Abstract
Cardiac malformations and disease are the leading causes of death in the United States in live-born infants and adults, respectively. In both of these cases, a decrease in the number of functional cardiomyocytes often results in improper growth of heart tissue, wound healing complications, and poor tissue repair. The field of cardiac tissue engineering seeks to address these concerns by developing cardiac patches created from a variety of biomaterial scaffolds to be used in surgical repair of the heart. These scaffolds should be fully degradable biomaterial systems with tunable properties such that the materials can be altered to meet the needs of both in vitro culture (e.g., disease modeling) and in vivo application (e.g., cardiac patch). Current platforms do not utilize both structural anisotropy and proper cell-matrix contacts to promote functional cardiac phenotypes and thus there is still a need for critically sized scaffolds that mimic both the structural and adhesive properties of native tissue. To address this need, we have developed a silk-based scaffold platform containing cardiac tissue-derived extracellular matrix (cECM). These silk-cECM composite scaffolds have tunable architectures, degradation rates, and mechanical properties. Subcutaneous implantation in rats demonstrated that addition of the cECM to aligned silk scaffold led to 99% endogenous cell infiltration and promoted vascularization of a critically sized scaffold (10 mm × 5 mm × 2.5 mm) after 4 weeks in vivo. In vitro, silk-cECM scaffolds maintained the HL-1 atrial cardiomyocytes and human embryonic stem cell-derived cardiomyocytes and promoted a more functional phenotype in both cell types. This class of hybrid silk-cECM anisotropic scaffolds offers new opportunities for developing more physiologically relevant tissues for cardiac repair and disease modeling.
Keywords: Cardiomyocytes, Integrins, Cell Infiltration, Acellular Scaffold
1. Introduction
Cardiac malformations and disease are the leading causes of death in the United States in live-born infants and adults, respectively [1]. In both of these cases, a decrease in the number of functional cardiomyocytes often results in improper growth of heart tissue, wound healing complications, and poor tissue repair. For example, after a myocardial infarction (MI), dead tissue is replaced with a collagenous scar composed of activated myofibroblasts that migrate into and remodel the damaged area, leading to the thinning of the ventricular wall and hypertrophy of the surviving cardiomyocytes [2–4]. This scarring and the associated diminished cardiac function can lead to arrhythmias, ventricular dilation, and heart failure, among other complications, which account for the high morbidity and mortality [5, 6]. Current therapies for MI repair leave much to be desired in terms of their ability to restore function to the injured adult heart. In terms of pediatric patients, treatment options for the most severe defects involve surgical procedures aimed at bridging the patient to an eventual transplant [7–9], but these temporary surgical options often do not fully restore the function of the heart. In both pediatric and adult disease, tissue engineering and regenerative medicine approaches have the potential to address these current needs, providing improved options to restore the function of damaged heart tissue.
Tissue engineering and regenerative strategies for repairing the heart have seen some success over the last few decades. For example, a number of different cell types (e.g. skeletal myoblasts [10, 11], mesenchymal stem cells [12, 13], cardiac progenitor cells [14–16], and differentiated embryonic and induced pluripotent stem cells [17–19]) have been utilized in cell therapy-based approaches for cardiac repair. However, while these studies have shown some functional benefits, the functional gains were not clinically significant. Two of the major reasons for this are the lack of cell retention [20–22] and the abhorrent effects of the infarct microenvironment on the cells. Tissue engineering strategies attempt to circumvent these issues by delivering the cells in a biomaterial scaffold that aids in cell retention and allows for the tuning of the cellular environment to optimize cellular function. Classically, two types of biomaterials have been utilized for delivery of cells or therapeutics: 1) hydrogels and injectable gels and 2) pre-formed scaffolds and matrices. Hydrogels such as fibrin [23–25] and collagen [26, 27] allow for encapsulation of cells at a high density and in a more native-like biophysical landscape based on cell-specific binding sites, but these biomaterials are difficult to control in terms of architecture and mechanics, and often have necrotic cores when the tissue thickness exceeds diffusion limits. Preformed scaffolds including biomaterials such as chitosan [28, 29] and collagen sponges [30, 31] allow for better control of mechanics and can be formatted with vascular conduits to culture critically sized tissues, however cell infiltration is more challenging and these material systems often lack the appropriate complexity of extracellular matrix (ECM)-based adhesive sites for the cells. Moreover, anisotropy is a critical component of functional cardiac tissue, yet preformed scaffolds are often isotropic in design. More useful preformed scaffolds would be a mix of these two types (gels and preformed), retaining tunability in architecture and mechanical properties, while also allowing for appropriate binding sites for cell infiltration and adhesion and improved cell density.
Recent work demonstrates the variety of scaffold architectures and material properties accessibly via alterations in pre- or post-processing techniques of silk protein based sponge scaffolds [32–34]. For example, aligned silk sponges are generated by directional freezing of an aqueous silk solution, with the pore size adjustable through manipulation of the freezing rate [34]. Similarly, scaffolds containing channels and arrays of channels have been designed to allow for proper fluid flow within critically sized silk scaffolds to provide avenues for control over vascularization of these silk sponges [32]. An additional benefit of these materials is that the elastic modulus can be tuned via optimization of properties such as molecular weight and polymer concentration. Furthermore, the degree to which silk scaffolds are insoluble in water, as well as their rate of degradation, correlates with β-sheet (crystalline) content. The β-sheet content can be controlled via exposure to solvents, temperature and pressure (e.g., autoclaving), or through water vapor annealing under negative pressure [35–37]. By adjusting these parameters (e.g., water annealing time and temperature), β-sheet content of the material is altered independently from concentration for polymer molecular weight and thus, in turn, altering the mechanics without altering the pore size [35]. While silk offers this versatile array of properties potentially useful for cardiac tissue engineering scaffolds, the drawback is that the native protein is non-adhesive to cells.
The composition of the ECM affects cardiac cell signaling, maturation, and differentiation of cardiac progenitors in vitro [25, 38, 39]. The complex composition of the ECM plays a critical role in the development of traction force of cells via integrin-based signaling [40, 41], which can affect everything from cell metabolism to gene expression and ECM production. Most commonly, decellularized heart tissue is digested via pepsin and utilized as a component of both 2D and 3D gels or scaffolds [38, 39] and this formulation has been shown to improve cardiac function when injected following MI in a pre-clinical porcine model [42]. Based on the versatility of silk biomaterials and recent advances in understanding the role of matrix composition on cell behavior, we pursued aligned silk-based scaffold systems which incorporate porcine left ventricle tissue-derived ECM (cardiac extracellular matrix, cECM). The goal of the present study was to determine if these composite silk-cECM scaffolds promoted native cell infiltration via both structural and adhesive cues. Such acellular matrix design approaches to cardiac repair are promising from both fundamental and practical (e.g., regulatory) perspectives.
