Abstract
The present study evaluates the effect of celastrol on the survival of retinal ganglion cells (RGCs) injured by optic nerve crush (ONC). Celastrol, a quinine methide triterpene extracted from the perennial vine Tripterygium wilfordii (Celastraceae), has been identified as a potential neuroprotective candidate in a comprehensive drug screen against various neurodegenerative diseases. Two weeks after ONC, the average density of remaining RGCs in retinas of animals treated with daily intraperitoneal (i.p.) injections of celastrol (1 mg/kg) was approximately 1332 cells/mm2, or 40.8% of that of the Celastrol/Control group. In retinas of the Vehicle/ONC group about 381 RGCs/mm2 were counted, which is 9.6% of the total number of RGCs in the DMSO/Control group. This corresponds to approximately a 250% increase in RGC survival mediated by celastrol treatment compared to control. Furthermore, the average RGC number in retinas of ONC animals treated with a single intravitreal injection of 1 mg/kg or 5 mg/kg of celastrol was increased by approximately 80% (760 RGCs/ mm2) and 78% (753 RGCs/mm2), respectively, compared to controls (422 cells/mm2). Injection of 0.2 mg/kg of celastrol had no significant effect on cell survival compared to DMSO-injected controls, with the average number of RGCs being 514 cells/mm2 in celastrol-treated animals versus 422 cells/mm2 in controls. The expression levels of Hsp70, Hsf1, Hsf2, HO-1 and TNF-alpha in the retina were analyzed to evaluate the roles of these proteins in the celastrol-mediated protection of injured RGCs. No statistically significant change in HO-1, Hsf1 and Hsp70 levels was seen in animals with ONC. An approximately 2 fold increase in Hsf2 level was observed in celastrol-treated animals with or without injury. Hsf2 level was also increased 1.8 fold in DMSO-treated animals with ONC injury compared to DMSO-treated animals with no injury suggesting that Hsf2 induction has an injury-induced component. Expression of TNF-alpha in retinas of celastrol-treated uninjured and ONC animals was reduced by approximately 2 and 1.5 fold compared to vehicle treated animals, respectively. The observed results point to the role of TNF-alpha in RGC degeneration following axonal injury, and that suggests mechanisms underlying celastrol’s RGC protective effect is associated with inhibition of TNF-alpha-mediated cell death.
Keywords: celastrol, retina, ganglion cells, optic nerve, neuroprotection
1. INTRODUCTION
Celastrol, a quinine methide triterpene extracted from the perennial vine Tripterygium wilfordii (Celastraceae), has been used in traditional Chinese medicine as a natural remedy for inflammation and a variety of autoimmune diseases for hundreds of years (Pinna et al., 2004; Tao et al., 2002). Celastrol has been identified as a potential neuroprotective candidate in a comprehensive drug screen against various neurodegenerative diseases and has since been demonstrated to have cell protective properties in animal models of “protein misfolding” neurodegenerative disorders such as Parkinson’s, Hungtington’s, Alzheimer’s diseases, and amyotrophic lateral sclerosis (Abbott, 2002; Allison et al., 2001; Cleren et al., 2005; Kiaei et al., 2005; Wang et al., 2005). The neuroprotective effects of celastrol are associated with the activation of heat shock and antioxidant responses that lead to an induction of an array of cytoprotective genes involved in protein folding/aggregation/turnover and the scavenging and export of reactive species (Wang et al., 2005; Trott etal., 2008; Westerheide et al., 2004). Furthermore, celastrol was shown to attenuate microglial activation and production of tumor necrosis factor (TNF)-alpha (Kiaei et al., 2005; Allison et al., 2001). Since protein misfolding/aggregation (Liu and Vollrath, 2004; Guo et al, 2007), oxidative damage (Kumar and Agarwal, 2007) and microglial activation (Neufeld and Liu, 2003; Tezel, 2008) have been associated with the pathogenesis of glaucoma, we hypothesized that celastrol could be a potent multifactorial therapeutic drug for the treatment of this disease.
The degeneration of retinal ganglion cells (RGCs) and their axons in the optic nerve is the cause of visual impairment in patients with optic neuropathies, including glaucoma, which affects more than 65 million people worldwide and is a leading cause of irreversible blinding. Chronic forms of the glaucoma usually progress over many years. Currently, reduction of intraocular pressure (IOP) remains the main strategy in slowing the progression of the disease. Yet, glaucomatous neuropathy often continues to progress even after IOP has been reduced, especially in advanced stages of disease. It is clear that it is necessary to develop new strategies to supplement or perhaps even to replace IOP reduction in some patients in order to reduce the number of degenerating neurons and preserve the surviving RGCs and their axons. However, this is extremely difficult since the exact molecular pathways of RGC death in glaucoma are not well understood, and the cellular damage in this disease may be caused by different molecular mechanisms that have the common characteristics of optic nerve damage and visual loss patterns. Since the pathophysiologic mechanisms underlying RGC loss in glaucoma remain unclear, several directions of RGC neuroprotection are being investigated, including blocking glutamate excitotoxicity (Hare et al., 2001; Schori 2001), stabilizing Ca2+ homeostasis (Zhang et al., 2003; Wood et al., 2003 Hains and Waxman, 2005), inhibiting nitric oxide production (Le and Lipton, 2001; Neufled et al., 2002), overexpressing proteins regulating the cellular redox state (Munemasa et al., 2009), supplying neurotrophins (Mansour-Robaey et al., 1994; Martin et al., 2003; Cheng et al., 2002), preventing apoptosis (McKinnon et al., 2002; Huang et al., 2005), inducing heat shock response (Park et al., 2001; Ishii et al., 2003; Kwong at al., 2015), and modulating the immunologic status (Moalem et al., 2000; Bakalash et al., 2003; Anderson et al., 2005). Each of these strategies increases the rate of RGC survival in the laboratory setting. However, their effect is limited due to the possible presence of diverse mechanisms involved in glaucomatous RGC degeneration, and none of these strategies have been evaluated clinically with the exception of memantine (NMDA receptor antagonist) trials which showed no significant beneficial effect compared to placebo (Allergan Press Release, 2008).
