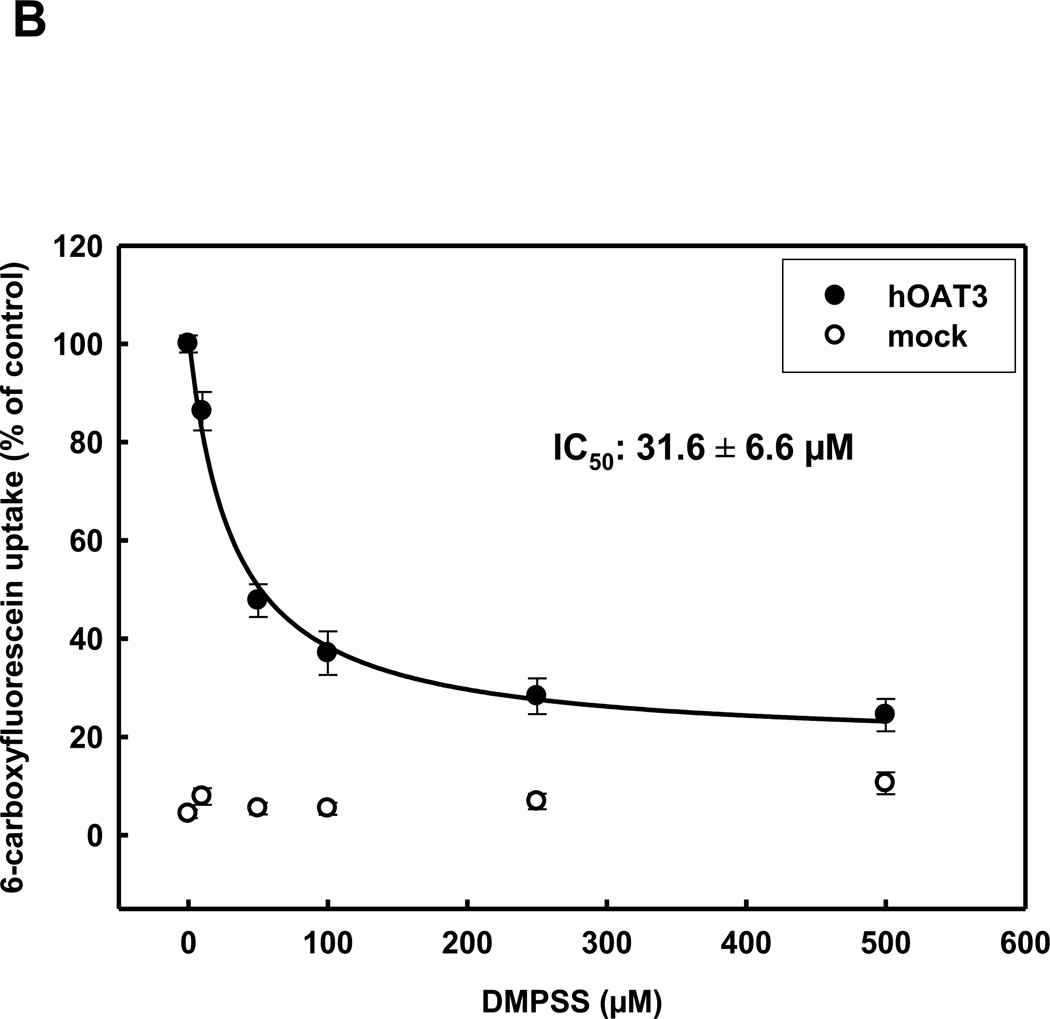
The interaction of OAT3 with the oxidized form of DMPS (DMPSS) is of importance, because DMPS is rapidly oxidized in the blood, thereby making DMPSS the principal form of the chelator to which the transporters are exposed in vivo. Inhibition of 6-carboxyfluorescein uptake by increasing concentrations of DMPSS in HEK293-hOAT1 and HEK293-rbOat1 cells resulted in IC50-values of 104.6±13.1 µM and 237.4±23 µM, respectively (table 1). However, both human and rabbit OAT3 orthologs displayed a higher apparent affinity for DMPSS (IC50-values of 31.6±6.6 µM and 52.4±7.6 µM, respectively), compared to OAT1, consistent with the evidence that OAT3 plays an important role in the basolateral uptake of DMPSS into the proximal tubule cell (Fig. 2A+B).
Fig. 2.
