Abstract
For more than a century, speech repetition has been used as an assay for gauging the integrity of the auditory-motor pathway in aphasia, thought classically to involve a linkage between Wernicke’s area and Broca’s area via the arcuate fasciculus. During the last decade, evidence primarily from functional imaging in healthy individuals has refined this picture both computationally and anatomically, suggesting the existence of a cortical hub located at the parietal-temporal boundary (area Spt) that functions to integrate auditory and motor speech networks for both repetition and spontaneous speech production. While functional imaging research can pinpoint the regions activated in repetition/auditory-motor integration, lesion-based studies are needed to infer causal involvement. Previous lesion studies of repetition have yielded mixed results with respect to Spt’s critical involvement in speech repetition. The present study used voxel-based lesion symptom mapping (VLSM) to investigate the neuroanatomy of repetition of both real words and non-words in a sample of 47 patients with focal left hemisphere brain damage. VLSMs identified a large voxel cluster spanning gray and white matter in the left temporal-parietal junction, including area Spt, where damage was significantly related to poor non-word repetition. Repetition of real words implicated a very similar dorsal network including area Spt. Cortical regions including Spt were implicated in repetition performance even when white matter damage was factored out. In addition, removing variance associated with speech perception abilities did not alter the overall lesion pattern for either task. Together with past functional imaging work, our results suggest that area Spt is integral in both word and non-word repetition, that its contribution is above and beyond that made by white matter pathways, and is not driven by perceptual processes alone. These findings are highly consistent with the claim that Spt is an area of sensory-motor translation in speech processing.
Keywords: sensory-motor integration, speech repetition, speech production, voxel-based lesion symptom mapping, area Spt
1. Introduction
In his classic 1885 article, “On Aphasia,” Lichtheim emphasized speech repetition as a critical factor in aphasia classification, that is, in identifying the source of a language deficit in the context of a model of language processing. In particular, within Lichtheim’s scheme, the ability to repeat speech differentiated transcortical motor aphasia (spared repetition) from motor or Broca’s aphasia (impaired repetition) and transcortical sensory aphasia (spared repetition) from sensory or Wernicke’s aphasia (impaired repetition). Although Lichtheim noted that repetition was impaired in many types of aphasia, today repetition deficits are most commonly associated, in the minds of most language scientists, with conduction aphasia. In fact, it is not uncommon to see conduction aphasia characterized as a disorder of repetition. It’s worth noting that this is not how Lichtheim, nor the discoverer of conduction aphasia, Wernicke, viewed the syndrome. To them, the presence of paraphasias in speech output was the critical symptom, which was evident in volitional speech as well as during speech repetition.
Of interest presently is the explanation that the classical aphasiologists put forward for the mechanism underlying both paraphasic errors in spontaneous speech and repetition deficits in conduction aphasia. Both Wernicke and Lichtheim agreed that the impairments were auditory-motor in nature and reflected a disruption of the normal auditory control over motor speech plans. In Wernicke’s words,
Observations of daily speech usage and the process of speech development indicates [sic] the presence of an unconscious, repeated activation and simultaneous mental reverberation of the acoustic image which exercises a continuous monitoring of the motor images. […This auditor-motor pathway] whose thousandfold use [during development] maintains a continuing significant control over the choice of the correct motor image. […] Apart from impairment in comprehension [in sensory/Wernicke’s aphasia], the patient also presents aphasic symptoms in speech produced by absence of the unconscious monitoring of the imagery of the spoken word. [p. 106–107, Eggert]
And in Lichtheim’s,
[Wernicke] assumes that the nervous influx descending along B M m [the concept to motor pathway] sends a branch current to A [the auditory center], and that this subconscious innervation of the auditory memories of words secures the correct choice and expression of them; and that irregularities occur as soon at the co-operation of these elements ceases to take place. I accept this interpretation, but with a modification, namely, […] that this [auditory] representation must enter into relationship with the concept [pp. 439–440]
Despite his agreement with Wernicke’s claim, Lichtheim found this mechanism “rather forced” (p. 439), reflecting the ad hoc stipulation generated by the data Wernicke was trying to explain.
Modern work on motor control provides a principled explanation for Wernicke’s stipulation that the auditory speech representation “maintains a continuing significant control” over motor speech planning. Specifically, the auditory representation serves as the target for motor speech acts (Guenther, Hampson, & Johnson, 1998; Perkell, 2012) and provides important sensory feedback information used in guiding them (Houde & Nagarajan, 2011; Tourville, Reilly, & Guenther, 2008). Such auditory targets function in just the same way as a sensory representation of, say, a cup defines the target for a reaching action; the only difference being that for auditory speech targets the sensory representation (i.e. the sound of a word) is stored in sensory memory rather than provided via immediate sensory stimulation. So, the reason why disruption of the auditory-motor pathway results in speech production disturbances is because such damage “obscures” the targets of speech acts, forcing affected patients to execute speech gestures based on stored motor memories alone, without auditory feedback. Just like one might be off target in reaching for an object in the dark, so too people with aphasia, who have a damaged auditory-motor pathway, will tend to be off target in their speech gestures and make paraphasic errors (Hickok, 2012a; Hickok, Houde, & Rong, 2011). Repetition will be affected as well by such a disruption for the same reason: the acoustically provided target will be less accessible to the motor system for reproduction (Hickok & Poeppel, 2004).
Thus, research in motor control has sharpened our understanding of the computational function of auditory-motor circuits for speech and sparked a renewed interest in mapping the neural circuits involved. Given that verbatim repetition, particularly of pseudowords, taps into this system relatively directly, repetition ability has become the focus of recent investigations.
A series of functional imaging studies in healthy participants has used covert repetition of pseudowords (or nonsense sentences) to map the cortical auditory-motor circuit for speech. This work has identified a left-dominant network of regions that includes the posterior superior temporal sulcus, a region in the posterior Sylvian fissure at the parietal-temporal boundary (termed, Spt), the pars opercularis of Broca’s area, and a more dorsal premotor site (Buchsbaum, Hickok, & Humphries, 2001; Buchsbaum, Olsen, Koch, Kohn, et al., 2005; Hickok, Buchsbaum, Humphries, & Muftuler, 2003). Spt has been a theoretical focus of this work because evidence points to it as a computational hub involved in auditory-motor integration: (i) it is located nearby known visuomotor integration regions in the parietal lobe;(ii) it appears to have functional-anatomical connections to the pars opercularis (Buchsbaum et al., 2001; Buchsbaum, Olsen, Koch, & Berman, 2005; Isenberg et al., 2012); (iii) it exhibits both auditory and motor response tuning (Hickok, Okada, & Serences, 2009); (iv) lesions associated with conduction aphasia overlap the location of Spt (Buchsbaum et al., 2011); and (v) direct cortical stimulation of the temporal-parietal junction has been found to cause symptoms of conduction aphasia, including repetition deficits (Anderson et al., 1999; Quigg & Fountain, 1999; Quigg, Geldmacher, & Elias, 2006).
Three recent large-scale voxel-based lesion-symptom mapping studies of repetition provide partial support for the view that Spt plays a critical role in auditory-motor integration. Baldo et al. (Baldo, Katseff, & Dronkers, 2012) studied 84 individuals with aphasia using real word and non-word repetition tasks as well as other short-term memory tasks. They report that the peak t-values for both repetition tasks are the posterior superior temporal region adjacent to the inferior parietal lobe, which corresponds well to Spt’s location. Baldo et al. also find that the arcuate fasciculus—the structure classically thought to mediate the relation between posterior auditory and anterior motor speech systems—was not implicated, suggesting that cortical damage, and not white matter disconnections, are the source of repetition deficits. Fridriksson et al. (2010) came to a slightly different conclusion in their study of 45 patients with aphasia who were tested for speech repetition ability. Both structural damage and blood perfusion was measured with MRI. Both measurements implicated tissue in the vicinity of Spt but not Spt directly. Structural damage predictive of repetition deficits was localized to the posterior arcuate fasciculus, while perfusion measures implicated cortical disruption in the supramarginal gyrus. Finally, in a larger-scale study involving 103 individuals with chronic aphasia, Dell and colleagues report that the left temporal-parietal boundary, including the Spt region, is associated with deficits on word and non-word repetition tasks (Dell et al., 2013). These authors report similar results when, instead of using raw repetition performance in generating the lesion maps, they used connection strength parameter values from a computational model of naming and repetition fit to each patient’s performance. The parameter that correlated with damaged voxels in and around Spt corresponded to weights mapping between auditory inputs and phonological output nodes (parameter nl in Dell et al.’s terminology), thus providing computational-anatomic evidence in favor of Spt’s proposed auditory-motor integration function.