In the present study, we evaluated the neuroprotective effect of celastrol on RGCs with axonal injury induced by optic nerve crush (ONC). In this model, both intraperitonial (i.p) and intravitreal treatment with celastrol stimulate survival of injured RGCs. Expression levels of Hsp70, Hsf1, Hsf2, heme oxigenase 1 (HO-1), and TNF-alpha were analyzed to gain insights into potential mechanisms underlying the observed celastrol-mediated RGC protection from axonal injury.
2. RESULTS
The effect of intraperitoneal injection of celastrol on RGC survival damaged by ONC
ONC was performed unilateraly with the contralateral eye remaining untreated. Celastrol (1 mg/kg) or vehicle (DMSO) was injected i.p. daily starting on the day of ONC and continued for 14 days, after which retinas were dissected and analyzed to determine the number of surviving RGCs. The dose of celastrol was chosen based on previous studies in in vivo model systems utilizing this drug (Venkatesha et al., 2011; Jiang et al., 2013; Kim et al, 2013). The number of surviving cells was determined by counting RGCs immunolabeled with Rbpms in flat-mount retinal preparations. Rbpms has characterized as a marker of RGCs in rodent retinas with a specificity matching that of retrograde labeling (Kwong et al., 2010; 2011). An extensive loss of RGCs (~87% RGC loss in DMSO-treated group) was observed in retinas subjected to ONC (Figs. 1A and B). The extent of RGC loss was similar across the superior, inferior and temporal quadrants that were sampled in this experiment. The average density of remaining RGCs in retinas of celastrol-treated animals was approximately 1332±152 cells/mm2, or 46% of that of the Celastrol/Control, whereas for the Vehicle/ONC group it was about 381±58 RGCs/mm2, or 12.5% of Vehicle/Control (n=6 per group; *P=0.004). These data indicate a more than 250% increase in RGC survival mediated by celastrol treatment compared to the Vehicle/ONC control group.
Figure 1.
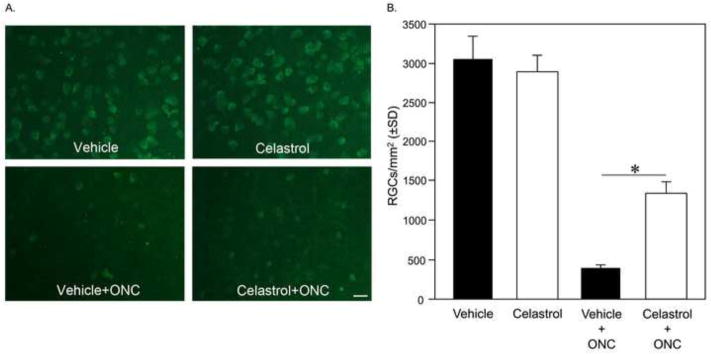
Systemic treatment of animals with celastrol protects RGCs from ONC injury. RGC densities in vehicle- and celastrol-treated animals. Celastrol (1 mg/kg) or vehicle was administered daily by i.p injection. RGCs were immunolabeled with antibodies against Rbpms and counted in inferior, superior, temporal and nasal retinal quadrants. A. Images represent inferior retina 3 mm from the center of the optic nerve head. B. Two weeks after ONC, the average density of remaining RGCs in the retinas of animals treated with celastrol was 1332±152 cells/mm2 compared to 381±58 RGCs/mm2 in the DMSO/ONC group (n=6 per group; *P=0.004).
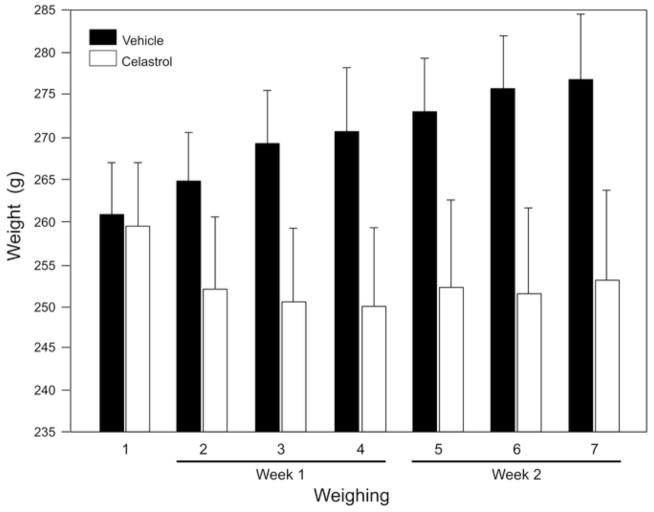
Body weight assessment of animals treated with celastrol
To administer an appropriate dose of celastrol, the body weight of all animals used in this study was closely monitored and measured before and during celastrol treatment (Fig. 2). The average weight of animals in the control group injected with vehicle (DMSO) increased from 260.8 g to 276.7 g within two weeks, which corresponds to approximately 6.1% weight gain. In the celastrol-injected group, the weight of animals remained relatively stable and no weight gain was observed. In fact, over the two week period, the average body weight of this group slightly decreased from 259.3 g to 253 g (2.4% weight loss). At the end of the experiment (two weeks after first injection of celastrol), the difference in body weight between DMSO- and celastrol-treated groups was 8.5% (n=6 per group; P=0.006).
Figure 2.
The effect of celastrol on animals’ body weight. The animals were weighed before (weighing 1) and 3 times per week after i.p. injections of celastrol (1 mg/kg) or DMSO (weighing 2–4 during first week and 5–7 during second week). The average weight of animals in the control group injected with DMSO increased from 260.8 g to 276.7 g within two weeks, whereas the weight of animals in the celastrol-injected group decreased from 259.3 g to 253 g. The difference in body weight between DMSO- and celastrol-treated groups was 8.5% at the time of last weighing (n=6 per group; P=0.006).
