Abstract
Background
The addition of interferon alpha (IFN) to adjuvant chemoradiotherapy regimens resulted in remarkable improvements in survival for pancreatic cancer patients. However, systemic toxicities and insufficient levels of IFN at the tumor sites have limited its widespread adoption in treatment schemes. We have previously developed an IFN-expressing conditionally replicative oncolytic adenovirus and demonstrated its therapeutic effects both in vitro and in vivo. Here, the same vectors were tested in a syngeneic and immunocompetent Syrian hamster model to better understand the roles of adenoviral replication and of IFN’s pleiotropic effects on pancreatic tumor growth suppression.
Methods
Oncolytic adenoviruses expressing human or hamster IFN were designed and generated. Viral vectors were tested in vitro to determine qualitative and quantitative cell viability, Cox2 promoter activity, and IFN production. For the in vivo studies, subcutaneous hamster pancreatic cancer tumors were treated with one intratumoral dose of virus. Similarly, one intraperitoneal dose of virus was used to prolong survival in a carcinomatosis model.
Results
All cell lines tested demonstrated Cox2 promoter activity. The oncolytic potential of a replication competent adenovirus expressing the IFN cytokine was clearly demonstrated. These viruses resulted in significant tumor growth suppression and survival increases compared to controls in a hamster model.
Conclusions
The profound therapeutic potential of an IFN-expressing oncolytic adenovirus for the treatment of pancreatic cancer was demonstrated in a syngeneic Syrian hamster model. These results strongly suggest the potential application of our viruses as part of combination regimens with other therapeutics.
Introduction
Pancreatic cancer is one of the most lethal types of solid tumors with an overall five year survival rate of approximately 6%1. These poor outcomes are in part due to the fact that many patients present with locally advanced or metastatic disease. In fact, less than 20% of patients are candidates for surgical resection at the time of initial presentation2. Unfortunately, the resulting overall median survival times are less than 8 months for stage III disease and less than 3 months for stage IV disease3.
Interferon alpha (IFN) is a cytokine with pleotropic effects. It not only has antiviral characteristics, but it also has antitumor, anti-angiogenic, and immunomodulatory properties4, 5. IFN is a well-studied and widely used cytokine that has been employed in the treatment of a variety of hematologic and solid malignancies. Furthermore, IFN’s role as a chemotherapy and radiotherapy sensitizer has been well documented4–10.
Adjuvant treatment regimens of chemotherapy with or without radiation have been the standard of care for pancreatic cancer11. Recent studies have combined these modalities with IFN and achieved encouraging results. The group at Virginia Mason Medical Center conducted a phase II clinical trial where they combined IFN with cisplatin, fluorouracil, and radiotherapy as part of their adjuvant treatment regimen. They reported a two year actuarial survival of 84%12. In a follow up study with higher patient enrollment, they achieved survival rates for 1, 2, and 5 years of 95%, 64%, and 55% respectively13.
While the potential benefits of using IFN as part of a multimodality approach are clear, it is not without its shortcomings. One of the main drawbacks of systemic IFN therapy has been the associated dose-limiting systemic side effects. Many studies, including the Virginia Mason trials, have had significant interruptions in treatment due to the toxicities caused by systemic IFN therapy12, 13. Additionally, in a recent phase III clinical trial from Germany examining adjuvant IFN-based chemoradiation, up to 85% of patients in the treatment arm reported a grade 3 or 4 toxicity14.
Adenovirus-based vectors are an emerging class of cancer therapeutics. Notably, IFN-expressing adenoviral vectors are being used with increasing frequency. Several research groups have demonstrated the practicality of this approach with replication deficient and replication competent adenoviral vector systems15–18. In particular, a group from the M.D. Anderson Cancer Center applied replication deficient IFN-expressing adenoviruses to urological cancers19. Additionally, the Aoki group was the first to employ a replication deficient adenovirus expressing an IFN transgene for the treatment of pancreatic adenocarcinoma20. To further enhance the effect of an IFN-expressing adenovirus, our group developed an infectivity-enhanced, conditionally replicative adenovirus (CRAd) that is designed to selectively replicate and spread within pancreatic cancer cells expressing cyclooxygenase 2 (Cox2). The incorporation of the human IFN transgene into the E3 region of the adenovirus genome allows for a high concentration of IFN to be locally produced at the tumor site with each successful cycle of viral replication21, 22. To overcome the low infectivity and improve upon the oncolysis of conventional CRAds, the virus was designed to include an Ad5/Ad3 chimeric fiber and overexpression of the adenovirus death protein (ADP). We have recently reported that this infectivity-enhanced, tumor-specific CRAd demonstrated a greatly improved oncolytic effect mediated by IFN overexpression. The virus was effective in both in vitro and in vivo settings, including a human pancreatic cancer xenograft model in nude mice22.
