Abstract
The membrane-associated protein, focal adhesion kinase (FAK), modulates cell-extracellular matrix interactions and also conveys pro-survival and proliferative signals. Notably, increased intraepithelial FAK levels accompany transformation of premalignant oral intraepithelial neoplasia (OIN) to oral squamous cell carcinoma (OSCC). OIN chemoprevention is a patient-centric, optimal strategy to prevent OSCC’s co-morbidities and mortality. The cancer chemopreventive and synthetic vitamin A derivative, fenretinide, has demonstrated protein-binding capacities e.g. mTOR and retinol binding protein interactions. These studies employed a continuum of human oral keratinocytes (normal-HPV E6/E7-transduced-OSCC) to assess potential fenretinide-FAK drug protein interactions and functional consequences on cellular growth regulation and motility. Molecular modeling studies demonstrated fenretinide has ~200-fold greater binding affinity relative to the natural ligand (ATP) at FAK’s kinase domain. Fenretinide also shows intermediate binding at FAK’s FERM domain and interacts at the ATP-binding site of the closest FAK analogue, Pyk2. Fenretinide significantly suppressed proliferation via induction of apoptosis and G2/M cell cycle blockade. Fenretinide-treated cells also demonstrated F-actin disruption, significant inhibition of both directed migration and invasion of a synthetic basement membrane, and decreased phosphorylation of growth-promoting kinases. A commercially available FAK inhibitor did not suppress cell invasion. Notably, while FAK’s FERM domain directs cell invasion, FAK inhibitors target the kinase domain. In addition, FAK-specific siRNA treated cells showed an intermediate cell migration capacity; data which suggest co-contribution of the established migrating-enhancing Pyk2. Our data imply that fenretinide is uniquely capable of disrupting FAK’s and Pyk2’s pro-survival and mobility-enhancing effects and further extend fenretinide’s chemopreventive contributions beyond induction of apoptosis and differentiation.
Keywords: Fenretinide, chemoprevention, oral squamous cell carcinoma, oral intraepithelial neoplasia, focal adhesion kinase
Introduction
Focal adhesion kinase (FAK) was originally identified as a Src oncogene substrate [1]. FAK is now known to also be activated by the SRC family kinases i.e. PLC, SOCS, GRB7, PI3K as well as bioactivated lipids such as lysophosphatidic acid [2]. In its mechanosensor capacity, FAK mediates cytoskeletal adaptations in response to extracellular matrix (ECM) interactions, regulates formation of cell membrane protrusions e.g. actin and matrix metalloproteinase-rich, extracellular matrix-degrading invadopodia and ultimately directs cell migration and invasion [1]. In a related role, FAK’s functions also extend to translocation of lipid raft components to the leading edge of motile cells thereby enabling the microtubule-cortical receptor stabilization that is essential for directed cell movement [1]. Furthermore, via its FERM domain, the membrane-spanning protein FAK serves as a chemosensor that links membrane-bound growth factor receptors such as EGFR and PDGFR, provides receptor cross-talk and ultimately signal transduction to the nucleus [3]. FAK’s FERM domain also directs FAK nuclear translocation enabling FAK-mediated p53 degradation and resultant increased cell survival and proliferation [4]. These abilities to promote cell survival/proliferation/angiogenesis while concurrently modulating ECM interactions and assisting invadopodia formation, make FAK an attractive cancer prevention therapeutic target [1, 5]. Notably, premalignant lesions that arise at visibly accessible sites, such as the mouth, are particularly well-suited for chemoprevention as treatment effects can be directly monitored.
Oral squamous cell carcinoma (OSCC) is a worldwide health problem that conveys significant socioeconomic impact [6]. Analogous to other surface origin cancers, OSCCs arise from malignant transformation of a precursor lesion i.e. oral intraepithelial neoplasia (OIN i.e. a white, red or mixed adherent lesion that possesses microscopically confirmed cytologic and maturational perturbations superior to the basement membrane). The poor prognosis of higher stage OSCCs, co-morbidities associated with vital tissue loss during surgical treatment, and visually accessible premalignant lesions combine to make chemoprevention the optimal OSCC treatment strategy [7].
Vitamin A and its derivatives have been regarded as promising OSCC chemopreventive agents for many years [8]. More recently, the synthetic analogue of all-trans retinoic acid, fenretinide (4-HPR), gained attention due to its reduced toxicity profile and its strong proapoptotic and prodifferentiation effects [9, 10, 11]. Additional studies demonstrated that 4-HPR disrupted cytoskeletal networks and suppressed migration of Kaposi’s sarcoma, ovarian cancer, and endothelial cells [12, 13]. Another investigation showed 4-HPR inhibited directed migration and invasion of prostate cancer cells; findings speculated by the investigators to reflect disruption of the FAK/AKT/GSK3β pathway and β-catenin stability [14].
This study investigated a spectrum of 4-HPR-FAK interactions including drug-protein interactions and functional consequences of these interactions on cellular growth state and motility. The final series of experiments introduced an additional chemopreventive shown in be clinically effective in OIN lesions i.e. freeze dried black raspberries (BRB) [15]. Concurrent 4-HPR + BRB administration provided additive invasion-inhibitory effects.
Materials and Methods
Cell Culture
OSCC cell lines CRL-2095, SCC-15 (American Type Cell Culture, human tongue primary tumor) and JSCC-1, JSCC-2 and JSCC-3 derived from human OSCC tumors of tonsil (JSCC-1), tongue (JSCC-2) and floor of mouth (JSCC3), a normal oral keratinocyte cell strain [ScienCell, Carlsbad, CA] HOK3437, and two immortalized cell lines [HPV E6/E7 transduced normal oral keratinocytes (HOK3437 E6/E7) and ethanol-treated HPV E6/E7-transduced normal oral keratinocytes (EPI)]. [16]. were used. All immortalized cells were cultured in Advanced DMEM supplemented with 1X Glutamax and 5% heat-inactivated fetal bovine serum (GIBCO, Life Technologies Grand Island, NY, “Complete Medium”) while normal oral keratinocytes were cultured in Keratinocyte Sera Free Medium + supplements (Gibco). Cells were cultured in a sera or growth factor free “Base” medium for chemoattractant-based experiments. Cell lines were authenticated by genomic analyses conducted by John Hopkins’ Genetic Resources Core Facility. With the exception of the HOK3437 E6/E7 strain and the EPI cell line (both transduced with HPV16 E6 and E7) cell lines were negative for HPV, as determined by PCR.
Cell Line Characterization
Formalin fixed cells were incubated with vimentin (1:200, Abcam, Cambridge, MA) or a pancytokeratin cocktail (AE1/AE3 + 5D3, 1:100, Abcam,) antibodies, followed by incubation with FITC or Texas Red conjugated secondary antibodies (Abcam, Cambridge, MA) [17]. Nuclei were stained with 4′,6′-Diaminidino-2-phenylindole dihydrochloride (DAPI, Abcam). Fluorescence microscopy images were obtained by using an Olympus BX51 microscope (Olympus, Japan), NikonDS-Fi1 digital camera (Nikon, Japan) and ImagePro 6.0 (Media-Cybernetics, Bethesda, MD). Immunoblot analyses were conducted to determine presence or absence of 4-HPR metabolizing enzymes (CYPs 3A4, 2C8, 26A1) and UDP glucuronosyl transferase 1A1 (UGT1A1) in accordance with our previously published method [18]. Additional characterization studies entailed a time-course assessment of intracellular levels of 4-HPR during 4-HPR treatment with concurrent 4-HPR medium evaluation using LC-MS/MS analyses as previously described [11].