2. Methods
2.1. Silk solution preparation
Silk fibroin solution was prepared as reported previously [43]. Briefly, pure silk fibroin was extracted from Bombyx mori cocoons by degumming 5 grams of fibers in 2 L of boiling sodium carbonate solution (0.02 M) for 30 min (Sigma-Aldrich, St. Louis, MO). Degummed fibers were collected and rinsed with distilled water three times, then air-dried. The pure silk fibroin was then solubilized in aqueous lithium bromide (9.3 M, Sigma-Aldrich, St. Louis, MO) at 60 °C for 4 hours. The solution was dialyzed using Slide-A-Lyzer Dialysis Cassettes (3,500 MWCO, ThermoScientific, Rockford, IL) against deionized water until the conductivity of the dialysis water was <10 mS cm−1 (indicative of complete lithium bromide removal). The solubilized silk protein solution was then centrifuged twice (9,700 RPM, 20 min, 4°C) to remove insoluble particulates. The concentration of the silk solution was determined by drying a known volume of the solution and massing the remaining solids. This protocol resulted in a 6–8% wt v−1 silk solution. Silk solutions were stored at 4°C for a maximum of 3 weeks.
2.2. Cardiac extracellular matrix (cECM)
Adult porcine hearts were obtained from the local abattoir. Left ventricular tissue was separated from the rest of the heart and utilized for matrix collection. The left ventricular tissue was decellularized and prepared as previously described [44, 45]. Briefly, the ventricular tissue was isolated and cut into small rectangular pieces, rinsed in phosphate buffered saline (PBS), and decellularized using 1% sodium dodecyl sulfate (SDS), until the tissue was white. To ensure that the decellularization process was complete, a small piece of the decellularized cECM was fixed in 10% buffered formalin, dehydrated, embedded in paraffin, sectioned into 7 μm slices, and stained with hematoxylin and eosin (H&E) to confirm decellularization. The resulting decellularized cECM was then rinsed with DI water overnight, lyophilized, and milled into a fine powder using a 40 mm mesh strainer and small tissue mill. The resulting milled powder was then solubilized by a pepsin-based enzymatic digestion in 0.1 M HCl for at least 48 hours. The solubilized cECM was adjusted to pH 7.4 with NaOH and lyophilized a second time. Solubilized cECM powder was reconstituted at 10–30 mg cECM/mL DI water prior to use in scaffolding systems. For incorporation into silk scaffolds, cECM was added to diluted silk solution and stirred slowly for 10 minutes prior to scaffold formation.
2.3. Collagen isolation
All animal experiments were carried out under protocols approved by the Institutional Animal Care and Use Committee at Tufts University and in accordance with the Guide for the Care and Use of Laboratory Animals (NIH, Bethesda MD). Rat tail collagen (RT-collagen) was isolated from the tails of adult female Sprague Dawley rats as previously described [46]. Following lyophilization, collagen was milled through a 20 mm mesh and then digested by pepsin in 0.1M HCl to mimic the processing that was utilized for the cECM. Following pepsin digestion, the sample was frozen at −80°C and lyophilized again resulting in a fine powder. Solubilized cECM powder was reconstituted at 10–30 mg cECM/mL DI water prior to use. For incorporation into silk scaffolds, cECM (either 0.2, 0.6, or 2.4 mg ECM protein/mL silk solution) was added to 3% silk solution and stirred slowly for 10 min prior to scaffold formation.
2.4. Scaffold formation
Scaffolds were prepared according to previously reported methods [32, 34]. Briefly, polydimethylsiloxane (PDMS) was utilized to secure a metal mold in a 150 mm petri dish so that the silk solution (3% wt. v−1) could be directionally frozen using a dry ice in a bath of 100% ethanol, resulting in a porous scaffold aligned in the direction of freezing . The scaffold was made insoluble by water annealing at room temperature [36, 37] to induce β-sheet formation. Scaffolds were rehydrated in sterile phosphate buffered saline containing 1% penicillin/streptomycin overnight and then trimmed using a sterile razor blade to 10 mm × 5 mm × 2.5 mm. Isotropic scaffolds were formed by pouring the silk solution into 3 wells of a 6 well plate and freezing the solution isotropically in a −20°C freezer overnight. Isotropic scaffolds were lyophilized and water annealed in the same way as the anisotropic scaffolds.
2.5. Mechanical properties
Unconfined compression tests were conducted to assess the effect of cECM addition on the mechanical properties of the silk sponges as well as the recovery of the sponges following multiple compression cycles. Aligned silk sponges were biopsy punched into cylinders (8 mm ø, 5 mm height, perpendicular to alignment) and allowed to equilibrate in DI water overnight. Samples were loaded in a TA Instruments RSA3 Dynamic Mechanical Analyzer (TA Instruments, New Castle, DE) between stainless steel parallel plates in an immersion bath. The upper plate was lowered until a compression force of ≈0.4 g was registered. The dynamic moduli were monitored over both constant frequency and constant strain.
2.6. Scanning Electron Microscopy
Scanning electron microscopy (SEM) (Supra55VP, Zeiss) was performed on scaffolds both before and after cell seeding for scaffolds with and without ECM. Cell-free scaffolds were dehydrated using a glass pipet and house vacuum. Scaffolds were then affixed to the SEM tabs using carbon tape and allowed to further air dry in the chemical hood for 4 days. For cell-seeded constructs, samples were first fixed with 2.5% glutaraldehyde and rinsed in PBS. Constructs were peeled open, along alignment to reveal the surface of the scaffold in the center and allowed to air dry in the chemical hood for over 1 week and then affixed to SEM tabs. The inner surface was imaged without further treatment to provide evidence of cellular matrix deposition and changes to the silk-cECM scaffold surface.
2.7. In vivo scaffold evaluation
All procedures were conducted under animal protocols approved by Tufts Institutional Animal Care and Use Committee. All animals used in this study were 6–7 week old Sprague Dawley rats (Charles River Laboratories, Wilmington, MA). Three scaffold types were used: aligned silk, aligned silk coated in fibrin, and aligned silk + cECM. Unseeded silk scaffolds (10 mm × 5 mm × 2.5 mm) were subcutaneously implanted in lateral pockets of each rat under general anesthesia of oxygen and isoflurane. Fibrin was prepared by mixing human fibrinogen (10 mg/mL) (EMD Millipore Chemicals, Billerica, MA) with human thrombin (5 U/mL) (Sigma-Aldrich, St. Louis, MO) in a 4:1 volume ratio. At week 4 post-surgery, animals were euthanized and the samples along with the overlaying tissue were collected for histological examination.
2.7.1. Histochemical analysis of in vivo samples
Samples were fixed with 10% neutral buffered formalin (NBF) and embedded in paraffin following a series of graded ethanol and xylene incubations. Samples were sectioned to 7–15 μm thickness and deparaffinized. Sections were stained with hematoxylin and (Sigma-Aldrich, St. Louis, MO) to visualize cell nuclei and Masson’s Trichrome (Sigma-Aldrich, St. Louis, MO) to visualize collagen deposition (blue) and muscle fibers (red).
