Abstract
Neuroinvasive viral infections invade the nervous system, often eliciting serious disease and death. Members of four viral families are both neuroinvasive and capable of transmitting progeny virions or virion components within long neuronal extensions known as axons. Axons provide physical structures to spread of viral infection within the host while avoiding extracellular immune responses. Technological advances in analysis of in vivo neural circuits, neuronal culturing, and live imaging of fluorescent fusion proteins have enabled an unprecedented view into the steps of virion assembly, transport, and egress involved in axonal spread. In this review, we will summarize the literature supporting anterograde (axon to cell) spread of viral infection, describe the various strategies of virion transport, and discuss the effects of spread on populations of neuroinvasive viruses.
Keywords: neuron, axon, anterograde directed spread, alphaherpes virus, rhabdovirus, picornavirus, flavivirus
Neuroinvasive viral infections and directional spread
The term “neuroinvasive” can be used to describe the capacity of some viral infections to invade and spread within the host nervous system (see Glossary). Some neuroinvasive viruses infect the peripheral nervous system (PNS) with limited spread to the central nervous system (CNS) while others often spread to the CNS eliciting significant disease [1,2]. Infection of the CNS may occur via the blood (hematogenous spread) or nerves (neurotropic spread). Many viral infections have the potential to be neuroinvasive, but only a limited number do so. The rarity of neuronal spread reflects many variables including strong intrinsic and innate defenses that protect the nervous system. However, one requirement to be neuroinvasive is that viral gene products, genomes, or virions must enter and move long distances in axons, a highly specialized neuronal compartment [3,4]. Since no viral genomes encode the molecular motors and cytoskeletal framework to move cargo in axons, they must encode adaptor or modifying gene products to repurpose neuronal components for axonal sorting and transport. In addition to directional movement in axons, for infection to spread to other neurons or cells, virions (or genomes) must leave the axon and enter cells in contact with the axons. As a result, spread of infections from axons tends to be localized; often taking apparent advantage of the tight associations of axonal membranes with their partner cells, including synaptic junctions, gap junctions, and glial/axonal interactions. The advantage of such focal axonal spread may be the avoidance of hostile extracellular immune effectors, including complement, antibodies, and phagocytes that have the potential to limit the intercellular spread of infection.
This review will focus on several neuroinvasive viruses with identified or potential for axonal spread of infection. We will review the data regarding virion component transport in axons, the sites of axonal egress, and the relationship to pathogenesis. Our goal is to identify properties shared by neuroinvasive viruses. We also will emphasize how technology has improved our capacity to study directional spread of viruses among synaptically connected neurons. Finally we will discuss recent data suggesting that spread of infection from axons represents a bottleneck that has the potential to limit diversity of the transmitted viral population.
Four well-studied neuroinvasive viruses
Many virus families and subfamilies can be neuroinvasive, but four groups are studied more intensely than others with in vivo and in vitro systems to identify anterograde spread. These groups include the Alpha herpesviridae, particularly, herpes simplex virus, varicella zoster virus and pseudorabies virus (HSV, VZV and PRV, respectively), Flaviviridae specifically West Nile virus (WNV), Rhabdoviridae including vesicular stomatitis virus (VSV) and rabies virus (RV), and Picornaviridae, specifically Theiler's murine encephalitis virus (TMEV).
Neuronal infections by the alpha herpesviruses, HSV-1 and PRV, have been studied using a combination of model systems, including in vivo infection of defined neuronal circuits and in vitro infection of neuronal cultures, capable of distinguishing the directionality of viral spread within neurons [5-7]. Alpha herpesvirus infections can spread from infected neurons in both anterograde and retrograde directions (Box 1), with viral gene products controlling directional spread. Early characterization of an attenuated vaccine strain of PRV, known as PRV Bartha, revealed that three gene products dictate the direction of PRV infection and spread. In the absence of the glycoproteins gE and gI and the membrane associated US9 protein, intracellular progeny virions do not enter axons, and, as a result, anterograde spread to synaptically connected post-synaptic neurons or to epithelial cell targets is completely abrogated. Retrograde spread is only modestly affected, indicative that progeny virions can spread from neuronal cell bodies and dendrites to synaptically connected axons [8-10]. These same genes are important for spread of HSV and VZV among connected neurons and cells, but the mechanism of spread may differ [11-13] In either case, anterograde spread of infection is important for diseases associated with both HSV and VZV infection [14,15].
