Abstract
Few studies have evaluated clinical outcomes following caries risk assessment in large datasets that reflect risk assessments performed during routine practice.
OBJECTIVE
From clinical records, compare 18-month caries incidence according to baseline caries risk designation.
METHODS
For this retrospective cohort study, data were collected from electronic records of non-edentulous adult patients who completed an oral examination and caries risk assessment (CRA) at a university instructional clinic from 2007 to 2012 (N=18,004 baseline patients). The primary outcome was the number of new decayed/restored teeth from the initial CRA to the ensuing oral examination, through June 30, 2013 (N=4468 patients with follow-up). We obtained doubly-robust estimates for 18-month caries increment by baseline CRA category (low, moderate, high, extreme), adjusted for patient characteristics (age, sex, payer type, race/ethnicity, number of teeth), provider type, and calendar year.
RESULTS
Adjusted mean decayed, restored tooth (DFT) increment from baseline to follow-up was greater with each rising category of baseline caries risk, from low (0.94), moderate (1.26), high (1.79), to extreme (3.26). The percentage of patients with any newly affected teeth (DFT increment >0) was similar among low-risk and moderate-risk patients (cumulative incidence ratio, RR: 1.01; 95% confidence interval, CI: 0.83, 1.23), but was increased relative to low-risk patients among high-risk (RR: 1.28; 95% CI: 1.10, 1.52), and extreme-risk patients (RR: 1.52; 95% CI: 1.23, 1.87).
CONCLUSIONS
These results lend evidence that baseline caries risk predicts future caries in this setting, supporting the use of caries risk assessment to identify candidate patients for more intensive preventive therapy.
Keywords: Caries Risk Assessment, Dental Caries, Epidemiology, Caries Management, Longitudinal Studies
Introduction
A widely supported expert- and evidence-based strategy for the treatment and prevention of dental caries involves collecting patient-specific caries risk information and using that information to guide individualized treatment decisions, with emphasis on minimally invasive and/or non-operative therapies, such as remineralizing or antibacterial agents, to manage caries as a disease process.1–3 However, there is not an extensive literature that evaluates the effectiveness of current clinical risk assessment strategies to classify patients into reliable risk categories. Prognostic stratification would allow the clinician to offer personalized caries prevention and management, with the most intensive preventive therapy reserved for those patients at the greatest caries risk.
A recent systematic review concluded that the evidence supporting the predictive ability of existing caries risk assessment (CRA) systems is limited and that whether identification of high-risk patients improves clinical outcomes is unknown.4 Of the few prospective studies to asses CRA-based caries prediction in adults, in one study of 100 young adults5 and in another of 148 older individuals,6 baseline caries risk was associated with future caries. In a seven-year retrospective analysis of 200 low-risk and 200 high-risk patients attending public clinics in Sweden (in which risk status was determined by the extent of carious lesions present at baseline), initially high-risk patients experienced a significantly greater increase in caries experience.7 Large-scale evaluation of systematic approaches to risk assessment is an essential step toward widespread incorporation of risk-based caries management into dental practice.
Caries Management by Risk Assessment (CAMBRA) is one approach for patient-specific caries management, of which the first step involves categorizing caries risk based on the clinician’s overall assessment of the patient’s disease indicators, caries protective factors, and caries predisposing factors.8,9 Thus, the CAMBRA approach considers both recent disease history (e.g., radiographically detectable lesions) and biological or behavioral predisposing conditions (e.g., salivary flow rate and snacking habits) as contributory factors to disease risk. CAMBRA clinical guidelines recommend that adults deemed at elevated caries risk are subsequently offered more intensive preventive treatment, such antibacterial therapy and remineralizing agents.10
In this study, we aimed to assess the predictive capacity of the CAMBRA caries risk assessment tool by using electronic patient records at a university clinic where CAMBRA is emphasized. Previously, in a retrospective cohort study at the same clinic, higher baseline caries risk designation was associated with the recording of cavitated lesions at subsequent caries risk assessments.11
Here, we assessed a more recent cohort of patients and compared caries occurrence by baseline caries risk category, using treatment and diagnostic codes entered into electronic patient records to measure caries outcomes. Specifically, we aimed to test the hypothesis that caries increment, defined as the number of new decayed/restored teeth from baseline CRA to the subsequent periodic oral evaluation, will be greater with each increasing category of baseline caries risk.