The cell protective effect of intravitreal injection of celastrol in ONC model
As the aim of the study was to determine the RGC protective effect of celastrol, we thought that a local delivery of this drug to the target cells could be more beneficial than a systemic treatment for at least two reasons: a) the concentration of the drug delivered to RGCs by intravitreal injection could be better controlled and easily adjusted to have the maximum neuroprotective effect; and b) intravitreal injection would minimize any potential side effects of the drug on other organs and tissues that are not targeted in this study. Since this is the first study on the effect of celastrol on the retina and no information on the dosage of this drug for intravitreal injection is available in the literature, three doses were used in these experiments: 1 mg/kg, 0.2 mg/kg and 5 mg/kg. 1 mg/kg concentration was used for the systemic treatment described above (Venkatesha et al., 2011; Jiang et al., 2013; Kim et al, 2013). A single injection of celastrol or DMSO at the time of the ONC procedure was performed to minimize the injury. Results of RGC counts showed that injection of 0.2 mg/kg of celastrol had no significant effect on cell survival compared to DMSO-injected controls: an average 514±82 cells survived in celastrol-treated animals compared to 422±55 cells/mm2 counted in controls (Fig. 3, n=4 per group). In retinas of animals treated with intravitreal injection of 1 mg/kg and 5 mg/kg of celastrol, the average number of RGCs was increased by approximately 80% (760±56 RGCs/ mm2) and 78% (753±164 RGCs/mm2), respectively, compared to ONC/vehicle controls (422±55 cells/mm2; n=4 per group; P<0.05).
Figure 3.

Intravitreal injection of celastrol stimulates survival of RGCs injured by ONC. RGCs were counted in inferior, superior and temporal retinal quadrants of animals without and with ONC injury treated with single injection of celastrol (0.2, 1 or 5 mg/kg) or DMSO. Injection of 0.2 mg/kg of celastrol had no statistically significant effect on cell survival compared to DMSO-injected controls (n=4 per group). The average number of RGCs in animals injected with 1 and 5 mg/kg of celastrol was increased by approximately 80% (760±56 RGCs/ mm2) and 78% (753±164 RGCs/mm2), respectively, compared to DMSO-treated ONC controls (422±55 cells/mm2; n=4 per group; *P<0.05).
Effect of celastrol on proteins of cellular heat shock response, HO-1 and TNF-alpha
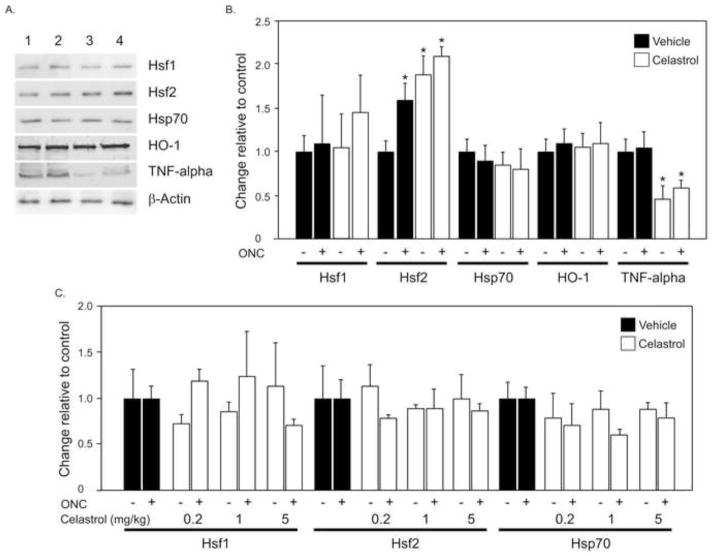
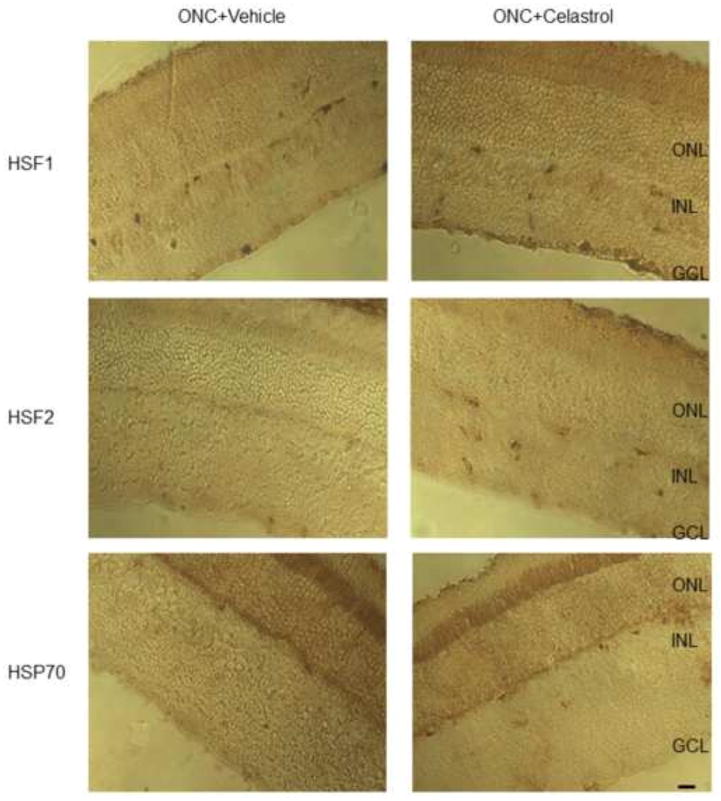
As mentioned above, celastrol-mediated cell protection has been commonly associated with activation of the cellular heat shock response, attenuation of oxidative stress and reduction in TNF-alpha production. First, we evaluated the levels of Hsf1, Hsf2 and Hsp70 expression in celastrol-treated (i.p.) retinas damaged by ONC with immunoblot. No significant changes in Hsf1 and Hsp70 expression in celastrol-treated compared to control retinas were detected (Figs. 4A and B). Hsf2 expression in celastrol-injected animals with and without ONC was upregulated by approximately 2.1 and 1.8 fold respectively compared to DMSO-treated uninjured controls (Figs. 4A and B; n=6 per group; *P<0.05). In animals receiving intravitreal injection of celastrol no change in Hsp70, Hsf1 or Hsf2 expression levels were observed. Immunohistochemical staining for Hsf1, Hsf2 and Hsp70 also showed no apparent change in the expression pattern of these proteins in retinas of DMSO or celastrol-treated animals (Fig. 5). The level of HO-1, which has been reported to be involved in attenuation of oxidative stress by celastrol, was similar in vehicle and drug treated animals. However, expression of TNF-alpha in retinas of celastrol-treated ONC and uninjured animals was reduced approximately 1.5 and 2 fold, respectively, compared to DMSO-injected rats (n=6 per group; *P<0.05).