Among rhabdoviruses, RV is the most extensively studied with regard to neuronal spread of infection. It is highly neuroinvasive and has often been said to be restricted to retrograde spread of infection [16,17]. There is evidence that RV is also capable of anterograde spread [18-20]. Using an in vitro system, researchers cultured dorsal root ganglia (DRG) neurons in a three-compartment device and observed RV transmission via axons to a distal compartment following infection of cell bodies [19]. Supporting in vivo evidence for RV anterograde spread comes from extensive antigenic RV reactivity in ipsilateral and contralateral DRG neurons following footpad injection [20]. This work suggests axonal transmission of either virions moving in the retrograde direction, or progeny virions spreading from the neuronal soma. Similarly, the rhabdovirus VSV is competent for bidirectional transport in axons and spread between neurons, though its exquisite sensitivity to interferon and other innate antiviral immune responses typically restricts viral replication to sites of primary replication. Using an intranasal in vivo model of VSV infection, researchers showed extensive neuroinvasive spread of VSV into olfactory bulb neurons via axons [21].
There is no consensus on how the flavivirus WNV infects and spreads in the nervous system [22,23]. However, an in vitro system of compartmentalized neuronal cultures may help to clarify this situation. In these compartmentalized cultures, embryonic superior cervical ganglia (SCG) neurons were grown such that cell bodies extended axons that penetrate underneath two physical barriers resulting in axonal termini in a separate hydrostatically isolated compartment. First pioneered in the 1970s to understand the role of nerve growth factor in neuronal development [24,25], the system was later adapted to study alpha herpesvirus transport and spread [26,27]. With this system, investigators showed that WNV infection can spread from axon terminals by long-distance axonal transport [28].
Persistent infection by the picornavirus TMEV is a remarkable example of specialized spread of infection from axons. TMEV infection results in a progressive disease of myelinating oligodendrocytes. This unusual mode of axonal spread of infection was identified using intravitreal eye infections, taking advantage of restricted spread of primary inoculum and direct infection of retinal ganglia cells that project axons into the optic nerve. In this model, infection spreads from infected axons to oligodendrocytes that myelinate and support the optic nerve [29]. The capacity of TMEV to infect and kill myelinating oligodendrocytes provides a disease model for the progressive demyelination of sensory and motor neurons [30,31]. In addition, the physical separation of primary inoculum in the eye from secondary spread events in distant axons is strong support for the model of axon to oligodendrocyte cell spread.
Moving virions and proteins into and out of axons
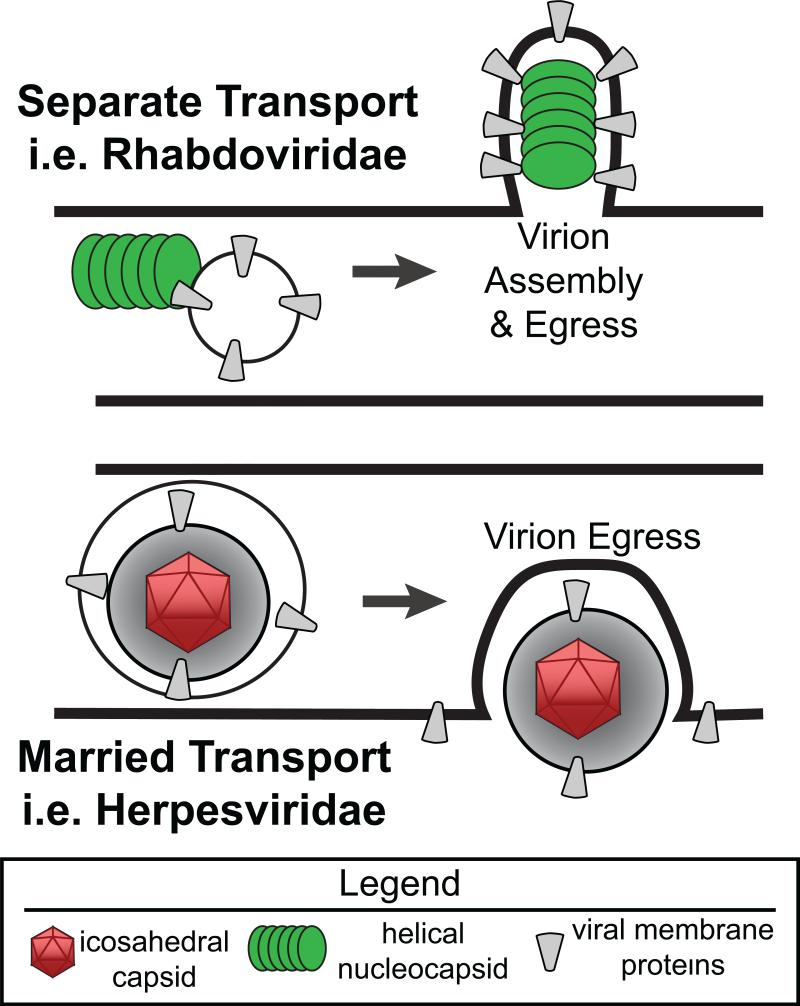
Even though axons contain more than 95% of the neuron's cytoplasm, they are highly specialized compartments [32,33]. They not only transmit nerve impulses, they also move a remarkable number of specialized cellular structures along microtubules including cytoskeletal elements, mitochondria, endosomes, lysosomes, and ribonucleoprotein complexes [34,35]. Axonal transport of virion components over long distances requires specificity in cargo loading and motor engagement. The subsequent egress of infectious particles and spread of viral infection then requires another level of specificity and targeting. For some viruses, particularly the rhabdoviridae, it may be that viral structural components are sorted into axons and move as separate units in or on axonal vesicles for assembly at distal sites of egress (Figure 1, Separate model). For other viruses, specifically PRV and potentially HSV, it may be that virions are assembled in the neuronal cell body and transit inside neuronal vesicles to axons for subsequent transport to distal egress sites (Figure 1, Married model).
