Abstract
Background
Mammographic density (MD) is a strong breast cancer risk factor. We previously reported associations of percent MD with larger and node-positive tumors across all ages, and estrogen receptor (ER)-negative status among women ages <55 years. To provide insight into these associations, we examined the components of percent MD (dense area (DA) and non-dense area (NDA) with breast cancer subtypes.
Methods
Data were pooled from six studies including 4095 breast cancers and 8558 controls. DA and NDA were assessed from digitized film-screen mammograms and standardized across studies. Breast cancer odds by density phenotypes and age according to histopathological characteristics and receptor status were calculated using polytomous logistic regression.
Results
DA was associated with increased breast cancer risk [odds ratios (OR) for quartiles: 0.65, 1.00(Ref), 1.22, 1.55; p-trend <0.001] and NDA was associated with decreased risk [ORs for quartiles: 1.39, 1.00(Ref), 0.88, 0.72; p-trend <0.001] across all ages and invasive tumor characteristics. There were significant trends in the magnitude of associations of both DA and NDA with breast cancer by increasing tumor size (p-trend<0.001) but no differences by nodal status. Among women <55 years, DA was more strongly associated with increased risk of ER+ vs. ER− tumors [p-heterogeneity (het) = 0.02] while NDA was more strongly associated with decreased risk of ER− vs. ER+ tumors [p-het = 0.03].
Conclusions
DA and NDA have differential associations with ER+ vs. ER− tumors that vary by age.
Impact
DA and NDA are important to consider when developing age- and subtype-specific risk models.
Keywords: Mammographic density, Breast density, Breast cancer, Tumor subtypes, Mammography, Epidemiology
Introduction
Mammographic density (MD) represents the variability of breast tissue composition on the mammogram image. Radiographically, there are two main components of breast tissue: fat, which appears dark on a mammogram and is considered “non-dense”, and fibroglandular tissue (i.e., epithelial cells and connective tissue), which appears white and is defined as “dense” tissue (1). Women in the highest quartile of percent MD (i.e., proportion of dense fibroglandular tissue within the total area of the breast) have about 4 times the risk of developing breast cancer compared to women in the lowest quartile, even after adjusting for other known breast cancer risk factors (2). The biological mechanism by which MD increases breast cancer risk, however, remains largely unknown.
We reported percent MD to be a breast cancer risk factor across tumor characteristics and age groups (3). We noted stronger associations for tumors of large size and positive lymph nodes across all ages, and ER-negative status among women ages <55 years, suggesting high MD may play an important role in tumor aggressiveness, especially in younger women. Recent evidence from a large meta-analysis suggests that dense and non-dense area may be independently associated with breast cancer risk (4–7). Few previous studies have evaluated the possible differential associations of dense and non-dense breast area by breast cancer subtype or tumor characteristics. Therefore, we investigated the underlying associations of dense (fibroglandular) or non-dense (adipose) area, or both, with tumor characteristics. Understanding these differential associations could provide insight into the mechanism by which percent density influences risk.
Materials and Methods
Study populations
Participating studies included the Mayo Mammography Health Study (MMHS) (8, 9), Mayo Clinic Breast Cancer Study (MCBCS) (10, 11), Nurses’ Health Study (NHS) and NHSII (12–14), Mayo Clinic Mammography Study (MCMAM) (15), and the San Francisco Bay Area Breast Cancer SPORE and San Francisco Mammography Registry (SFMR) (16–18) (Table 1). Details of the study populations are in Supplementary Table S1 and described in our earlier report (3); the present analysis includes additional cases and controls, primarily from the SFMR, which were not available at the time of our earlier analysis. Incident breast cancer cases were identified by self-report, linkage to clinic and/or statewide tumor registries, or death certificates with further confirmation by medical record review. Controls were selected from the underlying cohorts (MMHS, NHS, NHSII, SFMR) or from the source population (MCBCS, MCMAM) and typically matched to cases on age, menopausal status, and year of examination (MMHS, MCMAM, SFMR), blood draw (NHS, NHSII) or diagnosis (MCBCS) as described previously (3). From all studies, we excluded breast cancer cases diagnosed within 6 months of mammography and their matched controls, to minimize prevalent cancers at time of mammography. Covariate information was obtained from medical record review (MCMAM), self-administered questionnaires (NHS, NHSII), or both (MMHS, MCBCS) prior to (NHS, NHSII) or at the time of (MSBCS, MMHS, MCMAM, SFMR) mammography. In total, these analyses included 4095 breast cancer cases and 8558 controls.
Table 1.
Baseline characteristics of study population by age.
| Age <55 |
Age ≥55 |
|||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| N | 1884 | 4072 | 2211 | 4486 |
| Standardized % mammographic density Median (IQR) | 40.7 (30.2) | 32 (30.8) | 25 (26.1) | 19 (23.2) |
| Standardized Dense Area cm2 Median (IQR) | 51.9 (44.4) | 42.2 (41.8) | 41.1 (43.0) | 31.6 (37.3) |
| Standardized Nondense Area cm2 Median (IQR) | 79.6 (92.4) | 98 (100.7) | 130.7 (123.7) | 138.6 (119.9) |
| Mean age at mammogram (SD) | 47.2 (4.6) | 47.3 (4.5) | 64.9 (7.4) | 65.1 (7.4) |
| Mean age at diagnosis (SD) | 51.6 (5.5) | -- | 69 (7.6) | -- |
| Mean BMI (SD) | 24.2 (6.5) | 25.2 (6) | 25.6 (7.8) | 25.9 (5.5) |
| Body mass index categories, kg/m2 | ||||
| <25 | 1072 (56.9%) | 2352 (57.8%) | 909 (41.1%) | 2213 (49.3%) |
| 25–29 | 507 (26.9%) | 1007 (24.7%) | 701 (31.7%) | 1422 (31.7%) |
| 30–34 | 157 (8.3%) | 399 (9.8%) | 319 (14.4%) | 553 (12.3%) |
| 35+ | 85 (4.5%) | 275 (6.8%) | 177 (8%) | 275 (6.1%) |
| Unknown | 63 (3.3%) | 39 (1%) | 105 (4.7%) | 23 (0.5%) |
| Menopausal status | ||||
| Premenopausal | 1159 (61.5%) | 2629 (64.6%) | 16 (0.7%) | 44 (1%) |
| Postmenopausal | 556 (29.5%) | 1220 (30%) | 2191 (99.1%) | 4434 (98.8%) |
| Unknown | 169 (9%) | 223 (5.5%) | 4 (0.2%) | 8 (0.2%) |
| Parity | ||||
| Nulliparous | 419 (22.2%) | 901 (22.1%) | 330 (14.9%) | 624 (13.9%) |
| Parous | 1315 (69.8%) | 3046 (74.8%) | 1675 (75.8%) | 3526 (78.6%) |
| Unknown | 150 (8%) | 125 (3.1%) | 206 (9.3%) | 336 (7.5%) |
| Postmenopausal hormone therapya | ||||
| Not current user | 193 (41.1%) | 498 (45.8%) | 923 (51.6%) | 2403 (61.9%) |
| Current, estrogen | 90 (19.1%) | 256 (23.5%) | 272 (15.2%) | 561 (14.5%) |
| Current, estrogen + progestin | 155 (33%) | 290 (26.7%) | 373 (20.9%) | 574 (14.8%) |
| Unknown | 32 (6.8%) | 44 (4%) | 220 (12.3%) | 343 (8.8%) |
| Family history | ||||
| No | 1467 (77.9%) | 3588 (88.1%) | 1644 (74.4%) | 3745 (83.5%) |
| Yes | 315 (16.7%) | 466 (11.4%) | 463 (20.9%) | 706 (15.7%) |
| Unknown | 102 (5.4%) | 18 (0.4%) | 104 (4.7%) | 35 (0.8%) |
Among postmenopausal women in MMHS, NHS, NHSII, and UCSF.
IQR: interquartile range
This study was approved by the Institutional Review Boards at Mayo Clinic, Brigham and Women’s Hospital, the University of California, San Francisco (UCSF), and the Connecticut Department of Public Health Human Investigations Committee. Informed consent was obtained or implied by return of questionnaires (NHS, NHSII).
Assessment of mammographic density
As described previously (3), dense area (DA) and non-dense area (NDA), were measured using two computer-assisted threshold techniques (Cumulus (19) and UCSF custom mammographic density software) (20) from digitized images of pre-diagnostic film screening mammograms of the craniocaudal view. Percent MD was calculated as the proportion of absolute DA over total breast area (DA + NDA). With the exception of NHS and NHSII, for which average DA and NDA of both breasts was used, DA and NDA were estimated from the contralateral breast for cases and corresponding side for matched controls.
We standardized PMD, DA and NDA measurements made within each study to remove variability in measurements due to reader (1, 21), time of density assessment, and age distributions of different study populations for pooled analyses (Supplementary Figure S1). We have previously described and applied this method to percent MD using a logit transformation (3). For absolute dense and non-dense areas, an appropriate transformation was selected via the Box-Cox procedure. For DA, the square root transformation was selected while the 4th root was selected for non-dense area. Briefly the following procedure was implemented on each measure after appropriate transformation. First, we focused on women without breast cancer and estimated study-specific linear age trends in the medians of transformed MD (TMD) values using quantile regression. Study-specific age trends were removed by computing the difference between each individual’s observed TMD and the age-predicted median TMD from the corresponding study set. Variability was standardized across studies by dividing the residuals within each study by the corresponding inter-quartile range (IQR), and then multiplying these re-scaled residual values by the IQR of the original residuals from all studies. This ensured that the variability in standardized TMD was consistent across studies, and roughly equivalent to the observed variability in TMD. Finally, we estimated an overall age by TMD trend from the original data, and added the age-predicted median TMD to the rescaled residuals from each individual. This reincorporated the known age trend in MD into the standardized TMD measurements (Supplementary Figure S2). These TMD values were back-transformed to the original scale for use in analyses. Of note, variability in the tails of the smoother and limited data under age 40 (n=68 controls), resulted in an apparent difference in the distribution for DA for the NHS2 study (Supplementary Figure 2).
Assessment of tumor characteristics among cases
Information on tumor type, histology, grade, nodal involvement, tumor size, and ER, PR, and HER2 status was obtained from state-wide Surveillance Epidemiology and End Results programs (SFMR), pathology reports (NHS, NHSII), state and clinic cancer registries (MMHS, MCMAM, MCBCS), and medical records (MMHS, MCMAM, MCBCS). For 313 cases in NHS, 52 cases in NHS II, and 194 cases in MCMAM with missing receptor data on pathology reports, receptor status was obtained from immunohistochemical staining performed on paraffin sections of the tumor tissue microarray (TMA) according to a standard protocol (22). A proportion of cases (18%; N=624 cases ranging from 8% to 34% across studies) were still missing HER2 status after incorporating the TMA data and were excluded from HER2 analyses. Another 2% were cases with borderline HER2 results (2+ without available FISH) and not used in analyses.