To evaluate vascularization in the implanted scaffolds, we assessed PECAM-1 (Santa Cruz, sc13537) and von Willebrand factor (Sigma-Aldrich, F3520) expression. Briefly, sections were hydrated, unmasked using Vector Labs antigen unmasking solution (H-3300) and exogenous peroxide activity was quenched. Sections were blocked using 2.5% normal donkey serum, stained with primary antibody, rinsed, and stained with secondary via ImmPRESS HRP Anti-Rabbit Ig (Peroxidase) Polymer Detection Kit or ImmPRESS HRP Anti-Mouse Ig (Peroxidase) Polymer Detection Kit. Color (brown) was developed using the Vector Labs DAB substrate kit. Samples were counterstained in hematoxylin, dehydrated through ethanol and xylene. Following staining and dehydration, samples were embedded in DPX Mountant (Sigma Aldrich, St. Louis, MO) and imaged using a color camera.
2.7.2. Cell Infiltration
Cell infiltration was quantified from six images of H&E stained sections (10× magnification) per scaffold for total of eighteen images per condition (aligned, aligned coated in fibrin, aligned + cECM). Outlines were drawn around cellular areas of each section using the ‘free hand selection tool’ in ImageJ (National Institutes of Health) and total cellular area determined. Area of cell infiltration (%) was calculated as (total cellular area/total scaffold area) × 100.
2.8. In vitro scaffold evaluation
2.8.1. HL-1 cell seeding
HL-1 cells, a gift from Dr. William Claycomb in the Biochemistry and Molecular Biology Department and the Louisiana State University School of Medicine, are a continuously proliferating cardiomyocyte cell line derived from mouse atrial tumors [47, 48]. Cells were maintained according to the protocol provided via personal communication by the Claycomb laboratory. Briefly, Claycomb Medium (Sigma-Aldrich, St. Louis, MO) was supplemented with 10% FBS, 100 μg/mL penicillin/streptomycin, 2 mM l-glutamine, and 0.1 mM norepinephrine, which is necessary to maintain the beating cardiomyocyte phenotype [47, 48]. Cells from passages 40–45 were maintained on 0.02% gelatin/ 0.005% fibronectin-coated plates. HL-1 monolayers were contractile near 80% confluency.
To seed scaffolds, they were first dehydrated in a biological safety cabinet using a sterile glass pipet connected to house vacuum. The dried and dehydrated scaffolds resembled thick paper. For long term cultures it was important that the cells within the scaffold did not migrate out onto the tissue culture plastic of the plate. To prevent cell adhesion to the plates, 12-well plates were treated with 5% w/v Pluronic®F127 for at least two hours and then rinsed 3 times with sterile PBS and allowed to dry. Dehydrated scaffolds were cultured with one scaffold/well in the coated 12-well plates.
Scaffolds were seeded at 5 × 105 cells/mL of wet scaffold volume (scaffold volume is 125 mm3 or 0.125 mL). To deliver the cells, 100 μl of cell suspension was utilized to rehydrate the silk sponge by adding two 50 μl aliquots of concentrated cell suspension. The scaffold was then stored in the incubator for 15–20 minutes prior to adding enough media to each well so that the scaffolds floated (~3 mL). Well plates containing scaffolds were kept rotated in the incubator (37°C/5% CO2) to maintain proper diffusion for metabolic activity and cell culture media was exchanged every 4 days. HL-1 seeded scaffolds were harvested after 1 and 3 weeks for analysis.
2.8.2. Seeding of human embryonic stem cell-derived cardiomyocytes
HUES-9 human embryonic stem cells (hESCs) (a kind gift from Dr. Chad Cowen at the Harvard Stem Cell Institute) were cultured and differentiated using established protocols [49]. Briefly, hESCs were maintained in mTESR-1 stem cell maintenance medium (StemCell Technologies, Vancouver, British Columbia) in Matrigel (BD Biosciences, Franklin Lakes, NJ) coated polystyrene plates. hESCs were mechanically passaged every 5–7 days after reaching confluency. Cardiomyocytes were obtained by differentiating hESCs using small molecules [50]. Briefly, hESCs were treated with 10 μmol/L of CHIR99021, selective inhibitor of glycogen synthase kinase 3 (GSK-3), (Stemgent, Cambridge, MA) at day 0 and 5 μmol/L of IWP4, an inhibitor of Wnt, (Stemgent) at day 3 of the differentiation protocol. For the first 5 days, RPMI Medium 1640 (Life Technologies) with Gem21 NeuroPlex without Insulin (Gemini) was used. After day 7, media change was performed every 72 hours with RPMI Medium 1640 (Life Technologies) supplemented with Gem21 NeuroPlex (Gemini). Spontaneous contractions were first observed at day 8 of differentiation. Beating cardiomyocytes were dissociated after 15–20 days of differentiation as previously described [49].
Similar to the HL-1 protocol, scaffolds were dehydrated and placed in 5% Pluronic® F127-coated 12-well plates. hESC-derived cardiomyocytes were concentrated and seeded at a concentration of 1 × 106 cells /mL scaffold volume. This volume is twice that utilized for HL-1 cells because growth and doubling of the differentiated hESCs was not expected to match the rate of HL-1 growth. Similarly, to deliver the cells, 100 μl of cell suspension was used to rehydrate the silk sponges, delivered in two 50 μl aliquots, followed by reversibly deforming the scaffold sponge to promote cell infiltration. The scaffolds were stored in the cell culture incubator for 15–20 minutes prior to adding enough media to each well so that the scaffolds floated (~3 mL). Well plates containing scaffolds were kept rotated in the incubator (37°C/5% CO2) to maintain proper diffusion for metabolic activity and cell culture media was exchanged every 4 days. hESC-derived cardiomyocyte seeded scaffolds were harvested after 1 week in culture for further analysis.
2.8.3. Isolation of Protein, DNA, and RNA
BioReagents™ SurePrep™ RNA/DNA/Protein Purification Kit (Fisher Scientific, Waltham, MA) were utilized to collect RNA, DNA and protein from each of the scaffolds. Prior to isolation, scaffolds were flash frozen in liquid nitrogen and digested with lysis buffer, as directed for tissue samples. In some cases, extra time was added to the centrifugation steps in the protocol to ensure that the entire sample was processed in its entirety through the spin columns. In all cases, protein isolation was completed using only 200 μl of the cleared lysate collected during RNA extraction.
Total protein content in each sample was analyzed via a Pierce BCA Protein Assay (ThermoFisher Scientific, Waltham, MA) using BSA control solutions to determine the loading necessary for Western blot analysis. RNA purity and concentration were determined using a NanoDrop (ThermoFisher Scientific, Waltham, MA). Genomic DNA content was also measured on the NanoDrop. In all cases, relative amounts are reported, using the isotropic silk-only scaffold as the control for the HL-1 seeded constructs and anisotropic silk-only scaffold for the hESC-derived cardiomyocyte seeded constructs.
