Abstract
Background
Detecting behaviors related to orofacial pain in rodent models often relies on subjective investigator grades or methods that place the animal in a stressful environment. In this study, an operant-based behavioral assay is presented for the assessment of orofacial tactile sensitivity in the rat.
New Methods
In the testing chamber, rats are provided access to a sweetened condensed milk bottle; however, a 360° array of stainless steel wire loops impedes access. To receive the reward, an animal must engage the wires across the orofacial region. Contact with the bottle triggers a motor, requiring the animal to accept increasing pressure on the face during the test. To evaluate this approach, tolerated bottle distance was measured for 10 hairless Sprague-Dawley rats at baseline and 30 minutes after application of capsaicin cream (0.1%) to the face. The experiment was repeated to evaluate the ability of morphine to reverse this effect.
Results
The application of capsaicin cream reduced tolerated bottle distance measures relative to baseline (p<0.05). As long as morphine did not cause reduced participation due to sedation, subcutaneous morphine dosing reduced the effects of capsaicin (p<0.001).
Comparison with Existing Method
For behavioral tests, experimenters often make subjective decisions of an animal’s response. Operant methods can reduce these effects by measuring an animal’s selection in a reward-conflict decision. Herein, a method to measure orofacial sensitivity is presented using an operant system.
Conclusions
This operant device allows for consistent measurement of heightened tactile sensitivity in the orofacial regions of the rat.
Keywords: Orofacial, Allodynia, Operant, Behavior, Pain
1. Introduction
Orofacial pain can be caused by multiple disorders, including myofascial pain, headaches, temporomandibular joint disorders, and trigeminal neuralgia; combined, these conditions affect more than 20% of Americans (Lipton et al., 1993). Many orofacial pain conditions have overlapping symptomatic presentations, with symptoms including heightened sensitivity to temperature and touch in the orofacial region. This orofacial hypersensitivity to thermal and mechanical stimuli can be replicated in rodent orofacial pain models (Krzyzanowska and Avendaño, 2012; Kupers, 2001). Unfortunately, rodent orofacial pain models that are used to test emerging pain therapeutics are commonly evaluated with behavioral methods that have limited sensitivity and can be prone to experimenter bias. Accurate quantification of pain-related behaviors in rodent orofacial pain models is crucial for the preclinical evaluation of potential therapeutics and analgesics (Krzyzanowska and Avendaño, 2012).
Animal behavioral analyses can be used to assess the physical and symptomatic consequences of disease in the rodent. Behavioral assessments of orofacial pain in animals commonly include measuring pain-related response to heat (thermal hyperalgesia) or withdrawal response from the application of von Frey filaments (tactile allodynia). In addition, exploratory field behavior, freezing-like behavior, resting/sleeping time, grooming behavior, thigmotactic scanning, and dietary tracking may also be related to behavioral consequences of orofacial pain in animal models (Castonguay et al., 1986; Deseure and Adriaensen, 2002; Kerins et al., 2003; Kramer et al., 2012; Vos and Strassman, 1994). However, determining the sensitivity of the orofacial regions of a rodent can be difficult using some common methods. As an example, fixed stimulus and investigator driven devices can be used to determine tolerance thresholds in the orofacial region based on animal reaction to stimuli (Imamura et al., 1997; Krzyzanowska et al., 2011; Morris et al., 1982; Rosenfeld et al., 1978; Vos and Strassman, 1994); however, variations in reaction scoring criteria between experimenters, animal stress due to handling and restraint, and differences in protocols can limit the sensitivity of these methods and confound the results (Chesler et al., 2002; Hogan et al., 2004; Lambert et al., 2009).
Operant behavioral tests can combine the unobtrusive nature of observational tests with quantifiable thresholds to various stimuli (Mauderli et al., 2000). Existing operant tests for orofacial nociception include dietary and meal duration changes, as well as a device called the dolognawmeter that quantifies length of time to chew through plastic dowels (Dolan et al., 2010; Kerins et al., 2003; Kramer et al., 2012). These devices can describe pain-related behaviors in a variety of induced orofacial nociception models, but do not provide a withdrawal threshold associated with surface nociception similar to von Frey filament protocols. A commonly used operant test design uses a reward-conflict paradigm, in which the animal must accept a stimulus to receive a reward. Through the measurement of an animal’s participation, a threshold to the applied stimulus can be determined. Similar operant tests have previously been validated to describe thermal sensitivity (Neubert et al., 2005), and first-generation operant tests of tactile sensitivity have also been prototyped (Nolan et al., 2011). The primary output of these operant tests of orofacial sensitivity is bottle contact time, which is shown to be effective in differentiating naïve and experimental animals. In addition, facial contact with the stimulus and lick/pain indices can be constructed from these tests. However, this design is unable to quantify individual animal withdrawal thresholds. Additionally, in early prototype design, the animal could unequally apply the mechanical stimulus to the orofacial regions by tilting its head.