Statistical analyses
We categorized the standardized DA and NDA measurements into quartiles based on the control distribution across studies. We fit polytomous (multinomial) logistic regression models to estimate odds ratios (OR) and 95% confidence intervals (CI) for the associations of DA and NDA with risk of breast cancer overall as well as with breast cancer defined by tumor type (invasive or DCIS), histologic type (ductal or lobular), grade (well-differentiated, moderately differentiated, or poorly differentiated), tumor size (<1.1 cm, 1.1–2.0 cm, or 2.1+ cm), involvement of lymph nodes (positive or negative), and receptor status (ER+ or ER−; PR+ or PR−; HER2+ or HER2−). We also updated our prior analyses of percent MD (3) with this larger sample size for comparison purposes; percent MD categories were 0–10%, 11–25% (reference), 26%–50%, and 51%+.
We pooled data across the six studies and adjusted for study site, age (continuous), and body mass index (BMI, continuous) in multivariable models. We further considered potential confounding by parity (nulliparous, parous, or unknown) and first-degree family history of breast cancer (yes, no, or unknown) by evaluating the magnitude of change in ORs observed after including each potential confounder individually in the model. Postmenopausal hormone therapy (current estrogen alone, current estrogen plus progesterone, never/former, or unknown) was also evaluated as a confounder among postmenopausal women in the subset of studies for which this information was available (MMHS, NHS, NHSII, and SFMR). Addition of these variables to the models did not substantially change risk estimates and were not included in final models. In secondary analyses, we considered models that mutually adjusted for continuous measures of square root DA and NDA.
Because our previous findings suggested differences of percent MD and tumor characteristics primarily for younger women, we stratified by ages <55 years vs. ≥55 years only. We evaluated whether the associations between DA or NDA and breast cancer differed by specific tumor characteristics, both overall and within age groups, using polytomous logistic regression models (Pheterogeneity). For subtypes with a natural ordering, including tumor size and grade, tests of trend (Ptrend) across categories were used to assess significance. Formal tests of interaction (Page-interaction) assessed the significance of differential DA and NDA associations with each of the breast cancer characteristics and subtype by age groups.
Prior to pooling data across the six studies, study specific estimates were obtained by fitting separate models for each study and assessing individual associations between MD and each tumor subtype. We assessed the statistical significance of differences in associations by study site by testing for interactions between study group and DA or NDA category in the pooled analysis and, in general, found no evidence of differences across study (P-values >0.09) other than as noted in results below.
Analyses were performed using SAS software (version 9.3, SAS Institute, Cary, NC). All statistical tests were two-sided and P-values <0.05 were considered statistically significant.
Results
Overall, mean age at mammogram was 57 years among both cases and controls. Median time to diagnosis at mammogram was 4.1 years (interquartile range: 2.3–6.0) for cases. DA and NDA were not strongly correlated in the combined study population (r=0.07 based on continuous measure) or across individual study populations (correlations ranged from 0.06 (NHS) to 0.29 (NHS2)). Among both cases and controls, median percent MD and DA was lower while NDA was higher in women ages ≥55 years than women <55 years. Further, within each age group, median percent MD and DA was higher among cases vs. controls. Median NDA was lower among cases vs. controls in women ages <55 years but similar in cases and controls ≥55 years (Table 1). DCIS was more common among women ages <55 years (15.8%) vs. women ≥55 years (11.7%), while, among invasive cancers, more aggressive tumor characteristics were evident in women <55 years at mammography compared to women ≥55 years (Table 2).
Table 2.
Distribution (%) of breast cancer cases from six studies by age and tumor characteristics
| Age < 55 | Age ≥55 | |||
|---|---|---|---|---|
| N | % | N | % | |
|
|
|
|||
| Controls | 4072 | 68.4 | 4486 | 67 |
| Cases | 1884 | 31.6 | 2211 | 33 |
| Invasive | 1579 | 83.8 | 1944 | 87.9 |
| In situ | 297 | 15.8 | 259 | 11.7 |
| Unknown | 8 | 0.4 | 8 | 0.4 |
| Tumor characteristics | ||||
| Histology | ||||
| Ductal | 1277 | 80.9 | 1437 | 73.9 |
| Lobular | 156 | 9.9 | 265 | 13.6 |
| Mixed | 88 | 5.6 | 133 | 6.8 |
| Unknown/other | 58 | 3.7 | 109 | 5.6 |
| Histologic Grade | ||||
| Well differentiated | 393 | 24.9 | 622 | 32 |
| Moderately differentiated | 605 | 38.3 | 739 | 38 |
| Poorly differentiated | 447 | 28.3 | 373 | 19.2 |
| Unknown | 134 | 8.5 | 210 | 10.8 |
| Tumor size | ||||
| 0.1–1.0 cm | 488 | 30.9 | 701 | 36.1 |
| 1.1.–2.0 cm | 633 | 40.1 | 744 | 38.3 |
| 2.1+ cm | 409 | 25.9 | 435 | 22.4 |
| Unknown | 49 | 3.1 | 64 | 3.3 |
| Involvement of lymph nodes | ||||
| Negative | 1054 | 66.8 | 1323 | 68.1 |
| Positive | 445 | 28.2 | 422 | 21.7 |
| Unknown | 80 | 5.1 | 199 | 10.2 |
| Estrogen Receptor status | ||||
| Negative | 289 | 18.3 | 279 | 14.4 |
| Positive | 1236 | 78.3 | 1581 | 81.3 |
| Borderline/Unknown | 54 | 3.4 | 84 | 4.3 |
| Progesterone Receptor status | ||||
| Negative | 407 | 25.8 | 476 | 24.5 |
| Positive | 1114 | 70.6 | 1383 | 71.1 |
| Borderline/Unknown | 58 | 3.7 | 85 | 4.4 |
| HER2 status | ||||
| Negative | 1092 | 69.2 | 1268 | 65.2 |
| Positive | 231 | 14.6 | 223 | 11.5 |
| Borderline/Unknown | 256 | 16.2 | 453 | 23.3 |
In general, results of our updated analyses for percent MD were consistent with our previous report which included a large subset of these data (3) and are presented in Tables 3 and 4. However, our earlier report stratified age into three categories, instead of two as shown here (Table 4). Consistent with our earlier analyses, we found significant positive associations between percent MD and breast cancer risk. Briefly, with the addition of new cases (mostly invasive) and controls, we found similar or stronger associations than what we previously reported. In the updated analyses, we continue to observe stronger associations with increasing tumor size, positive nodal status, and lobular (vs. ductal) cancer)(Phet<0.02) across age groups (Table 3). Among women <55 years, there were stronger associations with node positive vs. node negative tumors (Table 4). Of note, the associations of percent MD with ER-negative vs. ER-positive tumors are not statistically significantly different across the two age groups examined here, <55 vs. 55+ (Page-interaction =0.12). However, when we analyzed by the original three age groups, the age-interaction remains (Page-interaction =0.048) suggesting it is partially driven by differential associations across the older age groups (Data not shown). Below, we focus on results for DA and NDA.
Table 3.
Associations of categoriesa of percent density, dense area, and non dense area with breast cancer overall and by morphological subtypes
| PERCENT DENSITY | DENSE AREA | NON DENSE AREA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. cases | No. controls | OR (95% CI)b | No. cases | No. controls | OR (95% CI)b | No. cases | No. controls | OR (95% CI)b | ||
| Overall breast cancer | ||||||||||
| Category 1 | 517 | 1784 | 0.63 (0.56, 0.72) | 618 | 2139 | 0.65 (0.58, 0.74) | 1280 | 2139 | 1.39 (1.25, 1.55) | |
| Category 2 (REF) | 1015 | 2597 | 1.00 (REF) | 910 | 2140 | 1.00 (REF) | 983 | 2140 | 1.00 (REF) | |
| Category 3 | 1626 | 2890 | 1.62 (1.47, 1.79) | 1134 | 2139 | 1.22 (1.10, 1.37) | 943 | 2139 | 0.88 (0.78, 0.98) | |
| Category 4 | 937 | 1287 | 2.34 (2.07, 2.65) | 1433 | 2140 | 1.55 (1.40, 1.73) | 889 | 2140 | 0.72 (0.63, 0.81) | |
| Invasiveness | ||||||||||
| In situ | ||||||||||
| Category 1 | 51 | 1784 | 0.51 (0.37, 0.72) | 73 | 2139 | 0.56 (0.42, 0.75) | 184 | 2139 | 1.19 (0.94, 1.51) | |
| Category 2 (REF) | 140 | 2597 | 1.00 (REF) | 135 | 2140 | 1.00 (REF) | 143 | 2140 | 1.00 (REF) | |
| Category 3 | 242 | 2890 | 1.58 (1.26, 1.98) | 167 | 2139 | 1.18 (0.93, 1.50) | 124 | 2139 | 0.85 (0.66, 1.10) | |
| Category 4 | 123 | 1287 | 1.88 (1.42, 2.49) | 181 | 2140 | 1.29 (1.02, 1.63) | 105 | 2140 | 0.75 (0.56, 1.00) | |
| Invasive | ||||||||||
| Category 1 | 461 | 1784 | 0.64 (0.56, 0.73) | 542 | 2139 | 0.67 (0.59, 0.76) | 1089 | 2139 | 1.42 (1.27, 1.60) | |