The evaluation of oncolytic adenoviruses with human cancer xenografts transplanted in immunodeficient mice does have limitations. To begin with, murine cells do not permit the replication of human adenovirus23, 24, which may affect the analyses of biodistribution and adenovirus-mediated toxicity. Moreover, it is impossible to assess the immunomodulatory effects of IFN in an animal model with an impaired immune system.
In this study, we investigated the anti-tumor potential of an IFN-expressing vector using an immunocompetent Syrian hamster model, which is capable of supporting the replication of human adenovirus25, 26. This syngeneic experimental system permits investigation of the therapeutic and immunomodulatory potential of IFN-expressing vectors. Therefore, analyses with this model provide critical information for the clinical translation of replicative adenovirus-mediated IFN therapy.
Materials and methods
Cell lines and culture conditions
The Syrian hamster pancreatic cancer cell lines Hap-T1, HP-1, Taka-1, and PC-1 (courtesy of Dr. M. A. Hollingsworth, University of Nebraska, USA) were cultured in Dulbecco’s modified Eagle medium (DMEM) (Mediatech, Herndon, VA). Syrian hamster PGHam-1 cells (courtesy of Dr. Uchida, Department of Surgery, Nippon Medical School, Tokyo, Japan) were propagated in Roswell Park Memorial Institute medium (RPMI) (Mediatech)27. The human pancreatic ductal adenocarcinoma (PDAc) cell line MIA PaCa-2 (American Type Culture Collection (ATCC), Manassas, VA) and the Cox2-positive lung cancer cell line A549 (ATCC) were grown in DMEM. All cell lines were supplemented with fetal bovine serum (10% for cell line wakeup, 5% for culture maintenance) and a 1% penicillin-streptomycin mixture (100 IU/mL and 100 µg/mL, respectively) and were maintained as adherent monolayers at 37°C in a humidified incubator with 5% CO2 in air.
Adenoviral vectors
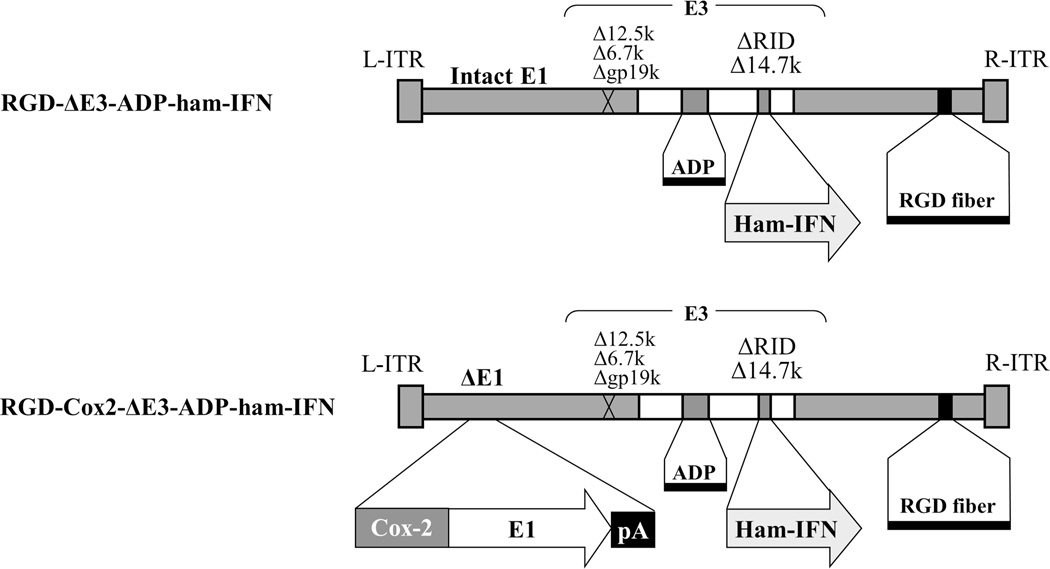
The IFN-expressing vectors (Figure 1) were based on adenovirus type 5 (Ad5) and contained the Ad-ΔE3-ADP structure as we have previously described21, 22, 28. Briefly, most of the non-essential adenovirus E3 genes have been deleted (with the exception of the adenovirus death protein (ADP) which is designed to facilitate viral spread and oncolysis) and replaced with the hamster IFN gene29, 30. All vectors involved in these studies included an RGD-modified fiber. In the CRAd (RGD-Cox2-ΔE3-ADP-ham-IFN), the adenovirus E1 region was placed under control of the Cox2 tumor-specific promoter.