2.8.4. Western Blot Analysis
In order to assess the potential cell signaling pathways involved in cECM incorporation into silk scaffolds, pathways previously implicated in proliferation and cell survival were evaluated by Western blot [23]. Equal amounts of protein (12 μg per lane) were loaded into pre-cast 4–15% polyacrylamide gels (cat # 456–1086, BioRad, Hercules, CA) and run before being transferred to a nitrocellulose membrane. A Western blot was conducted and primary antibodies were used to evaluate the expression of the various proteins over time and for each condition. GAPDH (G-9575 Sigma-Aldrich, St. Louis, MO) was utilized to normalize to total cell content. HL-1 proteins were stained with connexin 43 (1/200, #3512, Cell Signaling Technology®, Beverly, MA) and myosin heavy chain (MHC) (2 μg/ml, ALD-58, DSHB, University of Iowa). hESC-derived cardiomyocyte blots were stained with the same connexin 43 and MHC antibodies, along with Integrin β1 (2 μg/ml, AIIB2, DSHB, University of Iowa), Islet-1 (1/200, ab20670, Abcam, Cambridge, UK), cardiac troponin I (1/200, ab47003, Abcam, Cambridge, UK), and α-cardiac actin (1/200, sc-58670 Santa Cruz Biotechnology, Santa Cruz, CA). Species-specific secondary antibodies conjugated to HRP (656120 and 656520, Invitrogen, Carlsbad, CA) were used at 1/5000 dilutions for enhanced chemiluminescence. Images were acquired on the G:Box Chemi XR5 (Syngene, Cambridge, United Kingdom). Expression intensities were analyzed using ImageJ (NIH, Bethesda, MD). When multiple blots were required to ensure the appropriate sample size, expression intensities for each protein were normalized to the mean expression intensity per blot for that respective protein to allow for comparison between blots [51, 52].
2.9. Statistics
All results were analyzed with appropriate-sized analysis of variance with Student t test post hoc testing. p values less than 0.05 were considered statistically significant and are reported for each result.
3. Results
3.1. Cell seeding impacts long term structure of silk-cECM scaffolds
Silk-based scaffolds were formed via directional freezing to generate anisotropic features in the scaffold architecture [34]. ECM components, such as porcine derived cardiac ECM from the left ventricle of adult hearts (cECM) or collagen isolated from rat tail, were incorporated as cell adhesion moieties within these aligned silk scaffolds through mixing of the soluble ECM solution with the silk solution prior to scaffold formation (Figure 1A). SEM demonstrated that silk-cECM (Figure 1C) and silk collagen (Figure 2D) scaffolds had different surface structure when compared to silk alone (Figure 1B vs C,D), with pores in the silk layers leading to the potential for improved transport between these layers. Following 4 weeks in cell culture, SEM images showed evidence for both cell-based ECM deposition as well as remodeling of the incorporated cECM by seeded HL-1 cells, based on changes in silk architecture and increased microscale sized pores (arrows, Figure 1E–G).
Figure 1. Formation of cardiac extracellular matrix (cECM) – silk composite scaffolds.
A. Schematic of the processing techniques utilized to create structurally aligned silk scaffolds. Silk and cECM solutions are mixed and poured into PDMS molded fitted with a metal plate. On one side, dry ice and ethanol are mixed to provide a freezing source. The silk-cECM solution is poured on the other side of the plate and allowed to freeze. The water crystals that form direct the alignment achieved in the sponge. Then, lyophilization and water annealing set the form in place and create a water insoluble scaffold. SEM images show the structure of the silk and silk composites. B. Aligned silk scaffold, C. Aligned silk-cECM scaffold. D. Aligned silk-collagen scaffold. E, F, G. Dehydrated silk-cECM scaffolds following 4 weeks of cell culture. Matrix remodeling and new matrix deposition (E, arrow) are evident. Fig. 1G shows cell-based degradation of the silk-cECM matrix (arrows).
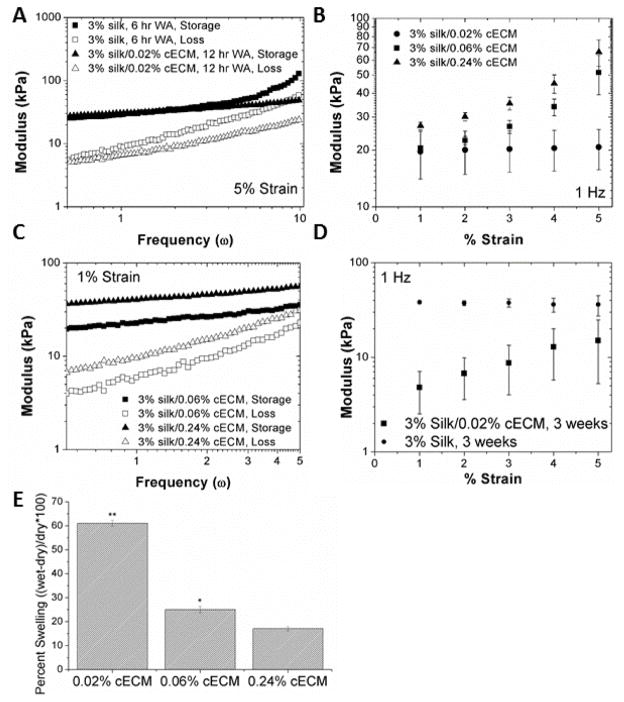
Figure 2. Mechanical Properties of cECM-silk composite scaffolds.
A. Prior to use, appropriate mechanical controls were identified such that the starting properties of all of the scaffolds were similar. B. Strain rate independence over frequencies of 0.5–5 Hz is necessary for proper cell function within the scaffold. Increasing the concentration of cECM to 0.06% introduces strain rate dependence in the material. C. Frequency dependence in the higher cECM concentrations is shown, demonstrating the complex interaction between the silk and cECM in these scaffolds. D. Following 3 weeks in culture with seeded HL-1 cells, the silk-cECM composite scaffolds exhibit increased degradation and strain rate dependence demonstrating that the cells are able to alter the properties of the material via remodeling and secretion of extracellular matrix. E. Swelling ratio of 3% silk scaffolds formed with 0.02, 0.06, and 0.24% cECM. The increase in cECM decreases the water absorption following water annealing.
3.2. Incorporation of cECM Impacts Silk Scaffold Mechanics
Given the changes to the architecture of the silk scaffolds in the presence of incorporated cECM, the mechanics of these scaffolds were investigated, both pre- and post-cell seeding. To arrive at physiologically relevant mechanical properties, many silk formulations were compared, until a set of experimental parameters was found that resulted in similar mechanical properties between both silk and silk-cECM scaffolds under physiologically relevant conditions through optimization of water annealing times and cECM concentration (Figure 2). First, we aimed to determine design parameters that resulted in similar dynamic moduli over a frequency of 0.5–10 Hz. Water annealing the silk-cECM scaffolds for twice as long (12 hours) in comparison to the silk only scaffolds (6 hours) resulted in similar dynamic moduli. At a strain of 5%, (Figure 2A) the addition of the cECM prevented frequency-dependent changes in dynamic moduli above 5 Hz, while under 5 Hz, the modulus was independent of both frequency and protein composition. Such similarities were not as evident in the dynamic modulus as a function of strain rate (constant frequency of 1 Hz).
3.3. The concentration of cECM incorporated into the scaffold significantly impacts mechanical properties
The data in Figure 2B,C show that addition of 200 μg cECM/mL to 3% silk solution was suitable for scaffold formulation with mechanical properties in the range of native tissue. At these ratios, the dynamic modulus of the biomaterial was unaffected by strain rate, while increasing cECM concentration yielded strain rate dependent materials, which are not ideal for cardiac applications (Figure 2B,C). Water re-uptake (rehydration potential) in silk-cECM composite scaffolds decreased as cECM concentration increased (Figure 2E). Data showed that tripling the cECM content while holding the silk content constant significantly increased the bulk elastic modulus. In addition, scaffolds containing 0.02% cECM (200 μg cECM/ mL 3% silk solution) were significantly (p < 0.05) more susceptible to cell-based degradation and remodeling after 3 weeks in culture (Figure 2D).