Concentration dependence of rbOAT3 (A) and hOAT3 (B) mediated uptake of 5 µM 6-CF into HEK293-OAT cells using various concentrations of DMPSS for 10 min at RT. Each point represents the mean of triplicate measurements from 3 separate experiments.
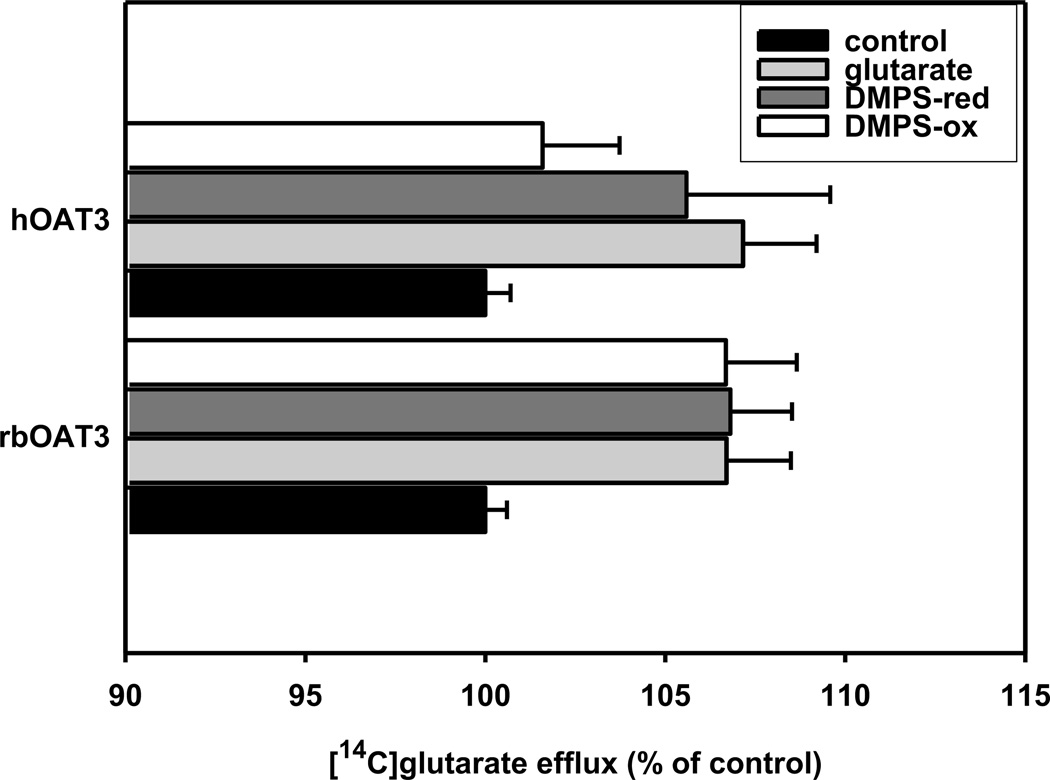
Effects of unlabeled DMPSH and DMPSS on the efflux of 14C-glutarate from hOAT3- and rbOat3-stably transfected HEK293-cells
Since the inhibition of 6-CF-uptake by DMPSH and DMPSS does not prove that either is transported via OAT3, we investigated the rate of [14C]glutarate efflux of cells, in the presence of inwardly-directed gradients of DMPSH, DMPSS or unlabeled glutarate. Efflux in the presence of mammalian Ringer was set to 100%. As expected extracellular glutarate trans-stimulated efflux of labelled (Fig. 3). Additionally, we observed significant stimulation of the efflux of [14C]glutarate by extracellular DMPSH (rbOat3: 6.8%, hOAT3: 8.6%) as well as by DMPSS (rbOat3: 6.7%, hOAT3: 4.6%), supporting the view that OAT3 may be able to translocate both substances.
Fig. 3.