Figure 1. Differences between models of virion transport and egress.
Two models of anterograde viral transport are depicted; Separate and Married. In the Separate model, the putative state of viral protein assemblies are shown for Rhabdoviridae, either RV or VSV. In this case, viral nucleocapsid assemblies transport in association with glycoprotein containing vesicles. At the point of egress, glycoproteins in the axonal membrane would direct nucleocapsid assembly into an infectious virion. In the Married model the assembly and egress of the alpha herpes virus PRV is depicted. In this circumstance, virion assembly occurs in the neuronal soma and undergoes axonal transport inside a transport vesicle. At sites of egress, this transport vesicle fuses with axonal membranes, releasing a fully infectious herpes virion into the extracellular space.
For PRV, the data indicates that virion assembly occurs in the neuronal cell body [36,37]. The PRV US9 protein in the transport vesicle membrane interacts with cytoplasmic components leading to the recruitment of kinesin-3 (also known as Kif1a) to both sort the virion containing vesicle into axons and to transport this vesicle to sites of egress [38]. There is evidence that HSV infections also use assembled virion transport within axons, similar to PRV, although alternative models have been proposed for the final assembly occurring at egress sites [39,40].
RV virions are taken up at neuromuscular junctions by endocytosis. The endocytosed virions are moved in the retrograde direction toward the cell body via dynein-mediated vesicular transport prior to the initiation of viral replication in the cell bodies [41]. Recently, anterograde transport of newly replicated virion components in axons were visualized using recombinant viruses expressing a GFP-P protein fusion [18]. Transport of these GFP-P assemblies was dependent upon the expression of the RV-G protein. Indeed, the GFP-P protein and RV-G protein co-localized in axons. Other studies have demonstrated that rhabdovirus glycoproteins dictate the directional transport and spread of virion components within the axons. Beier et al. constructed VSV recombinants expressing either native VSV-G or RV-G proteins. These recombinants had striking directional spread phenotypes in the mouse CNS [42]. The RV-G expressing VSV recombinants were unable to spread in the anterograde direction and were unable to infect presynaptic neurons from the site of injection. In contrast, the VSV-G expressing recombinants were capable of anterograde directed spread. It is possible that interactions between the viral genome nucleocapsid (NC) assemblies and the viral glycoproteins recruit cellular machinery to promote axonal trafficking and spread of viral infection. Research into the anterograde transport mechanisms of another member of the order Mononegavirales, Nipah virus, has identified functional interactions between the F and G proteins and the AP clathrin adaptor complexes [43]. While these live imaging studies of fluorescent fusion proteins identified anterograde motion of RV and Nipah virion components, their structure and composition are unknown. Similarly it is unclear what happens at sites of egress. It is not clear if exocytosis of fully assembled virions or budding of genome complexes into matrix/membrane protein assemblies occurs at egress site membranes. It also remains to be seen if these fluorescent structures represent infection competent NC complexes and whether the G protein promotes the anterograde spread of infection.
The technology used to study axonal sorting and transport of viral proteins often depends on fluorescent protein fusions to virion components to visualize the dependent or independent transport of specific viral protein assemblies. These fusion proteins enable analysis of sorting, transport, and egress dynamics. However, these fusion proteins are not the viral native proteins so care must be taken in interpreting all observations. Nevertheless, sensitive, rapid sequential imaging, and high-resolution live-cell video microscopy have enabled a quantitative description of the biophysics and dynamics of axonal transport and spread [44,45]. These same technologies are now being developed to understand the dynamics of axonal sorting and transport for other anterograde spreading viruses, including VZV [46].