Subjects and Methods
Population
This retrospective cohort study drew clinical data from electronic patient records at the student dental clinic of the University of California San Francisco (UCSF). This study received approval from the UCSF Committee on Human Research (IRB) to use retrospective patient data to evaluate clinical outcomes according to existing caries management practices.
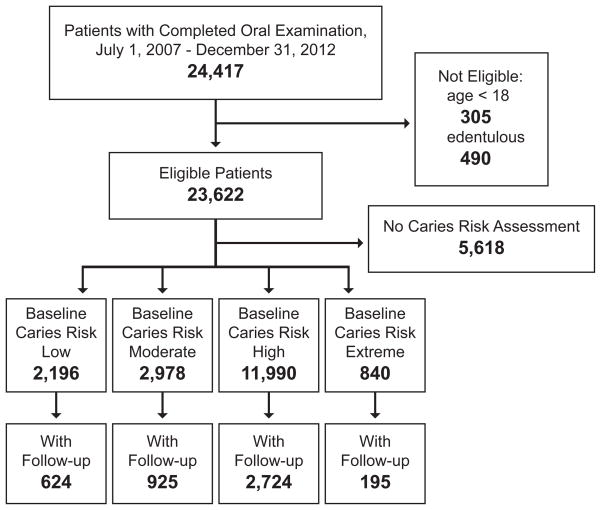
Eligible for the study were all non-edentulous patients (≥1 teeth, third molars excluded), age 18 years or older, who completed at least one full oral examination (new patient or recall) between July 1, 2007 and December 31, 2012 (N=23,622) (Figure 1). Included for analysis were those patients with a designated caries risk status category associated with the baseline examination (N=18,004). Of these patients, 4468 completed at least one follow-up periodic oral examination a minimum of 180 days after baseline (mean follow-up time: 539 days; SD: 257 days). Table 1 shows the characteristics of the baseline and follow-up samples.
Figure 1.
Participant flow diagram by inclusion criteria, caries risk assessment, and follow-up. Of all clinic patients with a completed oral examination from July 1, 2007 through December 31, 2012, the analytic sample included 18,004 eligible patients with a caries risk assessment.
Table 1.
Baseline characteristics of study population
| Characteristic | Initial Sample N=18,004 (%) |
Follow-up Sample N=4,468 (%) |
|---|---|---|
|
| ||
| Patient sex | ||
| Male | 46.9 | 49.9 |
| Female | 53.1 | 50.1 |
|
| ||
| Patient Age (years) | ||
| 18–34 | 29.5 | 19.3 |
| 35–44 | 15.8 | 13.1 |
| 45–54 | 18.2 | 19.3 |
| 55–64 | 18.4 | 22.4 |
| ≥65 | 18.1 | 25.9 |
|
| ||
| Patient payer type | ||
| Private insurance | 15.5 | 18.4 |
| Public program | 21.1 | 23.7 |
| Cash | 63.5 | 57.9 |
|
| ||
| Patient race/ethnicity | ||
| African American | 9.6 | 8.5 |
| Asian | 14.2 | 14.6 |
| Caucasian | 44.6 | 50.2 |
| Hispanic | 16.9 | 15.1 |
| Other or declined to state | 14.7 | 11.6 |
|
| ||
| Provider type | ||
| 4-year doctoral program | 77.2 | 77.8 |
| 2-year international dentist program | 22.8 | 22.2 |
|
| ||
| Provider year of training | ||
| Final year | 47.0 | 48.8 |
| Penultimate year | 53.0 | 51.2 |
Outcomes of Interest
Across the four caries risk categories (low, moderate, high, and extreme), we compared two caries incidence measures from baseline to follow-up: the number of new decayed and restored teeth (DFT increment) and the presence of any new decayed or restored teeth (DFT increment >0).
