Abstract
Isothiocyanates (ITCs) derived from cruciferous vegetables, including phenethyl isothiocyanate (PEITC) and sulforaphane (SFN), exhibit in vivo activity against prostate cancer in xenograft and transgenic mouse model, and thus are appealing for chemoprevention of this disease. Watercress constituent PEITC and SFN-rich broccoli sprout extract are under clinical investigations but the molecular mechanisms underlying their cancer chemopreventive effects are not fully understood. The present study demonstrates that chemokine receptor CXCR4 is a novel target of ITCs in prostate cancer cells. Exposure of prostate cancer cells (LNCaP, 22Rv1, C4-2, and PC-3) to pharmacologically applicable concentrations of PEITC, benzyl isothiocyanate (BITC), and SFN (2.5 and 5 μmol/L) resulted in downregulation of CXCR4 expression. None of the isothiocyanates affected secretion of CXCR4 ligand (stromal-derived factor-1). In vivo inhibition of PC-3 xenograft growth upon PEITC treatment was associated with a significant decrease in CXCR4 protein level. A similar trend was discernible in the tumors from SFN-treated TRAMP mice compared with those of control mice, but the difference was not significant. Stable overexpression of CXCR4 in PC-3 cells conferred significant protection against wound healing, cell migration, and cell viability inhibition by ITCs. Inhibition of cell migration resulting from PEITC and BITC exposure was significantly augmented by RNA interference of CXCR4. This study demonstrates, for the first time, that cancer chemopreventive ITCs suppress CXCR4 expression in prostate cancer cells in vitro as well as in vivo. These results suggest that CXCR4 downregulation may be an important pharmacodynamic biomarker of cancer chemopreventative ITCs in prostate adenocarcinoma.
Keywords: Isothiocyanates, CXCR4, Chemoprevention
Introduction
Cancer chemoprevention with edible plants and/or their bioactive constituents is appealing because of their safety, epidemiological evidence of risk reduction, preclinical indication of preventive efficacy, and cost-effectiveness. Cruciferous vegetables are a rich source of cancer chemopreventive phytochemicals collectively known as isothiocyanates (ITCs) (1,2). Cancer chemoprevention by ITCs, which occur naturally as thioglucoside conjugates in widely consumed vegetables such as watercress, garden cress, mustard, and broccoli, was first documented by Wattenberg more than three decades ago (3). Phenethyl isothiocyanate (PEITC) abundant in watercress and the garden cress constituent benzyl isothiocyanate (BITC) were shown to inhibit breast cancer induced by 7,12-dimethylbenz[a]anthracene in rats when administered 4 hour before the carcinogen treatment (3). Since then, the chemopreventive activity of ITCs was extended against other chemical carcinogens (1,2). Epidemiological association of cancer risk reduction with increasing intake of cruciferous vegetables provides additional support for their chemopreventive effect (4-6). PEITC and BITC have been studied extensively for their anticancer preventive efficacy in preclinical models as well as mechanistic characterization (1,2). The ClinicalTrials.gov lists 3 completed or ongoing clinical trials on PEITC or watercress juice. On the other hand, preclinical efficacy and mechanistic studies on SFN have primarily focused on the synthetic racemic (D,L-SFN) analogue of naturally-occurring L-isomer (2). Majority of the ongoing or completed clinical trials on SFN in healthy volunteers or cancer patients have used standardized broccoli sprout extract.
Our interest in ITCs was initially sparked by epidemiological studies suggesting an inverse relationship between intake of cruciferous vegetables and the risk of prostate cancer (7,8). Prostate adenocarcinoma remains a leading cause of cancer mortality among American men despite a comprehensive understanding of the underlying biology, genomic landscape, and the risk factors (9-12). Population-based evidence prompted us to test the efficacy of PEITC and SFN for prevention of prostate cancer using a transgenic mouse model (Transgenic Adenocarcinoma of Mouse Prostate; TRAMP) (13-15). Dietary administration of PEITC (3 μmol/g diet) for 19 weeks to male TRAMP mice resulted in a statistically significant decrease in the incidence of poorly-differentiated prostate cancer when compared to mice fed with basal diet (13). Oral SFN administration (6 μmol/mouse three times per week) inhibited the incidence of prostatic intraepithelial neoplasia and well-differentiated cancer, but not poorly-differentiated cancer in TRAMP mice (14). However, inhibition of poorly-differentiated prostate cancer by oral SFN was achievable with co-administration of an autophagy inhibitor (chloroquine) as SFN is known to induce cytoprotective autophagy (15,16). SFN was also effective in preventing pulmonary metastasis in the TRAMP mouse model (14). Feeding of 240 mg of broccoli sprout/day to TRAMP mice exhibited retardation of prostate tumor growth (17). The growth of PC-3 and LNCaP human prostate cancer cells subcutaneously implanted in athymic mice was retarded significantly by PEITC or SFN administration (18-20). PEITC also exhibited in vivo growth inhibitory activity against a cell line (TRAMP-C1) derived from a TRAMP tumor (21).
PEITC and SFN have been the focus of intense mechanistic studies to gain insights into the biology of prostate cancer chemoprevention by these agents (2,18,19,21-24). Mechanisms of prostate adenocarcinoma chemoprevention by PEITC and SFN include apoptotic or autophagic cell death induction (autophagy is cytoprotective for SFN), suppression of oncogenic pathways (e.g., nuclear factor-κB), and inhibition of cell proliferation (2,18,19,21-24). The present study explores the role of chemokine receptor CXCR4, which is implicated in prostate cancer progression and metastasis (25) in anticancer effects of PEITC, BITC, and SFN using human prostate cancer cells.