In this paper, an operant-based test is described for the detection of mechanical sensitivity in the orofacial region of rats. This device allows animals to participate independent of the experimenter, detecting rodent pain-related behaviors through a reward-conflict paradigm (Neubert et al., 2005). Our approach improves prior operant measures of tactile sensitivity in the orofacial region through two keys aspects: First, the stimulus required to receive the reward is increased during a trial, requiring the animal to tolerate increased mechanical force on the face to continue to receive the reward. Second, animal avoidance measures are minimized by incorporating a 360° array of stimulus that cannot be avoided by an animal making contact with the reward bottle. Increasing stimulus and minimizing avoidance errors allows for effective measurement of behavioral changes associated with surface nociception. To validate this approach, pain-related behaviors are quantified through individual animal’s tolerance for the mechanical stimulus under conditions of pain and analgesia. This approach could be used to detect changes due to induced pain or the efficacy of an analgesic and can provide a highly sensitive behavior method to screen the efficacy of emerging orofacial drugs and therapeutics.
2. Materials and Methods
2.1 Operant-based test of mechanical orofacial sensitivity
The testing procedures and general handling of animals described herein were approved by the Institutional Animal Care & Use Committee at the University of Florida and are in compliance with the ethical guidelines and standards established by the Guide for Care and Use of Laboratory Animals (National Research Council, 2011).
2.1.1 Device and function
An orofacial sensitivity device was designed to function on a reward-conflict paradigm, similar to that of previous operant devices (Mauderli et al., 2000; Neubert et al., 2005). The device consisted of a 7.5″×7.75″×5.75″ acrylic testing chamber with a 2.185″×2″ window and removable metal floor. A 360° array of looped 0.010″ diameter stainless steel wire with a 0.7″ opening at the center of the array was used to partially block the window (Figure 1a). A reward bottle filled with diluted sweetened condensed milk (2 Water: 1 Sweetened Condensed Milk, Nestlé, La Lechera) was placed inside the window, such that the 360° array of wires would impart a mechanical stimulus to the orofacial regions of a rat drinking from the reward bottle (Figure 1b). Initially, the reward bottle nozzle sits inside the array, such that initial contact with the bottle does not require contact with the mechanical stimulus. Contact with the reward bottle completes an electric circuit between the cage floor, animal, and bottle, allowing for the measurement of contact time (100Hz, custom LabVIEW 2013 module, National Instruments). In addition, continuous contact with the reward bottle, defined as 15 out of 30 milliseconds of contact time, initiates a stepper motor underneath the cage floor to move the bottle further from its initial position at a rate of 5.0 inches/minute (Figure 1c). This movement of the water bottle slowly increases the distance between the reward bottle and cage, requiring the animal to tolerate more mechanical force to continue to receive the reward. The bottle circuit remains dynamic for a period of 2 minutes, after which point the tolerated bottle distance is recorded and the bottle is automatically returned to its starting position. If the bottle position has changed from its initial position during a two minute time period, the period is considered a drinking event and indicates participation.
Figure 1. An operant device for the detection of orofacial sensitivity to mechanical stimuli.
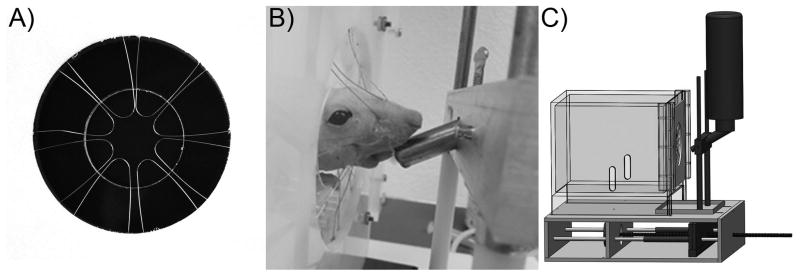
To engage with a reward bottle filled with sweetened condensed milk, an animal must engage with a 360° degree array of 0.010″ diameter looped stainless steel wires. This design minimizes error due to animal avoidance behavior (A). When the animal engages the array (B), a motor and threaded rod moves the reward bottle assembly farther from the cage (C). The animal must continue to move its head through mechanical stimulus wires to receive the reward bottle.
