Abstract
Background
In 2010, aztreonam for inhalation solution joined aminoglycosides and colistimethate as a new cystic fibrosis (CF) chronic inhaled antimicrobial therapy. We studied how introduction of this new inhaled antibiotic class changed management of US CF patients.
Methods
Use of inhaled aminoglycosides, colistimethate, and aztreonam among patients followed in the CF Foundation Patient Registry was analyzed by age group, lung disease stage, and microbiologic status both annually, and at individual visits between 2009 and 2012.
Results
The overall prevalence of inhaled antibiotic use did not change during the period, but the prevalence of annual and any visit treatment with >1 inhaled antibiotic class more than doubled. Adults, those with advanced lung disease, and those with >1 Pseudomonas aeruginosa respiratory culture were more likely to receive >1 antibiotic class.
Conclusions
Inhaled antibiotic management of US CF patients has dramatically changed in association with the introduction of a third inhaled antibiotic class.
Keywords: Cystic fibrosis, inhaled antibiotics, Pseudomonas aeruginosa, Patient registry, Observational research
INTRODUCTION
Chronic inhaled antipseudomonal antibiotic administration has become standard of care for the management of cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa airway infections, with the objective of preserving lung function and reducing the risk of pulmonary exacerbations [1,2]. Prior to 2010, inhaled antibiotic management opportunities were essentially limited to two antipseudomonal antibiotic classes: polymyxins (colistimethate) and aminoglycosides (tobramycin, gentamicin, and amikacin), although extemporaneous nebulization of other antibiotics undoubtedly occurred on occasion. In 2010, aztreonam for inhalation solution, a member of the beta-lactam antibiotic class, was approved by the US Food and Drug Administration (FDA) for management of CF patients with P. aeruginosa, creating commercial access to a preformulated and tested third inhaled antibiotic class for chronic maintenance of lung health. The most recent CF Foundation (CFF) pulmonary guidelines for chronic medications for maintenance of lung health recommended both inhaled tobramycin and aztreonam for patients with persistent airway P. aeruginosa, but did not make a specific statement regarding the incorporation of both medications into management [2].
The CFF Patient Registry (CFFPR), which tracks persons with CF treated at care centers in the US, has annually reported the prevalence of use of different inhaled antibiotics among patients aged ≥6 years who have had P. aeruginosa detected in their respiratory secretions. Between 2009 and 2012, prevalence of inhaled aztreonam use increased 35% among these patients, from approximately 4% to 39%, while the prevalence of inhaled tobramycin fell only 2%, from 71% to 69%, in the same group [3,4]. These data suggest that introduction of inhaled aztreonam changed the nature of inhaled antibiotic use among CFFPR patients between 2009 and 2012. However, the difference between treatment-naïve patients initiating inhaled anti-pseudomonal antibiotic therapy as opposed to patients already on therapy who receive a second inhaled anti-pseudomonal antibiotic class is difficult to ascertain with these data. A pilot analysis limited to data from our Adult CF program at University Hospitals Case Medical Center in Cleveland has suggested that the proportion of patients receiving rotating inhaled antibiotic classes (e.g., alternating beta-lactam/aminoglycoside) throughout the calendar year doubled between 2009 and 2012 [5]. Therefore, we used the CFFPR to test the hypothesis that the prevalence of patients reported to be receiving more than one inhaled anti-pseudomonal antibiotic class significantly increased nationwide from 2009 to 2012.