Materials and Methods
Ethics statement
The use of mice for the in vivo studies was approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Prostate tumor tissues from our previously published studies (15,26) were used to determine the in vivo effect of PEITC and SFN administration on CXCR4 protein expression. For the in vivo xenograft experiment with PEITC, PC-3 cells stably expressing luciferase were injected subcutaneously on flank of each mouse. Control mice were treated with phosphate-buffered saline (PBS; control) or 9 μmol PEITC (oral intubation) in 0.1 mL PBS five times per week for 38 days (26). PEITC treatment was started on the day of tumor cell injection (26). For the SFN-TRAMP study, 4 week old male TRAMP mice were treated with PBS (control) or 1 mg SFN in PBS three times/week for 15-18 weeks (15).
Reagents and cell lines
Majority of the cell culture reagents were purchased from Invitrogen-Life Technologies, whereas RPMI 1640 medium was from Mediatech. Sources of the antibodies were as follows: anti-CXCR4 antibody was from Abcam, an antibody specific for detection of S473 phosphorylated AKT was from Cell Signaling Technology; anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was from GeneTex; antibodies against phospho- and total extracellular-signal regulated kinases (ERK) were purchased from Santa Cruz Biotechnology, and anti-actin antibody was from Sigma-Aldrich. Transwell Permeable Support (8 µm polycarbonate membrane) chambers were purchased from Corning. Small interfering RNA for knockdown of CXCR4 was purchased from Santa Cruz Biotechnology. Stock solutions of PEITC, BITC, and SFN (purity ≥98%; structures are shown in Fig. 1A) were stored at −20°C and diluted immediately before use. LNCaP, C4-2, 22Rv1, and PC-3 human prostate cancer cells were acquired from the American Type Culture Collection and last authenticated in 2012. Each cell line was found to be of human origin and free of pathogen contamination. PC-3 cells stably transfected with CXCR4 plasmid (hereafter abbreviated as CXCR4_PC-3) or empty vector (Neo_PC-3) have been described previously (27).
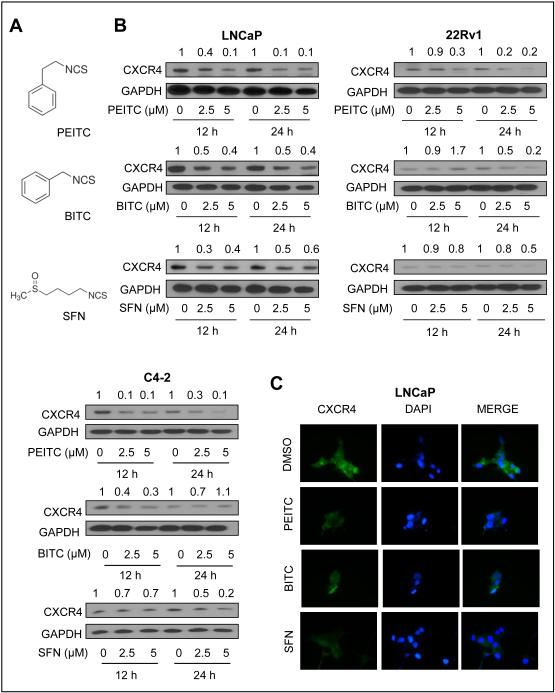
Figure 1.
ITCs downregulated CXCR4 protein level in prostate cancer cells. A, structures of PEITC, BITC, and SFN. B, western blots showing effect of ITC treatment on CXCR4 protein level in LNCaP cells, 22Rv1, and C4-2 cells. GAPDH was probed as a loading control. C, immunofluorescence microscopy for effect of ITC treatment (5 µmol/L, 24 hour) on CXCR4 protein level in LNCaP cells. Western blotting was performed 2-4 times using independently prepared lysates. Data on effect of SFN in 22Rv1 cell was inconsistent.
Western blotting
After treatment, cells were collected and lysed as described by us previously (28). TRAMP tumor tissues were processed as previously described (13). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and wet transferred onto a membrane. Western blotting was performed as described previously (28) except that dilution of CXCR4 antibody and membrane exposure time were optimized. Enhanced chemiluminescence reagent was used for immunodetection of the band.
Immunofluorescence microscopy
LNCaP or PC-3 cells were plated on coverslips in 12-well plates, allowed to attach overnight, and then exposed to PEITC, BITC, SFN or DMSO (control) for 24 hours. After washing with BD Perm/Wash™ buffer, cells were fixed with fixation/permeabilization solution supplied in the kit at 4°C for 10 minutes. Cells were washed again, blocked with 0.5% bovine serum albumin and 0.15% glycine in PBS for 1 hour, and incubated with anti-CXCR4 antibody overnight at 4°C or at room temperature for 1 hour. After washing with BD Perm/Wash™ buffer, Alexa Fluor 488-conjugated secondary antibody was added (1 hour at room temperature). Subsequently, cells were washed and treated with DAPI (10 ng/mL) for 5 minutes at room temperature to stain nuclear DNA. Cells were washed with PBS and examined under a fluorescence microscope at 100× objective magnification.
Reverse transcription-polymerase chain reaction (RT-PCR) and quantitative real-time PCR
Total RNA from dimethyl sulfoxide (DMSO)-treated control cells or those treated with the desired ITCs were isolated using RNeasy kit. The cDNA was synthesized with the use of SuperScript III reverse transcriptase and oligo(dT)20 primer. PCR was performed using specific primers: CXCR4 forward: 5’-GAAGCTGTTGGCTGAAAAGG-3’ and CXCR4 reverse: 5’-GAGTCGATGCTGATCCCAAT-3’ (PCR product size, 345 bp); with the following amplification conditions: 94ºC for 5 minutes, 28 cycles at 94ºC for 15 seconds, 54ºC for 30 seconds, 72ºC for 1 min and extension at 72 ºC 10 minutes. Quantitative PCR (qPCR) was done using 2x SYBR Green master mix (Applied Biosystems-Life Technologies) under same conditions, with the number of cycles changed to 40. Relative gene expression was calculated using the method described by Livak and Schmittgen (29).
