Abstract
Steroidogenesis begins with cholesterol transfer into mitochondria through the transduceosome, a complex composed of cytosolic proteins that include steroidogenesis acute regulatory protein (STAR), 14-3-3 adaptor proteins, and the outer mitochondrial membrane proteins Translocator Protein (TSPO) and Voltage-Dependent Anion Channel (VDAC). TSPO is a drug- and cholesterol- binding protein found at particularly high levels in steroid synthesizing cells. Its aberrant expression has been linked to cancer, neurodegeneration, neuropsychiatric disorders and primary hypogonadism. Brain steroids serve as local regulators of neural development and excitability. Reduced levels of these steroids have been linked to depression, anxiety and neurodegeneration. Reduced serum testosterone is common among subfertile young men and aging men, and is associated with depression, metabolic syndrome and reduced sexual function. Although testosterone-replacement therapy is available, there are undesired side-effects. TSPO drug ligands have been proposed as therapeutic agents to regulate steroid levels in the brain and testis.
Keywords: TSPO, VDAC, 14-3-3 proteins, mitochondria, cholesterol, steroidogenesis, adrenal, gonads, brain, hypogonadism, hypercortisolism, aging
1. Introduction to steroidogenesis
The steroidogenic process involves a series of substrates, the first being cholesterol, that are metabolized by enzymes distributed in the mitochondria and endoplasmic reticulum of steroid-forming cells (Payne and Hales, 2004). The first step occurs in mitochondria where the aliphatic tail of cholesterol is cleaved by the cytochrome P450 enzyme CYP11A1, localized on the matrix side of the inner mitochondrial membrane (IMM). Cholesterol is a highly lipophilic compound originating from various intracellular locations which cannot freely diffuse to the IMM. Thus, there are mechanisms to facilitate its movement to mitochondria, and its translocation to IMM and loading onto CYP11A1. The outer mitochondrial membrane (OMM) integral membrane protein translocator protein (18-kDa; TSPO) has been implicated in this process, owing to the ability of high-affinity TSPO ligands to stimulate steroidogenesis. The story of TSPO and its pharmacological targeting is the focus of this review.
2. Identification of TSPO
The benzodiazepine class of drugs exerts a number of psychoactive effects and operates through binding to central benzodiazepine binding sites in the central nervous system, principally at the GABAA receptor (Saari et al., 2011). The identification of diazepam binding sites in the kidney in 1977 initiated the search for a peripheral-type benzodiazepine receptor (Braestrup and Squires, 1977), later renamed TSPO (Papadopoulos et al., 2006). Snyder and his colleagues demonstrated the wide distribution of TSPO throughout the body (Anholt et al., 1985), its expression in particularly high levels in steroid-producing cells of the adrenal and testis (De Souza et al., 1985), and its localization at the outer mitochondrial membrane (OMM) (Anholt et al., 1986a). The large body of work generated by Gavish and colleagues, as well as other groups, demonstrated the multiple roles of this protein in mitochondrial and cell function in various tissues (Gavish et al., 1999; Papadopoulos et al., 2006; Veenman and Gavish, 2006). Various studies applying hypophysectomy, adrenalectomy, castration by surgical and chemical means, as well as hormone replacements, suggested hormonal regulation of TSPO expression and function and its possible association with the ability of the organs and tissues in question to synthesize steroids (Anholt et al., 1985; Veenman and Gavish, 2012).
3. The diazepam binding inhibitor and implication of TSPO in steroidogenesis
Although earlier studies had shown that diazepam and other benzodiazepines could modulate circulating steroid levels (Lacapere and Papadopoulos, 2003; Marc and Morselli, 1969), the identification of a natural polypeptide des-(Gly-Ile)-endozepine, also known as Diazepam Binding Inhibitor (DBI), that was able to stimulate cholesterol transport into isolated mitochondria, brought together the fields of steroidogenesis and benzodiazepines (Besman et al., 1989). DBI was originally characterized by Costa and Guidotti for its ability to displace diazepam from the benzodiazepine recognition site located on the type A receptor for gamma-aminobutyric acid (GABAA) (Costa and Guidotti, 1991) and later shown to physiologically modulate GABAA receptor function (Christian et al., 2013). The findings that TSPO was abundant in the OMM of adrenal mitochondria, and that DBI acts on the OMM to induce mitochondrial steroid formation, led to studies of the effects of well-characterized TSPO-binding families of compounds on corticosteroid and androgen formation by adrenal cortical and testicular Leydig cells, respectively (Krueger and Papadopoulos, 1990; Lacapere and Papadopoulos, 2003; Papadopoulos et al., 1991; Papadopoulos et al., 2006; Rupprecht et al., 2010). Exposure of structurally diverse TSPO ligands, including benzodiazepines, isoquinoline carboxamides and indole acetamides, to steroidogenic cell lines, primary steroidogenic cells and tissues, as well as isolated steroidogenic mitochondria, at concentrations close to their binding affinity consistently stimulates steroid synthesis 1.5–3.0-fold within minutes (Papadopoulos et al., 1990a; Romeo et al., 1993). Moreover, various TSPO ligands, namely flunitrazepam and 5-androsten-3β,17,19-triol, at nanomolar and low micromolar concentrations, respectively, were able to block hormone-induced steroid formation in both cell lines and primary cells (Midzak et al., 2011; Papadopoulos et al., 1991). Moreover, though not definitive proof of TSPO’s involvement in steroidogenesis, DBI was shown to increase steroid formation when applied to isolated mitochondria, while its oligodeoxynucleotide-based knockdown blocked steroidogenesis in Leydig cell lines (Boujrad et al., 1993). Interestingly, DBI levels in the Leydig cells were reduced in hypophysectomized animals (Schultz et al., 1992), suggesting a role in the hypothalamic-pituitary-gonadal endocrine signaling axis. Two knockout DBI (ACBD1/ACBP) mouse models have been generated, resulting in either an embryonic lethality (Landrock et al., 2010) or metabolic defects (Neess et al., 2011), suggesting a complex and as yet unclear phenotype.
4. TSPO drug-binding characteristics and relationship to steroidogenesis
Rat, human, mouse and bovine TSPO were cloned and expressed in various cells that contained low TSPO levels (Garnier et al., 1994; Parola et al., 1991; Riond et al., 1991b; Sprengel et al., 1989). The expressed TSPO was pharmacologically and biochemically characterized and found to bind TSPO drug ligands in a stereospecific manner. Earlier work had found that TSPO associated with the OMM integral membrane voltage-dependent anion channel (VDAC; McEnery et al. 1992) and reconstitution studies demonstrated that while TSPO was able to potently bind the isoquinoline carboxamide PK 11195, a TSPO-VDAC complex was necessary for binding of benzodiazepines (Garnier et al., 1994; Joseph-Liauzun et al., 1997). Elegant studies by Ferrara and colleagues characterized human TSPO expressed in S. cerevisiae, defined TSPO topography as a multispanning membrane protein consisting of a five α-helix bundle, and in silico studies demonstrated its ability to channel cholesterol through the OMM (Culty et al., 1999; Joseph-Liauzun et al., 1998; Riond et al., 1991a). We extended these biochemical studies in steroidogenic cell mitochondria (Culty et al., 1999), and further showed that hormonal stimulation of steroidogenic cells was able to alter TSPO drug-binding characteristics of steroidogenic mitochondria (Boujrad et al., 1994). TSPO has also been shown to be a high-affinity cholesterol binding protein, with cholesterol affinity localized to the C-terminal end of transmembrane helix 5 (TM5) at a conserved cholesterol recognition amino acid consensus (CRAC) domain ((Li and Papadopoulos, 1998; Li et al., 2001b); mutation of specific amino acids of TSPO’s CRAC domain has been shown to eliminate its ability to bind cholesterol (Jamin et al., 2005). Moreover, evidence indicates that hormone-mediated polymerization of TSPO in steroidogenic cell systems, in correlation with increased steroid production, increases TSPO ligand binding and decreases cholesterol binding, suggesting a mechanism of TSPO drug ligand and DBI-mediated stimulation of steroid synthesis in these cells ((Delavoie et al., 2003).
Cryo-electron microscopy studies at 10 Å resolution of a TSPO homolog from the bacterium Rhodobacter sphaeroides further supported the five-helix bundle model of the protein and its further association into a homodimer (Korkhov et al., 2010), similar to results observed for the mammalian TSPO (Delavoie et al., 2003). More recent NMR structural analysis of mouse TSPO in dodecylphosphocholine micelles indicated that the native protein is structurally dynamic, yielding a low signal dispersion (Jaremko et al., 2014). However, in the presence of the ligand PK 11195, high-resolution NMR spectra were obtained of the body of the protein, though the N- and C-terminal tails remained dynamic. This NMR structural work in the presence of TSPO ligand further validated the TSPO five transmembrane helix model binding drug ligands with a 1:1 stoichiometry at a binding pocket formed by the five transmembrane helices in the upper cytosolic part of the helix bundle (Farges et al., 1994; Jaremko et al., 2014; Li and Papadopoulos, 1998; ). The structural data indicates that the drug binding site and CRAC motif do not overlap, consistent with biochemical evidence (Li et al., 1998a). These studies were recently expanded with the determination of the crystal structure of TSPO (Guo et al., 2015; Li et al., 2015a). Furthermore, the structural effects of ligand binding, in conjunction with ligand association with Ala147 of TM5 just proximal to the CRAC motif, are consistent with biochemical evidence that drug ligand binding promotes cholesterol movement by this protein. Interestingly, a natural human polymorphism of TSPO at Ala147 (A147T) influences pregnenolone production in lymphocytes (Costa et al., 2009) as well as TSPO drug ligand binding (Owen et al., 2012), further supporting the importance of the C-terminal end of TM5 of TSPO in drug ligand binding and steroidogenesis. Interestingly, two recent studies confirmed the ability of TSPO to bind cholesterol, the presence of the CRAC domain, the ability of A147T polymorphism to affect cholesterol binding and demonstrated the presence of a cholesterol binding enhancement motif able to induce the ability of bacterial TSPO to bind cholesterol by 1000-fold (Li et al., 2015a; Li et al., 2015b).