2.1.2 Testing protocol
Animals were food fasted for 12–15 hr (overnight) prior to testing. The following morning, animals were placed in the acrylic testing chamber described in 2.1.1. When the animal made contact with the reward bottle, bottle movement was controlled and recorded as described in 2.1.1. The process was repeated until five measurements of tolerated bottle distance were collected or until 10 minutes of testing time had elapsed. Average tolerance distance was calculated on each testing day for each animal. At the end of this study, animals were transferred to an IACUC-approved experiment unrelated to the present study.
2.1.3 Training period
Acclimation to the testing chamber was conducted three times per week for two weeks with unhindered access to the reward bottle. On week 3 of acclimation, a 0.9″ aperture array was placed in front of the water bottle; testing continued three times per week with the maximum stop distance to the reward bottle increased from 0.0″, to 0.25″, then finally 0.5″. On week 4 of acclimation, the aperture was reduced to 0.7″ and the maximum stop distance to the reward bottle again increased 0.0″ to 0.25″ to 0.5″. On week 5 of acclimation, animals were allowed follow the reward bottle to their maximum threshold.
2.2 Capsaicin induced orofacial pain
Ten female hairless Sprague-Dawley rats (250–350g) were acquired from Charles River Laboratories and trained as described in 2.1.3. Following training, animals were anesthetized using isoflurane (1.0–2.5%, inhalation); then, capsaicin cream (0.1%, Capzacin-HP) was applied liberally on the skin covering the masseter up to the vibrissae, as described previously (Neubert et al., 2006). Heat was applied to the area treated with capsaicin with a warm water therapy pad (107°F). After 5 minutes, excess capsaicin was removed with a damp paper towel, and animals were allowed to recover for 30 minutes prior to mechanical sensitivity testing.
Orofacial mechanical sensitivity tests described in 2.1.2 were performed 1 week prior to capsaicin testing (Baseline), with capsaicin on Day 0 (Capsaicin #1), without capsaicin on Day 3 (Baseline #2), with capsaicin on Day 7 (Capsaicin #2), without capsaicin on Day 10 (Baseline #3), with capsaicin on Day 14 (Capsaicin #3), and then without capsaicin on Day 16 (Baseline #4), effectively separating capsaicin applications by 7 days. Unfortunately, a loose wire contact resulted in a device malfunction for Baseline #2. The loose contact was discovered and re-soldered after 4 rats had been tested, but as a result of the malfunction, data for 4 of the 10 rats were excluded from the analysis of the data on Baseline #2. Data on capsaicin effects were analyzed using a main effects analysis of variance (ANOVA) accounting for both treatment effects and an animal identifier. Post-hoc analyses included a Tukey’s HSD test to identify difference between testing days and a Bonferroni corrected Student’s t-test to investigate capsaicin effects within a specific animal (corrected for 10 planned comparisons).
2.3 Capsaicin and morphine administration
Animals were allowed to recover for two weeks after the capsaicin experiment described in 2.2. Then, animals received a subcutaneous bolus of morphine 15 minutes prior to the capsaicin application procedure (n=9, Sprague Dawley, 250–350g). Morphine doses included 0.1, 0.25, 0.5, 1, 5, 10, 15, 20 and 25 mg/kg. This experiment was repeated once per week for a period of 4 weeks, wherein animals randomly received one of the above doses over 4 weeks of testing (n=4 per morphine dose). Since Animal #7 did not show an effect to capsaicin in the experiment described in 2.2 (See Figure 2c in the Results), this animal was not included in the morphine study.
Figure 2. Reward bottle tolerance distance with and without the application of capsaicin cream to the orofacial regions of a Sprague-Dawley rat.
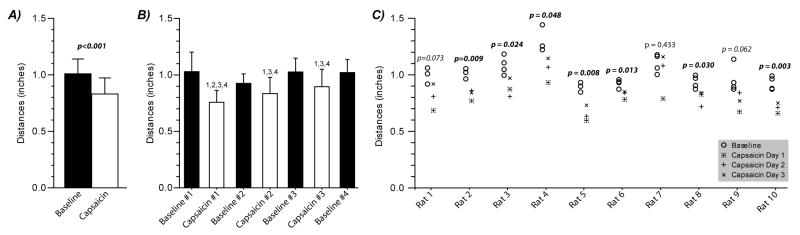
Following the application of capsaicin cream to the orofacial region of a rat’s face, reward bottle tolerance distances were reduced (Panel A, p-value associated with capsaicin treatment from 2-way Main-Effects ANOVA). Panel B shows that each application of capsaicin resulted in reduced tolerance distance relative to Baseline Day 1 (indicated by a “1”), Baseline Day 3 (indicated by a “3”), and Baseline Day 4 (indicated by a “4”); only the first application of capsaicin was different from Baseline Day 2 (indicated by a “2”, Tukey’s HSD post-hoc test). Data presented in Panel C demonstrate changes in individual animal tolerance distance resulting from capsaicin. Capsaicin application had a significant effect on tolerance distance in 7 of 10 animals (p<0.05, paired t-test) and was near significant in an additional 2 animals (0.05<p<0.10). With the exception of Animal 7, the application of capsaicin to the orofacial regions of the animal’s face consistently reduced an animal’s tolerance distance in our operant device of orofacial mechanical sensitivity. Data in Panel A and B are presented as mean ± standard deviation; data in Panel C are the average tolerance distance for a testing day based on 5 trials.