Measurement of CXCL12 secretion
Analysis of CXCL12 secretion was performed using a commercially available kit. PC-3 cells were plated into six-well plates, allowed to attach, and then exposed to PEITC, BITC, SFN or DMSO (control) for 24 hours. Conditioned medium was collected and spun down to remove debris and stored at −80°C. CXCL12 was measured in the medium by an immunoassay kit from R&D Systems. The concentration in the medium was calculated using a standard curve from serially diluted CXCL12 provided with the kit.
RNA interference
PC-3 cells were seeded in six-well plates and transfected at 70% confluency with a nonspecific (control) small interfering RNA (siRNA) or CXCR4-targeted siRNA (100 or 200 nmol) using Oligofectamine. Twenty-four hours post transfection cells were treated with DMSO or the test agent for an additional 24 hours. Cells were collected and processed for either immunoblotting, cell migration or wound healing assay. Knockdown of CXCR4 was confirmed by western blotting.
Wound healing assay
PC-3 cells stably transfected with either CXCR4 plasmid or empty vector were seeded in 6-well plates. For PC-3 cells transiently transfected with CXCR4 siRNA, 1×105 cells were placed in 48-well plates, and allowed to attach overnight. Cells were transfected with 200 nM control or CXCR4 siRNA for 24 hours. A wound in the confluent monolayer culture was created by scratching with a pipette tip. The wounded cells were washed with PBS and incubated with F12K medium containing 1% fetal bovine serum, puromycin (1 μg/mL), 1 mmol/L thymidine, and desired concentration of PEITC, BITC or SFN. Cells were allowed to migrate for 10 hours, fixed with methanol, and stained with Giemsa staining solution. Migration of cells was quantified by measuring distances between the borders of cells using Image J software. At least three non-overlapping areas per well were examined for wound healing.
Cell migration assay
PC-3 cells transfected with a control (nonspecific) siRNA or CXCR4-targeted siRNA or Neo_PC-3 or CXCR4_PC-3 cells were suspended in serum-free medium containing DMSO or the test agent (PEITC or BITC) and placed in the upper compartment of the Transwell chamber. After 24 hours of incubation, non-motile cells from the upper surface of the filter were removed using a cotton swab. The motile cells from the bottom face of the filter were fixed with methanol and stained with hematoxylin and eosin. At least 5 randomly selected areas were scored for cell migration.
Cell viability assay
Cell viability was determined by trypan blue dye exclusion assay as described by us previously (30).
Statistical analysis
One way-analysis of variance (ANOVA) with Dunnett’s adjustment was used to determine statistical significance of difference for dose-response studies whereas Bonferroni’s test was used for multiple comparisons (e.g., between Neo_PC-3 and CXCR4_PC-3 cells). Unpaired Student’s t-test was used for binary comparisons.
Results
ITCs downregulated CXCR4 expression in prostate cancer cells
We used androgen-sensitive (LNCaP and 22Rv1) and androgen-independent human prostate cancer cells (C4-2 and PC-3) and pharmacologically relevant concentrations of PEITC, BITC, and SFN (Fig. 1A) to determine their effect on CXCR4 protein. Level of CXCR4 protein was decreased after treatment with all three compounds in LNCaP cells (Fig. 1B). In LNCaP cells, this effect was most pronounced at the 5 μmol/L ITC dose. Suppression of CXCR4 protein after treatment with PEITC, BITC, and SFN was also evident in 22Rv1 and C4-2 cells (Fig. 1B). However, cell line-specific differences were also observed in the extent and kinetics of downregulation with each ITC compound. Data for 22Rv1 with SFN was also variable in five different experiments. Immunofluorescence microscopy confirmed ITC-mediated downregulation of the CXCR4 protein expression in LNCaP cells (Fig. 1C).
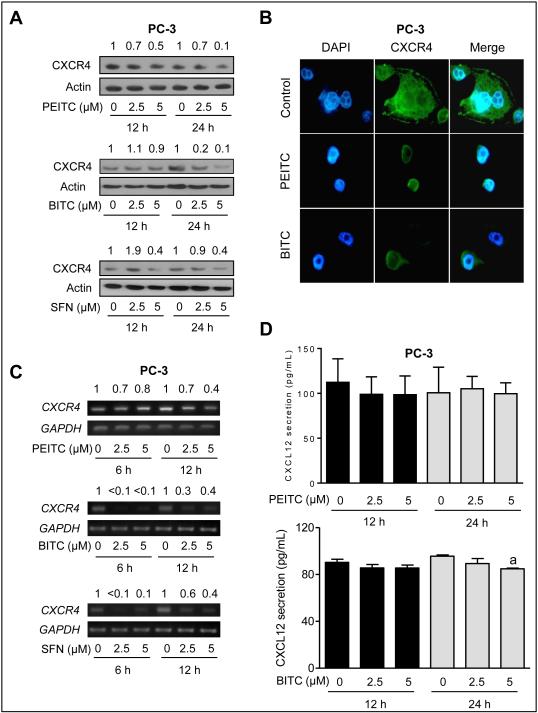
As can be seen in Fig. 2A, CXCR4 protein level was markedly decreased upon treatment with PEITC, BITC, and SFN in PC-3 cells. For PEITC, a 6 hour time point was also included but the results were inconsistent (results not shown). Immunofluorescence microscopy confirmed ITC-mediated downregulation of the CXCR4 protein expression in PC-3 cells (Fig. 2B). The suppression of CXCR4 protein was attributed to the transcriptional inhibition as revealed by RT-PCR (Fig. 2C) and quantitative real time PCR (Supplementary Fig. S1).
Figure 2.