5. TSPO-associated proteins and the transduceosome
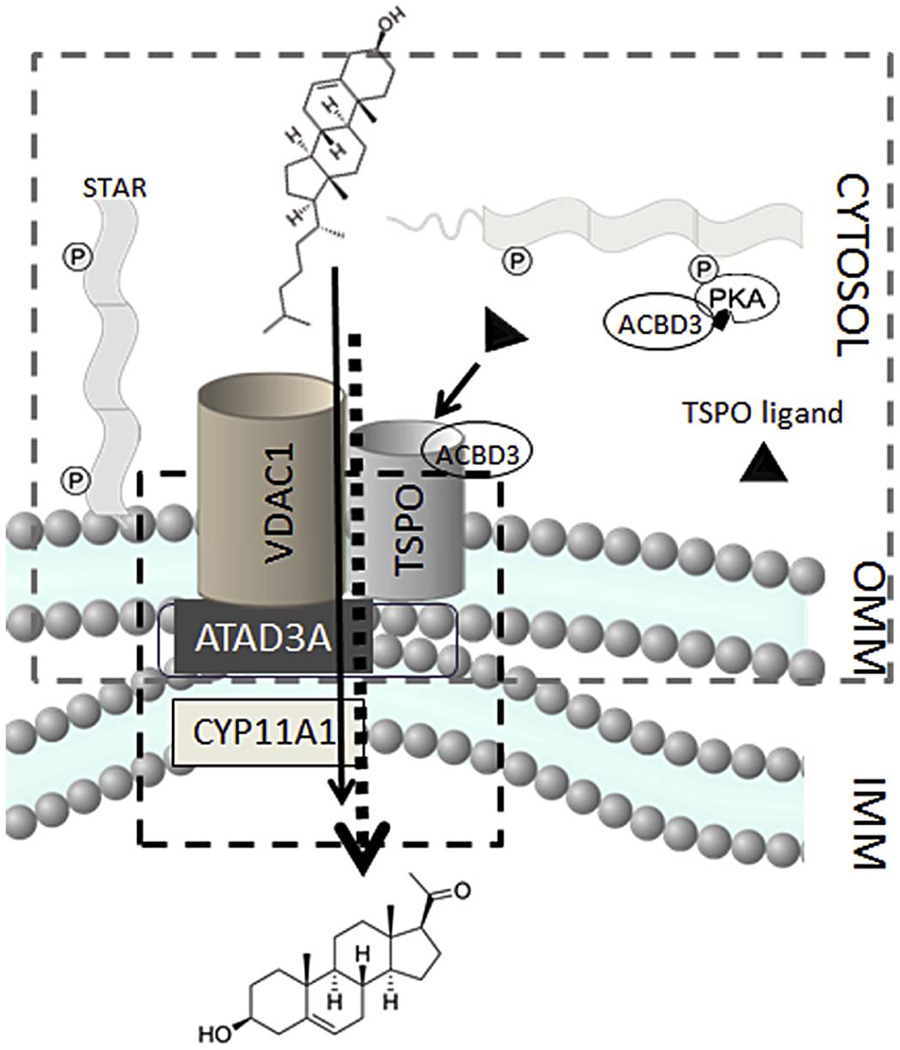
Work on identifying physical and functional partners of TSPO in steroidogenic cells has identified several proteins that have been hypothesized to play a role in steroidogenesis. These proteins include the above described VDAC (McEnery et al., 1992) and DBI (Besman et al., 1989), but also the acyl-CoA binding domain 3 (ACBD3) protein (Li et al., 2001a), a protein kinase A (PKA) anchor protein, as well as two members of the 14-3-3 protein family of cellular adaptors 14-3-3 γ and ε, Ywhag and Ywhae, respectively (Aghazadeh et al., 2012; Aghazadeh et al., 2014). Together, these proteins have been proposed to function as an outer mitochondrial signal transduction complex identified by protein crosslinking in steroidogenic cells, or “transduceosome,” linked to a mitochondrial “metabolon” (Liu et al., 2006; Rone et al., 2012). The term “metabolon” refers to multiprotein complexes that perform a series of reactions on a particular substrate. The components of metabolons are tethered at membranes, including mitochondrial membranes. Their main function is to optimally increase substrate concentration and targeting to certain enzymes for further actions (Issop et al., 2013). Recently, a native mitochondrial 800-kDa metabolon complex was identified and characterized, consisting of OMM and IMM proteins (Rone et al., 2012). This protein complex links the OMM transduceosome to the IMM CYP11A1, and therefore acts as an intermediate between cholesterol import into the mitochondria and its conversion to pregnenolone. The mitochondrial metabolon overlaps with the transduceosome at OMM as TSPO and VDAC1 are considered to be members of both complexes. Interestingly, other groups, utilizing similar methodologies, demonstrated a native STAR and VDAC interaction, suggesting a central role of VDAC in mediating mitochondrial cholesterol (Bose et al., 2008). The IMM enzyme, CYP11A1, is a part of the steroidogenic metabolon. Interactions between the IMM and OMM proteins are mediated through the IMM-OMM AAA+ ATPase protein, ATAD3 (Fig. 1) (Rone et al., 2012). Interestingly, ATAD3 has recently been reported to extend into the endoplasmic reticulum (ER), creating a bridge between the ER and mitochondria, through the formation of the mitochondrial-associated membrane fraction (Issop et al., 2015) (Figure 1). The precise intermolecular interactions of all these proteins remains an open area of research, with TSPO-VDAC interactions independently corroborated several times (Anholt et al., 1986b; Garnier et al., 1994), but investigations of TSPO-STAR molecular interactions yielding mixed results (Bogan et al., 2007; West et al., 2001).
Figure 1. Transduceosome and metabolon complexes.
The transduceosome consists of cytosolic and OMM proteins as indicated by grey margin. The metabolon, a protein complex that consists of OMM, IMS and IMM proteins, is indicated in red margin. VDAC1 and TSPO are members of both the transduceosome and metabolon. CYP11A1 enzyme, located at the mitochondrial matrix side of IMM, and ATAD3, present at the contact sites of IMM and OMM, are members unique to the metabolon complex. The transduceosome mediates the import of cholesterol from cytosolic sources to mitochondria, while the metabolon serves to direct the cholesterol molecule to CYP11A1 where it can be converted to pregnenolone.
6. Pharmacological targeting TSPO for stimulation of neurosteroid formation
An interesting development in the studies of steroids is the finding that steroids can be synthesized locally in the brain, leading to the coining of the term “neurosteroids” (Baulieu, 1997). These neurosteroids, as well as circulating neuroactive steroids (Paul and Purdy, 1992), have potent pharmacological properties, affecting neurological signaling at several neurotransmission receptors, including the GABA receptors (Chisari et al., 2010). As these steroids may mediate fundamental mechanisms that underlie behavioral symptoms in a variety of psychopathological conditions, including mood and anxiety disorders, (Rupprecht et al., 2010; Zorumski et al., 2013b) the physiological effects as well as the synthesis of these steroids have been areas of intense research; neurosteroids may prove a promising class of leads for psychological drug development (Zorumski and Mennerick, 2013a). For example, the correlation of decreased levels of GABAergic neurosteroids with manifestation of depression observed by a number of groups suggests that physiological changes in neurosteroid levels may contribute to mood disorders (Rasmusson et al., 2006; Romeo et al., 1998; Strohle et al., 1999; Uzunova et al., 1998). These lines of evidence all suggest that pharmacological stimulation of neurosteroid production may serve as a promising area for therapeutics.