Measurement
Student providers, with guidance from faculty dentists, determined patient caries risk status at baseline after completing a CRA form, which includes existing caries protective factors (e.g. fluoride exposure; antibacterial therapy), pathological factors (e.g. frequent consumption of fermentable carbohydrates; heavy plaque on the teeth; reduced saliva), and disease indicators (e.g. cavitation; “white spot” lesions; recently placed restorations). The CRA form used in the student clinic was based on forms initially proposed following a 2002 CAMBRA consensus conference8 and later updated at annual workshops of the CAMBRA Coalition, a national expert working group.9 The expert working group selected variables for inclusion in the CRA form that were assumed to be predictive of caries risk, proposing that those patients with a “balance” toward more pathological factors and disease indicators relative to protective factors should be classified at greatest risk.9 No fixed algorithm was applied to determine risk classification in the student clinic; providers worked with faculty dentists to categorized risk as either low, moderate, high, or extreme (“extreme high”), by weighing the combined contribution of protective and pathological factors and disease indicators, as described previously.9,11
DFT increment was considered the number of teeth (non-third molars) that were recorded as having carious decay or a dental restoration at the patient’s first post-baseline oral examination but that were not already affected by caries at baseline. Patient data were retrieved from the electronic patient record using practice management software (axiUm, Exan Group, Vancouver, Canada). Carious decay excluded white spot or arrested lesions and lesions confined to enamel, but included decay around existing restorations and root caries. Teeth designated for planned restorative treatment (amalgam, composite, glass ionomer, onlay/inlay, or crown) were considered decayed, unless abfraction, attrition, or erosion was noted without caries involvement. To avoid including teeth treated only for periodontal, orthodontic, or esthetic reasons, we excluded teeth designated for extraction, as well as anterior veneers and crowns, unless caries was noted. We made the assumption that any restorative treatment completed up to 180 days after baseline was in response to carious decay that existed at baseline rather than to emergent conditions and did not include these restorations in the DFT increment. The threshold 180 days was chosen based on observed patterns in the timing of treatments relative to baseline in the student clinic.
Adjustment variables
Analyses were adjusted for baseline covariates abstracted from patient charts: age (categorized as 18–34, 35–44, 45–54, 55–64, and ≥65), sex, payer type (private dental insurance, public dental benefits program, or cash), race/ethnicity (African American, Asian, Caucasian, Hispanic, or other/declined to state), number of teeth, calendar year, student provider type (4-year DDS program or 2-year program for internationally trained dentists), student provider year of training (final year or penultimate year), and follow-up time (in days). In this observational, retrospective cohort study, whether or not a patient received non-operative anti-caries therapy depended on treatment decisions made by patients and providers during the course of care. Our primary analysis did not include adjustment for preventive therapy. However, in a secondary analysis, we adjusted for whether any non-operative, anti-caries therapy was provided, which we defined as provision of chlorhexidine rinse (0.12% chlorhexidine gluconate), topical fluoride (e.g. fluoride toothpaste at 5000 ppm F, fluoride varnish), or xylitol products (e.g. mint-flavored tablets or gum), alone or in combination, between baseline and follow-up (yes/no).
Power Estimation
The number of patients attending the clinic from 2007 to 2012 determined the sample size used in analysis. Given 624 low-risk and 2724 high-risk patients with complete follow-up examinations, there would be 96% power to detect a 0.25 difference in DFT increment (0.75 versus 1.00, standard deviation 1.5 for each) and 95% power to detect a 1.2 ratio in caries incidence (DFT increment >0) (40% versus 48%), both tests using alpha=0.05 as the threshold for statistical significance. We deemed this sample size sufficient for multivariable analysis.