4-HPR’s induction of the execution phase of apoptosis
Cultured cells were treated with 1, 5, 10uM 4-HPR (0.1% DMSO, control cells received DMSO only) for 24 hours. Functional caspase 3 and 7 activities were determined by Caspase-Glo® 3/7 Assay (Promega, Madison, WI) according to the manufacturer’s protocol. Concurrent studies evaluated the effects of 4-HPR treatment on cell proliferation (CyQuant Assay, Invitrogen, Carlsbad, CA). Complementary FACS analyses, which used propridum iodide labelled DNA, were conducted to identify cell cycle distribution during 4-HPR challenge.
Immunocytochemical characterization of 4-HPR’s effects on F-actin and microtubules
Adherent cells were wounded with a sterile pipette tip, washed with PBS, followed by 5uM or 10uM 4-HPR for 24 hours in Complete Medium. Post-treatment, cells were extracted with 0.1% Triton X-100/PBS and fixed with 4% paraformaldehyde, permeabilized, blocked, and probed with Alexa Fluoro 488-conjugated phalloidin (Invitrogen, Carlsbad, CA) and DAPI (Vector Laboratories, Inc, Burlingame, CA). For co-localization studies, cells were first incubated with anti-tubulin antibody (1:500, Abcam, Cambridge, MA) and its Texas Red conjugated secondary antibody (1:1000, Abcam) for 1 hour at room temperature, and subsequently incubated with phalloidin and DAPI. Fluorescence microscopy images were obtained by using an Apotome Fluorescence Microscope (Carl Zeiss), and AxioVision software (Carl Zeiss).
Molecular Modeling of 4-HPR-FAK Interactions
Molecular modeling studies were conducted using AutoDock Vina software [19]. Initial 4-HPR binding studies used retinol binding protein (1BRP) [20] as a model binding protein.
A number of crystal structures exist for ligands bound to the FAK region of the protein. 2J0L was obtained and used for the AutoDock Vina binding study as it had the most complete structure (least disorder). An initial survey of the entire FAK protein surface for all ligands (retinol, ATP, an ATP-Mg complex, fenretinide) and ligands from the 2ETM and 2JKK crystal structures, as well as known agonists (PF228, TAE-226 and A18) revealed that all ligands could bind at the kinase domain ATP binding site. The calculations were then rerun to focus on the kinase (ATP) binding site while allowing for flexible amino acid side chains at the binding site (GLY 431, GLN 432, VAL 436, LYS 454, GLU 500, LEU 501, CYS 502, GLU 506, LEU 553, ASP 564 and LYS 583). Use of the flexible amino acid side chains resulted in marked improvement in calculated binding energies.
Modeling studies were also conducted to evaluate 4-HPR-FERM domain interactions. FAK’s FERM domain structure was acquired from the Protein Data bank (2AEH) [20]. All ligands were minimized using MMFF in Spartan 10 [21] while the protein structure was optimized via the default minimization protocol in Yasara [22]. Each ligand was run three times on a global search for the entire protein structure. 4-HPR interactions with the Fak family enzyme, protein tyrosine kinase 2 (PYK2) were also assessed. Analyses were conducted at the “closed” DFG and “DFG out” configurations which used 3FZR and 3FZT, respectively. All AutoDock Vina calculations were again repeated three times with an “exhaustiveness” of 100.
Assessment of 4-HPR’s effects on cell migration
Three complementary migration assays were used to assess 4-HPR’s effects on the diverse aspects of directed cell migration.
Scratch wound assay
Confluent cells were wounded by gently scratching the well surface with a sterile, cotton-tipped applicator, washed with PBS and treated with 1uM or 5uM 4-HPR for 24, 48, or 72 hours in Complete Medium with freshly prepared treatment supplied every 24 hours. At the end of each treatment period, three pictures were obtained for each well (left, middle, right) by using an Apotome Fluorescence Microscope (Carl Zeiss, Dublin, CA), and AxioVision software (Carl Zeiss, Dublin, CA). Immediately following the image capture, cell viability and proliferation were determined by using a hemocytometer. Quantitative image analysis of wound closure/cell migration was performed by utilizing ImagePro software (Media Cybernetics, Inc., Rockville, MD). Cells with a high migration rate (EPI) underwent FAK siRNA (5-AGCCAGUGAACCUCCUCUGACCGCAGG-3) (Integrated DNA Technologies Inc) treatment in accordance with standard procedures, with confirmation by immunoblotting [23].
Cell-free Zone Exclusion Assay
The cell-free zone exclusion assay was conducted using the Oris Cell Migration assay (Platypus Technologies, Madison, WI). Briefly, following gel plug removal, cells were treated with freshly prepared 1, 5, or 10uM 4-HPR (0.1% DMSO) or 0.1% DMSO, no 4-HPR (control) for 24 and 48 hours. Cells were stained with 0.5ug/mL Calcein AM in 1X PBS (Molecular Probes-Life Technologies, Grand Island, NY) for 30 minutes, followed by flurostar microplate reader (485nmEx/528nmEm) analyses.
Chemoattractant-initiated Transwell migration assays
96-well plates and 8μm pore membrane inserts were purchased from Trevigen (Gaithersburg, MD). JSCC-3 conditioned medium was determined to be the optimal chemoattactant relative to complete medium, or conditioned media from JSCC-1, JSCC-2 or 2095sc cells. 24 hour sera-starved cells were seeded into the top chamber with vehicle (0.1% DMSO), 1μM 4-HPR, or 5μM 4-HPR and were incubated for 16 hours. The bottom chamber contained either (1) sterile-filtered, conditioned JSCC-3 media or (2) base medium. Formalin fixed cells were stained with 0.1% v/v crystal violet solution, followed by removal of cells remaining in top chamber. Nikon DS-Ri1 using NIS Elements (Nikon, Melville, NY) was used to capture images, followed by target pixelation analyses by image segmentation [ImagePro software (Media Cybernetics, Inc., Rockville, MD)].
Assessment of 4-HPR, FAK Inhibitor II and freeze dried black raspberries’ effects on OSCC invasion of a synthetic basement membrane comprised of collagen type IV
Preliminary studies determined that only the JSCC-1, JSCC-2, EPI and SCC2095 cell lines successfully invaded the collagen type IV layer and the optimal chemoattractant was JSCC-3 conditioned medium. Fifty thousand 24-hour sera-starved cells/well were seeded onto type IV collagen-coated microporous polyester membrane (InnoCyte cell invasion kit, Calbiochem, San Diego, CA) with treatments (0, 5 μM 4-HPR, FAK II inhibitor (Calbiochem, San Diego, CA., CAS 869288-64-2, 500 nM and 2.5 μM), or freeze dried black raspberries (10 μM cyanidin 3-rutinoside equivalent in base medium [15]. After 16 hours of invasion (37°C, 5% CO2), cells were fixed and analyzed as described in migration assay.
Evaluation of treatment effects on phosphorylation status of proproliferative intracellular kinases
Cell lines with the greatest invasive capacities i.e. JSCC-2, EPI and 2095sc cells were pre-treated in sera-free media for 24 hours prior to 24-hour treatment in JSCC-3 conditioned medium. Experimental groups were: (1) Vehicle (0.1% DMSO, determined to have no deleterious effects on cell viabilities), (2) 5uM 4-HPR, (3) BRB (10uM cyanidin rutinoside equivalent), and (4) 4-HPR and BRB. Cells were harvested and analyzed in accordance with instructions (R & D Systems, Minneapolis, MN. Phospho-MAPK array kit #ARY002B) was used to extract proteins, which were quantified by a BCA assay (Pierce, Rockford, IL.). Equivalent input proteins [BCA assay, (Pierce, Rockford, IL.)] were incubated, images obtained with the Li-Cor Odyssey imager (Li-Cor Biosciences, Lincoln, NE) and analyzed by ImagePro software (Media Cybernetics, Inc., Rockville, MD).