Numerous laboratories have developed TSPO drug ligands, some of which have been used clinically (Caballero et al., 2013; Campiani et al., 2002; Da Settimo et al., 2008; Romeo et al., 1993; Rupprecht et al., 2009; Taliani et al., 2011). The pre-clinical and clinical use of these compounds required prior testing to be certain that they had no non-specific effects that were mediated by other receptors. We and others have shown, however, that nanomolar affinity TSPO drug ligands at micromolar concentrations can exert non-specific effects on steroid production and cell viability (Hans et al., 2005; Kletsas et al., 2004; Rupprecht et al., 2010; Veenman et al., 2007) (Figure 2). Steroids are known to act on neuronal GABAA and other receptors, thereby affecting neurotransmission and neuronal function. In light of the discovery that the brain has the ability to make steroids locally to regulate neuronal function (Schumacher et al., 2000), and with the knowledge that steroid synthesis in the brain is not regulated by any known hormone, we examined the possibility that TSPO drug ligands might be used to regulate neurosteroid formation. Using methodologies that had been used to examine gonadal and adrenal steroidogenesis, we demonstrated that brain neurosteroid synthesis was under the control of TSPO drug ligands and endogenous DBI (Lacapere and Papadopoulos, 2003; Papadopoulos et al., 2006). Thus, TSPO drug ligands offered the first pharmacological means to regulate steroid formation in the brain both in vitro and in vivo (Costa et al., 1994; Rupprecht et al., 2010). This field expanded to neuropsychiatric and neurodegenerative disorders as well as neurotrauma (Papadopoulos and Lecanu, 2009; Veenman and Gavish, 2012). Induction of neurosteroid formation in the brain by TSPO drug ligands has been used to alleviate neuropsychiatric disease symptoms (Rupprecht et al., 2010). Initially, the high affinity mixed TSPO and GABAA drug ligand alpidem (2-[6-chloro-2-(4 chlorophenyl)imidazo[1,2-a]pyridin-3-yl]-N,N-diethyl-acetamide) was approved and marketed in France as a non-sedative anxiolytic before its removal for liver toxicity (Skolnick, 2012). A clinical study showed that the high affinity TSPO drug ligand emapuril (N-benzyl-N-ethyl-2-(7-methyl-8-oxo-2-phenyl-purin-9-yl)acetamide) exerted antianxiety activity in humans and, in contrast to benzodiazepines, did not cause sedation and withdrawal symptoms (Rupprecht et al., 2009). Etifoxine (6-chloro-N-ethyl-4-methyl-4-phenyl-4H-benzo[d][1,3]oxazin-2-amine), a non-benzodiazepine, mixed TSPO and GABAA drug ligand with anxiolytic with anticonvulsant properties, was developed in the 60’s for the treatment of anxiety disorders. Today it is approved and marketed in France for the treatment of anxiety disorders, and is being studied for peripheral nerve injuries and axonal neuropathies, events shown to involve neurosteroid formation (Girard et al., 2012; Stein, 2015; Verleye et al., 2005). The precise mechanisms by which these TSPO drug ligands operate remain an open area of investigation, but are highly promising for psychiatric drug development.
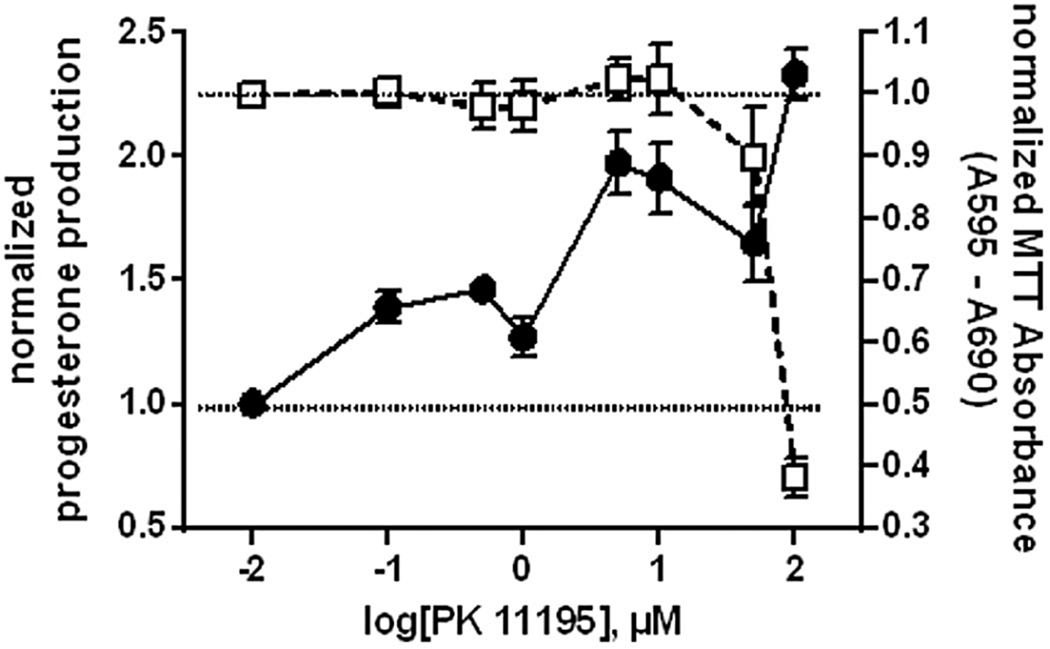
Figure 2. Relating nanomolar and micromolar concentration-based effects of TSPO ligands.
MA-10 Leydig cells were incubated with 0–100 µM of the isoquinaline carboxamide TSPO ligand PK 11195 for 2h. At the end of incubation, media was collected for analysis of steroid production by RIA, or cellular viability was assessed by MTT assay. PK 11195 began to significantly stimulate steroid production at nanomolar concentrations, and continued rising though high micromolar concentrations. However, high levels (>50µM) of PK 11195 were cytotoxic, without affecting steroid production, suggesting that such stimulation was nonspecific, and not due to stimulation of TSPO but rather to rupturing of mitochondrial membranes.
7. Pharmacological targeting TSPO for stimulation of HPG axis for hypogonadism
Reduced serum testosterone, or hypogonadism, is estimated to affect about 5 million American men, including both aging and young. The condition is common in aging men, with 20–50% of men over age 60 reported to have serum testosterone levels significantly below those of young men (age 20–30 years) (Feldman et al., 2002; Harman et al., 2001; Lutz et al., 1997; Oeppen and Vaupel, 2002). Administering exogenous testosterone, known as testosterone -replacement therapy (TRT), reverses many of the symptoms of low testosterone levels, but complications arise in the delivery and monitoring of optimal levels of supraphysiological testosterone administration. Increasing intratesticular and serum testosterone by stimulating the Leydig cells themselves could therefore have great advantages, and have been an active area of research.
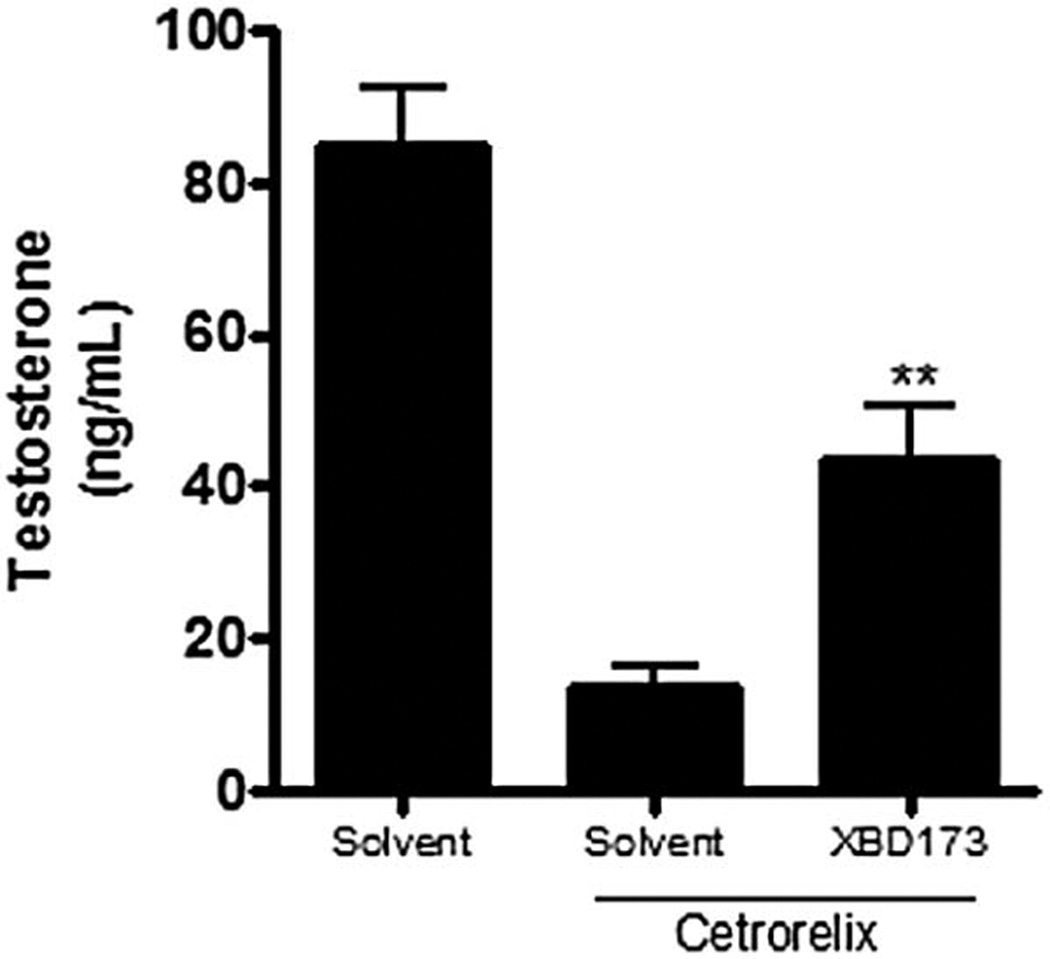
High affinity TSPO specific drug ligands have been shown to increase glucocorticoid levels in rats (reviewed in (Lacapere and Papadopoulos, 2003). The effects of these drug ligands on circulating corticosteroids were considerably more pronounced in hypophysectomized than in control animals (Cavallaro et al., 1992). Similarly, TSPO drug ligands have been used successfully to increase neurosteroid formation and thus rebalance neurological functions in cases in which neurosteroid levels were reduced (Costa et al., 1994; Rupprecht et al., 2010). These findings suggested that TSPO drug ligands may exert stimulatory effects on steroid formation by the adrenal and brain as well as by the gonads, but with effects that are relatively minor in normal (control) in vivo settings. This is likely due to the fact that in vivo, gonadal and adrenal cells are regulated by pituitary hormones, and brain cells by local factors, the same hormones and local factors that control TSPO activity. With reduced or dysfunctional central or local control, however, as for example in the case of hypogonadism, the direct activation of TSPO by its ligands could replace the effects of the natural stimulators and their second messengers, e.g. cAMP and its downstream effectors, on cholesterol import into mitochondria. Consistent with this, recent in vitro and in vivo studies of the Brown Norway rat model of aging demonstrated that the pharmacologic activation of TSPO with TSPO drug ligands is an approach that might be used therapeutically for the gonadotropin-independent induction of testosterone formation in cases of both primary and secondary hypogonadism (Chung et al., 2013). To test the ability of TSPO drug ligand to reverse hypogonadism in the young we used the GnRH antagonist (cetrorelix)-induced chemical castration. Treatment of cetrorelix-treated animals for 4 days with the TSPO specific drug ligand FGIN-1-27 resulted in the recovery of intratesticular testosterone production (Aghazadeh et al., 2014). These data were recently repeated using the TSPO ligand XBD173 with similar results (Figure 3), suggesting that these compounds can induce testosterone production by hypofunctional Leydig cells in an LH-independent manner.