Statistical Methods
We obtained doubly-robust adjusted estimates for caries outcomes using a combined approach of g-computation and inverse probability treatment weighting.12 We fitted a negative binomial regression model for DFT increment and a logistic regression model for caries incidence (DFT increment>0), both models using baseline CRA category as the exposure variable and the covariates age, sex, race/ethnicity, payer type, calendar year, provider type, provider years of training, and follow-up time. We then used those models to predict adjusted marginal outcomes under each baseline caries risk category and 18-months of follow-up time (548 days). Approachable overviews of this technique have been published elsewhere.13,14 Inverse probability treatment weights and inverse probability censoring weights were incorporated in the regression models to enhance the robustness of our estimates to model misspecification12,15 and to account for losses to follow-up, respectively. Missing baseline covariate data were multiply imputed (0.2% of covariate data among eligible participants) using a model-based approach. Point estimates were averaged over 25 imputations.
Our final adjusted estimates represent the expected average caries outcomes associated with each baseline caries risk category under the same distribution of patient and provider characteristics that was observed in the baseline population. As measures of association, we computed the difference in DFT increment and the ratio in caries incidence at moderate, high or extreme risk (low risk as the reference category). Corresponding 95% confidence intervals (CI) were obtained with the percentile bootstrap method from 3000 bootstrap re-samples to account for added variability from multiple imputation and weighting. Results were considered statistically significant at the 0.05 level if the 95% CI for measures of association excluded the null value. Analysis was completed using Stata 13.1 (College Station, USA) and R 3.1.2 (www.r-project.org). Study reporting followed STROBE guidelines.16
Results
New patients accounted for most baseline examinations (86.5%). Mean patient age was 47.3 years (standard deviation: 17.1; range: 18–99). The majority of patients lacked dental benefits coverage. Baseline and follow-up samples were similar in their measured characteristics; patients with follow-up examinations were somewhat more likely to be male, identify as Caucasian, and have private dental benefits than patients without a follow-up examination (Table 1).
The presence of caries protective factors, caries risk factors, and disease indicators, as recorded in the baseline CRA forms, was associated with the assigned caries risk category (Table 2). Low-risk patients were most likely to report twice-per-day use of fluoride toothpaste and least likely to report frequent snacking. High-risk and extreme-risk patients more commonly presented with existing carious decay or a recent history of dental restorations, with extreme-risk patients notable for the highest prevalence of inadequate salivary flow, exposed root surfaces, and heavy plaque (Table 2). At baseline, current use of preventive chemical therapies, such as high-concentration fluoride toothpaste, chlorhexidine rinse, or xylitol-containing products, was uncommon across all risk categories (Table 2).
Table 2.