A more recent lesion study, however, has questioned the claim that damage to Spt is responsible for repetition deficits. Parker Jones et al. (2014) also report on eight patients with repetition deficits. Each had involvement of the arcuate fasciculus and not the cortex in and around area Spt, consistent with Fridriksson et al.’s structural findings. This was interpreted as evidence in support of the classical model of the auditory-motor pathway implicating the arcuate fasciculus as the critical structure for auditory-motor integration rather than a cortically mediated circuit involving Spt.
Thus, although the functional evidence for Spt’s role in auditory-motor integration during speech production and repetition remains strong, lesion evidence is somewhat equivocal, particularly with respect to whether repetition deficits result from cortical damage involving Spt or white matter damage to the arcuate fasciculus. One potential limitation of all previous small and large-scale studies of repetition deficits following brain injury is that participant inclusion was limited to patients with clinically diagnosed aphasia. This could bias the sample toward larger lesion sizes and exclude patients with more focal, subclinical deficits, which in turn can complicate findings. The present study sought to address this issue by examining the neurobiology of speech repetition in a sample of individuals with a radiologically documented focal lesion independently of the presence of aphasia. Patient recruitment based on the presence of a brain lesion, not characteristic aphasic symptoms, improves sensitivity by maximizing variability in task performance, lesion size and lesion location. Voxel-based lesion symptom mapping was performed to identify regions associated with deficits on word and non-word repetition, which have been argued to involve potentially different routes (Dell, et al. 2013). We also explicitly examine the relative roles of the Spt region and the arcuate fasciculus.
2. Materials and Methods
2.1 Participants
118 patients were recruited via the Multi-site Aphasia Research Consortium (MARC) as part of an ongoing research program. Forty-seven of these patients were included in the present study based on the following criteria: (i) a chronic focal (6 months or more post-onset) lesion due to a stroke in the left hemisphere (n= 37; 20 female, mean age = 61 years, range = 43–81) or a unilateral left temporal lobectomy (n= 10; 7 female, mean age = 59, range = 35–77),1 (ii) no significant anatomical abnormalities other than the signature lesion of their vascular event (or evidence of surgery for the seizure subjects) nor signs of multiple strokes, and (iii) were administered both of the repetition tasks of interest. The stroke patients had no prior history of psychological or neurological disease.
The temporal lobectomy patients all had a seizure disorder resistant to medical management and, therefore, required surgical intervention. Patients were not selected based on a diagnosis of aphasia. Patients were all native speakers of English and the vast majority was strongly right-handed as determined by the Edinburgh Handedness Scale: one patient was left-handed and two were ambidextrous. Standard neuropsychological tests were administered to all subjects. Informed consent was obtained from each patient prior to participation in the study, and all procedures were approved by the Institutional Review Boards of UC Irvine, San Diego State University, University of Southern California, Arizona State University and University of Iowa.
2.2 Materials
To address our goal of characterizing the neuroanatomy supporting sensory-motor integration, data from two tasks will be examined: (i) the Boston Diagnostic Aphasia Exam (BDAE) Repetition sub-tests, as a measure of real word repetition, and (ii) a non-word repetition task. In addition, a syllable discrimination task (a speech perception task previously implicating sensory-motor networks (Schwartz, et al., 2012)), was analyzed and used as a covariate in some analyses. These tasks were administered as part of an extensive psycholinguistic test battery to assess speech perception and production abilities. Within the battery, individual tests themselves were presented in a non-fixed pseudorandom order. Items within a test were presented in a fixed random order.
2.2.3 Real Word Repetition Measure
The BDAE is a widely-used clinical and research tool (Goodglass & Kaplan, 1983); the repetition sub-tests include 36 words, phrases and sentences that subjects were asked to listen to and repeat. The stimuli include single high-frequency words, phrases of varying length (3–7 words), noun phrases (e.g. baseball player), and numbers (e.g. Eight forty-six). Normative data for the BDAE indicates that control subjects (i.e. older adults with no brain damage) perform at ceiling for the repetition subtests (Goodglass, Kaplan & Barresi, 2001).
2.2.4. Non-word repetition task
The non-word repetition task consisted of 30 trials in which subjects were asked to repeat a non-word presented via headphones at a volume level comfortable to each subject, while a fixation cross was presented on the computer screen. The task included ten two-syllable, ten three-syllable, and ten four-syllable non-words (Appendix A). All stimuli were recorded by a native English male speaker; all non-words are phonotactically legal in English.
2.2.5. Syllable discrimination task
The syllable discrimination task is modeled on previous discrimination tasks used with aphasic populations (Baker, Blumstein, & Goodglass 1981; Blumstein, Baker, & Goodglass 1977; Caplan & Waters, 1995; Caplan 1992). Our task involved the presentation of 64 pairs of consonant-voxel-consonant (CVC) syllables (non-words) via headphones while a fixation cross was presented on the computer screen. One male native English speaker recorded all of the non-words. Each non-word was generated by changing the vowel in a one-syllable real word to create a phonotactically legal (in English) non-word. There was a one second interval between each syllable in a pair, and five seconds between each pair (more time between pairs was given if necessary for the patient's comfort). Patients were instructed to determine if the two syllables presented were the same or different. “Different” trials contained two syllables that differed by one feature of the onset consonant (e.g. “mool” vs “nool”). The 64 trials comprised 16 non-word pairs, presented in four arrangements: A-B, B-A, A-A, and B-B such that for half of the trials the correct answer was "same", and for the other half the correct answer was "different". In the "same" trials, two different tokens of the same syllable were presented. Patients made their response either by speaking their answer or by pointing to the correct answer written in large print on a paper adjacent to the computer screen. Signal detection methods were used to determine how well subjects could discriminate between the same and different pairs (Swets, 1964).
2.3 Imaging & lesion analyses
High-resolution MPRAGE or SPGR MRIs were acquired for all participants, except for three patients for whom a CT scan was acquired due to their incompatibilities with the MR environment (pacemaker, metal clip, etc.).