| Category 2 (REF) | 875 | 2597 | 1.00 (REF) | 770 | 2140 | 1.00 (REF) | 836 | 2140 | 1.00 (REF) | |
| Category 3 | 1377 | 2890 | 1.62 (1.45, 1.80) | 961 | 2139 | 1.23 (1.10, 1.38) | 818 | 2139 | 0.89 (0.79, 1.00) | |
| Category 4 | 810 | 1287 | 2.40 (2.11, 2.74) | 1250 | 2140 | 1.61 (1.44, 1.80) | 780 | 2140 | 0.71 (0.62, 0.81) | |
| p-het | 0.18 | 0.25 | 0.40 | |||||||
| Histologyc | ||||||||||
| Ductal | ||||||||||
| Category 1 | 356 | 1784 | 0.67 (0.57, 0.77) | 433 | 2139 | 0.72 (0.63, 0.83) | 838 | 2139 | 1.38 (1.22, 1.56) | |
| Category 2 (REF) | 665 | 2597 | 1.00 (REF) | 581 | 2140 | 1.00 (REF) | 650 | 2140 | 1.00 (REF) | |
| Category 3 | 1073 | 2890 | 1.62 (1.44, 1.82) | 749 | 2139 | 1.27 (1.12, 1.44) | 635 | 2139 | 0.90 (0.79, 1.03) | |
| Category 4 | 620 | 1287 | 2.32 (2.01, 2.67) | 951 | 2140 | 1.61 (1.42, 1.82) | 591 | 2140 | 0.71 (0.61, 0.82) | |
| Lobular | ||||||||||
| Category 1 | 49 | 1784 | 0.51 (0.36, 0.72) | 51 | 2139 | 0.49 (0.35, 0.70) | 135 | 2139 | 1.59 (1.21, 2.10) | |
| Category 2 (REF) | 114 | 2597 | 1.00 (REF) | 96 | 2140 | 1.00 (REF) | 98 | 2140 | 1.00 (REF) | |
| Category 3 | 148 | 2890 | 1.44 (1.11, 1.87) | 107 | 2139 | 1.13 (0.85, 1.50) | 95 | 2139 | 0.82 (0.61, 1.11) | |
| Category 4 | 110 | 1287 | 3.00 (2.23, 4.04) | 167 | 2140 | 1.83 (1.41, 2.37) | 93 | 2140 | 0.65 (0.47, 0.89) | |
| p-het | 0.02 | 0.03 | 0.50 | |||||||
| Histologic grade | ||||||||||
| Well differentiated | ||||||||||
| Category 1 | 139 | 1784 | 0.62 (0.50, 0.77) | 157 | 2139 | 0.70 (0.56, 0.87) | 284 | 2139 | 1.25 (1.03, 1.51) | |
| Category 2 (REF) | 274 | 2597 | 1.00 (REF) | 215 | 2140 | 1.00 (REF) | 247 | 2140 | 1.00 (REF) | |
| Category 3 | 379 | 2890 | 1.44 (1.22, 1.71) | 283 | 2139 | 1.35 (1.11, 1.63) | 246 | 2139 | 0.91 (0.75, 1.10) | |
| Category 4 | 223 | 1287 | 2.23 (1.81, 2.74) | 360 | 2140 | 1.75 (1.45, 2.10) | 238 | 2140 | 0.77 (0.62, 0.95) | |
| Moderately differentiated | ||||||||||
| Category 1 | 177 | 1784 | 0.65 (0.53, 0.79) | 202 | 2139 | 0.69 (0.57, 0.84) | 398 | 2139 | 1.37 (1.16, 1.62) | |
| Category 2 (REF) | 327 | 2597 | 1.00 (REF) | 280 | 2140 | 1.00 (REF) | 313 | 2140 | 1.00 (REF) | |
| Category 3 | 523 | 2890 | 1.64 (1.40, 1.91) | 353 | 2139 | 1.25 (1.05, 1.48) | 329 | 2139 | 0.96 (0.81, 1.14) | |
| Category 4 | 317 | 1287 | 2.53 (2.10, 3.04) | 509 | 2140 | 1.81 (1.54, 2.13) | 304 | 2140 | 0.74 (0.61, 0.90) | |
| Poorly differentiated | ||||||||||
| Category 1 | 99 | 1784 | 0.65 (0.50, 0.84) | 120 | 2139 | 0.62 (0.48, 0.79) | 279 | 2139 | 1.55 (1.27, 1.90) | |
| Category 2 (REF) | 184 | 2597 | 1.00 (REF) | 188 | 2140 | 1.00 (REF) | 195 | 2140 | 1.00 (REF) | |
| Category 3 | 335 | 2890 | 1.79 (1.47, 2.17) | 221 | 2139 | 1.12 (0.91, 1.38) | 170 | 2139 | 0.80 (0.64, 0.99) | |
| Category 4 | 202 | 1287 | 2.62 (2.08, 3.30) | 291 | 2140 | 1.45 (1.19, 1.77) | 176 | 2140 | 0.65 (0.51, 0.83) | |
| p-het | 0.68 | 0.77 | 0.76 | |||||||
| Tumor size | ||||||||||
| <1.1 cm | ||||||||||
| Category 1 | 219 | 1784 | 0.90 (0.75, 1.09) | 244 | 2139 | 0.90 (0.74, 1.08) | 319 | 2139 | 1.19 (0.99, 1.42) | |
| Category 2 (REF) | 318 | 2597 | 1.00 (REF) | 262 | 2140 | 1.00 (REF) | 282 | 2140 | 1.00 (REF) | |
| Category 3 | 429 | 2890 | 1.33 (1.13, 1.56) | 314 | 2139 | 1.19 (1.00, 1.42) | 280 | 2139 | 0.94 (0.78, 1.12) | |
| Category 4 | 223 | 1287 | 1.70 (1.39, 2.07) | 369 | 2140 | 1.42 (1.20, 1.69) | 308 | 2140 | 0.93 (0.77, 1.14) | |
| 1.1 – 2.0 cm | ||||||||||
| Category 1 | 149 | 1784 | 0.52 (0.43, 0.64) | 188 | 2139 | 0.57 (0.47, 0.69) | 463 | 2139 | 1.62 (1.37, 1.90) | |
| Category 2 (REF) | 349 | 2597 | 1.00 (REF) | 320 | 2140 | 1.00 (REF) | 311 | 2140 | 1.00 (REF) | |
| Category 3 | 547 | 2890 | 1.60 (1.37, 1.86) | 388 | 2139 | 1.20 (1.02, 1.41) | 328 | 2139 | 0.96 (0.81, 1.14) | |
| Category 4 | 332 | 1287 | 2.45 (2.05, 2.93) | 481 | 2140 | 1.50 (1.29, 1.76) | 275 | 2140 | 0.68 (0.56, 0.82) | |
| 2.1+ cm | ||||||||||
| Category 1 | 74 | 1784 | 0.42 (0.32, 0.56) | 91 | 2139 | 0.51 (0.39, 0.66) | 273 | 2139 | 1.47 (1.21, 1.79) | |
| Category 2 (REF) | 189 | 2597 | 1.00 (REF) | 165 | 2140 | 1.00 (REF) | 214 | 2140 | 1.00 (REF) | |
| Category 3 | 349 | 2890 | 2.02 (1.67, 2.45) | 224 | 2139 | 1.32 (1.07, 1.64) | 186 | 2139 | 0.74 (0.60, 0.92) | |
| Category 4 | 232 | 1287 | 3.60 (2.88, 4.51) | 364 | 2140 | 2.13 (1.75, 2.60) | 171 | 2140 | 0.49 (0.39, 0.63) | |
| p-het | <0.001 | <0.001 | <0.001 | |||||||
| Involvement of lymph nodes | ||||||||||
| Negative | ||||||||||
| Category 1 | 314 | 1784 | 0.64 (0.55, 0.75) | 370 | 2139 | 0.68 (0.59, 0.79) | 722 | 2139 | 1.37 (1.20, 1.57) | |
| Category 2 (REF) | 609 | 2597 | 1.00 (REF) | 520 | 2140 | 1.00 (REF) | 565 | 2140 | 1.00 (REF) | |
| Category 3 | 908 | 2890 | 1.50 (1.33, 1.69) | 658 | 2139 | 1.24 (1.08, 1.41) | 568 | 2139 | 0.93 (0.81, 1.07) | |
| Category 4 | 546 | 1287 | 2.25 (1.95, 2.61) | 829 | 2140 | 1.58 (1.39, 1.79) | 522 | 2140 | 0.73 (0.63, 0.85) | |
| Positive | ||||||||||
| Category 1 | 89 | 1784 | 0.56 (0.43, 0.74) | 117 | 2139 | 0.61 (0.48, 0.78) | 300 | 2139 | 1.61 (1.32, 1.96) | |
| Category 2 (REF) | 189 | 2597 | 1.00 (REF) | 186 | 2140 | 1.00 (REF) | 202 | 2140 | 1.00 (REF) | |
| Category 3 | 369 | 2890 | 1.96 (1.62, 2.37) | 230 | 2139 | 1.18 (0.96, 1.45) | 191 | 2139 | 0.86 (0.70, 1.07) | |
| Category 4 | 220 | 1287 | 2.89 (2.31, 3.61) | 334 | 2140 | 1.70 (1.40, 2.07) | 174 | 2140 | 0.63 (0.49, 0.80) | |
| p-het | 0.01 | 0.39 | 0.11 | |||||||
| ER status | ||||||||||
| Negative | ||||||||||
| Category 1 | 69 | 1784 | 0.7 (0.51, 0.95) | 88 | 2139 | 0.57 (0.43, 0.75) | 209 | 2139 | 1.66 (1.31, 2.10) | |
| Category 2 (REF) | 126 | 2597 | 1.00 (REF) | 151 | 2140 | 1.00 (REF) | 136 | 2140 | 1.00 (REF) | |
| Category 3 | 236 | 2890 | 1.81 (1.43, 2.27) | 153 | 2139 | 0.96 (0.76, 1.21) | 113 | 2139 | 0.76 (0.59, 0.99) | |
| Category 4 | 137 | 1287 | 2.49 (1.89, 3.26) | 176 | 2140 | 1.11 (0.88, 1.40) | 110 | 2140 | 0.61 (0.45, 0.81) | |
| Positive | ||||||||||
| Category 1 | 373 | 1784 | 0.63 (0.54, 0.72) | 431 | 2139 | 0.69 (0.60, 0.79) | 837 | 2139 | 1.38 (1.22, 1.56) | |
| Category 2 (REF) | 716 | 2597 | 1.00 (REF) | 589 | 2140 | 1.00 (REF) | 667 | 2140 | 1.00 (REF) | |
| Category 3 | 1082 | 2890 | 1.57 (1.40, 1.76) | 764 | 2139 | 1.28 (1.13, 1.45) | 664 | 2139 | 0.90 (0.79, 1.03) | |
| Category 4 | 646 | 1287 | 2.40 (2.09, 2.76) | 1033 | 2140 | 1.74 (1.54, 1.97) | 649 | 2140 | 0.73 (0.64, 0.85) | |
| p-het | 0.64 | 0.003 | 0.05 | |||||||
| PR status | ||||||||||
| Negative | ||||||||||
| Category 1 | 119 | 1784 | 0.67 (0.53, 0.86) | 137 | 2139 | 0.59 (0.47, 0.73) | 277 | 2139 | 1.41 (1.16, 1.72) | |
| Category 2 (REF) | 220 | 2597 | 1.00 (REF) | 221 | 2140 | 1.00 (REF) | 215 | 2140 | 1.00 (REF) | |
| Category 3 | 349 | 2890 | 1.61 (1.34, 1.93) | 245 | 2139 | 1.08 (0.89, 1.31) | 191 | 2139 | 0.81 (0.66, 1.01) | |
| Category 4 | 195 | 1287 | 2.23 (1.79, 2.78) | 280 | 2140 | 1.24 (1.03, 1.50) | 200 | 2140 | 0.72 (0.57, 0.90) | |
| Positive | ||||||||||
| Category 1 | 324 | 1784 | 0.63 (0.54, 0.73) | 381 | 2139 | 0.70 (0.60, 0.82) | 762 | 2139 | 1.42 (1.24, 1.61) | |
| Category 2 (REF) | 619 | 2597 | 1.00 (REF) | 515 | 2140 | 1.00 (REF) | 588 | 2140 | 1.00 (REF) | |
| Category 3 | 971 | 2890 | 1.61 (1.43, 1.82) | 670 | 2139 | 1.28 (1.12, 1.47) | 590 | 2139 | 0.91 (0.79, 1.04) | |
| Category 4 | 583 | 1287 | 2.47 (2.14, 2.86) | 931 | 2140 | 1.79 (1.58, 2.04) | 557 | 2140 | 0.71 (0.61, 0.83) | |
| p-het | 0.70 | 0.007 | 0.74 | |||||||
| HER2 status | ||||||||||
| Negative | ||||||||||
| Category 1 | 325 | 1784 | 0.66 (0.57, 0.77) | 355 | 2139 | 0.65 (0.56, 0.76) | 686 | 2139 | 1.36 (1.19, 1.56) | |
| Category 2 (REF) | 591 | 2597 | 1.00 (REF) | 517 | 2140 | 1.00 (REF) | 543 | 2140 | 1.00 (REF) | |
| Category 3 | 898 | 2890 | 1.56 (1.38, 1.76) | 613 | 2139 | 1.17 (1.03, 1.34) | 566 | 2139 | 0.96 (0.83, 1.10) | |
| Category 4 | 546 | 1287 | 2.40 (2.07, 2.78) | 875 | 2140 | 1.68 (1.48, 1.91) | 565 | 2140 | 0.81 (0.70, 0.95) | |
| Positive | ||||||||||
| Category 1 | 52 | 1784 | 0.70 (0.49, 0.99) | 63 | 2139 | 0.67 (0.48, 0.93) | 154 | 2139 | 1.37 (1.06, 1.77) | |
| Category 2 (REF) | 92 | 2597 | 1.00 (REF) | 92 | 2140 | 1.00 (REF) | 121 | 2140 | 1.00 (REF) | |
| Category 3 | 204 | 2890 | 2.16 (1.67, 2.80) | 141 | 2139 | 1.46 (1.11, 1.92) | 93 | 2139 | 0.72 (0.54, 0.95) | |
| Category 4 | 106 | 1287 | 2.67 (1.96, 3.64) | 158 | 2140 | 1.64 (1.25, 2.14) | 86 | 2140 | 0.54 (0.39, 0.75) | |
| p-het | 0.07 | 0.21 | 0.04 | |||||||
Categories are 1: 0–10%, 2: 11–25%, 3: 26–50%, and 4: 51%+ for PMD and quartiles for DA and NDA.
adjusted for study site, age, BMI.
mixed and other histology categories are excluded
p-het: test for heterogeneity in association by subtype
Table 4.