Baseline caries risk assessment items, by designated risk status
| Low (%) | Moderate (%) | High (%) | Extreme (%) | p-value (Chi-square) | |
|---|---|---|---|---|---|
| Caries Pathological Factors | |||||
| Visible heavy plaque on teeth | 30.5 | 50.0 | 63.7 | 79.7 | <0.0005 |
| Frequent snacking (>3x daily) | 20.7 | 31.0 | 40.5 | 47.7 | <0.0005 |
| Deep pits and fissures | 1.9 | 2.8 | 2.5 | 0.6 | 0.001 |
| Recreational drug use | 5.3 | 7.5 | 8.6 | 13.3 | <0.0005 |
| Inadequate saliva flow | 4.0 | 7.7 | 7.4 | 61.1 | <0.0005 |
| Exposed roots | 35.3 | 47.0 | 45.0 | 68.8 | <0.0005 |
| Orthodontic appliances | 4.8 | 4.3 | 3.5 | 2.5 | 0.022 |
| Caries Protective Factors | |||||
| Home/work/school in fluoridated community | 84.0 | 80.3 | 79.8 | 71.6 | <0.0005 |
| Fluoride toothpaste (≥2x daily) | 83.2 | 77.2 | 70.6 | 61.7 | <0.0005 |
| Fluoride (0.05% NaF) mouthrinse daily | 1.6 | 1.8 | 1.5 | 1.6 | 0.002 |
| 5000 ppm F toothpaste daily | 2.4 | 2.0 | 1.9 | 4.5 | <0.0005 |
| Fluoride varnish in last 6 months | 2.0 | 1.1 | 0.9 | 1.3 | 0.002 |
| In-office topical fluoride in last 6 months | 1.5 | 0.7 | 0.6 | 0.7 | 0.008 |
| Chlorhexidine use in each of last 6 months | 1.1 | 1.5 | 1.3 | 3.6 | <0.0005 |
| Xylitol product 4x daily during last 6 months | 2.2 | 3.1 | 2.1 | 2.0 | 0.038 |
| Calcium phosphate paste during last 6 months | 0.5 | 0.7 | 0.6 | 1.2 | 0.143 |
| Adequate saliva flow (>1 ml/min stimulated) | 85.1 | 83.3 | 83.2 | 36.1 | <0.0005 |
| Disease Indicators | |||||
| Visible or radiographic cavities in dentin | 4.6 | 14.4 | 59.3 | 59.8 | <0.0005 |
| Radiographic approximal enamel lesion(s) | 1.1 | 4.8 | 17.0 | 16.4 | <0.0005 |
| White spot on smooth surfaces | 0.7 | 2.0 | 2.9 | 3.0 | <0.0005 |
| Restorations in last 3 years | 12.5 | 29.7 | 47.7 | 51.9 | <0.0005 |
Risk assessment items based on caries risk assessment form9 used in the university clinic. Baseline sample includes 18,004 patients, but sample size differs for some variables due to missing data.
Adjusted 18-month DFT increment and caries incidence were both greater with each rising category of baseline caries risk (Table 3). The difference in mean DFT increment was statistically significant between every risk category. The difference between low- and extreme-risk patients was more than two affected teeth per 18 months. However, the low and moderate risk groups did not differ significantly in the adjusted percentage of patients with DFT increment>0 (Table 3).
Table 3.
Caries increment from baseline to follow-up examination, by caries risk assessment
| Baseline Caries Risk Assessment | DFT Increment, Observed | DFT Increment, Adjusteda | DFT Increment Differencea (95% CI) | Caries Incidence (DFT>0), Observed, % | Caries Incidence (DFT>0), Adjusteda, % | Caries Incidence Risk Ratioa (95% CI) |
|---|---|---|---|---|---|---|
| Low | 1.01 | 0.94 | reference | 46.5 | 49.9 | reference |
| Moderate | 1.16 | 1.26 | 0.32 (0.02, 0.60) | 50.6 | 50.5 | 1.01 (0.83, 1.23) |
| High | 1.74 | 1.79 | 0.85 (0.63, 1.05) | 62.4 | 64.1 | 1.28 (1.10, 1.52) |
| Extreme | 2.71 | 3.26 | 2.32 (1.28, 3.49) | 71.3 | 76.0 | 1.52 (1.23, 1.87) |
Abbreviations: CI = confidence interval; DFT = decayed/restored teeth
Results based on 4,468 patients with observed follow-up from 2007 to 2012
Adjusted models account for patient age, sex, payer type, ethnicity, provider type and year in training, calendar year, baseline number of teeth, losses-to-follow-up, and set follow-up time to 18 months.
Most patients with a follow-up examination were not administered any non-operative anti-caries therapy. Yet, the percentage that was offered and accepted therapy between baseline and follow-up was greater with each increasing caries risk category (low: 12.0%; moderate: 21.3%; high: 44.9%; extreme: 72.3%). Adjustment for provision of preventive therapy did not substantially alter the association between baseline risk category and DFT increment, which maintained a graded relationship across the four risk categories (low: 0.89; moderate: 1.27; high: 1.80; extreme: 3.10).