Figure 4.
Quantitative analysis of Hsp70, Hsf1 and Hsf2 expression levels in ONC animals treated with celastrol. A. Representative western blot of Hsp70, Hsf1, Hsf2, HO-1 and TNF-alpha. Lanes contain protein from retinas of: 1, vehicle-treated uninjured; 2, celastrol-treated uninjured; vehicle-treated ONC and 4, celastrol-treated ONC animals. B No significant changes in expression levels of Hsf1 Hsp70, and HO-1 in celastrol-treated (1 mg/kg; i.p.) compared to control retinas were observed. The level of Hsf2 in ONC animals with DMSO injection was elevated approximately 1.6 fold compared to DMSO-injected uninjured animals. Injection of celastrol upregulated Hsf2 expression in animals with and without ONC by approximately 2.1 and 1.8 fold, respectively, compared to DMSO-treated uninjured controls (n=6 per group; *P<0.05). Expression of TNF-alpha in retinas of celastrol-treated ONC and uninjured animals was reduced approximately 1.5 and 2 fold, respectively, compared to DMSO-injected rats (n=6 per group; *P<0.05). C. Intravitreal injection of celastrol (0.2, 1 and 5 mg/kg) and expression of Hsp70, Hsf1 and Hsf2. A single intravitreal injection was performed on the day of ONC and protein levels were analyzed two weeks after ONC. No significant change in Hsf1, Hsf2 or Hsp70 levels was observed in celastrol-treated animals (n=4 per group).
Figure 5.
Representative images of Hsf1, Hsf2 and Hsp70 retinal immunostaining in ONC retinas. No significant change in staining patterns for Hsf1, Hsf2 and Hsp70 in celastrol- and DMSO-treated animals was observed.
DISCUSSION
A rationale for this study to investigate the neuroprotective effects of celastrol is based on the ability of this drug to target several essential mechanisms that have been implicated in glaucomatous RGC degeneration, including protein misfolding/aggregation, oxidative stress/damage, and microglia/astrocyte activation. The multipotent qualities of celastrol, together with its centuries long track record in oriental medicine make this drug an attractive candidate for an alternative glaucoma treatment that could replace or complement the current IOP reduction therapy.
In this study, we used the ONC model to evaluate the neuroprotective effects of celastrol. This is a commonly used model for RGC degeneration characterized by specific, fast and highly reproducible loss of RGCs. First, we evaluated the effect of i.p. injection of celastrol at a dose of 1 mg/kg in ONC animals. I.p. injection of celastrol at this dose has been successfully used in rat and mouse models of various human diseases with no side effects reported (Venkatesha et al., 2011; Jiang et al., 2013; Kim et al, 2013). However, at least two other studies observed a significant decrease in the body weight of animals treated with celastrol (Huang et al., 2012; Kim et al, 2013). Treatment of six-week-old diabetic db/db and non-diabetic db/m mice with 1 mg/kg/day of celastrol (i.p.) for two weeks was associated with lower body weight due to decrease in food intake, independent of diabetic status (Kim et al, 2013). Following celastrol administration into 4-week-old BALB/c mice for 5 days per week for 4 weeks, body weight decrease was also observed at a dose of 4 mg/kg/day, but not at 1 mg/kg/day (Huang et al., 2012). On the other hand, celastrol administered at 2 and 8 mg/kg/day doses in the diet to G93A SOD1 mice starting at 30 days of age significantly improved weight loss as well as motor performance and delayed the onset of ALS (Kiaei et al., 2005). No obvious toxicity or significant weight loss was seen in mice treated with 0.5 mg/kg of celastrol (i.p.) 4 times per week for 3 weeks (Shao et al., 2013). To avoid possible adverse effects and to ensure a proper drug dosage, we monitored the body weight of animals during a two week period following i.p. administration of celastrol. Compared to the weight gain of approximately 6.1% in the control group injected with DMSO, 2.4% weight loss was observed in the celastrol-injected group. The data from this and earlier studies suggest that the in vivo treatment with celastrol needs to be closely monitored for body weight loss, especially at drug doses higher than 1 mg/kg, probably due to decreased food intake.
The neuroprotective effect of celastrol in ONC animals was evaluated 2 weeks after ONC. Celastrol treatment led to more than a 250% increase in the number of RGCs in ONC retinas compared to the DMSO-treated group. After observing this impressive effect of celastrol on RGC survival, we decided to substitute a systemic treatment of animals with intravitreal administration of the drug. Local delivery of the drug may lead to better exposure of RGCs to the treatment, which consequently could translate into more efficient neuroprotection, and to fewer potential side effects on vital organs. However, the disadvantage of this approach is that in order to minimize injury to the eye, only a single injection was performed at the time of ONC procedure. Since, to our knowledge, this is the first experiment that employs intravitreal injection of celastrol, three different doses of the drug were evaluated. The lowest dose of 0.2 mg/kg had no effect on RGC survival. However, both 1 mg/kg and 5 mg/kg protected RGCs from ONC induced damage by approximately 80% compared to controls. The RGC protection mediated by a single intravitreal injection of celastrol was significantly lower than that observed in animals subjected to daily i.p. administration of this drug, suggesting that more frequent deliveries are required to achieve the full benefit of the treatment.