Effect of inwardly directed gradients of glutarate, DMPSH, or DMPSS (each in a concentration of 500 µM) on 14C-glutarate efflux in stably transfected HEK293 cells (rbOAT3, hOAT3). HEK293-OAT cells were preloaded with 14C-glutarate for 1h at 37°C. The efflux was calculated as percentage of 14C content recovered in the supernatant compared with the sum of total radioactivity recovered in the medium and that remaining in the cells at the conclusion of a 10 min efflux period at 37°C, where OAT-expressing cells in Ringer’s solution were set to 100%. Data are expressed as means SE of triplicate measurements from 3 separate experiments. If not otherwise noticed all tested substances showed a highly significant (P < 0.001) efflux of [14C]glutarate. *P < 0.05; **P < 0.01;
Discussion
Nephrotoxicity of mercury correlates well with the expression of the basolaterally localized organic anion transporters, OAT1 and OAT3, in renal proximal tubule cells (Lash et al. 2005a). Mercury ions conjugated with low-molecular weight thiols as well as methyl-conjugates are known substrates for OAT1, which facilitate an efficient renal accumulation of these toxic compounds (Aslamkhan et al. 2003; Zalups and Ahmad 2005). Interestingly, besides the involvement of OATs in mercury toxicity, there is increasing evidence that OATs and NaDC3 are also involved in detoxification processes of heavy metals (Zalups 1995; Bridges and Zalups 2005c; Burckhardt et al. 2002). In this context, medication with 2,3-dimercapto-1-propane-sulfonate (DMPS), an efficient metal chelator of low toxicity, is a well established clinical treatment for cases of mercury or arsenic poisonings, leading to a rapid mobilization of these heavy metals and subsequent secretion into the urine (Zalups et al. 1991). We have recently shown that OAT1 transports both forms of DMPS found in the body, i.e., the oxidized (DMPSS) and reduced (DMPSH) species. Using the rabbit kidney proximal tubule as a model, we found similar transport characteristics for DMPSH with the isolated rbOat1 clone and the non-perfused single proximal tubule S2 segment, indicating that DMPSH is a substrate for OAT1 in vivo (Bahn et al. 2002). This observation also holds true for the isolated human OAT1 clone (Islinger et al. 2001).
Functional characterizations of rbOat1 and rbOat3 resulted in clear-cut substrate specificities with exclusive affinities of rbOat1 for PAH, and of rbOat3 for estrone sulfate (ES, (Zhang et al. 2004)). Therefore, it became possible to discriminate between rbOat1 and rbOat3 function in vivo. To determine whether OAT3 is involved in renal handling of DMPS, Lungkaphin and coworkers examined PAH and ES transport in rabbit renal proximal tubule S2 segments as well as in tubule suspensions (Lungkaphin et al. 2004). It was found that DMPSH interacted with PAH (Kapp of 405 µM) and ES (Kapp of 320 µM) transport consistent with a possible contribution of OAT1 and OAT3 to DMPSH handling in vivo.
In terms of OAT1 this observation corresponds to previous reports (Islinger et al. 2001; Bahn et al. 2002) and the results of the present study, in which we determined the interaction of rbOat1 and hOAT1 with DMPSH in one assay system using stably transfected HEK293 cells and 6-carboxyfluorescein (6-CF), a substrate for OAT1 and OAT3 (Bahn et al. 2005). Our data confirm our previous determination of rbOat1 interaction with DMPSH (102 µM (Bahn et al. 2002) versus 123 µM, this study), validating the assay system. The similar interaction of DMPSH with both human and rabbit OAT1 is consistent with the view that OAT1 may play a similar role in both species mediating DMPS entry in renal proximal tubule cells. However, in rabbit proximal tubules, the marked difference in inhibition of fluorescein uptake versus PAH uptake produced by DMPSH (IC50 values of 76 µM (Bahn et al. 2002) and Kapp 405 µM (Lungkaphin et al. 2004)), respectively), leaves open the question of whether OAT1 is the main PAH-sensitive route for basolateral DMPSH uptake into proximal tubule cells. An additional candidate might be OAT2 which, in humans, also transports PAH (Sun et al. 2001). The rabbit Oat2 (rbOat2) ortholog sequence was recently identified (Acc.-No.: DQ146940), but its PAH-affinity has not been determined yet. We assume that rbOat2 is expressed at the basolateral side of proximal tubule cells like human OAT2 (Enomoto et al. 2002), and works in a concerted action together with OAT1 and OAT3 to mediate basolateral entry of organic anions (manuscript in preparation).
Rabbit Oat3 as well as hOAT3 exhibited a substantial interaction with DMPSH with IC50-values of 171 µM and 172 µM, respectively, indicating a species independent involvement of OAT3 in DMPSH transport. This observation is further supported by trans-stimulation experiments, providing the first evidence that OAT3 may support exchange of DMPSH for glutarate, a well-known substrate for OAT3 (Bakhiya et al. 2003; Sweet et al. 2002). The IC50-values for all four transporters for DMPSH are close to the apparent Km values for the single tubule measured with fluorescein (76 µM, (Bahn et al. 2002)) consistent with the evidence of a significant role of OAT1 and OAT3 in basolateral DMPSH uptake in vivo.