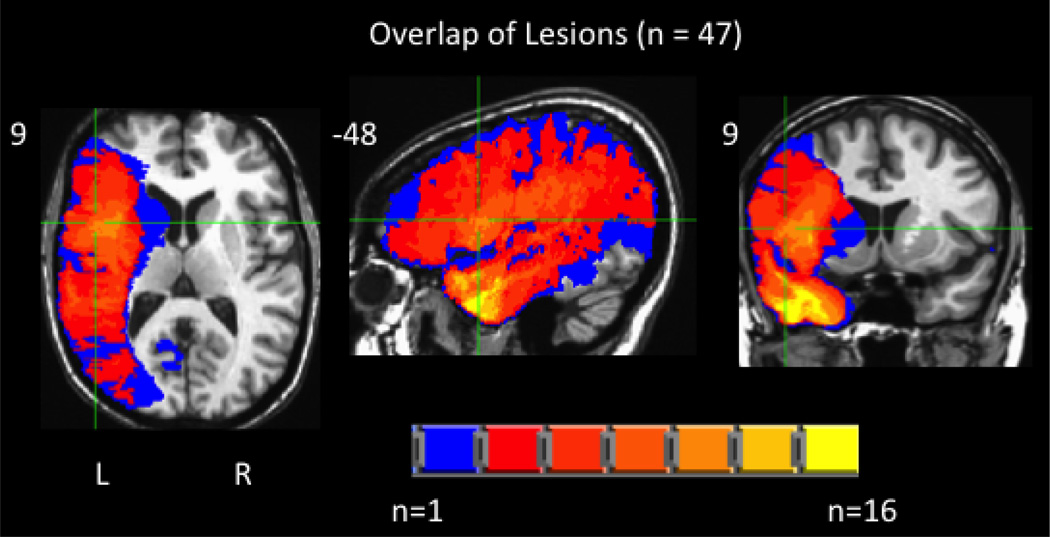
Lesion mapping was performed using MAP-3 lesion analysis methods (Damasio & Damasio 2003) implemented on Brainvox software (Frank, Damasio & Grabowski, 1997). This method is described in detail elsewhere (Damasio, 2000). Briefly, the method entails the transfer of the contour of the lesion seen in the patient's MRI/CT into the common space of a template brain. To do so, the template brain is resliced such that each slice corresponds to each slice in the lesion's native space, based on anatomical markers (sulci and subcortical structures). The lesion is then manually demarcated on the template brain's corresponding slice respecting the identifiable landmarks. Each lesion map was completed by an individual with extensive training in this technique, and supervised by an expert neuroanatomist (HD). The MAP-3 method has been shown to have high inter- and intra-rater reliability and in some cases higher accuracy than some automated methods (Fiez et al. 2000; Pantazis et al. 2010). Figure 1 depicts an overlay of all of the patients’ lesions maps on the template brain.
Figure 1.
Overlap of the patients’ left hemisphere lesions included in subsequent analyses (max overlap = 16).
Voxel-based lesion symptom mapping (VLSM; version 2.3, Bates, et al. 2003) was implemented in the 47 patients’ lesion maps. This VLSM analysis consisted of fitting a general linear model for each voxel, to determine the relationship between performance on each task and presence (or absence) of lesion in that voxel. A voxel-wise threshold of p < .001 was used. To correct for multiple comparisons, the following permutation (n =1000) method was implemented: in each voxel, patients’ behavioral scores were randomly reassigned 1000 times. For each permutation, the general linear model was refit, a statistical threshold of p < .001 identified significant voxels, and the size of the largest cluster of significant voxels was recorded. Voxels in the actual data were identified as significant if they reached a voxel-wise p value < .001 and were in a voxel cluster larger than 95% of the largest significant clusters identified in the permutated datasets. (This cluster-based permutation method threshold procedure is the “p < .001, corrected” referred to in the subsequent results section and figures). Variance due to lesion size (i.e. number of voxels marked as lesion on the template brain) was regressed out of all VLSM analyses via an Analysis of Covariance (ANCOVA). Voxel size was 1mm×1mm×1mm. Only voxels in which a minimum of five patients had damage were included in the VLSM analyses. All coordinates reported in the figures are in Talairach space (Talairach & Tournoux, 1988).
3. Results
3.1 Behavioral Performance
Performance was calculated based on proportion of correct syllables produced in each task. A syllable in a patient’s response was considered to be an “error” if one of the following was present: one or more incorrect phonemes or phonemes in an incorrect order, omissions, additions and articulatory distortions (articulatory distortions were defined as recognizable sounds but inaccurate articulation, e.g. the /s/ sound being produced with air escaping out of the side of the mouth). Overall, subjects performed significantly higher on real word repetition (M = 0.94, sd = 0.12) than non-word repetition (M = 0.82, sd = 0.24) (t = 4.09, p = .0002). Real word and non-word repetition performances were significantly correlated across subjects, r = .81, p < .0001.
3.2 VLSM Results
The VLSM for the real word repetition task performance (proportion correct) identified a large cluster of voxels spanning the left inferior parietal lobe and superior temporal regions, including Heschl’s gyrus and the supramarginal gyrus (18191 voxels; p < .001, corrected; Figures 2a and 4). The VLSM for the non-word repetition task (proportion correct) identified a similar, but larger voxel cluster in the inferior parietal and superior temporal lobe (34142 voxels; p< .001, corrected), extending more anteriorly into the postcentral gyrus and insula (Figures 3a and 4). Both VLSMs implicate gray matter and underlying white matter, consistent with previous work. These results also indicate that the neural circuits supporting real word and non-word repetition are highly overlapping, the difference being that real word repetition implicates a similar but smaller extent of voxels compared to non-word repetition.
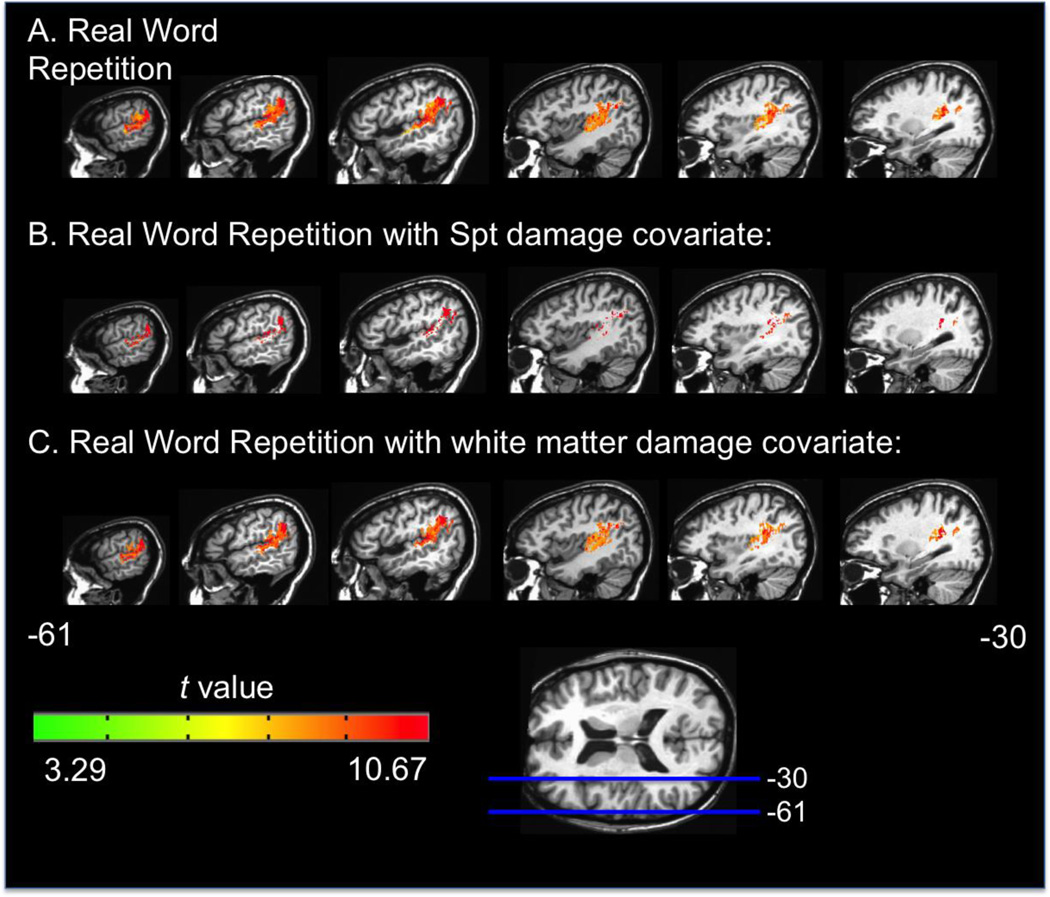
Figure 2.
Representative sagittal slices depicting the VLSM t-map for the real word repetition task alone (A), with Spt damage as a covariate (B), and with white matter damage as a covariate (C). All maps have a threshold of p < .001, corrected.
Figure 4.