Data for morphine effects were first analyzed using a nested ANOVA design; however, because sedative effects were identified for some morphine doses (5, 15, 20, and 25 mg/kg), the ANOVA was reevaluated using only animals that fully participated in the test. Provided a significant main effect was identified, a post-hoc Tukey’s HSD test was conducted to identify difference between groups and between morphine doses.
3. Results
3.1 Tolerated bottle distance in capsaicin-induced orofacial pain model
Application of capsaicin cream significantly reduced the distance animals were willing to push through mechanical stimulus for the reward (Figure 2a). While the magnitude of this change reduced over testing day, the resulting drop in tolerated bottle distance was significant relative to Baseline #1, #3, and #4 for each application of capsaicin (Figure 2b). Baseline #2 was significantly different from the first application of capsaicin #1, but not the second and third application, due in part to the reduced data set for this testing day (n=6, rather than n=10). Moreover, the baseline tolerated bottle distance was consistent across Baseline #1, #2, #3, and #4, indicating that the change between Capsaicin #1, #2, and #3 is likely due to a change in response to capsaicin and not a training effect.
Importantly, the drop in tolerated bottle distance was largely consistent in the animals tested, with capsaicin reducing the tolerated bottle distance by 0.175 ± 0.041 inches (mean ± standard deviation). The effect of capsaicin on the tolerated bottle distances was significant in 7 of the 10 animals (p<0.05), and shifts in an additional 2 animals demonstrated a similar non-significant tendency (0.05<p<0.10, Figure 2c). These data indicate the testing procedure is able to provide a consistent behavioral shift across the animals tested.
3.2 Morphine attenuation of capsaicin-induced orofacial pain
The effects of capsaicin treatment in conjunction with morphine are shown in Figure 3. Morphine doses above 15 mg/kg caused a sedative effect in most animals, reducing participation in the test (Figure 3a, see x’s and dotted line). As a result of this morphine sedation, animals treated with 15, 20, and 25 mg/kg morphine had lower tolerated bottle distances than baseline, and animals treated with 20 and 25 mg/kg morphine were lower than all other groups (p <0.001, Figure 3a).
Figure 3. Morphine attenuation of capsaicin-induced orofacial hypersensitivity.
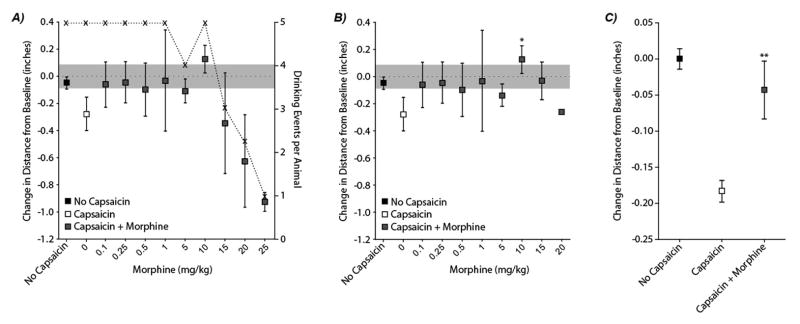
Average tolerated bottle distances are shown in Figure 3, Panel A. At high morphine doses, animals reduced their participation in the test (see x’s and dotted line). At 5 mg/kg, 3 of 4 animals fully participated, while 2 of 4 animals fully participated at 15 mg/kg and 1 of 4 animals fully participated at 20 mg/kg. No animal fully participated when receiving 25 mg/kg. In Panel B, trials where animals failed to fully participate are removed. With this exclusion, treatment with morphine demonstrated an ability to reduce the effects of capsaicin application to the orofacial regions (p<0.001, main effect in ANOVA, Panel C). While all morphine doses visually appear to reduce the shift in tolerated bottle distance to near baseline levels (grey squares are closer to zero than the white square), only morphine doses of 10 mg/kg resulted significant changes in bottle distance relative to no morphine treatment controls (*, p<0.001, Panel B).