Discussion
In this patient population, baseline caries risk was associated with future caries, including after adjustment for other patient characteristics. Clinically, the ability to stratify patients according to risk status is a pivotal element of targeted, patient-focused treatment17 and increasingly a point of emphasis for dental education and practice.18 Our results demonstrate the predictive validity of the multi-component caries risk assessment approach used in CAMBRA9 and confirm the conclusions drawn following analysis of a prior set of patients.11
Not surprisingly, the distribution of several known caries risk factors, such as recent disease history, frequent snacking, inadequate oral hygiene practices, and reduced salivary flow-rate, differed sharply over the caries risk categories. This suggests that student dental providers in this clinic were reasonably adept at assigning a risk designation based on these factors. We did not adjust the analysis for any of these individual potential predictors of caries incidence, as these factors directly influence the clinical decision-making driving assignment of a caries risk category, and therefore, are contained in the overall caries risk designation.
In a retrospective study of administrative data from two large US group practices, higher baseline caries risk, in one practice based only on existing disease and in another based on both disease indicators and additional risk factors, was associated with greater subsequent caries-related restorative treatment.19 Much like in the present analysis, caries incidence in these practices followed a gradient from low- to high-risk groups, yet a meaningful percentage of patients experienced incident tooth decay in the low (17% – 34%) and moderate (30% – 51%) categories.19 These results, along with those of the present study, highlight that existing methods for caries risk assessment are not able to predict of future carious decay with perfect accuracy, as would an ideal diagnostic tool. However, as an instrument for risk stratification, CRA appears to be well suited.
Student providers in this university clinic stratified patients into risk categories that were strongly associated with subsequent caries occurrence. Patients classified as high or extreme caries risk were notable for a higher baseline prevalence of caries pathological factors (e.g., heavy dental plaque, frequent snacking) and disease indicators (e.g., cavitated lesions, recently placed restorations), suggesting that student providers largely followed CAMBRA guidelines in assigning risk categories.9 At baseline, several of the caries protective factors were uncommon across all risk categories, specifically chemical therapies, such as fluoride varnish and chlorhexidine rinses, that are typically dispensed at dental practices. This is not surprising for a population of predominantly new patients seeking dental care after a period of potentially limited dental care utilization.
Although caries increment increased with each rising caries risk category, about half of low- and moderate-risk patients in this study experienced decay at follow-up. This could be a result of general underestimation of caries risk on the part of student providers, leading to misclassification of baseline caries risk categories, as reported in other educational settings.20 It is also possible that some teeth included in the “F” component of the DFT increment were treated for reasons other than caries, such as replacement of defective restorations, leading to overestimation of caries occurrence in all risk categories.
A recent study evaluated the predictive validity of four CRA systems side-by-side among kindergarten children in Hong Kong.21 Reason-based/checklist approaches, including CAMBRA, demonstrated greater sensitivity but lower specificity than computer-based algorithms in identifying children who would develop future decay, although all approaches demonstrated a gradient in caries incidence over baseline risk categories.21 Further research to compare the performance of new and/or existing caries risk assessment systems in adult populations would help clinicians to select the most appropriate risk assessment tool for use in practice.
In some clinical settings, completion of caries risk assessment has not necessarily resulted in delivery of preventive therapy in accordance with designated caries risk.22 In this study, patients categorized at elevated caries risk were more likely to be offered and accept non-operative anti-caries therapy, as recently reported.23 However, more than half of high-risk patients were not treated with any form of anti-caries agent. It is unknown whether less than universal use of non-operative therapies reflects reticence on the part of providers, patients, or both. For this analysis, we did not consider the type, intensity, or periodicity of therapy delivery to these patients: factors that plausibly influence prevention effectiveness and that merit further study.