Effect of ITCs on CXCR4 protein and mRNA expression in PC-3 cells. A, effect of ITC treatment on protein levels of CXCR4 by western blotting. Samples after 6 hour treatment were also used for western blotting for PEITC, but these data are not shown because of inconsistency. B, immunofluorescence microscopy for effect of ITCs (5 µmol/L) on CXCR4 protein level in PC-3 cells after 24 hour treatment with DMSO or specified ITC compound. C, RT-PCR for analysis of CXCR4 mRNA after treatment with DMSO or specified ITC compound D, quantitation of CXCL12 secretion in culture media of PC-3 cells after 12 or 24 hour treatment with DMSO or ITCs (PEITC or BITC). The results shown (mean ± SD) for PEITC are combined from two independent experiments (n = 5). Analysis of CXCL12 secretion in culture media of BITC-treated cells was done once (n = 3). aSignificantly different compared with control by one-way ANOVA with Dunnett’s adjustment.
ITCs had no effect on CXCL12 expression or secretion
We next determined the effect of ITCs on expression and secretion of CXCR4 ligand CXCL12. Expression of CXCL12 was not affected by PEITC, BITC or SFN at least in PC-3 cells as determined by immunofluorescence microscopy (data not shown). As shown in Fig. 2D, secretion of CXCL12 was not affected either except for a modest decrease by BITC at the 24 hour time point. Similarly, SFN treatment failed to alter CXCL12 secretion in PC-3 cells (data not shown). Collectively, these results indicated transcriptional suppression of CXCR4 by aromatic ITCs (PEITC and BITC) and a thioalkyl ITC (SFN).
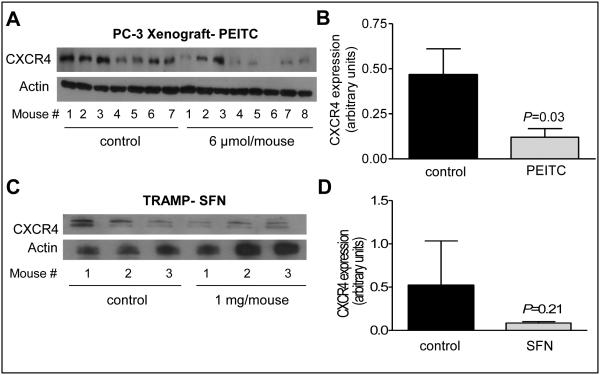
Effects on PEITC and SFN administration on tumor CXCR4 protein level in vivo
In a PC-3 xenograft study with 5 times/week oral administration of 9 μmol PEITC, the average tumor volume (mean ± SD) in control mice (879.2 ± 284.5) was about 1.9-fold higher compared with PEITC-treated mice (P=0.068) (26). Fresh frozen tumor tissues from this study (26) were used to determine the effect of PEITC administration on CXCR4 protein level in vivo. As shown in Fig. 3A, CXCR4 protein was detectable in most of the control PC-3 xenografts. The level of CXCR4 protein was lower by about 74% in the PC-3 xenografts from PEITC-treated mice when compared with controls with a P= 0.03 by unpaired Student’s t-test (n = 7 for control and n = 8 for PEITC treatment group) (Fig. 3B). We have shown previously that the incidence of well-differentiated cancer in the dorsolateral prostate of TRAMP mice is decreased by about 25% upon oral intubation with 1 mg SFN three times/week (15). Western blotting for CXCR4 protein also showed its suppression in the tumors from SFN-treated TRAMP mice relative to control (Fig. 3C) but the difference was insignificant possibly due to small sample size (Fig. 3D). Nevertheless, these results provided in vivo evidence for suppression of CXCR4 expression in prostate tumors upon treatment with PEITC and SFN.
Figure 3.
PEITC administration downregulated CXCR4 protein expression in vivo in PC-3 xenografts. A, western blotting for CXCR4 expression in tumor lysates from PC-3 xenografts from control and PEITC-treated mice. B, densitometric quantitation of CXCR4 protein in PC-3 tumors; control (n = 7) and PEITC (n = 8). C, western blotting for CXCR4 protein using tumor lysates from control and SFN-treated TRAMP mice. D, densitometric quantitation of CXCR4 protein in TRAMP tumors (n = 3). The results shown are mean ± SD. Statistical significance was determined by unpaired Student’s t-test.