To evaluate the ability of morphine to reverse the effects of capsaicin, animals that did not fully participate in the behavioral test were excluded from the statistical analysis (See Figure 3b). With this exclusion, treatment with morphine demonstrated an ability to reduce the effects of capsaicin application to the orofacial regions (p<0.001, main effect in ANOVA, Figure 3c). While all morphine doses appear to reduce the shift in tolerated bottle distance to near baseline levels, only morphine doses of 10 mg/kg resulted significant changes in bottle distance relative to no morphine treatment controls (*, p<0.001).
4. Discussion
Operant testing combines the unobtrusive nature of observational testing with the quantitative nature of threshold testing. Here, we describe a device to measure an animal’s sensitivity to orofacial mechanical stimuli through operant testing methods. Our device successfully captured a decrease in tolerated bottle distance between no treatment and capsaicin treated animals. This significant decrease can be associated with heightened sensitivity related to the application of capsaicin (p<0.05). Moreover, trials without capsaicin treatment (baseline trials) were consistent across experiments, if trials that are associated with decreased participation due to the sedative effects of morphine are excluded from the statistical analysis, the effects of capsaicin application were significantly reduced by morphine treatment. When placed in the context of individual animals, the response to capsaicin treatment was reasonably robust, with 9 of 10 animals showing significant (p<0.05) or near-significant (0.05<p<0.10) responses to capsaicin application over 3 repeated trials.
To validate the efficacy of our proposed device, several orofacial pain models were considered, including nerve injury (Bennett and Xie, 1988; Jeon et al., 2012; Vos and Strassman, 1994) and neurogenic inflammation (Okumura et al., 2010; Pelissier et al., 2002; Poh et al., 2009; Ro et al., 2009, 2007; Xu et al., 2010). Of these, capsaicin injections are particularly useful in modeling temporary heightened nociception, as sensitivity decreases within a few hours, allowing for subsequent baseline testing several days after experimental testing. Capsaicin, an ingredient in hot peppers, binds to vanilloid receptors (TRPV1) in afferent nerve endings, which excites C-fiber nociceptors causing a burning sensation, mechanical hyperalgesia, and thermal hyperalgesia (Boudreau et al., 2009; Caterina and Julius, 2001; Caterina et al., 1997; Gilchrist et al., 1996; LaMotte et al., 1991; Park et al., 1995; Simone et al., 1989; Wasner et al., 1999). Acute application of capsaicin increases heat sensitivity, detected by a decreased thermal sensitivity threshold, while repeated chronic exposure to capsaicin decreases heat sensitivity (Fuchs et al., 2000; Simone and Ochoa, 1991). At low doses, topical capsaicin cream application to the facial area has also been shown to produce repeatable, rescindable orofacial sensitivity; this response is similar to capsaicin injection, but removes potential irritation at the injection site caused by the fluid bolus (Kupers et al., 1997; Neubert et al., 2006). Moreover, the heightened sensitivity observed using the capsaicin cream application closely resembles the decrease in orofacial sensitivity thresholds, both thermal and mechanical, in similar models (Neubert et al., 2006, 2005). As such, we selected a capsaicin cream model to validate our device. In addition, the behaviors associated with capsaicin cream application are also likely to be more subtle than multiple other orofacial pain models; and as such, we anticipate the effective change in tolerated bottle distance will be larger in many other orofacial pain models. However, it should be noted that capsaicin application to the skin will sensitize surface nociceptors, and it is not yet clear whether this technique will demonstrate similar utility for nociceptive changes in deeper tissues, such as those of the temporomandibular joint.
This experiment was restricted to female hairless Sprague Dawley animals. This strain and gender was selected based upon prior work investigating the thermal sensitivity of rats, where hairless rat strains are advantageous for the detection of facial contact with the stimulus (Neubert et al., 2005). The methods described herein should be capable of investigating the orofacial sensitivities in other rodent genders and strains; however, as with most tests of rodent behavior, different responses should be anticipated between male and female rats and for rats of different strains (LaCroix-Fralish et al., 2005; Mogil et al., 2000).
