Abstract
Objective
Our primary goal was to examine the clinical characteristics of a series of patients with urinary bladder paragangliomas (UBPGLs), focusing particularly on their genetic backgrounds.
Materials and methods
We analyzed the medical records of patients who presented to the National Institutes of Health with UBPGL from 2000 to 2013 to determine their clinical characteristics and outcomes, biochemical phenotype, tumor size, and genetic background.
Results
Of the 27 patients with UBPGLs who were identified, 17 (63%) had underlying genetic mutations. Overall, 14 (51.9%) patients had a germline mutation in the succinate dehydrogenase subunit B gene (SDHB), and 3 (11.1%) had in the von Hippel-Lindau gene (VHL). Of the 21 patients who had biochemical data available before their first operation, 19 (90.5%) presented with a noradrenergic biochemical phenotype; 7 (33.3%) patients had tumors that also secreted dopamine. In addition, 1 patient (4.8%) had elevated metanephrine levels, and 2 (9.5%) had normal biochemical data. In total, 13 (48.1%) patients in the series were diagnosed with metastatic disease, at either first presentation or follow-up; 6 of these patients (46.1%) had SDHB mutations.
Conclusions
UBPGLs typically present with a noradrenergic phenotype and are frequently associated with underlying germline mutations. Patients presenting with these rare neuroendocrine tumors should be screened for these mutations. In addition, patients with UBPGLs should be followed up closely for metastatic development regardless of genetic background, as almost half of the patients in this series presented with metastatic disease and less than half of them had SDHB mutations.
Keywords: Paraganglioma, Succinate dehydrogenase, SDHB, Urinary bladder, von Hippel-Lindau
1. Introduction
Pheochromocytomas (PHEOs) and paragangliomas (PGLs) are neuroendocrine tumors of chromaffin cells in the adrenal medulla (for PHEOs) or sympathetic or parasympathetic paraganglia (for PGLs). Extra-adrenal PGLs account for 15% to 20% of PHEO/PGLs and may be located anywhere from the bladder to the base of the skull [1]. PHEOs/PGLs secrete, synthesize, and metabolize catecholamines; measurement of catecholamines and their metabolites, metanephrines, is important both for diagnosis and biochemical phenotype determination. Secretory PHEOs/PGLs can be adrenergic (predominantly epinephrine and its metabolite metanephrine), noradrenergic (predominantly norepinephrine/normetanephrine), or dopaminergic (predominantly dopamine and its newly discovered metabolite methoxytyramine) [2]. PHEOs/PGLs can also be biochemically silent [3].
Approximately one-third of PHEOs/PGLs are associated with inherited mutations in 17 genes. The most important include the von Hippel-Lindau (VHL); rearranged during transfection (RET); neurofibromatosis 1 (NF1); succinate dehydrogenase (SDH) subunits A, B, C, and D; SDH complex assembly factor 2 (SDHAF2); transmembrane protein 127 (TMEM127); and myc-associated factor X (MAX) genes [4]. Of these, mutations in SDHB, SDHC, and SDHD, which encode subunits of mitochondrial complex II, are strongly associated with the development of extra-adrenal PGLs [4–8]. SDH is involved in the Krebs cycle, oxidative phosphorylation, and electron transport chain. Particular subunits are strongly associated with certain PGL locations; for example, SDHD mutations are often linked to head and neck PGLs, and SDHB-related tumors are commonly extra-adrenal [3–5,7,8]. PHEOs/PGLs are also found in 10% to 20% of patients with VHL mutations [4]. Although PHEOs are more common in VHL, extra-adrenal PGLs have been identified, including 1 reported case of a bladder tumor [9].
PGLs of the urinary bladder (UBPGLs) are rare, accounting for less than 6% of PGLs and 0.06% of bladder tumors [10]. UBPGLs often present with hypertension, hematuria, postmicturition syncope, or other symptoms due to increased catecholamines (e.g., headaches, palpitations, blurred vision, flushing, and sweating). However, approximately 17% to 39% are biochemically silent [11,12]. Although there have been several reports on UBPGLs, there has been no study focusing on their genetic characteristics. In the present study, we aimed to describe carefully the clinical characteristics, biochemical phenotypes, and genetic backgrounds of UBPGLs.
2. Materials and methods
2.1. Subjects
A retrospective medical record review of patients with PHEOs/PGLs seen at the National Institutes of Health (NIH) in Bethesda, Maryland, USA, from 2000 to 2013 was conducted. Only those with pathologically confirmed UBPGL were included. Imaging, biochemical, operative, and pathology reports were reviewed. Patients were imaged using a combination of computed tomography (CT), magnetic resonance imaging (MRI), 123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy, octreotide (OCT) scans, and positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) and 18F-fluorodopamine (FDA). Clinical presentations and patient outcomes were also recorded, including metastases development, defined as the presence of disease in sites where chromaffin cells are not normally present (e.g., lymph nodes, bones, liver, and lungs).
This study was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the NIH. Each patient gave informed written consent upon enrollment in the study.
