Abstract
Purpose
To investigate the clinical relevance of PTEN in HER2-amplified and HER2-non-amplified disease.
Experimental Design
We assessed PTEN status in two large adjuvant breast cancer trials (BCIRG-006 and BCIRG-005) using a PTEN IHC assay that was previously validated in a panel of 33 breast cancer cell lines and prostate cancer tissues with known PTEN gene deletion.
Results
In the HER2-positive patient population, absence of tumor cell PTEN staining occurred at a rate of 5.4% and was independent of ER/PR status. In contrast, 15.9% of HER2-negative patients exhibited absence of PTEN staining with the highest frequency seen in triple negative breast cancer (TNBC) subgroup versus ER/PR-positive patients (35.1% vs. 10.9%). Complete absence of PTEN staining in tumor cells was associated with poor clinical outcome in HER2-positive disease. Those patients whose cancers demonstrated absent PTEN staining had a significant decrease in disease-free survival (DFS) and overall survival (OS) compared to patients with tumors exhibiting any PTEN staining patterns (low, moderate or high). Trastuzumab appeared to provide clinical benefit even for patients lacking PTEN staining. In the HER2-negative population there were no statistically significant differences in clinical outcome based on PTEN status.
Conclusions
This study is the largest to date examining PTEN status in breast cancer and the data suggest that the rate and significance of PTEN status differ between HER2-positive and HER2-negative disease. Furthermore, the data clearly suggest that HER2-positive patients with PTEN loss still benefit from trastuzumab.
Introduction
The HER2 (ERBB2) gene is a member of the human type 1 receptor tyrosine kinase family. In approximately 20%–25% of breast cancers, this gene is amplified resulting in HER2 protein overexpression and oncogenic transformation (1–3). Under normal conditions, HER2 is activated only when ligand binds with one of the other three HER family members (EGFR/HER1, HER3, or HER4), causing heterodimer formation with HER2 and activation of its kinase activity (4, 5). However, when overexpressed, HER2 is able to associate with itself and other HER family members in a ligand-independent manner (6, 7). The most potent oncogenic unit in the setting of HER2 amplification is the HER2/HER3 heterodimer (3, 8, 9) and involvement of HER3 is particularly significant given its role in potent activation of the PI3K pathway (10, 11).
Prior to the approval of trastuzumab (12) and lapatinib (13), HER2 amplification in breast cancer was associated with poor clinical outcomes (1, 2, 14, 15). More recently additional anti-HER2 agents like pertuzumab (16) and trastuzumab emtansine (17) have also been introduced into treatment regimens. The most commonly used agent, trastuzumab, is a humanized monoclonal antibody that extends progression-free survival (PFS) and overall survival (OS) in the HER2-positive metastatic breast (12) and gastric cancer settings (18) and significantly improves disease-free and overall survival in the adjuvant HER2-positive breast cancer setting (19–21). There are several possible mechanisms for trastuzumab’s therapeutic effect, one of which is the ability of the drug to disrupt the ligand-independent association between HER2 and HER3 (22) that in turn mitigates oncogenic signaling through the PI3K pathway.
Despite this significant clinical benefit in HER2-positive patients, not every individual in this subgroup benefits equally despite having confirmed HER2-positive disease. In the metastatic setting, approximately 50% of patients do not exhibit an objective response with trastuzumab plus chemotherapy (12). Moreover, many of those who do exhibit an objective response will eventually acquire resistance and progress on therapy (12). In the adjuvant setting, there are many HER2-positive patients who develop disease recurrence despite one year of trastuzumab therapy (19–21). While there are several proposed mechanisms of resistance to HER2-targeted therapies, one of the most favored hypotheses is that the molecular alterations resulting in activation of the PI3K pathway renders cells independent of the abnormal HER2 signaling and circumventing inhibition of this pathway upstream through the altered HER2 receptor levels (23–25).
Here we address the role of PTEN (phosphatase and tensin homolog) loss in trastuzumab resistance. We first validate the IHC assay we used to measure PTEN in formalin-fixed paraffin-embedded (FFPE) tissue by demonstrating agreement between the IHC assay and Western blot results across a panel of 33 breast cancer cell lines. We also confirm that PTEN staining status using this assay was consistent with silver in situ hybridization (SISH) data on PTEN status in prostate cancer samples. Using this validated PTEN IHC assay, we then examined PTEN status in two large adjuvant breast cancer trials in HER2-positive and HER2-negative disease (BCIRG 006 and BCIRG 005, respectively).
Materials and Methods
Patients and tissues
The Breast Cancer International Research Group (BCIRG)-005 and BCIRG-006 adjuvant breast cancer trials were conducted between August 2000 and March 2004 and accrued 3,298 and 3,222 patients, respectively. BCIRG-005 enrolled early breast cancer patients with HER2-non-amplified, node-positive, early breast cancer who were randomized to receive adjuvant treatment with one of two anthracycline-containing regimens, either four cycles of doxorubicin plus cyclophosphamide given every three weeks followed by four cycles of docetaxel given every three weeks (AC→T) or alternatively six cycles of docetaxel plus doxorubicin plus cyclophosphamide given every three weeks (TAC)(26). BCIRG-006 enrolled early breast cancer patients with node-positive or high-risk, node-negative, invasive HER2-amplified disease. This trial compared two different experimental trastuzumab plus chemotherapy regimens (one with and one without anthracyclines) and compared each to anthracycline-based chemotherapy alone (21). Both studies followed patients for disease-free survival (DFS) and overall survival (OS). Patients in both studies provided consent for centralized molecular analysis of their primary tumors and paraffin blocks of tumor tissue were submitted to one of two central laboratories to determine HER2 status by fluorescence in situ hybridization (FISH) that was subsequently confirmed in a blinded re-analysis of the tissue (27). Cores from available tissue blocks were placed into tissue microarrays for subsequent exploratory analyses. Breast carcinoma specimens were available from the primary cancers as tissue microarrays as described elsewhere (27). Eight replica blocks, each with one core per tumor, were created, and one of the replica blocks was used for this investigation. The study was approved by the USC Institutional Research Board (IRB).
Immunohistochemistry
The PTEN immunohistochemistry analyses for this study were conducted using formalin-fixed, paraffin-embedded tissue sections with automated immunostaining platforms. The assay was developed on the Ventana Discovery XT platform and subsequently adapted to the Ventana Benchmark platform (Ventana Medical Systems, Inc., Tucson, AZ). The primary rabbit monoclonal anti-PTEN antibody (Cell Signaling Technology, Cat#9559, lot 2; Danvers, MA) was used at a 1:25 dilution for one hour at room temperature. On the Discovery XT platform, heat-induced, antigen retrieval was conducted using the CC1 standard program and a high pH Tris/borate/EDTA buffer (VMSI, Catalog No. 950-124). Primary antibody was detected using the ChromoMap™ diaminobenzidine detection kit (VMSI, Catalog No. 760-159) and UltraMap™ anti-rabbit horseradish peroxidase (VMSI, Catalog No. 760-4315). The anti-rabbit horseradish peroxidase secondary antibody was applied for 32 minutes at room temperature. Slides were counterstained with hematoxylin (VMSI, Catalog No. 790-2208) for 8 minutes at 37C. An identical program was written for the Benchmark XT platform using a PTEN primary antibody at a concentration of 1:20 and the UltraViewTM Universal DAB detection Kit (Catalog No. 760-500).
