Abstract
Membrane association of estrogen receptors (ER) depends on cysteine palmitoylation and two leucines in the ligand binding domain (LBD), conserved in most steroid receptors. The role of this region, corresponding to helix 8 of the glucocorticoid receptor (GR) LBD, on membrane association of GR was studied in 4B cells, expressing endogenous GR, and Cos-7 cells transfected EGFP-GR constructs. 4B cells preloaded with radiolabeled palmitic acid showed no radioactivity incorporation into immunoprecipitated GR. Moreover, mutation C683A (corresponding to ER palmitoylation site) did not affect corticosterone-induced membrane association of GR. Mutations L687-690A, L682A, E680G and K685G, prevented membrane and also nuclear localization through reduced ligand binding. L687-690A mutation decreased association of GR with heat shock protein 90 and transcriptional activity, without overt effects on receptor protein stability. The data demonstrate that palmitoylation does not mediate membrane association of GR, but that the region 680-690 (helix 8), is critical for ligand binding and receptor function.
Keywords: Glucocorticoid receptor, palmitoylation, ligand binding domain, helix 8, dexamethasone binding, HSP90
1. INTRODUCTION
Adrenal glucocorticoids, the end product of hypothalamic pituitary adrenal axis activation, are critical for normal metabolic activity and stress adaptation. Glucocorticoids exert their effects by binding to intracellular receptors belonging to the nuclear receptor family. In basal conditions Glucocorticoid receptors (GR) are associated with heat shock protein 90 (HSP90), within a protein complex in the cytoplasm. Upon ligand binding, GR and other proteins in the scaffolding complex translocate to the nucleus where GR regulates gene transcription (Dean et al. 2001; Zanchi et al. 2010; Vandevyver et al. 2013). Although changes in transcription are rapid, the biological actions of glucocorticoids require time for protein synthesis and can take hours following GR activation by the ligand (Jensen 2005; O’Malley 2005). However, some effects of glucocorticoids, including negative feedback on HPA axis activity, are too rapid (i.e. within minutes) to be attributed to the classical genomic actions (Losel et al. 2003; Watson & Gametchu 2003; Norman et al. 2004; Acconcia et al. 2005).
Several mechanisms have been postulated to explain non-genomic effects of glucocorticoids, including non-specific interaction of the ligand with membrane proteins, a yet unidentified plasma membrane receptor, and non-genomic effects mediated by the classical GR (Orchinik et al. 1991; Gametchu et al. 1999; Song & Buttgereit 2006; Roozendaal et al. 2010; Stojadinovic et al. 2013). Consistent with the latter, immunohistochemical studies have shown GR association to the plasma membrane (Liposits & Bohn 1993; Johnson et al. 2005; Komatsuzaki et al. 2005; Samarasinghe et al. 2011). In recent western blot studies we have demonstrated rapid association and dissociation of irGR to membrane fractions, with kinetics that parallel rapid inhibition of ACTH release by low physiological levels of the natural glucocorticoid, corticosterone, in perifused rat anterior pituitary cells (Deng et al. 2014). There is evidence that the estrogen receptor (ER) can associate with the plasma membrane through palmitoylation of cysteine 447, and the participation of two leucines at positions 453 and 454 (Pedram et al. 2007). Interestingly, this sequence is highly conserved for a number of nuclear receptors, including human and rat GR (Marino et al. 2006).
The aim of this study is to test the hypothesis that this conserved region plays a role in the mechanism of membrane association of GR. We used the hypothalamic cell line 4B, which contains endogenous GR, and Cos-7 cells transfected with wild type and mutant GR constructs to examine the role of cysteine 683 palmitoylation and the leucine repeat 687 to 690 on membrane association of GR.
2. MATERIALS AND METHODS
2.1 Constructs
An amino terminus fusion construct of the rat GR with EGFP (EGFP-GR) was created by cloning the entire coding sequence of the rat GR, into the BamH1 and XhoI sites of pEGFP-C1 (Addgene, Cambridge, MA). A 4686 bp DNA fragment encoding the GR was obtained by PCR using cDNA from the rat hypothalamic cell line 4B and the following primers with added BamH1 and XhoI ends: forward, 5′GAGGCGAAAGGGTGGCTCTGTGTAGCACTG3′; reverse, 5′AGCAAATATAAGGCAGCAAGCAGGTTAAGC 3′. The wild type GR construct, pSG5/GR, was kindly provided by Dr Stoney Simons (NIDDK, NIH). The mutant EGFP-GR constructs shown in Table 1, were created by site directed mutagenesis (Epoch Life Science, Missouri City, Texas). The ability of GR to exert positive regulation of gene expression was studied by examining the effect of 10nM corticosterone on luciferase activity driven by a tyrosine kinase promoter containing a glucocorticoid responsive element (GRE-TK), also provided by Dr Stoney Simons, NIDDK, NIH).
Table 1.
EGFP-GR mutant constructs used in the study
| Constructs | Sequence | |
|---|---|---|
| 1 | EGFP-GR (Wild Type) | 674LQVSYEEYLCMKTLLLLSSVP694 |
| 2 | EGFP-GR C683A |
|
| 3 | EGFP-GR C683A, L687-690A |
|
| 4 | EGFP-GR L689-690A |
|
| 5 | EGFP-GR L687-690A |
|
| 6 | EGFP-GR L688-690A |
|
| 7 | EGFP-GR L687-688A |
|
| 8 | EGFP-GR C683A, L689-690A |
|
| 9 | EGFP-GR L682A |
|
| 10 | EGFP-GR L687A |
|
| 11 | EGFP-GR L688A |
|
| 12 | EGFP-GR L689A |
|
| 13 | EGFP-GR L690A |
|
| 14 | EGFP-GR E680G |
|
| 15 | EGFP-GR K685G |
|
| 16 | EGFP-GR S692G |
|
2.2 Cell culture and transfections
The rat hypothalamic cell line 4B (provided by Dr. John Kasckow, VA Pittsburgh Health Care System, Pittsburgh, PA) which expresses endogenous GR was used to examine GR trafficking and GR palmitoylation. Cells were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Aliquots of 5 million cells were transfected with 5 μg of empty plasmid, pEGFP-C1, or the constructs mentioned above by electroporation using a Nucleofector (Lonza Walkersville, Inc., Walkersville, MD) and Amaxa Cell Line Nucleofector Kit V (Lonza). After transfection, cells were resuspended in DMEM containing 10% fetal bovine serum and plated into 60cm2 tissue culture dishes (Falcon) at a density of 33,000/cm2 for western blotting, or immunoprecipitation. Experiments were performed 24 h after transfection when cells reached approximately 90% confluence.
Cos 7 cells (purchased from American Tissue Culture Collection, Manassas, VA), which have negligible endogenous GR were used for assessing biological activity of GR mutants in reporter gene assays and ligand binding assays. Cells were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Aliquots containing 5 million cells were transfected with 5μg EGFP-GR wild type or mutant constructs by electroporation using a Nucleofector (Lonza Walkersville, Inc., Walkersville, MD) and Kit R (Lonza) and program W-001. Cells were plated in 24-well plates, 200,000 cells/well for luciferase activity and 15 cm culture dishes, 5 million cells per dish for binding assays.
2.3 Palmitic acid incorporation into GR
Cells were labeled with [3H]- or [14C]-palmitic acid (0.5 mCi/ml) for 4 h before 30 min treatment with 10nM corticosterone or vehicle. Cytoplasmic and membrane fractions were obtained using kit reagents from Pierce Subcellular fractionation kit (Life Technologies, Grand Island, NY). Five hundred μl aliquots containing 500–1000 μg of protein were subjected to immunoprecipation using 10 μg of rabbit polyclonal antibody, anti-GR H300, Santa Cruz Biotechnology, Santa Cruz, CA. After separation of immunoprecipitated proteins using protein A/G Dynabeads, pellets were resuspended in 30μl of SDS loading buffer, and aliquots used for counting radioactivity in a liquid scintillation counter (5 μl), western blot analysis (5 μl) and the rest (20 μl) was separated in a 10% Tris-glycine gel followed by film exposure for up to 120 days.