Figure 1. Schematic of oncolytic adenoviruses expressing interferon alpha.
Both viruses have a RGD-4C motif incorporated into the fiber knob region for infectivity enhancement. Additionally, an expression cassette containing the adenovirus death protein (ADP) and the hamster interferon alpha transgene (IFN) were placed into the adenovirus E3 region. In the CRAd, the Cox2 promoter was inserted into the 5’-side of the adenovirus E1 region to serve as a tissue-specific promoter.
The human IFN coding plasmid was kindly provided by Dr. Aoki (Genetics Division, National Cancer Center Research Institute, Tokyo, Japan)18. As control vectors, the identical adenoviral vectors expressing the firefly luciferase (Luc) gene have been used21, 31, 32.
All viruses were propagated in the 911 cell line, purified by double cesium chloride density gradient ultracentrifugation, and dialyzed against phosphate-buffered saline (PBS) with 10% glycerol. The vectors were titrated by plaque forming assay, and the viral particle (vp) number was measured spectrophotometrically. Viral quality was confirmed by polymerase chain reaction for the presence of the Cox2 promoter, the fiber structure, and the absence of contamination with a mutant replication competent adenovirus as described previously21, 22, 31, 33.
In vitro analysis of Cox2 promoter activity
Five different hamster pancreatic cancer cell lines (Hap-T1, HP-1, Taka-1, PGHAM-1, and PC-1) were plated in 24-well plates (5×104 cells/well). Subsequently, they were infected with replication-deficient, Cox2 promoter-controlled, luciferase expressing vectors at 100 vp/cell. Two days after infection, the cells were lysed with 100 µl of cell culture lysis buffer, and the luciferase activity was determined with the Luciferase Assay System (Promega, Madison, WI). Results were standardized with protein concentration quantitated by the DC protein assay (Bio-Rad, Hercules, CA). The A549 cell line was used as a Cox2 positive control22.
Qualitative evaluation of in vitro cytocidal effect of oncolytic adenoviruses
The in vitro cytocidal effect was analyzed via crystal violet staining. One day after 2×105 cells/well were grown in a 12-well plate, the RGD-Cox2-ΔE3-ADP-Luc virus was added at strengths of 1, 10, 100, and 1000 vp/cell. After 10 days of cultivation, the cells were fixed with 10% buffered formalin for 10 minutes and stained with 1% crystal violet in 70% ethanol for 20 minutes. Thereafter, the plates were washed 3 times with tap water and allowed to air dry.
In vitro quantitative cell viability analysis
3,000 cells/well were cultured in 96-well plates and subsequently infected with adenoviral vectors at strengths of 100, 1000, or 10,000 vp/cell. The number of surviving cells was analyzed by a colorimetric method using the Cell Titer Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI) as described by the manufacturer. Absorbance was measured at a wavelength of 490 nm in a FLUOstar Omega spectrophotometer (BMG Labtech, Ortenberg, Germany). The proportion of living cells at each time point was normalized to the number of living uninfected cells.
In vitro IFN production by ELISA
HP-1 cells (5×104 cells/well) were cultured in 24-well plates and infected with adenoviral vectors at a dose of 10 vp/cell. At serial time points, cell culture supernatant was collected and centrifuged to remove cell debris. Samples were analyzed for IFN concentration by the use of a commercial mouse IFN ELISA kit (4211-1, PBL Interferonsource, Piscataway, NJ) according to the manufacturer’s instructions.