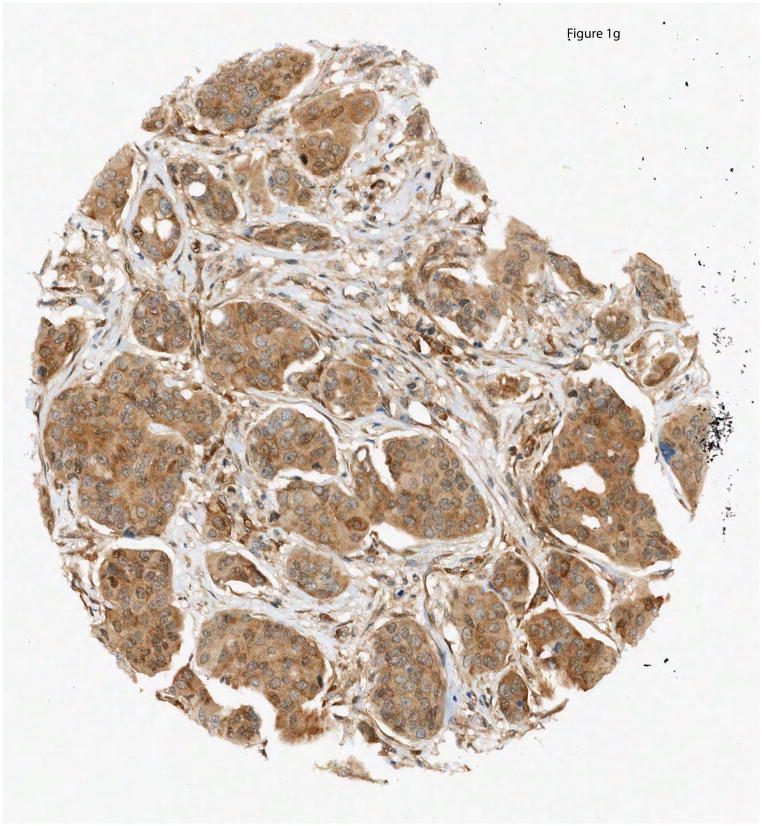
PTEN IHC was subjectively interpreted as absent (0) if absolutely no immunostaining was detectable in breast carcinoma cells but was present in adjacent benign stromal cells; as weak but reduced (1*) if cytoplasmic immunostaining was detectable but less intense in the carcinoma cells than in adjacent benign stromal cells; as weak (1+) if cytoplasmic staining was low but of similar intensity to that observed in adjacent benign stromal cells; as moderate (2+) if cytoplasmic immunostaining in carcinoma cells was intermediate between weak and strong; and as strong (3+) if the cytoplasmic immunostaining was intense (Figure 1). Two pathologists (WE and MFP) interpreted the IHC of the TMAs. Discrepancies were resolved by consensus over a multi-headed microscope.
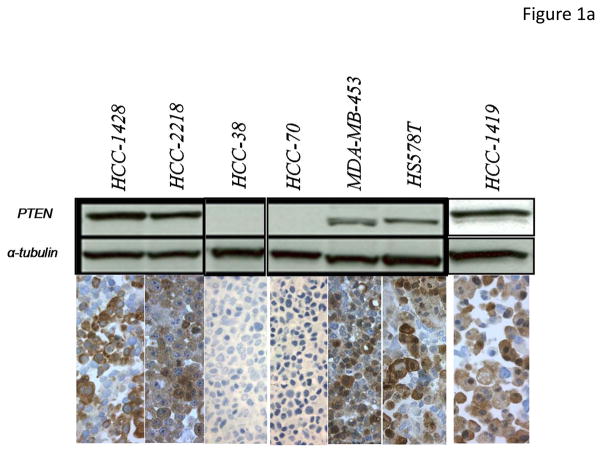
Figure 1. PTEN IHC assay validation and scoring.
PTEN IHC was performed on a panel of FFPE breast cancer cell pellets of known PTEN status confirmed by western immunoblot. (A.) Western immunoblot analyses for HCC-1428, HCC-2218, HCC-38, HCC-70, MDA-MB-453, Hs578T and HCC-1419 human breast cancer cell lines are illustrated above with PTEN IHC illustrated below; (B.) Comparison of western immunoblot results and PTEN IHC in HCC-1937, HCC-70, HDQ-P1, AU565, MDA-MB-231, CAMA-1, and DU4475 human breast cancer cell lines. (C–G.) PTEN IHC was performed on breast cancer TMA’s containing tissue from the BCIRG 006 and BCIRG 005 clinical trials. Tissues for which PTEN was present in stroma and could be evaluated in tumor tissue were grouped into 5 categories: Strong cytoplasmic immunostaining in carcinoma cells (3+) (G), Moderate cytoplasmic immunostaining in carcinoma cells (2+) (F), Weak cytoplasmic immunostaining in carcinoma cells, comparable to the immunostaining observed in the benign stromal cells (1+) (E), Weak cytoplasmic immunostaining in carcinoma cells, but weaker than the immunostaining observed in benign stromal cells (1*) (D), and no immunostaining detectable in carcinoma cells, but with distinct immunostaining observed only in the benign stromal cells (0) (C).
Cell lines
Human breast cancer cell lines (AU565, BT474, BT549, CAL-51, CAL-120, CAL-148, CAMA-1, DU4475, EFM192A, EFM-19, HCC-38, HCC-70, HCC-1419, HCC-1428, HCC-1569, HCC-1937, HCC-1954, HCC-2218, HS578T, HDQ-P1, KPL-1, MCF7, MDA-MB-231, MDA-MB-361, MDA-MB-415, MDA-MB-453, MDA-MB-468, MFM-223, and MDA-MB-436 and ZR-75-1) and MT-3 which was originally described as a breast cancer line but subsequently determined by fingerprinting to be a human colon cancer cell line were obtained from American Type Culture Collection (ATCC) and authenticated via Affymetrix gene expression profiling and single nucleotide polymorphism genotyping arrays. Cell lines were maintained in RPMI supplemented with 10% fetal bovine serum (Sigma-Aldrich), nonessential amino acids, and 2 mM L-glutamine. Western blots were performed using the following primary antibodies: rabbit monoclonal anti-PTEN clone 138G6 (Cell Signaling Technology) and mouse monoclonal anti-α-tubulin clone B-5-1-2 (Sigma-Aldrich). PTEN IHC on cell lines was performed using a tissue microarray of formalin fixed paraffin embedded breast cancer cell lines prepared separately from the BCIRG clinical trials patient samples as previously described (28). The optimal immunohistochemistry conditions determined from cell line TMAs were used for the BCIRG patient sample TMAs.