2.4 Western blot
Twenty μg of total cytoplasmic, membrane or nuclear proteins, or 20 μl of GR-immunoprecipited proteins, were subjected to SDS-PAGE on 10% tricine-glycine gels and transferred onto polyvinyl difluoride membranes (Millipore). After blocking nonspecific protein-binding sites by incubation for 1 h at room temperature in 50 mM Tris-HCl (pH 8), 150 mM NaCl and 0.1% Tween 20 (TBST) containing 5% nonfat dry milk, membranes were incubated overnight with anti GR antibody (Rabbit polyclonal, 1:1000, H-300, Santa Cruz Biotechnology), or anti-HSP90 (rabbit polyclonal, 1:1000, Cell Signaling #4874, or mouse monoclonal, AC-16, 1:1000, Santa Cruz Biotechnology). After washing in TBST 3 × for 5 min, membranes were incubated for 1 h at room temperature with the second antibody in 5% nonfat dry milk in TBST. The specific complexes were detected using the enhanced chemiluminescence (ECL) system from GE health. After film exposure, blots were stripped and assayed for HDAC1 (Goat polyclonal, 1:1000, Santa Cruz) in the nucleus pan-cadherin (Mouse polyclonal, 1:5000, Abcam, Cambridge, MA) for membrane and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Goat polyclonal, 1:1000, Santa Cruz) for cytoplasm as loading controls. In some experiments in transfected cells membranes were exposed to anti-GFP (Rabbit polyclonal, 1:1000, Santa Cruz sc-8334) as control for the EGFP-GR band.
2.4 Luciferase activity
Eighteen hours after transfection with wild type and mutant constructs, Cos-7 cells were cultured overnight in medium supplemented with 10% stripped fetal bovine serum, washed with PBS and preincubated in serum free medium (0.1% BSA) for 2h before addition of 10 nM or 1 μM corticosterone. After 6h incubation and removal of the medium, cells were lysed by addition of 100 μl of passive lysis buffer (Promega, Madison, WI). Luciferase activity was measured using the Dual Luciferase System (Promega, Madison, Wisconsin), as previously described (Liu et al. 2008).
2.5 Binding of [3H]dexamethasone to cytosolic proteins
COS-7 cells transfected with the different constructs were collected in 1.5 ml microcentrifuge tubes, pelleted and resuspended in 150μl of binding buffer, containing 25 mM Tris (pH 7.4),1mM EDTA, 20mM NaMoO4, 10% glycerol, 5mM DTT, and protease inhibitor cocktail. After freezing and thawing three times, cells were homogenized by sonication for 30 seconds at the high setting in a Bioruptor (Diogenode, Denville, NJ), and centrifuged for 30 minutes at 100,000 × g in an Airfuge (Beckman Coulter, Indianapolis, IN). Aliquots containing 10 to 20 μg of protein were incubated with 25 to 100nM [3H]-dexamethasone in a total volume of 50 μl, for 2.5 h. Bound radioactivity was separated from free ligand by addition of 7 mg of dextran activated charcoal. Forty μl of supernatant containing bound radioactivity were counted in a liquid scintillation counter. The remaining cytosolic proteins were run through Zeba Desalt Spin Columns (Thermo Scientific, Rockford, IL) for determination of protein concentration using the bicinchoninic acid (BCA) method (Life Technologies, Pierce, Grand Island, NY). Bound disintegrations per minute (dpm) were corrected by the concentration of cytosolic GR measured by western blot.
2.6 Confocal microscopy imaging
4B cells plated on coverslips were transfected with EGFP-GR constructs and the nuclear marker H2B-mCherry using Lipofectamine 2000 and cultured for 18h in standard conditions, before incubating in medium supplemented with stripped serum overnight. Cells were then washed and preincubated in medium with 0.1% BSA before addition of corticosterone at concentrations from 10nM to 1 μM. After 30 minute incubation, cells were fixed for 14 minutes with 4% formaldehyde, washed with PBS, treated 2 × 5min with 30mM glycine to quench fixation, and washed again with PBS for imaging. Images composed of 13 slices (with a 0.3 μm step size) were acquired using a Nikon spinning disc microscope. Nuclear translocation of EGFP-GR constructs was assessed by the co-localization of EGFP-GR and H2B-mCherry in 10 or more cells for each condition. The Pearson Correlation Coefficient was used to quantify the colocalization of the two signals in each z-stack image.
2.7 Statistics
Data are represented as mean ± SEM from the values in the number of observations indicated in the text and legend to the figures. The statistical significance of the differences between groups was determined by one- or two-way ANOVA followed by Fisher protected least-significant difference post hoc test unless specified in Results or figure legends. Statistical significance was set at P < 0.05.
3. RESULTS
3.1 Palmitoylation does not mediate membrane association of GR in 4B cells
BLAST analysis of the ER region responsible for membrane association confirmed a high homology with other steroid receptors (Table 2). This region comprises helix 8 of the GR and it is identical across the species, including human, rat, mouse, ovine, bovine, rabbit, guinea pig, chicken, squirrel monkey and wild boar (Table 2). Computer analysis of cysteine palmitoylation probabilities of the amino acid sequences of GR using the program CSS-Palm 3.0 (freely available at http://csspalm.biocuckoo.org/archive/prediction, (Ren et al. 2008), revealed 4 potential palmitoylation sites at cysteines 169, 656, 683 and 754 (Table 3). The highest score (1.29) was found for cysteine 683, located within the conserved sequence corresponding to the palmitoylation site of the estrogen receptor (Table 2). Analysis of the ER sequence, under the same parameters, showed a single potential palmitoylation site, corresponding to cysteine 447, with a score of 2.97. This suggests that cysteine 683 is a potential palmitoylation site for the GR though with lower possibility than the equivalent site in ER.
Table 2.
Amino acid sequence alignment of the conserved region of the human estrogen receptor alpha containing palmitoylation site (c447) with other steroid receptors. The cysteine is showed in red, the leucine repeat in green and other conserved amino acids in blue.
| 443 | 447 | 453 | 456 | |||||||||||
| hERα | E | E | F | V | C | L | K | S | I | I | L | L | N | S |
| 816 | 820 | 824 | 829 | |||||||||||
| hPR | E | E | F | L | C | M | K | V | L | L | L | L | N | T |
| 867 | 875 | 880 | ||||||||||||
| hMR | E | E | Y | T | I | M | K | V | L | L | L | L | S | T |
| 515 | 519 | 528 | ||||||||||||
| hAR | P | S | P | T | C | V | K | S | E | M | G | P | W | M |
| 661 | 665 | 669 | 674 | |||||||||||
| hGRα | E | E | Y | L | C | M | K | T | L | L | L | L | S | S |
| 661 | 665 | 669 | 692 | |||||||||||
| hGRβ | E | E | Y | L | C | M | K | T | L | L | L | L | S | S |
| 679 | 683 | 687 | 692 | |||||||||||
| rGRα | E | E | Y | L | C | M | K | T | L | L | L | L | S | S |
| 676 | 680 | 684 | 689 | |||||||||||
| mGRα | E | E | Y | L | C | M | K | T | L | L | L | L | S | S |
hERα, human estrogen receptor alpha; hPR, human progesterone receptor; hMR, human mineralocorticoid receptor; hAR, human androgen receptor; GRα, human glucocorticoid receptor alpha (h, human; r, rat; m, mouse). In a number of species (not shown in the table) GR share identical sequence in all species examined, including bovine, ovine, guinea pig, rabbit, wild boar and chicken. Comparison of the ERα sequence with other steroid receptors showed a homologous region in the ligand binding domain, sharing the features required for membrane association of the ER. This includes, the cysteine shown in red (except the MR), and the leucine repeat shown in green (except the AR). The isoleucines preceding the leucine pair in hERα are replaced by leucine in GR. Other shared aminoacid in this region of the ER and other receptors are shown in blue. GenBank accession codes: hERa (NP_000116.2), hGRa (ADP91253.1), rGRa (CAA72938.1), hPR (NP_000917), hMR (NP_000892), hAR (NP_000035), hGRβ (NP_001018661), mGRα (NP_032199).