Celastrol’s cytoprotective effect is largely attributed to its antioxidant potency, induction of heat shock response and downregulation of pro-inflammatory cytokine TNF-alpha (Allison et al, 2001; Westerheide et al., 2004; Kiaei et al. 2005; Zhang and Sarge, 2007; Trott et al., 2008; Salminen A et al., 2010). Celastrol and its ability to stimulate Hsp70 expression was identified by screening a library of FDA-approved or biologically active drugs that suppress the expression of mutant Huntingtin and superoxide dismutase, followed by screening of positive compounds to induce expression of the luciferase reporter gene, driven by Hsp70.1 promoter (Abbott, 2002; Westerheide et al., 2004). Of the several compounds with a positive effect, celastrol was of particular interest because it was also identified independently in five laboratories using six different cell-based screens for Huntingtin aggregation and neurotoxicity. The induction of Hsp70 gene expression by celastrol has been shown to be due to the activation of Hsf1 (Westerheide et al., 2004). In this study, the effect of celastrol on the expression of Hsf1, Hsf2 and Hsp70 was analyzed. No significant change in Hsf1 and Hsp70 levels was observed suggesting that celastrol-mediated RGC protection from ONC injury is not associated with induction of Hsp70. Hsf2 was upregulated in celastrol-treated animals with or without injury as well as to a lesser degree in DMSO-treated animals with ONC injury suggesting that Hsf2 induction has an injury-induced component. To determine whether the RGC protective effect of celastrol involves attenuation of oxidative stress, the expression level of HO-1 was analyzed. However, no change in HO-1 levels in drug-treated compared to the vehicle-treated animals was detected. With respect to the effect of celastrol on the expression of TNF-alpha, celastrol-treated ONC and uninjured animals showed approximately 1.5 and 2 fold decrease in the level of TNF-alpha, respectively, compared to vehicle-injected rats. Our data is in agreement with many other studies showing the association of TNF-alpha with neuronal death in various neurological diseases including Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease (Montgomery and Bowers, 2012), as well as with RGC degeneration in glaucomatous neuropathy and following optic nerve injury (Tezel and Wax, 2000; Yan et al., 2000; Yuan and Neufeld, 2000; Tezel et al., 2001, 2004; Yoshida et al., 2002; Nakazawa et al., 2006; Kitaoka et al., 2006; Tezel, 2008; Yang et al 2011; Roh et al., 2012). Downregulation of TNF-alpha in celastrol-treated animals suggests that the observed RGC protective effect of this drug involves inhibition of TNF-alpha-mediated cell death pathways.
In conclusion, both i.p. and intravitreal injection of celastrol protects RGCs from ONC-induced degeneration. Two weeks after ONC, the average density of remaining RGCs in the retinas of animals treated with celastrol (1 mg/kg; daily i.p. injection) was increased by approximately 250%. The average number of RGCs in retinas of ONC animals treated with a single intravitreal injection of 1 mg/kg or 5 mg/kg of celastrol was increased by approximately 80% compared to controls. Injection of 0.2 mg/kg of celastrol had no significant effect on cell survival compared to controls. Hsp70 and HO-1 levels in celastrol- and vehicle-treated retinas were similar, whereas the expression of TNF-alpha was significantly reduced by celastrol, suggesting that celastrol-mediated RGC protection from ONC involves mechanisms controlling cell death pathways associated with TNF-alpha.
4. EXPERIMENTAL PROCEDURES
Ethics Statement
The use of animals and the procedures involving animals were approved by the Animal Research Committee of the University of California at Los Angeles and were in compliance with the National Institutes of Health Guide for the Care and Use of Animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Animals
Adult Brown Norway rats (3 month-old; 250–300μg) were used to generate ONC model. Animals were housed with standard food and water provided ad libitum in a room with the temperature set at 21°C and illuminated with fluorescent lights (330 lux) automatically turned on at 03:00 am and off at 03:00 pm. The animals were kept at least 1 week in this environment before surgical procedures. Surgical procedures (described below) were performed on one eye of each rat. Celastrol or vehicle (DMSO) was administered by i.p. or intravitreal injection.
Rat ONC injury model of RGC degeneration
ONC injury model was generated as described previously with minor modifications (Nadal-Nicolás et al., 2009). Briefly, the optic nerve was exposed and crushed with fine forceps approximately 2 mm behind the posterior pole of the eye for 5 sec. Care was taken not to damage the blood circulation. Animals were excluded from the study if any obvious changes in the retina and lens had occurred due to interrupted blood supply after ONC. The right eyes were not used as normal controls because crushing one optic nerve has been demonstrated to affect the morphology of the contralateral retina (Panagis et al., 2005). The optic nerve in the untreated rat was used as a normal control. To evaluate the neuroprotective effect of celastrol after ONC, the drug or vehicle was administered by daily i.p. injection or single intravitreal injection. For i.p. injections, animals were divided into 4 groups (n=6 per group): vehicle treated; vehicle treated ONC; celastrol treated; and celastrol treated ONC. For intravitreal injections, animals were divided into 8 experimental groups (n=4 per group): 1) vehicle; 2) vehicle+ONC; 3) celastrol (0.2 mg/kg); 4) celastrol (1 mg/kg); 5) celastrol (5 mg/kg); 6) celastrol (0.2 mg/kg) +ONC; 7) celastrol (1 mg/kg) +ONC; and 8) celastrol (5 mg/kg) +ONC.