3.4. cECM incorporation improves cell infiltration over silk alone
To investigate the ability of host cells to infiltrate within the aligned silk scaffolds in an in vivo setting, subcutaneous implants of critically sized (10 mm × 5 mm × 2.5 mm) scaffolds were performed in rats. Representative images of silk (Figure 3A–C) and silk-cECM (Figure 3D–F) scaffolds removed after 4 weeks are shown. In the scaffolds containing cECM, both cell-mediated degradation and remodeling of the scaffold were visible, as the silk structure looks different from the silk alone scaffolds, containing significantly more cells as noted by the increase in cytoskeletal components (purple) and nuclei (dark brown). Analysis of cell infiltration depth in silk, fibrin-coated silk, and silk-cECM scaffolds revealed that the addition of cECM to the scaffold formulation significantly improved cell infiltration over silk and fibrin-coated silk scaffolds (p≪0.001) (Figure 3G). Even in the presence of fibrin, cell infiltration in aligned silk scaffolds was 56 ± 1%, while the addition of 0.2 mg cECM/ml at a concentration of 0.02% to silk solution resulted in 99 ± 1%. These results demonstrated that cECM incorporation into the silk scaffold formulation improved cell infiltration even over the addition of a degradable matrix coating, such as fibrin.
Figure 3. Infiltration and degradation of the scaffolds in vivo.
Hematoxylin and Masson’s Trichrome staining of a silk only (A–C) and silk-cECM (D–F) aligned scaffolds, demonstrating that cell infiltration within the silk only scaffold is limited to the edges. Representative images of the center of the scaffold are shown in B and E, while C and F show the edge of the scaffolds. Initial vascularization of the silk-cECM scaffolds is evident, given the staining for muscle fibers in red surrounded by the start of organized cell structures (arrows, E,F). This is particularly evident near the top left arrow in panel F. To confirm vascularization, staining for von Willebrand factor (G) and PECAM-1 (H) were performed on silk-cECM sections. Positive staining is shown in brown and highlighted by the black arrows; sections are counterstained with hematoxylin. Image analysis (I) demonstrates that cell infiltration was significantly improved in samples containing cECM (p < 0.001). Green dashed line represents the interface between the fascia and silk scaffold.
Significantly, cECM incorporation also promoted vascularization near the edges of the scaffold (Figure 3C), as demonstrated Masson’s Trichrome staining (Figure 3E,F). Near the edges, organized cell and muscle fiber structures were present. Such structures were not present in any of the silk only samples. The fibrin coated silk sponges were previously reported to only demonstrate evidence of vascularization (via positive PECAM-1 (CD31) and α-smooth muscle actin staining) in thin silk sponges containing large channels [32]. Here, we demonstrate positive staining for von Willebrand factor and PECAM-1, demonstrating that angiogenesis is present within silk-cECM scaffolds after 4 weeks (Figure 3G,H, respectively).
3.5. Extracellular Matrix Proteins Improve Cell Seeding
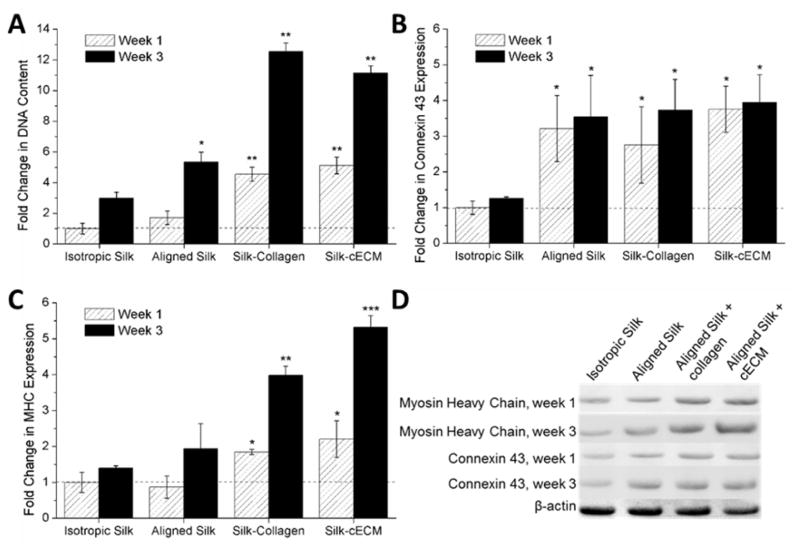
HL-1 cells were utilized to evaluate scaffold architecture and in vitro culture conditions for critically sized scaffolds. To determine the role of alignment and protein coating, we evaluated scaffolds with isotropic and anisotropic (aligned) pore architectures. The results demonstrated that cell attachment and infiltration in aligned scaffolds was improved (> 8-fold higher DNA content in the aligned scaffolds after 3 weeks compared to a 3-fold increase in the isotropic scaffolds). In addition, both pepsin digested rat-tail collagen and pepsin digested decellularized left ventricle tissue (cECM) were studied. Figure 4A shows that initial adhesion and growth in the scaffolds improved with the addition of the collagen or ECM (> 4-fold greater DNA content for both silk-collagen and silk-cECM scaffolds after 1 week). After 3 weeks, the scaffolds contained significantly more cells in samples containing both collagen and cECM, while the plain silk scaffolds did not provide an optimal system for growth and attachment.
Figure 4. Silk-cECM sponges provide and optimal environment for growth and maintenance of cardiac phenotype.
A. Fold change in DNA content compared to week 1 DNA content in isotropic silk sponges (no ECM). A greater than 10-fold increase in DNA content was measured for silk sponges containing either collagen or cECM after 3 weeks in culture. B. Connexin 43 content in silk sponges represented as a fold-change comparison to content in isotropic sponges after 1 week in culture. Significant differences in connexin 43 were measured for aligned constructs at all time points (*p < 0.001). C. Myosin Heavy Chain (MHC) content in silk sponges represented as a fold-change comparison to content in isotropic sponges after 1 week in culture. Significant differences from isotropic sponges were measured for all constructs containing ECM components at 1 week (*p < 0.001). After 3 weeks, silk-collagen and silk-cECM sponge constructs were significantly greater than isotropic and aligned silk samples (**p < 0.05) and the silk-cECM scaffold is significantly greater than the silk-collagen scaffold (***p < 0.05). D. Representative western blots for the data presented in sections B and C.