Figure 3.
The effect of TSPO drug ligand XBD173 in vivo is LH-independent. Adult Sprague-Dawley rats were given i.p. injections of H2O or the GnRH antagonist Cetrorelix (0.71 mg/kg/day) for 5 days. On day 5, one testis per animal was given a bolus injection of 10 mg/Kg XBD173 or just solvent used to dissolve the drug to induce acute steroidogenesis in the absence or presence of LH signaling. Intratesticular testosterone of the injected testes was measured 2 hrs post injection by radioimmunoassay. Results shown are means ± SD (n= 5).
Tu et al recently claimed that the effect of PK 11195 on steroidogenesis is not mediated by TSPO (Tu et al., 2015). These authors reported that PK 11195 at a single dose, out of the 3 tested, increased progesterone production in MA-10 Leydig cells where Tspo gene expression was apparently abolished. Previous studies performed by various groups, cited earlier in this review, using distinct classes of TSPO drugs ligands, PK 11195 stereoisomers, photoactivatable isoquinolines and displacement studies all showed a TSPO-mediated mechanism. These results were further supported by NMR studies demonstrating the PK 11195-TSPO interaction (Jaremko et al., 2014). It is evident that like many lipophilic compounds PK 11195 could exert non-specific membrane effects that could have an effect on steroid formation. However, such effects will not be stereospecific or dose-dependent, and will not be replicated by other classes of compounds of distinct chemistry.
In addition to targeting TSPO for pharmacological stimulation of steroidogenesis, other members of the mitochondrial transduceosome complex may serve as useful pharmacological targets for stimulation of steroidogenesis as well. Work from our laboratory has demonstrated that 14-3-3γ is able to bind to the Ser194 phosphorylation site of the STAR protein (Aghazadeh et al., 2012), a phosphorylation site important for STAR steroidogenic activity. Additionally, 14-3-3ε binds to the Ser167 of VDAC1 (Aghazadeh et al., 2014), a proposed dimerization and protein association site. Reducing 14-3-3 protein association with STAR and VDAC in model cell systems, either through genetic knockdown or pharmacologically with small transducible peptides, stimulated steroid production. Administration of 14-3-3 competing peptides directly into the testes of male Sprague-Dawley rats resulted in increases in testicular and plasma testosterone levels in a dose-dependent manner (Aghazadeh et al., 2012; Aghazadeh et al., 2014). These biological fusion peptides thus present useful lead compounds in the treatment of hypogonadism in men.
8. Pharmacological targeting of TSPO for inhibition of steroidogenesis
Just as pharmacological targeting of TSPO has provided evidence that small molecules and proteins can stimulate steroidogenesis in a TSPO-dependent manner, work over the years has indicated that the reverse is possible as well, i.e., TSPO can be targeted for inhibition of steroidogenesis. Early indications of this came from work with the benzodiazepine flunitrazepam. Like other benzodiazepines, flunitrazepam is able to bind to TSPO with high affinity (Papadopoulos et al., 1990b). However, flunitrazepam inhibited hormone-stimulated steroidogenesis in both adrenal and Leydig cell lines, reducing the efficacy, but not the potency, of the hormones (Papadopoulos et al., 1991). Subsequent studies (Gazouli et al., 2002) identified peptides able to displace the benzodiazepine drug ligand Ro5-4864 (4’-chlorodiazepam). This displacement was associated with inhibition of steroidogenesis in a Leydig tumor cell line.
As noted above, TSPO possesses a cholesterol ligand binding site, its CRAC motif, in addition to its well-characterized drug ligand binding site. To explore the possibility of pharmacologically targeting this CRAC site on TSPO, we have computationally modeled and screened TSPO and its CRAC motif against large chemical databases. This has led to the identification and experimental validation of several alternative steroidal CRAC domain ligands (Midzak et al., 2011) able to potently inhibit steroidogenesis in mouse and rat Leydig tumor cell lines. These results indicate that not only is the drug-binding site a pharmacological target for control of steroidogenesis, but that the C-terminal CRAC motif of the protein is as well (Midzak et al., 2011; Midzak et al., 2012). Compounds targeting TSPO’s CRAC motif may thus serve as pharmacological leads for the treatment of diseases of steroidal excess, such as Cushing’s disease in humans and animals (Biller et al., 2008), as well as a variety of endocrine tumors (Freeman, 1986).