2.2. Genetic studies
Genomic DNA was extracted from whole blood samples for genetic testing of SDHB, SDHC, SDHD, and VHL. Polymerase chain reaction–based bidirectional sequencing was performed by Mayo Medical Laboratories, Rochester, MN, or by the Division of Molecular Diagnostics, University of Pittsburgh Medical Center, as previously described [13]. Large deletions were detected using multiplex ligation-dependent probe amplification and Luminex Flex-Map Technologies [14]. None of the patients had clinical presentations suggestive of RET or NF1 mutations. Testing for MAX, TMEM127, SDHA, and SDHAF2 genes was not performed.
2.3. Catecholamine and metanephrine assays
Plasma or urinary catecholamine and metanephrine levels were measured using standard high-pressure liquid chromatography with electrochemical detection at the NIH or Mayo Medical Laboratories, Rochester, MN. Forearm blood samples were drawn with patients in supine position at least 20 minutes after an intravenous catheter was inserted, as previously described [15].
2.4. Statistical analysis
A statistical analysis of the metastatic rates in the SDHB and non-SDHB groups was done using the Fisher exact test.
3. Results
Of the 531 patients with PHEO/PGL seen at NIH, 27 were treated for UBPGL. Of them, 15 were women (55.6%) and 12 (44.4%) were men, with a mean age at initial diagnosis of 29.5 ± 14.7 years (range: 6–58 y). Overall, 24 (88.9%) patients initially presented with UBPGL. The remaining 3 presented with UBPGL after previous resections of other primary PHEO or PGL. At UBPGL diagnosis, 19 (70.4%) patients were hypertensive, and 22 (81.5%) presented with signs and symptoms of catecholamine excess. Detailed clinical profiles are summarized in Table 1.
Table 1.
Patient information and clinical data
| Patient | Sex | Age at presentation, y |
Age at diagnosis, y |
HTN | Symptoms of CA excess |
Micturition- related symptoms |
Hematuria | Tumor size, cm | Imaging studiesa | Biochemistry | Other tumors at diagnosis |
Sites of metastases |
Postoperative treatments |
Recurrence in bladder |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urine | Plasma | ||||||||||||||
| 1 | F | 8 | 9 | + | + | − | − | 7.5 × 5.0 × 4.5 | CT, MRI, FDG, 123I-MIBG, and FDA |
NA | NE, NMN, and DA |
Mlt | – | – | − |
| 2 | F | 32 | 33 | + | + | − | + | 5.0 × 5.0 × 4.5 | MRI, 123I-MIBG, and OCT |
NMN and VMA |
NMN | – | Lung and lymph nodes |
CVD, radiation, and RFA |
+ |
| 3 | F | 30 | 31(34)b | − | + | − | − | 2.0 × 2.0 × 1.7 | CT, MRI, 123I- MIBG, and OCT |
NA | NMN | Mlt | – | – | − |
| 4 | M | 29 | 30 | + | + | + | − | 1.8 × 1.9 | CT, US, and FDG | NA | NE | Mlt | – | – | − |
| 5 | M | 8 | 8(16)b | + | + | − | + | 3.5 × 4.5 × 2.0 | MRI and 123I- MIBG |
NMN and VMA |
NA | Mlt/Metc | Bone and lymph nodes |
MIBG | − |
| 6 | M | 10 | 11 | + | + | − | − | NA | Unknown | MN, NMN, and VMA |
NA | – | Bone | – | − |
| 7 | M | 29 | 30 | − | − | − | − | 2.7 × 2.6 × 2.1; 2.6 × 2.42.3 |
CT | NA | NA | Mlt | – | – | − |
| 8 | F | 17 | 18 | + | + | − | − | 4.0 × 3.0 × 3.0 | CT, MRI, 123I- MIBG, and OCT |
NMN and VMA |
NE and NMN | Met | Bone and lymph nodes |
– | − |
| 9 | F | 25 | 34 | + | + | + | − | 0.4 × 4.0 | CT, MRI, and 123I-MIBG |
NE and NMN |
NA | – | – | – | − |
| 10 | M | 50 | 50 | + | − | − | − | 3.5 | CT, MRI, 123I- MIBG, and OCT |
NA | NMN | Met | Bone, liver, and lymph nodes |
CVD | − |
| 11 | F | 6 | 6 | + | + | + | − | 3.3 × 2.0 × 2.0 | CT and 123I-MIBG | NA | NE and NMN | – | – | – | − |
| 12 | F | 43 | 43 | – | + | + | + | 2.8 × 1.8 × 1.6 | CT and US | NE and NMN |
NMN | – | – | – | − |
| 13 | F | 11 | 12 | + | + | − | + | 3.5 × 3.9 × 0.4 | CT, MRI, FDG, and 123I-MIBG |
NE, NMN, and DA |
NE, NMN, DA, MTX, and CgA |
Met | Bone and lymph nodes |
– | − |
| 14 | F | 50 | 50 | + | + | − | + | NA | CT, MRI, FDG, 123I-MIBG, and FDAd |
NE and NMNd |
NE, NMN, DA, and CgAd |
– | Bone and lymph nodes |
sunitinib and MIBG |