For oxidized DMPS (DMPSS) we found a clear difference in the interaction between rabbit and human OAT1, suggesting different contributions of these transporters in these species in vivo. Both an IC50-value of 105 µM for DMPSS inhibition of hOAT1, and the fact that hOAT1 facilitates DMPSS/glutarate exchange, provide evidence of an involvement of hOAT1 in DMPSS uptake in vivo, which is not reflected by the high Kapp value found for the PAH-dependent transport system in single rabbit tubules (Lungkaphin et al. 2004). We observed a similar discrepancy in DMPSS interaction with the isolated OAT3 clones studies here and the ES-transporting systems in vivo measured by Lungkaphin and co-workers. Whereas a Kapp value of 696 µM for single rabbit tubule segments suggests a limited involvement of OAT3 in DMPSS uptake in vivo, we found on the other hand a relatively high, and species-independent, affinity of OAT3 for DMPSS. Additionally, the significant trans-stimulation of glutarate efflux of OAT3-expressing cells induced by DMPSS suggests that OAT3 may be an important mediator of DMPSS uptake at the basolateral side of proximal tubule cells.
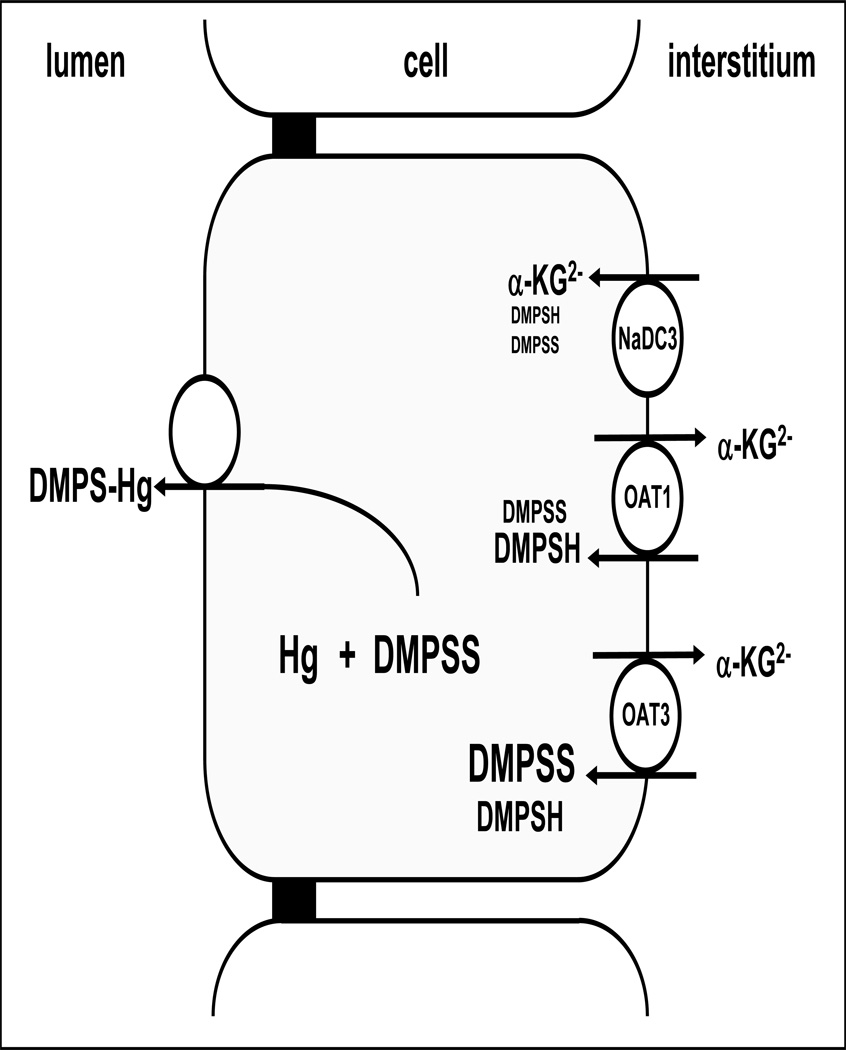
A reason for the high Kapp values in single rabbit tubules compared to the low IC50 values for the interaction of DMPS determined in this study may be the involvement of other, not yet identified, low affinity transport systems, or differences in expression levels of OAT1 and OAT3 in different systems. Along this line, with a plasma concentration of 70 µM detected in patients treated with DMPS (Maiorino et al. 1991), we suggest that OAT3 is most probably the main basolateral route for excretion of both forms of DMPS (Fig. 4), particularly because OAT3 expression exceeds that of OAT1 in human kidney ((Motohashi et al. 2002; Groves et al. 2006; Lungkaphin et al. 2006)).