Three orthogonal views of the real word and non-word repetition VLSMs (e.g. Figures 2a & 3a) to emphasize significant cortical and white matter voxels.
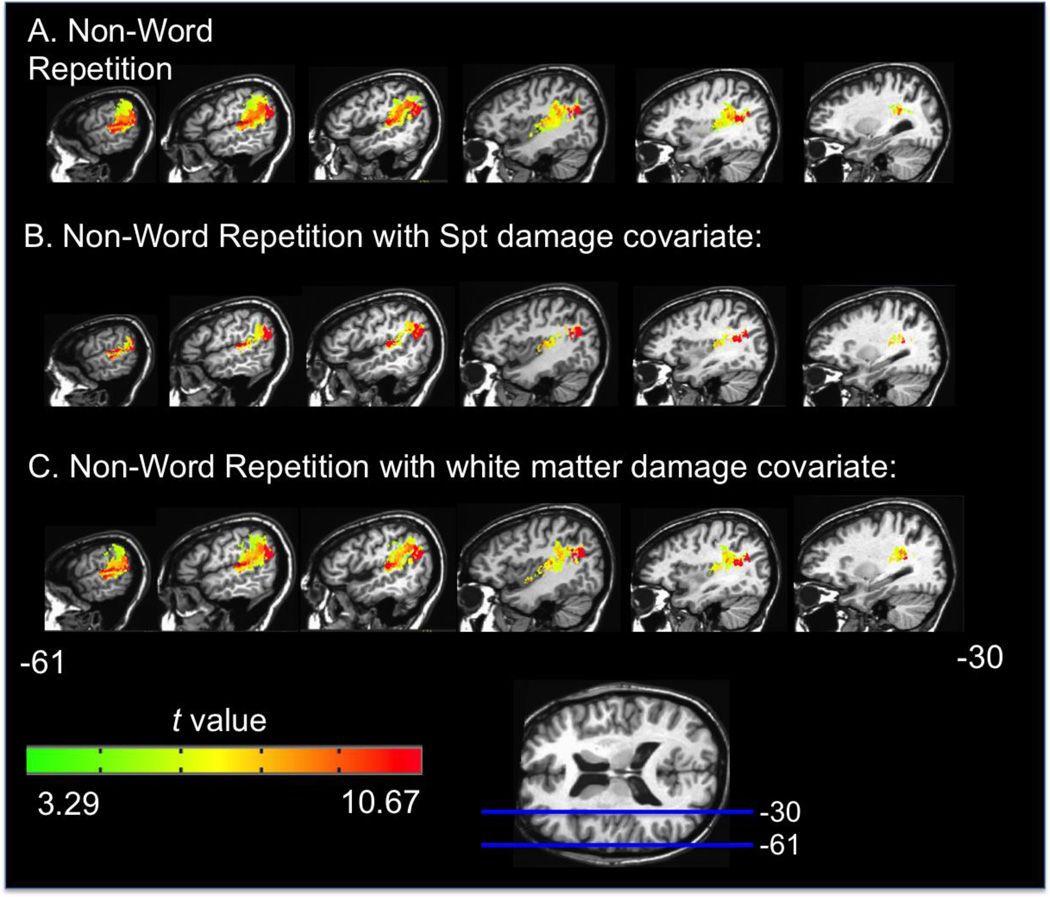
Figure 3.
Representative sagittal slices depicting the VLSM t-map for the non-word repetition task alone (A), with Spt damage as a covariate (B), and with white matter damage as a covariate (C). All maps have a threshold of p < .001, corrected.
3.3 Regions of interest ANCOVA analyses
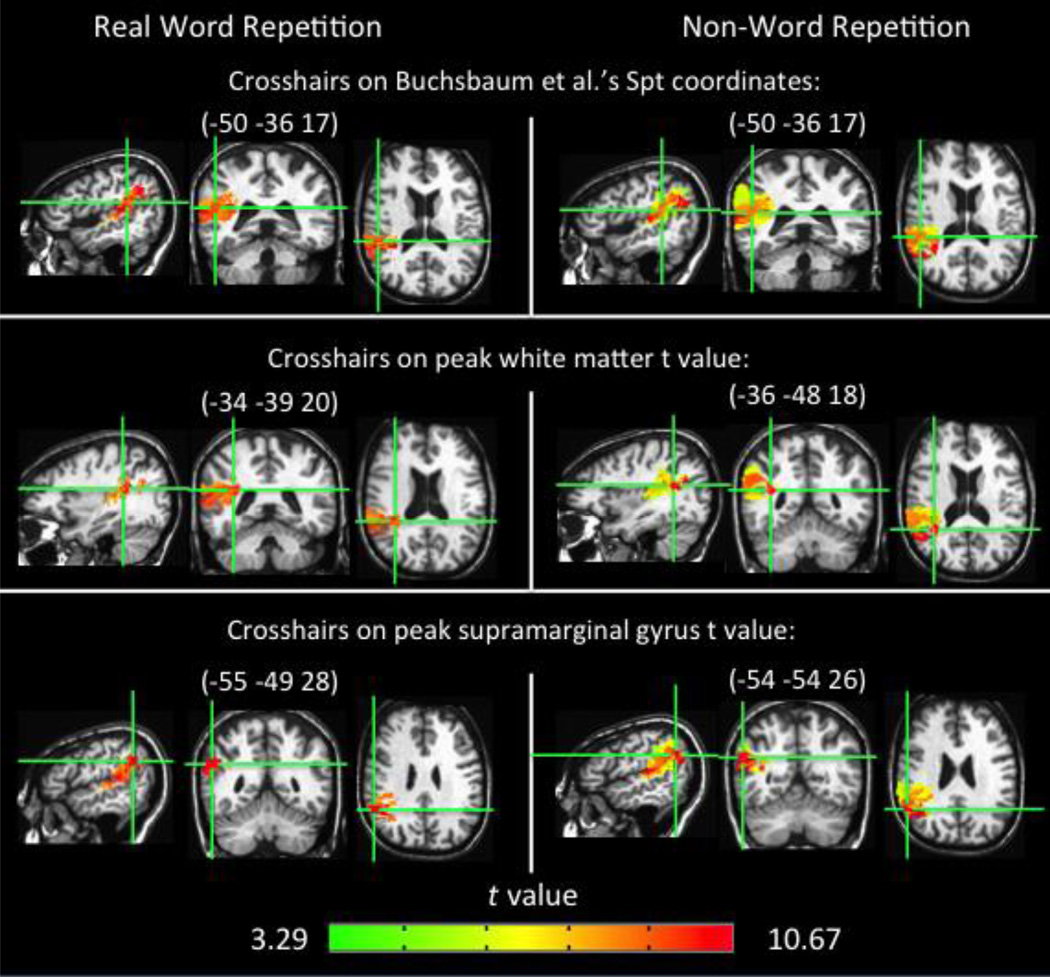
The above VLSM results implicate cortical regions (e.g. Spt, superior temporal gyrus, inferior parietal lobe, supramarginal gyrus) as well as dorsal white matter tracts, including the arcuate fasciculus in speech repetition performance. Our participant sample did not include any patients with lesions selectively in cortical Spt but not in the underlying white matter; a limitation of almost any study with naturally occurring lesions. Thus, to attempt to understand the relative contributions of Spt versus underlying white matter damage to repetition deficits, we performed a series of Analysis of Covariance (ANCOVA) VLSM analyses (Bates et al. 2003). The ANCOVA procedure can determine if a brain region plays a direct causal role in a decrease in task performance or is affecting performance due to highly correlated damaged regions elsewhere in the brain (Bates et al. 2003). In each ANCOVA, the covariate was the presence (or not) of damage in Spt or the white matter implicated in the original VLSMs. Each covariate was binary: 1 = the subject has at least one voxel lesioned in the ROI, or 0 = no voxels lesioned in the ROI.2 The Spt ROI was defined as a sphere with a 5mm diameter around the coordinates of maximal overlap in the posterior planum temporale reported in Buchsbaum et al.'s (2011) meta-analysis of more than 100 fMRI subjects in whom Spt had been individually localized (MNI -51 -42 21). Only gray matter voxels within the sphere were included in the ROI. The white matter ROI was determined by generating a mask from the significant voxels containing white matter in the non-word repetition VLSM results (p< .001, corrected; as can be seen in Figures 3A & 4). The white matter ROI was identified from within the significant voxels to provide the strongest possible test of Spt’s involvement in repetition independent of the contributions of the white matter implicated. In effect we are asking the question, does Spt damage contribute to repetition deficits above and beyond those white matter regions that are significantly implicated in repetition deficits?