Quantification of RGCs immunolabeled with Rbpms
Animals were deeply anesthetized with intramuscular injections of 80 mg/kg sodium pentobarbitual and then transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. The eyes were enucleated, immersed in fixative for 0.5 hours, and the lenses were removed. The eyecups were postfixed for another 0.5 hours. Retinas were used for immunohistochemistry with a custom-made antibody against Rbpms according to a published protocol (Kwong et al., 2010; 2011). Briefly, the retinas were washed with PBS, incubated with 10% fetal bovine serum for 1 hour to block nonspecific staining, followed by incubation with the primary antibody against Rbpms in PBS containing 1% triton, 0.5% BSA, and 0.9% sodium chloride (PBS-T-BSA) overnight at 4°C. The retinas were washed in PBS-T-BSA and incubated with secondary Alexa Fluor 488 goat anti-rabbit IgG antibody (1/1000) overnight at 4°C. Retinas were then washed with PBS, flat mounted with several radial cuts on a glass slide, air dried and covered with a cover-slip in an aqueous mount. Topographical analysis of RGC density was performed under a fluorescence microscope (LSM410; Carl Zeiss, Oberkochen, Germany). Three areas (0.32 × 0.24 mm each) per retinal quadrant (superior, temporal, inferior and nasal) at 1, 2 and 3 mm from the center of optic nerve head were analyzed yielding 27 separate retinal areas for RGC counting. RGCs were counted by one observer masked to the protocol used for the eyes.
Immunoblot analysis
Protein concentrations were determined with a BCA Protein Assay Kit (Pierce, Rockford, IL). Aliquots of 1 μg of detergent-soluble retinal proteins were separated on precast 12% SDS-polyacrylamide gels (Mini-Protein TGX™, BIO-RAD, Hercules, CA) and transferred to nitrocellulose membranes (Immobuline-P, Millipore, Billerica, MA). After blocking with 5% non-fat milk, membranes were incubated at 4°C overnight with primary antibodies against Hsf1 (1:1000; Enzo, Farmingdale, NY), Hsf2 (1:5000; Enzo), Hsp70 (1:5000; Enzo), HO-1 (1:250; Enzo), iNOS (1:100; Abcam, Cambridge, MA), and TNF-α (1:100; Abcam). Membranes were subsequently incubated for 1 hour at 4°C with species-specific biotynilated antibodies (1:500; Enzo) and horseradish peroxidase (1:400; Enzo). Immunoreactive bands were detected using ECL western blotting detection reagents (Amersham/GE Healthcare, Piscataway, NJ). Quantification was carried out with NIH image software. To confirm equal loading of samples, membranes were further reprobed with antibodies against beta-actin (1:10000; Sigma, St. Louis, MO).
Immunohistochemistry
Immunohistochemistry was performed according to a standard procedure published elsewhere. Briefly, enucleated eyeballs were immersed in fixative for 1 hour, bisected, and postfixed for 3 hours. The eye cups were incubated with 30% sucrose at 4°C overnight and embedded in optical cutting temperature compound (O.C.T.; Sakura Finetec, Torrance, CA). Sections (10 μm thick) were obtained along the vertical meridian through the optic nerve head. The sections were washed in 0.01M PBS and twice in 0.01M PBS with Triton-X (T-PBS), followed by a 1-hour blocking at 4°C (T-PBS containing 20% bovine serum albumin and 5% goat serum). After blocking, tissues were incubated in T-PBS with primary antibodies for Hsf1 (1:500; Enzo), Hsf2 (1:500; Enzo), or Hsp70 (1:500; Enzo) at 4°C overnight following VECTASTAIN ABC kit protocol (Vector Labs, Burlingame, CA). Sections were labeled with DAB Peroxidase Substrate Kit (Vector Labs) and mounted.
Statistical Analysis
Data are presented as the mean ± SD. Differences among groups were analyzed by the Mann-Whitney test. P<0.05 was considered statistically significant.
We evaluated the effect of celastrol on RGC survival after optic nerve crush.
Systemic and intravitreal injection of celastrol protect RGCs from axonal injury.
Celastrol-mediated RGC protection is associated with downregulation of TNF-alpha.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) Grant EY018644 (NP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott A. Neurologists strike gold in drug screen effort. Nature. 2002;417:109. doi: 10.1038/417109a. [DOI] [PubMed] [Google Scholar]
- Allergan Press Release. Trials, Fourth Quarter Operating Results. 2008 Jan 30; Available at: http://agn.client.shareholder.com/releasedetail.cfm?ReleaseID=290764.
- Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Libby RT, Gould DB, Smith RS, John SW. High-dose radiation with bone marrow transfer prevents neurodegeneration in an inherited glaucoma. Proc Natl Acad Sci U S A. 2005;102:4566–4571. doi: 10.1073/pnas.0407357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalash S, Kessler A, Mizrahi T, Nussenblatt R, Schwartz M. Antigenic specificity of immunoprotective therapeutic vaccination for glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3374–3381. doi: 10.1167/iovs.03-0080. [DOI] [PubMed] [Google Scholar]
- Moalem G, Yoles E, Leibowitz-Amit R, Muller-Gilor S, Mor F, Cohen IR, Schwartz M. Autoimmune T cells retard the loss of function in injured rat optic nerves. J Neuroimmunol. 2000;106:189–197. doi: 10.1016/s0165-5728(00)00240-x. [DOI] [PubMed] [Google Scholar]
- Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci. 2002;22:3977–3986. doi: 10.1523/JNEUROSCI.22-10-03977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleren C, Calingasan NY, Chen J, Beal MF. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem. 2005;94:995–1004. doi: 10.1111/j.1471-4159.2005.03253.x. [DOI] [PubMed] [Google Scholar]
- Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, Ferrari G, Russo-Marie F, Sillito AM, Cheetham ME, Moss SE, Fitzke FW, Cordeiro MF. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci U S A. 2007;104:13444–13449. doi: 10.1073/pnas.0703707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare W, WoldeMussie E, Lai R, Ton H, Ruiz G, Feldmann B, Wijono M, Chun T, Wheeler L. Efficacy and safety of memantine, an NMDA-type open-channel blocker, for reduction of retinal injury associated with experimental glaucoma in rat and monkey. Surv Ophthalmol. 2001;45:S284–289. doi: 10.1016/s0039-6257(01)00200-4. [DOI] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Neuroprotection by sodium channel blockade with phenytoin in an experimental model of glaucoma. Invest Ophthalmol Vis Sci. 2005;46:4164–4169. doi: 10.1167/iovs.05-0618. [DOI] [PubMed] [Google Scholar]
- Heemskerk J, Tobin AJ, Bain LJ. Teaching old drugs new tricks. Trends Neurosci; Meeting of the Neurodegeneration Drug Screening Consortium; 7–8 April 2002; Washington, DC, USA. 2002. pp. 494–496. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhou D, Hang T, Wu Z, Liu J, Xu Q, Xie X, Zuo J, Wang Z, Zhou Y. Preparation, characterization, and assessment of the antiglioma effects of liposomal celastrol. Anticancer Drugs. 2012;23:515–524. doi: 10.1097/CAD.0b013e3283514b68. [DOI] [PubMed] [Google Scholar]
- Huang W, Fileta JB, Dobberfuhl A, Filippopolous T, Guo Y, Kwon G, Grosskreutz CL. Calcineurin cleavage is triggered by elevated intraocular pressure, and calcineurin inhibition blocks retinal ganglion cell death in experimental glaucoma. Proc Natl Acad Sci US A. 2005;102:12242–12247. doi: 10.1073/pnas.0505138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Kwong JM, Caprioli J. Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003;44:1982–1992. [PubMed] [Google Scholar]
- Jiang HL, Jin JZ, Wu D, Xu D, Lin GF, Yu H, Ma DY, Liang J. Celastrol exerts synergistic effects with PHA-665752 and inhibits tumor growth of c-Met-deficient hepatocellular carcinoma in vivo. Mol Biol Rep. 2013;40:4203–4209. doi: 10.1007/s11033-013-2501-y. [DOI] [PubMed] [Google Scholar]
- Jung HW, Chung YS, Kim YS, Park YK. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-kappaB in LPS-stimulated BV-2 microglial cells. Exp Mol Med. 2007;39:715–721. doi: 10.1038/emm.2007.78. [DOI] [PubMed] [Google Scholar]
- Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- Kim JE, Lee MH, Nam DH, Song HK, Kang YS, Lee JE, Kim HW, Cha JJ, Hyun YY, Han SY, Han KH, Han JY, Cha DR. Celastrol, an NF-κB Inhibitor, improves insulin resistance and attenuates renal iInjury in db/db mice. PLoS One. 2013;28:e62068. doi: 10.1371/journal.pone.0062068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaoka Y, Kitaoka Y, Kwong JM, Ross-Cisneros FN, Wang J, Tsai RK, Sadun AA, Lam TT. TNF-alpha-induced optic nerve degeneration and nuclear factor-kappaB p65. Invest Ophthalmol Vis Sci. 2006;47:1448–1457. doi: 10.1167/iovs.05-0299. [DOI] [PubMed] [Google Scholar]
- Kwong JM, Quan A, Kyung H, Piri N, Caprioli J. Quantitative analysis of retinal ganglion cell survival with Rbpms immunolabeling in animal models of optic neuropathies. Invest Ophthalmol Vis Sci. 2011;52:9694–9702. doi: 10.1167/iovs.11-7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JM, Caprioli J, Piri N. RNA binding protein with multiple splicing: a new marker for retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010;51:1052–1058. doi: 10.1167/iovs.09-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JM, Gu L, Nassiri N, Bekerman V, Kumar-Singh R, Rhee KD, Yang XJ, Hauswirth WW, Caprioli J, Piri N. AAV-mediated and pharmacological induction of Hsp70 expression stimulates survival of retinal ganglion cells following axonal injury. Gene Ther. 2015;22:138–145. doi: 10.1038/gt.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar DM, Agarwal N. Oxidative stress in glaucoma: a burden of evidence. J Glaucoma. 2007;16:334–343. doi: 10.1097/01.ijg.0000243480.67532.1b. [DOI] [PubMed] [Google Scholar]
- Le DA, Lipton SA. Potential and current use of N-methyl-D-aspartate (NMDA) receptor antagonists in diseases of aging. Drugs Aging. 2001;18:717–724. doi: 10.2165/00002512-200118100-00001. [DOI] [PubMed] [Google Scholar]
- Liu Y, Vollrath D. Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum Mol Genet. 2004;13:1193–1204. doi: 10.1093/hmg/ddh128. [DOI] [PubMed] [Google Scholar]
- Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KR, Quigley HA, Zack DJ, Levkovitch-Verbin H, Kielczewski J, Valenta D, Baumrind L, Pease ME, Klein RL, Hauswirth WW. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003;44:4357–4365. doi: 10.1167/iovs.02-1332. [DOI] [PubMed] [Google Scholar]
- McKinnon SJ, Lehman DM, Tahzib NG, Ransom NL, Reitsamer HA, Liston P, LaCasse E, Li Q, Korneluk RG, Hauswirth WW. Baculoviral IAP repeat-containing-4 protects optic nerve axons in a rat glaucoma model. Mol Ther. 2002;5:780–787. doi: 10.1006/mthe.2002.0608. [DOI] [PubMed] [Google Scholar]
- Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol. 2012;7:42–59. doi: 10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- Munemasa Y, Ahn JH, Kwong JM, Caprioli J, Piri N. Redox proteins thioredoxin 1 and thioredoxin 2 support retinal ganglion cell survival in experimental glaucoma. Gene Ther. 2009;16:17–25. doi: 10.1038/gt.2008.126. [DOI] [PubMed] [Google Scholar]
- Nadal-Nicolás FM, Jiménez-López M, Sobrado-Calvo P, Nieto-López L, Canovas-Martinez I, Salinas-Navarro M, Vidal-Sanz M, Agudo M. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve–injured retinas. Invest Ophthalmol Vis Sci. 2009;50:3860–3868. doi: 10.1167/iovs.08-3267. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, Benowitz LI. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006;26:12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld AH, Das S, Vora S, Gachie E, Kawai S, Manning PT, Connor JR. A prodrug of a selective inhibitor of inducible nitric oxide synthase is neuroprotective in the rat model of glaucoma. J Glaucoma. 2002;11:221–225. doi: 10.1097/00061198-200206000-00010. [DOI] [PubMed] [Google Scholar]