METHODS
This was a descriptive cohort study of individuals followed in the CFFPR at any time between January 1, 2009 and December 31, 2012. The CFFPR employs a standardized data collection form to capture demographic and clinical data, including the use of three classes of inhaled antibiotics: 1) aminoglycosides (tobramycin and other aminoglycosides), 2) polymyxins (colistimethate), and 3) beta-lactams (aztreonam) as chronic pulmonary medications (i.e., not prescribed to treat a pulmonary exacerbation) [4]. Patients were included in the current analysis if they were followed in the CFFPR in any year from 2009 through 2012, had a diagnosis of CF, and were recorded to have received any inhaled antibiotics as chronic pulmonary medications at any visit during the period, irrespective of their age or their microbiologic history. Patients were excluded from analyses beginning in the year of solid organ transplantation. Treatment prevalences were determined among all patients in the CFFPR receiving any chronic inhaled antibiotics, as well as by a priori subgroups of age (<6, 6–12, 13–18, and >18 years of age), lung disease stage categorized by mean forced expiratory volume in 1 second (FEV1) (<40% predicted, 40% – <70% predicted, 70% – <100% predicted, and ≥ 100% predicted) for patients with FEV1 data available, and by microbiologic culture results available during the calendar year. The de-identified dataset included the year of birth for each patient, therefore age was an integer that was calculated by subtracting the year of birth from the cohort year. Mean annual FEV1 % predicted was calculated using the reference equations of Wang [6] for children and Hankinson [7] for males >17 years of age and females > 15 years of age.
Two outcomes were studied: 1) the change in the annual prevalence of patients receiving more than one inhaled antibiotic class from 2009 to 2012. This outcome captures individual patient exposure to different inhaled antibiotic classes in a given year. 2) The change in annual prevalence of patients reported to be receiving multiple classes of inhaled antibiotics at any single visit between 2009 and 2012. This outcome is an attempt to capture individuals in which different antibiotic classes were given in ‘combination’, either continuously or by rotation/alternation in a systematic fashion at some point during the year. The second outcome listed above was used to power the study.
Means, standard deviations, and medians were calculated for continuous variables and proportions were calculated for categorical variables. Comparisons between the prevalence of patients receiving treatment with multiple inhaled antibiotics either annually or at a single visit within a year in 2009 and 2012 were conducted using the chi-squared test. A p-value < .05 was considered statistically significant for all analyses. No corrections were made for multiple comparisons. Area-proportional 3-Venn diagrams using ellipses were generated using an online applet at http://www.eulerdiagrams.org/eulerAPE [8]. Because of our large sample size, we anticipated >90% power to detect a 10% absolute increase in the prevalence of multiple inhaled antibiotic class use between 2009 and 2012. Analyses were performed using STATA version 10.0. Patients in the CFFPR (or guardians for minors) gave informed consent permitting their de-identified records to be used for research purposes. The study was reviewed and approved by the institutional review board at University Hospitals Case Medical Center and Rainbow Babies and Children’s Hospital and the Cystic Fibrosis Foundation Patient Registry Committee.
RESULTS
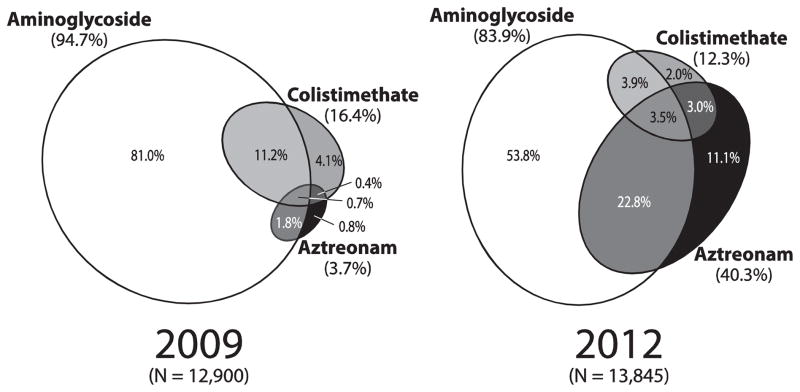
Between 2009 and 2012, the number of individuals in the CFFPR who met our inclusion and exclusion criteria increased from 25,080 to 26,487. The prevalence of inhaled antibiotic treatment among all pre-transplant CFFPR patients as well as among subgroups of age, FEV1% predicted (for those with FEV1 measures), and Pseudomonas aeruginosa culture status are provided in Table 1. Between 2009 and 2012, the percentage of patients in the CFFPR recorded as receiving any inhaled antibiotics (irrespective of their age or microbiologic status) had a non-significant increase from 51.4% to 52.3% (P = .0577). In contrast, between 2009 and 2012, patients in the CFFPR receiving >1 inhaled antibiotic class annually increased from 7.3% to 17.3% (Table 1) and the prevalence of patients receiving >1 class of inhaled antibiotics at any visit increased from 6.5% to 15.7% (Table 1). Area-proportional Venn diagrams, where the overlap of ellipses identifies patients who received more than one inhaled antibiotic class during the year, allow discrimination of inhaled antibiotic class combinations occurring in 2009 and 2012 (Figure 1). A sensitivity analysis in which inhaled tobramycin was the only aminoglycoside included did not materially change these results.