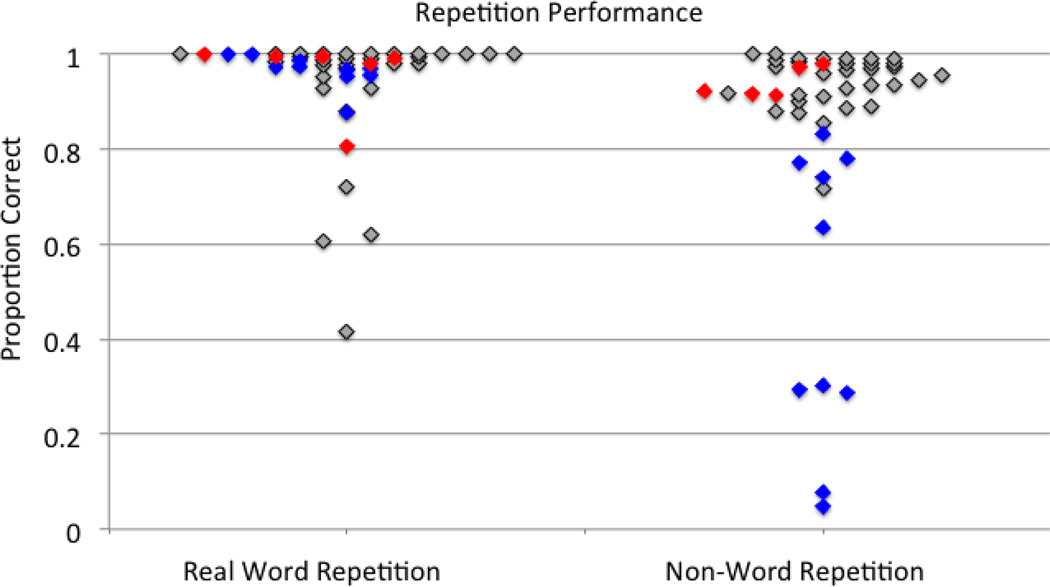
The results of the ANCOVA were as follows: the ANCOVAs using the Spt and white matter ROIs, respectively, all resulted in t maps and significant clusters in a general pattern similar to the original VLSM results, but with a few important exceptions (p< .001 corrected, Figures 2b,c & 3b,c). For real word repetition, the Spt ANCOVA resulted in a greatly reduced number of white matter voxels implicated, while the white matter ANCOVA yielded results nearly identical to the original real word repetition VLSM. This result indicates that once Spt damage is factored out, there is a greatly reduced apparent contribution of white matter damage to real word repetition performance. For non-word repetition, both the Spt and white matter ANCOVA VLSMs, respectively, yielded results nearly identical to the original non-word repetition VLSM. In other words, in non-word repetition, the relative contributions of Spt versus underlying white matter cannot be separated; both appear to make their own contribution to repetition. Figure 5 shows word and non-word repetition performance for patients with white matter damage only, with white matter plus Spt damage, and no Spt or white matter damage. Note that nonword performance is more affected in the sample and that the combined effects of white matter plus Spt damage yielded the most dramatic deficits.
Figure 5.
Plots of real word and non-word repetition. Blue = patients with damage in Spt & the white matter ROI, red = white matter ROI damage only, gray = no SPT or white matter ROI damage. No patients had damage in SPT but not in the white matter ROI.
3.4 Task-related ANCOVAs
The ROI ANCOVAs indicate that cortical regions, including Spt and the supramarginal gyrus are implicated in speech repetition. To further investigate the nature of the contributions of these cortical regions, two additional ANCOVA VLSMs were conducted, one targeting the potential role of semantic information on real word repetition and another targeting the contribution of potential speech perception deficits on repetition.
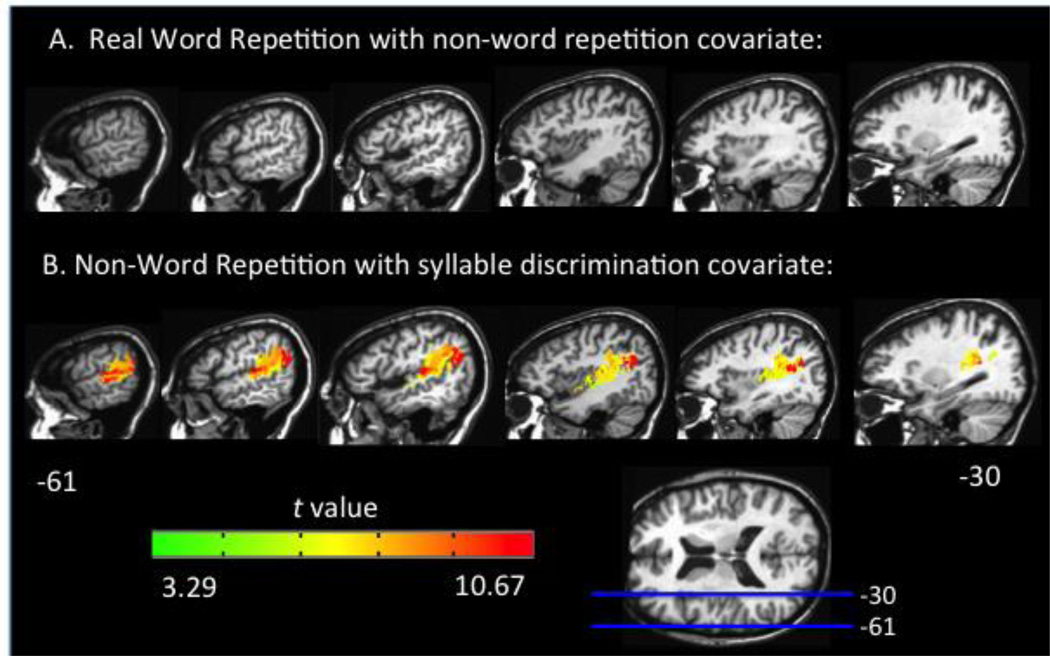
To assess the role of semantic information on real word repetition an ANCOVA VLSM for real word repetition was performed using non-word repetition performance as a regressor. The logic here is that non-word repetition must rely on phonological information, thus, by factoring out regions implicated non-word repetition we might uncover regions that can support repetition via an alternative semantic route. This ANCOVA resulted in no significant voxels, however (at p < .001, corrected; Figure 6a) indicating that semantic information is not contributing substantially to performance on the real word repetition task. The strong correlation between real and nonword tasks (see above) is consistent with this conclusion.
Figure 6.
Representative sagittal slices depicting: (A) the VLSM t-map for real word repetition with non-word repetition performance as a covariate which yields no significant voxels and (B) the VLSM t-map for nonword repetition with syllable discrimination as a covariate. Both maps have a threshold of p < .001, corrected.