Table 3.
Cysteine palmitoylation prediction sites for the rat glucocorticoid and estrogen receptors, using the program CCS-Palm 3.0
| Human Estrogen Receptor alpha | Rat Glucocorticoid receptor | ||||||
|---|---|---|---|---|---|---|---|
| Position | Peptide | Score | Cluster | Position | Peptide | Score | Cluster |
|
|
|
||||||
| 447 |
|
2.97 | C | 169 |
|
0.69 | B |
| 656 |
|
0.76 | B | ||||
| 683 |
|
1.29 | C | ||||
| 754 |
|
0.21 | A | ||||
Analysis was performed using the program CSS-Palm 3.0, available in the internet at: http://csspalm.biocuckoo.org/archive/prediction. Scores over 0 indicate palmitoylation potential and higher scores within a cluster indicates higher palmitoylation potential. Cysteine (C) 683 in GR and 447 in ER fall within the same cluster suggesting higher probability of palmitoylation for the ER.
To determine whether the GR can be palmitoylated, we examined the incorporation of tritiated palmitic acid into GR in 4B cells, which express endogenous GR. Western blot analysis of cytoplasmic and membrane protein fractions of 4B cells preloaded with tritiated palmitic acid before immunoprecipitation revealed the expected 90 kDa band corresponding to immunoreactive GR. Consistent with observations in unlabeled cells (Deng Q. et al. 2014) there were converse changes in irGR content following 30 min incubation of the cells with 10 nM corticosterone, with a decrease in cytosol and an increase in membrane protein fractions (Figure 1-A). Western blot in protein aliquots after immunoprecipitation with GR antibody showed marked irGR bands in cytoplasm and membranes (Fig 1-B). No beta actin or pan cadherin bands were detected after GR immunoprecipitation (not shown). Measurement of radioactivity in aliquots of total cytoplasmic and membrane proteins showed marked incorporation of palmitic acid into proteins (Fig, 1-C). However, no [3H] counts above background were found in aliquots of the immunoprecipitation products. The table in Fig 1-C shows the amount of incorporated [3H]-palmitic acid in cytoplasm and membrane proteins of 4B cells in total protein fractions and after GR immunoprecipitation. Thirty min incubation with corticosterone had no effect on the amount of radioactivity incorporated into proteins. Similar negative results were found using 4B cells preloaded with [14C]-labeled palmitic acid (not shown). Consistent with the absence of radioactivity over background absence of autoradiographic band, in two experiments using [3H]-labeled palmitic acid, film exposure for 60 or 120 days of electrophoresis gels of immunoprecipitated GR revealed no specific bands corresponding to palmitoylated proteins (not shown).
Figure 1. [3H]-palmitic acid incorporation into proteins in the hypothalamic cell line 4B.
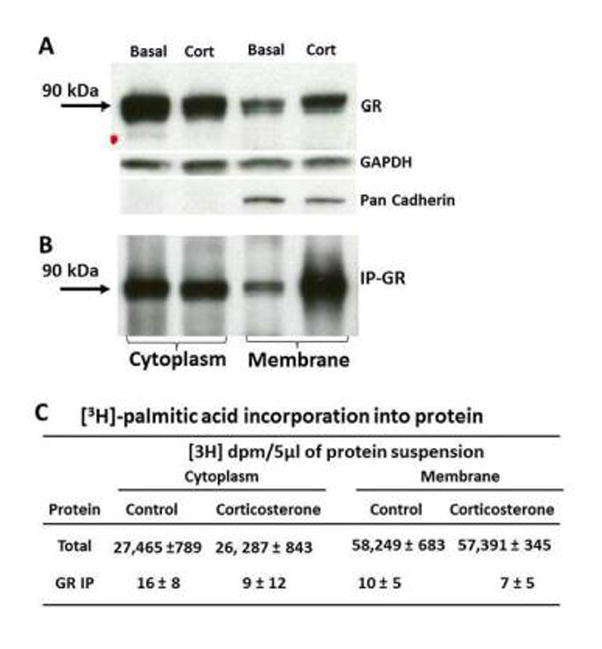
The film images show representative western blot analysis for GR in cytoplasmic and membrane protein fractions of 4B cells preloaded with [3H]-palmitic acid, before (A) and after (B) immunoprecipitation with an anti-GR antibody GAPDH and Pan Cadherin were used as housekeeping controls for cytoplasm and membrane, respectively. [3H]-palmitic acid incorporation into total cytoplasmic and membrane proteins and after immunoprecipitation with anti-GR antibody (H300, Santa Cruz Biotechnology) in 4B cells treated with 10nM corticosterone of vehicle (C). IP-GR immunoprecipitated GR; GAPDH, glycerol 3-phosphate dehydrogenase.
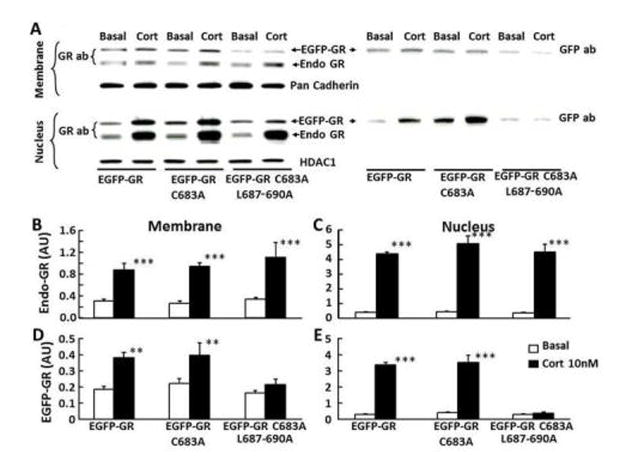
3.2 Conserved cysteine 683 does not influence membrane association of GR
The role of cysteine 683 on membrane association of GR was examined in cells transfected with EGFP-GR constructs, wild type or bearing a C683A mutation. As shown in Figure 2-A, western blot analysis with the anti-GR antibody, GR H-300, showed two bands, a 90 kDa band corresponding to the endogenous GR and a ~120k Da band corresponding to the product of the transfected EGFP-GR constructs. A single band in the same position was present after reblotting of the membrane with GFP antibody (Fig 2-A). The endogenous GR band shows the typical translocation to nucleus and membrane following incubation of the cells for 30 minutes with 10nM corticosterone Fig 2-A, B and C). Similar increases in nuclear and membrane GR following incubation with corticosterone were observed in the higher molecular weight band corresponding to the transfected with EGFP-GR (wild type) or EGFP-GRC683A mutant constructs, indicating that cysteine 683 is not involved on GR trafficking (Fig 2-A, D and E). Since in addition to the palmitoylated cysteine 447, leucines 453 and 454 have been shown to be necessary for membrane association of the human ER, we examined the effect of C683A with additional mutations L687-690A (EGFP-GR C683A/L687-690A) on membrane association of the GR. Western blot for GR in cells transfected with EGFP-GR C683A/L687-690A showed the expected nuclear (8.7 ± 1.9 fold) and membrane (2.7 ± 0.7 fold) translocation of the 90 kDa band corresponding to the endogenous GR after incubation of the cells with 10 nM corticosterone (Fig 2-A, B and C). However, corticosterone treatment had no effect on the intensity of the 120 kDa band corresponding to EGFP-GR C683A/L687-690A in either membrane or nuclear proteins, suggesting that the leucine repeat is essential for GR trafficking (Figure 2-A, D and E).
Figure 2. Effect of C683A and L687-690A on GR trafficking.
Representative western blot for GR in membrane and nuclear fractions from 4B cells transfected either with EGFP-GR (wild type), or EGFP-GR C683A, or EGFP-GR C683A/L687-690A constructs incubated for 30 min with vehicle or 10 nM corticosterone (A). The images to the left corresponds to the blot using GR antibody, and the image to the right corresponds to the western blot using GFP antibody, after stripping the same membrane. Both antibodies revealed superimposed bands consistent with the molecular size of EGFP-GR (A). The bar graphs represent the content of endogenous GR [Endo GR](B and C), and EGFP-GR (D and E) in membrane (B and D) and nuclear (C and E) proteins as the mean and SE of pooled data from 3 experiments. ** p<0.01 higher than the respective basal; ***, P<0.001 higher than the respective basal.