3.6. Protein expression by HL-1s improves with extracellular matrix addition to aligned silk scaffolds
To evaluate the response of HL-1s to the different 3D culture conditions, protein expression of the cells seeded in the different culture conditions was compared across conditions after 1 and 3 weeks in culture via Western blot analysis. All data was normalized to isotropic silk scaffolds after 1 week in culture. Figure 4B shows that connexin 43 expression was improved in scaffolds with anisotropic pore architectures (p < 0.001), but expression was not significantly improved when ECM proteins were added to the silk formulation. Figure 4C shows that myosin heavy chain expression significantly increased in the presence of ECM protein addition (p < 0.001). After 1 week in culture both the silk-collagen scaffolds and the silk-cECM scaffolds had greater than 2-fold increase in MHC content. After 3 weeks, these significant differences from ECM-free scaffolds remained (p < 0.05), where silk-cECM scaffolds had an ~5-fold increase in MHC content, which was also significantly greater than the 4-fold increase in MHC content in the silk-collagen scaffolds after 3 weeks (p < 0.05). Figure 4D shows representative western blots for the data presented.
3.7. Silk-cECM scaffolds promote cardiomyocyte phenotype in differentiated hESCs
To evaluate the potential of silk-cECM scaffolds with a more clinically relevant cell population [49, 53], hESCs differentiated to a cardiomyocyte lineage following a previously described protocol [49, 50] were seeded into the silk scaffolds. After 1 week, the protein content was analyzed via Western blot to determine if the addition of left ventricle derived ECM (cECM) maintained and promoted the growth of the differentiated cardiomyocytes as well as the progenitor cell population that exist within this heterogeneous cell population (Figure 5A). In comparison to aligned silk scaffolds without cECM, expression of both connexin 43 and α-cardiac actin remained the same (p > 0.2) after week 1 in culture. However, a significant increase in integrin β1, islet-1, and cardiac troponin I were measured (p < 0.05) in the aligned silk-cECM scaffolds compared to aligned silk scaffolds without cECM. Figure 5B shows a representative western blot for the samples presented in Figure 5A.
Figure 5. hESC-derived CMs in Silk-cECM sponges.
A. Western blot analysis normalized to protein content in aligned silk scaffolds. Results demonstrate that the addition of cECM improved significantly improved the expression of Integrin β1, Islet-1, and cardiac troponin I by hESC-derived CMs (*p < 0.05). B. Representative western blot for data shown in (A), representing expression of hESC-CMs in aligned silk with cECM and aligned silk alone.
4. Discussion
In order for 3D scaffolds for cardiac tissue engineering to become a viable clinical option for cardiac repair and regeneration, scaffold design and fabrication must be optimized to enhance host cell infiltration, tunable degradation, and matrix remodeling are critical, as is the ability of the scaffold to promote or maintain functional cardiac cell phenotypes of both cells that may be seeded in the scaffold or host cells that migrate into the scaffold. Recent work has demonstrated that adhesive interactions related to properties such as specific integrin binding, ligand density, and matrix composition, play key roles in cardiomyocyte phenotype and function. We have developed a silk-based scaffold that addresses these needs through the incorporation of cECM. The resulting scaffold platform is multifaceted with the potential to function as an acellular cardiac patch and/or as a platform for in vitro analysis of stem or progenitor cell metabolism.
The addition of cECM is advantageous for engineering critically sized tissue constructs both in vitro and in vivo. cECM enhances cell attachment and viability, while promoting functional phenotypes. In addition, cECM derived from porcine left ventricular tissues has been evaluated in preclinical trials [42, 54, 55], demonstrating the ability to aid in functional cardiac repair by promoting vascularization and progenitor cell homing to the site of injury. The silk-cECM composite materials described herein present a useful approach to achieve desired material properties for cardiac tissue engineering needs. Silk is an abundant protein for biomaterials and can be processed using aqueous-based methods and a single pig heart can provide adequate amounts of cECM for hundreds of scaffolds, leading to the potential scalability of the silk-cECM systems described here.
Initial investigations with these materials aimed to achieve mechanical properties that were similar between the two scaffold formulations, while maintaining a dynamic modulus in the range of healthy cardiac tissue (25–50 kPa). It was also necessary to make sure these material properties did not change significantly over small strains, as beating cells are able to compact the material. Based on the above conditions we utilized a material consisting of 30 minute extracted silk containing 0.2 mg of decellularized left ventricular porcine ECM (cECM).
Previous work has demonstrated that silk sponges with aligned scaffold architecture can be formed with a variety of pore sizes and tunable mechanical properties, and with arrayed hollow channels to improve diffusion and vascularization within the bulk architecture [32, 34]. However, most of these studies required the incorporation of fibrin gel to allow for in vitro and in vivo cell infiltration and adhesion. While fibrin is critical during wound healing, its relevance for cardiac tissue engineering strategies is minimal. The composition of cECM changes with developmental age [25, 38] and it has been demonstrated that substrate composition alters the maturation and proliferation of cardiomyocytes as well as other cells utilized for cardiac tissue engineering [25, 56–58]. Therefore, development of scaffolds for in vivo needs following open-heart surgery or for reconstruction in newborns with congenital heart defects requires matrices with specific composition. The present study addresses these issues of host cell infiltration and specific cell response through incorporation of tissue-derived matrix into silk solution, generating silk-cECM composite scaffolds which should be highly reproducible using the methods described. These scaffold systems represent an efficient approach to significantly improve cell seeding (Figure 4), enhancing cell phenotype (Figure 4,5), and promoting in vivo cell infiltration (Figure 3) in critically-sized scaffolds (10 mm × 5 mm × 2.5 mm).
It has been demonstrated that mechanical properties of the heart vary with disease and age, and therefore it is important that 3D scaffolds also properly recapitulate the desired native mechanical properties, regardless of cECM incorporation [59]. As such, it was critical that the addition of matrix proteins into the silk solution allowed for reproducible mechanical properties that matched the in vivo mechanical environment (Figure 2). It is important to note that water annealing the scaffolds containing cECM or collagen for 6 hours or less resulted in disintegration of the scaffolds within 48 hours, due to the disruption of the silk β-sheet formation. In addition, the results showed that an increase in modulus was achieved with an increase in cECM concentration (Figure 2). During this optimization process, increasing cECM content was expected to lead to more elastic scaffolds with lower moduli, as the cECM would interfere with silk β-sheet formation. However, in these systems, the silk and cECM proteins are frozen in place, not covalently cross-linked as in other systems [60]. Therefore, the water annealing method used to induce β-sheet formation in the silk can affect both the silk protein structure, as well as the structure of the cECM. This results in the measured change in swelling ratio (Figure 2E), leading to the loss of some of the bulk structure. The merging of the originally formed pore architecture generated a scaffold which cannot expand in water as much as the scaffolds containing only 0.02% cECM.
In addition to improved adhesion features achieved by the addition of the cECM, the results demonstrated that silk-cECM scaffolds were degraded more rapidly in cell culture (Figure 2). The modulus of the material decreased significantly after 4 weeks, but not to the point of disintegration or below normal tissue modulus (~10–25 kPa). Since these scaffold systems are readily tunable in terms of initial mechanical properties and bulk architecture, systems can be developed with a variety of shapes and sizes (See Figure 1). The tenability of the scaffold’s material properties increases the utility of these systems and demonstrates the ability to scale these scaffolds to fit defects of various morphologies in order to meet a patient’s specific needs.