Data analyzed in this study were not collected specifically for use in research. For example, student providers, although undergoing the same educational program, were not explicitly calibrated for conducting caries risk assessments. Such limitations were balanced in part by access to a large and representative analytic sample. Even without calibration of student providers, caries risk assessments, assigned with faculty guidance, were strongly correlated with future caries. This is meaningful for clinical practice, where providers do not necessarily follow identical criteria to determine patients’ caries risk.
Our analysis accounted for a number of presumptive confounding factors; however, it remains possible that unmeasured differences in patient characteristics could have influenced the results. We applied inverse probability censoring weighting to account for losses to follow-up, and the initial and follow-up sample were reasonably similar in their measured characteristics. However, the large portion of patients who failed to return for a later visit would limit the generalizeability of our findings if such patients differed considerably in unmeasured characteristics for which we could not adjust. Finally, the results were drawn from a predominantly high-risk population served by student providers at a university clinic. While the conclusions were consistent with results drawn from private practice,19 additional research involving more established practitioners is needed.
Conclusions
This analysis was among the largest longitudinal studies to evaluate clinical caries outcomes following caries risk assessment. The results add evidence that patient-focused risk assessment can validly separate patients into groups with greater or lesser potential for future tooth decay. These findings support the use of a patient-specific approach to caries risk assessment and risk-guided clinical decision-making as the future standard of care for caries management in dental practice.
Clinical Significance Statement.
Identification of patients at greater risk for future caries helps clinicians to plan appropriate personalized care. In this study, a multifactorial approach to caries risk assessment effectively stratified patients into groups of higher or lower caries propensity. Dentists can apply risk assessment in practice antecedent to patient-tailored caries management.
Acknowledgments
Thank you to Bing Espiritu and Tom Ferris of UCSF for technical assistance in abstraction of clinical data. Support was provided from the NIH National Center for Advancing Translational Sciences (KL2TR000143).
Footnotes
The authors declare that they have no conflicts of interest related to this research.
The information presented is solely the responsibility of the authors and does not necessarily represent the official views of the supporting organization.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Benjamin W. Chaffee, Email: benjamin.chaffee@ucsf.edu.
Jing Cheng, Email: jing.cheng@uscf.edu.
John D. B. Featherstone, Email: jdbf@ucsf.edu.
References
- 1.Fontana M, Young DA, Wolff MS. Evidence-based caries, risk assessment, and treatment. Dental Clinics of North America. 2009;53:149–161. doi: 10.1016/j.cden.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Hallett KB. The application of caries risk assessment in minimum intervention dentistry. Australian Dental Journal. 2013;58 (Suppl 1):26–34. doi: 10.1111/adj.12047. [DOI] [PubMed] [Google Scholar]
- 3.Lallam C, Decup F. Minimal intervention dentistry II: part 2. Management of caries and periodontal risks in general dental practice. British Dental Journal. 2014;216:179–85. doi: 10.1038/sj.bdj.2014.143. [DOI] [PubMed] [Google Scholar]
- 4.Tellez M, Gomez J, Pretty I, Ellwood R, Ismail A. Evidence on existing caries risk assessment systems: are they predictive of future caries? Community Dentistry and Oral Epidemiology. 2013;41:67–78. doi: 10.1111/cdoe.12003. [DOI] [PubMed] [Google Scholar]