To confirm the western blot results, we used confocal microscope to visualize 4B cells transfected with wild type and mutant constructs. As shown in Fig 3, under basal conditions EGFP fluorescence was present predominantly in the cytoplasm. Consistent with the western blot results after 30 min incubation with 10 nM corticosterone most of the fluorescence was found in the nucleus in cells transfected with the wild type and EGFP-GR C683A constructs but no nuclear translocation was observed in cells transfected with EGFP-GR C683A/L687-690A. Using fixed- and live-cell spinning disk microscopy, as well as Total Internal Reflection Flourescence (TIRF) microscopy no increases in EGFP-GR fluorescence were observed on the membrane either in basal conditions or after corticosterone exposure (not shown).
Figure 3. Confocal microscopy imaging of the effect of corticosterone on intracellular trafficking of fusion proteins of EGFP with wild type and mutant GR.
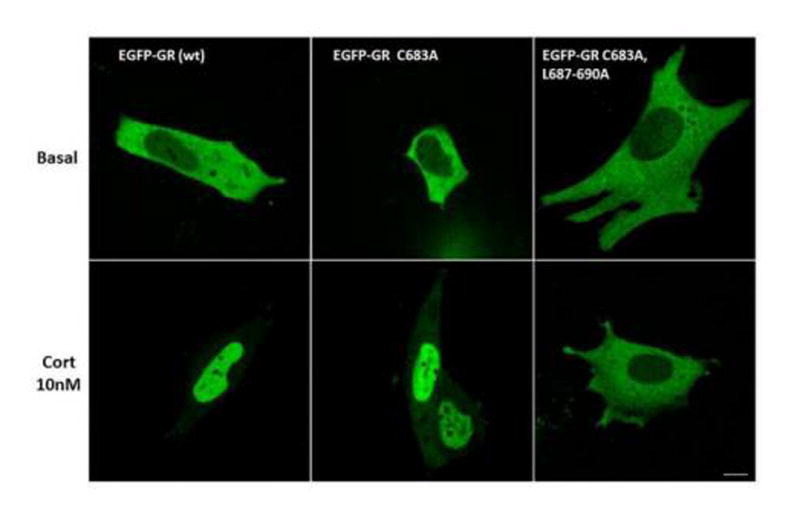
Cells transfected with either wild type (EGFP-GR) or EGFP-GR C683A, or EGFP-GR C683A/L687-690A were incubated with 10 nM corticosterone (Cort) for 30 min, before fixation and confocal microscopy examination. The magnification line at the bottom represents 10 μm.
3.3 Leucine repeat 687-690 is essential for transcriptional activity and association with HSP-90
Reporter gene assays conducted in Cos-7 cells (which express little endogenous GR) co-transfected with a GRE-luciferase reporter gene and GR expression vectors showed that the fusion protein EGFP-GR has similar basal and glucocorticoid-stimulated luciferase activity to the non-EGFP tagged construct (Fig 4-A). When compared with cells transfected with the wild type EGFP-GR, mutations EGFP-GR C683A, EGFP-GR C683A/L687-690A or EGFP-GR L687-690A had no significant effect on basal GRE-driven luciferase activity (Fig 4-B). Exposure to corticosterone increased luciferase activity 2.2 ± 0.2-fold over basal values in cells transfected with the wild type EGFP-GR construct. A similar increase of 1.9 ± 0.1-fold over the respective basal, p<0.01, was observed for the C683A mutation. However, in keeping with the lack of nuclear translocation, 10nM corticosterone failed to stimulate luciferase activity in cells transfected with EGFP-GR C683A/L687-690A or EGFP-GR L687-690A (Fig 4-B).
Figure 4. Effect of 10 nM corticosterone on GRE-dependent luciferase activity.
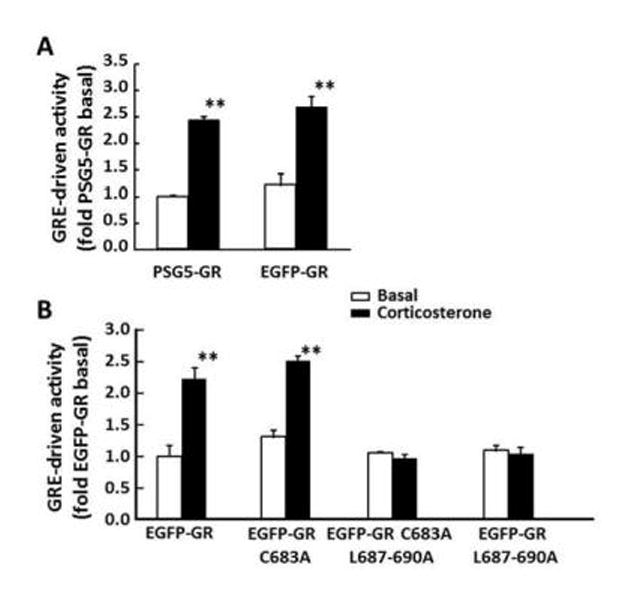
Cos-7 cells were co-transfected with GRE-TK-luc and PSG-GR (non-EGFP-tagged), or EGFP-GR wild type or mutant constructs. (A) The comparison between EGFP-tagged (EGFP-GR) and untagged (PSG5-GR) GR shows that the amino terminus EGFP tag does not affect the transcriptional activity of the receptor. (B) Effect of C683A and C683A/L687-690 mutations of GR on corticosterone-stimulated luciferase activity. Bars are the mean ± SE in 3 experiments. **, P<0.001.
To determine whether the leucine repeat influences the association of GR with the scaffolding protein, HSP90, we performed co-immunoprecipitation of GR and HSP90 in Cos-7 cells transfected with EGFP-GR wild type and mutant constructs. Western blot analysis of HSP90 immunoprecipitated with anti-GR antibody showed bands corresponding to the molecular size of HSP90 of similar intensity in cells transfected with wild type EGFP-GR and EGFP-GR C683A, and a much weaker band in cells transfected with EGFP-GR C683A/L687-690 (Fig 5). Western blot for GR after stripping the membranes showed bands corresponding to GR of similar magnitude in cells transfected with either one of the 3 constructs. The bar graph shows the HSP90 co-immunoprecipitated with GR (expressed as HSP90/GR ratio) in 3 experiments. No significant change in HSP90/GR ratio was observed in cells transfected with C683A compared with cells transfected with the wild type EGFP-GR. In contrast, a marked decrease of 62.7 ± 3.0% (p<0.001) was observed in cells transfected with EGFP-GR C683A/L677-690A (Fig 5).
Figure 5. Effect of cysteine 683 and leucines 687-690 mutations in the rat GR on GR association with HSP90.
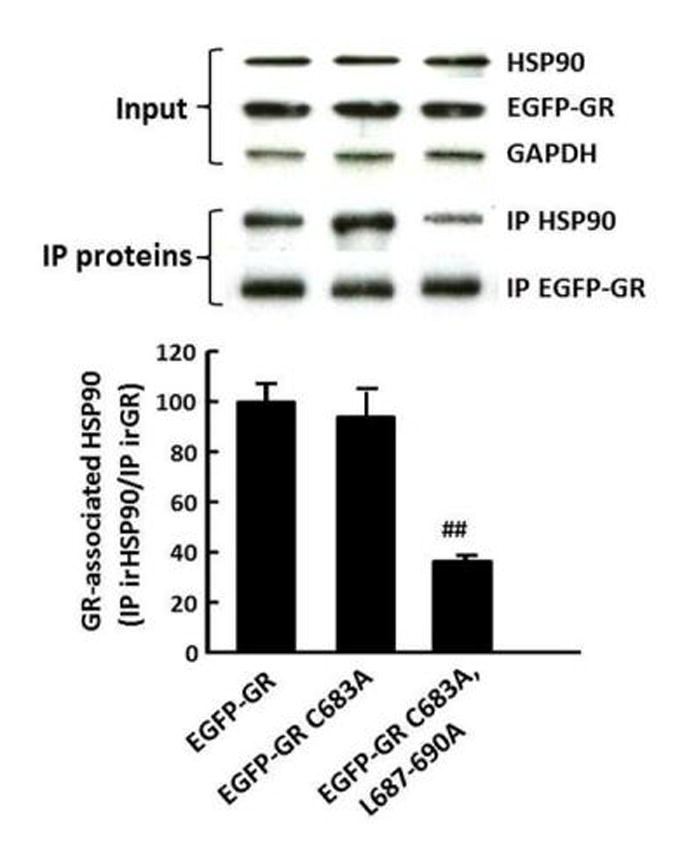
Representative western blots for GR and HSP90 in total cell proteins of Cos-7 cells transfected with EGFP-GR, wild type or mutants, before (input) and after immunoprecipitation with anti-GR antibody (IP proteins). Bars represent the mean ± SE of the ratio HSP90/GR in 3 experiments. ##, p<0.01, for EGFP-GR C683A/L687-690A compared with EGFP-GR.