Exit from axons
Egress of virus particles from axons to infect adjacent cells is an understudied aspect of neuroinvasive viral infection. The capacity to spread from peripheral axons to epithelial cells is a major step in inter-host transmission of alpha herpesviruses after reactivation of the latent infection. We know remarkably little about the axonal exit process. In particular, the molecular composition of egress sites is poorly defined. It is unclear if any axonal membrane site will suffice or if egress only occurs at sites of functional contact with another neuron or cell (e.g., sites of synapse formation, gap junctions, and glial/neuronal contact sites)[47]. For viruses that may directly assemble at egress sites (e.g., rhabdoviruses), the transport and targeting of virion envelope protein components and NC complexes remains poorly understood [18,48]. For viruses such as PRV and HSV, one model suggests that fully assembled virions are transported in axons within transport vesicles. The transport vesicle must fuse at the egress site to release the mature virion. It is unknown if the egressing alpha herpes virion is free to diffuse or if it immediately enters a cell that is in functional contact. In this case, the existence of an extra-axonal virion is transient. The fusion and release events can occur in the absence of the viral fusion proteins, suggesting that unidentified cellular exocytosis machinery is required.
The assembly state of virions prior to sorting into axons for long distance transport has direct implications for both transport mechanisms and the steps required for virion delivery to susceptible cells localized at or near axonal structures. Recent work examining the involvement of axon specific kinesin-3 motors and dense core vesicles has demonstrated that these moving vesicles avoid synaptic sites mid axon and are preferentially transported to axon termini [49,50]. It is possible that any virion cargo recruiting the kinesin-3 motor for axonal transport would follow similar mechanisms [38]. This targeting scenario suggests that limited numbers of virions would be observed egressing mid-axon and would initially accumulate at termini. We do not know if all neurotropic viruses utilize synaptic junctions specifically or simply spread into cells that have close associations with axonal membranes. PRV and HSV egress events have been associated with axonal varicosities, areas of axons with accumulations of synaptic vesicles, transport machinery, and pre-synaptic markers observed in both cultured axons and in vivo [51-53]. In fact, De Regge et al. suggested that the PRV receptor binding protein, glycoprotein D, promoted membrane deformations related to varicosities [54]. It may be, at least for herpesviruses, that viral glycoproteins play a role in establishing virion egress sites. To complicate matters, infected axons usually contain large populations of nonvirion, viral protein assemblies moving within axons [55,56]. These assemblies include herpesvirus L-particles (virions lacking capsids and DNA) and glycoprotein rich vesicles with no other virion structural proteins. These assemblies may interact with axonal membranes, preparing virion egress sites [57,58]. Neuroinvasive viruses that transport virion structural components separately in axons (as has been suggested for RV, VSV, and WNV) present problems of coordinated assembly. In these models, nucleocapsid complexes must associate with surface glycoproteins in the appropriate stoichiometry at sites of axonal egress. Identification of the structure of transport and assembly intermediates during axonal spread of infection for viruses that undergo separate transport remain incomplete at this time.
Quantification: how many virions leave axons to infect cells?
The question is simple, but technological advances were required to examine how many virions leave axons to infect connected cells. The number of PRV and HSV particles transmitted from axons into populations of susceptible cells was determined using live-imaging microscopy, fluorescent proteins, and modified compartmentalized cultures of PNS neurons [59,60]. Using a mixed infection model where co-infection by multiple viruses can be tracked by fluorescent protein expression, we observed that anterograde spread predominantly produced cells expressing only one fluorescent protein. The distribution of co-expression was indicative that anterograde spread resulted in the expression of fewer than 2 viral genomes per cell. This observation was supported by time-lapse microscopy of fluorescent viral particles that accumulated in infected cells following anterograde spread. A limited number of fluorescently tagged PRV capsids (often only 1) accumulated on nuclear pores of infected cells [59]. These two methods supported the conclusion that on average, one viral genome is successfully delivered by axonal spread of infection to adjacent epithelial cells. Rarely, epithelial cells were infected with more than five capsids or those that co-expressed all three markers of co-infection. This restricted spread marks a previously unknown bottleneck on viral populations that spread out of axons and into susceptible cells. These observations suggest individual HSV lesions, following viral reactivation, are initiated from a limited number of viral genomes. Limiting axonal spread to one or two virions reduces the probability of trans-complementation of defective viral genomes by co-infection [61]. Single virion infection and subsequent amplification may increase the overall fitness of the viral populations by limiting co-infection and complementation of less fit genomes [62-64]. It seems that for RNA viruses such as RV, WNV, or TMEV, such a restriction to single particle axonal transmission would be deleterious. The high mutation rate and low abundance of wild-type genomes in any replicating population suggest that co-infection might be necessary to maintain fitness and pathogenecity of viral populations [65-67]. Nevertheless, population bottlenecks do occur during neuroinvasive spread of poliovirus [68,69].