Statistical analyses
Initial analyses confirmed that all data sets demonstrated a Gaussian distribution. A one-way ANOVA followed by Bonferroni’s multiple comparisons post hoc test was used to assess 4-HPR’s effects on caspase 3/7 activation and accompanying FACS analyses, and also to determine the effects of 4-HPR, BRB, or combined treatments on cell invasion. 4-HPR’s effects on cell migration in the cell-free zone exclusion assay and the scratch wound assay were evaluated by the two-way ANOVA followed by Bonferroni’s multiple comparisons post hoc test.
Results
Cell lines co-express cytokeratin and vimentin and possess 4-HPR metabolizing enzymes
Similar to our previous ATCC OSCC cell characterization studies [17], JSCC1, JSCC2 and JSCC3 cell cultures uniformly demonstrated strong cytokeratin staining along with coexpression of cytokeratin and vimentin in cellular subpopulations (Supplemental Figure S1).
Time course cell-4-HPR incubation studies revealed intracellular 4-HPR levels were higher than media levels during both the single and multiple dosing experiments (Supplemental Table S1).
Furthermore, two of the three enzymes responsible for oxidative bioactivation of 4-HPR to 4-oxo-HPR i.e. cytochrome P450 (CYP) CYP3A4 and CYP26A1 were present in all the cell lines evaluated i.e. EPI, 2095sc, JSCC1, JSCC2, and JSCC3 cell lines. CYP2C8 and the Phase II enzyme capable of 4-HPR glucuronidation (UGT1A1) were not present.
4-HPR treatment activated caspases 3 and 7 and perturbed F-actin organization
4-HPR treatment activated caspases 3 and 7 in a dose-dependent fashion in 6 of the 8 evaluated cell lines. While 1 μM 4-HPR significantly increased caspase activity in the HOK3437E6/E7, JSCC1, and SCC15 cell lines, the HOK3437, EPI, and JSCC-2 cells only showed caspase induction with higher (5 μM) 4-HPR treatment (Figure 1.A.). The JSCC3 and 2095sc cells were refractory to 4-HPR mediated caspase activation. Cell viabilities were comparable in all treatment groups. Corresponding FACS analyses, conducted in caspase-responsive (EPI) and caspase-refractory (2095sc) cell lines revealed increases in the sub-G1 (EPI) and G2/M (EPI and 2095sc) cell populations, respectively, during 4-HPR treatment (5 μM, 24 h treatment) (Figure 1.B). Somewhat paradoxically, the 2095sc cells showed a proapoptotic DNA profile with the lower 1 μM 4-HPR dose (Figure 1.B.).
Figure 1. Activation of caspase 3/7 and cell cycle modulations by 4-HPR.
(A) 4HPR induced activation of the execution phase apoptotic enzymes, caspase 3/7, in HOK3437 (a), HOK3437 E6/E7 (b), EPI (c), JSCC1 (d), and JSCC2 (e), and SCC15 (g) cell lines. JSCC-3 (f) and SCC2095sc (h) did not show caspase 3/7 activation during treatment with any of the 4-HPR doses. Cells were seeded at 1×105/well in 96 well plates and treated in serum-free media for 24 hours before measurement. Data are represented as means ± SEM of 7 replicates (c,d,e,f) or of 4 replicates (a,b,g,h). Asterisks indicate a significant difference from cell line matched vehicle control (B) FACS analyses demonstrated 4-HPR treatment perturbed cell cycle kinetics by increasing sub-G1 and G2/M DNA distribution in both a caspase induced (EPI) and caspase refractory (2095sc) cell lines (n=2). (*p<0.05, **p<0.01, ****p<0.0001).
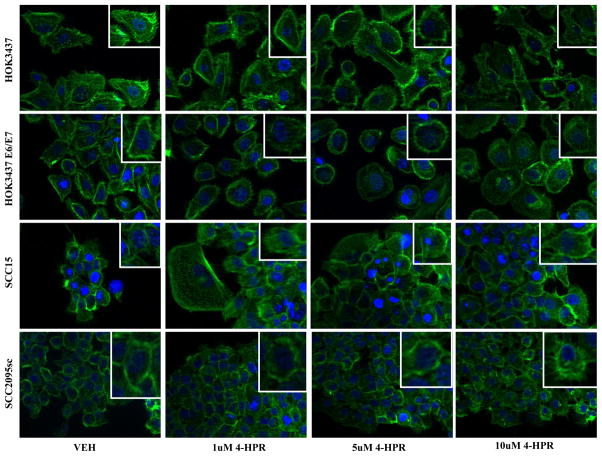
4-HPR treatment also elicited distinct qualitative effects. 4-HPR challenge disrupted actin filament polymerization and intercellular adhesion as shown by loss of cellular polarity and dissipation of F-actin-cell membrane interactions (Figure 2).
Figure 2. 4-HPR disrupts actin cytoskeleton organization.
Actin filaments (F-actin) were labeled with a rhodamine fluorescent probe and visualized under 400X image scale via fluorescent microscopy. 4-HPR-treated samples showed (1) loss of cellular polarity, (2) dissipation of cortical actin networks, and (3) loosening of intercellular junctions. HOK3437, HOK3437 E6/E7 and SCC2095sc all exhibited 4-HPR’s dose-escalating effects manifesting as cytoskeletal rearrangement and/or destabilization of actin filaments. SCC15 appeared to be largely unaffected by 4-HPR. A DAPI counterstain identifies the nuclei.
4-HPR interacts with FAK’s kinase and FERM domains and also FAK’s closest homologue, Pyk2
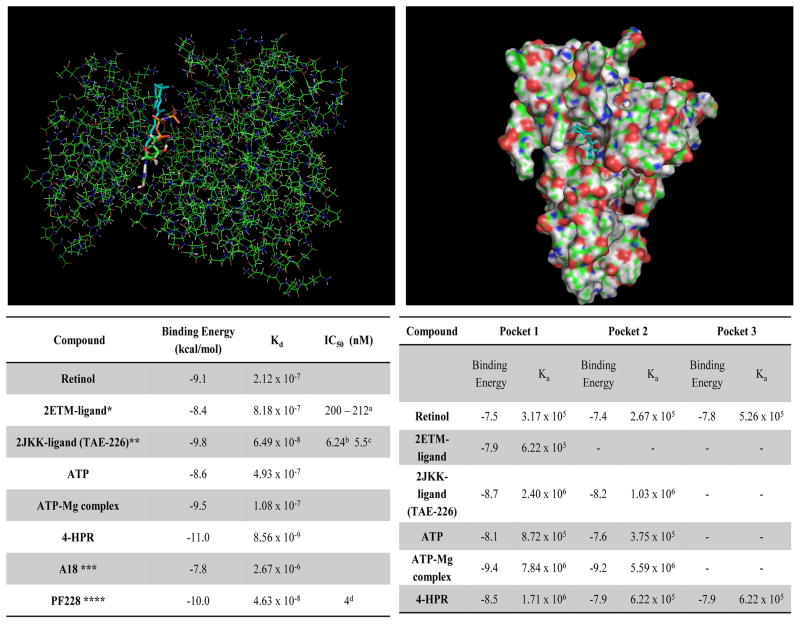
4-HPR demonstrates the highest binding affinity of all ligands at FAK’s kinase ATP-binding site (Figure 3.A.). While a direct comparison between the binding affinity and an IC50 is not possible, these data imply that 4-HPR has a lower IC50 than any of the other compounds including the natural ligand ATP (See Figure 3.A.).