3.4 Dose response for corticosterone-induced GR translocation in wild type and mutant GR
To determine the relative importance of different amino acids within the conserved sequence containing C683 and the leucine repeat on GR trafficking, we created additional EGFP-GR constructs with mutations in selected amino acids within the region and examined nuclear trafficking by confocal microscopy. 4B cells co-transfected with different EGFP-GR constructs and the nuclear marker H2B-mCherry were treated with increasing concentrations of corticosterone (10nm to 1μM) for 30 min before processing for imaging. The Pearson’s Correlation Coefficient of the two signals was used to measure the degree of GR nuclear translocation. As shown in Fig 6, 10 nM corticosterone is sufficient for full nuclear translocation of the GR. Consistent with western blot results, mutation of the 4-leucines, 687–690, completely impaired nuclear translocation of the GR following corticosterone exposure, even at the highest concentration of 1 μM. Mutation of single leucines in the repeat, with exception of L690, decreased the sensitivity to corticosterone, with nuclear localization becoming apparent with 100 nM corticosterone. As seen in the Figure, corticosterone-induced nuclear localization and the Pearson’s Coefficient for EGFP-GR L690A was no different from the wild type EGFP-GR. However, L690A mutation synergized the inhibitory effect of the other leucines in the repeat since the double mutation EGFP-GR L688-690A showed nuclear trafficking impairment similar to that of EGFP-GR L687-690A. The double mutant EGFP-GR C683A/L687-688A showed nuclear localization with 1 μM corticosterone. Other amino acids tested outside the leucine repeat also affected sensitivity to corticosterone. Mutation of L682A showed nuclear localization only with 1 μM corticosterone, while mutations of lysine 685 to glycine (EGFP-GR K685G) or glutamic acid 680 to glycine (EGFP-GR E680G) were completely unresponsive to corticosterone. Mutation of serine 692 to glycine (EGFP-GR S692G) showed responses to corticosterone similar to the wild type receptor (Fig 6).
Figure 6. Confocal microscopy gray scale images for the dose-response of the effect of corticosterone on the nuclear translocation of EGFP-GR wild type and mutants.
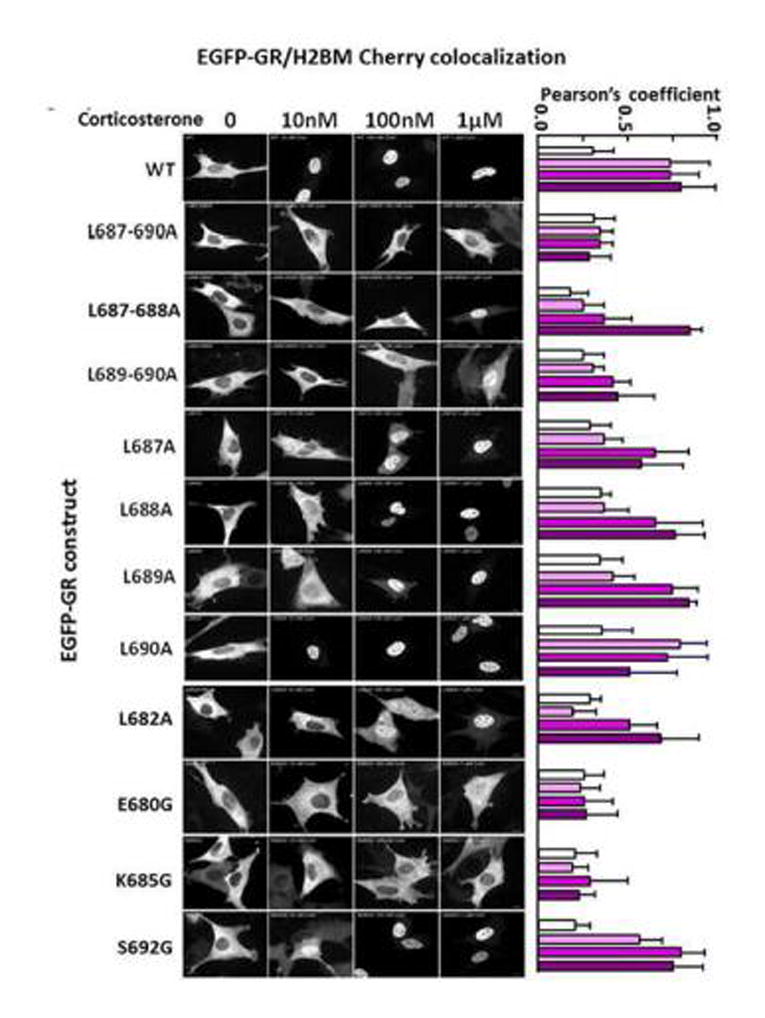
4B cells transfected with EGFP-GR constructs and the nuclear marker H2B-mCherry were treated with 10nm to 1μM corticosterone for 30 min before processing for imaging. The bars to the left of each image represent the Pearson’s coefficients for EGFP/H2BM Cherry colocalization in the average of 10 or more cells per corticosterone concentration for the corresponding construct in the images.
3.5 Leucine repeat 687-690 is essential for GR ligand binding
To determine whether the impaired GR trafficking following mutations in the leucine repeat region is due to altered ligand binding, we measured [3H]-dexamethasone binding to cytosol of Cos-7 cells transfected with the different constructs. Using a single ligand concentration (50nM), binding was negligible in Cos-7 cells transfected with the empty EGFP vector compared with cell transfected with EGFP-GR (0.1 ± 0.03 and 4.1 ± 0.2 nM/mg protein, respectively). As seen in Fig 7-A, mutation of the 4 leucines 687–690 to alanine, with or without mutation of cysteine 683 completely abolished [3H]-dexamethasone binding with 100.9 ± 0.6% and 99.5 ± 0.7% inhibition, respectively. Almost complete reduction in [3H]-dexamethasone binding of 92.8 ± 1.5% and 89.6 ± 0.7% was also observed for the paired leucine mutations, EGFP-GR L687-688A and EGFP-GR L689-690 A, respectively. Mutations of leucine 682, lysine 685 or glutamic acid 680 also markedly decreased [3H]-dexamethasone binding by 81.8±5.7%, 90.9 ± 2.0% and 96.9 ± 0.5%,, respectively, while mutation of serine 692 had no effect.
Figure 7.