RNA virus invasion of the CNS from the PNS
Spread of infection from the PNS into the CNS can be common for viruses that infect peripheral tissues such as the flavivirus, WNV, and the rhabdoviruses RV and VSV. For RV, CNS invasion by retrograde axonal spread is well studied. However, the contribution of axonal spread to WNV CNS disease is less clear [23]. The timing of CNS invasion correlates with the destruction of the blood brain barrier (BBB). The BBB regulates the exchange of nutrients and gases while preventing large protein complexes, bacteria and viruses from entering the CNS [1]. One hypothesis is that WNV mediated destruction of the blood brain barrier allows the direct transmission of free virions in the blood or in infected macrophages and monocytes to the brain [22,70]. It remains to be seen if axonal spread of infection contributes to CNS invasion and pathogenesis. It will be important to determine if the mechanism of WNV transport is determined both by viral and cellular gene products and whether or not these viral encoded elements are important in producing CNS disease in experimental system and external models.
Concluding remarks
Viral genetics, in vivo models of viral spread, and in vitro compartmentalized neuronal culture systems coupled with fluorescent fusion proteins and advances in live cell imaging have increased our understanding of neuroinvasive infections and the role of axonal spread of infection. These techniques are now regularly used to characterize directional transport and spread of neuroinvasive infections. Knowledge of the mechanisms of neuroinvasive spread of infection may provide better treatments and antiviral targets. By understanding how viral genomes and virion components move in axons and between axons and cells, we will learn more about viral fitness, transmission efficiency, and evolution. The continued investigation of axonal spread mechanisms and their ramifications for any virus group requires the integration of virology and neuroscience (Box 2). The highly specific spread of RV, VSV, and alpha herpesvirus infections in neurons is being used for tracing neuronal circuitry [16,71,72]. Exploiting knowledge of transport, egress, and spread of viral components from axons will enhance our ability to control these devastating infections and to use them for studying the nervous system.
BOX 1. Distinguishing directional spread and virion transport in neurons.
The directional transport of progeny virions within a neuron underlies the directional spread of infection with the peripheral and central nervous system. Directional spread of infection in circuits of neurons is defined as follows: Retrograde spread of infection proceeds from a post-synaptic neuron to uninfected pre-synaptic neurons. Virions attach to and enter axons, where virus particles undergo retrograde transport on microtubules toward the cell body using dynein motor complexes. Retrograde spread continues by transport of progeny virions into neuronal dendrites or cell bodies, with subsequent virion transmission to the pre-synaptic cell. Anterograde spread of infection proceeds from a pre-synaptic neuron to an uninfected post-synaptic neuron. Progeny virions or virion components are sorted into axons and undergo anterograde transport away from the cell body on microtubules using kinesin motor complexes. Infection spreads to post-synaptic cells from axons. This review is focused upon those viral infections with the demonstrated capacity for anterograde transport and spread.
Box 2. Outstanding questions.
Axonal spread: How does virion assembly impact spread from axons? How are assembly and spread from axons coordinated?
Axonal transport: How are motors and viral cargo assembled? How does transport of viral assemblies relate to normal axonal transport of dense core vesicles and neurotransmitter vesicles?
Axonal egress: How does infection spread from axons to closely engaged cells? Do virus particles move through or near synapses and other functional sites like gap junctions?
Article Highlights for “Axonal spread of neuroinvasive viral infections” by Taylor and Enquist.
Four viral families have members with a demonstrated capacity for axonal spread of infection.
Anterograde axonal spread of infection requires the transport of virions or virion components from the cell body toward distal axon egress sites.
The axonal spread of infection to connected cells restricts the number of virions transmitted, reducing the diversity of viral genomes.
Restrictions on axonal spread may impact the fitness and virulence of transmitted viral populations.
Glossary
- Axon
a specialized extension from neuronal cell bodies that facilitate the propagation of electrical signals.
- Capsid/nucleocapsid
an assembly of protein that directly interacts with viral genomes. The structure often has have icosahedral or helical symmetry. Enveloped viruses wrap capsids with host cell membranes either during intracellular assembly or through budding of virions from the plasma membrane.
- Dynein
a family of molecular motors that facilitate minus-end transport of cargo along microtubules. These motors are involved in transporting cargo, including retrograde transporting viruses, from synapses back to the neuronal cell body.
- Kinesin
a family of molecular motors that facilitate plus-end directed transport of cargo along microtubules. Certain kinesins have specialized functions within the cell, such as kinesin-3 that is associated with moving neurovesicles from the cell body to axon termini.
- Neuroinvasive
viral infections with the capacity to infect neurons and spread to the central nervous system.
- Synapse
specialized point of contact between an axon and a dendrite or cell body. Defined by closely apposed membranes containing synaptic vesicles (in the axon) and neurotransmitter receptors (on the dendrite/cell body).
- Varicosities
a thickening of the axon often associated with disrupted microtubule tracks and accumulations of axonal vesicles.