The analgesic effect of morphine has been demonstrated in several other pain models, where heightened mechanical and thermal sensitivity is reduced within one hour of morphine injections in other pain models (Neubert et al., 2006; Nolan et al., 2011; Rosenfeld and Stocco, 1981; Saloman et al., 2011). Our data demonstrate behaviors measured by our device can be modulated by morphine; however, it is important to note that opiates, as well as opiates in conjunction with food deprivation, may affect food intake (Sanger and McCarthy, 1980). In addition to motivation for food, opiates can cause anesthetic responses. This effect was seen in our data for morphine doses of 15 mg/kg or higher; at these morphine levels, animal participation in the test decreased. As such, the changes in tolerated bottle distance caused by morphine could be alternatively related to changes in feeding behavior and not specifically the attenuation of orofacial sensitivity.
Separating behavioral changes due to nociception, feeding, and anesthetic/sedative responses to a therapeutic is challenging, but tracking food consumption in addition to the bottle distance measures detected by our device may help to decipher these interacting behaviors. Milk consumption was not measured in this study. Moreover, because the motor speed was held constant in this study, bottle distance and bottle contact time were not independent measures. However, varying the motor speed while measuring bottle distance, contact time, and milk consumption with our device could help to decipher nociceptive, feeding, and sedative effects in future testing of opiate-based and other pain relieving drugs. Nonetheless, the application of capsaicin is not considered to be an appetite suppressor; and while the reversal of changes in tolerated bottle distance due to morphine may be explained by competing behaviors, changes in tolerated bottle distance independent of morphine application shown in Figure 2 are still highly likely to be due to surface nociception changes in the facial region.
While statistically non-significant on dose basis, morphine doses from 0.1–5.0 mg/kg were near baseline levels for tolerated bottle distance. Our experiment was not designed to identify an effective morphine dose, but was instead designed to validate a behavioral testing method. These results align, to some degree, with our previous work on orofacial thermal sensitivity following application of capsaicin cream, where morphine doses of 0.5–2 mg/kg were shown to be effective (Neubert et al., 2005). However, to determine an effective morphine dose, larger sample sizes, testing across multiple rat strains, and different animal models with larger tolerated bottle distance effect sizes are necessary.
Measuring behavioral responses in individual animals can increase the sensitivity of the behavioral device by accounting for inter-subject variability. While traditional von Frey testing can also estimate inter-subject variability in principal (Gilchrist et al., 1996; Honda et al., 2008), the discrete nature of the data set, experimenter bias in grading, and animal stress due to handling and restraint can reduce the sensitivity of von Frey-based techniques. Our operant approach to measuring mechanical orofacial sensitivity can acquire quantitative data for individual animals and should reduce animal stress and experimenter bias by reducing animal-experimenter interaction. Thus, this approach should allow for more consistent, comparable results between individual animals than traditional von Frey testing.
Conclusions
The described device allows for quantitative measurement of orofacial mechanical sensitivity using an operant-based method that is free from experimenter bias and should provide repeatable comparisons across different animal models, experimenters, and labs. The data contained herein demonstrate the sensitivity of our method to a relatively subtle model of orofacial pain, low-dose application of capsaicin cream. This device can also capture differences in individual animal sensitivity, allowing inter-subject variability to be incorporated into the statistical analyses. This type of quantitative preclinical behavioral analyses provides the foundation to further investigate other preclinical models of clinical disorders that exhibit orofacial pain symptoms, in an effort to increase our understanding of pain in these models. The results from this experiment can assist in future investigations of orofacial pain, allowing operant-based behavioral responses to be integrated into pre-clinical studies of diagnostics and therapeutics for orofacial pain.
Supplementary Material
A novel operant device was used to measure orofacial mechanical allodynia in rats.
Our device was validated using a capsaicin model of orofacial neuropathy.
Individual animal behavioral measurements were assessed.
Morphine attenuation was successfully detected by our device.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health under award numbers K99/R00AR057426.
Footnotes
Conflict of Interest
Robert M. Caudle and John K. Neubert are employees and founders of Velocity Laboratories, a company that provides fee-for-service behavioral testing using operant pain assays. The other authors do not have a conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Eric L. Rohrs, Email: Rohrs.eric@ufl.edu.
Heidi E. Kloefkorn, Email: hkloefkorn@ufl.edu.
Emily H. Lakes, Email: elakes@ufl.edu.
Brittany Y. Jacobs, Email: starjacobs@ufl.edu.
John K. Neubert, Email: jneubert@dental.ufl.edu.
Robert M. Caudle, Email: caudle@ufl.edu.
Kyle D. Allen, Email: kyle.allen@bme.ufl.edu.