+ |
| 15 | M | 15 | 15 | + | + | − | − | 1.7 × 1.5 × 1.5 | CT, MRI, FDG, 123I-MIBG, and FDA |
NE and NMN |
NE, NMN, DA, and CgA |
Met | Bone | – | − |
| 16 | M | 23 | 25 | + | + | + | − | 4.5 × 5 | MRI and 123I- MIBG |
NMN | NA | – | – | – | − |
| 17 | F | 34 | 37 | − | + | − | − | 3.5 × 2.5 × 0.7 | CT, MRI, US, and FDG |
WNL | WNL | – | – | – | − |
| 18 | M | 22 | − | + | + | − | NA | MRI | NE | NE | Met | – | − | ||
| 19 | M | 42 | + | + | + | NA | CT and MRI | NA | NA | – | Lung | chemotherapy (unspecified agents) |
− | ||
| 20 | F | 23 | + | + | − | − | NA | CT, MRI, and 123I-MIBG |
WNL | WNL | – | – | – | − | |
| 21 | F | 37 | − | − | + | + | NA | CT | NA | NA | – | – | – | − | |
| 22 | M | 6 (12)b | + | + | + | − | 3.4 × 2.8 × 1.0 | CT, MRI, FDG, and 123I-MIBG |
NE and NMN |
NMN | – | – | – | − | |
| 23 | F | Asymptomatic | 58 | − | − | − | − | 1.8 × 2.5 | CT | NA | NA | – | – | – | − |
| 24 | F | 50 | 50 | + | + | − | − | 0.8 | CT and US | NA | NA | – | – | – | − |
| 25 | F | Asymptomatic | 32 | − | − | − | − | NA | Unknown | NA | NA | – | – | – | − |
| 26 | M | 46 | 46 | + | + | − | 1.2 × 0.8 × 0.4; | ||||||||
1.5 × 1.0 × 0.5 CTNMN and VMANE, DAMltLiver, lung, and lymphnodes––
27M2828++−−6.5 × 6.3 × 3.8CT, MRI,FDG, 123I-MIBG, OCT, and FDANE, NMN, DA, and VMAMN, NE, NMN, DA, and CgAMetBone CVD (no surgery) No surgery CA = catecholamine; CgA = chromogranin A; CVD = cyclophosphamide/vincristine/dacarbazine chemotherapy; DA = dopamine; HTN = hypertension; 131I-MIBG = 131I-metaiodobenzylguanidine treatment; Met = metastatic; Mlt = multiple; NA = not available; NE = norepinephrine; NMN = normetanephrine; RFA = radiofrequency ablation; US = ultrasound; VMA = vanillylmandelic acid; WNL = within normal limits.
Scan findings in italics were negative for the UBPGL.
Patient initially presented with other PHEO/PGLs; UBPGL was diagnosed on later follow-up. Age in parentheses is age of diagnosis with the UBPGL.
Patient was initially diagnosed with multiple tumors; at the time of diagnosis with UBPGL, the patient presented with metastatic disease.
Reports not available from before first surgery for UBPGL; data reflect diagnostic studies done during evaluation of recurrent UBPGL.
Most patients had elevated plasma/urine catecholamines/metanephrines. Of the 21 patients with available preoperative biochemical data, 19 (90.5%) had elevated norepinephrine/normetanephrine 7 (36.8%) had both elevated norepinephrine/normetanephrine and dopamine/methoxytyramine, and 1 patient (5.3%) had elevated metanephrine levels in addition to norepinephrine/normetanephrine and dopamine. There were 2 patients who showed normal finding on biochemistry (Table 1).
Overall, 6 (22.2%) patients presented with metastases at diagnosis, and 6 patients (22.2%) developed metastases later. There was 1 patient (patient 5) who presented with metastases at the time of UBPGL diagnosis, 8 years after diagnosis with multiple PGLs. Therefore, 13 patients (48.1%) developed metastases. Additionally, 7 patients (25.9%) had multiple primary lesions at the time of UBPGL diagnosis, and 2 (7.4%) were later diagnosed with additional primary PGLs. Moreover, 3 (11.1%) patients had previous primary tumors, and 2 patients (7.4%) had recurrent UBPGLs.
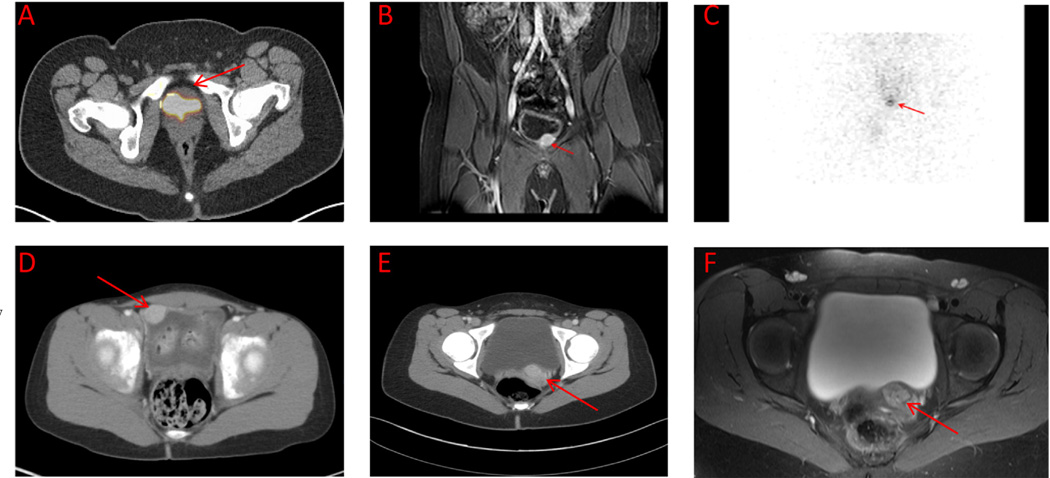
CT scan results were positive for UBPGL in 19 of 21 (90.5%) patients who underwent this imaging modality, and 17 patients had MRI scanning in the area of the bladder; all 17 UBPGLs were visualized. Similarly, FDG-PET scans identified UBPGLs in all 7 patients who underwent this technique. A total of 4 patients had FDA-PET scans—3 at first presentation and 1 on presentation with a recurrent UBPGL; only 1 was positive. In addition, 15 patients underwent 123I-MIBG scintigraphy; only 6 (40%) identified UBPGL. Moreover, 4 patients underwent OCT; none had positive uptake in UBPGL. Representative scan images of UBPGLs are shown in Fig. 1.