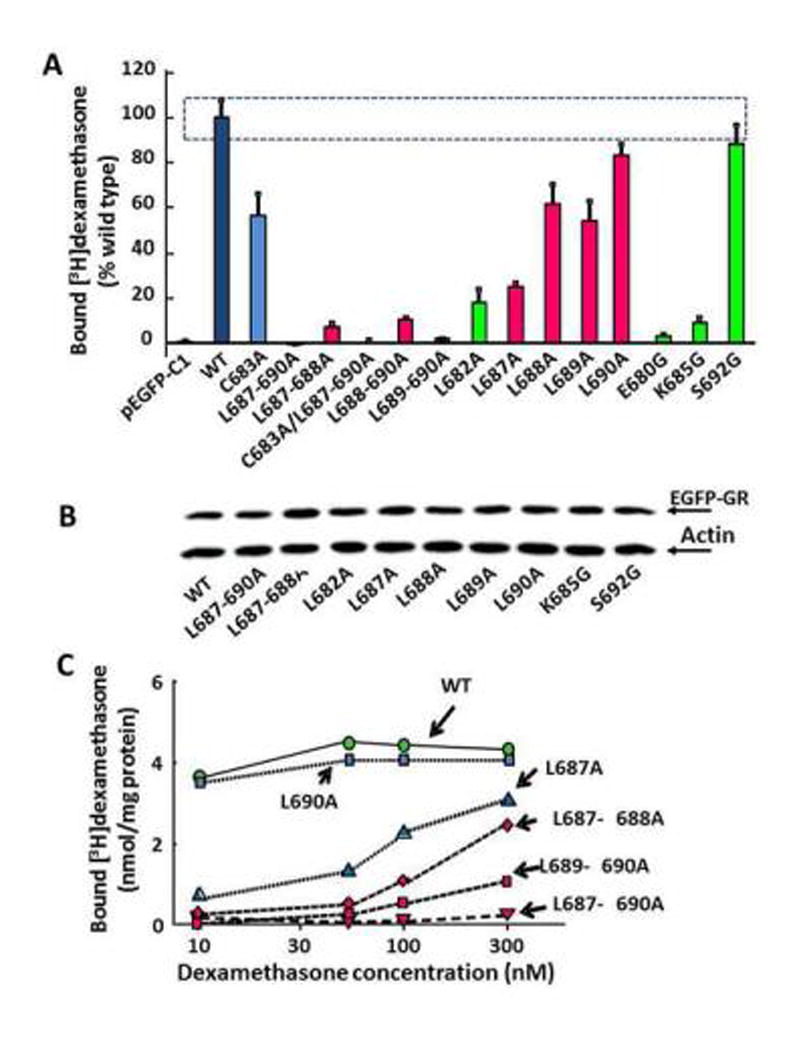
Binding of [3H]-dexamethasone in COS7 cells transfected with constructs wild type and mutant EGFP-GR constructs. (A) [3H]-Dexamethasone binding using a single ligand concentration (50nM) in cytosolic fractions of cells transfected with the different constructs. Binding values for EGFP-GR (WT) were assigned 100% and data for the mutants were expressed as percent of WT. The SE for the WT is shown by the dotted box. Binding for the WT, was 4.2 ± 0.1 nmol/mg of protein and for the empty vector (pEGFP-C1) was 0.04±0.02 nmol/mg of protein, indicating negligible endogenous GR content in Cos 7 cells. All constructs shown in the graph were EGFP-tagged GR. Binding for the non-tagged receptor PSG5-GR was no different from EGFP-GR (not shown). (B) Representative western blot image showing similar EGFP-GR content in the cytosol of Cos-7 cells transfected with the different EGFP-GR constructs. Bound radioactivity for each construct was corrected by EGFP-GR content in the respective cytosol. (C) Effect of increasing [3H]-dexamethasone concentrations on binding to selected constructs in duplicate determinations. Negligible increments in binding with increasing ligand concentrations from 10 to 50 nM were observed for EGFP-GR (WT) and L690A, while binding increased at higher ligand concentrations for L687A or double leucine mutations, reflecting reduced binding affinities. Mutation of all 4 leucines in the repeat completely suppressed binding.
To determine whether the changes in ligand binding observed for the mutant GR was due to changes in receptor number or affinity we measured binding for some of the constructs using concentrations of ligand from 10 to 300 nM. As shown in Fig 7-B, binding in cytosol from cells transfected with the wild type EGFP-GR or EGFP-GR L690A had already reached a plateau with 10 nM dexamethasone. In contrast, binding remained almost undetectable with all ligand concentrations in cells transfected with EGFP-GR L687-690A. In cells transfected with EGFP-GR L687A and double leucine mutations, binding increased with the higher ligand concentration suggesting a decrease in receptor affinity.
As shown in Fig 8, there was a strong positive relationship between ligand binding and the ability of corticosterone to induce nuclear translocation of the different GR mutants. Correlation analysis between [3H]-dexamethasone binding and Pearson’s coefficients for nuclear localization of GR with 100nM corticosterone for each of the EGFP-GR wild type and mutant constructs showed a positive correlation between both parameters (R=0.9).
Figure 8.
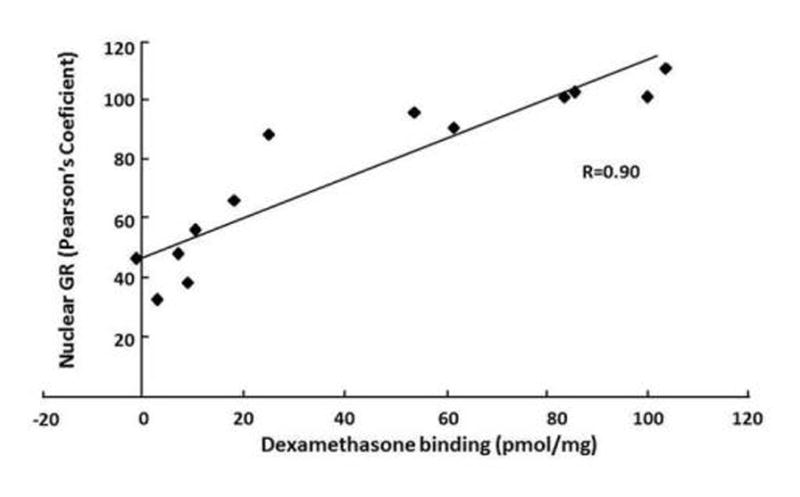
Correlation between [3H]-dexamethasone binding (nmol/mg at 50nM dexamethasone) and nuclear localization of wild type and mutant EGFP-GR constructs (Pearson’s coefficient at 100nM corticosterone). Each data point represents a different construct, from left to right: L687-690A; E680G; L687-688A; K685G; L689-690A; L682A; L687A; L690A; L688A; L689A; S692G; wild type (WT).
4. DISCUSSION
The aim of the present study was to determine whether, as for the ER, cysteine palmitoylation in conjunction with a leucine repeat located in a conserved region of the ligand binding domain mediates membrane association of the GR. The data demonstrate that in spite of the sequence homology of this region with the ER, the GR does not undergo palmitoylation, and that the equivalent cysteine (positions 683 and 665, for rat and human GR, respectively) is not required for GR function. However, the study demonstrates that the leucine repeat and other amino acids within the conserved region between glutamate 678 and leucine 690 for the rat GR, corresponding to helix 8 of the ligand binding domain, is critical for GR ligand binding.
The present demonstration that immunoreactive GR is present in membrane protein fractions by western blot is consistent with previous observations in our laboratory (Deng Q. et al. 2014) and with reports showing immunoreactive GR in the membrane by immunohistochemistry (Liposits & Bohn 1993; Johnson et al. 2005; Komatsuzaki et al. 2005; Samarasinghe et al. 2011). Moreover, the consistent increase in immunoreactive GR in the membrane following exposure of the cells to basal concentrations of the natural glucocorticoid, corticosterone, suggests that membrane association of the GR is a ligand-dependent dynamic process with potential physiological significance. The absence of nuclear markers in the membrane protein fractions indicates that the increase in immunoreactive GR in membrane proteins does not reflect contamination with nuclear proteins. The commonly used cytoplasmic markers, including beta actin and GAPDH, are not exclusive for cytosol (Hoock et al. 1991; Raje et al. 2007). Although an extra washing step was introduced in the membrane preparation procedure to minimize cross-contamination of the fractions, the presence of some cytosolic contamination in membrane fractions cannot be ruled out. However, this is unlikely since cytoplasmic and membrane GR consistently underwent converse changes following corticosterone treatment.