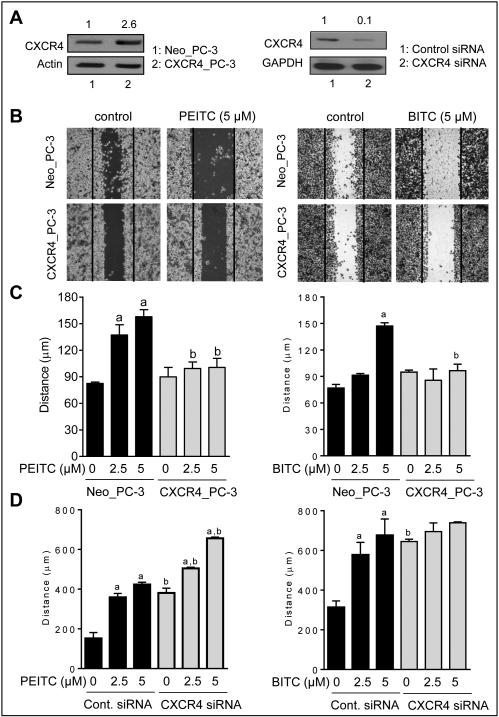
Overexpression of CXCR4 conferred significant protection against ITCs-mediated inhibition of wound healing
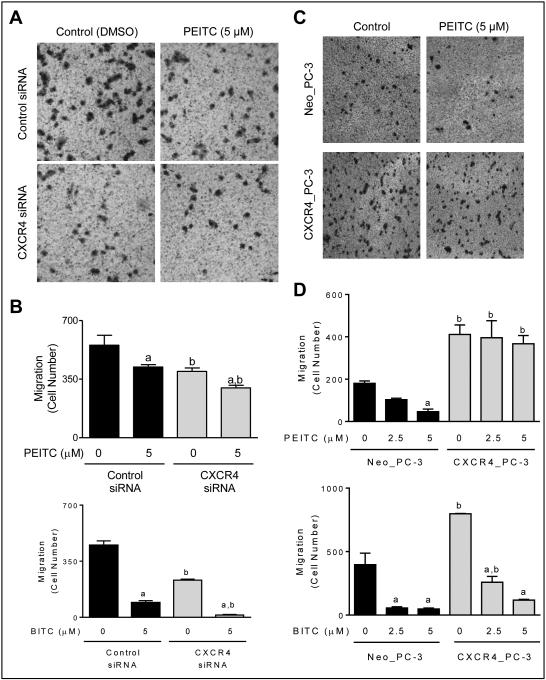
A scratch wound healing assay was performed to determine the functional significance of CXCR4 downregulation by ITCs. Expression of CXCR4 protein was 2.6-fold higher in CXCR4_PC-3 cells in comparison with empty vector transfected Neo_PC-3 cells (Fig. 4A). PEITC and BITC treatments inhibited wound healing in Neo-PC-3 cells, but this effect was nearly fully abolished by CXCR4 overexpression (Fig. 4B,C). Similar experiments were performed using SFN, but the results were inconclusive (data not shown). We also determined the effect of CXCR4 knockdown in PC-3 cells using a siRNA (Fig. 4A) on wound healing inhibition by PEITC and BITC (Fig. 4D). The wound healing inhibition by PEITC, but not BITC was significantly augmented by CXCR4 knockdown. Difference between PEITC and BITC is not surprising as these compounds are known to exhibit mechanistic differences despite close structural similarity (1,2).
Figure 4.
Stable overexpression of CXCR4 conferred protection against wound healing inhibition by PEITC and BITC. A, western blots show overexpression (left panel) or knockdown (right panel) of CXCR4 protein in PC-3 cells. B, results of scratch assay showing the effect of PEITC and BITC treatments (10 hour treatment) on wound healing in PC-3 cells transfected with CXCR4 plasmid or empty vector. C, quantitation of wound healing. The results shown (mean ± SD) are representative of two independent experiments (n = 3). Significantly different compared with acorresponding control, and bbetween Neo_PC-3 and CXCR4_PC-3 cells by one-way ANOVA followed by Bonferroni’s test. D, bar graphs showing effect of CXCR4 knockdown using a siRNA on wound healing inhibition by PEITC or BITC. The results shown are mean ± SD (n = 2). Significantly different compared with acorresponding control, and bbetween control siRNA and CXCR4 siRNA by one-way ANOVA followed by Bonferroni’s test.
RNA interference of CXCR4 augmented PEITC- and BITC-mediated inhibition of PC-3 cell migration
Fig 5A depicts PC-3 cell migration that was decreased by 28% (P < 0.05) by CXCR4 knockdown alone in the absence of PEITC treatment. Exposure to PEITC also resulted in a significant inhibition of migration capacity of PC-3 cells (Fig. 5B). PEITC-mediated inhibition of PC-3 cell migration was modestly but statistically significantly augmented by RNA interference of CXCR4 but this effect was relatively more pronounced for BITC (Fig. 5B).
Figure 5.
Cell migration inhibition by PEITC and BITC was augmented by CXCR4 knockdown in PC-3 cells. A, representative images showing the effect of PEITC treatment (24 hours) on PC-3 cell migration. A decrease in cell migration by PEITC treatment as well as CXCR4 knockdown was clearly visible. B, bar graphs show quantitation of cell migration by PC-3 cells transfected with control siRNA or CXCR4-specific siRNA after 24 hour treatment with DMSO or the specified ITC compound. C, microscopic images depicting migration by Neo_PC-3 and CXCR4_PC-3 cells after 24 hour treatment with DMSO or PEITC. D, bar graphs show quantitation of cell migration. The results shown are mean ± SD (n = 2-3). Significantly different compared with acorresponding control, and bbetween Neo_PC-3 and CXCR4_PC-3 by one-way ANOVA followed by Bonferroni’s test. Each experiment was repeated for 2-3 times and representative data from one such experiment are shown.
Inhibition of cell migration and cell viability by ITCs in PC-3 cells was attenuated by ectopic expression of CXCR4
Cell migration was more intense for CXCR4 overexpressing cells compared with Neo_PC-3 cells (Fig. 5C). PEITC treatment decreased cell migration in Neo_PC-3 cells but this effect was abolished in CXCR4 overexpressing cells (Fig. 5D). CXCR4 overexpression also conferred partial but significant protection against BITC-mediated inhibition of PC-3 cell migration (Fig. 5D).