References
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Boudreau SA, Wang K, Svensson P, Sessle BJ, Arendt-Nielsen L. Vascular and psychophysical effects of topical capsaicin application to orofacial tissues. J Orofac …. 2009;23:253–264. [PMC free article] [PubMed] [Google Scholar]
- Castonguay TW, Kaiser LL, Stern JS. Meal pattern analysis: artifacts, assumptions and implications. Brain Res Bull. 1986;17:439–43. doi: 10.1016/0361-9230(86)90252-2. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher Ma, Tominaga M, Rosen Ta, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev. 2002;26:907–23. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Deseure KR, Adriaensen HF. Comparison between two types of behavioral variables of non-evoked facial pain after chronic constriction injury to the rat infraorbital nerve. Comp Med. 2002;52:44–9. [PubMed] [Google Scholar]
- Dolan JC, Lam DK, Achdjian SH, Schmidt BL. The dolognawmeter: a novel instrument and assay to quantify nociception in rodent models of orofacial pain. J Neurosci Methods. 2010;187:207–15. doi: 10.1016/j.jneumeth.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PN, Campbell JN, Meyer Ra. Secondary hyperalgesia persists in capsaicin desensitized skin. Pain. 2000;84:141–9. doi: 10.1016/s0304-3959(99)00194-3. [DOI] [PubMed] [Google Scholar]
- Gilchrist HD, Allard BL, Simone Da. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67:179–88. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
- Hogan Q, Sapunar D, Modric-Jednacak K, Mccallum JB. Detection of Neuropathic Pain in a Rat Model of peripheral nerve injury. Anesthesiology. 2004:476–487. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- Honda K, Kitagawa J, Sessle BJ, Kondo M, Tsuboi Y, Yonehara Y, Iwata K. Mechanisms involved in an increment of multimodal excitability of medullary and upper cervical dorsal horn neurons following cutaneous capsaicin treatment. Mol Pain. 2008;4:59. doi: 10.1186/1744-8069-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y, Kawamoto H, Nakanishi O. Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Exp Brain Res. 1997;116:97–103. doi: 10.1007/pl00005748. [DOI] [PubMed] [Google Scholar]
- Jeon HJ, Han SR, Park MK, Yang KY, Bae YC, Ahn DK. A novel trigeminal neuropathic pain model: compression of the trigeminal nerve root produces prolonged nociception in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:149–58. doi: 10.1016/j.pnpbp.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Kerins Ca, Carlson DS, McIntosh JE, Bellinger LL. Meal pattern changes associated with temporomandibular joint inflammation/pain in rats; analgesic effects. Pharmacol Biochem Behav. 2003;75:181–189. doi: 10.1016/S0091-3057(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Kramer PR, He J, Puri J, Bellinger LL. A non-invasive model for measuring nociception after tooth pulp exposure. J Dent Res. 2012;91:883–7. doi: 10.1177/0022034512454297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzanowska A, Avendaño C. Behavioral testing in rodent models of orofacial neuropathic and inflammatory pain. Brain Behav. 2012;2:678–97. doi: 10.1002/brb3.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzanowska A, Pittolo S, Cabrerizo M, Sánchez-López J, Krishnasamy S, Venero C, Avendaño C. Assessing nociceptive sensitivity in mouse models of inflammatory and neuropathic trigeminal pain. J Neurosci Methods. 2011;201:46–54. doi: 10.1016/j.jneumeth.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Kupers R. Facial pain: from animal models to functional neuroimaging studies. Acta Neurol Belg. 2001;101:32–8. [PubMed] [Google Scholar]
- Kupers RC, Chen CC, Bushnell MC. A model of transient hyperalgesia in the behaving monkey induced by topical application of capsaicin. Pain. 1997;72:269–75. doi: 10.1016/s0304-3959(97)00052-3. [DOI] [PubMed] [Google Scholar]
- LaCroix-Fralish ML, Rutkowski MD, Weinstein JN, Mogil JS, Deleo Ja. The magnitude of mechanical allodynia in a rodent model of lumbar radiculopathy is dependent on strain and sex. Spine (Phila Pa 1976) 2005;30:1821–1827. doi: 10.1097/01.brs.0000174122.63291.38. [DOI] [PubMed] [Google Scholar]