In vivo analyses in a Syrian hamster model
Female Syrian (golden) hamsters (Mesocricetus auratus, HsdHan: AURA; 6–7 weeks of age) were obtained from Harlan Sprague Dawley (Indianapolis, IN). Before procedures, the animals were anesthetized with isoflurane. Hamster pancreatic cancer cells (HP-1, 2×106 cells in 100ul of PBS) were subcutaneously inoculated into both flanks of each animal. When the tumor nodules achieved a diameter of approximately 15mm, a single dose (2×1011 vp/tumor) of virus was injected into the tumors. The tumor diameter was measured twice per week with calipers. The tumor volume was calculated using the following formula: Tumor Volume = (Width2 × Length)/2. The hamsters were sacrificed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota.
For the survival study in a peritoneal dissemination model, the hamsters were anesthetized with isoflurane, tilted steeply head-down, and HP-1 cells (1×106 cells in 500 µl of PBS) were injected into the peritoneal cavity at a 30 degree angle. Seven days after tumor inoculation, the hamsters received a single intraperitoneal injection (2×1011 vp in 1.5 mL of PBS) of virus or were left untreated. The endpoint of the study was either the natural death of the animals or the IACUC criteria.
For in vivo imaging of adenovirus replication, HP-1 cells (2×106 cells) were subcutaneously injected into the flanks of the hamsters. When tumor nodules reached a diameter of 15 mm, the luciferase-expressing oncolytic adenovirus (RGD-Cox2-ΔE3-ADP-Luc) was injected intratumorally (1×1010 vp/tumor). Bioluminescence was assessed over a 5 week time-course with an optical imaging system as described previously28, 34. The imaging data were displayed as a pseudocolored luminescence intensity image overlaid on a brightfield image of the entire hamster body. Index color image overlays were performed in Photoshop 7.0 (Adobe, Seattle, WA, USA).
All procedures were carried out according to protocols approved by the IACUC of the University of Minnesota.
Statistical methods
Statistical analyses of in vitro and in vivo viral effects were carried out with Excel (Microsoft, Redmond, WA). The Student’s t test was used to compare the percentage of living cells between treatment groups, the relative units of IFN production, and the relative volumes of in vivo treatment groups. The results were considered statistically significant when a two-tailed P value was less than 0.05. Data are expressed as a mean ± standard deviation, unless otherwise noted.
In the survival study, the Kaplan-Meier method was used to estimate survival from the day of tumor inoculation to natural death or sacrifice. Treatment groups were compared using the log-rank test with a Tukey-Kramer adjustment for multiple pairwise comparisons.
Results
Selectivity of oncolytic adenoviruses in a Syrian hamster model
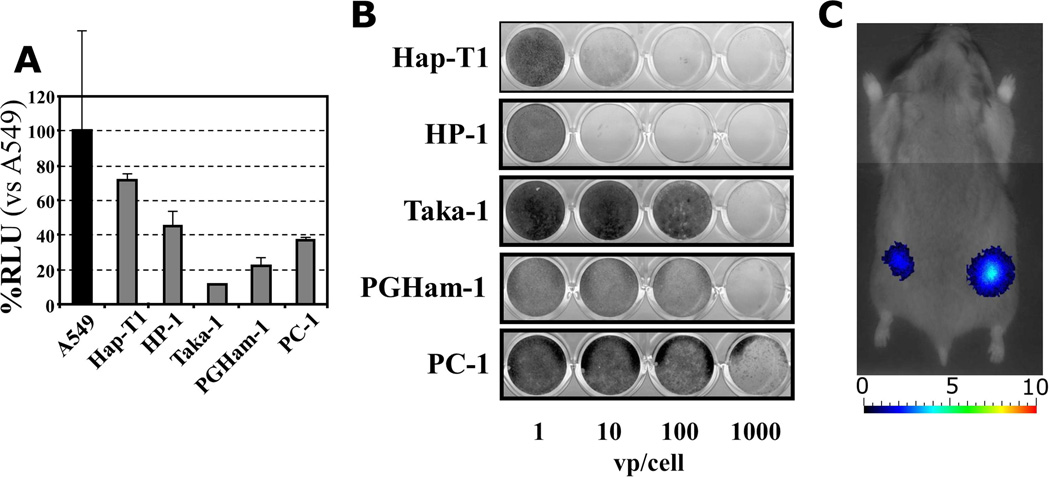
Replication deficient vectors driven by the Cox2 promoter and expressing the luciferase transgene were used to analyze the Cox2 promoter activity in five hamster pancreatic cancer cell lines (Figure 2A). A549 (human lung adenocarcinoma) was used as a Cox2-positive cell line22, 31. Hap-T1 and HP-1 demonstrated the highest Cox2 promoter activity with over 70% and 40% RLU respectively when compared to the A549 control cell line.