This IHC assay was also tested and optimized in human tissues. Prostate cancer samples with PTEN deletion by quantum-dot fluorescence in situ hybridization (QD-FISH) were used as tissue controls. After examining a series of archival breast cancer tissues as well as some from the BCIRG adjuvant trials, a scoring algorithm was developed to capture the various staining patterns observed (Figure 1, C–G). Non-malignant tissue, such as stroma, was used as an internal positive control for each sample.
Statistical analysis
Observed proportions were compared by using the chi-square test. OS was computed from the date of randomization to the date of death or the last observation (whichever occurred first). DFS was computed from the date of randomization to the date of death, recurrence, or the last observation (whichever occurred first).
Survival curves were estimated by using the Kaplan-Meier estimator. Hazard ratios for PTEN staining levels were estimated based on a multivariable Cox model stratified by the ER/PR status and adjusted for treatment, age, performance status, tumor site, T-stage, histological type, number of positive nodes, grade, and menopausal status. Comparisons based on the model were conducted by using the likelihood ratio test. Results of all statistical significance tests were evaluated by using the 5% significance level (two-sided).
Results
PTEN IHC assay development and validation
An IHC assay to assess PTEN status was developed and validated utilizing formalin fixed paraffin embedded (FFPE) cell pellets of known PTEN status as controls. Cell lines that were positive for PTEN by Western blot were clearly positive by IHC and those that were negative for PTEN by Western blot were negative by IHC (Figure 1, A and B). This analysis was expanded to a panel of 30 breast cancer cell lines and a colon cancer cell line, assessed by both Western blot and IHC (Figure 1 and Supplementary Figure 3). The cell lines were defined as positive or negative based on whether the expected PTEN protein band was detectable by Western blot as previously reported (29). The IHC was quantified in the same cell lines using fixed and paraffin-embedded pellets with image analysis. Ten cell lines contained no detectable PTEN by either western immunoblot or IHC (BT549, CAL-148, CAL-51, HCC-38, HCC-70, HCC-1569, HCC1937, MDA-MB-436, MDA-MB-468 and ZR-75-1), while 20 cell lines demonstrated detectable PTEN by both western immunoblot and IHC (AU565, BT474, CAL-120, CAMA-1, DU4475, EFM-19, EFM192A, HDQ-P1, HCC-1419, HCC-1428, HCC-1954, HCC-2218, HS578T, KPL-1, MCF7, MDA-MB-231, MDA-MB-361, MDA-MB-453, MFM-223 and MT-3). One cell line (MDA-MB-415) showed a barely detectable PTEN band but IHC failed to demonstrate any immunostaining. Therefore the sensitivity (95.2%) and specificity (100%) for IHC compared to western immunoblot analysis were both considered acceptable with a high overall agreement rate (97%). These data support the conclusion that the PTEN IHC assay subsequently used for this study was both sensitive and specific for PTEN.
Description of the study population
The PTEN IHC assay and scoring algorithm was applied to tissues from the BCIRG-006 and BCIRG-005 adjuvant trials (see clinical trial schemata in Supplementary Fig. 1).
PTEN IHC was performed on tissue microarrays representing 4587 patients across both BCIRG trials. For 2211 patients the cores were missing or insufficient tissue was present to generate a PTEN score. In another 12 patients, there was insufficient staining in non-malignant stromal cells thus precluding assessment of the tumor tissue. Accordingly, PTEN scores were successfully generated from a total of 2364 patients of which 1201 were from BCIRG-006 and 1163 from BCIRG-005. The characteristics of the patients for whom PTEN data were obtained were balanced across treatment arms (Table 1). Clinical data from each trial demonstrated that neither DFS nor OS of patients for whom PTEN data were available differed from without available PTEN analyses (Supplementary Fig. 2).
Table 1.
Characteristic of Patients with PTEN data
| Characteristic | HER2-amplified (BCIRG 006) N = 1201 |
HER2 Not Amplified (BCIRG 005) N = 1163 |
|||
|---|---|---|---|---|---|
|
| |||||
| AC->T (394) | AC->TH (402) | TCH (405) | AC -> T (566) | TAC (597) | |
|
| |||||
| Age at randomization | |||||
| < 35 year | 36 (9.1%) | 33 (8.2%) | 31 (7.7%) | 39 (6.9%) | 26 (4.4%) |
| 35–49 yr | 178 (45.2%) | 171 (42.5%) | 190 (46.9%) | 255 (45.1%) | 271 (45.4%) |
| 50–65 yr | 165 (41.9%) | 185 (46.0%) | 167 (41.2%) | 242 (42.8%) | 273 (45.7%) |
| ≥ 65 yr | 15 (3.8%) | 13 (3.2%) | 17 (4.2%) | 30 (5.3%) | 27 (4.5%) |
|
| |||||
| Number of positive nodes | |||||
| Unknown | 0 | 0 | 0 | 0 | 0 |
| 0 | 97 (24.6%) | 105 (26.1%) | 99 (24.4%) | 0 | 0 |
| 1–3 | 156 (39.6%) | 154 (38.3%) | 172 (42.5%) | 342 (60.4%) | 355 (59.5%) |
| 4–10 | 94 (23.9%) | 105 (26.1%) | 95 (23.5%) | 166 (29.3%) | 168 (28.1%) |
| ≥ 11 | 47 (11.9%) | 38 (9.5%) | 39 (9.6%) | 58 (10.3%) | 74 (12.4%) |
|
| |||||
| ER status | |||||
| Missing | 3 (0.8%) | 3 (0.8%) | 5 (1.2%) | 16 (2.8%) | 16 (2.7%) |
| Negative | 197 (50.0%) | 200 (49.8%) | 194 (47.9%) | 146 (25.8%) | 150 (25.1%) |
| Positive | 194 (49.2%) | 199 (49.5%) | 206 (50.9%) | 404 (71.4%) | 431 (72.2%) |
|
| |||||
| PR status | |||||
| Missing | 3 (0.8%) | 3 (0.8%) | 5 (1.2%) | 16 (2.8%) | 16 (2.7%) |
| Negative | 239 (60.7%) | 229 (57.0%) | 227 (56.1%) | 182 (32.2%) | 200 (33.5%) |
| Positive | 152 (38.6%) | 170 (42.3%) | 173 (42.7%) | 368 (65.0%) | 381 (63.8%) |
|
| |||||
| Tumor size | |||||
| Missing | 0 | 0 | 0 | 0 | 0 |
| ≤ 2.0 cm (pT1) | 145 (36.8%) | 127 (31.6%) | 136 (33.6%) | 232 (41.0%) | 221 (27.0%) |
| 2.1–5.0 cm (pT2) | 221 (56.1%) | 241 (60.0%) | 235 (58.0%) | 297 (52.5%) | 330 (55.3%) |
| ≥ 5.1 cm (pT3) | 28 (7.1%) | 34 (8.5%) | 32 (7.9%) | 37 (6.5%) | 44 (7.4%) |
| Any (pT4) | 0 | 0 | 2 (0.5%) | 0 | 2 (0.3%) |
|
| |||||
| Tumor grade | |||||
| Missing | 0 | 0 | 0 | 1 (0.2%) | 1 (0.2%) |
| Unknown | 18 (4.6%) | 22 (5.5%) | 23 (5.7%) | 45 (8.0%) | 50 (8.4%) |
| Well | 6 (1.5%) | 1 (0.3%) | 7 (1.7%) | 45 (8.0%) | 48 (8.0%) |
| Moderately | 93 (23.6%) | 106 (26.4%) | 106 (26.2%) | 260 (45.9%) | 254 (42.6%) |
| Poorly | 275 (69.8%) | 273 (67.9%) | 268 (66.2%) | 213 (37.6%) | 242 (40.5%) |
| Undifferentiated | 2 (0.5%) | 0 | 1 (0.3%) | 2 (0.4%) | 2 (0.3%) |
Absence of PTEN staining is more common in HER2-negative disease
The percentage of patients in each of five different PTEN cytoplasmic staining categories for both trials is summarized in Table 2. PTEN scores for nuclear staining were also determined and were found to significantly agree with cytoplasmic staining results (Supplementary Table 1). Given this staining concordance and the fact that a mechanistic understanding of PTEN function is more in keeping with cytoplasmic expression, the remainder of the analyses for this study focused on cytoplasmic IHC scores. There was a statistically significant difference in the percentage of patients with complete absence of PTEN staining in tumor cells when comparing the HER2-positive and HER2-negative population (5.4% in BCIRG-006 versus 15.9% in BCIRG-005, p<0.0001) indicating that absence of PTEN expression is more common in HER2-negative (non-amplified) disease.