9. Pharmacological targeting of TSPO expression for inhibition of steroidogenesis
In addition to the focus on the regulation of steroid formation through modulation of TSPO function, the pharmacological control of TSPO expression also represents an active area of research. In search of a pharmacological approach to modulating Tspo expression and glucocorticoid biosynthesis, we examined the effect of Ginkgo biloba extract EGb 761 and its isolated components, ginkgolides. EGb 761 has been shown to have beneficial effects on memory, vigilance, cognitive functions associated with aging, dementia, and the ability to cope with daily stressors (Defeudis et al., 2003). The anti-stress action of EGb 761 differs from that of classical anxiolytics or antidepressants (Porsolt et al., 1990). Because of the well-known pathogenic potential of glucocorticoid excess on the hippocampus (McEwen, 1994; Sapolsky, 1996), we examined whether EGb 761 and its bioactive terpene trilactones ginkgolides A and B (GKA, GKB) exert their beneficial effect by regulating glucocorticoid levels. Treatment of adult rats with EGb 761, GKA or GKB decreased serum corticosterone levels (Amri et al., 1996). We demonstrated that EGb 761 induced a 50% decrease of the 18-kDa TSPO protein and mRNA expression, accompanied by a similar decrease in the number of adrenal TSPO ligand binding sites. No changes occurred either in renal or testicular TSPO ligand binding characteristics or protein expression. Analysis of metabolically radiolabeled proteins obtained from adult rat adrenocortical cells and treated ex-vivo with EGb 761 or GKB confirmed the reduction of the 18-kDa TSPO protein levels. Corticosterone production was reduced by 80% in these cells in response to ACTH (Amri et al., 1997). Similarly, treatment of the H-295 human adrenal cells with GKB resulted in reduced production of cortisol levels in response to cAMP (Amri et al., 1996). These findings suggested that GKA and GKB, components of EGb 761, exert specific effects on adrenocortical cells by inhibiting Tspo mRNA and protein expression (Amri et al., 2003), thus limiting the amount of mitochondrial cholesterol available to the P450scc for corticosteroid synthesis. Interestingly, GKB treatment reduced the ACTH-stimulated corticosteroid production, without affecting basal glucocorticoid and aldosterone formation, suggesting a role in hormone-stimulated steroidogenesis
10. Genetic targeting of TSPO expression
In contrast to pharmacological targeting of TSPO, for which much is known and agreed upon, the genetic manipulation of TSPO is far more controversial. Knockdown of TSPO in tumor cell models, through homologous recombination (Papadopoulos et al., 1997b), treatment with antisense oligodeoxynucleotides (Hauet et al., 2005) or by antisense knockdown (Kelly-Hershkovitz et al., 1998), was shown to affect steroid biosynthesis in steroidogenic cells. However, it has been technically challenging to genetically separate the effects of TSPO on steroidogenesis from those on cell viability (Kelly-Hershkovitz et al., 1998). Complete deletion of the 11kb Tspo gene led to arrest of cell growth and cells could not be maintained in culture (Amri et al., 1999; Lacapere and Papadopoulos, 2003; Papadopoulos et al., 1997c). Further, we co-authored industry-led studies reporting that deletion of the entire Tspo gene in ES cells did not result in viable mice, suggesting embryonic lethality (Papadopoulos et al., 1997a). Although this work was not followed up at that time, an examination of the entire genome area of the Tspo locus reveals that two genes, tubulin tyrosine ligase-like family member 12 (Ttll12) and mitochondrial malonyl-CoA:ACP acyltransferase (Mcat), have partial overlap at this locus (http://www.ncbi.nlm.nih.gov/mapview/maps.cgi?taxid=10090&chr=15&query=uid(-2121628991,-1265745590,-2146573800)&QSTR=12257%5Bgene%5Fid%5D&maps=gene_set&cmd=focus). In addition, the Tspo gene locus encodes 48 predicted miRNAs (http://mirbase.org) and the Tspo coding DNA sequence is the target of 23 miRNAs (http://informatics.jax.org). In light of the fact that a number of biological systems are networked, it is possible that changes in any of those genes or miRNAs may have led to the observed embryonic lethality. The necessity of TSPO for embryonic development and steroidogenesis have been recently challenged, however, as testis-specific (Morohaku et al., 2014) and whole body (Banati et al., 2014; Tu et al., 2014) TSPO knockout mice were generated. In these animals, TSPO was reported to not be present in genetically modified tissue, and no observable effect on steroid levels was found. The authors concluded that TSPO is neither essential nor involved in steroidogenesis, contending that if TSPO is a key rate-limiting step in cholesterol movement into mitochondria, a substantial decrease should have had some effect on the testis and adrenal. In contrast to these findings, we observed murine embryonic lethality in our own recent conditional TSPO knockout studies targeting the region between exons 2 and 3, suggesting that TSPO plays an important role in embryogenesis and hormone-induced steroidogenesis (Campioli et al., 2015). That said, gene knockouts are becoming increasing difficult to interpret; different substrains of the same mouse can yield different results; breeding schemes affect the selection process and Mendelian ratios are rarely reported. It is likely that the embryonic lethality observed when deleting the entire 11kb Tspo gene, where the first intron covers more that 8-kb in the 5’-untranslated region, may be due to elements within the gene that may affect the expression of other genes important for survival as indicated above. This would not occur when floxing Tspo exons 2 to 3, although our recent data shows that even removal of these exons led to a major embryonic lethality (Campioli et al., 2015). Nonetheless, these findings raise questions regarding mitochondrial TSPO’s exact function in physiological steroidogenesis. Though no physiological effect of TSPO deletion on gonadal and adrenal steroidogenesis was observed in these studies, the effect of TSPO ligands on steroid levels in these genetically modified animals was not examined.
Interestingly, mice null for the OMM voltage-dependent anion channel (Vdac), a nucleus-encoded ubiquitous protein, major component of the OMM, implicated in numerous biological processes, are viable and display little overt phenotypical changes (Messina et al., 2012; Raghavan et al., 2012). Moreover, mitochondria from Vdac1−/− and Vdac3-null mice as well as Vdac1, 2 and 3 null cell lines failed to show changes in mitochondrial permeability transition pore activity and Bcl-2 family member-driven cell death compared to wild-type mitochondria (Baines et al., 2007; Craigen et al., 2008), processes in which VDAC had ascribed a key role. These results suggest that, despite VDAC’s apparent importance, other proteins may take over its functions after deletion. The same is true for the adenine nucleotide translocator 1 (ANT1) a nucleus-encoded protein inserted into IMM of many tissues, including heart, muscle, brain, eye, lungs, kidney, and testis, and serving as a critical component of mitochondrial oxidative phosphorylation driving the ATP-ADP exchange (Graham et al., 1997). Homozygous Ant1−/−mice were viable, fertile and exhibited normal growth characteristics when compared to wild-type mice (Graham et al., 1997). Thus, functional redundancies may exist for mitochondrial proteins including VDAC1, ANT1 and TSPO because of their importance in general cell and animal physiology, in addition to steroidogenesis.
With the increased use of genetic mouse models we appear to be entering an exciting era in the study of TSPO and mitochondrial membrane proteins, one that should prove fruitful in the discovery of new pharmacological tools and leads for therapeutics.
11. Conclusions
Numerous in vitro and in vivo findings (outlined above) indicate TSPO drug ligands induce steroid formation in the steroidogenic tissues of the adrenal, gonad, brain, placenta and liver (reviewed in (Lacapere and Papadopoulos, 2003), suggesting that TSPO is important in the control of steroid formation in a manner independent of hormones. Indeed, TSPO provides a physical mechanism since it binds cholesterol, is located in the OMM, and physically associates (directly or indirectly) with the steroidogenic machinery. Recent structural studies confirmed the proposed structure and function of TSPO (Guo et al., 2015; Jaremko et al., 2014; Li et al., 2015a). TSPO upregulation has been connected to several diseases, including cancer (Batarseh and Papadopoulos, 2010), neuronal damage, neurodegeneration, and inflammation, making the protein an important marker for glial cell activation and neuroinflammation (Dickens et al., 2014; Harberts et al., 2013).TSPO has also attracted attention as a possible molecular target for tumor imaging and chemotherapy (Austin et al., 2013). Preclinical and clinical studies have indicated that TSPO ligands are able to restore normal rates of steroid formation in cases of reduced androgen synthesis, adrenal function and neurosteroid formation and might be valuable in the treatment of hypogonadism, neurological and psychiatric disorders. Through the use of drug antagonists or CRAC ligands, TSPO also is a target for the reduction of steroid formation in cases of excessive steroid synthesis, including Cushing’s syndrome (Biller et al., 2008) and gonadal and adrenal steroid- producing tumors (Freeman, 1986).
Data generated from over 30 years of work by numerous laboratories around the world, in academia and in industry, have clearly shown that the manipulation of TSPO by pharmacological means can stimulate steroidogenesis in cell lines, primary cells, and organisms. However, the recent genetic studies in mice do raise questions about whether all steroidogenesis is “monothetic”, i.e. the result of one mechanism.
To conclude, answers to biological questions typically are obtained by combining a multitude of positive and negative experiments and by using various methodologies and animal models. Of course, the ideal model is the human. There is no single experiment that can provide the “definitive” answer to any research question, but rather guide us toward the next steps. With this in mind, the present paper calls for further studies to identify the cellular and molecular mechanisms driving cholesterol import into mitochondria for gonadal steroidogenesis. There is much left to learn.
Highlights.
Historical perspective on pharmacological targeting of TSPO in steroidogenesis
Targeting mitochondrial transduceosome for control of steroidogenesis
Review of recent genetic engineering work targeting transduceosome components
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (MOP 102647 and MOP 125983) and a Canada Research Chair in Biochemical Pharmacology to VP, and by a grant from the National Institutes of Health (R37 AG21092) to BZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: V.P., YA, JF and AM are named inventors on patents related to drugs regulating steroid production.