Table 1.
Inhaled antibiotic class utilization, 2009 to 2012
| Patients | 2009 (N=25080) | 2010 (N=25343) | 2011 (N=25966) | 2012 (N=26487) | Absolute Prevalence Change, 2009 – 2012a | P valueb |
|---|---|---|---|---|---|---|
| Receiving inhaled antibiotics, N (%) | 12900 (51.4%) | 13112 (51.7%) | 13554 (52.2%) | 13845 (52.3%) | 0.8% | .0577 |
| >1 class annually, N (%)c | 1822 (7.3%) | 3410 (13.5%) | 4222 (16.3%) | 4575 (17.3%) | 10.0% | <.0001 |
| >1 class at any visit, N (%)c | 1639 (6.5%) | 2825 (11.1%) | 3778 (14.5%) | 4152 (15.7%) | 9.1% | <.0001 |
| Age <6 years, N (%)c | 4312 (17.2%) | 4476 (17.7%) | 4587 (17.7%) | 4549 (17.2%) | ||
| Receiving inhaled antibiotics, N (%)d | 1011 (28.3%) | 947 (25.3%) | 923 (23.4%) | 917 (23.4%) | −4.9% | <.0001 |
| >1 class annually, N (%)d | 20 (0.5%) | 35 (0.8%) | 38 (0.8%) | 50 (1.1%) | 0.6% | .0007 |
| >1 class at any visit, N (%)c | 13 (0.3%) | 24 (0.5%) | 27 (0.6%) | 32 (0.7%) | 0.4% | .0078 |
| Age 6 to 12 years, N (%)c | 5426 (21.6%) | 5385 (21.2%) | 5378 (20.7%) | 5454 (20.6%) | ||
| Receiving inhaled antibiotics, N (%)d | 1075 (24.9%) | 1011 (22.6%) | 972 (21.2%) | 969 (21.3%) | −3.6% | <.0001 |
| >1 class annually, N (%)d | 170 (3.1%) | 379 (7.0%) | 423 (7.9%) | 488 (8.9%) | 5.8% | <.0001 |
| >1 class at any visit, N (%)d | 145 (2.7%) | 290 (5.4%) | 348 (6.5%) | 420 (7.7%) | 5.0% | <.0001 |
| Age 13 to 18 years, N (%)c | 4852 (19.3%) | 4804 (19%) | 4824 (18.6%) | 4867 (18.4%) | ||
| Receiving inhaled antibiotics, N (%)d | 3016 (62.2%) | 3016 (62.8%) | 2990 (62.0%) | 2975 (61.1%) | −1.0% | .2946 |
| >1 class annually, N (%)d | 450 (9.3%) | 816 (17%) | 982 (20.4%) | 985 (20.2%) | 11.0% | <.0001 |
| >1 class at any visit, N (%)d | 402 (8.3%) | 659 (13.7%) | 854 (17.7%) | 876 (18%) | 9.7% | <.0001 |
| Age > 18 years, N (%)c | 10490 (41.8%) | 10678 (42.1%) | 11177 (43.0%) | 11617 (43.9%) | ||
| Receiving inhaled antibiotics, N (%)d | 6341 (60.4%) | 6739 (63.1%) | 7329 (65.6%) | 7651 (65.9%) | 5.4% | <.0001 |
| >1 class annually, N (%)d | 1182 (11.3%) | 2180 (20.4%) | 2779 (24.9%) | 3052 (26.3%) | 15.0% | <.0001 |
| >1 class at any visit, N (%)c | 1079 (10.3%) | 1852 (17.3%) | 2549 (22.8%) | 2824 (24.3%) | 14.0% | <.0001 |
| With FEV1 data, N (%)c | 19380 (77.3%) | 19748 (77.9%) | 20368 (78.4%) | 20928 (79%) | ||
| FEV1 ≥ 100% predicted, N (%)c | 3864 (15.4%) | 4028 (15.9%) | 4142 (16.0%) | 4308 (16.3%) | ||
| Receiving inhaled antibiotics, N (%)e | 1498 (38.8%) | 1519 (37.7%) | 1547 (37.3%) | 1625 (37.7%) | −1.0% | .3305 |