Pooled associations of categoriesa of percent density, dense area, and non dense area for morphological subtypes of invasive breast cancer by age
| PERCENT DENSITY | DENSE AREA | NONDENSE AREA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age < 55 | Age ≥55 | Age < 55 | Age ≥55 | Age < 55 | Age ≥55 | ||||||||||||||
| No. cases | No. controls |
OR (95% CI)b | No. cases | No. controls |
OR (95% CI)b | No. cases | No. controls |
OR (95% CI)b | No. cases | No. controls |
OR (95% CI)b | No. cases | No. controls |
OR (95% CI)b | No. cases |
No. controls |
OR (95% CI)b | ||
| Overall breast cancer | |||||||||||||||||||
| Category 1 | 109 | 530 | 0.60 (0.47, 0.77) | 408 | 1254 | 0.61 (0.53, 0.71) | 191 | 759 | 0.65 (0.53, 0.80) | 427 | 1380 | 0.64 (0.55, 0.75) | 822 | 1357 | 1.44 (1.24, 1.66) | 458 | 782 | 1.33 (1.13, 1.56) | |
| Category 2 (REF) | 316 | 1016 | 1.00 (REF) | 699 | 1581 | 1.00 (REF) | 376 | 948 | 1.00 (REF) | 534 | 1192 | 1.00 (REF) | 456 | 1087 | 1.00 (REF) | 527 | 1053 | 1.00 (REF) | |
| Category 3 | 809 | 1604 | 1.68 (1.44, 1.97) | 817 | 1286 | 1.60 (1.41, 1.83) | 553 | 1135 | 1.22 (1.04, 1.43) | 581 | 1004 | 1.26 (1.09, 1.46) | 362 | 878 | 0.96 (0.81, 1.14) | 581 | 1261 | 0.82 (0.71, 0.96) | |
| Category 4 | 650 | 922 | 2.40 (2.02, 2.86) | 287 | 365 | 2.16 (1.79, 2.61) | 764 | 1230 | 1.56 (1.34, 1.81) | 669 | 910 | 1.58 (1.37, 1.83) | 244 | 750 | 0.70 (0.57, 0.86) | 645 | 1390 | 0.67 (0.57, 0.79) | |
| Invasiveness | |||||||||||||||||||
| In situ | |||||||||||||||||||
| Category 1 | 15 | 530 | 0.64 (0.35, 1.18) | 36 | 1254 | 0.43 (0.29, 0.64) | 33 | 759 | 0.79 (0.50, 1.24) | 40 | 1380 | 0.42 (0.28, 0.62) | 129 | 1357 | 1.14 (0.84, 1.54) | 55 | 782 | 1.21 (0.82, 1.78) | |
| Category 2 (REF) | 44 | 1016 | 1.00 (REF) | 96 | 1581 | 1.00 (REF) | 56 | 948 | 1.00 (REF) | 79 | 1192 | 1.00 (REF) | 81 | 1087 | 1.00 (REF) | 62 | 1053 | 1.00 (REF) | |
| Category 3 | 138 | 1604 | 1.96 (1.37, 2.81) | 104 | 1286 | 1.40 (1.04, 1.89) | 91 | 1135 | 1.33 (0.94, 1.88) | 76 | 1004 | 1.10 (0.79, 1.54) | 58 | 878 | 0.86 (0.60, 1.23) | 66 | 1261 | 0.86 (0.60, 1.24) | |
| Category 4 | 100 | 922 | 2.38 (1.60, 3.54) | 23 | 365 | 1.31 (0.80, 2.13) | 117 | 1230 | 1.60 (1.15, 2.24) | 64 | 910 | 1.06 (0.75, 1.49) | 29 | 750 | 0.57 (0.35, 0.91) | 76 | 1390 | 0.81 (0.55, 1.19) | |
| Invasive | |||||||||||||||||||
| Category 1 | 92 | 530 | 0.58 (0.45, 0.76) | 369 | 1254 | 0.63 (0.54, 0.74) | 158 | 759 | 0.63 (0.51, 0.79) | 384 | 1380 | 0.68 (0.58, 0.80) | 688 | 1357 | 1.49 (1.28, 1.74) | 401 | 782 | 1.35 (1.14, 1.59) | |
| Category 2 (REF) | 272 | 1016 | 1.00 (REF) | 603 | 1581 | 1.00 (REF) | 317 | 948 | 1.00 (REF) | 453 | 1192 | 1.00 (REF) | 374 | 1087 | 1.00 (REF) | 462 | 1053 | 1.00 (REF) | |
| Category 3 | 667 | 1604 | 1.62 (1.37, 1.92) | 710 | 1286 | 1.63 (1.42, 1.87) | 458 | 1135 | 1.19 (1.01, 1.42) | 503 | 1004 | 1.29 (1.10, 1.51) | 304 | 878 | 0.99 (0.82, 1.19) | 514 | 1261 | 0.82 (0.70, 0.96) | |
| Category 4 | 548 | 922 | 2.39 (1.99, 2.88) | 262 | 365 | 2.27 (1.87, 2.76) | 646 | 1230 | 1.56 (1.33, 1.83) | 604 | 910 | 1.68 (1.44, 1.95) | 213 | 750 | 0.72 (0.58, 0.90) | 567 | 1390 | 0.65 (0.55, 0.77) | |
| p-het | 0.55 | 0.08 | 0.78 | 0.03 | 0.40 | 0.58 | |||||||||||||
| Histologyc | |||||||||||||||||||
| Ductal | |||||||||||||||||||
| Category 1 | 82 | 530 | 0.65 (0.49, 0.86) | 274 | 1254 | 0.65 (0.54, 0.77) | 135 | 759 | 0.66 (0.52, 0.83) | 298 | 1380 | 0.75 (0.63, 0.90) | 546 | 1357 | 1.47 (1.24, 1.73) | 292 | 782 | 1.29 (1.07, 1.55) | |
| Category 2 (REF) | 216 | 1016 | 1.00 (REF) | 449 | 1581 | 1.00 (REF) | 260 | 948 | 1.00 (REF) | 321 | 1192 | 1.00 (REF) | 302 | 1087 | 1.00 (REF) | 348 | 1053 | 1.00 (REF) | |
| Category 3 | 544 | 1604 | 1.67 (1.39, 2.00) | 529 | 1286 | 1.61 (1.38, 1.88) | 374 | 1135 | 1.19 (0.99, 1.42) | 375 | 1004 | 1.35 (1.14, 1.61) | 253 | 878 | 1.02 (0.84, 1.24) | 382 | 1261 | 0.83 (0.69, 0.98) | |
| Category 4 | 435 | 922 | 2.41 (1.97, 2.95) | 185 | 365 | 2.12 (1.71, 2.63) | 508 | 1230 | 1.50 (1.26, 1.78) | 443 | 910 | 1.75 (1.47, 2.07) | 176 | 750 | 0.74 (0.58, 0.93) | 415 | 1390 | 0.65 (0.53, 0.78) | |
| Lobular | |||||||||||||||||||
| Category 1 | 5 | 530 | 0.29 (0.11, 0.78) | 44 | 1254 | 0.53 (0.36, 0.78) | 12 | 759 | 0.59 (0.29, 1.18) | 39 | 1380 | 0.45 (0.30, 0.68) | 75 | 1357 | 1.55 (1.03, 2.33) | 60 | 782 | 1.55 (1.06, 2.26) | |
| Category 2 (REF) | 27 | 1016 | 1.00 (REF) | 87 | 1581 | 1.00 (REF) | 26 | 948 | 1.00 (REF) | 70 | 1192 | 1.00 (REF) | 39 | 1087 | 1.00 (REF) | 59 | 1053 | 1.00 (REF) | |
| Category 3 | 57 | 1604 | 1.43 (0.89, 2.30) | 91 | 1286 | 1.47 (1.08, 2.01) | 42 | 1135 | 1.31 (0.80, 2.17) | 65 | 1004 | 1.08 (0.76, 1.54) | 22 | 878 | 0.65 (0.38, 1.12) | 73 | 1261 | 0.90 (0.62, 1.29) | |
| Category 4 | 67 | 922 | 3.14 (1.91, 5.17) | 43 | 365 | 2.73 (1.83, 4.08) | 76 | 1230 | 2.22 (1.41, 3.51) | 91 | 910 | 1.68 (1.21, 2.32) | 20 | 750 | 0.58 (0.32, 1.08) | 73 | 1390 | 0.64 (0.43, 0.95) | |
| p-het | 0.07 | 0.27 | 0.18 | 0.08 | 0.31 | 0.79 | |||||||||||||
| Histologic grade | |||||||||||||||||||
| Well differentiated | |||||||||||||||||||
| Category 1 | 19 | 530 | 0.48 (0.29, 0.82) | 120 | 1254 | 0.62 (0.49, 0.80) | 39 | 759 | 0.72 (0.48, 1.07) | 118 | 1380 | 0.67 (0.52, 0.87) | 170 | 1357 | 1.31 (1.00, 1.71) | 114 | 782 | 1.16 (0.89, 1.52) | |
| Category 2 (REF) | 72 | 1016 | 1.00 (REF) | 202 | 1581 | 1.00 (REF) | 72 | 948 | 1.00 (REF) | 143 | 1192 | 1.00 (REF) | 103 | 1087 | 1.00 (REF) | 144 | 1053 | 1.00 (REF) | |
| Category 3 | 159 | 1604 | 1.41 (1.05, 1.90) | 220 | 1286 | 1.48 (1.20, 1.83) | 111 | 1135 | 1.29 (0.95, 1.76) | 172 | 1004 | 1.41 (1.11, 1.80) | 75 | 878 | 0.91 (0.66, 1.25) | 171 | 1261 | 0.90 (0.71, 1.15) | |
| Category 4 | 143 | 922 | 2.31 (1.67, 3.18) | 80 | 365 | 2.02 (1.51, 2.72) | 171 | 1230 | 1.86 (1.39, 2.48) | 189 | 910 | 1.70 (1.34, 2.16) | 45 | 750 | 0.62 (0.41, 0.92) | 193 | 1390 | 0.78 (0.60, 1.01) | |
| Moderately differentiated | |||||||||||||||||||
| Category 1 | 44 | 530 | 0.77 (0.53, 1.13) | 133 | 1254 | 0.59 (0.47, 0.75) | 65 | 759 | 0.74 (0.54, 1.03) | 137 | 1380 | 0.65 (0.51, 0.83) | 254 | 1357 | 1.50 (1.19, 1.89) | 144 | 782 | 1.26 (0.98, 1.61) | |