A recent hypothesis holds that Spt is not an auditory-motor integration area but rather a purely auditory area (Parker Jones et al., 2014). On this view, Spt might be implicated in repetition deficits because damage to this area results in perceptual deficits that impact repetition accuracy. To assess this possibility we carried out an ANCOVA VLSM for non-word repetition using performance (d’) on a non-word syllable discrimination task as a regressor (syllable discrimination performance mean d’ = 4.07, range = 1.57–5.15, see Appendix B for a scatterplot of syllable discrimination performance in relation to repetition performance). Previous lesion mapping work using such a task (and the current data set, see Appendix B for VLSM results) has found that it implicates the region around Spt (Schwartz et al., 2012), making it a strong test of the auditory interpretation of Spt. This ANCOVA VLSM resulted in a large significant cluster including in and surrounding Spt (26735 voxels; p < .001 corrected, Figure 6b) associated with repetition deficits—almost identical to the original VLSM result—even with auditory discrimination factored out.3 We also carried out an ANCOVA VLSM for real word repetition with the same syllable discrimination task as a regressor, and the same pattern holds: the results did not differ qualitatively from the original real word repetition results. These results suggest that perceptual processing alone cannot account for Spt’s contribution to speech repetition.
4. Discussion
Using VLSM methods in a sample of focal lesion patients we found an association between speech repetition and the posterior temporal-parietal boundary. A very similar lesion distribution was found for word and non-word repetition. This region included area Spt as determined using previously published average coordinates for Spt’s location (Buchsbaum et al., 2011). The VLSMs also implicated white matter structures including the arcuate fasciculus, which is classically associated with repetition deficits. However, cortical regions, including Spt, were still associated with repetition deficits even after white matter damage was covaried out of the analysis. Finally, the involvement of cortical structures including Spt in repetition deficits cannot be attributed to auditory perception deficits: factoring out performance on a syllable discrimination task did not change the findings. We conclude that Spt is indeed implicated in repetition ability and auditory-motor integration, likely in concert with underlying white matter pathways which link the Spt hub to frontal motor speech regions.
Qualitatively, we found little difference in the lesion maps associated with repetition of words versus non-words, although the maps for non-words were more robust and expansive. A similar effect was reported by Baldo et al. (Baldo et al., 2012), who suggested that non-word repetition may recruit more neural tissue because, unlike words, they are not overlearned and cannot rely on semantic information for repetition. Another way to frame this idea from a motor control standpoint is that non-word repetition requires the temporary storage of a novel auditory target and the transformation of that target into a set of motor plans thus heavily taxes the auditory-motor circuit. In repeating words, conversely, well-learned motor plans can be activated via an indirect route (perhaps involving the basal ganglia) (Dell et al., 2013; Nozari, Kittredge, Dell, & Schwartz, 2010), with the direct auditory-motor route serving to ensure, via internal feedback, that the activated motor plan will indeed hit its target (Hickok, 2012a). The load on the auditory-motor circuit is therefore less for word than non-word repetition resulting in fewer errors, even when the circuit is damaged, and therefore weaker associations between word repetition and lesions to the auditory-motor circuit. A semantic route for real word repetition is also likely available based on previous work (Baldo, et al. 2012; Dell, et al. 2013), although we found little evidence for it in our study. This could be due to the nature of our real word stimuli that included some complex phrases and sentences.
Overall, our findings on the lesion correlates of repetition deficits are highly consistent with the two previous large-scale studies of repetition impairment in aphasia. Baldo et al. (Baldo et al., 2012)(N=84) and Dell, et al. (N=106) report very similar findings including both cortical and subcortical regions implicated. Thus a convergence of results is emerging. Our study adds to previous work in that it specifically tailored the analyses to assess the contribution of cortical versus white matter tissue to address the classical view, and recent claim, that damage to the arcuate fasciculus is primarily responsible for repetition deficits (Geschwind, 1965, 1971; Parker Jones et al., 2014). As noted, the present study provided clear evidence that cortical damage indeed makes a substantial contribution to both word and non-word repetition deficits even after the extent of white matter involvement is factored out, consistent with evidence from direct cortical stimulation studies (Anderson et al., 1999; Quigg & Fountain, 1999; Quigg et al., 2006). These findings suggest a role for both structures in non-word repetition, one that performs the necessary computation that translates between auditory and motor representations and the other transports the information, likely via the arcuate fasciculus primarily (Isenberg et al., 2012).
As argued in the introduction, we believe that these findings are relevant not only for illuminating the neural circuits underlying speech repetition but also those underlying volitional speech production. A relatively coherent and convergent story is starting to emerge from functional imaging (Buchsbaum et al., 2001; Hickok et al. 2003; Wise et al. 2001), cytoarchitectonics (Galaburda & Sanides, 1980; Galaburda, 1982), lesion-symptom mapping (present study, Baldo et al. 2012; Fridriksson et al. 2010; etc.), connectivity (Isenberg et al. 2012), direct cortical stimulation (Anderson et al., 1999), and computational modeling (Hickok, 2012a) suggesting that Spt is a hub in an auditory-motor network for speech motor control (Hickok, 2012a, 2012b; Hickok et al., 2011). Functional imaging work has provided information (i) on the precise distribution of a circuit with auditory-motor response properties (Buchsbaum et al., 2001; Hickok et al., 2003; Wise et al., 2001); (ii) that within that network, Spt is comprised of interdigitated units (voxels) differentially tuned to auditory versus motor components of speech repetition (Hickok et al., 2009) similar to what is found in primate visuomotor regions in parietal cortex (Sakata, Taira, Murata, & Mine, 1995); (iii) that Spt is preferentially tuned to vocal tract actions (Pa & Hickok, 2008); and (iv) that it activates more strongly during demanding feedback control situations (altered feedback) (Tourville et al., 2008). Cytoarchitectonically, area Spt falls within area Tpt in the planum temporale/parietal operculum region which is not cellularly characteristic of nearby auditory cortex (Galaburda & Sanides, 1980), but rather shares features with Broca’s area (Galaburda, 1982). Lesion-symptom and lesion-parameter mapping work has implicated the temporal-parietal junction in speech repetition (present study and reviewed above), as well as in phonological-level aspects of speech production (Dell et al., 2013; Schwartz et al., 2012). Both functional (Buchsbaum et al., 2001; Buchsbaum, Olsen, Koch, & Berman, 2005) and structural connectivity (Isenberg et al., 2012) studies have suggested a tight relation between Spt and the pars opercularis of Broca’s area. Direct cortical stimulation mapping has shown that cortical disruption alone is sufficient to induce repetition impairment, without impairing speech perception (Anderson et al., 1999). And computational modeling work has demonstrated the feasibility of the proposed auditory-motor network (Hickok, 2012a).
In this context it is worth commenting on a recent functional imaging study that has questioned Spt’s role in auditory-motor integration. Using overt speech production, Parker Jones et al. (2014) compared activity in Spt during pseudoword repetition and animal sound naming (e.g., listening to a “meow” and saying cat). The authors reasoned that pseudoword repetition should tax the auditory-motor system more so than animal sound naming because the former involves direct auditory-motor translation whereas the latter involves a semantic mediation. They report that Spt was more strongly activated during animal sound naming, suggesting to them that the region is not particularly involved in auditory-motor integration. There are two problems with this conclusion. One is that, according to current models, any speech production task will involve some level of auditory-motor integration because the targets of speech acts are auditory, as noted above. Therefore Parker Jones et al.’s test is not a strong one. The second problem is that the pseudowords and animal sounds were not matched for length (sounds were longer than pseudowords), which resulted in more than double the acoustic response in primary auditory regions to the animal sounds. Since Spt also responds vigorously to acoustic stimulation, this confound gives the animal sound naming a strong response amplitude advantage over pseudoword repetition. It is no wonder, then, that Spt did not respond more to pseudoword repetition4. Interestingly, the difference in response amplitude between the animal sound and pseudoword condition was smaller in Spt than in auditory cortex, suggesting a relative increase for the pseudoword task in Spt, as predicted by the auditory-motor integration hypothesis.
4.1 Conclusion
Word and non-word repetition recruit highly overlapping left inferior parietal-superior temporal networks. Analyses of covariance indicate that damage to underlying white matter does not fully account for these findings nor is Spt’s involvement in speech repetition accounted for by auditory perceptual processes alone, as measured in a syllable discrimination task previously found to implicate Spt. The present findings highlight the motor planning properties of area Spt, which serves as an auditory-motor integration hub.