References
- Aghazadeh Y, Martinez-Arguelles DB, Fan J, Culty M, Papadopoulos V. Induction of androgen formation in the male by a TAT-VDAC1 fusion peptide blocking 14-3-3varepsilon protein adaptor and mitochondrial VDAC1 interactions. Mol.Ther. 2014;22:1779–1791. doi: 10.1038/mt.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghazadeh Y, Rone MB, Blonder J, Ye X, Veenstra TD, Hales DB, Culty M, Papadopoulos V. Hormone-induced 14-3-3gamma adaptor protein regulates steroidogenic acute regulatory protein activity and steroid biosynthesis in MA-10 Leydig cells. J.Biol.Chem. 2012;287:15380–15394. doi: 10.1074/jbc.M112.339580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri H, Li H, Culty M, Gaillard J-L, Teper G, Papadopoulos V. The peripheral-type benzodiazepine receptor and adrenal setroidogenesis. Curr.Opin.Endo.Diab. 1999;6:179–184. [Google Scholar]
- Amri H, Drieu K, Papadopoulos V. Ex vivo regulation of adrenal cortical cell steroid and protein synthesis, in response to adrenocorticotropic hormone stimulation, by the Ginkgo biloba extract EGb 761 and isolated ginkgolide B. Endocrinology. 1997;138:5415–5426. doi: 10.1210/endo.138.12.5604. [DOI] [PubMed] [Google Scholar]
- Amri H, Drieu K, Papadopoulos V. Transcriptional suppression of the adrenal cortical peripheral-type benzodiazepine receptor gene and inhibition of steroid synthesis by ginkgolide B. Biochem.Pharmacol. 2003;65:717–729. doi: 10.1016/s0006-2952(02)01603-9. [DOI] [PubMed] [Google Scholar]
- Amri H, Ogwuegbu SO, Boujrad N, Drieu K, Papadopoulos V. In vivo regulation of peripheral-type benzodiazepine receptor and glucocorticoid synthesis by Ginkgo biloba extract EGb 761 and isolated ginkgolides. Endocrinology. 1996;137:5707–5718. doi: 10.1210/endo.137.12.8940403. [DOI] [PubMed] [Google Scholar]
- Anholt RR, De Souza EB, Kuhar MJ, Snyder SH. Depletion of peripheral-type benzodiazepine receptors after hypophysectomy in rat adrenal gland and testis. Eur.J.Pharmacol. 1985;110:41–46. doi: 10.1016/0014-2999(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Anholt RR, Pedersen PL, De Souza EB, Snyder SH. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. Journal of Biological Chemistry. 1986a;261:576–583. [PubMed] [Google Scholar]
- Anholt RR, Pedersen PL, De SE, Snyder SH. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J Biol Chem. 1986b;261:576–583. [PubMed] [Google Scholar]
- Austin CJ, Kahlert J, Kassiou M, Rendina LM. The translocator protein (TSPO): a novel target for cancer chemotherapy. Int.J.Biochem.Cell Biol. 2013;45:1212–1216. doi: 10.1016/j.biocel.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat.Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB, Middleton RJ, Chan R, Hatty CR, Kam WW, Quin C, Graeber MB, Parmar A, Zahra D, Callaghan P, Fok S, Howell NR, Gregoire M, Szabo A, Pham T, Davis E, Liu GJ. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat.Commun. 2014;5:5452. doi: 10.1038/ncomms6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batarseh A, Papadopoulos V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol.Cell Endocrinol. 2010;327:1–12. doi: 10.1016/j.mce.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog.Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- Besman MJ, Yanagibashi K, Lee TD, Kawamura M, Hall PF, Shively JE. Identification of des-(Gly-Ile)-endozepine as an effector of corticotropin-dependent adrenal steroidogenesis: stimulation of cholesterol delivery is mediated by the peripheral benzodiazepine receptor. Proc.Natl.Acad.Sci.U.S.A. 1989;86:4897–4901. doi: 10.1073/pnas.86.13.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M. Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. J.Clin.Endocrinol.Metab. 2008;93:2454–2462. doi: 10.1210/jc.2007-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan RL, Davis TL, Niswender GD. Peripheral-type benzodiazepine receptor (PBR) aggregation and absence of steroidogenic acute regulatory protein (StAR)/PBR association in the mitochondrial membrane as determined by bioluminescence resonance energy transfer (BRET) Journal of Steroid Biochemistry Molecular Biology. 2007;104:61–67. doi: 10.1016/j.jsbmb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Bose M, Whittal RM, Miller WL, Bose HS. Steroidogenic activity of StAR requires contact with mitochondrial VDAC1 and phosphate carrier protein. J.Biol.Chem. 2008;283:8837–8845. doi: 10.1074/jbc.M709221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boujrad N, Gaillard JL, Garnier M, Papadopoulos V. Acute action of choriogonadotropin on Leydig tumor cells: induction of a higher affinity benzodiazepine-binding site related to steroid biosynthesis. Endocrinology. 1994;135:1576–1583. doi: 10.1210/endo.135.4.7925120. [DOI] [PubMed] [Google Scholar]
- Boujrad N, Hudson JR, Jr, Papadopoulos V. Inhibition of hormone-stimulated steroidogenesis in cultured Leydig tumor cells by a cholesterol-linked phosphorothioate oligodeoxynucleotide antisense to diazepam-binding inhibitor. Proc.Natl.Acad.Sci.U.S.A. 1993;90:5728–5731. doi: 10.1073/pnas.90.12.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braestrup C, Squires RF. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc.Natl.Acad.Sci.U.S.A. 1977;74:3805–3809. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero B, Veenman L, Gavish M. Role of mitochondrial translocator protein (18 kDa) on mitochondrial- related cell death processes. Recent Pat Endocr.Metab Immune.Drug Discov. 2013;7:86–101. doi: 10.2174/1872214811307020002. [DOI] [PubMed] [Google Scholar]
- Campiani G, Ramunno A, Fiorini I, Nacci V, Morelli E, Novellino E, Goegan M, Mennini T, Sullivan S, Zisterer DM, Williams CD. Synthesis of new molecular probes for investigation of steroid biosynthesis induced by selective interaction with peripheral type benzodiazepine receptors (PBR) J Med.Chem. 2002;45:4276–4281. doi: 10.1021/jm020849l. [DOI] [PubMed] [Google Scholar]
- Campioli E, Fan J, Midzak A, Culty M, Papadopoulos V. Global knockout and steroidogenic cell-targeted deletion of the translocator protein (18-kDa) unveil its crucial role in viability and hormone-dependent steroid formation. San Diego, CA, USA. Endocrine Society Annual Meeting; 2015. Mar 5–8, [Google Scholar]
- Cavallaro S, Korneyev A, Guidotti A, Costa E. Diazepam-binding inhibitor (DBI)-processing products, acting at the mitochondrial DBI receptor, mediate adrenocorticotropic hormone-induced steroidogenesis in rat adrenal gland. Proc.Natl.Acad.Sci.U.S.A. 1992;89:10598–10602. doi: 10.1073/pnas.89.22.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABA(A) receptors. Trends Neurosci. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Herbert AG, Holt RL, Peng K, Sherwood KD, Pangratz-Fuehrer S, Rudolph U, Huguenard JR. Endogenous positive allosteric modulation of GABA(A) receptors by diazepam binding inhibitor. Neuron. 2013;78:1063–1074. doi: 10.1016/j.neuron.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Chen H, Midzak A, Burnett AL, Papadopoulos V, Zirkin BR. Drug ligand-induced activation of translocator protein (TSPO) stimulates steroid production by aged brown Norway rat Leydig cells. Endocrinology. 2013;154:2156–2165. doi: 10.1210/en.2012-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Pini S, Gabelloni P, Da PE, Abelli M, Lari L, Preve M, Lucacchini A, Cassano GB, Martini C. The spontaneous Ala147Thr amino acid substitution within the translocator protein influences pregnenolone production in lymphomonocytes of healthy individuals. Endocrinology. 2009;150:5438–5445. doi: 10.1210/en.2009-0752. [DOI] [PubMed] [Google Scholar]
- Costa E, Auta J, Guidotti A, Korneyev A, Romeo E. The pharmacology of neurosteroidogenesis. J.Steroid Biochem.Mol.Biol. 1994;49:385–389. doi: 10.1016/0960-0760(94)90284-4. [DOI] [PubMed] [Google Scholar]
- Costa E, Guidotti A. Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life Sciences. 1991;49:325–344. doi: 10.1016/0024-3205(91)90440-m. [DOI] [PubMed] [Google Scholar]
- Craigen WJ, Graham BH. Genetic strategies for dissecting mammalian and Drosophila voltage-dependent anion channel functions. J.Bioenerg.Biomembr. 2008;40:207–212. doi: 10.1007/s10863-008-9146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culty M, Li H, Boujrad N, Amri H, Vidic B, Bernassau JM, Reversat JL, Papadopoulos V. In vitro studies on the role of the peripheral-type benzodiazepine receptor in steroidogenesis. J.Steroid.Biochem.Mol.Biol. 1999;69:123–130. doi: 10.1016/s0960-0760(99)00056-4. [DOI] [PubMed] [Google Scholar]
- Da Settimo F, Simorini F, Taliani S, La Motta C, Marini AM, Salerno S, Bellandi M, Novellino E, Greco G, Cosimelli B, Da PE, Costa B, Simola N, Morelli M, Martini C. Anxiolytic-like effects of N,N-dialkyl-2-phenylindol-3-ylglyoxylamides by modulation of translocator protein promoting neurosteroid biosynthesis. J.Med.Chem. 2008;51:5798–5806. doi: 10.1021/jm8003224. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Anholt RR, Murphy KM, Snyder SH, Kuhar MJ. Peripheral-type benzodiazepine receptors in endocrine organs: autoradiographic localization in rat pituitary, adrenal, and testis. Endocrinology. 1985;116:567–573. doi: 10.1210/endo-116-2-567. [DOI] [PubMed] [Google Scholar]