| >1 class annually, N (%)e | 63 (1.6%) | 189 (4.7%) | 253 (6.1%) | 294 (6.8%) | 5.2% | <.0001 |
| >1 class at any visit, N (%)e | 53 (1.4%) | 134 (3.3%) | 199 (4.8%) | 241 (5.6%) | 4.2% | <.0001 |
| FEV1 70 – <100% predicted, N (%)c | 8303 (33.1%) | 8443 (33.3%) | 8800 (33.9%) | 9057 (34.2%) | ||
| Receiving inhaled antibiotics, N (%)e | 4658 (56.1%) | 4754 (56.3%) | 4965 (56.4%) | 5023 (55.5%) | −0.6% | .3961 |
| >1 class annually, N (%)e | 422 (5.1%) | 850 (10.1%) | 1257 (14.3%) | 1383 (15.3%) | 10.2% | <.0001 |
| >1 class at any visit, N (%)e | 367 (4.4%) | 744 (8.8%) | 1096 (12.5%) | 1234 (13.6%) | 9.2% | <.0001 |
| FEV1 40 – <70% predicted, N (%)c | 5052 (20.1%) | 5151 (20.3%) | 5234 (20.2%) | 5372 (20.3%) | ||
| Receiving inhaled antibiotics, N (%)e | 3699 (73.2%) | 3854 (74.8%) | 4009 (76.6%) | 4150 (77.3%) | 4.0% | <.0001 |
| >1 class annually, N (%)e | 748 (14.8%) | 1374 (26.7%) | 1640 (31.3%) | 1783 (33.2%) | 18.4% | <.0001 |
| >1 class at any visit, N (%)e | 683 (13.5%) | 1160 (22.5%) | 1492 (28.5%) | 1637 (30.5%) | 17.0% | <.0001 |
| FEV1 <40% predicted, N (%)c | 2161 (8.6%) | 2126 (8.4%) | 2192 (8.4%) | 2191 (8.3%) | ||
| Receiving inhaled antibiotics, N (%)e | 1739 (80.5%) | 1745 (82.1%) | 1833 (83.6%) | 1831 (83.6%) | 3.1% | .0078 |
| >1 class annually, N (%)e | 539 (24.9%) | 825 (38.8%) | 969 (44.2%) | 1006 (45.9%) | 21.0% | <.0001 |
| >1 class at any visit, N (%)e | 497 (23.0%) | 732 (34.4%) | 908 (41.4%) | 951 (43.4%) | 20.4% | <.0001 |
Abbreviations: FEV1, forced expiratory volume in the first second;
Absolute change is 2012 prevalence – 2009 prevalence. Values in this column may differ slightly from those obtained by subtraction of 2012 from 2009 (rounded) prevalences provided in the table.
Chi-squared test for difference in prevalence between 2009 and 2012. Values <.05 are bolded.
proportion of all CFFPR patients
proportion of patients within age subgroup
proportion of patients within FEV1 subgroup
Figure 1. Annual prevalence of treatment with three inhaled antibiotic classes among CFFPR patients receiving inhaled antibiotics.
Area-proportional Venn diagrams showing inhaled antibiotic use prevalence in 2009 (left) and 2012 (right) among all patients receiving inhaled antibiotics as chronic pulmonary medications. Each figure shows three ellipses depicting the relative exposure of treated patients to aminoglycosides, colistimethate, and aztreonam. Areas in which ellipses overlap identify patients receiving more than one inhaled antibiotic class.