Fig. 1.
(A–C) Fluorodeoxyglucose positron emission tomography/computed tomography (CT) overlay, T2-weighted magnetic resonance imaging (MRI), and 123I-metaiodobenzylguanidine (MIBG) scintigraphy showing a tumor in the anterior bladder wall of a 12-year-old male patient (patient 22). (D) Contrast CT in a 6-year-old female patient with tumor in the right lateral bladder (patient 11). (E and F) Contrast CT and T2-weighted MRI in a 13-year-old female patient showing an enhancing mass at bladder base (patient 13). (Color version of the figure is available online)
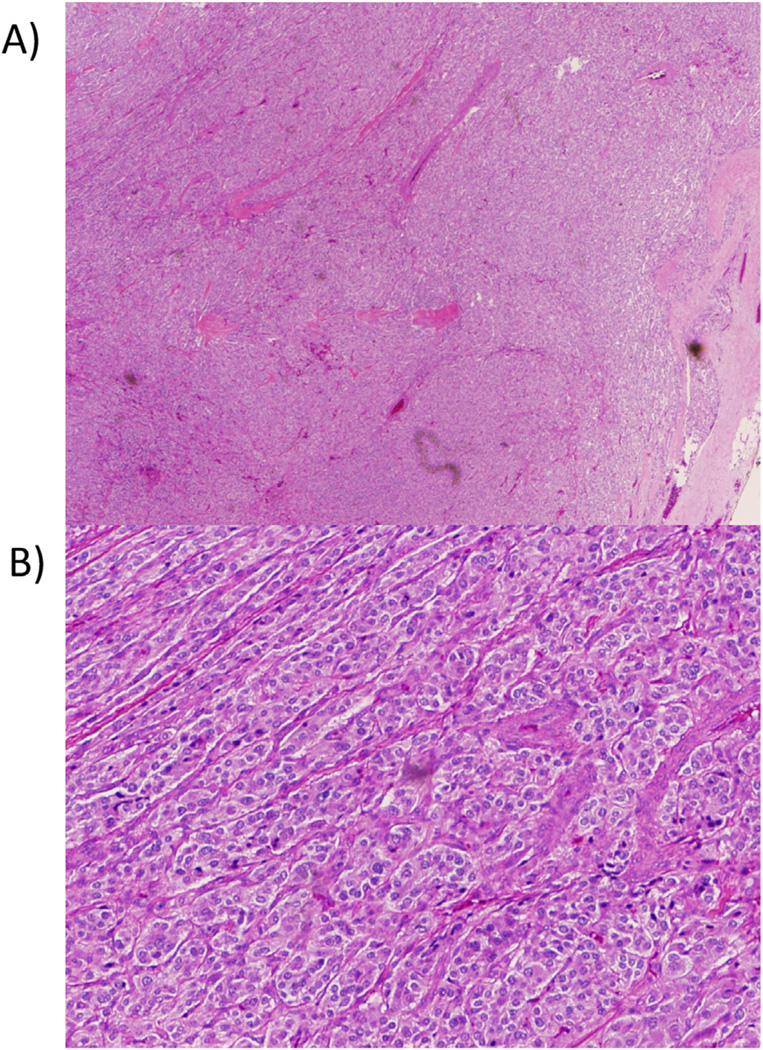
All patients except 1 (patient 27) underwent tumor resection with transurethral resection of bladder tumor, partial cystectomy, or radical cystectomy. A representative image of a resected tumor is shown in Fig. 2. Patients whose surgeries were performed at the NIH underwent preoperative blockade with phenoxybenzamine, and, in most cases, metyrosine. Surgical procedure information was available for 12 patients. Tumors were removed successfully by transurethral resection of bladder tumor in 5 patients, partial cystectomy in 5, and radical cystectomy in 2. A robotic approach was successfully employed in 2 patients who underwent partial cystectomy. Surgical management was largely dictated by the size and location of the tumor. Surgical success was defined as the normalization of catecholamine level or metanephrine level or both (in patients with a single tumor) and absence of UBPGL on follow-up imaging.
Fig. 2.
Representative images of a resected urinary bladder paraganglioma under low-power (A) and high-power (B) microscopy. (Color version of the figure is available online)
Patient 27, who did not undergo surgical resection owing to widespread metastatic disease, received chemotherapy with cyclophosphamide, vincristine, and dacarbazine (CVD). In some patients, further treatment was done postoperatively for metastatic disease. This included chemotherapy with CVD in 2 patients, sunitinib in 1 patient, and unspecified agents in 1 patient; radioactive 131I-MIBG therapy in 2 patients; external beam radiation therapy for bone metastases in 2 patients; and radiofrequency ablation of osseous metastases in 1 patient.