Figure 2. Syngeneic immunocompetent hamster model.
A. The Cox2 promoter activity of five hamster pancreatic cancer cell lines (Hap-T1, HP-1, Taka-1, PGHam-1, and PC-1) was analyzed. The A549 (human lung adenocarcinoma) cell line was used as a Cox2-positive control, and results were reported in relative light units (RLU) with respect to the A549 cells. All cell lines demonstrated Cox2 promoter activity, with the highest being in the Hap-T1 and HP-1 cells.
B. Ability of human adenovirus to replicate in hamster pancreatic cancer cell lines. The RGD-Cox2-ΔE3-ADP-Luc virus was added at various concentrations and the crystal violet assay was used to qualitatively analyze cell viability. In the HP-1 and Hap-T1 cell lines, even low titers of 10 vp/cell were able to induce total cell death. At high viral titers, the virus achieved a complete cytocidal effect in all cell lines.
C. Active replication of a Cox2 promoter controlled adenovirus (RGD-Cox2-ΔE3-ADP-Luc) in a hamster model was demonstrated via bioluminescent imaging. Following a single intratumoral injection, a signal was evident as early as the day after inoculation and persisted for up to 4 weeks (day 5 image shown).
To confirm the ability of human adenovirus to replicate in hamster pancreatic cancer cell lines, the in vitro cytocidal effects of a replication competent adenovirus (RGD-Cox2-ΔE3-ADP-Luc) were tested (Figure 2B). In the HP-1 and Hap-T1 cell lines, low concentrations of virus (10 vp) had a complete cytocidal effect. At high viral titers, complete oncolysis was achieved in all tested cell lines.
Additionally, the RGD-Cox2-ΔE3-ADP-Luc virus was used to demonstrate active in vivo replication via non-invasive bioluminescent imaging (Figure 2C). Signal was recorded as early as the day after a single intratumoral injection into a subcutaneous HP-1 tumor, and persisted for up to four weeks. Given that the luciferase transgene from the adenoviral E3 region is only expressed during viral replication, this provided evidence of effective replication of our virus in a Cox2-expressing tumor. The background signals of other organs (indicating ectopic viral replication) were minimal, especially within the liver.
This series of experiments demonstrated the Cox2 promoter activity of five hamster pancreatic cancer cell lines as well as the ability of our adenoviral vectors to replicate within them in both in vitro and in vivo settings.
Gene transfer of hamster and human IFN via replication deficient vectors to analyze direct cytotoxic effect and species specificity
Human and hamster IFN were cloned into replication deficient adenoviral vectors to enable the analysis of the therapeutic effect of IFN in both human and hamster cell lines. Quantitative cell viability assays were performed to study the direct cytotoxic effect of IFN expression. A luciferase expressing adenovirus was used as a control vector (Figure 3).
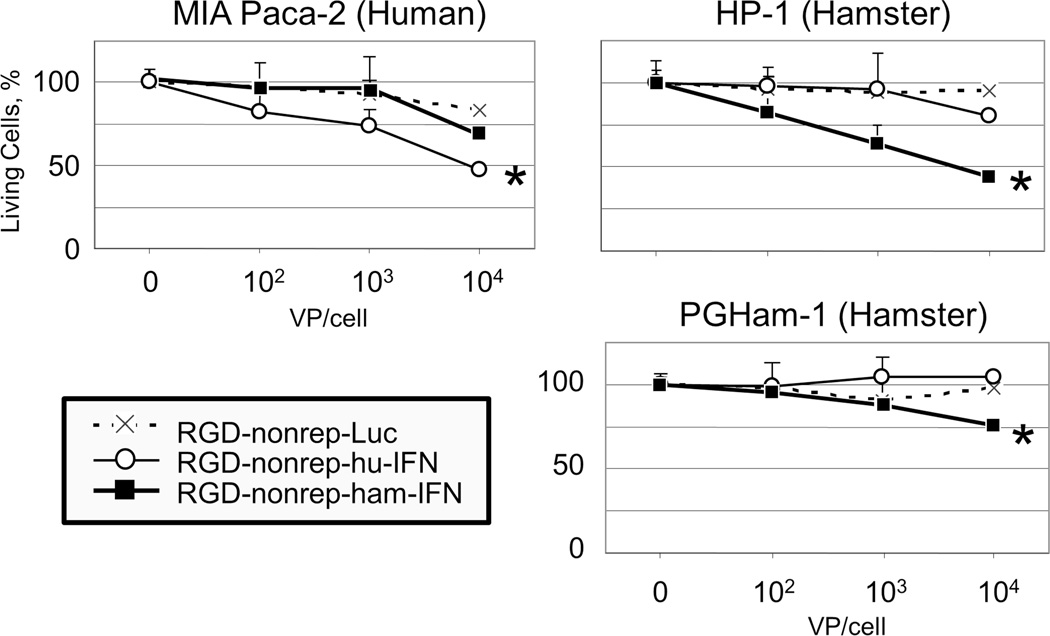
Figure 3. Cytocidal effect and species specificity of IFN-expressing vectors.