The results presented here further demonstrate the addition of cECM to the silk scaffold maintains the phenotype of seeded cells via Western blot analysis. HL-1 cardiomyocytes were first utilized to optimize seeding and culturing protocols, demonstrating that the addition of ECM proteins improved cell seeding and promoted functional phenotypes (Figure 3). Further utilization of these scaffolds with a clinically relevant cell type demonstrated that when a heterogeneous population of differentiated hESCs was seeded within the scaffolds, the addition of cECM promoted interactions with the scaffolds (integrin B1), maintenance of cardiac progenitor phenotypes (islet-1), and definitive myocardial phenotypes (cardiac troponin I). Specifically, the increase in TNI gene expression suggests that cECM incorporation maintains a more favorable cardiogenic milieu compared to silk alone. DNA content in the silk-cECM scaffolds was 2-fold greater than the silk scaffolds (p < 0.05), but it is unclear if this was due to initial cell attachment, maintenance of viability, or proliferation of cardiac progenitors within the construct. An increase in integrin β1 expression demonstrates that cells were able to bind to and access the cECM in the silk scaffold, which influenced both mature myocyte and progenitor phenotypes.
Overall, the in vitro analysis demonstrated that the incorporation of pepsin-digested cECM maintained hESC-CM phenotypes and promoted integrin signaling over the ECM-free scaffolds. While this work supports the continued investigation and utilization of these systems, it does not demonstrate that these scaffold systems are necessarily optimized for in vivo use in cardiac applications. Future work aims to look at small molecule delivery, incorporation of large channels for promotion of vascular network formation, and differentiation of cardiac progenitors within the 3D scaffold systems, as recent work demonstrates that cECM can alter the fate of cardiac progenitors to yield more homogenous and mature cell types [38, 39]. Ultimately we seek to translate the use of this scaffold system to in vivo studies in both cell encapsulated cardiac patches and as an acellular biomaterial for cardiac reconstruction.
5. Conclusions
A versatile scaffold system was developed for in vitro 3D culture of cells and for use as cell-free patches from silk and decellularized cardiac tissue matrix. Using directional freezing, the architecture of these materials was regulated, resulting in bulk anisotropic pore architectures that mimic heart tissue. Both in vitro and in vivo results demonstrated the potential of this material for cell-based or cell-free implantation. The results demonstrate that alignment improved cell infiltration in vivo and that addition of cECM further improved cell infiltration and vascularization of the cell-free scaffolds as well as the phenotype of seeded cardiac cells.
Acknowledgments
The authors would like to acknowledge Dr. William Claycomb, professor in the department of Biochemistry and Molecular Biology at the Louisiana State University School of Medicine for his kind donation of HL-1 cells for use in this work. In addition, WLS would like to acknowledge funding from the National Institutes of Health Institutional Research and Academic Career Development Awards program at Tufts University (K12GM074869, Training in Education and Critical Research Skills (TEACRS)). We also thank the NIH P41 (EB002520) for support of this work.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics—2015 Update: A Report From the American Heart Association. Circulation. 2014 doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Eschenhagen T, Zimmermann WH. Engineering myocardial tissue. Circulation Research. 2005;97:1220–31. doi: 10.1161/01.RES.0000196562.73231.7d. [DOI] [PubMed] [Google Scholar]
- 3.Garbern Jessica C, Lee Richard T. Cardiac Stem Cell Therapy and the Promise of Heart Regeneration. Cell Stem Cell. 2013;12:689–98. doi: 10.1016/j.stem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacology & Therapeutics. 2009;123:255–78. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics—2013 Update: A Report From the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics—2010 update. A report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 7.Brown JW, Ruzmetov M, Vijay P, Rodefeld MD, Turrentine MW. Right ventricular outflow tract reconstruction with a polytetrafluoroethylene monocusp valve: A twelve-year experience. The Journal of Thoracic and Cardiovascular Surgery. 2007;133:1336–43. doi: 10.1016/j.jtcvs.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 8.Hauser M, Eicken A, Kuehn A, Hess J, Fratz S, Ewert P, et al. Managing the right ventricular outflow tract for pulmonary regurgitation after tetralogy of Fallot repair. Heart Asia. 2013;5:106–11. doi: 10.1136/heartasia-2013-010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreutziger KL, Murry CE. Engineered Human Cardiac Tissue Pediatric Cardiology. 2011;32:334–41. doi: 10.1007/s00246-011-9888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durrani S, Konoplyannikov M, Ashraf M, Haider KH. Skeletal myoblasts for cardiac repair. Regen Med. 2010;5:919–32. doi: 10.2217/rme.10.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Formigli L, Zecchi-Orlandini S, Meacci E, Bani D. Skeletal Myoblasts for Heart Regeneration and Repair: State of the Art and Perspectives on the Mechanisms for Functional Cardiac Benefits. Current Pharmaceutical Design. 2010;16:915–28. doi: 10.2174/138161210790883390. [DOI] [PubMed] [Google Scholar]
- 12.Osterziel KJ. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1 year results of the REPAIR-AMI trial. European heart journal. 2007;28:638. doi: 10.1093/eurheartj/ehl515. [DOI] [PubMed] [Google Scholar]
- 13.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart circulation. 2002;105:93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 14.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, et al. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123:364–73. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nature methods. 2013;10:781–7. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20:616–23. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turnbull IC, Karakikes I, Serrao GW, Backeris P, Lee JJ, Xie C, et al. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. Faseb J. 2014;28:644–54. doi: 10.1096/fj.13-228007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malliaras K, Marban E. Cardiac cell therapy: where we’ve been, where we are, and where we should be headed. Br Med Bull. 2011;98:161–85. doi: 10.1093/bmb/ldr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WY, Wei HJ, Lin WW, Yeh YC, Hwang SM, Wang JJ, et al. Enhancement of cell retention and functional benefits in myocardial infarction using human amniotic-fluid stem-cell bodies enriched with endogenous ECM. Biomaterials. 2011;32:5558–67. doi: 10.1016/j.biomaterials.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 22.Tran N, Li Y, Maskali F, Antunes L, Maureira P, Laurens MH, et al. Short-term heart retention and distribution of intramyocardial delivered mesenchymal cells within necrotic or intact myocardium. Cell Transplant. 2006;15:351–8. doi: 10.3727/000000006783981918. [DOI] [PubMed] [Google Scholar]