Figure 3. 4-HPR interacts with FAK’s kinase and FERM domains.
Molecular modeling studies were conducted using AutoDock Vina software [19]. Initial 4-HPR binding studies used retinol binding protein as a model binding protein. (A) Molecular modeling image depicting 4-HPR (blue and white) interacting with the FAK kinase ATP binding site (orange, green and red). The accompanying table compares ligand binding affinities for the kinase domain of FAK. [* 7-PYRIDIN-2-YL-N-(3,4,5-TRIMETHOXYPHENYL)- 7H-PYRROLO[2,3-D]PYRIMIDIN-2-AMINE; ** 2-({5-CHLORO-2-[(2-METHOXY-4-MORPHOLIN-4- YLPHENYL)AMINO]PYRIMIDIN-4-YL}AMINO)-N-METHYLBENZAMIDE; *** 1,4-bis(diethylamino)-5,8-dihydroxyanthraquinone; **** 6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluoromethyl)pyrimidin-2-ylamino-3,4-dihydroquinolin-2(1H)-one] (B) 4-HPR (blue and white) depicted binding in FERM domain’s pocket 1. Ligand binding affinities at FAK’s FERM domain pockets are listed in the table below.
- The Protein Data Bank: http://www.rcsb.org/pdb/home/home.do: 2ETM
- The Protein Data Bank: http://www.rcsb.org/pdb/home/home.do: 2JKK
- Shi, Q, et al. Molecular Carcinogenesis, 46: 488-96 (2007)
- Slack-Davis, J.K. et al J. Biol. Chem. 2007, 282: 14845-52. doi: 10.1074/jbc.M606695200
Three distinct binding pockets are located in the FERM domain. Pocket 1 is in a deep cleft between the F1, F2 and F3 domains of FERM, pocket 2 is in a deep cleft in the F2 domain and pocket 3 is on the surface on the “backside” to the other two pockets and spans F1 and F2 (Figure 1.B.). 4-HPR shows an intermediate binding affinity with pockets 1 and 2 (3rd of 6 and 3rd of 5, respectively) relative to the other ligands evaluated (Figure 3.B.). Retinol and 4-HPR were the exclusive ligands capable of binding in FERM pocket 3 and 4-HPR demonstrated a slightly higher binding affinity. ATP binding at the FERM domain was restricted to pockets 1 and 2, while 2ETM binds only in pocket 1 (Figure 3.B.).
Pyk2 modeling studies evaluated 4-HPR’s interactions with its kinase catalytic site using the “closed” DFG (3FZR) and “DFG out” (3FZT) configurations (Supplemental Table S2). All compounds were determined to bind with a higher affinity to the DFG out conformer (3FZT). Results indicated that 4-HPR binds to both conformers of Pyk2 with affinities comparable to recognized Pyk2 inhibitors such as PF-4618433 (Supplemental Table S2).
4-HPR significantly inhibits cell migration
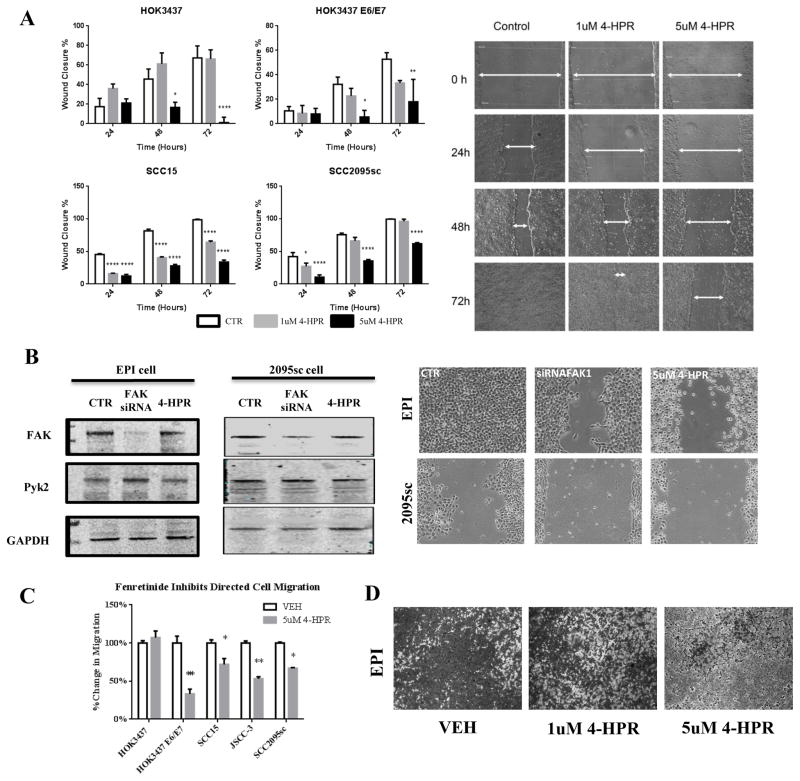
4-HPR inhibited scratch wound healing in a cell line, dose and time dependent fashion (Figure 4.A). 5μM 4-HPR significantly suppressed both SCC15 and SCC2095sc cell line migration (p<0.0001, n=6, at 24, 48 and 72 h time points). In contrast, cell migration in normal oral keratinocytes (HOK3437) and the transduced HOK3437E6/E7 cells was only significantly affected at the 48 and 72 h time point when using the 5μM 4-HPR dose (p<0.0001, n=6). Scratch wound cell viabilities were comparable among all cell lines and treatment groups at every time point. Furthermore, FAK-targeted siRNA treated cells demonstrated wound healing that was intermediate between control and 4-HPR treated cultures (Figure 4.B.). Corresponding Western immunoblotting confirmed FAK siRNA treatment reduced endogenous cellular FAK levels while Pyk2 protein levels remained unchanged or slightly increased. Also apparent was a distinct transition in cellular morphology from a flattened shape to a more rounded, less adherent phenotype in 4-HPR-treated cultures (Figure 4.B.).
Figure 4. Evaluation of 4-HPR’s effects on directed cell migration.
(A) HOK3437, HOK3437 E6/E7, SCC15, and SCC2095sc cells were treated with 1 and 5uM 4-HPR over 72 hours with fresh drug and media replenished every 24 hours. 4-HPR significantly attenuated directed cell migration in a dose- and time-dependent manner. (n=6). (B) FAK-targeted siRNA treated cells showed an intermediate cell migration capacity between control and 4-HPR-treated samples. Western blot analyses demonstrate decreased levels of FAK protein in EPI and SCC2095sc cell after FAK siRNA transfection while Pyk2 levels remained constant or were slightly higher in the FAK siRNA-treated samples. (C) Normal, HPV E6/E7 immortalized, and oral squamous cell carcinoma cell lines were treated with 5uM 4-HPR for 24 hours in a cell-free zone exclusion cell migration assay. While 4-HPR significantly inhibited cell migration in HOK3437 E6/E7 (n=8), SCC15 (n=8), JSCC-3 (n=7), and SCC2095sc (n=7), no anti-migratory effects were noted on normal HOK3437 cells. (*p<0.05, **p<0.01, ****p<0.0001) (D) Directed migration of EPI cells was evaluated by using Boyden chambers. 1uM and 5uM 4-HPR treatment for 24 hours resulted in a dose dependent inhibition of directed cell migration. Comparable results were obtained in SCC2095sc. These migration assays showed 4-HPR’s consistent inhibitory effects on all aspects of migration
Zone exclusion assays demonstrated that 5 μM 4-HPR significantly inhibited every cell line relative to its matched control cultures at 24h with the exception of normal keratinocytes (HOK3437) (Figure 4.C.). By 48 h 4-HPR significantly inhibited migration of all cell lines (normal HOK 3437, HOKE6/E7 cells, SCC15, and SCC2095sc cells [p<0.001, n=8].