Table 2.
PTEN IHC score distribution
| Score | BCIRG 006 (HER2-amplified) | BCIRG 005 (HER2-not-amplified) | Overall |
|---|---|---|---|
|
| |||
| Absent in tumor1 (0) | 65 (5.4%) | 185 (15.9%) | 250 (10.6%) |
| Weak in tumor2 (1*) | 85 (7.1%) | 88 (7.6%) | 173 (7.3%) |
| Weak overall3 (1+) | 501 (41.7%) | 443 (38.1%) | 944 (39.9%) |
| Moderate overall4 (2+) | 486 (40.5%) | 387 (33.3%) | 873 (36.9%) |
| Strong in tumor5 (3+) | 64 (5.3%) | 60 (5.2%) | 124 (5.2%) |
|
| |||
| Total scorable | 1201 | 1163 | 2364 |
No detectable staining in tumor cells but with positive staining present in stroma.
Weak staining present in tumor cells; however, this staining is weaker than the immunostaining observed in adjacent benign stromal cells.
Weak staining (1+) present in tumor cells with similar intensity to that observed in adjacent stromal cells.
Moderate immunostaining (2+) with immunostaining also present in stromal cells.
Strong staining (3+) in tumor cells with staining also present in stromal cells.
Prior studies have indicated that loss of PTEN is most common in triple-negative breast cancer (30–33). To determine whether this observation could be confirmed in the BCIRG adjuvant trial populations, PTEN status was examined based on ER/PR status (Table 3). In the BCIRG-006 population (HER2-positive), 548 (45.6%) of 1201 patients with available PTEN data were classified as ER/PR-negative. The percentage of patients lacking PTEN staining in tumor cells was similar in the ER/PR-negative population compared to the ER/PR-positive population (6.4% versus 4.6%).
Table 3.
PTEN by ER/PR status
| Trial | PTEN | ER− and PR− | ER+ and/or PR+ |
|---|---|---|---|
|
| |||
| BCIRG 006 n = 1201 HER2 amplified |
Absent in tumor | 35 (6.4%) | 30 (4.6%) |
| Weak in tumor | 52 (9.5%) | 33 (5.1%) | |
| Weak overall | 257 (46.9%) | 244 (37.4%) | |
| Moderate overall | 184 (33.6%) | 302 (46.2%) | |
| Strong in tumor | 20 (3.6%) | 44 (6.7%) | |
|
| |||
| Total | 548 (100%) | 653 (100%) | |
|
| |||
| BCIRG 005 n = 1163 HER2 not amplified |
Absent in tumor | 86 (35.1%) | 99 (10.8%) |
| Weak in tumor | 28 (11.4%) | 60 (6.5%) | |
| Weak overall | 76 (31.0%) | 367 (40.0%) | |
| Moderate overall | 43 (17.6%) | 344 (37.5%) | |
| Strong in tumor | 12 (4.9%) | 48 (5.2%) | |
|
| |||
| Total | 245 (100%) | 918 (100%) | |
PTEN status with respect to ER/PR in BCIRG-005 (HER2-negative) exhibits some important differences compared to BCIRG-006. In BCIRG-005, 245 (21.1%) of 1163 patients with available PTEN data were ER/PR-negative (vs. 45.6% in BCIRG 006). The percentage of patients in BCIRG-005 lacking PTEN staining in tumor cells was unevenly distributed with 35.1% having an ER/PR-negative status, and, therefore, defined as “triple-negative” 10.9% of ER/PR-positive samples lacked PTEN staining (p<0.0001). Together, these data confirm that the frequency of tumors lacking PTEN expression in tumor cells is highest in the triple-negative population which is followed by the ER/PR-positive, HER2-negative populations and is lowest in the HER2-positive population.
Clinical outcome based on PTEN status
All five PTEN staining patterns were independently examined and analyzed for correlation with DFS and OS in the HER2-positive and HER2-negative populations. In women with HER2-positive breast cancer, the subpopulation of patients lacking PTEN staining exhibited a decrease in both DFS and OS compared to all other PTEN staining categories; however, the effect of PTEN absence did not reach statistical significance (p=0.09). However, the hazard ratios for very weak, weak, moderate, and strong staining versus absent PTEN staining were 0.57 (95% CI, {0.30,1.08}), 0.56 (95% CI, {0.35,0.89}), 0.48 (95% CI, {0.30,0.78}), and 0.48 (95% CI, {0.22, 1.02}), respectively, and consistently indicated a trend toward a negative prognostic effect of absent PTEN staining (Figure 2A). The similarity of hazard ratio values for different categories of PTEN staining is also noteworthy. Conversely, for the OS, the effect of PTEN staining was strongly significant (p=0.002), with the hazard ratios equal to 0.35 (95% CI, {0.16,0.79}), 0.35 (95% CI, {0.20,0.61}), 0.26 (95% CI, {0.15,0.46}), and 0.30 (95% CI, {0.11, 0.83}) respectively, for the other four levels of staining compared to absent PTEN staining, again consistently indicating a similar, negative prognostic effect of absence of PTEN staining (Figure 2B).
Figure 2. Disease free survival and overall survival based on PTEN IHC score.
For all 5 PTEN IHC scoring categories, Kaplan-Meier curves for disease free survival (A, C) and overall survival (B, D) were analyzed for BCIRG 006 (HER2-amplified) (A, B) and BCIRG 005 (HER2-non-amplified) (C, D).