- Virion
the minimal infectious unit of a virus. It is a combination of proteins and sometimes host cell membrane that protects and transmits viral nucleic acids.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- 1.Koyuncu OO, et al. Virus Infections in the Nervous System. Cell Host & Microbe. 2013;13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin DE. Viral Encephalomyelitis. PLoS Pathog. 2011;7:e1002004. doi: 10.1371/journal.ppat.1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salinas S, et al. A hitchhiker's guide to the nervous system: the complex journey of viruses and toxins. Nat. Rev. Microbiol. 2010;8:645–655. doi: 10.1038/nrmicro2395. [DOI] [PubMed] [Google Scholar]
- 4.Greber UF, Way M. A Superhighway to Virus Infection. Cell. 2006;124:741–754. doi: 10.1016/j.cell.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Ekstrand MI, et al. The alpha-herpesviruses: molecular pathfinders in nervous system circuits. Trends in Molecular Medicine. 2008;14:134–140. doi: 10.1016/j.molmed.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enquist LW, et al. Directional spread of an α-herpesvirus in the nervous system. Veterinary Microbiology. 2002;86:5–16. doi: 10.1016/s0378-1135(01)00486-2. [DOI] [PubMed] [Google Scholar]
- 7.Diefenbach RJ, et al. Transport and egress of herpes simplex virus in neurons. Rev Med Virol. 2007;18:35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- 8.Lomniczi B, et al. Genetic basis of the neurovirulence of pseudorabies virus. J. Virol. 1984;52:198–205. doi: 10.1128/jvi.52.1.198-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card JP, et al. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J. Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curanovic D, et al. Repair of the UL21 Locus in Pseudorabies Virus Bartha Enhances the Kinetics of Retrograde, Transneuronal Infection In Vitro and In Vivo. J. Virol. 2009;83:1173–1183. doi: 10.1128/JVI.02102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaVail JH, et al. Viral regulation of the long distance axonal transport of herpes simplex virus nucleocapsid. Neuroscience. 2007;146:974–985. doi: 10.1016/j.neuroscience.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGraw HM, et al. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not Us9. J. Virol. 2009;83:8315–8326. doi: 10.1128/JVI.00633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard PW, et al. Herpes Simplex Virus Membrane Proteins gE/gI and US9 Act Cooperatively To Promote Transport of Capsids and Glycoproteins from Neuron Cell Bodies into Initial Axon Segments. J. Virol. 2012;87:403–414. doi: 10.1128/JVI.02465-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagel MA, Gilden D. Complications of Varicella Zoster Virus Reactivation. Curr Treat Options Neurol. 2013;15:439–453. doi: 10.1007/s11940-013-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koelle DM, Corey L. Herpes Simplex: Insights on Pathogenesis and Possible Vaccines. Annu. Rev. Med. 2008;59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 16.Ugolini G. Advances in viral transneuronal tracing. Journal of Neuroscience Methods. 2009 doi: 10.1016/j.jneumeth.2009.12.001. DOI: 10.1016/j.jneumeth.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 17.McGovern AE, et al. Anterograde neuronal circuit tracing using a genetically modified herpes simplex virus expressing EGFP. Journal of Neuroscience Methods. 2012;209:158–167. doi: 10.1016/j.jneumeth.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 18.Bauer A, et al. Anterograde Glycoprotein Dependent Transport of Newly Generated Rabies Virus in Dorsal Root Ganglion Neurons. J. Virol. 2014;88:14172–14183. doi: 10.1128/JVI.02254-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsiang H, et al. The anterograde transport of rabies virus in rat sensory dorsal root ganglia neurons. J. Gen. Virol. 1989;70:2075–2085. doi: 10.1099/0022-1317-70-8-2075. [DOI] [PubMed] [Google Scholar]
- 20.Velandia-Romero ML, et al. In vivo differential susceptibility of sensory neurons to rabies virus infection. J Neurovirol. 19:367–375. doi: 10.1007/s13365-013-0179-5. [DOI] [PubMed] [Google Scholar]
- 21.Detje CN, et al. Local Type I IFN Receptor Signaling Protects against Virus Spread within the Central Nervous System. The Journal of Immunology. 2009;182:2297–2304. doi: 10.4049/jimmunol.0800596. [DOI] [PubMed] [Google Scholar]
- 22.Roe K, et al. West Nile virus-induced disruption of the blood-brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. Journal of General Virology. 2012;93:1193–1203. doi: 10.1099/vir.0.040899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neal JW. Flaviviruses are neurotropic, but how do they invade the CNS? J. Infect. 2014;69:203–215. doi: 10.1016/j.jinf.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Campenot RB. Local control of neurite development by nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campenot RB, et al. Production of compartmented cultures of rat sympathetic neurons. Nature Protocols. 2009;4:1869–1887. doi: 10.1038/nprot.2009.210. [DOI] [PubMed] [Google Scholar]