Overall, 14 patients (51.9%) had SDHB mutations (Table 2), 5 (35.7%) had a relative with PHEO/PGL, and 3 patients (11.1%) had VHL mutations. Metastatic disease developed in 6 of the patients with SDHB mutations (42.8%), whereas none of patients with VHL mutations had metastases. Additionally, 9 patients with SDHB mutations (64.2%) presented with multiple tumors, with 6 (6/9, 66.7%) presenting with multiple concurrent tumors at first presentation; 1 patient with VHL mutation (33.3%) had multiple tumors, though not concurrently. Moreover, 4 patients with SDHB mutations (28.5%) presented with tumors that secreted both norepinephrine/normetanephrine and dopamine/methoxytyramine; 1 (7.1%) had a biochemically silent tumor. Finally, 1 patient with VHL mutation (33.3%) also had a biochemically silent UBPGL.
Table 2.
Genetic profile of the patients
| Patient | Mutation | Nucleotide change | Protein change | Family Hx of PHEO/PGL |
|---|---|---|---|---|
| 1 | SDHB | Exon 1 deletion | Exon 1 deletion | − |
| 2 | SDHB | c.343C>T | p.Arg115X | − |
| 3 | – | – | – | − |
| 4 | SDHB | Promoter and exon 1 deletion | Promoter and exon 1 deletion | + |
| 5 | SDHB | c.418G>T | p.Val140Phe | − |
| 6 | – | – | – | − |
| 7 | SDHB | c.380T>G | p.Ile127Ser | + |
| 8 | SDHB | c.380T>G | p.Ile127Ser | − |
| 9 | SDHB | c.136C>T | p.Arg46X | − |
| 10 | – | – | – | − |
| 11 | – | – | – | − |
| 12 | SDHB | c.286+1G>A | Splice site mutation | − |
| 13 | SDHB | c.418G>T | p.Val140Phe | − |
| 14 | – | – | – | − |
| 15 | SDHB | c.445–447delCAinsGGTATCT | p.Gln149LeufsX159 | − |
| 16 | SDHB | c.540G>A | p.Leu180Leu (mutation affects transcript splicing) | + |
| 17 | SDHB | c.343C>T | p.Arg115X | + |
| 18 | – | – | – | − |
| 19 | – | – | – | − |
| 20 | VHL | Partial deletion | Partial deletion | − |
| 21 | VHL | c.337C>T | p.Arg113X | + |
| 22 | VHL | c.371C>T | p.Thr124Ile | + |
| 23 | – | – | – | − |
| 24 | – | – | – | − |
| 25 | SDHB | c.553G>T | p.Glu185X | − |
| 26 | – | – | – | − |
| 27 | SDHB | c.587G>A | p.Cys196Tyr | − |
NA = not available.
4. Discussion
SDHB mutations have previously been associated with extra-adrenal PGLs. In the present study, for the first time, we show a high correlation between UBPGLs and SDHB mutations, with 51.9% of patients diagnosed with an SDHB mutation, several in the absence of a family history of PHEOs/PGLs. SDHB mutations are frequently found in the absence of a family history, most likely owing to low penetrance [3,4]. The high rate of SDHB mutations in our study suggests that all patients with UBPGLs should be tested for SDHB mutations. As 3 patients (11.1%) presented with VHL mutations, testing for VHL could be considered if SDHB mutation analysis finding is negative or if clinical or family history is suggestive of VHL disease.
SDHB mutations are associated with a higher incidence of metastases [3]. In our series, the rate of metastases was high for both SDHB and apparently sporadic tumors. This suggests that the metastatic potential of UBPGLs may not be owing to the presence of SDHB mutations but rather to their extra-adrenal location, which is another known independent predictor of malignancy [15,16]. The difference between the rate of metastases in patients with SDHB mutations and patients with no known genetic mutation was not statistically significant (P = 0.24). Overall, the metastatic rate in our series (48.1%) is much higher than that previously reported (10%–15%) [12,17]. Thus, our findings suggest that patients with UBPGLs may be at a higher risk of developing metastases, regardless of genetic background, and should undergo close clinical follow-up. However, none of the 3 patients with VHL mutations developed metastatic disease, consistent with the known metastatic rate of <5% for VHL PGLs [4].
In addition to the high rate of metastatic disease, almost half of the patients in this series had multiple primary tumors. Both SDHB and VHL mutations, which were highly represented in these patients, are known to predispose patients to the development of multiple PGLs [4].
Although metastatic disease rates were high regardless of SDHB status, genetic testing is a crucial component of patient management. Although all patients with UBPGL should undergo careful follow-up, the appropriate imaging technique can vary based on genetic background. Patients with SDHB mutations should be screened periodically with FDG-PET, as this is the most sensitive technique for identifying lesions, particularly metastases, in this population [18]. For patients without SDHB mutations, FDG-PET may not always be the best choice for detecting additional primary or metastatic PGL [19,20]. Furthermore, knowing a patient's genetic status can guide appropriate treatment strategies when metastases are present. For example, CVD chemotherapy may be more effective in the treatment of patients with SDHB mutations (unpublished observations). Future treatment options may also become available that target specific cellular pathways, such as the Krebs cycle, which will require knowledge of a patient's genetic status. In addition, by discovering familial mutations, testing can be offered to relatives, leading to earlier identification and screening of mutation carriers and better outcomes for at-risk relatives.