| Category 2 (REF) | 97 | 1016 | 1.00 (REF) | 230 | 1581 | 1.00 (REF) | 111 | 948 | 1.00 (REF) | 169 | 1192 | 1.00 (REF) | 137 | 1087 | 1.00 (REF) | 176 | 1053 | 1.00 (REF) | |
| Category 3 | 254 | 1604 | 1.74 (1.35, 2.25) | 269 | 1286 | 1.61 (1.32, 1.97) | 167 | 1135 | 1.25 (0.97, 1.62) | 186 | 1004 | 1.27 (1.01, 1.60) | 126 | 878 | 1.13 (0.87, 1.47) | 203 | 1261 | 0.86 (0.68, 1.07) | |
| Category 4 | 210 | 922 | 2.64 (2.00, 3.48) | 107 | 365 | 2.43 (1.85, 3.18) | 262 | 1230 | 1.81 (1.43, 2.30) | 247 | 910 | 1.85 (1.49, 2.30) | 88 | 750 | 0.82 (0.60, 1.12) | 216 | 1390 | 0.65 (0.51, 0.83) | |
| Poorly differentiated | |||||||||||||||||||
| Category 1 | 23 | 530 | 0.53 (0.32, 0.86) | 76 | 1254 | 0.65 (0.48, 0.89) | 39 | 759 | 0.52 (0.35, 0.77) | 81 | 1380 | 0.67 (0.49, 0.91) | 197 | 1357 | 1.47 (1.14, 1.90) | 82 | 782 | 1.63 (1.18, 2.25) | |
| Category 2 (REF) | 71 | 1016 | 1.00 (REF) | 113 | 1581 | 1.00 (REF) | 95 | 948 | 1.00 (REF) | 93 | 1192 | 1.00 (REF) | 109 | 1087 | 1.00 (REF) | 86 | 1053 | 1.00 (REF) | |
| Category 3 | 202 | 1604 | 1.87 (1.40, 2.50) | 133 | 1286 | 1.69 (1.29, 2.22) | 142 | 1135 | 1.24 (0.94, 1.64) | 79 | 1004 | 0.97 (0.71, 1.33) | 76 | 878 | 0.85 (0.62, 1.16) | 94 | 1261 | 0.77 (0.56, 1.05) | |
| Category 4 | 151 | 922 | 2.53 (1.84, 3.49) | 51 | 365 | 2.49 (1.73, 3.59) | 171 | 1230 | 1.36 (1.04, 1.78) | 120 | 910 | 1.59 (1.19, 2.12) | 65 | 750 | 0.73 (0.51, 1.05) | 111 | 1390 | 0.56 (0.40, 0.79) | |
| p-het | 0.48 | 0.91 | 0.36 | 0.40 | 0.70 | 0.15 | |||||||||||||
| Tumor size | |||||||||||||||||||
| <1.1 cm | |||||||||||||||||||
| Category 1 | 46 | 530 | 0.94 (0.65, 1.37) | 173 | 1254 | 0.85 (0.68, 1.06) | 70 | 759 | 0.94 (0.68, 1.30) | 174 | 1380 | 0.86 (0.68, 1.08) | 195 | 1357 | 1.28 (1.00, 1.65) | 124 | 782 | 1.09 (0.85, 1.41) | |
| Category 2 (REF) | 96 | 1016 | 1.00 (REF) | 222 | 1581 | 1.00 (REF) | 98 | 948 | 1.00 (REF) | 164 | 1192 | 1.00 (REF) | 114 | 1087 | 1.00 (REF) | 168 | 1053 | 1.00 (REF) | |
| Category 3 | 196 | 1604 | 1.25 (0.96, 1.63) | 233 | 1286 | 1.40 (1.14, 1.72) | 134 | 1135 | 1.13 (0.86, 1.49) | 180 | 1004 | 1.27 (1.01, 1.60) | 97 | 878 | 1.12 (0.84, 1.50) | 183 | 1261 | 0.84 (0.67, 1.06) | |
| Category 4 | 150 | 922 | 1.63 (1.21, 2.17) | 73 | 365 | 1.62 (1.20, 2.18) | 186 | 1230 | 1.47 (1.14, 1.91) | 183 | 910 | 1.41 (1.12, 1.78) | 82 | 750 | 1.14 (0.82, 1.59) | 226 | 1390 | 0.79 (0.62, 1.01) | |
| 1.1 – 2.0 cm | |||||||||||||||||||
| Category 1 | 32 | 530 | 0.50 (0.33, 0.76) | 117 | 1254 | 0.51 (0.40, 0.65) | 57 | 759 | 0.53 (0.39, 0.74) | 131 | 1380 | 0.58 (0.45, 0.74) | 288 | 1357 | 1.68 (1.34, 2.10) | 175 | 782 | 1.56 (1.23, 1.98) | |
| Category 2 (REF) | 110 | 1016 | 1.00 (REF) | 239 | 1581 | 1.00 (REF) | 136 | 948 | 1.00 (REF) | 184 | 1192 | 1.00 (REF) | 139 | 1087 | 1.00 (REF) | 172 | 1053 | 1.00 (REF) | |
| Category 3 | 271 | 1604 | 1.63 (1.28, 2.07) | 276 | 1286 | 1.59 (1.31, 1.93) | 189 | 1135 | 1.15 (0.91, 1.46) | 199 | 1004 | 1.26 (1.01, 1.57) | 128 | 878 | 1.12 (0.86, 1.45) | 200 | 1261 | 0.86 (0.69, 1.08) | |
| Category 4 | 220 | 922 | 2.39 (1.83, 3.11) | 112 | 365 | 2.43 (1.87, 3.17) | 251 | 1230 | 1.42 (1.13, 1.78) | 230 | 910 | 1.59 (1.28, 1.97) | 78 | 750 | 0.71 (0.52, 0.99) | 197 | 1390 | 0.61 (0.47, 0.78) | |
| 2.1+ cm | |||||||||||||||||||
| Category 1 | 12 | 530 | 0.28 (0.15, 0.54) | 62 | 1254 | 0.44 (0.32, 0.61) | 28 | 759 | 0.49 (0.31, 0.77) | 63 | 1380 | 0.51 (0.36, 0.71) | 183 | 1357 | 1.47 (1.13, 1.91) | 90 | 782 | 1.42 (1.05, 1.92) | |
| Category 2 (REF) | 60 | 1016 | 1.00 (REF) | 129 | 1581 | 1.00 (REF) | 71 | 948 | 1.00 (REF) | 94 | 1192 | 1.00 (REF) | 107 | 1087 | 1.00 (REF) | 107 | 1053 | 1.00 (REF) | |
| Category 3 | 176 | 1604 | 2.10 (1.53, 2.88) | 173 | 1286 | 2.00 (1.56, 2.56) | 120 | 1135 | 1.40 (1.03, 1.91) | 104 | 1004 | 1.28 (0.95, 1.71) | 70 | 878 | 0.74 (0.54, 1.03) | 116 | 1261 | 0.75 (0.56, 1.00) | |
| Category 4 | 161 | 922 | 3.77 (2.69, 5.28) | 71 | 365 | 3.24 (2.33, 4.51) | 190 | 1230 | 2.02 (1.51, 2.70) | 174 | 910 | 2.30 (1.76, 3.01) | 49 | 750 | 0.45 (0.30, 0.67) | 122 | 1390 | 0.48 (0.35, 0.66) | |
| p-het | <0.001 | <0.001 | 0.002 | <0.001 | 0.001 | 0.006 | |||||||||||||
| Involvement of lymph nodes | |||||||||||||||||||
| Negative | |||||||||||||||||||
| Category 1 | 64 | 530 | 0.57 (0.42, 0.77) | 250 | 1254 | 0.65 (0.54, 0.78) | 110 | 759 | 0.66 (0.52, 0.86) | 260 | 1380 | 0.68 (0.56, 0.82) | 458 | 1357 | 1.53 (1.28, 1.83) | 264 | 782 | 1.24 (1.02, 1.50) | |
| Category 2 (REF) | 198 | 1016 | 1.00 (REF) | 411 | 1581 | 1.00 (REF) | 211 | 948 | 1.00 (REF) | 309 | 1192 | 1.00 (REF) | 240 | 1087 | 1.00 (REF) | 325 | 1053 | 1.00 (REF) | |
| Category 3 | 427 | 1604 | 1.41 (1.17, 1.71) | 481 | 1286 | 1.58 (1.35, 1.85) | 309 | 1135 | 1.21 (0.99, 1.47) | 349 | 1004 | 1.29 (1.08, 1.54) | 210 | 878 | 1.08 (0.87, 1.33) | 358 | 1261 | 0.84 (0.70, 1.00) | |
| Category 4 | 365 | 922 | 2.16 (1.75, 2.66) | 181 | 365 | 2.23 (1.79, 2.78) | 424 | 1230 | 1.54 (1.28, 1.86) | 405 | 910 | 1.64 (1.38, 1.95) | 146 | 750 | 0.81 (0.63, 1.04) | 376 | 1390 | 0.64 (0.53, 0.78) | |
| Positive | |||||||||||||||||||
| Category 1 | 23 | 530 | 0.62 (0.37, 1.02) | 66 | 1254 | 0.51 (0.38, 0.70) | 38 | 759 | 0.53 (0.35, 0.78) | 79 | 1380 | 0.66 (0.48, 0.91) | 195 | 1357 | 1.47 (1.13, 1.90) | 105 | 782 | 1.81 (1.34, 2.45) | |
| Category 2 (REF) | 59 | 1016 | 1.00 (REF) | 130 | 1581 | 1.00 (REF) | 91 | 948 | 1.00 (REF) | 95 | 1192 | 1.00 (REF) | 109 | 1087 | 1.00 (REF) | 93 | 1053 | 1.00 (REF) | |
| Category 3 | 205 | 1604 | 2.36 (1.73, 3.22) | 164 | 1286 | 1.75 (1.36, 2.24) | 123 | 1135 | 1.11 (0.83, 1.48) | 107 | 1004 | 1.28 (0.96, 1.72) | 84 | 878 | 0.91 (0.67, 1.24) | 107 | 1261 | 0.85 (0.63, 1.14) | |
| Category 4 | 158 | 922 | 3.40 (2.43, 4.76) | 62 | 365 | 2.48 (1.77, 3.48) | 193 | 1230 | 1.60 (1.22, 2.09) | 141 | 910 | 1.83 (1.39, 2.42) | 57 | 750 | 0.60 (0.41, 0.87) | 117 | 1390 | 0.61 (0.44, 0.84) | |
| p-het | 0.02 | 0.28 | 0.56 | 0.78 | 0.54 | 0.06 | |||||||||||||
| ER status | |||||||||||||||||||
| Negative | |||||||||||||||||||
| Category 1 | 14 | 530 | 0.53 (0.29, 1.00) | 55 | 1254 | 0.73 (0.51, 1.04) | 28 | 759 | 0.47 (0.30, 0.74) | 60 | 1380 | 0.65 (0.45, 0.92) | 139 | 1357 | 1.72 (1.26, 2.34) | 70 | 782 | 1.64 (1.15, 2.35) | |