In women with HER2-negative breast cancer (BCIRG-005), there was a marginally significant effect of PTEN staining on DFS (p=0.01). The hazard ratios for very weak, weak, moderate, and strong staining patterns, respectively, versus absent PTEN were 1.19 (95% CI, {0.75,1.90}), 0.71 (95% CI, {0.49,1.03}), 1.23 (95% CI, {0.85,1.76}), and 0.85 (95% CI, {0.46, 1.61}) demonstrating no consistent pattern beyond a trend toward correlation between lower PTEN staining levels and a worse outcome (Figure 2C). The effect of PTEN staining on OS was not statistically different (p=0.15; see Figure 2D). These data suggest that the clinical significance of PTEN staining differs in HER2-positive and HER2-negative disease and that in the HER2-positive setting only complete absence of PTEN staining in tumor cells is clinically significant.
To determine whether PTEN status was associated with both clinical outcome and response to trastuzumab therapy in HER2-positive patients, we examined DFS and OS in the trastuzumab-containing arms of BCIRG-006 versus the chemotherapy alone arm. Because only those patients lacking PTEN staining had a significantly different clinical outcome, we looked at the trastuzumab treatment effects in patients lacking PTEN staining versus patients having any other PTEN staining category. Among the patients lacking PTEN staining, trastuzumab treatment appeared to provide a trend towards benefit in terms of DFS and OS compared to patients receiving chemotherapy alone (Fig. 3A and B). Statistically, however, there was no significant interaction effect between PTEN status and clinical outcome (p=0.68 for OS, p=0.41 for DFS), indicating no evidence between PTEN status and response to trastuzumab. These data suggest that while lack of PTEN is associated with poor outcome, it is not associated with resistance to trastuzumab.
Figure 3. Disease free survival and overall survival based on PTEN status and treatment arm.
Patients were grouped by treatment arm into those lacking PTEN staining in tumor cells and those with tumor PTEN staining present. A Kaplan-Meier analysis was performed for disease free survival (A, C) and overall survival (B, D) on BCIRG 006 (HER2-amplified) (A, B) and BCIRG 005 (HER2-non-amplified) (C, D).
In the HER2-non-amplified population, there were no statistically significant differences in either DFS or OS based on PTEN status, despite a trend towards worse outcome in patients lacking PTEN staining (Fig. 3C and D). Consistent with analysis of outcome data for the entire clinical trial population, TAC and ACT were equivalent in clinical outcome (interaction test, p=0.69 and p=0.32 for OS and DFS, respectively).
Discussion
Several published studies have hypothesized that PTEN loss could be a resistance factor for trastuzumab treatment (34–37). From a signaling perspective, a logical hypothesis is that downstream activation of a relevant pathway might likely lead to resistance to upstream inhibition (38, 39). There are both pre-clinical and clinical data supporting this hypothesis. First, it has been pre-clinically shown both in vitro and in vivo that introduction of PIK3CA activating mutations and/or PTEN loss can mitigate the growth inhibitory effects of trastuzumab (29, 34–36). Second, cell lines that are resistant to trastuzumab have been found to respond to the potent and selective PI3K inhibitor GDC-0941 both in vitro and in vivo (22, 37). Third, several retrospective studies of PTEN and PIK3CA in tumors from HER2-positive, metastatic breast cancer patients suggest that PTEN loss and/or PIK3CA activating mutations are associated with poorer clinical outcomes despite trastuzumab treatment (23, 34, 36, 40–42). However most of these data were generated in small clinical cohorts with limited follow-up and most were from studies that did not contain a control (no trastuzumab) arm. In addition, several different assay methods to evaluate PTEN status have been used with varying success. These shortcomings in the reported literature make it difficult to determine whether PI3K pathway activation is a prognostic marker associated generally with poor outcomes or is a predictive marker of trastuzumab resistance. Furthermore, examination of this question has largely been in patients with metastatic disease who may not be ideal from the perspective that the assessment of PI3K pathway status is done on the primary tumor that may or may not reflect the status of the subsequent metastases.
One study using the N9831 trial did involve an assessment of PTEN in large numbers of patients with HER2-positive breast cancers who were randomized to trastuzumab therapy versus no trastuzumab combination chemotherapy regimens in the adjuvant setting (43). This trial did not show any association between PTEN status and response to trastuzumab. Given these somewhat conflicting data, it seems clear that evaluation of the hypothesis that downstream PI3K pathway activation can cause resistance to HER2-targeted agents in the clinic would benefit from examination in an additional large data set containing an appropriate control population with long follow-up.
The goal of the present study is to validate and utilize a reproducible PTEN IHC assay to test the impact of PTEN status on clinical outcome in two large adjuvant cohorts including HER2-positive breast cancer patients treated with and without trastuzumab as well as in HER2-negative breast cancer patients treated with combination versus sequential chemotherapy (21, 26).
Decreased PTEN expression has been reported to occur in 15 to 50 percent of breast cancers (23, 34, 36, 40–44). A potential short-coming of these studies is that decreased PTEN expression is variably defined but generally reflects a reduction in PTEN staining in tumor cells compared to non-malignant tissue elements – usually stromal cells. Although no studies directly compare the frequency of decreased PTEN in HER2-positive versus HER2-negative disease, the reported PTEN loss data in HER2-positive studies spans a similar range. Combining our PTEN IHC categories “absent in tumor” and “weak in tumor,” allows comparison of our data to published studies looking at decreased PTEN expression. In that regard, we observed a decreased PTEN rate of 12.5% in the HER2-positive study cohort and 23.5% in the HER2-negative cohort. The data in HER2-negative patients is similar to the rates observed in published studies, but the rate in the HER2-positive patients is lower than previously reported. It is not clear if this discrepancy reflects differences in the assay or scoring or alternatively in real biological differences in the various study populations.
A key question when considering the significance of decreased PTEN expression is “how low does PTEN expression need to go to exert a biologically and clinically meaningful effect?” Interestingly, our data revealed that in the HER2-positive population, those patients with absent PTEN expression exhibit a significantly worse outcome than those that exhibit weak staining in tumor cells. These data suggest that a substantial decrease in PTEN expression is necessary before becoming biologically significant. It should be noted that absence of staining must be interpreted as “below the limit of detection of the assay” and does not necessarily mean that PTEN protein is completely absent. This point is important to consider in the event that more sensitive detection methods might be developed. With this caveat in mind, an interesting finding from this study is that absence of PTEN staining appears to have a more profound significance in the HER2-positive as opposed to HER2-negative breast cancers. In HER2-positive patients whose breast cancers lack PTEN staining there is a statistically significant reduction in DFS and OS, while this is not the case in the HER2-negative patient population lacking PTEN staining. In contrast to smaller studies (43), in the HER2-positive group we find no substantial difference in the rate of PTEN loss in ER/PR-positive versus ER/PR-negative cancers. However, among HER2-negative patients, there was a significant association between PTEN loss and ER/PR-negative status. The data in the HER2-negative setting are consistent with published literature indicating that PTEN loss is most common in the triple-negative or basal-like subtype of breast cancer (30–33).