Most UBPGLs reported in the literature present with symptoms such as paroxysmal hypertension, palpitations, micturition syncope, and hematuria. Hypertension is one of the most common symptoms, previously reported in 54.7% of patients with UBPGL [11]. Painless hematuria has also been reported frequently, in an estimated 33% to 60% of cases [11,21–23]. In our study, 70.4% were hypertensive on diagnosis, but only 25.9% had documented hematuria. Furthermore, 81.5% presented with symptoms of catecholamine excess. In 40% of these patients, the symptoms were associated with urination, most likely owing to catecholamine release by the tumor after contraction of the urinary bladder.
In addition to symptomatic presentations, functional UBPGLs, estimated to represent 61% to 83% of UBPGLs, present with elevated catecholamine/metanephrine levels [11,12,24]. In our study, 90.5% had elevated biochemistry. All had elevated norepinephrine/normetanephrine level in the plasma/urine, consistent with previous reports of noradrenergic phenotypes in UBPGLs. Only 1 patient with widespread metastatic disease had elevated metanephrine. The high prevalence of noradrenergic phenotypes is most likely owing to a lack of phenylethanolamine N-methyltransferase, the enzyme necessary for the conversion of norepinephrine to epinephrine [2,22]. One-third of the UBPGLs also secreted dopamine. Noradrenergic and dopaminergic phenotypes have previously been linked to SDHB mutations [15], which were prevalent in this study. Indeed, 5 of the 7 (71.4%) patients with elevated norepinephrine/normetanephrine and dopamine/methoxytyramine levels had SDHB mutations.
Several imaging studies have been used in localizing and characterizing UBPGLs. In many bladder tumors, cystoscopy is used in localizing the tumors. However, this procedure is not recommended without adequate perioperative blockade, as distention of the bladder with fluid or manipulation of the tumor or both can induce catecholamine release and episodic spells [25]. CT has a high sensitivity for detecting PHEOs/PGLs. However, UBPGLs can be hard to visualize on CT owing to density and enhancement similar to soft tissue on plain images or surgical clip artifacts [23,26]. MRI may be favorable for visualizing UBPGLs owing to its higher soft tissue contrast and decreased interference from artifacts because of surgical clips [1,23,26]. On T1-weighted images, UBPGLs are not very intense, but on T2-weighted images, UBPGLs, like many PGLs, are moderately to highly intense [26]. In our patients, CT scan results were positive in 90.5% of patients, whereas MRI finding was positive for all the patients who underwent these scans.
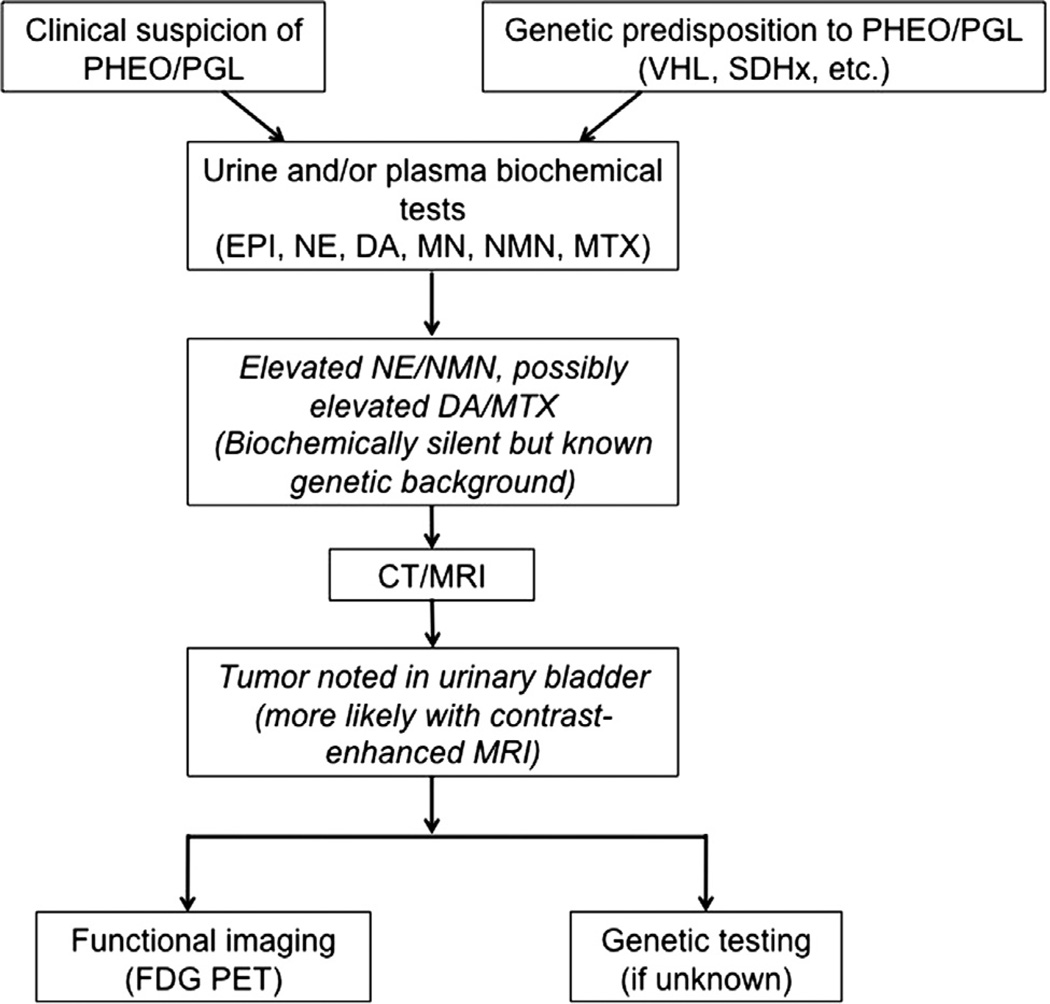