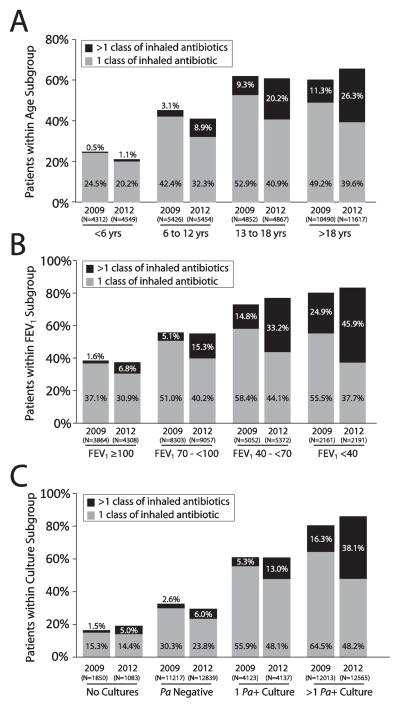
Changes in inhaled antibiotic class use prevalence between 2009 and 2012 differed by age subgroup (Figure 2a) and by FEV1 subgroup (among patients that had an FEV1 % predicted value recorded during the calendar year) (Figure 2b). In patients up to 18 years of age, the overall prevalence of inhaled antibiotic use actually decreased between 2009 and 2012 while the prevalence of use of more than 1 inhaled antibiotic class increased (Figure 2a). The overall prevalence of patients >18 years of age receiving inhaled antibiotics increased from 60.4% to 65.9% and the prevalence of those receiving greater than one class annually more than doubled (11.3% to 26.3%). There were no notable changes in the prevalence of inhaled antibiotic use between 2009 and 2012 among different lung function subgroups. However, the prevalence of patients receiving more than one class of inhaled antibiotics between 2009 and 2012 at least doubled in nearly all lung function subgroups, with the greatest overall prevalence being noted in the <40% predicted subgroup (45.9% of patients on multiple inhaled antibiotics in 2012) (Figure 2b). We also performed an analysis generally mirroring the CFF recommendations for maintenance of chronic lung health [2]. In 2012, 4589 CFFPR patients had >1 positive P. aeruginosa culture, were 6 years of age or older, and had an FEV1% predicted between 25% and 75% predicted. Among these patients, 46.6% received >1 inhaled antibiotic class during the year and 43.4% had >1 inhaled antibiotic class recorded as a chronic therapy at a single visit.
Figure 2. Inhaled antibiotic class utilization among CFFPR patients stratified by age subgroup, lung disease stage, and P. aeruginosa culture status.
Panel A: All CFFPR patients stratified by age group. Panel B: Patients with available FEV1 data stratified by FEV1% predicted group. Panel C: Patients stratified by P. aeruginosa (Pa) culture status during the year. Patients who received only 1 inhaled antibiotic class during the calendar year are shown with gray bars and those receiving >1 inhaled antibiotic class are shown with black bars. For all groups, increases in the proportion of patients receiving >1 inhaled antibiotic class from 2009 to 2012 were statistically significant (P<.001; Table 1).
As might be anticipated, the prevalence of inhaled antibiotic use among patients who had P. aeruginosa isolated from their respiratory secretions during a calendar year was higher than among those patients where P. aeruginosa was not isolated, as well as those where no culture results were recorded (Figure 2c). Interestingly, the proportion of patients in each of these groups receiving more than one inhaled antibiotic class increased significantly between 2009 and 2012. Among the subgroup of patients, of any age, that cultured Pseudomonas aeruginosa two or more times in a given year, the prevalence of >1 class of inhaled antibiotics increased from 16.3% in 2009 to 38.1% in 2012.
Changes in multiple inhaled antibiotic class treatment prevalence at the visit level essentially mirrored those observed for annualized treatment prevalence among every subgroup. On average, about 90% of patients who were identified as having received >1 inhaled antibiotic class during a calendar year were also found to have had >1 inhaled antibiotic class recorded as chronic pulmonary medication at the same visit at least once during that year. (Table 1).