Supplementary Material
Highlights.
Auditory-motor brain networks are investigated using speech repetition.
Cortical area Spt, white matter and parietal cortex are implicated in repetition.
Spt contributes to repetition above and beyond the white matter contribution.
These results hold with perceptual processing factored out.
Findings are highly consistent with Spt being an area of sensory-motor translation.
Acknowledgments
This work was supported by grant NIH-DC03681 (G.Hickok).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
It should be noted that the temporal lobectomies were performed on patients with medically intractable epilepsy. We have chosen to include these patients to include lesions localized in the anterior temporal lobe, a region frequently left intact by all but the most severe middle cerebral artery strokes (Holland & Lambon Ralph 2010). However, it is quite possible that the epileptic brains have abnormal functional organization, particularly regarding laterality of language processing (e.g. Swanson et al. 2002, 2007; Hertz-Pannier et al. 2002) and any findings resulting from this population should be interpreted carefully. Note that the present results do not change if this patient group is excluded, but their inclusion allows us to examine (albeit cautiously) the possible involvement of the anterior temporal lobe in speech processing.
ANCOVA analyses using number of voxels lesioned, instead of the binary covariate of damage or no damage, were also calculated. The results are qualitatively the same.
One may wonder why a syllable discrimination task implicates an auditory-motor integration region in the first place and whether this undermines the use of such a task to assess speech perception deficits. Syllable discrimination indeed implicates auditory-motor integration areas (including Spt) as Hickok & Poeppel (2000) predicted due to a mild short-term working memory load involved in performing this task (maintaining and comparing two stimulus items), which recruits auditory-motor circuits. In contrast, the repetition task imposes a substantial load on auditory-motor integration such that deficits on the repetition task are not fully accounted for by deficits on the easier syllable discrimination. But does this undermine the point of using syllable discrimination as a covariate? Not at all: the point of using the syllable discrimination task is to assess whether the perception of speech sounds is what’s driving the effect for repetition. And while discrimination is a relatively weak assay for auditory-motor integration deficits, it is a robust assay for speech sound perception deficits, which typically require bilateral lesions (Hickok & Poeppel, 2007). The covariate in fact does its job despite having some task-dependent overlap with the repetition task.
Another likely reason for greater activation to animal sound naming versus pseudoword repetition is the difference in computing demands for the two tasks: both the naming and pseudoword repetition require sensorimotor processes, but the naming task also requires sensorimotor processes to interact with sound identification and categorization, followed by retrieval of lexical-semantic and phonological information to correctly articulate the name of the animal.
References
- Anderson JM, Gilmore R, Roper S, Crosson B, Bauer RM, Nadeau S, et al. Conduction aphasia and the arcuate fasciculus: A reexamination of the Wernicke-Geschwind model. Brain and Language. 1999;70(1):1–12. doi: 10.1006/brln.1999.2135. [DOI] [PubMed] [Google Scholar]
- Axer H, von Keyserlingk AG, Berks G, von Keyserlingk DG. Supra- and infrasylvian conduction aphasia. Brain and Language. 2001;76(3):317–331. doi: 10.1006/brln.2000.2425. [DOI] [PubMed] [Google Scholar]
- Baker E, Blumstein SE, Goodglass H. Interaction between phonological and semantic factors in auditory comprehension. Neuropsychology. 1981;19(1):1–15. doi: 10.1016/0028-3932(81)90039-7. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Klostermann EC, Dronkers NF. It’s either a cook or a baker: patients with conduction aphasia get the gist but lose the trace. Brain and Language. 2008;105(2):134–140. doi: 10.1016/j.bandl.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Katseff S, Dronkers NF. Brain regions underlying repetition and auditory-verbal short-term memory deficits in aphasia: Evidence from voxel-based lesion symptom mapping. Aphasiology iFirst. 2011:1–17. doi: 10.1080/02687038.2011.602391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha L, Benke T. Acute conduction aphasia: an analysis of 20 cases. Brain and Language. 2003;85(1):93–108. doi: 10.1016/s0093-934x(02)00502-3. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Blumstein SE, Baker E, Goodglass H. Phonological factors in auditory comprehension in aphasia. Neuropsychologia. 1977;15:19–30. doi: 10.1016/0028-3932(77)90111-7. [DOI] [PubMed] [Google Scholar]
- Buchsbaum B, Hickok G, Humphries C. Role of Left Posterior Superior Temporal Gyrus in Phonological Processing for Speech Perception and Production. Cognitive Science. 2001;25:663–678. [Google Scholar]
- Buchsbaum BR, Baldo J, Okada K, Berman KF, Dronkers N, D'Esposito M, Hickok G. Conduction aphasia, sensory-motor integration, and phonological short-term memory - an aggregate analysis of lesion and fMRI data. Brain and Language. 2011;119(3):119–128. doi: 10.1016/j.bandl.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, Olsen RK, Koch P, Berman KF. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron. 2005;48(4):687–697. doi: 10.1016/j.neuron.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Olsen RK, Koch PF, Kohn P, Kippenhan JS, Berman KF. Reading, hearing, and the planum temporale. Neuroimage. 2005;24(2):444–454. doi: 10.1016/j.neuroimage.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Caplan D. Language: Structure, processing and disorders. Cambridge, MA: MIT Press (Bradford Books); 1992. [Google Scholar]
- Caplan D, Waters GS. On the nature of the phonological output planning processes involved in verbal rehearsal: evidence from aphasia. Brain Lang. 1995;48(2):191–220. doi: 10.1006/brln.1995.1009. [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. The anatomical basis of conduction aphasia. Brain: A Journal of Neurology. 1980;103(2):337–350. doi: 10.1093/brain/103.2.337. [DOI] [PubMed] [Google Scholar]
- Damasio H. The lesion method in cognitive neuroscience. In: Boller F, Graffman J, Rizzolati G, editors. Handbook of neuropsychology. 2nd ed. Philadelphia: Elsevier; 2000. pp. 77–102. [Google Scholar]
- Damasio H, Damasio AR. The lesion method in behavioral neurology and neuropsychology. In: Feinberg TE, Farah MJ, editors. Behavioral neurology and neuropsychology. 2nd ed. New York: McGraw Hill; 2003. pp. 71–83. [Google Scholar]
- Dell GS, Schwartz MF, Nozari N, Faseyitan O, Branch Coslett H. Voxel-based lesion-parameter mapping: Identifying the neural correlates of a computational model of word production. Cognition. 2013;128(3):380–396. doi: 10.1016/j.cognition.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert GH. Wernicke's work on aphasia: a sourcebook and review. The Hague: Mouton; 1977. [Google Scholar]
- Feinberg TE, Gonzalez Rothi LJ, Heilman KM. “Inner speech” in conduction aphasia. Archives of Neurology. 1986;43(6):591–593. doi: 10.1001/archneur.1986.00520060053017. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Damasio H, Grabowski TJ. Lesion segmentation and manual warping to a reference brain: Intra- and interobserver reliability. Human Brain Mapping. 2000;9(4):192–211. doi: 10.1002/(SICI)1097-0193(200004)9:4<192::AID-HBM2>3.0.CO;2-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5(1):13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Kjartansson O, Morgan PS, Hjaltason H, Magnusdottir S, Bonilha L, Rorden C. Impaired speech repetition and left parietal lobe damage. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2010;30(33):11057–11061. doi: 10.1523/JNEUROSCI.1120-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda A, Sanides F. Cytoarchitectonic organization of the human auditory cortex. Journal of Comparative Neurology. 1980;190:597–610. doi: 10.1002/cne.901900312. [DOI] [PubMed] [Google Scholar]