- 23.Morgan KY, Black LD., III Mimicking isovolumic contraction with combined electromechanical stimulation improves the development of engineered cardiac constructs. Tissue engineering Part A. 2014;20:1654–67. doi: 10.1089/ten.tea.2013.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black LD, III, Meyers JD, Weinbaum JS, Shvelidze YA, Tranquillo RT. Cell-Induced Alignment Augments Twitch Force in Fibrin Gel–Based Engineered Myocardium via Gap Junction Modification. Tissue Engineering Part A. 2009;15:3099–108. doi: 10.1089/ten.tea.2008.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams C, Budina E, Stoppel WL, Sullivan KE, Emani S, Emani SM, et al. Cardiac extracellular matrix-fibrin hybrid scaffolds with tunable properties for cardiovascular tissue engineering. Acta biomaterialia. 2015;14:84–95. doi: 10.1016/j.actbio.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nature Medicine. 2006;12:452–8. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 27.Fink C, Ergun S, Kralisch D, Remmers U, Weil J, Eschenhagen T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. Faseb Journal. 2000;14:669–79. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- 28.Martins AM, Eng G, Caridade SG, Mano JF, Reis RL, Vunjak-Novakovic G. Electrically Conductive Chitosan/Carbon Scaffolds for Cardiac Tissue Engineering. Biomacromolecules. 2014;15:635–43. doi: 10.1021/bm401679q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pok S, Benavides OM, Hallal PA, Jacot J. Use of myocardial matrix in a chitosan-based full thickness heart patch. Tissue Engineering. 2014 doi: 10.1089/ten.tea.2013.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyagi Y, Chiu LL, Cimini M, Weisel RD, Radisic M, Li RK. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials. 2011;32:1280–90. doi: 10.1016/j.biomaterials.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Radisic M, Park H, Martens TP, Salazar-Lazaro JE, Geng W, Wang Y, et al. Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue. Journal of Biomedical Materials Research Part A. 2008;86A:713–24. doi: 10.1002/jbm.a.31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rnjak-Kovacina J, Wray LS, Golinski JM, Kaplan DL. Arrayed Hollow Channels in Silk-Based Scaffolds Provide Functional Outcomes for Engineering Critically Sized Tissue Constructs. Advanced Functional Materials. 2014;24:2188–96. doi: 10.1002/adfm.201302901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wray LS, Hu X, Gallego J, Georgakoudi I, Omenetto FG, Schmidt D, et al. Effect of processing on silk-based biomaterials: reproducibility and biocompatibility. Journal of Biomedical Materials Research: Part B Applied Biomaterials. 2011;99:89–101. doi: 10.1002/jbm.b.31875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wray LS, Rnjak-Kovacina J, Mandal BB, Schmidt DF, Gil ES, Kaplan DL. A silk-based scaffold platform with tunable architecture for engineering critically-sized tissue constructs. Biomaterials. 2012;33:9214–24. doi: 10.1016/j.biomaterials.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockwood DN, Preda RC, Yucel T, Wang XQ, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nature Protocols. 2011;6:1612–31. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin HJ, Park J, Karageorgiou V, Kim UJ, Valluzzi R, Kaplan DL. Water-stable silk films with reduced beta-sheet content. Advanced Functional Materials. 2005;15:1241–7. [Google Scholar]
- 37.Hu X, Shmelev K, Sun L, Gil ES, Park SH, Cebe P, et al. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules. 2011;12:1686–96. doi: 10.1021/bm200062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams C, Quinn KP, Georgakoudi I, Black LD., III Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomaterialia. 2014;10:194–204. doi: 10.1016/j.actbio.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.French KM, Boopathy AV, DeQuach JA, Chingozha L, Lu H, Christman KL, et al. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomaterialia. 2012;8:4357–64. doi: 10.1016/j.actbio.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gershlak JR, Black LD., 3rd Beta 1 integrin binding plays a role in the constant traction force generation in response to varying stiffness for cells grown on mature cardiac extracellular matrix. Exp Cell Res. 2014 doi: 10.1016/j.yexcr.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Gershlak JR, Resnikoff JIN, Sullivan KE, Williams C, Wang RM, Black LD. Mesenchymal stem cells ability to generate traction stress in response to substrate stiffness is modulated by the changing extracellular matrix composition of the heart during development. Biochemical and Biophysical Research Communications. 2013;439:161–6. doi: 10.1016/j.bbrc.2013.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Science translational medicine. 2013;5:173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rockwood DN, Preda RC, Yucel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nature Protocols. 2011;6:1612–31. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–16. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seif-Naraghi S, Singelyn J, DeQuach J, Schup-Magoffin P, Christman K. Fabrication of Biologically Derived Injectable Materials for Myocardial Tissue Engineering. Journal of visualized experiments: JoVE. 2010 doi: 10.3791/2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajan N, Habermehl J, Coté M-F, Doillon CJ, Mantovani D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nature protocols. 2007;1:2753–8. doi: 10.1038/nprot.2006.430. [DOI] [PubMed] [Google Scholar]
- 47.White SM, Constantin PE, Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. American Journal of Physiology - Heart and Circulatory Physiology. 2004;286:H823–H9. doi: 10.1152/ajpheart.00986.2003. [DOI] [PubMed] [Google Scholar]
- 48.Claycomb WC, Lanson NA, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, et al. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proceedings of the National Academy of Sciences. 1998;95:2979–84. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atmanli A, Hu D, Domian IJ. Molecular etching: a novel methodology for the generation of complex micropatterned growth surfaces for human cellular assays. Advanced Healthcare Materials. 2014;3:1759–64. doi: 10.1002/adhm.201400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1848–57. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morgan KY, Black LD., III Mimicking isovolumic contraction with combined electromechanical stimulation improves the development of engineered cardiac constructs. Tissue Engineering Part A. 2014;20:1654–67. doi: 10.1089/ten.tea.2013.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lan JY, Williams C, Levin M, Black LD., 3rd Depolarization of Cellular Resting Membrane Potential Promotes Neonatal Cardiomyocyte Proliferation In Vitro. Cellular and molecular bioengineering. 2014;7:432–45. doi: 10.1007/s12195-014-0346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buikema JW, Mady AS, Mittal NV, Atmanli A, Caron L, Doevendans PA, et al. Wnt/beta-catenin signaling directs the regional expansion of first and second heart field-derived ventricular cardiomyocytes. Development. 2013;140:4165–76. doi: 10.1242/dev.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson TD, Braden RL, Christman KL. Injectable ECM scaffolds for cardiac repair. Methods in molecular biology (Clifton, NJ) 2014;1181:109–20. doi: 10.1007/978-1-4939-1047-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson TD, Christman KL. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin Drug Deliv. 2013;10:59–72. doi: 10.1517/17425247.2013.739156. [DOI] [PubMed] [Google Scholar]
- 56.Ye KY, Sullivan KE, Black LD. Encapsulation of cardiomyocytes in a fibrin hydrogel for cardiac tissue engineering. Journal of visualized experiments: JoVE. 2011 doi: 10.3791/3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sullivan KE, Quinn KP, Tang KM, Georgakoudi I, Black LD. Extracellular matrix remodeling following myocardial infarction influences the therapeutic potential of mesenchymal stem cells. Stem cell research & therapy. 2014;5:14. doi: 10.1186/scrt403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.French KM, DeQuach JA, Christman KL, Davis ME. Enhanced Proliferation and Cardiogenic Differentiation of Cardiac Progenitor Cells Treated with a Naturally Derived Cardiac Extracellular Matrix. Faseb J. 2012;26 [Google Scholar]
- 59.Buckberg G, Hoffman JIE, Mahajan A, Saleh S, Coghlan C. Cardiac Mechanics Revisited: The Relationship of Cardiac Architecture to Ventricular Function. Circulation. 2008;118:2571–87. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- 60.Partlow BP, Hanna CW, Rnjak-Kovacina J, Moreau JE, Applegate MB, Burke KA, et al. Highly Tunable Elastomeric Silk Biomaterials. Advanced Functional Materials. 2014;24:4615–24. doi: 10.1002/adfm.201400526. [DOI] [PMC free article] [PubMed] [Google Scholar]