Western blotting for CXCR4 was performed using lysates from Neo_PC-3 and CXCR4_PC-3 cells after 24 hour treatment with DMSO (control) or 2.5 and 5 μmol/L PEITC. Generally consistent with the results in un-transfected PC-3 cells (Fig. 2A), expression of CXCR4 protein was decreased by 50-70% after PEITC treatment in Neo_PC-3 cells (Fig. 6A). PEITC treatment was relatively less effective in downregulating CXCR4 protein in CXCR4_PC-3 cells in comparison with Neo_PC-3 cells (Fig. 6A). Viability of Neo_PC-3 cells was significantly decreased after 24 hour treatment with PEITC, BITC, and SFN; albeit in the order of PEITC > BITC > SFN (Fig. 6B). Similar to cell migration assay, CXCR4 overexpression conferred significant protection against cell viability inhibition by each compound (Fig. 6B). Previous studies have shown that CXCR4/CXCL12 signaling activates AKT, which is a pro-survival signal (31). In agreement with these results, S473 phosphorylation of AKT was >2-fold higher in CXCR4_PC-3 cells when compared with empty vector transfected control cells (Fig. 6C). Phosphorylation of AKT was decreased to varying extent upon treatment of CXCR4_PC-3 and Neo_PC-3 cells with each compound. A modest attenuation of pAKT suppression upon CXCR4 overexpression in the presence of 2.5 µmol/L PEITC or BITC was not consistent in different experiments. Previous studies have also shown ERK activation in CXCR4 signaling (25). Level of phospho-ERK2 was modestly higher in CXCR4_PC-3 cells than in Neo_PC-3 (Fig. 6D), but ERK activation was not affected by ITCs in either cell type (Fig. 6D). These results indicated that CXCR4-mediated protection against cell proliferation inhibition by ITCs was independent of AKT or ERK activation.
Figure 6.
Cell viability inhibition by ITCs was significantly attenuated by CXCR4 overexpression in PC-3 cells. A, western blot showing effect of PEITC treatment (24 hours) on CXCR4 protein level in Neo_PC-3 and CXCR4_PC-3 cells. B, Effects of PEITC, BITC, and SFN treatments (24 hours) on viability of Neo_PC-3 and CXCR4_PC-3 cells. The results shown are mean ± SD (n = 3). Significantly different compared with acorresponding control, and bbetween Neo_PC-3 and CXCR4_PC-3 cells by one-way ANOVA followed by Bonferroni’s test. C, western blotting for S473 phosphorylated AKT using lysates from Neo_PC-3 or CXCR4_PC-4 after 24 hour treatment with DMSO or specified ITC compound. D, western blotting for phospho-ERK and total ERK using lysates from Neo_PC-3 or CXCR4_PC-4 after 4 hour treatment with DMSO or specified ITC compound. The blots were stripped and re-probed with GAPDH to ensure equal protein loading. Each experiment was repeated at least twice.
Discussion
The present study reveals that CXCR4 is a novel target of widely-studied cancer chemopreventive ITCs at least in prostate cancer cells. Despite a close structural similarity between PEITC and BITC (Fig. 1A), marked differences have been documented in their cancer protective effect as well as associated mechanisms (2,3). However, CXCR4 seems to be a common target of both these compounds as well as for a commonly studied thioalkyl-type ITC. We also show that CXCR4 downregulation by ITCs is not a cell line-specific effect as both androgen-sensitive and androgen-independent cells are susceptible to CXCR4 down-regulation by ITCs. It is important to point out that downregulation of CXCR4 protein upon 48 h treatment of PC-3 and DU145 cells with 10 µmol/L SFN was documented previously but functional significance of its suppression was not studied (32). Since submission of this manuscript, BITC-mediated downregulation of CXCR4 was shown in a human glioma cell line but the functional significance of this observation was not studied (33).
Even though expression of CXCR4 mRNA is decreased after treatment with ITCs (Fig. 2C), the upstream mediator(s) of transcriptional repressors remain unknown. It is plausible that CXCR4 downregulation by ITCs is mediated by nuclear factor-κB as this transcription factor, which is a known regulator of CXCR4 expression, is inhibited by ITC treatment in prostate cancer cells (22,32). Recent studies have also implicated ERG (ETS-related gene) in transcriptional regulation of CXCR4 (34,35), and the possibility of ERG suppression by ITCs needs to be explored in future studies. In this context, it is interesting to note that the androgen-sensitive LNCaP cell line is relatively more sensitive to CXCR4 downregulation by ITCs compared with PC-3 cells especially at the 12 hour time point. Repression of androgen receptor expression and inhibition of synthetic androgen-stimulated proliferation of prostate cancer cells after PEITC and/or SFN treatment has also been documented (36,37). Other possible molecular mediators in ITC-mediated downregulation of CXCR4 include hypoxia-inducible factor 1α, Ets1, CREBP3, Krüppel-like factor 2, and microRNA 494-3p (38-42). However, more work is needed to determine which of these mechanism(s) accounts for ITC-mediated downregulation of CXCR4.
Downregulation of CXCR4 protein following treatment with PEITC and SFN is also observed in in vivo. The PC-3 xenografts from PEITC-treated mice clearly exhibit statistically significant decrease in CXCR4 protein expression in comparison with control. A similar trend is observed from prostate tumors of SFN-treated TRAMP mice, but the difference did not reach statistical significance due to small sample size.. It is also possible that a more intense SFN dosing regimen (e.g., daily treatment or higher dose) may be required for in vivo suppression of CXCR4 expression. Nevertheless, these results are encouraging and suggest that CXCR4 may be a predictive pharmacodynamic biomarker of ITCs in prostate adenocarcinoma.
Several studies have evaluated CXCR4 overexpression in prostate and other tumors (25,43,44). Analysis of CXCR4 expression in humans using high-density tissue microarrays from a cohort of over 600 patients revealed significantly elevated levels of this chemokine receptor in localized cancer and metastatic disease (43). High expression of CXCR4 in metastatic prostate cancers from patients on androgen deprivation therapy was associated with poor survival, but not predictive of clinical response to hormonal therapy (44). In a mouse model, a positive correlation was observed for CXCL12 levels in tissues with metastatic lesions (45). Several studies have suggested a role for CXCR4 in metastasis of prostate cancer (46,47). Because treatment with PEITC and SFN results in inhibition of distant site metastasis (13,14,15) it is reasonable to consider that anti-metastatic activity of ITCs is mediated, at least in part, via downregulation of CXCR4.