Preliminary studies confirmed that JSCC-3 conditioned media was the optimal chemoattractant relative to 10% FBS or conditioned media from any other cell lines. Media protein array analyses revealed that JSCC3 conditioned medium contained appreciably higher levels of the established chemoattractant, IL-8, relative to either conditioned media or 10% FBS. As the JSCC-2, EPI, and SCC2095sc cells demonstrated the greatest motility, these lines were selected for the Boyden chamber assays. The chemotaxis-directed migration study results were comparable to our other migration data as 4-HPR suppressed cell migration in a dose-dependent fashion (Figure 4.D.).
While 4-HPR suppresses invasion, concurrent 4-HPR + BRB treatment provides additional invasion-suppressive effects
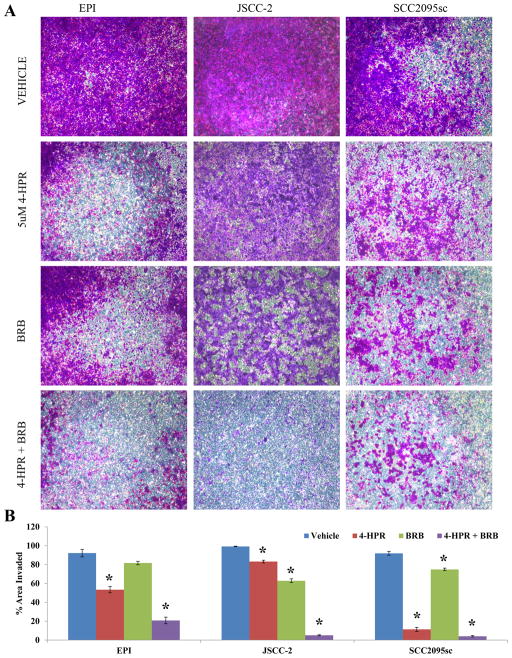
Pilot studies revealed that only the EPI, 2095sc and JSCC2 cells were reproducibly invasion-competent. Treatment with 5 μM 4-HPR significantly suppressed collagen type IV membrane invasion all three tested cell lines (Figure 5.A. and 5.B.). Furthermore, although solitary BRB treatment produced modest anti-invasion effects, concurrent 5μM 4-HPR+ BRB (10μM cyanidin-3-rutinoside equivalent) treatment of all cell lines demonstrated an additive anti-invasive effect in the EPI, 2095sc cells and synergistic effects in the JSCC2 line (Figure 5.B.). Inclusion of the FAK inhibitor II (0.5 and 2.5 μM final concentrations) had no anti-invasive effects on any cell lines.
Figure 5. Evaluation of the effects of 4-HPR and freeze dried blackraspberries (BRB) on cell invasion.
(A) The effects of 4-HPR and BRB of directed cell invasion were evaluated by using collagen IV-coated transwell membrane (8 micron pores). Cells were stained with 0.1% v/v crystal violet after 4% paraformaldehyde fixation. 5uM 4-HPR significantly inhibited invasion in all cell lines with 2095sc cells showing most 4-HPR responsiveness. While single agent treatment with BRB significantly suppressed invasion in the JSCC2 and 2095sc cell lines, concurrent 4-HPR + BRB demonstrated additive (EPI and 2095sc) or synergistic (JSCC-2) anti-invasive effects. (B) Histogram depiction of 4-HPR and BRB’s effects on cell invasion of a synthetic basement membrane. (n=8, error bars represent SEM, * p<0.0001). Data were normalized to the total area of the field. Introduction of the FAK inhibitor II (0.5 and 2.5 μM), which inhibits FAK’s kinase function, had no effects on cell invasion (Supplemental Figure S2).
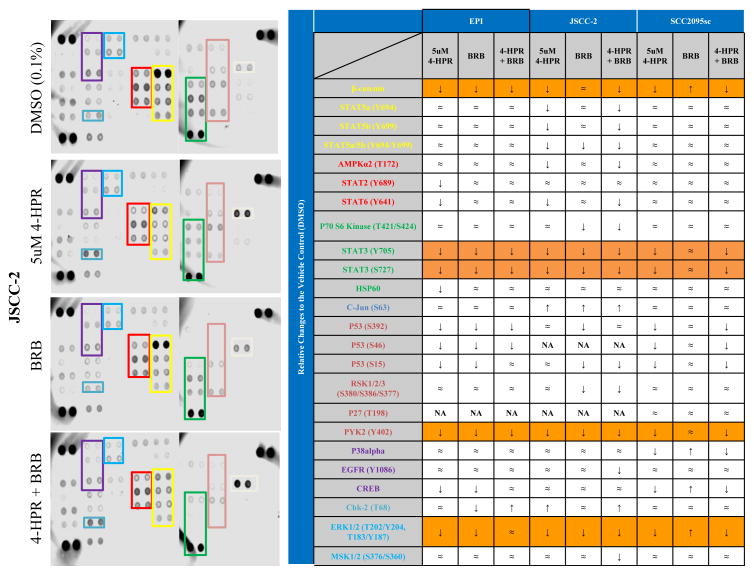
Treatment with 4-HPR and BRB, singularly and in combination, reduced phosphorylation status of kinases associated with cell proliferation, survival, and apoptosis
Singular and combined treatment with 4-HPR and BRB affected kinase phosphorylation status (Figure 6). The JSCC2 cells experienced the greatest therapeutic effects relative to the other invasion-competent 2095sc or EPI cells. Of the 24 proteins evaluated in the JSCC2 cells, 4-HPR suppressed phosphorylation in 10, and had no effect on 10. Notably, the two proteins that showed increased phosphorylation in JSCC2 cells i.e. c-Jun and Chk-2 are associated with stress-induced apoptosis and cell cycle arrest, respectively. p53 (S46) and p27 (T198) were not detected. While singular BRB treatment also decreased phosphorylation levels, its effects were not as pervasive as 4-HPR. Notably, only combination 4-HPR + BRB treatment was able to decrease phosphorylation of the proliferation and migration enabling EGFR and the transcriptional activator MSK1/2.
Figure 6. Modulation of phosphorylation status of kinases associated with cell migration, proliferation, survival, and apoptosis by 4-HPR and BRB.
Proteome profiles were currently conducted on the 3 highly invasive cell lines depicted in Figure 5. Of the 24 proteins evaluated in the panel of serine/threonine/tyrosine phosphorylation residues, 4-HPR consistently suppressed phosphorylation at 5 target residues (i.e. β-catenin, STAT3 Y705, STAT3 S727, PYK2, and Erk1/2; orange-colored cells) in all cell lines tested. Furthermore, 4-HPR treatment increased phosphorylation of 2 (c-Jun, which is associated with stress-induced apoptosis and Chk-2 which leads to cell cycle arrest) in the JSCC-2 cells.
Discussion
Clinical evidence implicates FAK in the development and progression of OSCC [24]. While FAK expression is restricted to the proliferative basal cell layer in healthy human oral epithelia, full-thickness FAK protein is present in premalignant OIN lesions [24]. Notably, FAK contributes to essential aspects of OIN malignant transformation by facilitating basement membrane invasion and inappropriately sustaining proliferation [25]. Our data demonstrate that local delivery achievable levels of 4-HPR [11] inhibit FAK’s prosurvival, mobility-enhancing functions in a spectrum of cultured oral human keratinocytes that range from normal to HPV E6/E7-transduced to malignant to metastatic.