DISCUSSION
We performed an analysis of the United States CFFPR to determine whether the prevalence of patients recorded as taking more than one inhaled antipseudomonal antibiotic class significantly increased after FDA approval of aztreonam for inhalation solution, representing a new inhaled antibiotic class. The most important finding from this study was that although the proportion of patients treated with inhaled antibiotics did not appreciably change between 2009 and 2012, inhaled antibiotic treatment complexity increased substantially over the period. This change in treatment complexity is highlighted by the fact that even in the most conservative analyses, approximately 1 in 5 of all pre-transplant patients in the CFFPR (irrespective of age, lung function, or underlying microbiologic status) were receiving 2 or more inhaled antibiotic classes in 2012, which is more than double the prevalence in the year prior to the introduction of aztreonam for inhalation solution. Furthermore, these were not patients receiving one inhaled antibiotic for several months and then switching to another inhaled antibiotic later in the year; rather, the data suggest that these were patients periodically rotating inhaled antibiotics in some fashion. Finally, among the subgroup of patients 6 years of age and older with moderate to severe lung disease and P. aeruginosa persistently present in the airways, almost half were receiving two or more inhaled antibiotic classes in 2012. These findings suggest that the use of multiple inhaled antibiotic classes for patients with chronic P. aeruginosa airway infections is becoming increasingly common in the US.
Introduction of an additional inhaled antibiotic class might be hypothesized to provide multiple benefits with respect to patient management. First, patients who do not tolerate, have a contra-indication (i.e. pregnancy), or are allergic to existing inhaled antibiotic classes might benefit from a new antibiotic class. Second, patients who may have become refractory to existing inhaled antibiotic classes following repeated exposure (as evidenced by no improvement in symptoms or lung function during an “on” month compared to an “off” month), might derive greater relative benefit in symptoms and lung function through treatment with a new antibiotic class. Finally, rotation of different inhaled antibiotic classes might be perceived to extend the effective life of all inhaled antibiotic classes; a patient might respond to inhaled antibiotic class ‘A’ monotherapy for X years and class ‘B’ monotherapy for X years but respond to rotation of ‘A’ and ‘B’ classes for greater than 2X years [9].
Previously, the annual prevalence of inhaled antibiotic use among patients enrolled in the Epidemiologic Study of CF was observed to significantly increase between 1996 and 2005 following the 1997 commercial introduction of tobramycin inhalation solution [10]. Between 2003 and 2005, 8.1% of patients receiving inhaled antibiotics were treated at least once with 2 inhaled antibiotic classes (aminoglycoside and polymyxin) [10]. In contrast, our data show that commercial registration of inhaled aztreonam in 2010 was not associated with a significant increase in the prevalence of CFFPR patients receiving inhaled antibiotics as chronic pulmonary medications between 2009 and 2012 (Table 1), suggesting that increased treatment among previously inhaled antibiotic-intolerant patients did not occur to any great degree. However, the prevalence of CFFPR patients receiving >1 inhaled antibiotic class in a calendar year more than doubled over the period, as did the prevalence of treatment with >1 inhaled antibiotic class at individual visits (Table 1). These changes did not occur immediately upon commercial availability of inhaled aztreonam, but rather occurred incrementally over the study period, presumably as a result of clinicians and third-party providers gradually becoming more comfortable with inhaled aztreonam use and the recognition that their sickest patients required management during “off” months of chronic intermittent therapy. A transient inhaled aztreonam shortage following commercial launch may also have affected the rate at which it was incorporated into chronic regimens. Consistent with this hypothesis, increased treatment with multiple inhaled antibiotic classes was associated with advancing disease stage: more patients with FEV1 <40% predicted were treated with at least two inhaled antibiotic classes than were treated with a single inhaled antibiotic class in 2012 (Table 1, Figure 2).