CXCR4/CXCL12 signaling is mechanistically linked to AKT-1, EGFR, ERK, and MMP-9 in prostate cancer cells (31,48). CXCR4 is localized in lipid rafts of prostate cancer cells and initiates AKT phosphorylation (31). Loss of PTEN results in induction of both CXCR4 and CXCL12 expression and these effects are reversed by AKT inhibition (31). Expression of CXCR4 is also increased by overexpression of AKT-1 in DU145 cells (48). The present study reveals that CXCR4-mediated attenuation of cell proliferation inhibition by ITCs is independent of AKT or ERK activation status. Other possibilities need to be explored to explain these observations. For example, forced expression of zinc-finger transcription factor SLUG in prostate cancer cells causes induction of CXCR4 and CXCL12 leading to promotion of cell migration (49). The effect of PEITC or SFN on SLUG is not yet known in prostate or other cancers, but we previously reported downregulation of this transcription factor in BITC-treated breast cancer cells (50). Thus, it is reasonable to conclude that suppression of SLUG likely contributes to cell migration and proliferation inhibition by ITCs.
In conclusion, the present study shows that CXCR4, but not CXCL12, is transcriptionally downregulated after treatment with PEITC, SFN and/or BITC in prostate tumor cells. We show further that CXCR4 downregulation is functionally important as inhibition of cell viability and migration by ITCs is attenuated by its overexpression.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by the grant RO1 CA101753-11 and CA115498-09 awarded by the National Cancer Institute (S.V. Singh). This research used the Animal Facility supported in part by a grant from the National Cancer Institute at the National Institutes of Health (P30 CA047904).
Footnotes
Conflict of Interest: None of the authors has any conflict of interest.
References
- 1.Hecht SS. Chemoprevention of cancer by isothiocyanates, modifiers of carcinogen metabolism. J Nutr. 1999;129:768–74. doi: 10.1093/jn/129.3.768S. suppl. [DOI] [PubMed] [Google Scholar]
- 2.Singh SV, Singh K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis. 2012;33:1833–42. doi: 10.1093/carcin/bgs216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst. 1977;58:395–8. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- 4.Ketterer B. Dietary isothiocyanates as confounding factors in the molecular epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:645–6. [PubMed] [Google Scholar]
- 5.van Poppel G, Verhoeven DT, Verhagen H, Goldbohm RA. Brassica vegetables and cancer prevention. Epidemiology and mechanisms. Adv Exp Med Biol. 1999;472:159–68. doi: 10.1007/978-1-4757-3230-6_14. [DOI] [PubMed] [Google Scholar]
- 6.Liu B, Mao Q, Cao M, Xie L. Cruciferous vegetables intake and risk of prostate cancer: a meta-analysis. Int J Urol. 2012;19:134–41. doi: 10.1111/j.1442-2042.2011.02906.x. [DOI] [PubMed] [Google Scholar]
- 7.Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- 8.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–8. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 9.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 10.Drudge-Coates L, Turner B. Prostate cancer overview. Part 1: non-metastatic disease. Br J Nurs. 2012;21:23–8. suppl. [PubMed] [Google Scholar]
- 11.Roobol MJ, Carlsson SV. Risk stratification in prostate cancer screening. Nat Rev Urol. 2013;10:38–48. doi: 10.1038/nrurol.2012.225. [DOI] [PubMed] [Google Scholar]
- 12.Spans L, Clinckemalie L, Helsen C, Vanderschueren D, Boonen S, Lerut E, et al. The genomic landscape of prostate cancer. Int J Mol Sci. 2013;14:10822–51. doi: 10.3390/ijms140610822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powolny AA, Bommareddy A, Hahm ER, Normolle DP, Beumer JH, Nelson JB, et al. Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer. J Natl Cancer Inst. 2011;103:571–84. doi: 10.1093/jnci/djr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh SV, Warin R, Xiao D, Powolny AA, Stan SD, Arlotti JA, et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009;69:2117–25. doi: 10.1158/0008-5472.CAN-08-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vyas AR, Hahm ER, Arlotti JA, Watkins S, Stolz DB, Desai D, et al. Chemoprevention of prostate cancer by D,L-sulforaphane is augmented by pharmacological inhibition of autophagy. Cancer Res. 2013;73:5985–95. doi: 10.1158/0008-5472.CAN-13-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome c and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:5828–35. doi: 10.1158/0008-5472.CAN-06-0139. [DOI] [PubMed] [Google Scholar]
- 17.Keum YS, Khor TO, Lin W, Shen G, Kwon KH, Barve A, et al. Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase pathway. Pharm Res. 2009;26:2324–31. doi: 10.1007/s11095-009-9948-5. [DOI] [PubMed] [Google Scholar]
- 18.Xiao D, Lew KL, Zeng Y, Marynowski SW, Dhir R, et al. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006;27:2223–34. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- 19.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 20.Hudson TS, Perkins SN, Hursting SD, Young HA, Kim YS, Wang TC, et al. Inhibition of androgen-responsive LNCaP prostate cancer cell tumor xenograft growth by dietary phenethyl isothiocyanate correlates with decreased angiogenesis and inhibition of cell attachment. Int J Oncol. 2012;40:1113–21. doi: 10.3892/ijo.2012.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao D, Zeng Y, Choi S, Lew KL, Nelson JB, Singh SV. Caspase-dependent apoptosis induction by phenethyl isothiocyanate, a cruciferous vegetable-derived cancer chemopreventive agent, is mediated by Bak and Bax. Clin Cancer Res. 2005;11:2670–9. doi: 10.1158/1078-0432.CCR-04-1545. [DOI] [PubMed] [Google Scholar]