| Category 2 (REF) | 42 | 1016 | 1.00 (REF) | 84 | 1581 | 1.00 (REF) | 74 | 948 | 1.00 (REF) | 77 | 1192 | 1.00 (REF) | 70 | 1087 | 1.00 (REF) | 66 | 1053 | 1.00 (REF) | |
| Category 3 | 130 | 1604 | 2.14 (1.48, 3.09) | 106 | 1286 | 1.63 (1.21, 2.21) | 88 | 1135 | 0.97 (0.70, 1.35) | 65 | 1004 | 0.95 (0.67, 1.34) | 47 | 878 | 0.76 (0.51, 1.12) | 66 | 1261 | 0.79 (0.55, 1.13) | |
| Category 4 | 103 | 922 | 3.16 (2.13, 4.70) | 34 | 365 | 1.86 (1.21, 2.86) | 99 | 1230 | 1.01 (0.74, 1.39) | 77 | 910 | 1.24 (0.89, 1.73) | 33 | 750 | 0.48 (0.30, 0.77) | 77 | 1390 | 0.67 (0.46, 0.99) | |
| Positive | |||||||||||||||||||
| Category 1 | 76 | 530 | 0.60 (0.45, 0.80) | 297 | 1254 | 0.61 (0.51, 0.72) | 125 | 759 | 0.68 (0.54, 0.87) | 306 | 1380 | 0.68 (0.57, 0.82) | 527 | 1357 | 1.46 (1.23, 1.74) | 310 | 782 | 1.27 (1.06, 1.53) | |
| Category 2 (REF) | 221 | 1016 | 1.00 (REF) | 495 | 1581 | 1.00 (REF) | 234 | 948 | 1.00 (REF) | 355 | 1192 | 1.00 (REF) | 287 | 1087 | 1.00 (REF) | 380 | 1053 | 1.00 (REF) | |
| Category 3 | 509 | 1604 | 1.51 (1.26, 1.82) | 573 | 1286 | 1.62 (1.40, 1.88) | 348 | 1135 | 1.23 (1.02, 1.48) | 416 | 1004 | 1.36 (1.15, 1.61) | 247 | 878 | 1.06 (0.87, 1.30) | 417 | 1261 | 0.81 (0.68, 0.96) | |
| Category 4 | 430 | 922 | 2.30 (1.88, 2.81) | 216 | 365 | 2.32 (1.89, 2.86) | 529 | 1230 | 1.73 (1.45, 2.07) | 504 | 910 | 1.79 (1.52, 2.11) | 175 | 750 | 0.80 (0.63, 1.01) | 474 | 1390 | 0.65 (0.54, 0.77) | |
| p-het | 0.22 | 0.47 | 0.02 | 0.09 | 0.02 | 0.48 | |||||||||||||
| PR status | |||||||||||||||||||
| Negative | |||||||||||||||||||
| Category 1 | 23 | 530 | 0.55 (0.33, 0.89) | 96 | 1254 | 0.69 (0.53, 0.92) | 39 | 759 | 0.51 (0.34, 0.75) | 98 | 1380 | 0.63 (0.47, 0.83) | 181 | 1357 | 1.57 (1.20, 2.06) | 96 | 782 | 1.25 (0.94, 1.68) | |
| Category 2 (REF) | 72 | 1016 | 1.00 (REF) | 148 | 1581 | 1.00 (REF) | 96 | 948 | 1.00 (REF) | 125 | 1192 | 1.00 (REF) | 96 | 1087 | 1.00 (REF) | 119 | 1053 | 1.00 (REF) | |
| Category 3 | 180 | 1604 | 1.70 (1.27, 2.28) | 169 | 1286 | 1.54 (1.21, 1.96) | 131 | 1135 | 1.13 (0.86, 1.50) | 114 | 1004 | 1.03 (0.79, 1.36) | 74 | 878 | 0.91 (0.66, 1.26) | 117 | 1261 | 0.76 (0.58, 1.00) | |
| Category 4 | 132 | 922 | 2.29 (1.66, 3.17) | 63 | 365 | 2.13 (1.53, 2.96) | 141 | 1230 | 1.13 (0.86, 1.49) | 139 | 910 | 1.38 (1.06, 1.79) | 56 | 750 | 0.69 (0.47, 1.01) | 144 | 1390 | 0.69 (0.51, 0.92) | |
| Positive | |||||||||||||||||||
| Category 1 | 67 | 530 | 0.61 (0.45, 0.83) | 257 | 1254 | 0.61 (0.51, 0.73) | 112 | 759 | 0.69 (0.53, 0.88) | 269 | 1380 | 0.70 (0.58, 0.85) | 479 | 1357 | 1.46 (1.23, 1.75) | 283 | 782 | 1.35 (1.11, 1.63) | |
| Category 2 (REF) | 188 | 1016 | 1.00 (REF) | 431 | 1581 | 1.00 (REF) | 209 | 948 | 1.00 (REF) | 306 | 1192 | 1.00 (REF) | 261 | 1087 | 1.00 (REF) | 327 | 1053 | 1.00 (REF) | |
| Category 3 | 462 | 1604 | 1.61 (1.33, 1.96) | 509 | 1286 | 1.64 (1.41, 1.92) | 303 | 1135 | 1.19 (0.98, 1.46) | 367 | 1004 | 1.39 (1.17, 1.66) | 222 | 878 | 1.05 (0.85, 1.29) | 368 | 1261 | 0.82 (0.69, 0.98) | |
| Category 4 | 397 | 922 | 2.49 (2.02, 3.08) | 186 | 365 | 2.28 (1.83, 2.83) | 490 | 1230 | 1.79 (1.49, 2.15) | 441 | 910 | 1.82 (1.53, 2.16) | 152 | 750 | 0.75 (0.59, 0.96) | 405 | 1390 | 0.63 (0.52, 0.77) | |
| p-het | 0.73 | 0.64 | 0.007 | 0.15 | 0.68 | 0.73 | |||||||||||||
| HER2 status | |||||||||||||||||||
| Negative | |||||||||||||||||||
| Category 1 | 69 | 530 | 0.63 (0.46, 0.85) | 256 | 1254 | 0.64 (0.53, 0.77) | 106 | 759 | 0.61 (0.47, 0.79) | 249 | 1380 | 0.66 (0.55, 0.80) | 451 | 1357 | 1.39 (1.17, 1.67) | 235 | 782 | 1.27 (1.03, 1.55) | |
| Category 2 (REF) | 186 | 1016 | 1.00 (REF) | 405 | 1581 | 1.00 (REF) | 221 | 948 | 1.00 (REF) | 296 | 1192 | 1.00 (REF) | 262 | 1087 | 1.00 (REF) | 281 | 1053 | 1.00 (REF) | |
| Category 3 | 455 | 1604 | 1.64 (1.35, 1.99) | 443 | 1286 | 1.53 (1.30, 1.80) | 294 | 1135 | 1.10 (0.91, 1.34) | 319 | 1004 | 1.26 (1.05, 1.51) | 219 | 878 | 1.03 (0.84, 1.26) | 347 | 1261 | 0.91 (0.75, 1.09) | |
| Category 4 | 382 | 922 | 2.52 (2.04, 3.13) | 164 | 365 | 2.12 (1.69, 2.65) | 471 | 1230 | 1.63 (1.35, 1.95) | 404 | 910 | 1.75 (1.47, 2.09) | 160 | 750 | 0.79 (0.62, 1.01) | 405 | 1390 | 0.77 (0.63, 0.93) | |
| Positive | |||||||||||||||||||
| Category 1 | 10 | 530 | 0.54 (0.26, 1.12) | 42 | 1254 | 0.71 (0.47, 1.07) | 20 | 759 | 0.68 (0.39, 1.19) | 43 | 1380 | 0.63 (0.42, 0.96) | 106 | 1357 | 1.48 (1.06, 2.08) | 48 | 782 | 1.24 (0.83, 1.84) | |
| Category 2 (REF) | 31 | 1016 | 1.00 (REF) | 61 | 1581 | 1.00 (REF) | 38 | 948 | 1.00 (REF) | 54 | 1192 | 1.00 (REF) | 59 | 1087 | 1.00 (REF) | 62 | 1053 | 1.00 (REF) | |
| Category 3 | 115 | 1604 | 2.43 (1.60, 3.67) | 89 | 1286 | 2.00 (1.42, 2.82) | 82 | 1135 | 1.79 (1.21, 2.66) | 59 | 1004 | 1.24 (0.84, 1.81) | 40 | 878 | 0.83 (0.54, 1.26) | 53 | 1261 | 0.64 (0.44, 0.94) | |
| Category 4 | 75 | 922 | 2.82 (1.78, 4.45) | 31 | 365 | 2.51 (1.58, 3.99) | 91 | 1230 | 1.82 (1.23, 2.69) | 67 | 910 | 1.55 (1.07, 2.24) | 26 | 750 | 0.54 (0.32, 0.91) | 60 | 1390 | 0.50 (0.33, 0.75) | |
| p-het | 0.11 | 0.54 | 0.07 | 0.92 | 0.38 | 0.16 | |||||||||||||
Categories are 1: 0–10%, 2: 11–25%, 3: 26–50%, and 4: 51%+ for PMD and quartiles for DA and NDA.
adjusted for study site, age, BMI.
mixed and other histology categories are excluded
p-het: test for heterogeneity in association by subtype
Overall and invasive breast cancer and DCIS
Overall, DA was significantly positively associated with breast cancer risk while NDA was significantly inversely associated with breast cancer risk (Table 3) and across age groups (Table 4). Specifically, the ORs for overall breast cancer associated with DA were: Q1 vs. Q2, 0.65; Q3 vs. Q2, 1.22; Q4 vs. Q2, 1.55 (p-trend <0.001) and the ORs for overall breast cancer associated with NDA were: Q1 vs. Q2, 1.39; Q3 vs. Q2, 0.88; Q4 vs. Q2, 0.72 (p-trend <0.001). For DA, associations were similar by age; for NDA, however, the interaction with age was statistically significant (Page-interaction <0.01) although the differences in associations by age were not clinically meaningful: <55 years (OR for Q1 vs. Q2, 1.44; 95% CI, 1.24–1.66) compared to those ≥55 years (corresponding OR, 1.33; 95% CI: 1.13–1.56) (Table 4).