- 26.Ch'ng TH, Enquist LW. An in vitro system to study trans neuronal spread of pseudorabies virus infection. Veterinary Microbiology. 2006;113:193–197. doi: 10.1016/j.vetmic.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Curanovic D, et al. Compartmented neuron cultures for directional infection by alpha herpesviruses. Curr Protoc Cell Biol. 2009;26 doi: 10.1002/0471143030.cb2604s43. Unit 26.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuel MA, et al. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:17140–17145. doi: 10.1073/pnas.0705837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roussarie J-P, et al. Axon Myelin Transfer of a Non-Enveloped Virus. PLoS ONE. 2007;2:e1331. doi: 10.1371/journal.pone.0001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roussarie JP, et al. The role of myelin in Theiler's virus persistence in the central nervous system. PLoS Pathog. 2007;3:e23. doi: 10.1371/journal.ppat.0030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brahic M, Roussarie J-P. Axon–Myelin Interactions during a Viral Infection of the Central Nervous System. PLoS Pathog. 2009;5:e1000519. doi: 10.1371/journal.ppat.1000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones SL, et al. Axon initial segment cytoskeleton comprises a multiprotein submembranous coat containing sparse actin filaments. The Journal of Cell Biology. 2014;2:89. doi: 10.1083/jcb.201401045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis TL, et al. Cell biology in neuroscience: Cellular and molecular mechanisms underlying axon formation, growth, and branching. The Journal of Cell Biology. 2013;202:837–848. doi: 10.1083/jcb.201305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enquist LW. Five Questions about Viral Trafficking in Neurons. PLoS Pathog. 2012;8:e1002472. doi: 10.1371/journal.ppat.1002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maday S, et al. Axonal Transport: Cargo-Specific Mechanisms of Motility and Regulation. Neuron. 2014;84:292–309. doi: 10.1016/j.neuron.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kratchmarov R, et al. Making the case: Married versus Separate models of alphaherpes virus anterograde transport in axons. Rev Med Virol. 2012;22:378–391. doi: 10.1002/rmv.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunningham A, et al. Letter in response to: Making the case: Married versus Separate models of alphaherpes virus anterograde transport in axons. Rev Med Virol. 2013;23:414–418. doi: 10.1002/rmv.1760. [DOI] [PubMed] [Google Scholar]
- 38.Kramer T, et al. Kinesin-3 mediates axonal sorting and directional transport of alphaherpesvirus particles in neurons. Cell Host & Microbe. 2012;12:806–814. doi: 10.1016/j.chom.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howard PW, et al. Herpes Simplex Virus gE/gI Extracellular Domains Promote Axonal Transport and Spread from Neurons to Epithelial Cells. J. Virol. 2014;88:11178–11186. doi: 10.1128/JVI.01627-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsalenchuck Y, et al. Different modes of herpes simplex virus type 1 spread in brain and skin tissues. J Neurovirol. 2014;20:18–27. doi: 10.1007/s13365-013-0224-4. [DOI] [PubMed] [Google Scholar]
- 41.Gluska S, et al. Rabies Virus Hijacks and accelerates the p75NTR retrograde axonal transport machinery. PLoS Pathog. 2014;10:e1004348. doi: 10.1371/journal.ppat.1004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beier KT, et al. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proceedings of the National Academy of Sciences. 2011;108:15414–15419. doi: 10.1073/pnas.1110854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattera R, et al. Co-assembly of Viral Envelope Glycoproteins Regulates Their Polarized Sorting in Neurons. PLoS Pathog. 2014;10:e1004107. doi: 10.1371/journal.ppat.1004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith GA, et al. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3466. doi: 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wisner TW, et al. Anterograde transport of herpes simplex virus capsids in neurons by both separate and married mechanisms. J. Virol. 2011;85:5919–5928. doi: 10.1128/JVI.00116-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grigoryan S, et al. Retrograde axonal transport of VZV: kinetic studies in hESC-derived neurons. J Neurovirol. 2012;18:462–470. doi: 10.1007/s13365-012-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curanovic D, Enquist LW. Virion-incorporated glycoprotein B mediates transneuronal spread of pseudorabies virus. J. Virol. 2009;83:7796–7804. doi: 10.1128/JVI.00745-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finke S, et al. Tracking fluorescence-labeled rabies virus: enhanced green fluorescent protein-tagged phosphoprotein P supports virus gene expression and formation of infectious particles. J. Virol. 2004;78:12333–12343. doi: 10.1128/JVI.78.22.12333-12343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moughamian AJ, Holzbaur ELF. Synaptic Vesicle Distribution by Conveyor Belt. Cell. 2012;148:849–851. doi: 10.1016/j.cell.