Our study has some inherent limitations. Our analyses are dependent on accurate capture of chronic inhaled antibiotic regimens by CF care centers within the CFFPR case report form, and we were unable to randomly audit or query data entries for accuracy. However, there is no basis to believe that CFFPR data integrity had changed meaningfully between 2009 and 2012, and thus we are confident that the changes in treatment patterns we report across the period are real. In addition, we have employed treatment with two or more classes of inhaled antibiotic at a single visit as a surrogate for ‘combination’ of inhaled antibiotic classes for patient management, without discrimination between regimens such as continuous treatment with one or more classes or various possible rotation or cycling regimens (with or without drug holidays). We have chosen not to characterize how specific treatment regimens recorded in the case report form (alternating months, continuous, ‘other’) may have been related to antibiotic class choice, as the number and type of combinations encountered was surprisingly large and we were unable to satisfactorily characterize this complex array of treatment regimens, a challenge recognized previously [10]. Thus, although we can say with certainty that there has been a consistent expansion in the prevalence of patients receiving two or more inhaled antibiotic classes as part of their pulmonary management, exactly how the two or more antibiotic classes are administered is not clear and probably varies. In addition, we did not characterize the extemporaneous administration of other antibiotics (e.g., meropenem, ceftazidime, vancomycin, or liposomal amphotericin) as inhaled aerosols. Thus, our results likely represent a conservative estimate of the proportion of CFFPR patients that are receiving multiple inhaled antibiotic classes. Finally, we have not attempted to determine whether the observed increase in prevalence of combination inhaled antibiotic class patient management was associated with changes in health outcomes, as complex statistical modeling would be needed to handle the indication biases associated with these observational data (Figure 2).
Inhaled antibiotic monotherapies have been both studied in and recommended for management of patients with CF, chronic P. aeruginosa airway infection, and some degree of lung disease [1,2]. To date, there has been no rigorous objective evidence that combination inhaled antibiotic therapy improves outcomes relative to intermittent inhaled monotherapy, although the trend towards increasing management by combination appears undeniable, especially as additional antibiotic classes are being formulated and studied as inhaled therapies.
Recently, a blinded randomized controlled trial evaluating the risks and benefits of continuous rotation of inhaled tobramycin and inhaled aztreonam compared to traditional every-other-month inhaled tobramycin monotherapy stopped recruiting participants prematurely (NCT01641822) [11]. The clinician prescribing habits we observed in 2012 may have contributed to poor enrollment in this study (initiated in late 2012): among the 4589 CFFPR patients meeting NCT01641822 age and FEV1 inclusion criteria that had >1 positive P. aeruginosa culture during 2012, 46.6% received >1 inhaled antibiotic class during the year and 43.4% had >1 inhaled antibiotic class recorded as a chronic therapy at a same visit. It is likely that many study candidates that met inclusion criteria (age ≥6 years, FEV1 ≥ 25% and ≤75% predicted, chronic P. aeruginosa airway infection, and at least one pulmonary exacerbation requiring hospitalization or intravenous antibiotics in the prior year) were already receiving combination inhaled antibiotic therapy and might have been unwilling to risk randomization to a treatment group receiving intermittent placebo treatment. The failure of NCT01641822 to fully enroll does not lessen the need for evaluation of the safety and efficacy of inhaled antibiotic class rotation, but ‘simple’ comparison of what appears to be a waning standard of care (intermittent monotherapy) to an emerging standard of care seems infeasible, as the community appears to have lost equipoise on the matter. However, it may be possible to compare the effectiveness of inhaled antibiotic monotherapy to rotation of inhaled antibiotic classes using the CFFPR and CF registry data from other countries with differing inhaled antibiotic usage patterns.
The ramifications of our observations for CF clinical investigators are clear: they should anticipate a high prevalence of study subjects rotating inhaled antibiotic classes; identification of inhaled antibiotic regimens during screening and adequate randomization will be key to prevent potential confounding from differential use of inhaled antibiotics. Today, many clinicians and their patients with chronic P. aeruginosa infection may not feel comfortable participating in an inhaled antibiotic trial with a placebo-controlled component of any extended duration. Given our results, it further appears that it may also be infeasible to propose extended studies in which the control group is not receiving some form of continuous inhaled antibiotic treatment in populations most likely to benefit from a new antibiotic: patients with advanced lung disease and substantial experience with other inhaled antibiotics. Ironically, these are exactly the patients for whom an inhaled antibiotic treatment response in the form of sustained FEV1 improvement or reduced risk of exacerbation are most likely to be demonstrated [12].
Highlights.