Fig. 4.
Model for basolateral entry of DMPSH and DMPSS via OATs and NaDC-3.
acknowledgements
We thank Mrs. A. Hillemann, Mrs. G. Dallmeyer and Mr. S. Petzke for excellent technical assistance. This work was supported in part by NIH grant DK074022.
abbreviations
- OAT
organic anion transporter
- 6-CF
6-carboxyfluorescein
- DMPS
2,3-Dimercapto-1-propane-sulfonate
- DMPSH
reduced form of DMPS
- DMPSS
oxidized form of DMPS
Reference List
- Aslamkhan AG, Han YH, Yang XP, Zalups RK, Pritchard JB. Human renal organic anion transporter 1-dependent uptake and toxicity of mercuric-thiol conjugates in Madin-Darby canine kidney cells. Mol. Pharmacol. 2003;63:590–596. doi: 10.1124/mol.63.3.590. [DOI] [PubMed] [Google Scholar]
- Bahn A, Knabe M, Hagos Y, Rodiger M, Godehardt S, Graber-Neufeld DS, Evans KK, Burckhardt G, Wright SH. Interaction of the metal chelator 2,3-dimercapto-1-propanesulfonate with the rabbit multispecific organic anion transporter 1 (rbOAT1) Mol. Pharmacol. 2002;62:1128–1136. doi: 10.1124/mol.62.5.1128. [DOI] [PubMed] [Google Scholar]
- Bahn A, Ljubojevic M, Lorenz H, Schultz C, Ghebremedhin E, Ugele B, Sabolic I, Burckhardt G, Hagos Y. Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am. J. Physiol Cell Physiol. 2005;289:C1075–C1084. doi: 10.1152/ajpcell.00619.2004. [DOI] [PubMed] [Google Scholar]
- Bakhiya N, Bahn A, Burckhardt G, Wolff N. Human organic anion transporter 3 (hOAT3) can operate as an exchanger and mediate secretory urate flux. Cell Physiol Biochem. 2003;13:249–256. doi: 10.1159/000074539. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol. 2005a;204:274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol. 2005b;204:274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol. 2005c;204:274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt BC, Burckhardt G. Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev. Physiol Biochem. Pharmacol. 2003;146:95–158. doi: 10.1007/s10254-002-0003-8. [DOI] [PubMed] [Google Scholar]
- Burckhardt BC, Drinkuth B, Menzel C, König A, Steffgen J, Wright SH, Burckhardt G. The renal Na(+)-dependent dicarboxylate transporter, NaDC-3, translocates dimethyl- and disulfhydryl-compounds and contributes to renal heavy metal detoxification. J. Am. Soc. Nephrol. 2002;13:2628–2638. doi: 10.1097/01.asn.0000033463.58641.f9. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Takeda M, Shimoda M, Narikawa S, Kobayashi Y, Kobayashi Y, Yamamoto T, Sekine T, Cha SH, Niwa T, Endou H. Interaction of human organic anion transporters 2 and 4 with organic anion transport inhibitors. J. Pharmacol. Exp. Ther. 2002;301:797–802. doi: 10.1124/jpet.301.3.797. [DOI] [PubMed] [Google Scholar]
- Groves CE, Suhre WB, Cherrington NJ, Wright SH. Sex differences in the mRNA, protein, and functional expression of organic anion transporter (Oat) 1, Oat3, and organic cation transporter (Oct) 2 in rabbit renal proximal tubules. J. Pharmacol. Exp. Ther. 2006;316:743–752. doi: 10.1124/jpet.105.094979. [DOI] [PubMed] [Google Scholar]
- Hurlbut KM, Maiorino RM, Mayersohn M, Dart RC, Bruce DC, Aposhian HV. Determination and metabolism of dithiol chelating agents. XVI: Pharmacokinetics of 2,3-dimercapto-1-propanesulfonate after intravenous administration to human volunteers. J. Pharmacol. Exp. Ther. 1994;268:662–668. [PubMed] [Google Scholar]
- Islinger F, Gekle M, Wright SH. Interaction of 2,3-dimercapto-1-propane sulfonate with the human organic anion transporter hOAT1. J. Pharmacol. Exp. Ther. 2001;299:741–747. [PubMed] [Google Scholar]