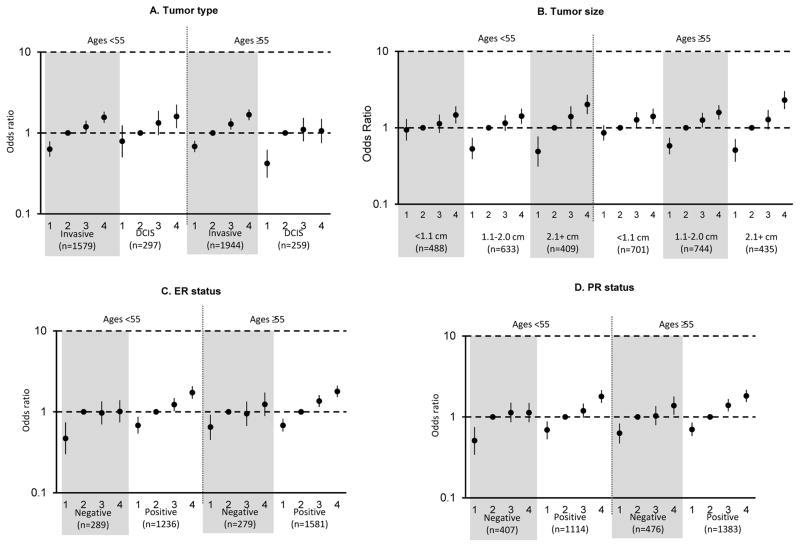
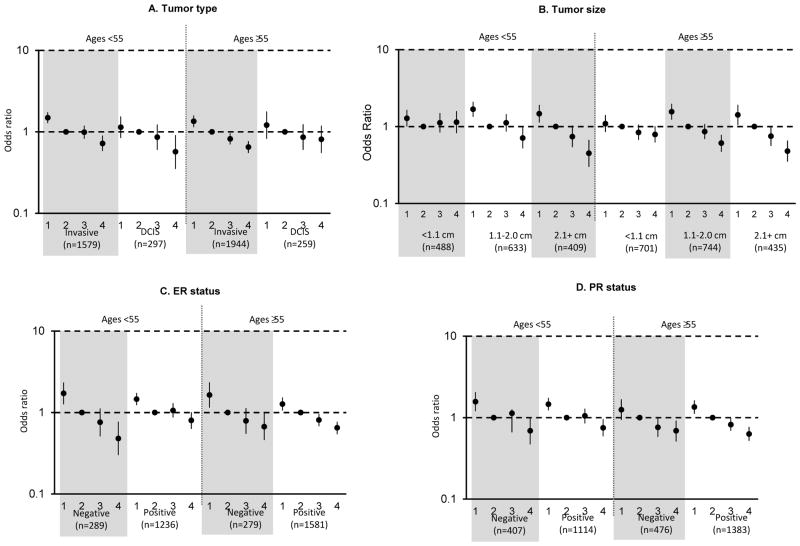
DA was significantly positively associated with both invasive breast cancer and DCIS across all age groups (Tables 3 and 4; Figure 1). Among women ≥55 years, this association was stronger for invasive tumors than DCIS (Pheterogeneity = 0.03; Table 4; Figure 1); however, there was no evidence of a significant interaction between age and DA for associations with tumor type (Page-interaction = 0.41). Again, even though a statistically significant association was seen by age (Page-interaction =0.02), NDA was significantly inversely associated with risk of both invasive breast cancer and DCIS among both younger and older women (Tables 3 and 4; Figure 2).
Figure 1. Associations of categorical dense area (DA) for breast cancer tumor type and selected tumor characteristics of invasive breast cancer, by age.
dds ratios and 95% confidence intervals, adjusted for age, body mass index, and study, are shown for quartiles of DA. A) Tumor type, B) Tumor size, C) ER status, D) PR status.
Figure 2. Associations of categorical non dense area (NDA) for breast cancer tumor type and selected tumor characteristics of invasive breast cancer, by age.
Odds ratios and 95% confidence intervals, adjusted for age, body mass index, and study, are shown for quartiles of NDA. A) Tumor type, B) Tumor size, C) ER status, D) PR status.
Grade, invasive histology, size and nodal status
DA was significantly positively associated with all invasive tumor characteristics evaluated while NDA was significantly inversely associated with these characteristics (Tables 3 and 4). While there were no differences in the magnitude of associations of DA or NDA with tumor histology, grade, or nodal involvement, we did observe heterogeneity of associations with tumor size. Specifically, DA was positively associated with invasive tumors of all sizes; however, stronger positive associations of DA and breast cancer were noted for larger tumors ≥2.1 cm compared to smaller tumors across all ages (Ptrend <0.01) (Figure 1). For example, the overall ORs comparing women in Q4 of DA vs. Q2 were 1.42, 1.50, and 2.13 for tumors <1.1 cm, 1.1–2.0 cm, ≥2.1 cm, respectively (Table 3) and findings were similar among ages <55 and ≥55 years (Table 4; Figure 1; Page-interaction =0.91). The opposite trend was observed for associations of NDA with tumor size, with a stronger inverse association noted for larger tumors compared to smaller tumors across age groups (Ptrend <0.01), with the strongest associations most apparent for tumors 1.1–2.0 cm and 2.1+ cm in women ages <55 and ≥55 years (Table 4; Figure 2). This trend was also similar across age (Page-interaction =0.30).
ER, PR and HER2 receptor status
Among women of all ages, stronger associations of DA were noted for ER+ and PR+ tumors compared to hormone receptor negative tumors (Pheterogeneity <0.01). Although there was no significant evidence of differences by age (Page-interaction >0.38), among women <55 years, stronger associations were observed for ER+ (OR for Q4 vs. Q2, 1.73; 95% CI, 1.45–2.07) vs. ER− (corresponding OR, 1.01; 95% CI, 0.74–1.39) (Pheterogeneity =0.02; Table 4; Figure 1) and PR+ (OR for Q4 vs. Q2, 1.79; 95% CI, 1.49–2.15) vs. PR− (corresponding OR, 1.13; 95% CI, 0.86, 1.49) (Pheterogeneity =0.01; Table 4; Figure 1). Similarly, although not significantly different by age group (Page-interaction = 0.08), among women <55 years, NDA was more strongly inversely associated with ER− tumors (OR for Q4 vs. Q2, 0.48; 95% CI, 0.30, 0.77) than with ER+ tumors (corresponding OR, 0.8; 95% CI, 0.63–1.01) (Pheterogeneity =0.03; Table 4; Figure 2). In contrast, among women ages ≥55, DA and NDA were similarly associated with tumors defined by ER or PR status (Pheterogeneity >0.08; Table 4; Figure 2). Finally, DA and NDA were similarly associated with tumors defined by HER2 status (Tables 3 and 4).
Results were not materially changed in models that included mutual adjustment for DA and NDA (Data not shown). Finally, there was little evidence of differences across study (majority of P-values >0.09). Between study heterogeneity was noted, however, for associations of DA with overall breast cancer (P=0.02) and tumor histology (P=0.04), suggesting caution when interpreting these results.
Discussion
In this large study, the positive associations between percent MD and breast cancer overall and by tumor characteristics were similar or stronger than in our first paper based on a subset of these data (23). In analyses of DA and NDA, we found that DA was significantly associated with increased breast cancer risk and NDA was significantly associated with decreased risk and that these were independent risk factors for breast cancer. Further, statistically significant associations of the absolute DA and NDA measures with breast cancer were apparent for all tumor characteristics evaluated. Our findings suggest greater magnitude of association for DA with ER+ vs. ER− disease and PR+ vs. PRv disease and stronger associations of NDA with ER− vs. ER+ disease in women <55 years. We also observed significant positive and inverse trends for associations of DA and NDA, respectively, with tumor size across all ages.
Our findings of opposing associations of DA and NDA with breast cancer risk generally agree with most of the existing literature in this area, including a recent large meta-analysis that included several of the studies here (7). However, while the meta-analysis found that associations for NDA were attenuated in many studies upon adjustment for absolute DA (7), we did not observe attenuation in mutually adjusted models, possibly because correlations between DA and NDA were low (0.06–0.29) and similar across studies or because we adjusted for BMI, which is a surrogate for NDA. We conclude DA and absolute NDA are independent risk factors associated with breast cancer risk.
Few previous studies reported associations of absolute DA or NDA with breast cancer according to specific tumor characteristics. Consistent with our findings, in 601 cases and 667 controls from the Multiethnic Cohort, absolute DA was associated with both invasive breast cancer and DCIS (24); although in the present analysis, there was suggestion of a stronger association for invasive cancers vs. DCIS among women ≥55 years. Also in the Multiethnic Cohort, stronger associations of DA with ER+/PR+ vs. ER−/PR− tumors were observed (25). Like us, Eriksson et al. (26) reported stronger associations of absolute DA with ER+ vs. ER− tumors (p-value =0.065) and with PR+ vs. PR− (p-value = 0.099) in a case-only study of 110 breast cancer patients. Positive associations of absolute DA with ER+ vs. ER− tumors and larger vs. smaller tumors were also observed in recent UK case-control study (27). Similar to our findings, a case-only study among postmenopausal women (n=286) reported a non-significant positive trend of DA with tumor size and a non-significant trend of NDA with tumor size as well as significant positive associations between DA and ER and PR positivity (28). Our study is among the first to comprehensively explore associations of absolute NDA with breast tumor characteristics and, to our knowledge, is the largest to date. Current hypotheses to explain associations between increased MD and breast cancer risk have been reviewed recently (29) and include the higher amount of fibroglandular tissue “at risk” of transformation into cancer (30) and the increased epithelial and fibroblast cellular activity and interaction between stroma and epithelium in dense tissue (31, 32) as well as hormonal mechanisms, including the influence of sex steroid hormones and growth factors on density and breast cancer risk (33). Evaluating associations by tumor characteristics can provide insight into these hypothesized mechanisms. If the mechanism of action were purely through hormonal influences, then we might expect to observe associations of DA with ER+ tumors only; however, we observed significant positive associations of DA with both ER+ and ER− tumors, although the magnitude of association was greater for ER+ tumors among women <55 years. Moreover, we observed strong inverse associations of NDA with ER− tumors in this age group, independent of DA. Our findings of independent associations of DA and NDA with breast cancer risk across tumor characteristics suggest that several causal pathways may play a role in associations with risk. Petterson and Tamimi (34) propose several mechanisms by which breast fat (nondense area) may lead to reduced risk of breast cancer, including the possible direct effect of adipose tissue on normal breast development, indirect effects of adipose tissue in regard to the endocrine environment of the breast, or via lobular involution, which is positively correlated with NDA and inversely associated with breast cancer risk (35). On the other hand, some studies have suggested breast fat as a risk factor for breast cancer (6, 36).
As in our previously published analysis based on a subset of these data (23), we found that percent MD was more strongly associated with risk of ER-negative breast cancer than with ER-positive breast cancer among women < 55 years of age. Our current findings of the MD area phenotypes further suggest that the positive association observed between percent MD and ER-negative disease among women <55 years is driven by the inverse association of non-dense area with ER-negative disease in this group, rather than by a positive association with absolute dense breast area. Based on the results of our analyses and considering the current body of published literature on this topic, it appears that breast density (including percent and area measures) plays an important role in tumor aggressiveness, especially in younger women, giving differential associations observed with respect to tumor size, nodal status as well as ER-status. In light of the lack of significant age-interaction, however, we cannot discount an association of MD phenotypes with tumor aggressiveness among older women.