One human pancreatic cancer cell line (MIA PaCa-2) and two hamster pancreatic cancer cell lines (HP-1 and PGHam-1) were infected with three non-replicating adenoviruses that only differed in the transgene that they expressed (RGD-nonrep-Luc, RGD-nonrep-hu-IFN, RGD-nonrep-ham-IFN). Gradually increasing doses of virus were used (102, 103, and 104 vp/cell), and the percentages of living cells were quantified using the MTS assay four days after viral infection. Results are shown as a percentage of living cells.
In the MIA PaCa-2 cell line, the human IFN-expressing adenovirus demonstrated a significant cytocidal effect when compared to the luciferase control vector (* p<0.05), while the hamster IFN-expressing vector demonstrated minimal cell death. Conversely, in the HP-1 and PGHam-1 cell lines, the hamster IFN-expressing adenovirus resulted in significant cell death (* p<0.05) and greatly outperformed its human IFN-expressing counterpart.
It was clear that the IFN-expressing viruses outperformed their luciferase expressing counterparts in all cell lines. Given that these two vectors only differed in their transgene expression, the clearly apparent increase in cell death provided evidence of the IFN cytokine’s direct anti-tumor effects.
The efficacy of our vectors was compared in human and hamster cell lines. In human MIA Paca-2 cells, the human IFN-expressing virus outperformed its hamster IFN-expressing virus counterpart by killing approximately 25% more cells. The reverse was true in the hamster HP-1 cell line where the hamster IFN-expressing virus had a much stronger cytocidal effect resulting in 35% more cell death. By confirming the augmented cytotoxic effect of human and hamster IFN in their corresponding cell lines, the species-specific nature of the IFN cytokine was demonstrated. Of note, the syngeneic IFN-expressing viruses demonstrated significant cytocidal effects compared to the luciferase vector (p<0.05).
Delivery of IFN with a replication competent adenoviral system resulted in improved in vitro oncolysis and IFN production
The percentages of living cells after treatment with a low dose of replication deficient and replication competent IFN-expressing vectors were compared (Figure 4A). At each time point in both cell lines, the replication competent vector was clearly superior and resulted in statistically significant cell death (p<0.05). Notably, at days 5 and 6 in the HP-1 cell line, the replicating IFN virus killed almost all of the cells while the non-replicating counterpart killed only 40–50% of the cells.