- 5.Celik EU, Gokay N, Ates M. Efficiency of caries risk assessment in young adults using Cariogram. European Journal of Dentistry. 2012;6:270–279. [PMC free article] [PubMed] [Google Scholar]
- 6.Hänsel Petersson G, Fure S, Bratthall D. Evaluation of a computer-based caries risk assessment program in an elderly group of individuals. Acta Odontologica Scandinavica. 2003;61:164–171. doi: 10.1080/00016350310002261. [DOI] [PubMed] [Google Scholar]
- 7.Söderström U, Johansson I, Sunnegårdh-Grönberg K. A retrospective analysis of caries treatment and development in relation to assessed caries risk in an adult population in Sweden. BMC Oral Health. 2014;14:126. doi: 10.1186/1472-6831-14-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Featherstone JD, Adair SM, Anderson MH, Berkowitz RJ, Bird WF, Crall JJ, et al. Caries management by risk assessment: consensus statement, April 2002. Journal of the California Dental Association. 2003;31:257–269. [PubMed] [Google Scholar]
- 9.Featherstone JD, Doméjean-Orliaguet S, Jenson L, Wolff M, Young DA. Caries risk assessment in practice for age 6 through adult. Journal of the California Dental Association. 2007;35:703–707. 710–713. [PubMed] [Google Scholar]
- 10.Jenson L, Budenz AW, Featherstone JD, Ramos-Gomez FJ, Spolsky VW, Young DA. Clinical protocols for caries management by risk assessment. Journal of the California Dental Association. 2007;35:714–723. [PubMed] [Google Scholar]
- 11.Doméjean S, White JM, Featherstone JD. Validation of the CDA CAMBRA caries risk assessment--a six-year retrospective study. Journal of the California Dental Association. 2011;39:709–715. [PubMed] [Google Scholar]
- 12.Vansteelandt S, Keiding N. Invited Commentary: G-computation – lost in translation? American Journal of Epidemiology. 2011;173:739–742. doi: 10.1093/aje/kwq474. [DOI] [PubMed] [Google Scholar]
- 13.Ahern J, Hubbard A, Galea S. Estimating the effects of potential public health interventions on population disease burden: a step-by-step illustration of causal inference methods. American Journal of Epidemiology. 2009;169:1140–1147. doi: 10.1093/aje/kwp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snowden JM, Rose S, Mortimer KM. Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. American Journal of Epidemiology. 2011;173:731–738. doi: 10.1093/aje/kwq472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funk MJ, Westreich D, Wiesen C, Sturmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. American Journal of Epidemiology. 2011;173:761–767. doi: 10.1093/aje/kwq439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 17.Pitts NB, Richards D International Caries Detection and Assessment System Committee. Personalized treatment planning. Monographs in Oral Science. 2009;21:128–143. doi: 10.1159/000224217. [DOI] [PubMed] [Google Scholar]
- 18.Pitts N. Preventive and minimal intervention dentistry in the undergraduate curriculum. Journal of Dentistry. 2011;39 (Suppl 2):S41–8. doi: 10.1016/j.jdent.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Bader JD, Perrin NA, Maupomé G, Rindal B, Rush WA. Validation of a simple approach to caries risk assessment. Journal of Public Health Dentistry. 2005;65:76–81. doi: 10.1111/j.1752-7325.2005.tb02790.x. [DOI] [PubMed] [Google Scholar]
- 20.Teich ST, Demko C, Al-Rawi W, Gutberg T. Assessment of implementation of a CAMBRA-based program in a dental school environment. Journal of Dental Education. 2013;77:438–47. [PubMed] [Google Scholar]
- 21.Gao X, Di Wu I, Lo EC, Chu CH, Hsu CY, Wong MC. Validity of caries risk assessment programmes in preschool children. Journal of Dentistry. 2013;41:787–95. doi: 10.1016/j.jdent.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Rindal DB, Rush WA, Perrin NA, Maupomé G, Bader JD. Outcomes associated with dentists’ risk assessment. Community Dentistry and Oral Epidemiology. 2006;34:381–6. doi: 10.1111/j.1600-0528.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- 23.Chaffee BW, Featherstone JDB. Long-Term Adoption of Caries Management by Risk Assessment Among Dental Students in a University Clinic. Journal of Dental Education. In Press. [PMC free article] [PubMed] [Google Scholar]