US CF patient registry inhaled antibiotic use was studied from 2009–2012
A new class of CF inhaled antibiotic was approved in 2010: inhaled aztreonam
Fraction of patients treated with inhaled antibiotics didn’t change, 2009 to 2012
Patients treated with >1 inhaled antibiotic class doubled from 2009 to 2012
Use of >1 inhaled antibiotic class was more common in older, sicker patients
Acknowledgments
The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. Additionally, we would like to thank the patients, care providers, and clinic coordinators at CF Centers throughout the United States for their contributions to the CF Foundation Patient Registry.
Footnotes
CONFLICTS OF INTEREST
ECD was supported by the Cystic Fibrosis Foundation (DASENB13A0, CC007-14, and CFFT KONSTA09Y0), is the Associate Chair, CF Foundation Patient Registry Committee and has served in the past year as an advisor/consultant for Savara Pharmaceuticals, AbbVie, and Enanta Pharmaceuticals DRV has served in the past year as an advisor/consultant for Affinium Pharmaceuticals, Aptalis Pharma, Aradigm, Baxter Healthcare, CFF, CURx Pharma, Forest Pharmaceuticals, Genentech, Gilead Sciences, Glycomimetics, ICON Clinical Sciences, Kalobios, MedImmune, and Vertex. MWK was supported by the National Institutes of Health (P30 DK27651) and the Cystic Fibrosis Foundation (CFFT KONSTA09Y0), and has served as an advisor/consultant for Aradigm, Celtaxsys, Digestive Care Inc, Genentech, Gilead Sciences, Insmed, Novartis Pharmaceuticals, Savara Pharmaceuticals, and Vertex. No commercial parties had any role in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elliott C. Dasenbrook, Email: elliott.dasenbrook@case.edu.
Michael W. Konstan, Email: michael.konstan@case.edu.
Donald R. VanDevanter, Email: drv15@case.edu.
References
- 1.Doring G, Flume P, Heijerman H, Elborn JS. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461–79. doi: 10.1016/j.jcf.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Resp Crit Care Med. 2013;187:680–9. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- 3.Cystic Fibrosis Foundation Patient Registry. 2009 Annual Data Report to the Center Directors. Bethesda, Maryland: Cystic Fibrosis Foundation; 2010. [Google Scholar]
- 4.Cystic Fibrosis Foundation Patient Registry. 2012 Annual Data Report to the Center Directors. Bethesda, Maryland: Cystic Fibrosis Foundation; 2013. [Google Scholar]
- 5.VanDevanter DR, Dasenbrook EC, Konstan MW. Incorporation of a third inhaled antipseudomonal antibiotic class into the management of patients at an adult CF care center. J Cystic Fibros. 2014;13:S16. [Google Scholar]
- 6.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 7.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 8.Micallef L, Rodgers P. eulerAPE: Drawing area-proportional 3-Venn diagrams using ellipses. PLoS ONE. 9(7):e101717. doi: 10.1371/journal.pone.0101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VanDevanter DR, Ballmann M, Flume PA. Applying clinical outcome variables to appropriate aerosolized antibiotics for the treatment of patients with cystic fibrosis. Resp Med. 2011;105 (Suppl 2):S18–23. doi: 10.1016/S0954-6111(11)70023-3. [DOI] [PubMed] [Google Scholar]
- 10.Moskowitz SM, Silva SJ, Mayer-Hamblett N, Pasta DJ, Mink DR, Mabie JA, et al. Shifting patterns of inhaled antibiotic use in cystic fibrosis. Pediatr Pulmonol. 2008;43:874–881. doi: 10.1002/ppul.20873. [DOI] [PubMed] [Google Scholar]
- 11.Bresnik M. Clinicaltrials.Gov [internet] Bethesda (md): National library of medicine (us); 2012. Phase 3 study of aztreonam for inhalation solution (AZLI) in a continuous alternating therapy regimen for the treatment of chronic Pseudomonas aeruginosa infection in patients with CF (AZLI CAT) [cited 2014 aug 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01641822 nlm identifier: NCT01641822. [Google Scholar]
- 12.VanDevanter DR, Konstan MW. Outcome measures for clinical trials assessing treatment of cystic fibrosis lung disease. Clin Invest. 2012;2(2):163–175. doi: 10.4155/cli.11.174. [DOI] [PMC free article] [PubMed] [Google Scholar]