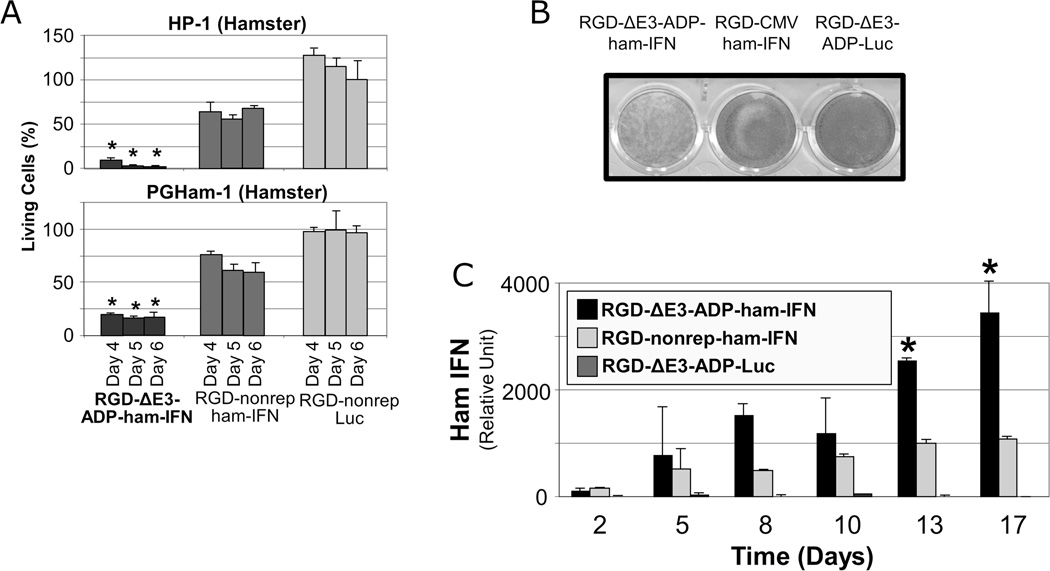
Figure 4. Production of IFN with a replication competent adenoviral system resulted in improved in vitro oncolysis and elevated levels of IFN.
A. Superior oncolysis of IFN-expressing replication competent vectors. HP-1 and PGHam-1 hamster pancreatic cancer cells were infected with a replicating IFN-expressing virus (RGD-ΔE3-ADP-ham-IFN), a non-replicative IFN-expressing virus (RGD-nonrep-ham-IFN), or a non-replicative luciferase virus (RGD-nonrep-Luc) at 1000 vp/cell. The percentage of living cells was determined using the MTS assay at days 4,5, and 6 following viral infection. The oncolytic effect of the replication competent IFN-expressing adenovirus was significantly better than either of the non-replicative vectors (*p<0.05).
B. Crystal violet assay demonstrates the greatest degree of cell death with replication competency and IFN expression via the RGD-ΔE3-ADP-ham-IFN virus. 1×105 cells were cultured in a 12 well plate and subsequently infected with viruses at a strength of 100 vp/cell. Crystal violet staining was performed eight days following viral inoculation.
C. Hamster pancreatic cancer cells (HP-1) were infected at 10 vp/cell, and the IFN level in the culture supernatant was determined by ELISA. At day 13 following viral inoculation and until the end of the experiment, the replicating IFN virus (RGD-ΔE3-ADP-ham-IFN) showed significantly higher IFN expression compared to its non-replicative counterpart (RGD-nonrep-ham-IFN) and the luciferase control (RGD-ΔE3-ADP-Luc) (* p<0.05).
The cytocidal effects of IFN-expressing vectors were qualitatively studied with a crystal violet assay (Figure 4B). Not surprisingly, the replicating IFN-expressing vector (RGD-ΔE3-ADP-ham-IFN) provided the strongest cytocidal effect.
IFN production was measured after infection with replication competent and replication deficient vector systems (Figure 4C). The replication competent vector had three times more IFN expression compared to the non-replicating vector (p<0.05).
Replication competent adenoviruses can be designed to express transgenes in a replication-dependent manner. Here, our replicating IFN-expressing virus not only demonstrated improved direct cell lysis but also superior IFN transgene expression.