For functional imaging, 123I-MIBG scintigraphy has long been considered the gold standard for PHEO/PGL, owing to its structural similarity to norepinephrine allowing its uptake by catecholamine-secreting tumors [19,27]. In our study, only 40% of UBPGLs showed positive results on 123I-MIBG scintigraphy, suggesting that this technique may be suboptimal for these tumors. OCT scanning has also been used in diagnosing PHEOs/PGLs, though with low sensitivity [27]. In our series, none of the patients who underwent this imaging modality had positive scan results. This modality is being replaced by newly developed 68Ga-DOTA-peptide PET/CT scans, which appear to be highly sensitive (unpublished observations). In contrast, PET imaging may be more sensitive. FDG-PET scanning has been shown to have high sensitivity for PHEO/PGL, particularly for SDHB-related metastatic tumors [18–20]. All 7 of our patients who had FDG-PET scans had positive UBPGL uptake. Moreover, 4 patients also underwent PET scanning with FDA, an analog of norepinephrine. In these patients, 1 had positive UBPGL uptake, and the other 3 were not visible owing to contrast excretion in the urinary bladder. Overall, FDG-PET imaging seems to be the most sensitive functional test for UBPGL, but further study with a larger cohort of patients will be necessary. A suggested diagnostic algorithm for UBPGL is presented in Fig. 3.
Fig. 3.
Diagnostic algorithm for urinary bladder paraganglioma. Boxes in italics represent typical findings in patients with urinary bladder paraganglioma. DA = dopamine; EPI = epinephrine; MTX = methoxytyramine; NE = norepinephrine; NMN = normetanephrine.
The main treatment strategy for UBPGL is surgical resection. The surgical approach should be dictated by the size and location of the tumor. The high rate of metastatic disease in this study suggests that follow-up imaging should be performed periodically on patients with UBPGL. If metastases can be caught early, curative surgical resection may be possible; if not possible, treatment with chemotherapeutic agents like CVD may help prolong survival [28]. CVD is especially effective in the management of SDHB patients (unpublished observations).
5. Conclusions
UBPGLs are frequently associated with a noradrenergic biochemical phenotype and SDHB mutations. Thus, patients with UBPGLs should be tested for SDHB mutations. In addition, a high metastatic rate was found in our patient series, with no statistically significant difference between patients with SDHB or no known mutations, suggesting a need for regular follow-up with biochemical testing and imaging studies regardless of genetic background. Anatomic imaging with contrast-enhanced pelvic MRI and functional imaging with FDG-PET appear to have the greatest sensitivity for detecting UBPGLs and may be best for diagnosis and follow-up.
Acknowledgments
Zarina Lorenzo, a visiting fellow at NIH, acknowledges the staff of the Section of Endocrinology and Metabolism, Department of Medicine, University of Santo Tomas Hospital, Manila, Philippines, and the Thomasian Endocrine Progress, Inc. for their unwavering support during their training at the National Institutes of Health.
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Cancer Institute, National Institutes of Health, Bethesda, MD.
Contributor Information
Piyush K. Agarwal, Email: piyush.agarwal@nih.gov.
Karel Pacak, Email: karel@mail.nih.gov.