All cell lines used in this study contained subpopulations that co-expressed cytokeratin and vimentin; findings consistent with the epithelial-to mesenchymal transition [26]. Our migration and invasion data show 4-HPR suppressed this mobile phenotype. Also, 4-HPR treatment resulted in an intracellular gradient that was appreciably higher than 4-HPR media levels. These findings suggest that intracellular 4-HPR retention is sustainable and at least energetically neutral-potentially via phospholipid and protein binding. Our previous in vivo studies, which showed a time-dependent increase in target tissue 4-HPR levels following sequential 4-HPR topical dosing, support this premise [11].
4-HPR, at levels comparable to those used in this study, induced apoptosis in a variety of cultured human cancer cells including head and neck, ovary, and small cell lung carcinomas [27–29]. Following 4-HPR treatment in the current studies, execution phase caspase induction occurred in half of the cell lines. Treated cell DNA content showed increases in the sub-G1 and G2/M populations, even in those cell lines that did not show 4-HPR mediated caspase induction. These findings are consistent with 4-HPR and 4-oxo-HPR’s pro-apoptotic effects and 4-oxo-HPR’s mitotic arrest capabilities, respectively [30]. This premise is substantiated by the intracellular presence of cytochrome P450s (CYPs) capable of oxidative bioactivation of 4-HPR to 4-oxo-HPR i.e. 3A4 (consistent with human oral epithelia) and CYP26A1 [18]. Further, cell-ECM interactions are integral for both cell survival and induction of apoptosis [31]. FAK’s dual capacity as a signaling kinase and adaptor/scaffold protein enables modulation of cell-ECM interactions and ultimately cell survival [1]. Our data, which showed disruption of actin filaments and transition to tall, rounded cells, confirmed 4-HPR disrupted cytoskeletal-ECM interactions [30]. Although cell-ECM disruptions generally trigger apoptosis, upregulated FAK activates constitutive cell survival pathways and apoptosis-resistance [31]. Notably, concurrent upregulation of FAK and oncogenic transformation of formerly cell adhesion-based survival signaling pathways occurs in a variety of human cancers [31]. We speculate that transformation of ECM-associated survival pathways was at least partially responsible for the failure of caspase activation in some of the OSCC cell lines.
4-HPR demonstrated the highest binding affinity-including the endogenous ligand ATP-at the FAK-kinase domain ATP-binding site. These findings recapitulate another 4-HPR-natural ligand interaction i.e. nyctalopia induced by 4-HPR’s displacement of vitamin A on retinol binding protein [32]. 4-HPR also interacted, albeit at a reduced affinity, with FAK-FERM’s 1, 2 and 3 pockets. FAK’s FERM domain links FAK to plasma membrane-associated growth factors, regulates FAK’s tyrosine kinase activity and facilitates FAK nuclear translocation [3]. In addition, the FERM domain binds to the Arp2/3 complex, a key mediator in actin nucleation, and regulates lamellipodia formation, cell spreading, and ultimately cell movement [33]. Consequently, 4-HPR-FERM interactions could significantly abate FAK’s proliferative, survival and pro-migratory functions [3]. Finally, an additional therapeutic effect is achieved via 4-HPR’s interaction with FERM’s pocket 2 [1, 3]. 4-HPR’s occupancy of pocket 2 will block its associated Lys152, prevent a key FAK post-translational modification i.e. sumoylation and subsequently suppress FAK autophosphorylation at Tyr397 (integral in FAK kinase activation) and inhibit FAK nuclear translocation [1, 3].
4-HPR also interacts with FAK’s closest homologue, proline rich tyrosine kinase 2 (Pyk2), at its kinase catalytic site. Because Pyk2 can also contribute to p53 degradation and enable invasion and migration, it is regarded as a “FAK-alternative enzyme” [34]. Consequently, exclusive reliance on a FAK-only blockade can be at least partially overcome by Pyk2 [35]. FAK’s and Pyk2’s kinase sites contain a uniquely conserved glycine residue immediately adjacent their N terminals; a feature speculated to convey compound binding specificity [35]. In addition, 4-HPR’s capacity to bind more efficiently to Pyk2’s “out” DFG conformation corresponds to more selective kinase inhibitors [5]. Previous modeling studies by Xie et al. demonstrated 4-HPR interactions at the ATP-binding pocket of mammalian target of rapamycin (mTOR) [36]. The reduced phosphorylation of mTOR’s downstream target proteins following 4-HPR treatment supported these modeling studies [36]. Collectively, these data along with our kinase profiling results imply a predilection for 4-HPR binding at kinase ATP binding pockets which perturbs kinase function.
All migratory functions were significantly inhibited by 4-HPR. These findings likely reflect a dual mechanism of action i.e. disrupted actin microtubule assembly with concurrent reduction in cell proliferation via apoptosis or mitotic blockade. Our F-actin, caspase activation and flow cytometry data all support this mechanistic combination. Our migration inhibition results compare favorably to other studies that demonstrated comparable 4-HPR levels inhibited migration of cultured Kaposi’s sarcoma cells and androgen-independent prostate cancer cells [12, 14]. Also, FAK siRNA treatment intermediately suppressed scratch wound closure. These results are consistent with the co-contribution of Pyk2 in directed cell migration and support the modeling studies that implied 4-HPR perturbs both FAK and Pyk2 functions [34]. Notably, FAK translocates to the lipid raft components (an ideal milieu for retention of lipophilic 4-HPR) of migrating cells’ leading edges. This intracellular proximity increases prospects for 4-HPR-FAK interactions.
Basement membrane invasion by transformed keratinocytes defines OIN malignant transformation to OSCC. Invading cancer cells generate actin rich cellular protrusions “invadopodia” that contain a variety of proteins including cortactin, β1 integrin and matrix metalloproteinases (MMPs) [37]. The coordinated efforts of proteins such as FAK that modulate signaling, cytoskeletal-ECM interactions and actin stabilization are integral for invadopodia formation [38]. 4-HPR, putatively via perturbations in FAK and Pyk2 functions, significantly inhibited invasion in all 3 invasion-competent cell lines. The ineffectiveness of the FAK kinase targeted FAK inhibitor II to suppress invasion suggests that 4-HPR exerts its anti-migratory/-invasive effects via interference with the FERM domain. Furthermore, concurrent treatment with BRB + 4-HPR augmented the inhibitory effects. Intracellular reactive species levels, which are elevated in many cancers, provide a plausible mechanism for these observations [39]. Reactive species mobilize MMPs via zymogen pro-domain cleavage and protease catalytic domain activation [39, 40]. BRB contain numerous redox-active compounds e.g. anthocyanins proficient in reactive species scavenging [41]. Complementary proteome profiling analyses revealed 4-HPR singularly and in combination with BRB reduced phosphorylation status of 8 proteins which are integral for pro-proliferative signaling, cell adhesion and mobility. As reactive species also contribute to activation of kinase signaling cascades, these findings are consistent with the established redox-active functions of both BRB and 4-HPR [42, 43].