- Defeudis FV, Papadopoulos V, Drieu K. Ginkgo biloba extracts and cancer: a research area in its infancy. Fundam.Clin.Pharmacol. 2003;17:405–417. doi: 10.1046/j.1472-8206.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- Delavoie F, Li H, Hardwick M, Robert JC, Giatzakis C, Peranzi G, Yao ZX, Maccario J, Lacapere JJ, Papadopoulos V. In vivo and in vitro peripheral-type benzodiazepine receptor polymerization: functional significance in drug ligand and cholesterol binding. Biochemistry. 2003;42:4506–4519. doi: 10.1021/bi0267487. [DOI] [PubMed] [Google Scholar]
- Dickens AM, Vainio S, Marjamaki P, Johansson J, Lehtiniemi P, Rokka J, Rinne J, Solin O, Haaparanta-Solin M, Jones PA, Trigg W, Anthony DC, Airas L. Detection of microglial activation in an acute model of neuroinflammation using PET and radiotracers 11C-(R)-PK11195 and 18F–GE-180. J.Nucl.Med. 2014;55:466–472. doi: 10.2967/jnumed.113.125625. [DOI] [PubMed] [Google Scholar]
- Farges R, Joseph-Liauzun E, Shire D, Caput D, Le FG, Ferrara P. Site-directed mutagenesis of the peripheral benzodiazepine receptor: identification of amino acids implicated in the binding site of Ro5-4864. Mol Pharmacol. 1994;46:1160–1167. [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J.Clin.Endocrinol.Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- Freeman DA. Steroid hormone-producing tumors in man. Endocr.Rev. 1986;7:204–220. doi: 10.1210/edrv-7-2-204. [DOI] [PubMed] [Google Scholar]
- Garnier M, Dimchev AB, Boujrad N, Price JM, Musto NA, Papadopoulos V. In vitro reconstitution of a functional peripheral-type benzodiazepine receptor from mouse Leydig tumor cells. Mol.Pharmacol. 1994;45:201–211. [PubMed] [Google Scholar]
- Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, Weisinger G, Weizman A. Enigma of the Peripheral Benzodiazepine Receptor. Pharmacol.Rev. 1999;51:629–650. [PubMed] [Google Scholar]
- Gazouli M, Han Z, Papadopoulos V. Identification of a peptide antagonist to the peripheral-type benzodiazepine receptor that inhibits hormone-stimulated leydig cell steroid formation. J.Pharmacol.Exp.Ther. 2002;303:627–632. doi: 10.1124/jpet.102.039388. [DOI] [PubMed] [Google Scholar]
- Girard C, Liu S, Adams D, Lacroix C, Sineus M, Boucher C, Papadopoulos V, Rupprecht R, Schumacher M, Groyer G. Axonal regeneration and neuroinflammation: roles for the translocator protein 18 kDa. J.Neuroendocrinol. 2012;24:71–81. doi: 10.1111/j.1365-2826.2011.02215.x. [DOI] [PubMed] [Google Scholar]
- Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, Wallace DC. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat.Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- Guo G, Kalathur RC, Liu Q, Kloss B, Ginter C, Kloppmann E, Rost B, Hendrickson WA. Structure and activity of tryptophan-rich TSPO proteins. Science. 2015;347:551–555. doi: 10.1126/science.aaa1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans G, Wislet-Gendebien S, Lallemend F, Robe P, Rogister B, Belachew S, Nguyen L, Malgrange B, Moonen G, Rigo JM. Peripheral benzodiazepine receptor (PBR) ligand cytotoxicity unrelated to PBR expression. Biochemical Pharmacology. 2005;69:819–830. doi: 10.1016/j.bcp.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Harberts E, Datta D, Chen S, Wohler JE, Oh U, Jacobson S. Translocator protein 18 kDa (TSPO) expression in multiple sclerosis patients. J.Neuroimmune.Pharmacol. 2013;8:51–57. doi: 10.1007/s11481-012-9397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J.Clin.Endocrinol.Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Hauet T, Yao ZX, Bose HS, Wall CT, Han Z, Li W, Hales DB, Miller WL, Culty M, Papadopoulos V. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into leydig cell mitochondria. Mol Endocrinol. 2005;19:540–554. doi: 10.1210/me.2004-0307. [DOI] [PubMed] [Google Scholar]
- Issop L, Fan J, Lee S, Rone MB, Basu K, Mui J, Papadopoulos V. Mitochondria-associated membrane formation in hormone-stimulated Leydig cell steroidogenesis: Role of ATAD3. Endocrinology. 2015;156:334–345. doi: 10.1210/en.2014-1503. [DOI] [PubMed] [Google Scholar]
- Issop L, Rone MB, Papadopoulos V. Organelle plasticity and interactions in cholesterol transport and steroid biosynthesis. Mol.Cell Endocrinol. 2013;371:34–46. doi: 10.1016/j.mce.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Jamin N, Neumann JM, Ostuni MA, Vu TK, Yao ZX, Murail S, Robert JC, Giatzakis C, Papadopoulos V, Lacapere JJ. Characterization of the cholesterol recognition amino acid consensus sequence of the peripheral-type benzodiazepine receptor. Mol.Endocrinol. 2005;19:588–594. doi: 10.1210/me.2004-0308. [DOI] [PubMed] [Google Scholar]
- Jaremko L, Jaremko M, Giller K, Becker S, Zweckstetter M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science. 2014;343:1363–1366. doi: 10.1126/science.1248725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph-Liauzun E, Delmas P, Shire D, Ferrara P. Topological analysis of the peripheral benzodiazepine receptor in yeast mitochondrial membranes supports a five-transmembrane structure. Journal of Biological Chemistry. 1998;273:2146–2152. doi: 10.1074/jbc.273.4.2146. [DOI] [PubMed] [Google Scholar]
- Joseph-Liauzun E, Farges R, Delmas P, Ferrara P, Loison G. The Mr 18,000 subunit of the peripheral-type benzodiazepine receptor exhibits both benzodiazepine and isoquinoline carboxamide binding sites in the absence of the voltage-dependent anion channel or of the adenine nucleotide carrier. J Biol Chem. 1997;272:28102–28106. doi: 10.1074/jbc.272.44.28102. [DOI] [PubMed] [Google Scholar]
- Kelly-Hershkovitz E, Weizman R, Spanier I, Leschiner S, Lahav M, Weisinger G, Gavish M. Effects of peripheral-type benzodiazepine receptor antisense knockout on MA-10 Leydig cell proliferation and steroidogenesis. J Biol Chem. 1998;273:5478–5483. doi: 10.1074/jbc.273.10.5478. [DOI] [PubMed] [Google Scholar]
- Kletsas D, Li W, Han Z, Papadopoulos V. Peripheral-type benzodiazepine receptor (PBR) and PBR drug ligands in fibroblast and fibrosarcoma cell proliferation: role of ERK, c-Jun and ligand-activated PBR-independent pathways. Biochem Pharmacol. 2004;67:1927–1932. doi: 10.1016/j.bcp.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Korkhov VM, Sachse C, Short JM, Tate CG. Three-dimensional structure of TspO by electron cryomicroscopy of helical crystals. Structure. 2010;18:677–687. doi: 10.1016/j.str.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger KE, Papadopoulos V. Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. Journal of Biological Chemistry. 1990;265:15015–15022. [PubMed] [Google Scholar]
- Lacapere JJ, Papadopoulos V. Peripheral-type benzodiazepine receptor: structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids. 2003;68:569–585. doi: 10.1016/s0039-128x(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Landrock D, Atshaves BP, McIntosh AL, Landrock KK, Schroeder F, Kier AB. Acyl-CoA binding protein gene ablation induces pre-implantation embryonic lethality in mice. Lipids. 2010;45:567–580. doi: 10.1007/s11745-010-3437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Liu J, Zhen Y, Garavito RM, Ferguson-Miller S. Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism. Science. 2015a;347:555–558. doi: 10.1126/science.1260590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Liu J, Valls L, Hiser C, Ferguson-Miller S. Identification of a Key Cholesterol Binding Enhancement Motif in Translocator Protein 18 kDa. Biochemistry. 2015b doi: 10.1021/bi5015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Degenhardt B, Tobin D, Yao ZX, Tasken K, Papadopoulos V. Identification, localization, and function in steroidogenesis of PAP7: a peripheral-type benzodiazepine receptor- and PKA (RIalpha)-associated protein. Mol.Endocrinol. 2001a;15:2211–2228. doi: 10.1210/mend.15.12.0736. [DOI] [PubMed] [Google Scholar]
- Li H, Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139:4991–4997. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- Li H, Yao Z, Degenhardt B, Teper G, Papadopoulos V. Cholesterol binding at the cholesterol recognition/ interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc.Natl.Acad.Sci.U.S.A. 2001b;98:1267–1272. doi: 10.1073/pnas.031461598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Rone MB, Papadopoulos V. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. Journal of Biological Chemistry. 2006;281:38879–38893. doi: 10.1074/jbc.M608820200. [DOI] [PubMed] [Google Scholar]
- Lutz W, Sanderson W, Scherbov S. Doubling of world population unlikely. Nature. 1997;387:803–805. doi: 10.1038/42935. [DOI] [PubMed] [Google Scholar]
- Marc V, Morselli PL. Effect of diazepam on plasma corticosterone levels in the rat. J.Pharm.Pharmacol. 1969;21:784–786. doi: 10.1111/j.2042-7158.1969.tb08173.x. [DOI] [PubMed] [Google Scholar]
- McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc.Natl.Acad.Sci.U.S.A. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Corticosteroids and hippocampal plasticity. Ann.N.Y.Acad.Sci. 1994;746:134–142. doi: 10.1111/j.1749-6632.1994.tb39223.x. [DOI] [PubMed] [Google Scholar]