- Neufeld AH, Liu B. Glaucomatous optic neuropathy: when glia misbehave. Neuroscientist. 2003;9:485–495. doi: 10.1177/1073858403253460. [DOI] [PubMed] [Google Scholar]
- Panagis L, Thanos S, Fischer D, Dermon CR. Unilateral optic nerve crush induces bilateral retinal glial cell proliferation. Eur J Neurosci. 2005;21:2305–2309. doi: 10.1111/j.1460-9568.2005.04046.x. [DOI] [PubMed] [Google Scholar]
- Park KH, Cozier F, Ong OC, Caprioli J. Induction of heat shock protein 72 protects retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2001;42:1522–1530. [PubMed] [Google Scholar]
- Pinna GF, Fiorucci M, Reimund JM, Taquet N, Arondel Y, Muller CD. Celastrol inhibits pro-inflammatory cytokine secretion in Crohn’s disease biopsies. Biochem Biophys Res Commun. 2004;322:778–786. doi: 10.1016/j.bbrc.2004.07.186. [DOI] [PubMed] [Google Scholar]
- Roh M, Zhang Y, Murakami Y, Thanos A, Lee SC, Vavvas DG, Benowitz LI, Miller JW. Etanercept, a widely used inhibitor of tumor necrosis factor-alpha (TNF-alpha): prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS One. 2012;7:e40065. doi: 10.1371/journal.pone.0040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Lehtonen M, Paimela T, Kaarniranta K. Celastrol: molecular targets of thunder god vine. Biochem Biophys Res Commun. 2010;394:439–442. doi: 10.1016/j.bbrc.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Schori H, Kipnis J, Yoles E, WoldeMussie E, Ruiz G, Wheeler LA, Schwartz M. Vaccination for protection of retinal ganglion cells against death from glutamate cytotoxicity and ocular hypertension: implications for glaucoma. Proc Natl Acad Sci U S A. 2001;98:3398–3403. doi: 10.1073/pnas.041609498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Zhou Z, Cai Y, Castro P, Dakhov O, Shi P, Bai Y, Ji H, Shen W, Wang J. Celastrol suppresses tumor cell growth through targeting an AR-ERG-NF-κB pathway in TMPRSS2/ERG fusion gene expressing prostate cancer. PLoS One. 2013;8:e58391. doi: 10.1371/journal.pone.0058391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Younger J, Fan FZ, Wang B, Lipsky PE. Benefit of an extract of Tripterygium Wilfordii Hook F in patients with rheumatoid arthritis: a double-blind, placebo-controlled study. Arthritis Rheum. 2002;46:1735–1743. doi: 10.1002/art.10411. [DOI] [PubMed] [Google Scholar]
- Tezel G, Wax MB. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci. 2000;20:8693–8700. doi: 10.1523/JNEUROSCI.20-23-08693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Li LY, Patil RV, Wax MB. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2001;42:1787–1794. [PubMed] [Google Scholar]
- Tezel G, Yang X, Yang J, Wax MB. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 2004;996:202–212. doi: 10.1016/j.brainres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Tezel G. TNF-alpha signaling in glaucomatous neurodegeneration. Prog Brain Res. 2008;173:409–421. doi: 10.1016/S0079-6123(08)01128-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott A, West JD, Klaic L, Westerheide SD, Silverman RB, Morimoto RI, Morano KA. Activation of heat shock and antioxidant responses by the natural product celastrol: transcriptional signatures of a thiol-targeted molecule. Mol Biol Cell. 2008;19:1104–1112. doi: 10.1091/mbc.E07-10-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesha SH, Yu H, Rajaiah R, Tong L, Moudgil KD. Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J Biol Chem. 2011;286:15138–15146. doi: 10.1074/jbc.M111.226365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gines S, Macdonald ME, Gusella JF. Reversal of a full-length mutant hungtingtin neuronal phenotype by chemical inhibitors of polyglutamine-mediated aggregation. BMC Neurosci. 2005;6:1–12. doi: 10.1186/1471-2202-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, Bosman JD, Mbadugha BN, Kawahara TL, Matsumoto G. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- Wood JP, Schmidt KG, Melena J, Chidlow G, Allmeier H, Osborne NN. The beta-adrenoceptor antagonists metipranlol and timolol are retinal neuroprotectants: comparison with betaxolol. Exp Eye Res. 2003;76:505–516. doi: 10.1016/s0014-4835(02)00335-4. [DOI] [PubMed] [Google Scholar]
- Yan X, Tezel G, Wax MB, Edward DP. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch Ophthalmol. 2000;118:666–673. doi: 10.1001/archopht.118.5.666. [DOI] [PubMed] [Google Scholar]
- Yang X, Luo C, Cai J, Powell DW, Yu D, Kuehn MH, Tezel G. Neurodegenerative and inflammatory pathway components linked to TNF-alpha/TNFR1 signaling in the glaucomatous human retina. Invest Ophthalmol Vis Sci. 2011;52:8442–8454. doi: 10.1167/iovs.11-8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Behrens A, Le-Niculescu H, Wagner EF, Harada T, Imaki J, Ohno S, Karin M. Amino-terminal phosphorylation of c-Jun regulates apoptosis in the retinal ganglion cells by optic nerve transection. Invest Ophthalmol Vis Sci. 2002;43:1631–1635. [PubMed] [Google Scholar]
- Yuan L, Neufeld AH. Tumor necrosis factor-alpha: a potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia. 2000;32:42–50. [PubMed] [Google Scholar]
- Zhang J, Wu SM, Gross RL. Effects of beta-adrenergic blockers on glutamate-induced calcium signals in adult mouse retinal ganglion cells. Brain Res. 2003;959:111–119. doi: 10.1016/s0006-8993(02)03735-6. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Sarge KD. Celastrol inhibits polyglutamine aggregation and toxicity though induction of the heat shock response. J Mol Med. 2007;85:1421–1428. doi: 10.1007/s00109-007-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]