- Lash LH, Hueni SE, Putt DA, Zalups RK. Role of organic anion and amino acid carriers in transport of inorganic mercury in rat renal basolateral membrane vesicles: influence of compensatory renal growth. Toxicol. Sci. 2005a;88:630–644. doi: 10.1093/toxsci/kfi328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, Hueni SE, Putt DA, Zalups RK. Role of organic anion and amino acid carriers in transport of inorganic mercury in rat renal basolateral membrane vesicles: influence of compensatory renal growth. Toxicol. Sci. 2005b;88:630–644. doi: 10.1093/toxsci/kfi328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungkaphin A, Chatsudthipong V, Evans KK, Groves CE, Wright SH, Dantzler WH. Interaction of the metal chelator DMPS with OAT1 and OAT3 in intact isolated rabbit renal proximal tubules. Am. J. Physiol Renal Physiol. 2004;286:F68–F76. doi: 10.1152/ajprenal.00075.2003. [DOI] [PubMed] [Google Scholar]
- Lungkaphin A, Lewchalermwongse B, Chatsudthipong V. Relative contribution of OAT1 and OAT3 transport activities in isolated perfused rabbit renal proximal tubules. Biochim. Biophys. Acta. 2006;1758:789–795. doi: 10.1016/j.bbamem.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Maiorino RM, Dart RC, Carter DE, Aposhian HV. Determination and metabolism of dithiol chelating agents. XII. Metabolism and pharmacokinetics of sodium 2,3-dimercaptopropane-1-sulfonate in humans. J. Pharmacol. Exp. Ther. 1991;259:808–814. [PubMed] [Google Scholar]
- Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, Fukatsu A, Ogawa O, Inui K. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J. Am. Soc. Nephrol. 2002;13:866–874. doi: 10.1681/ASN.V134866. [DOI] [PubMed] [Google Scholar]
- Rizwan AN, Burckhardt G. Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm. Res. 2007;24:450–470. doi: 10.1007/s11095-006-9181-4. [DOI] [PubMed] [Google Scholar]
- Sabolic I. Common mechanisms in nephropathy induced by toxic metals. Nephron Physiol. 2006;104:107–114. doi: 10.1159/000095539. [DOI] [PubMed] [Google Scholar]
- Sun W, Wu RR, van Poelje PD, Erion MD. Isolation of a family of organic anion transporters from human liver and kidney. Biochem. Biophys. Res. Commun. 2001;283:417–422. doi: 10.1006/bbrc.2001.4774. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Chan LM, Walden R, Yang XP, Miller DS, Pritchard JB. Organic anion transporter 3 is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am. J. Physiol Renal Physiol. 2002;284:F763–F769. doi: 10.1152/ajprenal.00405.2002. [DOI] [PubMed] [Google Scholar]
- Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev. 2004;84:987–1049. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Organic anion transport and action of gamma-glutamyl transpeptidase in kidney linked mechanistically to renal tubular uptake of inorganic mercury. Toxicol. Appl. Pharmacol. 1995;132:289–298. doi: 10.1006/taap.1995.1110. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Transport of N-acetylcysteine s-conjugates of methylmercury in Madin-Darby canine kidney cells stably transfected with human isoform of organic anion transporter 1. J. Pharmacol. Exp. Ther. 2005;314:1158–1168. doi: 10.1124/jpet.105.086645. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Gelein RM, Cernichiari E. DMPS as a rescue agent for the nephropathy induced by mercuric chloride. J. Pharmacol. Exp. Ther. 1991;256:1–10. [PubMed] [Google Scholar]
- Zhang X, Groves CE, Bahn A, Barendt WM, Prado MD, Rodiger M, Chatsudthipong V, Burckhardt G, Wright SH. Relative contribution of OAT and OCT transporters to organic electrolyte transport in rabbit proximal tubule. Am. J. Physiol Renal Physiol. 2004;287:999–1010. doi: 10.1152/ajprenal.00156.2004. [DOI] [PubMed] [Google Scholar]