- Galaburda AM. Histology, architectonics, and asymmetry of language areas. In: Arbib MA, Caplan D, Marshall JC, editors. Neural models of language processes. San Diego: Academic Press; 1982. pp. 435–445. [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. Brain. 1965;88:237–294. 585–644. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Aphasia. New England Journal of Medicine. 1971;284:654–656. doi: 10.1056/NEJM197103252841206. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination (BDAE) 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1983. [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. The Assessment of Aphasia and Related Disorders (3rd ed.) Austin, TX: PRO-ED, Inc.; 2001. [Google Scholar]
- Guenther FH, Hampson M, Johnson D. A theoretical investigation of reference frames for the planning of speech movements. Psychol Rev. 1998;105:611–633. doi: 10.1037/0033-295x.105.4.611-633. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Chiron C, Jambaque I, Renaux-Kieffer V, Van de Moortele PF, Delalande O, et al. Late plasticity for language in a child's non-dominant hemisphere: a pre- and post-surgery fMRI study. Brain. 2002;125(Pt 2):261–272. doi: 10.1093/brain/awf020. [DOI] [PubMed] [Google Scholar]
- Hickok G. Computational neuroanatomy of speech production. Nature Reviews Neuroscience. 2012a;13(2):135–145. doi: 10.1038/nrn3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. The cortical organization of speech processing: feedback control and predictive coding the context of a dual-stream model. J Commun Disord. 2012b;45(6):393–402. doi: 10.1016/j.jcomdis.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Buchsbaum B, Humphries C, Muftuler T. Auditory-motor interaction revealed by fMRI: Speech, music, and working memory in area Spt. Journal of Cognitive Neuroscience. 2003;15:673–682. doi: 10.1162/089892903322307393. [DOI] [PubMed] [Google Scholar]
- Hickok G, Houde J, Rong F. Sensorimotor integration in speech processing: computational basis and neural organization. Neuron. 2011;69(3):407–422. doi: 10.1016/j.neuron.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Okada K, Serences JT. Area Spt in the human planum temporale supports sensory-motor integration for speech processing. J Neurophysiol. 2009;101(5):2725–2732. doi: 10.1152/jn.91099.2008. [DOI] [PubMed] [Google Scholar]
- Holland R, Lambon Ralph MA. The anterior temporal lobe semantic hub is a part of the language neural network: selective disruption of irregular past tense verbs by rTMS. Cerebral Cortex. 2010;20(12):2771–2775. doi: 10.1093/cercor/bhq020. [DOI] [PubMed] [Google Scholar]
- Houde JF, Nagarajan SS. Speech production as state feedback control. Frontiers in Human Neuroscience. 2011;5 doi: 10.3389/fnhum.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg AL, Vaden KI, Jr, Saberi K, Muftuler LT, Hickok G. Functionally distinct regions for spatial processing and sensory motor integration in the planum temporale. Hum Brain Mapp. 2012;33(10):2453–2463. doi: 10.1002/hbm.21373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempler D, Metter EJ, Jackson CA, Hanson WR, Riege WH, Mazziotta JC, Phelps ME. Disconnection and cerebral metabolism. The case of conduction aphasia. Archives of Neurology. 1988;45(3):275–279. doi: 10.1001/archneur.1988.00520270049020. [DOI] [PubMed] [Google Scholar]
- Lichtheim L. On aphasia. Brain. 1885;7:433–485. [Google Scholar]
- Leeper HA, Shewan CM, Booth JC. Altered acoustic cue discrimination in Broca’s and conduction aphasics. Journal of Communication Disorders. 1986;19(2):83–103. doi: 10.1016/0021-9924(86)90013-4. [DOI] [PubMed] [Google Scholar]
- McCarthy RA, Warrington EK. Understanding: a function of short-term memory? Brain: A Journal of Neurology. 1987;110(Pt 6):1565–1578. doi: 10.1093/brain/110.6.1565. [DOI] [PubMed] [Google Scholar]
- McCarthy R, Warrington EK. A two-route model of speech production. Evidence from aphasia. Brain: A Journal of Neurology. 1984;107(Pt 2):463–485. doi: 10.1093/brain/107.2.463. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Benson DF. Atypical conduction aphasia. A disconnection syndrome. Archives of Neurology. 1985;42(9):886–891. doi: 10.1001/archneur.1985.04060080068018. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Kempler D, Jackson C, Hanson WR, Mazziotta JC, Phelps ME. Cerebral glucose metabolism in Wernicke’s, Broca’s, and conduction aphasia. Archives of Neurology. 1989;46(1):27–34. doi: 10.1001/archneur.1989.00520370029014. [DOI] [PubMed] [Google Scholar]
- Nozari N, Kittredge AK, Dell GS, Schwartz MF. Naming and repetition in aphasia: Steps, routes, and frequency effects. J Mem Lang. 2010;63(4):541–559. doi: 10.1016/j.jml.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pa J, Hickok G. A parietal-temporal sensory-motor integration area for the human vocal tract: Evidence from an fMRI study of skilled musicians. Neuropsychologia. 2008;46:362–368. doi: 10.1016/j.neuropsychologia.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantasiz D, Joshi A, Shattuck DW, Bernstein LE, Damasio H, Leahy RM. Comparison of landmark-based and automatic methods for cortical surface registration. Neuroimage. 2010;49(3):2479–2493. doi: 10.1016/j.neuroimage.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker Jones, OŌiwi, Prejawa S, Hope T, Oberhuber M, Seghier ML, Leff AP, Price CJ. Sensory-to-motor integration during auditory repetition: a combined fMRI and lesion study. Frontiers in Human Neuroscience. 2014;8:24. doi: 10.3389/fnhum.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkell JS. Movement goals and feedback and feedforward control mechanisms in speech production. J Neurolinguistics. 2012;25(5):382–407. doi: 10.1016/j.jneuroling.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncet M, Habib M, Robillard A. Deep left parietal lobe syndrome: conduction aphasia and other neurobehavioural disorders due to a small subcortical lesion. Journal of Neurology, Neurosurgery, and Psychiatry. 1987;50(6):709–713. doi: 10.1136/jnnp.50.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigg M, Fountain N. Conduction aphasia elicited by stimulation of the left posterior superior temporal gyrus. Journal of Neurology, Neurosurgery, and Psychiatry. 1999;66(3):393–396. doi: 10.1136/jnnp.66.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigg M, Geldmacher DS, Elias WJ. Conduction aphasia as a function of the dominant posterior perisylvian cortex. Report of two cases. Journal of Neurosurgery. 2006;104(5):845–848. doi: 10.3171/jns.2006.104.5.845. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex. 1995;5(5):429–438. doi: 10.1093/cercor/5.5.429. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135(Pt 12):3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuren JE, Schefft BK, Yeh HS, Privitera MD, Cahill WT, Houston W. Repetition and the arcuate fasciculus. Journal of Neurology. 1995;242(9):596–598. doi: 10.1007/BF00868813. [DOI] [PubMed] [Google Scholar]
- Starr A, Barrett G. Disordered auditory short-term memory in man and event-related potentials. Brain: A Journal of Neurology. 1987;110(Pt 4):935–959. [PubMed] [Google Scholar]
- Swanson SJ, Sabsevitz DS, Hammeke TA, Binder JR. Functional magnetic resonance imaging of language in epilepsy. Neuropsychol Rev. 2007;17:491–504. doi: 10.1007/s11065-007-9050-x. [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Binder JR, Possing ET, Hammeke TA, Sabsevitz DS, Spanaki M, et al. FMRI language laterality during a semantic decision task: age of onset and side of seizure focus effects. Journal of the International Neuropsychological Society. 2002;8:222. [Google Scholar]
- Swets JA. Signal detection and recognition by human observers. New York: Wiley; 1964. [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. p. 122. [Google Scholar]
- Tourville JA, Reilly KJ, Guenther FH. Neural mechanisms underlying auditory feedback control of speech. Neuroimage. 2008;39(3):1429–1443. doi: 10.1016/j.neuroimage.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RJS, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural sub-systems within "Wernicke's area". Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.