Limitations of the study have been described (3) and include variation in study design and populations, use of clinical pathology as opposed to central pathology review; changes in diagnostic criteria over time that may influence tumor characteristics and receptor status, in particular, and generalizability of results primarily to Caucasian women. Even with 4000 cases, power to detect age-interactions remained limited. Detection bias is also a potential limitation, given that extent of breast density may make earlier tumors more difficult to detect on screening mammogram (37). While we were not able to evaluate the influence of detection bias directly in this analysis due to the lack of high-quality data regarding interval vs. screen-detected cancers for most included studies, in the Breast Cancer Surveillance Consortium, Kerlikowske et al. reported that higher breast density in premenopausal women was more strongly related to aggressive tumors and that this finding persisted in analyses restricted to screen-detected cases only (38). We did find evidence of study heterogeneity for the analyses of DA with overall breast cancer and by invasive vs. in situ status, so these results should be cautiously interpreted. However, our associations of these absolute measures with overall breast cancer were consistent with the literature. Finally, this study relied on digitized film mammograms vs. more contemporary full field digital mammograms.
Strengths of this pooled analysis include the large sample size with mammograms available years prior to the cancer (for cases), standardized estimates of NDA and DA, detailed information on covariates and tumor characteristics from pathology reports, supplemented with information from TMAs, and screening mammograms assessed in a generally systematic fashion.
In summary, we found that percent MD and absolute dense breast area were associated with increased breast cancer risk while non-dense area was associated with decreased risk across all ages and invasive tumor characteristics. Among women <55 years, dense area was more strongly associated with an increased risk for ER+ vs. ER− tumors [p-heterogeneity (het) = 0.02] while non-dense area was more strongly associated with a decreased risk for ER− vs. ER+ tumors [p-het = 0.03]. Dense area was similarly associated with increased risk (and non-dense area decreased risk) of both node-positive and node-negative tumors, while significant trends in the magnitude of these associations were observed with increasing tumor size.
Our results suggest DA is positively associated (and NDA, inversely associated) with breast cancer across tumor characteristics. Further, these results suggest differential associations for these phenotypes with ER+ vs. ER− tumors, particularly in younger women. As such, DA and NDA may be important to consider when developing age- and subtype-specific risk models for breast cancer. Further research is warranted to clarify the possible differential associations of DA and NDA on breast cancer risk according to tumor characteristics.
Supplementary Material
Acknowledgments
Financial Support:
This work was supported in part by the National Institutes of Health, National Cancer Institute (NCI) grants listed below:
R01 CA140286 R01 CA128931 and R01 CA97396 to Dr. Vachon
R01 CA124865 and R01 CA131332 to Dr. Tamimi;
P50 CA116201 to Dr. Ingle (Dr. Couch has Project on SPORE);
R01 CA140286, P50 CA58207, U01 CA63740 and P01 CA154292 to Dr. Kerlikowske;
R01 CA116167 to Dr. Couch;
P01CA087969 and UM1 CA186107 to Dr. Stampfer (supporting NHS); and
R01 CA050385, UM1 CA176726 and Breast Cancer Research Foundation to Dr. Willett (supporting NHS2).
Additional support was provided by the Simeon J. Fortin Charitable Foundation, Bank of America, N.A., Co-Trustee to Dr. Bertrand; Department of Defense (DAMD 17-00-1-033) to Dr. Vachon;
We would like to thank the participants and staff of all the studies for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WI, WY. We also thank Fang Fang Wu who provided percent mammographic density estimates on all Mayo studies. The authors assume full responsibility for analyses and interpretation of these data.
Abbreviations
- MD
Mammographic density
- MMHS
Mayo Mammography Health Study
- MCBCS
Mayo Clinic Breast Cancer Study
- NHS and NHSII
Nurses’ Health Study
- MCMAM
Mayo Clinic Mammography Study
- SFMR
San Francisco Bay Area Breast Cancer SPORE and San Francisco Mammography Registry
- SEER
Surveillance Epidemiology and End Results
- BMI
body mass index
- OR
odds ratios
- CI
confidence intervals
Footnotes
Disclosure of Potential Conflicts of Interest:
The authors declare that they have no conflicts of interests to disclose.
References
- 1.Yaffe MJ. Mammographic density. Measurement of mammographic density. Breast Cancer Res. 2008;10:209. doi: 10.1186/bcr2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 3.Bertrand KA, Tamimi RM, Scott CG, Jensen MR, Pankratz VS, Visscher D, et al. Mammographic density and risk of breast cancer by age and tumor characteristics. Breast Cancer Res. 2013;15:R104. doi: 10.1186/bcr3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pettersson A, Hankinson SE, Willett WC, Lagiou P, Trichopoulos D, Tamimi RM. Nondense mammographic area and risk of breast cancer. Breast Cancer Res. 2011;13:R100. doi: 10.1186/bcr3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone J, Ding J, Warren RM, Duffy SW, Hopper JL. Using mammographic density to predict breast cancer risk: dense area or percentage dense area. Breast Cancer Res. 2010;12:R97. doi: 10.1186/bcr2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lokate M, Peeters PH, Peelen LM, Haars G, Veldhuis WB, van Gils CH. Mammographic density and breast cancer risk: the role of the fat surrounding the fibroglandular tissue. Breast Cancer Res. 2011;13:R103. doi: 10.1186/bcr3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettersson A, Graff RE, Ursin G, Santos Silva ID, McCormack V, Baglietto L, et al. Mammographic Density Phenotypes and Risk of Breast Cancer: A Meta-analysis. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heine JJ, Scott CG, Sellers TA, Brandt KR, Serie DJ, Wu FF, et al. A novel automated mammographic density measure and breast cancer risk. J Natl Cancer Inst. 2012;104:1028–37. doi: 10.1093/jnci/djs254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olson JE, Sellers TA, Scott CG, Schueler BA, Brandt KR, Serie DJ, et al. The influence of mammogram acquisition on the mammographic density and breast cancer association in the Mayo Mammography Health Study Cohort. Breast Cancer Res. 2012;14:R147. doi: 10.1186/bcr3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelemen LE, Couch FJ, Ahmed S, Dunning AM, Pharoah PD, Easton DF, et al. Genetic variation in stromal proteins decorin and lumican with breast cancer: investigations in two case-control studies. Breast Cancer Res. 2008;10:R98. doi: 10.1186/bcr2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Goode EL, Fredericksen ZS, Vierkant RA, Pankratz VS, Liu-Mares W, et al. Association of genetic variation in genes implicated in the beta-catenin destruction complex with risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2101–8. doi: 10.1158/1055-9965.EPI-08-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamimi RM, Hankinson SE, Colditz GA, Byrne C. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:2641–7. doi: 10.1158/1055-9965.EPI-05-0558. [DOI] [PubMed] [Google Scholar]
- 13.Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006;66:2476–82. doi: 10.1158/0008-5472.CAN-05-3369. [DOI] [PubMed] [Google Scholar]
- 14.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 15.Vachon CM, Brandt KR, Ghosh K, Scott CG, Maloney SD, Carston MJ, et al. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:43–9. doi: 10.1158/1055-9965.EPI-06-0738. [DOI] [PubMed] [Google Scholar]
- 16.Kerlikowske K, Carney PA, Geller B, Mandelson MT, Taplin SH, Malvin K, et al. Performance of screening mammography among women with and without a first-degree relative with breast cancer. Ann Intern Med. 2000;133:855–63. doi: 10.7326/0003-4819-133-11-200012050-00009. [DOI] [PubMed] [Google Scholar]
- 17.Kerlikowske K, Shepherd J, Creasman J, Tice JA, Ziv E, Cummings SR. Are breast density and bone mineral density independent risk factors for breast cancer? J Natl Cancer Inst. 2005;97:368–74. doi: 10.1093/jnci/dji056. [DOI] [PubMed] [Google Scholar]
- 18.Ziv E, Tice J, Smith-Bindman R, Shepherd J, Cummings S, Kerlikowske K. Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:2090–5. [PubMed] [Google Scholar]
- 19.Boyd NF, Stone J, Martin LJ, Jong R, Fishell E, Yaffe M, et al. The association of breast mitogens with mammographic densities. Br J Cancer. 2002;87:876–82. doi: 10.1038/sj.bjc.6600537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shepherd JA, Kerlikowske K, Ma L, Duewer F, Fan B, Wang J, et al. Volume of mammographic density and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1473–82. doi: 10.1158/1055-9965.EPI-10-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prevrhal S, Shepherd JA, Smith-Bindman R, Cummings SR, Kerlikowske K. Accuracy of mammographic breast density analysis: results of formal operator training. Cancer Epidemiol Biomarkers Prev. 2002;11:1389–93. [PubMed] [Google Scholar]
- 22.Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10:R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertrand KA, Tamimi RM, Scott CG, Jensen MR, Pankratz VS, Visscher D, et al. Mammographic density and risk of breast cancer by age and tumor characteristics. Breast Cancer Res. 2013;15:R104. doi: 10.1186/bcr3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill JK, Maskarinec G, Pagano I, Kolonel LN. The association of mammographic density with ductal carcinoma in situ of the breast: the Multiethnic Cohort. Breast Cancer Res. 2006;8:R30. doi: 10.1186/bcr1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conroy SM, Pagano I, Kolonel LN, Maskarinec G. Mammographic density and hormone receptor expression in breast cancer: the Multiethnic Cohort Study. Cancer Epidemiol. 2011;35:448–52. doi: 10.1016/j.canep.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eriksson L, Hall P, Czene K, Dos Santos Silva I, McCormack V, Bergh J, et al. Mammographic density and molecular subtypes of breast cancer. Br J Cancer. 2012;107:18–23. doi: 10.1038/bjc.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding J, Warren R, Girling A, Thompson D, Easton D. Mammographic density, estrogen receptor status and other breast cancer tumor characteristics. Breast J. 2010;16:279–89. doi: 10.1111/j.1524-4741.2010.00907.x. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh K, Brandt KR, Sellers TA, Reynolds C, Scott CG, Maloney SD, et al. Association of mammographic density with the pathology of subsequent breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:872–9. doi: 10.1158/1055-9965.EPI-07-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettersson A, Tamimi R. Breast density and breast cancer risk: understanding of biology and risk. Curr Epidemiol Rep. 2014;1:120–9. [Google Scholar]
- 30.Trichopoulos D, Lipman RD. Mammary gland mass and breast cancer risk. Epidemiology. 1992;3:523–6. doi: 10.1097/00001648-199211000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:343–9. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 32.Guo YP, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E, et al. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev. 2001;10:243–8. [PubMed] [Google Scholar]
- 33.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pettersson A, Tamimi RM. Breast fat and breast cancer. Breast Cancer Res Treat. 2012;135:321–3. doi: 10.1007/s10549-012-2186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh K, Hartmann LC, Reynolds C, Visscher DW, Brandt KR, Vierkant RA, et al. Association between mammographic density and age-related lobular involution of the breast. J Clin Oncol. 2010;28:2207–12. doi: 10.1200/JCO.2009.23.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beer AE, Billingham RE. Adipose tissue, a neglected factor in aetiology of breast cancer? Lancet. 1978;2:296. doi: 10.1016/s0140-6736(78)91694-x. [DOI] [PubMed] [Google Scholar]
- 37.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 38.Kerlikowske K, Cook AJ, Buist DS, Cummings SR, Vachon C, Vacek P, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830–7. doi: 10.1200/JCO.2009.26.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.