FAK dysregulation-such as observed in some premalignant oral lesions-can promote progression to OSCC. Our data, which imply 4-HPR is uniquely capable of perturbing FAK and Pyk2 survival and mobility enhancing effects, expand 4-HPR’s chemopreventive range beyond induction of apoptosis and differentiation. Although this investigation focused on 4-HPR-FAK interactions, virtually every bioactive compound elicits multiple cellular effects. As previously mentioned, 4-HPR’s proapoptotic effects likely co-contributed to inhibition of cell migration. To preserve the 4-HPR-FAK emphasis, this study concentrated on experimental parameters that were FAK-function based i.e. F-actin organization, cell-ECM interaction-based migration assays, formation of invadopodia, digestion of type IV collagen and invasion.
While this study focused on 4-HPR and to a lesser extent BRB, a variety of other OSCC chemopreventives, with varied mechanisms of action, have been identified. Among natural products, green tea extract (GTE), whose bioactive constituents include polyphenols (including epigallocatechin-3-gallate) and alkaloids (caffeine, theophylline and theobromine) has shown promising chemopreventive effects at both the in vitro and in vivo levels [44]. Although expression of the high output cyclooxygenase isoform COX-2 had been implicated in OSCC development, negative results from a celecoxib oral premalignant lesion trial [45] combined with associated adverse cardiac events have eliminated COX-2 inhibitors from further OSCC chemopreventive considerations. Recently, signaling pathway monoclonal antibodies and small molecule growth factor inhibitors that target either the receptor or associated tyrosine kinases have been introduced as therapeutic agents with chemopreventive potential [46]. Notably, the “bench” chemopreventive success of 4-HPR has not translated to clinical oral cancer prevention [45]. This disconnect likely reflects poor bioavailability and significant first pass metabolism of systemically administered 4-HPR. To address this challenge, our labs developed a 4-HPR releasing mucoadhesive patch for direct application to OIN lesions [11]. In vivo studies confirmed patch-released 4-HPR provided therapeutically-relevant levels to the treatment site, did not elicit any local or systemic toxicity, increased enzymes associated with keratinocyte differentiation and Phase II drug detoxification and also increased apoptosis [11]. We are, therefore, optimistic that targeted local delivery will enable 4-HPR to fulfill its chemopreventive potential.
Although selectively targeting pathways that are overexpressed in cancer cells is a compelling treatment concept, clinical use has revealed a range of side effects and eventual development of redundant signaling pathways in treated cancers [46, 47]. As recently discussed by a well-recognized oral cancer chemoprevention researcher, despite extensive efforts we still do not have an effective oral cancer chemoprevention strategy [48]. Provided the extensive inter-patient heterogeneity of premalignant oral epithelial lesions [15], agent combinations based on complementary mechanisms of actions may be necessary. Therefore, continued elucidation of agent(s)’ chemopreventive mechanisms combined with development of refined delivery formulations to address bioavailability issues appears timely and warranted.
Supplementary Material
Acknowledgments
Financial support: NIH grants R01 CA129609 and R01 CA171329 to Susan R. Mallery. NIH T32DE14320 to Byungdo Brian Han, Graduate Fellow.
Footnotes
Conflict of interest statement: The authors have no conflict of interest to disclose
References
- 1.Mitra AK, Hanson DA, Schlaepfer DD. Focal Adhesion kinase: In Command and Control of Cell Motility. Nature Rev. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 2.Liao Y, Mu G, Zhang L, Zhou W, Zhang J, Yu H. Lysophosphatidic Acid Stimulate Activation of Focal Adhesion Kinase and Paxillin and Promotes Cell Motility, via LPA1-3, in Human Pancreatic Cancer. Dig Dis Sci. 2013;58:3524–3533. doi: 10.1007/s10620-013-2878-4. [DOI] [PubMed] [Google Scholar]
- 3.Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nature Rev. 2010;11:802–814. doi: 10.1038/nrm2996. [DOI] [PubMed] [Google Scholar]
- 4.Lim S-TS. Nuclear FAK: a New Mode of Gene Regulation from Cellular Adhesions. Mol Cells. 2013;36:1–6. doi: 10.1007/s10059-013-0139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultze A, Fiedler W. Therapeutic potential and limitations of new FAK inhibitors in the treatment of cancer. Expert Opin Investig Drugs. 2010;19:777–788. doi: 10.1517/13543784.2010.489548. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson JJ, Epstein JB, Eichmiller FC, Gibson TB, Carls GS, Vogtmann E, et al. The cost burden of oral, oral pharyngeal and salivary gland cancers in three groups: commercial insurance, Medicare and Medicaid. Head & Neck Oncology. 2012;4:1–17. doi: 10.1186/1758-3284-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho MW, Field EA, Field JK, Risk JM, Rajlawat BP, Rogers SN, et al. Outcomes of oral squamous cell carcinoma arising from oral epithelial dysplasia: rationale for monitoring premalignant oral lesions in a multidisciplinary clinic. Br J Oral Maxillofac Surg. 2013;51:594–599. doi: 10.1016/j.bjoms.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–5. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 9.Chiesa F, Tradati N, Grigolato R, Boracchi P, Biganzoli E, Crose N, et al. Randomized trial of fenretinide (4-HPR) to prevent recurrences, new localizations and carcinomas in patients operated on for oral leukoplakia: long-term results. Int J Cancer. 2005;115:625–29. doi: 10.1002/ijc.20923. [DOI] [PubMed] [Google Scholar]
- 10.Ulukay E, Pirianov G, Kurt MA, Wood EJ, Mehmet H. Fenretinide induces cytochrome c release, caspase 9 activation and apoptosis in the absence of mitochondrial membrane depolarization. Cell Death and Differentiation. 2003;10:856–859. doi: 10.1038/sj.cdd.4401242. [DOI] [PubMed] [Google Scholar]
- 11.Holpuch AS, Phelps MP, Desai K-GD, Chen W, Koutras GM, Han BB, et al. Evaluation of a mucoadhesive fenretinide patch for local intraoral delivery: a strategy to reintroduce fenretinide for oral cancer chemoprevention. Carcinogenesis. 2012;33:1098–1105. doi: 10.1093/carcin/bgs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari N, Morini M, Pfeffer U, Minghelli S, Noonan DM, Albini A. Inhibition of Kaposi’s Sarcoma in Vivo by Fenretinide. Clin Cancer Res. 2003;9:6020–6029. [PubMed] [Google Scholar]
- 13.Golubkov V, Garcia A, Markland FS. Action of Fenretinide (4-HPR) on Ovarian Cancer and Endothelial Cells. Anticancer Res. 2005;25:249–253. [PubMed] [Google Scholar]
- 14.Benelli R, Monteghirfo S, Vene R, Tosetti F, Ferrari N. The chemopreventive retinoid 4HPR impairs prostate cancer cell migration and invasion by interfering with FAK/AKT/GSK3β pathway and β-catenin stability. Mol Cancer. 2010;9:1–13. doi: 10.1186/1476-4598-9-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallery SR, Tong M, Shumway BS, Curran AE, Larsen PI, Ness GM, et al. Topical Application of a Mucoadhesive Freeze-Dried Black Raspberry Gel Induces Clinical and Histologic Regression and Reduces Loss of Heterozygosity Events in Premalignant Oral Intraepithelial Lesions: Results from a Multicentered, Placebo-Controlled Clinical Trial. Clin Cancer Res. 2014;20:1910–1924. doi: 10.1158/1078-0432.CCR-13-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müssig E, Steinberg T, Kohl A, Chamulitrat W, Komposch G, Tomakidi P. Discrimination of epithelium-like and fibroblast-like phenotypes derived from ethanol-treated immortalized human gingival keratinocytes in Epithelial equivalents. Cell Tissue Res. 2008;332:57–71. doi: 10.1007/s00441-007-0551-y. [DOI] [PubMed] [Google Scholar]