- Lambert Ga, Mallos G, Zagami AS. Von Frey’s hairs--a review of their technology and use--a novel automated von Frey device for improved testing for hyperalgesia. J Neurosci Methods. 2009;177:420–6. doi: 10.1016/j.jneumeth.2008.10.033. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone Da, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993;124:115–21. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- Mauderli aP, Acosta-Rua a, Vierck CJ. An operant assay of thermal pain in conscious, unrestrained rats. J Neurosci Methods. 2000;97:19–29. doi: 10.1016/s0165-0270(00)00160-6. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–389. doi: 10.1016/S0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- Morris R, Cahusac PM, Salt TE, Morris RG, Hill RG. A behavioural model for the study of facial nociception and the effects of descending modulatory systems in the rat. J Neurosci Methods. 1982;6:245–52. doi: 10.1016/0165-0270(82)90087-5. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. The National Academies Press; Washington, DC: 2011. [Google Scholar]
- Neubert JK, Rossi HL, Malphurs W, Vierck CJ, Caudle RM. Differentiation between capsaicin-induced allodynia and hyperalgesia using a thermal operant assay. Behav Brain Res. 2006;170:308–15. doi: 10.1016/j.bbr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–95. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Nolan Ta, Hester J, Bokrand-Donatelli Y, Caudle RM, Neubert JK. Adaptation of a novel operant orofacial testing system to characterize both mechanical and thermal pain. Behav Brain Res. 2011;217:477–80. doi: 10.1016/j.bbr.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura M, Iwata K, Yasuda K, Inoue K, Shinoda M, Honda K, Shibuta K, Yasuda M, Kondo E. Alternation of gene expression in trigeminal ganglion neurons following complete Freund’s adjuvant or capsaicin injection into the rat face. J Mol Neurosci. 2010;42:200–9. doi: 10.1007/s12031-010-9348-7. [DOI] [PubMed] [Google Scholar]
- Park KM, Max MB, Robinovitz E, Gracely RH, Bennett GJ. Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain. 1995;63:163–72. doi: 10.1016/0304-3959(95)00029-R. [DOI] [PubMed] [Google Scholar]
- Pelissier T, Pajot J, Dallel R. The orofacial capsaicin test in rats: effects of different capsaicin concentrations and morphine. Pain. 2002;96:81–7. doi: 10.1016/s0304-3959(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Poh KW, Lutfun N, Manikandan J, Ong WY, Yeo JF. Global gene expression analysis in the mouse brainstem after hyperalgesia induced by facial carrageenan injection--evidence for a form of neurovascular coupling? Pain. 2009;142:133–41. doi: 10.1016/j.pain.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Ro JY, Lee J, Capra NF, Zhang Y. Role of soluble guanylate cyclase in the trigeminal subnucleus caudalis in capsaicin-induced muscle hypersensitivity. Brain Res. 2007;1184:141–8. doi: 10.1016/j.brainres.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144:270–7. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld J, Broton J, Clavier R. A reliable, facial nociception device for unrestrained, awake animals: effects of morphine and trigeminal complex lesions. Physiol Behav. 1978;21:287–290. doi: 10.1016/0031-9384(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JP, Stocco S. Effects of midbrain, bulbar and combined morphine microinjections and systemic injections on orofacial nociception and rostral trigeminal stimulation: independent midbrain and bulbar opiate analgesia systems? Brain Res. 1981;215:342–8. doi: 10.1016/0006-8993(81)90514-x. [DOI] [PubMed] [Google Scholar]
- Saloman JL, Niu KY, Ro JY. Activation of peripheral delta-opioid receptors leads to anti-hyperalgesic responses in the masseter muscle of male and female rats. Neuroscience. 2011;190:379–85. doi: 10.1016/j.neuroscience.2011.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger DJ, McCarthy PS. Differential effects of morphine on food and water intake in food deprived and freely-feeding rats. Psychopharmacology (Berl) 1980;72:103–6. doi: 10.1007/BF00433813. [DOI] [PubMed] [Google Scholar]
- Simone Da, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Simone DA, Ochoa J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain. 1991;47:285–94. doi: 10.1016/0304-3959(91)90217-L. [DOI] [PubMed] [Google Scholar]
- Vos BP, Strassman AM. Behavioral Evidence Chronic Constriction of Trigeminal Neuropathic Pain Following Injury to the Rat’s lnfraorbital Nerve. J Neurosci. 1994;74:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasner G, Baron R, Jänig W. Dynamic mechanical allodynia in humans is not mediated by a central presynaptic interaction of A beta-mechanoreceptive and nociceptive C-afferents. Pain. 1999;79:113–9. doi: 10.1016/s0304-3959(98)00159-6. [DOI] [PubMed] [Google Scholar]
- Xu W, Wu Y, Bi Y, Tan L, Gan Y, Wang K. Activation of voltage-gated KCNQ/Kv7 channels by anticonvulsant retigabine attenuates mechanical allodynia of inflammatory temporomandibular joint in rats. Mol Pain. 2010;6:49. doi: 10.1186/1744-8069-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