References
- 1.Whalen RK, Althausen AF, Daniels GH. Extra-adrenal pheochromocytoma. J Urol. 1972;147:1–10. doi: 10.1016/s0022-5347(17)37119-7. [DOI] [PubMed] [Google Scholar]
- 2.Eisenhofer G, Pacak K, Huynh TT, Qin N, Bratslavsky G, Linehan WM, et al. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer. 2011;18:97–111. doi: 10.1677/ERC-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmers HJ, Gimenez-Roqueplo AP, Mannelli M, Pacak K. Clinical aspects of SDHx-related pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2009;16:391–400. doi: 10.1677/ERC-08-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44:328–333. doi: 10.1055/s-0031-1301302. [DOI] [PubMed] [Google Scholar]
- 5.Ghayee HK, Havekes B, Corssmit EP, Eisenhofer G, Hammes SR, Ahmad Z, et al. Mediastinal paragangliomas: association with mutations in the succinate dehydrogenase genes and aggressive behavior. Endocr Relat Cancer. 2009;16:291–299. doi: 10.1677/ERC-08-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havekes B, Corssmit EP, Jansen JC, van der Mey AG, Vriends AH, Romijn JA. Malignant paragangliomas associated with mutations in the succinate dehydrogenase D gene. J Clin Endocrinol Metab. 2007;92:1245–1248. doi: 10.1210/jc.2006-1993. [DOI] [PubMed] [Google Scholar]
- 7.Kantorovich V, King KS, Pacak K. SDH-related pheochromocytoma and paraganglioma. Best Pract Res Clin Endocrinol Metab. 2010;24:415–424. doi: 10.1016/j.beem.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodish MB, Adams KT, Huynh TT, Prodanov T, Ling A, Chen C, et al. Succinate dehydrogenase gene mutations are strongly associated with paraganglioma of the organ of Zuckerkandl. Endocr Relat Cancer. 2010;17:581–588. doi: 10.1677/ERC-10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Athyal RP, Al-Khawari H, Arun N, Abul F, Patrick J. Urinary bladder paraganglioma in a case of von Hippel-Lindau disease. Australas Radiol. 2007;51:B67–B70. doi: 10.1111/j.1440-1673.2007.01758.x. (Spec No.) [DOI] [PubMed] [Google Scholar]
- 10.Leestma JE, Price EB. Paraganglioma of the urinary bladder. Cancer. 1971;28:1063–1073. doi: 10.1002/1097-0142(1971)28:4<1063::aid-cncr2820280433>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Beilan J, Lawton A, Hajdenberg J, Rosser CJ. Pheochromocytoma of the urinary bladder: a systematic review of the contemporary literature. BMC Urol. 2013;13:22. doi: 10.1186/1471-2490-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halefoglu AM, Miroglu C, Uysal V, Mahmutoglu A. Malignant paraganglioma of the urinary bladder. Eur J Radiol Extra. 2006;58:53–58. [Google Scholar]
- 13.Brouwers FM, Elkahloun AG, Munson PJ, Eisenhofer G, Barb J, Linehan WM, et al. Gene expression profiling of benign and malignant pheochromocytoma. Ann N Y Acad Sci. 2006;1073:541–556. doi: 10.1196/annals.1353.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monico CG, Rossetti S, Schwanz HA, Olson JB, Lundquist PA, Dawson DB, et al. Comprehensive mutation screening in 55 probands with type 1 primary hyperoxaluria shows feasibility of a gene-based diagnosis. J Am Soc Nephrol. 2007;18:1905–1914. doi: 10.1681/ASN.2006111230. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhofer G, Lenders JW, Timmers H, Mannelli M, Grebe SK, Hofbauer LC, et al. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011;57:411–420. doi: 10.1373/clinchem.2010.153320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2010;96:717–725. doi: 10.1210/jc.2010-1946. [DOI] [PubMed] [Google Scholar]
- 17.Ansari MS, Goel A, Goel S, Durairajan LN, Seth A. Malignant paraganglioma of the urinary bladder. A case report. Int Urol Nephrol. 2001;33:343–345. doi: 10.1023/a:1015253427161. [DOI] [PubMed] [Google Scholar]
- 18.Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, et al. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007;25:2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- 19.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, et al. Comparison of 18F-fluoro-l-DOPA, 18F-fluorodeoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, et al. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst. 2012;104:700–708. doi: 10.1093/jnci/djs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng L, Leibovich BC, Cheville JC, Ramnani DM, Sebo TJ, Neumann RM, et al. Paraganglioma of the urinary bladder: can biologic potential be predicted? Cancer. 2000;88:844–852. doi: 10.1002/(sici)1097-0142(20000215)88:4<844::aid-cncr15>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Dong SG, Dong Z, Mao X, Shi XY. Diagnosis and treatment of pheochromocytoma in urinary bladder. J Zhejiang Univ Sci B. 2007;8:435–438. doi: 10.1631/jzus.2007.B0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thrasher JB, Rajan RR, Perez LM, Humphrey PA, Anderson EE. Pheochromocytoma of urinary bladder: contemporary methods of diagnosis and treatment options. Urology. 1993;41:435–439. doi: 10.1016/0090-4295(93)90503-3. [DOI] [PubMed] [Google Scholar]
- 24.Deng JH, Li HZ, Zhang YS, Liu GH. Functional paragangliomas of the urinary bladder: a report of 9 cases. Chin J Cancer. 2010;29:729–734. doi: 10.5732/cjc.009.10703. [DOI] [PubMed] [Google Scholar]
- 25.Wong-You-Cheong JJ, Woodward PJ, Manning MA, Sesterhenn IA. From the archives of the AFIP: neoplasms of the urinary bladder: radiologic-pathologic correlation. Radiographics. 2006;26:553–580. doi: 10.1148/rg.262055172. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Ye H, Guo A, Wei Z, Zhang X, Zhong Y, et al. Bladder paraganglioma in adults: MR appearance in four patients. Eur J Radiol. 2011;80:e217–e220. doi: 10.1016/j.ejrad.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Havekes B, King K, Lai EW, Romijn JA, Corssmit EPM, Pacak K. New imaging approaches to phaeochromocytomas and paragangliomas. Clin Endocrinol (Oxf) 2010;72:137–145. doi: 10.1111/j.1365-2265.2009.03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez C, Rohren E, Habra MA, Rich T, Jimenez P, et al. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr Oncol Rep. 2013;15:356–371. doi: 10.1007/s11912-013-0320-x. [DOI] [PubMed] [Google Scholar]