- 17.Tong M, Han BB, Holpuch AS, Pei P, He L, Mallery SR. Inherent phenotypic plasticity facilitates progression of head and neck cancer: Endotheliod characteristics enable angiogenesis and invasion. Exp Cell Res. 2013;319:1028–1042. doi: 10.1016/j.yexcr.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallery SR, Tong M, Michaels GC, Kiyani AR, Hecht SS. Clinical and Biochemical Studies Support Smokeless Tobacco’s Carcinogenic Potential in the Human Oral Cavity. Cancer Prev Res. 2014;7:23–32. doi: 10.1158/1940-6207.CAPR-13-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trott O, Olson AJJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Computational Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berman Helen M, Westbrook John, Feng Zukang, Gilliland Gary, Bhat TN, Weissig Helge, et al. The Protein Data Bank. Nucl Acids Res. 2000;18 (1):235–242. doi: 10.1093/nar/28.1.235. http://www.rcsb.org/pdb/home/home.do. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spartan’10, Wavefunction, Inc. Irvine CA, Shao Y, Molnar LF, Jung Y, Kussmann J, Ochsenfeld C, Brown ST, Gilbert ATB, et al. Phys Chem Chem Phys. 2006;8:3172. [Google Scholar]
- 22.Krieger E, Koraimann G, Vriend G. Increasing the precision of comparative models with YASARA NOVA—a self-parameterizing force field. Proteins. 2002;47:393–402. doi: 10.1002/prot.10104. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Zhang H, Li M, Frid MG, Flockton AR, McKeon BA, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res. 2014;114:67–78. doi: 10.1161/CIRCRESAHA.114.301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canel M, Secades P, Rodrigo JP, Cabanillas R, Herrero A, Suarez C, et al. Overexpression of Focal Adhesion Kinase in Head and Neck Squamous Cell Carcinoma Is Independent of fak Gene Copy Number. Clin Cancer Res. 2006;12:3272–3279. doi: 10.1158/1078-0432.CCR-05-1583. [DOI] [PubMed] [Google Scholar]
- 25.Siesser PM, Hanks SK. The Signaling and Biological Implications of FAK Overxpression in Cancer. Clin Cancer Res. 2006;12:3233–3237. doi: 10.1158/1078-0432.CCR-06-0456. [DOI] [PubMed] [Google Scholar]
- 26.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scher RL, Saito W, Dodge RK, Richtsmeier WJ, Fine RL. Fenretinide-induced apoptosis of human head and neck squamous carcinoma cell lines. Otolaryngology. 1998;118:464–471. doi: 10.1177/019459989811800406. [DOI] [PubMed] [Google Scholar]
- 28.Supino R, Crosti M, Clerici M, Warlters A, Cleris L. Induction of apoptosis by fenretinide (4HPR) in human ovarian carcinoma cells and its association with retinoic acid receptor expression. Int J Cancer. 1996:491–497. doi: 10.1002/(SICI)1097-0215(19960208)65:4<491::AID-IJC17>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 29.Kalemkerian GP, Slusher R, Ramalingam S, Gadgeel S, Mabry M. Growth Inhibition and Induction of Apoptosis by Fenretinide in Small-Cell Lung Cancer Cell Lines. J Natl Cancer Inst. 1995;22:1674–1680. doi: 10.1093/jnci/87.22.1674. [DOI] [PubMed] [Google Scholar]
- 30.Tiberio P, Cavadini E, Abolafio G, Formelli F, Appierto V. 4-oxo-N-(4-hydroxyphenyl)retinamide: two independent ways to kill cancer cells. PLoS One. 2010;5:1–11. doi: 10.1371/journal.pone.0013362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Nimwegen MF, Huigsloot M, Camier Am, Tijdens IB, Van de Water B. Focal Adhesion Kinase and Protein Kinase B Cooperate to Suppress Doxorubicin-Induced Apoptosis of Breast Tumor Cells. Mol Pharmacol. 2006;70:1330–1339. doi: 10.1124/mol.106.026195. [DOI] [PubMed] [Google Scholar]
- 32.Garaventa A, Luksch R, LoPicolo MS, Cavadini E, Montaldo PG, Pizzitola MR, et al. Phase I Trial and Pharmacokinetics of Fenretinide in Children with Neuroblastoma. Clin Cancer Res. 2003;9:2032–2039. [PubMed] [Google Scholar]
- 33.Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, Jones GE, Frame MC. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nature Cell Biology. 2007;9:1046–1056. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- 34.Lipinski CA, Tran NL, Menashi E, Rohl C, Kloss J, Bay RC, et al. The Tyrosine Kinase Pyk2 Promotes Migration and Invasion of Glioma cells. Neoplasia. 2005;7:435–445. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipinski CA, Loftus JC. Targeting Pyk2 for therapeutic intervention. Expert Opin Ther Targets. 2010;14:95–108. doi: 10.1517/14728220903473194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie H, Zju F, Huang Z, Lee M-H, Kin DJ, Li X, et al. Identification of mammalian target of rapamycin as a direct target of fenretinide both in vitro and in vivo. Carcinogenesis. 2012;33:1814–1821. doi: 10.1093/carcin/bgs234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi H. Pathological roles of invadopodia in cancer invasion and metastasis. Eur J Cell Biol. 2012;91:902–907. doi: 10.1016/j.ejcb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol. 2012;196:375–385. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storz P. Reactive Oxygen Species in Tumor Progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 40.Chiu WT, Shen SC, Chow JM, Lin CW, Shia LT, Chen TC. Contribution of reactive oxygen species to migration/invasion of human glioblastoma cells U87 via ERK-dependent COX-2/PGE2 activation. Neurobiol Dis. 2010;37:118–129. doi: 10.1016/j.nbd.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29:1665–1674. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCubrey JA, LaHair MM, Franklin RA. Reactive Oxygen Species-Induced Activation of the MAP Kinase Signaling Pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 43.Wang K, Fang H, Xiao D, Zhu X, He M, Pan X, et al. Converting Redox Signaling to Apoptotic Activities by Stress-Responsive Regulators HSF1 and NRF2 in Fenretinide Treated Cancer Cells. PLoS ONE. 2009;4:e7538, 1–13. doi: 10.1371/journal.pone.0007538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen D, Wan SB, Yang H, Yuan J, Chan TH, Dou QP. EGCG, Green Tea Polyphenols and their Synthetic Analogs and Prodrugs for Human Cancer Prevention and Treatment. Adv Clin Chem. 2011;53:155–177. doi: 10.1016/b978-0-12-385855-9.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papadimitrakopoulou VA, William WN, Jr, Dannenberg AJ, Lippman SM, Lee JJ, Ondrey FG, et al. Pilot randomized phase II study of celecoxib in oral premalignant lesions. Clin Cancer Res. 2008;14:2095–2101. doi: 10.1158/1078-0432.CCR-07-4024. [DOI] [PubMed] [Google Scholar]
- 46.Widakowich C, De Castro G, De Azambuja E, Dinh P, Awada A. Review: Side Effects of Approved Molecular Targeted Therapies in Solid Cancers. The Oncologist. 2007;12:1443–1455. doi: 10.1634/theoncologist.12-12-1443. [DOI] [PubMed] [Google Scholar]
- 47.Widakowich, Logue JS, Morrison DK. Complexity in the signaling network: insights from the use of targeted inhibitors in cancer therapy. Genes Dev. 2012;26:641–650. doi: 10.1101/gad.186965.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.William AN., Jr Oral premalignant lesions: any progress with systemic therapies? Curr Opin Oncol. 2012;24:205–210. doi: 10.1097/CCO.0b013e32835091bd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.