It is important to note that we evaluated PTEN status by immunohistochemistry and any molecular alteration leading to loss of PTEN function other than by reduced protein levels would be missed by an IHC assay. For example, mutations in the PTEN gene are associated with loss of function even though PTEN protein is expressed (45). Although PTEN mutations are common in some cancers such as glioblastomas and endometrial carcinomas, they are infrequent (approximate 3.5%) in breast cancers and have little overlap with HER2 gene amplification (45, 46). Other genetic changes in PTEN such as deletions (including PTEN haplo-insufficiency from loss of heterozygosity {LOH} at the PTEN locus), reduced RNA or protein levels due to transcriptional dysregulation from epigenetic down-modulation of PTEN or increased protein degradation due to increased ubiquitination would be expected to be identified by IHC since these are ultimately mediated through reduced protein expression. Our use of western immunoblot analyses of PTEN expression in a variety of breast cancer cell lines is a useful demonstration that the IHC methods we have employed recognize PTEN in formalin-fixed, paraffin-embedded samples with a high level of both sensitivity and specificity. Although we have used the same commercially available primary anti-PTEN antibody in our IHC as used by others (43), our optimal IHC conditions involved a higher concentration of anti-PTEN antibody (1:20 versus 1:250 dilutions) as well as different antigen retrieval conditions (pH=9.0 versus pH=6.0). These differences in method could be responsible for higher rates of PTEN expression (82.1% for our 1+/2+/3+ combined, Table 2) observed in our study compared to a previous retrospective study of PTEN in specimens from the N9831 trial of adjuvant trastuzumab (74% for 1+/2+/3+ combined)(43). Of note, our study does reach the same conclusion with regard to the lack of a role for PTEN IHC loss (as determined by IHC) in treatment resistance to adjuvant trastuzumab. However, an important difference in the studies is that we find PTEN loss to be associated with significantly worse DFS and OS outcomes in the overall study population irrespective of treatment arm, while this was not observed in the N9831 trial.
We find that absence of PTEN staining is a prognostic factor for poor outcome in the HER2-positive population. However, the evidence from our study as well as N9831 (43) indicates that patients with absence of PTEN expression still exhibit clinical benefit from trastuzumab. This finding suggests that PTEN loss may identify patients who could benefit from additional therapy, but does not identify patients resistant to trastuzumab.
Although an initial prediction was that downstream activation of the PI3K pathway might cause upstream resistance to trastuzumab, there may be a biological rationale to suggest that PI3K pathway activation is not a substitute for HER2-amplification. First, in at least 5% of cases, absent PTEN expression occurs in combination with HER2-amplification without any prior HER2 directed therapy. There is also evidence in the literature that PIK3CA activating mutations occur at a rate of approximately 25%, including in HER2-positive patients (34, 47–51). Because these PI3K pathway molecular alterations are not mutually exclusive with HER2 amplification, it is reasonable to speculate that perhaps they are not entirely redundant, in which case inhibition upstream at the receptor level may still be beneficial despite downstream PI3K pathway activation. Furthermore, PIK3CA activating mutations can up-regulate signaling through HER3, suggesting that the pathway may not be linear, and may involve upstream feedback loops (52). In addition, even in the setting of downstream PI3K activation, trastuzumab may still be able to inhibit signaling through PI3K-independent pathways. For example, there is clear evidence that HER2 also signals through the MAPK pathway (53–55). Finally, the trastuzumab mechanism of action may include the ability to engage immune effector cells and mediate tumor cell destruction via antibody-dependent cellular cytotoxicity (ADCC)(56, 57), a process that may be independent of PI3K pathway signaling.
In summary, we have validated a method for assessing PTEN status by IHC and find that absence of PTEN expression by IHC assay is less common in HER2-amplified compared to HER2-non-amplified disease. Nevertheless, a significant association with poor clinical outcome is only observed in patients with HER2-amplified breast cancers, not in patients with HER2-non-amplified breast cancers. Furthermore, absence of PTEN staining in tumor cells was not associated with resistance to trastuzumab. These data suggest that clinical strategies combining trastuzumab with potent, selective PI3K inhibitors such as GDC-0941 (22, 58) might be beneficial in the treatment of HER2-amplified breast cancer patients with absent PTEN expression.
Supplementary Material
Supplementary Table 1: Nuclear PTEN IHC compared to cytoplasmic PTEN IHC
Diagrammatic representation of the BCIRG 005 (A) and BCIRG 006 (B) adjuvant breast cancer clinical trials.
Kaplan Meier analysis was performed for patients with PTEN data available compared to patients without PTEN data available. Disease free survival (A, C) and overall survival (B, D) are shown for BCIRG 006 (HER2-amplified) (A, B) and BCIRG 005 (HER2-non-amplified) (C, D).
PTEN IHC was performed on a panel of FFPE breast cancer cell pellets of known PTEN status confirmed by western immunoblot. (A.) Western immunoblot analyses for MDA-MB-468, CAL-148, MDA-MB-361, MT-3, and MDA-MB-436 human breast cancer cell lines are illustrated above with PTEN IHC illustrated below; (B.) Comparison of western immunoblot results and PTEN IHC in CAL-51, MDA-MB-451, HCC-1954, ZR-75-1, MCF7, CAL-120, and BT474 human breast cancer cell lines; and (C.) Comparison of western immunoblot results and PTEN IHC in EFM-19, CAMA-1, MDA-MB-415, MFM-223, HCC-1569, EFM192A, KPL-1, and BT549 human breast cancer cell lines.
A. Hematoxylin-and-eosin stained section of human prostate shows a focus of early prostatic adenocarcinoma. B. A serial section shows the same microscopic field after processing for PTEN localization by immunohistochemistry. Note the lack of immunostaining in the tumor cells. C. QD-FISH for PTEN gene and chromosome 10 centromere demonstrate a loss of the PTEN gene in tumor cells.
Statement of Translational Relevance.
The humanized anti-HER2 monoclonal antibody trastuzumab provides clinical benefit for patients with HER2-positive breast cancer. Despite this proven efficacy, some patients with HER2-positive breast cancer exhibit intrinsic resistance to trastuzumab, and, in the metastatic setting many develop acquired resistance. While loss of PTEN (phosphatase and tensin homolog) expression is hypothesized to be a trastuzumab resistance factor, clinical studies to date have been largely limited to small metastatic cohorts. This study explores PTEN status in a large adjuvant population consisting of 1201 HER2-amplified and 1163 HER2-non-amplified breast cancer patients. The data demonstrate that the rate and significance of PTEN loss differs between HER2-amplified and HER2-non-amplified cancers. Furthermore, absence of PTEN staining was a poor prognostic indicator in HER2-amplified disease but was not predictive of trastuzumab resistance. These findings suggest that a combination of trastuzumab plus PI3K pathway inhibitors may be effective in HER2-amplified patients whose breast cancers lack PTEN staining.