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Wong MY, et al. Neuropeptide Delivery to Synapses by Long-Range Vesicle Circulation and Sporadic Capture. Cell. 2012;148:1029–1038. doi: 10.1016/j.cell.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mikloska Z, et al. Neutralizing antibodies inhibit axonal spread of herpes simplex virus type 1 to epidermal cells in vitro. J. Virol. 1999;73:5934–5944. doi: 10.1128/jvi.73.7.5934-5944.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saksena MM, et al. Herpes Simplex Virus Type 1 Accumulation, Envelopment, and Exit in Growth Cones and Varicosities in Mid-Distal Regions of Axons. 2006 doi: 10.1128/JVI.80.7.3592-3606.2006. DOI: 10.1128/JVI.80.7.3592–3606.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ibiricu I, et al. Cryo Electron Tomography of Herpes Simplex Virus during Axonal Transport and Secondary Envelopment in Primary Neurons. PLoS Pathog. 2011;7:e1002406. doi: 10.1371/journal.ppat.1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Regge N, et al. Alpha-herpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. The Journal of Cell Biology. 2006;174:267–275. doi: 10.1083/jcb.200510156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor MP, et al. Visualization of an alphaherpesvirus membrane protein that is essential for anterograde axonal spread of infection in neurons. MBio. 2012;3:e00063–12. doi: 10.1128/mBio.00063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibiricu I, et al. Characterization of herpes simplex virus type 1 L-particle assembly and egress in hippocampal neurones by electron cryo-tomography. Cellular Microbiology. 2013;15:285–291. doi: 10.1111/cmi.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carpenter JE, et al. Egress of Light Particles among Filopodia on the Surface of Varicella-Zoster Virus-Infected Cells. J. Virol. 2008;82:2821–2835. doi: 10.1128/JVI.01821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mingo RM, et al. Replication of herpes simplex virus: egress of progeny virus at specialized cell membrane sites. J. Virol. 2012;86:7084–7097. doi: 10.1128/JVI.00463-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor MP, et al. Alphaherpesvirus axon-to-cell spread involves limited virion transmission. Proceedings of the National Academy of Sciences. 2012;109:17046–17051. doi: 10.1073/pnas.1212926109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor MP, et al. Live cell imaging of alphaherpes virus anterograde transport and spread. JoVE (Journal of Visualized Experiments) 2013 doi: 10.3791/50723. DOI: 10.3791/50723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu CA, et al. Molecular basis for interference of defective interfering particles of pseudorabies virus with replication of standard virus. J. Virol. 1986;59:308–317. doi: 10.1128/jvi.59.2.308-317.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaramillo N, et al. Evidence of Muller's ratchet in herpes simplex virus type 1. Journal of General Virology. 2013;94:366–375. doi: 10.1099/vir.0.044685-0. [DOI] [PubMed] [Google Scholar]
- 63.Zwart MP, et al. One Is Enough: In Vivo Effective Population Size Is Dose-Dependent for a Plant RNA Virus. PLoS Pathog. 2011;7:e1002122. doi: 10.1371/journal.ppat.1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Roossinck MJ. Genetic bottlenecks reduce population variation in an experimental RNA virus population. J. Virol. 2004;78:10582–10587. doi: 10.1128/JVI.78.19.10582-10587.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Domingo E, et al. Viral Quasispecies Evolution. Microbiology and Molecular Biology Reviews. 2012;76:159–216. doi: 10.1128/MMBR.05023-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deardorff ER, et al. West Nile virus experimental evolution in vivo and the trade-off hypothesis. PLoS Pathog. 2011;7:e1002335. doi: 10.1371/journal.ppat.1002335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lauring AS, Andino R. Quasispecies Theory and the Behavior of RNA Viruses. PLoS Pathog. 2010;6:e1001005. doi: 10.1371/journal.ppat.1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfeiffer JK. Bottleneck-mediated quasispecies restriction during spread of an RNA virus from inoculation site to brain. Proceedings of the National Academy of Sciences. 2006;103:5520–5525. doi: 10.1073/pnas.0600834103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lancaster KZ, Pfeiffer JK. Limited Trafficking of a Neurotropic Virus Through Inefficient Retrograde Axonal Transport and the Type I Interferon Response. PLoS Pathog. 2010;6:e1000791. doi: 10.1371/journal.ppat.1000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beasley DWC, et al. Mouse Neuroinvasive Phenotype of West Nile Virus Strains Varies Depending upon Virus Genotype. Virology. 2002;296:17–23. doi: 10.1006/viro.2002.1372. [DOI] [PubMed] [Google Scholar]
- 71.Card JP, Enquist LW. Neuromethods. Vol. 70. Humana Press; 2012. [Google Scholar]
- 72.Haberl MG, et al. An anterograde rabies virus vector for high- resolution large-scale reconstruction of 3D neuron morphology. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0730-z. DOI: 10.1007/s00429-014-0730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]