- Messina A, Reina S, Guarino F, De PV. VDAC isoforms in mammals. Biochim.Biophys.Acta. 2012;1818:1466–1476. doi: 10.1016/j.bbamem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Midzak A, Akula N, Lecanu L, Papadopoulos V. Novel androstenetriol interacts with the mitochondrial translocator protein and controls steroidogenesis. J.Biol.Chem. 2011;286:9875–9887. doi: 10.1074/jbc.M110.203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzak A, Rammouz G, Papadopoulos V. Structure-activity relationship (SAR) analysis of a family of steroids acutely controlling steroidogenesis. Steroids. 2012;77:1327–1334. doi: 10.1016/j.steroids.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Morohaku K, Pelton SH, Daugherty DJ, Butler WR, Deng W, Selvaraj V. Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology. 2014;155:89–97. doi: 10.1210/en.2013-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neess D, Bloksgaard M, Bek S, Marcher AB, Elle IC, Helledie T, Due M, Pagmantidis V, Finsen B, Wilbertz J, Kruhoffer M, Faergeman N, Mandrup S. Disruption of the acyl-CoA-binding protein gene delays hepatic adaptation to metabolic changes at weaning. J.Biol.Chem. 2011;286:3460–3472. doi: 10.1074/jbc.M110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J.Cereb.Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, Hardwick M, Li H, Vidic B, Brown AS, Reversa JL, Bernassau JM, Drieu K. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997a;62:21–28. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J.Biol.Chem. 1997b;272:32129–32135. doi: 10.1074/jbc.272.51.32129. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J.Biol.Chem. 1997c;272:32129–32135. doi: 10.1074/jbc.272.51.32129. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol.Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Lecanu L. Translocator protein (18 kDa) TSPO: An emerging therapeutic target in neurotrauma. Experimental Neurology. 2009;217:53–57. doi: 10.1016/j.expneurol.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Mukhin AG, Costa E, Krueger KE. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J.Biol.Chem. 1990a;265:3772–3779. [PubMed] [Google Scholar]
- Papadopoulos V, Mukhin AG, Costa E, Krueger KE. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J.Biol.Chem. 1990b;265:3772–3779. [PubMed] [Google Scholar]
- Papadopoulos V, Nowzari FB, Krueger KE. Hormone-stimulated steroidogenesis is coupled to mitochondrial benzodiazepine receptors. Tropic hormone action on steroid biosynthesis is inhibited by flunitrazepam. J.Biol.Chem. 1991;266:3682–3687. [PubMed] [Google Scholar]
- Parola AL, Stump DG, Pepperl DJ, Krueger KE, Regan JW, Laird HE. Cloning and expression of a pharmacologically unique bovine peripheral-type benzodiazepine receptor isoquinoline binding protein. J.Biol.Chem. 1991;266:14082–14087. [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB Journal. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr.Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Martin P, Lenegre A, Fromage S, Drieu K. Effects of an extract of Ginkgo Biloba (EGB 761) on “learned helplessness” and other models of stress in rodents. Pharmacol.Biochem.Behav. 1990;36:963–971. doi: 10.1016/0091-3057(90)90107-s. [DOI] [PubMed] [Google Scholar]
- Raghavan A, Sheiko T, Graham BH, Craigen WJ. Voltage-dependant anion channels: novel insights into isoform function through genetic models. Biochim.Biophys.Acta. 2012;1818:1477–1485. doi: 10.1016/j.bbamem.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol.Psychiatry. 2006;60:704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Riond J, Leplatois P, Laurent P, Le FG, Caput D, Loison G, Ferrara P. Expression and pharmacological characterization of the human peripheral-type benzodiazepine receptor in yeast. Eur.J.Pharmacol. 1991a;208:307–312. doi: 10.1016/0922-4106(91)90076-t. [DOI] [PubMed] [Google Scholar]
- Riond J, Mattei MG, Kaghad M, Dumont X, Guillemot JC, Lefur G, Caput D, Ferrara P. Molecular Cloning and Chromosomal Localization of a Human Peripheral-type Benzodiazepine Receptor. Eur J Biochem. 1991b;195:305–311. doi: 10.1111/j.1432-1033.1991.tb15707.x. [DOI] [PubMed] [Google Scholar]
- Romeo E, Cavallaro S, Korneyev A, Kozikowski AP, Ma D, Polo A, Costa E, Guidotti A. Stimulation of brain steroidogenesis by 2-aryl-indole-3-acetamide derivatives acting at the mitochondrial diazepam-binding inhibitor receptor complex. J.Pharmacol.Exp.Ther. 1993;267:462–471. [PubMed] [Google Scholar]
- Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am.J.Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Rone MB, Midzak AS, Issop L, Rammouz G, Jagannathan SYX, Fan J, Ye X, Blonder J, Veenstra TD, Papadopoulos V. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol.Endocrinol. 2012;26:1868–1882. doi: 10.1210/me.2012-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat.Rev.Drug Discov. 2010;9:971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schule C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, Uzunov V, McAllister KH, Bertaina-Anglade V, La Rochelle CD, Tuerck D, Floesser A, Kiese B, Schumacher M, Landgraf R, Holsboer F, Kucher K. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325:490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Saari TI, Uusi-Oukari M, Ahonen J, Olkkola KT. Enhancement of GABAergic activity: neuropharmacological effects of benzodiazepines and therapeutic use in anesthesiology. Pharmacological Reviews. 2011;63:243–267. doi: 10.1124/pr.110.002717. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Schultz R, Pelto-Huikko M, Alho H. Expression of diazepam binding inhibitorlike immunoreactivity in rat testis is dependent on pituitary hormones. Endocrinology. 1992;130:3200–3206. doi: 10.1210/endo.130.6.1597138. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Akwa Y, Guennoun R, Robert F, Labombarda F, Desarnaud F, Robel P, De Nicola AF, Baulieu EE. Steroid synthesis and metabolism in the nervous system: trophic and protective effects. J.Neurocytol. 2000;29:307–326. doi: 10.1023/a:1007152904926. [DOI] [PubMed] [Google Scholar]
- Skolnick P. Anxioselective anxiolytics: on a quest for the Holy Grail. Trends Pharmacol.Sci. 2012;33:611–620. doi: 10.1016/j.tips.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R, Werner P, Seeburg PH. Molecular Cloning and Expression of cDNA Encoding a Peripheral-type Benzodiazepine Receptor. J Biol Chem. 1989;264:20415–20421. [PubMed] [Google Scholar]
- Stein DJ. Etifoxine versus alprazolam for the treatment of adjustment disorder with anxiety: a randomized controlled trial. Adv.Ther. 2015;32:57–68. doi: 10.1007/s12325-015-0176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohle A, Romeo E, Hermann B, Pasini A, Spalletta G, di MF, Holsboer F, Rupprecht R. Concentrations of 3 alpha-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol.Psychiatry. 1999;45:274–277. doi: 10.1016/s0006-3223(98)00328-x. [DOI] [PubMed] [Google Scholar]
- Taliani S, Pugliesi I, Da SF. Structural requirements to obtain highly potent and selective 18 kDa Translocator Protein (TSPO) Ligands. Curr.Top.Med.Chem. 2011;11:860–886. doi: 10.2174/156802611795165142. [DOI] [PubMed] [Google Scholar]
- Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM, Selvaraj V. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. Journal of Biological Chemistry. 2014;289:27444–27454. doi: 10.1074/jbc.M114.578286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu LN, Zhao AH, Stocco DM, Selvaraj V. PK11195 effect on steroidogenesis is not mediated through the translocator protein (TSPO) Endocrinology. 2015;156:1033–1039. doi: 10.1210/en.2014-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc.Natl.Acad.Sci.U.S.A. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenman L, Gavish M. The peripheral-type benzodiazepine receptor and the cardiovascular system. Implications for drug development. Pharmacology Therapeutics. 2006;110:503–524. doi: 10.1016/j.pharmthera.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Veenman L, Gavish M. The role of 18 kDa mitochondrial translocator protein (TSPO) in programmed cell death, and effects of steroids on TSPO expression. Curr.Mol.Med. 2012;12:398–412. doi: 10.2174/1566524011207040398. [DOI] [PubMed] [Google Scholar]
- Veenman L, Papadopoulos V, Gavish M. Channel-like functions of the 18-kDa translocator protein (TSPO): regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr.Pharm.Des. 2007;13:2385–2405. doi: 10.2174/138161207781368710. [DOI] [PubMed] [Google Scholar]
- Verleye M, Akwa Y, Liere P, Ladurelle N, Pianos A, Eychenne B, Schumacher M, Gillardin JM. The anxiolytic etifoxine activates the peripheral benzodiazepine receptor and increases the neurosteroid levels in rat brain. Pharmacology Biochemistry Behavior. 2005;82:712–720. doi: 10.1016/j.pbb.2005.11.013. [DOI] [PubMed] [Google Scholar]
- West LA, Horvat RD, Roess DA, Barisas BG, Juengel JL, Niswender GD. Steroidogenic acute regulatory protein and peripheral-type benzodiazepine receptor associate at the mitochondrial membrane. Endocrinology. 2001;142:502–505. doi: 10.1210/endo.142.1.8052. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S. Neurosteroids as therapeutic leads in psychiatry. JAMA Psychiatry. 2013a;70:659–660. doi: 10.1001/jamapsychiatry.2013.245. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci.Biobehav.Rev. 2013b;37:109–122. doi: 10.1016/j.neubiorev.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]