Acknowledgments
The authors would like to thank Xander Munroe (Genentech, Inc.) for his expert technical assistance. C. O’Brien and M.R. Lackner are current employees of Genentech, Inc. H.M. Stern was an employee of Genentech Research and Early Development and a shareholder in Roche Holding AG at the time the work was completed. G. Pistano was an employee of Ventana Medical Systems, Inc. and a shareholder in Roche Holding AG at the time the work was completed. H.M. Stern is currently an employee and a shareholder of Infinity Pharmaceuticals. G. Pistano is currently an employee and shareholder in Biodesix, Inc., 2970 Wilderness Place, Boulder, CO. H. Gardner was an employee of Novartis at the time the work was completed and is currently an employee of AstraZeneca. M.F. Press was supported in part by a grant from “The Breast Cancer Research Foundation”, California Breast Cancer Research Program (14NB-0179), Entertainment Industry Foundation and Genentech Inc./F. Hoffmann- La Roche Ltd.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. Epub 1987/01/09. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12. doi: 10.1126/science.2470152. Epub 1989/05/12. [DOI] [PubMed] [Google Scholar]
- 3.Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA, et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10(9):1813–21. Epub 1995/05/04. [PubMed] [Google Scholar]
- 4.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. Embo J. 2000;19(13):3159–67. doi: 10.1093/emboj/19.13.3159. Epub 2000/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern HM. EGFR family heterodimers in cancer pathogenesis and treatment. In: Gullick JDHaW., editor. EGFR signaling networks in cancer therapy. Humana Press; 2008. [Google Scholar]
- 6.Boccaccio C, Gaudino G, Cilli M, Mondino A, Comoglio PM. Ligand-independent tyrosine phosphorylation of the receptor encoded by the c-neu oncogene. Growth Factors. 1991;5(3):233–42. doi: 10.3109/08977199109000287. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 7.Penuel E, Akita RW, Sliwkowski MX. Identification of a region within the ErbB2/HER2 intracellular domain that is necessary for ligand-independent association. J Biol Chem. 2002;277(32):28468–73. doi: 10.1074/jbc.M202510200. Epub 2002/05/10. [DOI] [PubMed] [Google Scholar]
- 8.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100(15):8933–8. doi: 10.1073/pnas.1537685100. Epub 2003/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68(14):5878–87. doi: 10.1158/0008-5472.CAN-08-0380. Epub 2008/07/18. [DOI] [PubMed] [Google Scholar]
- 10.Hellyer NJ, Cheng K, Koland JG. ErbB3 (HER3) interaction with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem J. 1998;333 (Pt 3):757–63. doi: 10.1042/bj3330757. Epub 1998/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones RB, Gordus A, Krall JA, MacBeath G. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature. 2006;439(7073):168–74. doi: 10.1038/nature04177. Epub 2005/11/08. [DOI] [PubMed] [Google Scholar]
- 12.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. Epub 2001/03/15. [DOI] [PubMed] [Google Scholar]
- 13.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. New England Journal of Medicine. 2006;355(26):2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 14.Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. Journal of Clinical Oncology. 1997;15(8):2894–904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 15.Press MF, Pike MC, Chazin VR, Hung G, Udove JA, Markowicz M, et al. Her-2/neu expression in node-negative breast cancer: direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res. 1993;53(20):4960–70. [PubMed] [Google Scholar]
- 16.Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. The lancet oncology. 2013;14(6):461–71. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. doi: 10.1016/S0140-6736(10)61121-X. Epub 2010/08/24. [DOI] [PubMed] [Google Scholar]
- 19.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. Epub 2005/10/21. [DOI] [PubMed] [Google Scholar]
- 20.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. Epub 2005/10/21. [DOI] [PubMed] [Google Scholar]
- 21.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83. doi: 10.1056/NEJMoa0910383. Epub 2011/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15(5):429–40. doi: 10.1016/j.ccr.2009.03.020. Epub 2009/05/05. [DOI] [PubMed] [Google Scholar]
- 23.Esteva FJ, Guo H, Zhang S, Santa-Maria C, Stone S, Lanchbury JS, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177(4):1647–56. doi: 10.2353/ajpath.2010.090885. Epub 2010/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esteva FJ, Yu D, Hung MC, Hortobagyi GN. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010;7(2):98–107. doi: 10.1038/nrclinonc.2009.216. Epub 2009/12/23. [DOI] [PubMed] [Google Scholar]
- 25.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21(2):177–84. doi: 10.1016/j.ceb.2008.12.010. Epub 2009/02/12. [DOI] [PubMed] [Google Scholar]
- 26.Eiermann W, Pienkowski T, Crown J, Sadeghi S, Martin M, Chan A, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. Journal of Clinical Oncology. 2011;29(29):3877–84. doi: 10.1200/JCO.2010.28.5437. Epub 2011/09/14. [DOI] [PubMed] [Google Scholar]
- 27.Press MF, Sauter G, Buyse M, Bernstein L, Guzman R, Santiago A, et al. Alteration of topoisomerase II-alpha gene in human breast cancer: association with responsiveness to anthracycline-based chemotherapy. Journal of Clinical Oncology. 2011;29(7):859–67. doi: 10.1200/JCO.2009.27.5644. Epub 2010/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zha J, O’Brien C, Savage H, Huw LY, Zhong F, Berry L, et al. Molecular predictors of response to a humanized anti-insulin-like growth factor-I receptor monoclonal antibody in breast and colorectal cancer. Mol Cancer Ther. 2009;8(8):2110–21. doi: 10.1158/1535-7163.MCT-09-0381. Epub 2009/08/13. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9(6):1489–502. doi: 10.1158/1535-7163.MCT-09-1171. Epub 2010/05/27. [DOI] [PubMed] [Google Scholar]
- 30.Dourdin N, Schade B, Lesurf R, Hallett M, Munn RJ, Cardiff RD, et al. Phosphatase and tensin homologue deleted on chromosome 10 deficiency accelerates tumor induction in a mouse model of ErbB-2 mammary tumorigenesis. Cancer Res. 2008;68(7):2122–31. doi: 10.1158/0008-5472.CAN-07-5727. Epub 2008/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu X, Stern HM, Ge L, O’Brien C, Haydu L, Honchell CD, et al. Genetic alterations and oncogenic pathways associated with breast cancer subtypes. Molecular Cancer Research. 2009;7(4):511–22. doi: 10.1158/1541-7786.MCR-08-0107. Epub 2009/04/18. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Knowles E, O’Toole SA, McNeil CM, Millar EK, Qiu MR, Crea P, et al. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. International Journal of Cancer. 2010;126(5):1121–31. doi: 10.1002/ijc.24831. Epub 2009/08/18. [DOI] [PubMed] [Google Scholar]
- 33.Saal LH, Gruvberger-Saal SK, Persson C, Lovgren K, Jumppanen M, Staaf J, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40(1):102–7. doi: 10.1038/ng.2007.39. Epub 2007/12/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. Epub 2007/10/16. [DOI] [PubMed] [Google Scholar]