In spite of the clear ligand-induced increase in endogenous GR in membrane fractions by western blot, the imaging studies failed to detect EGFP fluorescence at the cell membrane. Consistent with previous reports, the amino terminus fusion protein EGFP-GR exhibited similar dexamethasone binding, binding to HSP90, glucocorticoid induced nuclear translocation and transcriptional activity compared with the non-EGFP tagged receptor. EGFP-GR also showed ligand induced membrane association as shown by the higher molecular weight band in the western blot detected with both GR and GFP antibodies. Several reasons could be considered for the lack of visualization of fluorescent EGFP-GR by confocal microscopy. It is known that different sample preparation and fixation methods can greatly influence protein visualization by immunodetection. However, equal lack of membrane localization, was seen in live-cell imaging experiments (not shown). A possible factor to be considered is the relative lower abundance of GR in membrane fractions compared with the cytoplasmic and nuclear GR. In addition, the western blot also shows that the amount of EGFP-GR on the membrane is less than endogenous GR. Thus, it is possible that preferential association of endogenous GR with the membrane prevents a robust membrane localization of EGFP-GR. Lastly, the membrane fraction is by no means a plasma membrane preparation. Although not fully investigated in this study, it is possible that GR localizes to another membrane compartment other than the plasma membrane. Such localization would be even more difficult to image in the setting of high cytoplasmic signal.
The lack of radiolabeled palmitic acid incorporation into protein fractions immunoprecipitated with GR antibody demonstrates that, in contrast to ER, the GR does not undergo palmitoylation. The expression of S-palmitoyltransferase in 4B cells was not measured, but the marked labeling of total proteins in these experiments suggests that mechanisms required for palmitoylation exists in these cells. There is no consensus sequence for cysteine palmitoylation and the prediction algorithm in CSS-Palm is based on the comparison of the sequence context of each cysteine in a given sequence, e.g., GR or ER, with 263 peptides experimentally proven to be palmitoylated. Analysis of GR shows that the cysteine within the conserved region has the highest palmitoylation probability within the molecule. However, this was lower than that for the equivalent cysteine in the ER, which is known to be palmitoylated. The fact that mutation of C683A had no effect on EGFP-GR content in membrane protein fractions is consistent with a lack of involvement of palmitoylation of the conserved cysteine on membrane association of GR. These data indicates that in spite of the sequence homology between this conserved region in the ER and GR, the mechanism for membrane association of GR differs from that described for the ER.
Since in addition to C447 palmitoylation, membrane association of the ER requires leucines 453 and 454, further studies were performed with EGFP-GR constructs bearing mutations of an equivalent four leucine repeat, L687-690A. However, the effect of the leucine repeat on membrane association of the GR could not be interpreted since the additional L687-690A mutation obliterated not only membrane but also corticosterone-induced nuclear translocation and transcriptional activity of the GR. Since association to the scaffolding protein HSP90 confers stability to the GR, the reduced association of the L687-690A mutant to HSP90 shown by the co-immunoprecipitation raises the possibility that an increase in receptor degradation contributes to the loss in receptor function. However, the similar protein expression for the different constructs shown by western blot and immunofluorescence indicates that receptor protein stability is not majorly affected. Interaction of GR with HSP90 involves helix 1 of the ligand binding domain (Xu et al. 1998) and the mutations in helix 8 could destabilize the receptor association to HSP90, impairing complete immunoprecipitation, but still being capable to protect the receptor from degradation. Although not evaluated in the present study, it is possible that the leucine repeat mutation also affect the receptor association to other chaperone and co-chaperone proteins. Further analysis of individual leucines in the repeat indicated that each one contributes to the GR sensitivity to glucocorticoids, and that they have a synergist action in determining GR activity. However, the fact that mutation of other conserved amino acids within the region also affected nuclear translocation of GR indicates a broader participation of this region on receptor activity. Interestingly, mutations of leucine 690 alone, as well as serine 692, which are outside helix 8, have no effect on GR translocation but single mutations within the helix had marked effects.
Since the present amino acid mutations resulting in loss of function of GR are within the ligand binding domain of the GR, we sought the possibility that the mechanism leading to loss of function involves impaired ability of the receptor to bind glucocorticoids. A number of studies have demonstrated that amino acid mutations within the carboxy terminus result in altered ligand binding. For example, mutation of the 532–536 LXXLL motif of the human GR to LXXAA suppresses dexamethasone induced transcription and ligand binding, while the 718–722 LXXAA mutant is fully active at high ligand concentrations (Dong et al. 2006). A number of additional mutations in the ligand binding domain have been shown to decrease or suppress ligand binding affinity (Chakraborti et al. 1991; Garabedian & Yamamoto 1992; Schmitt & Stunnenberg 1993; Lanz et al. 1994), for review see (Simons 1994). On the other hand, mutant human GR with C656G or C656S have higher affinities than the wild type receptors (Chakraborti et al. 1991).
The present studies show a good correlation between corticosterone-induced nuclear translocation and [3H]-dexamethasone binding for the individual mutants, indicating that the loss in receptor function is due to impaired ligand binding. Although the amounts of protein available did not allow performing full binding curves for all mutants, the increases in binding with increasing ligand concentrations for L687A and double leucine mutants indicate that the mutations cause a decrease in binding affinity. Consistent with the inability to undergo nuclear translocation, mutation of the leucine repeat or single mutations of glutamic acid 680, leucine 682, and lysine 685, all within helix 8, completely disrupted ligand binding. Since the binding data were corrected by GR protein expression after transfection, it is unlikely that receptor stability plays a role on the altered ligand binding. The mechanism by which mutations within helix 8 abrogate ligand binding is not clear, since none of these residues have been shown to interact with the ligand and helix 8 is not part of the binding pocket (Dey et al. 2001; Bledsoe et al. 2002). Thus, it is likely that helix 8 region of the GR containing the leucine repeat is important for maintaining the receptor conformation allowing interaction of the ligand with the binding pocket. In this regard, the LXXLL motifs in helixes 1 and 10 shown to affect GR binding affinity for the ligand are also outside the binding pocket (Dong et al. 2006). A recognized factor determining structural stability of the binding pocket and ligand affinity is the interaction of the GR with HSP90 (Morishima et al. 2000; Fuller et al. 2004; Nettles et al. 2004). Not all mutations were tested for HSP90 interaction but there was a marked reduction of HSP90 co-imunoprecipitated with GR in cells transfected with the L687-690A mutant. Therefore, it is possible that the disrupted GR/HSP90 interaction plays a role in the altered binding affinity of the mutants.
5. CONCLUSIONS
The study demonstrates that the glucocorticoid receptor does not undergo palmitoylation and that C683 located in a conserved region of the ligand binding domain, implicated in membrane localization of the ER, does not influence membrane association or biological activity of the GR. Although the mechanisms for membrane association of the GR remain to be elucidated, the study provides evidence that the leucine repeat 687 to 690 and selected residues within helix 8 of the ligand binding domain are critical for ligand binding, and in consequence receptor function.
Highlights.
Helix 8 in LBD of GR matches conserved region mediating membrane association of ER
Unlike the ER, cysteine 683 in helix 8 of rat GR does not undergo palmitoylation
The leucine repeat and selected amino acids in Helix 8 of the LBD are critical for ligand binding
Acknowledgments
The authors are indebted to Dr Stoney Simons, NIDDK, NIH, for helpful discussions and for providing reagents. We also thank Dr Jennifer Lippincott-Schwartz, Section on Section on Organelle Biology, NICHD, for her advice and support for the imaging work. The work was funded by the Intramural Research Program of the National Institute of Child Health and Human Development, and the China Scholarship Council, China.