- 22.Xu C, Shen G, Chen C, Gelinas C, Kong ANT. Suppression of NF-kB and NF-kB-regulated gene expression by sulforaphane and PEITC through IkBα, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–95. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 23.Bommareddy A, Hahm ER, Xiao D, Powolny AA, Fisher AL, Jiang Y, et al. Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells. Cancer Res. 2009;69:3704–12. doi: 10.1158/0008-5472.CAN-08-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahm ER, Singh SV. Sulforaphane inhibits constitutive and interleukin 6 induced activation of signal transducer and activator of transcription 3 in prostate cancer cells. Cancer Prev Res (Phila) 2010;3:484–94. doi: 10.1158/1940-6207.CAPR-09-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furusato B, Mohamed A, Uhlén M, Rhim JS. CXCR4 and cancer. Pathol Int. 2010;60:497–505. doi: 10.1111/j.1440-1827.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 26.Xiao D, Singh SV. Phenethyl isothiocyanate sensitizes androgen-independent human prostate cancer cells to docetaxel-induced apoptosis in vitro and in vivo. Pharm Res. 2010;27:722–31. doi: 10.1007/s11095-010-0079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinni SR, Yamamoto H, Dong Z, Sabbota A, Bonfil RD, Cher ML. CXCL12/CXCR4 transactivates HER2 in lipid rafts of prostate cancer cells and promotes growth of metastatic deposits in bone. Mol Cancer Res. 2008;6:446–57. doi: 10.1158/1541-7786.MCR-07-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–7. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Xiao D, Choi S, Johnson DE, Vogel VG, Johnson CS, Trump DL, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 31.Chinni SR, Sivalogan S, Dong Z, Filho JCT, Deng X, Bonfil RD, et al. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate. 2006;66:32–48. doi: 10.1002/pros.20318. [DOI] [PubMed] [Google Scholar]
- 32.Labsch S, Liu L, Bauer N, Zhang Y, Aleksandrowicz E, Gladkich J, et al. Sulforaphane and TRAIL induce a synergistic elimination of advanced prostate cancer stem-like cells. Int J Oncol. 2014;44:1470–80. doi: 10.3892/ijo.2014.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Y, Zhang L, Zhang GD, et al. Potential mechanisms of benzyl isothiocyanate suppression of invasion and angiogenesis by the U87MG human glioma cell line. Asian Pac J Cancer Prev. 2014;15:8225–8. doi: 10.7314/apjcp.2014.15.19.8225. [DOI] [PubMed] [Google Scholar]
- 34.Cai J, Kandagatla P, Singareddy R, Kropinski A, Sheng S, Cher ML, et al. Androgens Induce Functional CXCR4 through ERG Factor Expression in TMPRSS2-ERG Fusion-Positive Prostate Cancer Cells. Transl Oncol. 2010;3:195–203. doi: 10.1593/tlo.09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singareddy R, Semaan L, Conley-Lacomb MK, St John J, Powell K, Iyer M, et al. Transcriptional regulation of CXCR4 in prostate cancer: significance of TMPRSS2-ERG fusions. Mol Cancer Res. 2013;11:1349–61. doi: 10.1158/1541-7786.MCR-12-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang LG, Liu XM, Chiao JW. Repression of androgen receptor in prostate cancer cells by phenethyl isothiocyanate. Carcinogenesis. 2006;27:2124–32. doi: 10.1093/carcin/bgl075. [DOI] [PubMed] [Google Scholar]
- 37.Kim SH, Singh SV. D,L-Sulforaphane causes transcriptional repression of androgen receptor in human prostate cancer cells. Mol Cancer Ther. 2009;8:1946–54. doi: 10.1158/1535-7163.MCT-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maroni P, Bendinelli P, Matteucci E, Desiderio MA. HGF induces CXCR4 and CXCL12-mediated tumor invasion through Ets1 and NF-κB. Carcinogenesis. 2007;28:267–79. doi: 10.1093/carcin/bgl129. [DOI] [PubMed] [Google Scholar]
- 40.Kim HC, Choi KC, Choi HK, et al. HDAC3 selectively represses CREB3-mediated transcription and migration of metastatic breast cancer cells. Cell Mol Life Sci. 2010;67:3499–510. doi: 10.1007/s00018-010-0388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchida D, Onoue T, Begum NM, et al. Vesnarinone downregulates CXCR4 expression via upregulation of Krüppel-like factor 2 in oral cancer cells. Mol Cancer. 2009;8:62. doi: 10.1186/1476-4598-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen PF, Chen XQ, Liao YC, et al. MicroRNA-494-3p targets CXCR4 to suppress the proliferation, invasion, and migration of prostate cancer. Prostate. 2014;74:756–67. doi: 10.1002/pros.22795. [DOI] [PubMed] [Google Scholar]
- 43.Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89:462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 44.Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99:539–42. doi: 10.1111/j.1349-7006.2007.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–29. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 46.Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004;18:1240–2. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 47.Arya M, Patel HR, McGurk C, Tatoud R, Klocker H, Masters J, et al. The importance of the CXCL12-CXCR4 chemokine ligand-receptor interaction in prostate cancer metastasis. J Exp Ther Oncol. 2004;4:291–303. [PubMed] [Google Scholar]
- 48.Conley-LaComb MK, Saliganan A, Kandagatla P, Chen YQ, Cher ML, Chinni SR. PTEN loss mediated Akt activation promotes prostate tumor growth and metastasis via CXCL12/CXCR4 signaling. Mol Cancer. 2013;12:85. doi: 10.1186/1476-4598-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uygur B, Wu WS. SLUG promotes prostate cancer cell migration and invasion via CXCR4/CXCL12 axis. Mol Cancer. 2011;10:139. doi: 10.1186/1476-4598-10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sehrawat A, Kim SH, Vogt A, Singh SV. Suppression of FOXQ1 in benzyl isothiocyanate-mediated inhibition of epithelial-mesenchymal transition in human breast cancer cells. Carcinogenesis. 2013;34:864–73. doi: 10.1093/carcin/bgs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.