- 35.Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68(22):9221–30. doi: 10.1158/0008-5472.CAN-08-1740. Epub 2008/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–27. doi: 10.1016/j.ccr.2004.06.022. Epub 2004/08/25. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien C, Wallin JJ, Sampath D, GuhaThakurta D, Savage H, Punnoose EA, et al. Predictive biomarkers of sensitivity to the phosphatidylinositol 3′ kinase inhibitor GDC-0941 in breast cancer preclinical models. Clinical Cancer Research. 2010;16(14):3670–83. doi: 10.1158/1078-0432.CCR-09-2828. Epub 2010/05/11. [DOI] [PubMed] [Google Scholar]
- 38.De P, Hasmann M, Leyland-Jones B. Molecular determinants of trastuzumab efficacy: What is their clinical relevance? Cancer treatment reviews. 2013;39(8):925–34. doi: 10.1016/j.ctrv.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497–510. doi: 10.1038/onc.2008.245. Epub 2008/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabi A, Metro G, Di Benedetto A, Nistico C, Vici P, Melucci E, et al. Clinical significance of PTEN and p-Akt co-expression in HER2-positive metastatic breast cancer patients treated with trastuzumab-based therapies. Oncology. 2010;78(2):141–9. doi: 10.1159/000312656. Epub 2010/04/15. [DOI] [PubMed] [Google Scholar]
- 41.Fujita T, Doihara H, Kawasaki K, Takabatake D, Takahashi H, Washio K, et al. PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br J Cancer. 2006;94(2):247–52. doi: 10.1038/sj.bjc.6602926. Epub 2006/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gori S, Sidoni A, Colozza M, Ferri I, Mameli MG, Fenocchio D, et al. EGFR, pMAPK, pAkt and PTEN status by immunohistochemistry: correlation with clinical outcome in HER2-positive metastatic breast cancer patients treated with trastuzumab. Annals of Oncology. 2009;20(4):648–54. doi: 10.1093/annonc/mdn681. Epub 2009/02/04. [DOI] [PubMed] [Google Scholar]
- 43.Perez EA, Dueck AC, McCullough AE, Chen B, Geiger XJ, Jenkins RB, et al. Impact of PTEN protein expression on benefit from adjuvant trastuzumab in early-stage human epidermal growth factor receptor 2-positive breast cancer in the North Central Cancer Treatment Group N9831 trial. Journal of Clinical Oncology. 2013;31(17):2115–22. doi: 10.1200/JCO.2012.42.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faratian D, Goltsov A, Lebedeva G, Sorokin A, Moodie S, Mullen P, et al. Systems biology reveals new strategies for personalizing cancer medicine and confirms the role of PTEN in resistance to trastuzumab. Cancer Res. 2009;69(16):6713–20. doi: 10.1158/0008-5472.CAN-09-0777. Epub 2009/07/30. [DOI] [PubMed] [Google Scholar]
- 45.Rexer BN, Shyr Y, Arteaga CL. Phosphatase and tensin homolog deficiency and resistance to trastuzumab and chemotherapy. Journal of Clinical Oncology. 2013;31(17):2073–5. doi: 10.1200/JCO.2012.48.5243. [DOI] [PubMed] [Google Scholar]
- 46.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64(21):7678–81. doi: 10.1158/0008-5472.CAN-04-2933. Epub 2004/11/03. [DOI] [PubMed] [Google Scholar]
- 48.Li SY, Rong M, Grieu F, Iacopetta B. PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res Treat. 2006;96(1):91–5. doi: 10.1007/s10549-005-9048-0. Epub 2005/12/01. [DOI] [PubMed] [Google Scholar]
- 49.Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65(7):2554–9. doi: 10.1158/0008-5472-CAN-04-3913. Epub 2005/04/05. [DOI] [PubMed] [Google Scholar]
- 50.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. doi: 10.1126/science.1096502. Epub 2004/03/16. [DOI] [PubMed] [Google Scholar]
- 51.Wu G, Xing M, Mambo E, Huang X, Liu J, Guo Z, et al. Somatic mutation and gain of copy number of PIK3CA in human breast cancer. Breast Cancer Res. 2005;7(5):R609–16. doi: 10.1186/bcr1262. Epub 2005/09/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chakrabarty A, Rexer BN, Wang SE, Cook RS, Engelman JA, Arteaga CL. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene. 2010;29(37):5193–203. doi: 10.1038/onc.2010.257. Epub 2010/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amin DN, Sergina N, Ahuja D, McMahon M, Blair JA, Wang D, et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci Transl Med. 2010;2(16):16ra7. doi: 10.1126/scitranslmed.3000389. Epub 2010/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vijapurkar U, Cheng K, Koland JG. Mutation of a Shc binding site tyrosine residue in ErbB3/HER3 blocks heregulin-dependent activation of mitogen-activated protein kinase. J Biol Chem. 1998;273(33):20996–1002. doi: 10.1074/jbc.273.33.20996. Epub 1998/08/08. [DOI] [PubMed] [Google Scholar]
- 55.Vijapurkar U, Kim MS, Koland JG. Roles of mitogen-activated protein kinase and phosphoinositide 3′-kinase in ErbB2/ErbB3 coreceptor-mediated heregulin signaling. Exp Cell Res. 2003;284(2):291–302. doi: 10.1016/s0014-4827(02)00040-x. Epub 2003/03/26. [DOI] [PubMed] [Google Scholar]
- 56.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–6. doi: 10.1038/74704. Epub 2000/03/31. [DOI] [PubMed] [Google Scholar]
- 57.Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, et al. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res. 2010;70(11):4481–9. doi: 10.1158/0008-5472.CAN-09-3704. Epub 2010/05/21. [DOI] [PubMed] [Google Scholar]
- 58.Yao E, Zhou W, Lee-Hoeflich ST, Truong T, Haverty PM, Eastham-Anderson J, et al. Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clinical Cancer Research. 2009;15(12):4147–56. doi: 10.1158/1078-0432.CCR-08-2814. Epub 2009/06/11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Nuclear PTEN IHC compared to cytoplasmic PTEN IHC
Diagrammatic representation of the BCIRG 005 (A) and BCIRG 006 (B) adjuvant breast cancer clinical trials.
Kaplan Meier analysis was performed for patients with PTEN data available compared to patients without PTEN data available. Disease free survival (A, C) and overall survival (B, D) are shown for BCIRG 006 (HER2-amplified) (A, B) and BCIRG 005 (HER2-non-amplified) (C, D).
PTEN IHC was performed on a panel of FFPE breast cancer cell pellets of known PTEN status confirmed by western immunoblot. (A.) Western immunoblot analyses for MDA-MB-468, CAL-148, MDA-MB-361, MT-3, and MDA-MB-436 human breast cancer cell lines are illustrated above with PTEN IHC illustrated below; (B.) Comparison of western immunoblot results and PTEN IHC in CAL-51, MDA-MB-451, HCC-1954, ZR-75-1, MCF7, CAL-120, and BT474 human breast cancer cell lines; and (C.) Comparison of western immunoblot results and PTEN IHC in EFM-19, CAMA-1, MDA-MB-415, MFM-223, HCC-1569, EFM192A, KPL-1, and BT549 human breast cancer cell lines.
A. Hematoxylin-and-eosin stained section of human prostate shows a focus of early prostatic adenocarcinoma. B. A serial section shows the same microscopic field after processing for PTEN localization by immunohistochemistry. Note the lack of immunostaining in the tumor cells. C. QD-FISH for PTEN gene and chromosome 10 centromere demonstrate a loss of the PTEN gene in tumor cells.