Abbreviations
- GR
glucocorticoid receptor
- ER
estrogen receptor
- LBD
ligand binding domain
- EGFP
enhanced green fluorescent protein
- DMEM
Dulbecco’s Modified Eagle medium
- TBST
Tris-Buffered Saline/0.1% Tween 20
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- BSA
bovine serum albumin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–7. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe RK, Montana VG, Stanley TB, Delves CJ, Apolito CJ, McKee DD, Consler TG, Parks DJ, Stewart EL, Willson TM, Lambert MH, Moore JT, Pearce KH, Xu HE. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell. 2002;110:93–105. doi: 10.1016/s0092-8674(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Chakraborti PK, Garabedian MJ, Yamamoto KR, Simons SS., Jr Creation of “super” glucocorticoid receptors by point mutations in the steroid binding domain. J Biol Chem. 1991;266:22075–8. [PubMed] [Google Scholar]
- Dean DA, Urban G, Aragon IV, Swingle M, Miller B, Rusconi S, Bueno M, Dean NM, Honkanen RE. Serine/threonine protein phosphatase 5 (PP5) participates in the regulation of glucocorticoid receptor nucleocytoplasmic shuttling. BMC Cell Biol. 2001;2:6. doi: 10.1186/1471-2121-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Aguilera G. Rapid Glucocorticoid Feedback Inhibition of ACTH Secretion Involves Ligand-Dependent Membrane Association of Glucocorticoid Receptors and Inhibition of Src Phosphorylation. In. The Endocrine Society 96th Annual Meeting.; Chicago. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey R, Roychowdhury P, Mukherjee C. Homology modelling of the ligand-binding domain of glucocorticoid receptor: binding site interactions with cortisol and corticosterone. Protein Eng. 2001;14:565–71. doi: 10.1093/protein/14.8.565. [DOI] [PubMed] [Google Scholar]
- Dong DD, Jewell CM, Bienstock RJ, Cidlowski JA. Functional analysis of the LXXLL motifs of the human glucocorticoid receptor: association with altered ligand affinity. J Steroid Biochem Mol Biol. 2006;101:106–17. doi: 10.1016/j.jsbmb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Fuller PJ, Smith BJ, Rogerson FM. Cortisol resistance in the New World revisited. Trends Endocrinol Metab. 2004;15:296–9. doi: 10.1016/j.tem.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Gametchu B, Chen F, Sackey F, Powell C, Watson CS. Plasma membrane-resident glucocorticoid receptors in rodent lymphoma and human leukemia models. Steroids. 1999;64:107–19. doi: 10.1016/s0039-128x(98)00097-x. [DOI] [PubMed] [Google Scholar]
- Garabedian MJ, Yamamoto KR. Genetic dissection of the signaling domain of a mammalian steroid receptor in yeast. Mol Biol Cell. 1992;3:1245–57. doi: 10.1091/mbc.3.11.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoock TC, Newcomb PM, Herman IM. Beta actin and its mRNA are localized at the plasma membrane and the regions of moving cytoplasm during the cellular response to injury. J Cell Biol. 1991;112:653–64. doi: 10.1083/jcb.112.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EV. The contribution of “alternative approaches” to understanding steroid hormone action. Mol Endocrinol. 2005;19:1439–42. doi: 10.1210/me.2005-0154. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136:289–99. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Komatsuzaki Y, Murakami G, Tsurugizawa T, Mukai H, Tanabe N, Mitsuhashi K, Kawata M, Kimoto T, Ooishi Y, Kawato S. Rapid spinogenesis of pyramidal neurons induced by activation of glucocorticoid receptors in adult male rat hippocampus. Biochem Biophys Res Commun. 2005;335:1002–7. doi: 10.1016/j.bbrc.2005.07.173. [DOI] [PubMed] [Google Scholar]
- Lanz RB, Hug M, Gola M, Tallone T, Wieland S, Rusconi S. Active, interactive, and inactive steroid receptor mutants. Steroids. 1994;59:148–52. doi: 10.1016/0039-128x(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Bohn MC. Association of glucocorticoid receptor immunoreactivity with cell membrane and transport vesicles in hippocampal and hypothalamic neurons of the rat. J Neurosci Res. 1993;35:14–9. doi: 10.1002/jnr.490350103. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kamitakahara A, Kim AJ, Aguilera G. Cyclic adenosine 3′,5′-monophosphate responsive element binding protein phosphorylation is required but not sufficient for activation of corticotropin-releasing hormone transcription. Endocrinology. 2008;149:3512–20. doi: 10.1210/en.2008-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, Wehling M. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- Marino M, Ascenzi P, Acconcia F. S-palmitoylation modulates estrogen receptor alpha localization and functions. Steroids. 2006;71:298–303. doi: 10.1016/j.steroids.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Murphy PJ, Li DP, Sanchez ER, Pratt WB. Stepwise assembly of a glucocorticoid receptor.hsp90 heterocomplex resolves two sequential ATP-dependent events involving first hsp70 and then hsp90 in opening of the steroid binding pocket. J Biol Chem. 2000;275:18054–60. doi: 10.1074/jbc.M000434200. [DOI] [PubMed] [Google Scholar]
- Nettles KW, Sun J, Radek JT, Sheng S, Rodriguez AL, Katzenellenbogen JA, Katzenellenbogen BS, Greene GL. Allosteric control of ligand selectivity between estrogen receptors alpha and beta: implications for other nuclear receptors. Mol Cell. 2004;13:317–27. doi: 10.1016/s1097-2765(04)00054-1. [DOI] [PubMed] [Google Scholar]
- Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- O’Malley BW. A life-long search for the molecular pathways of steroid hormone action. Mol Endocrinol. 2005;19:1402–11. doi: 10.1210/me.2004-0480. [DOI] [PubMed] [Google Scholar]
- Orchinik M, Murray TF, Moore FL. A corticosteroid receptor in neuronal membranes. Science. 1991;252:1848–51. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–88. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- Raje CI, Kumar S, Harle A, Nanda JS, Raje M. The macrophage cell surface glyceraldehyde-3-phosphate dehydrogenase is a novel transferrin receptor. J Biol Chem. 2007;282:3252–61. doi: 10.1074/jbc.M608328200. [DOI] [PubMed] [Google Scholar]
- Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X. CSS-Palm 2.0: an updated software for palmitoylation sites prediction. Protein Eng Des Sel. 2008;21:639–44. doi: 10.1093/protein/gzn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci. 2010;30:5037–46. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarasinghe RA, Di Maio R, Volonte D, Galbiati F, Lewis M, Romero G, DeFranco DB. Nongenomic glucocorticoid receptor action regulates gap junction intercellular communication and neural progenitor cell proliferation. Proc Natl Acad Sci U S A. 2011;108:16657–62. doi: 10.1073/pnas.1102821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J, Stunnenberg HG. The glucocorticoid receptor hormone binding domain mediates transcriptional activation in vitro in the absence of ligand. Nucleic Acids Res. 1993;21:2673–81. doi: 10.1093/nar/21.11.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons SS., Jr Function/activity of specific amino acids in glucocorticoid receptors. Vitam Horm. 1994;49:49–130. doi: 10.1016/s0083-6729(08)61146-2. [DOI] [PubMed] [Google Scholar]
- Song IH, Buttgereit F. Non-genomic glucocorticoid effects to provide the basis for new drug developments. Mol Cell Endocrinol. 2006;246:142–6. doi: 10.1016/j.mce.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Stojadinovic O, Sawaya A, Pastar I, Tomic-Canic M. Glucocorticoid receptor localizes to adherens junctions at the plasma membrane of keratinocytes. PLoS One. 2013;8:e63453. doi: 10.1371/journal.pone.0063453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevyver S, Dejager L, Tuckermann J, Libert C. New insights into the anti-inflammatory mechanisms of glucocorticoids: an emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology. 2013;154:993–1007. doi: 10.1210/en.2012-2045. [DOI] [PubMed] [Google Scholar]
- Watson CS, Gametchu B. Proteins of multiple classes may participate in nongenomic steroid actions. Exp Biol Med (Maywood) 2003;228:1272–81. doi: 10.1177/153537020322801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Dittmar KD, Giannoukos G, Pratt WB, Simons SS., Jr Binding of hsp90 to the glucocorticoid receptor requires a specific 7-amino acid sequence at the amino terminus of the hormone-binding domain. J Biol Chem. 1998;273:13918–24. doi: 10.1074/jbc.273.22.13918. [DOI] [PubMed] [Google Scholar]
- Zanchi NE, Filho MA, Felitti V, Nicastro H, Lorenzeti FM, Lancha AH., Jr Glucocorticoids: extensive physiological actions modulated through multiple mechanisms of gene regulation. J Cell Physiol. 2010;224:311–5. doi: 10.1002/jcp.22141. [DOI] [PubMed] [Google Scholar]