In vivo tumor suppression with IFN-expressing adenoviruses
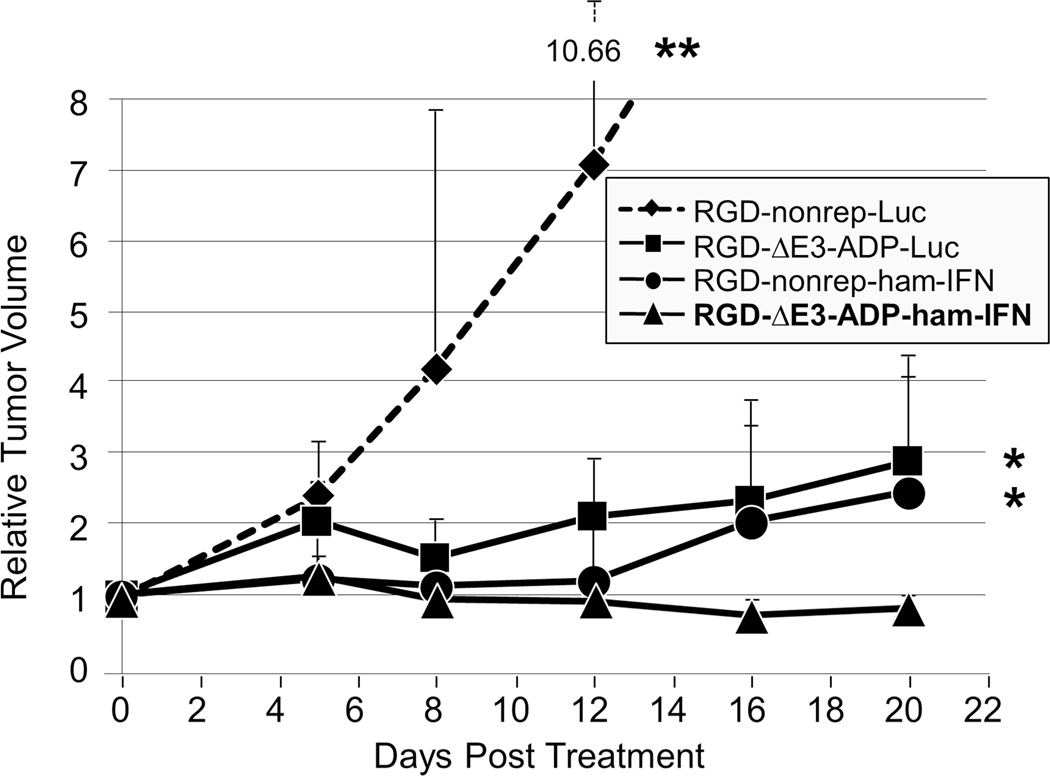
The antitumor effects of replication-deficient and replication-competent IFN-expressing adenoviruses were compared in vivo in a syngeneic subcutaneous pancreatic cancer model (Figure 5). From early time points, it was clear that both IFN-expressing viruses demonstrated a significant therapeutic effect compared to the replication deficient luciferase control vector (p≤0.01 for both comparisons at day 12). In fact, the animals in the group treated with the luciferase control vector had to be sacrificed at day 12 due to increased tumor size (relative volume of 7.10). The non-replicating IFN and luciferase viruses were identical except for the transgene that they expressed. Therefore, the great difference in therapeutic effect can be attributed to the strong cancer-killing capabilities of the IFN cytokine in this immunocompetent model.
Figure 5. In vivo antitumor effect of IFN-expressing oncolytic adenovirus.
Hamsters bearing subcutaneous HP-1 tumors were treated with a single intratumoral injection of adenovirus (2×1011 vp/tumor). The replication competent IFN-expressing virus (RGD-ΔE3-ADP-ham-IFN, n=10 tumors) significantly outperformed its non-replicative counterpart (RGD-nonrep-ham-IFN, n=8 tumors) and an otherwise identical virus that expressed luciferase (RGD-ΔE3-ADP-Luc, n=4 tumors) (*p<0.05). The RGD-ΔE3-ADP-ham-IFN virus also demonstrated a significant oncolytic effect when compared to the non-replicative luciferase expressing adenovirus (RGD-nonrep-Luc, n=6 tumors) (**p<0.01). Tumor size is shown as a relative volume compared to day 0.
By the end of the experiment, the replication competent IFN virus had caused tumor regression (final relative volume of 0.82) which was in stark contrast to the group treated with the non-replicating IFN virus that allowed tumor re-growth (p<0.05 at day 20). Given that the only difference between these two viruses is the ability to deliver replication dependent IFN expression, it provided support of the oncolytic adenovirus’ ability to augment IFN concentration locally at the tumor site.
The replication competent IFN-expressing virus outperformed its luciferase counterpart at all time points (p<0.05) and further supported the improved anti-tumor effects of the IFN-expressing viruses. Additionally, while it was not statistically significant, the replication competent luciferase expressing vector also showed anti-tumor effect when compared to non-replicative luciferase vector.
This experiment used a syngeneic subcutaneous tumor model to demonstrate the improved cytocidal effect when replication competent vectors are combined with IFN expression.
Survival study in a hamster peritoneal dissemination model following systemic adenovirus delivery
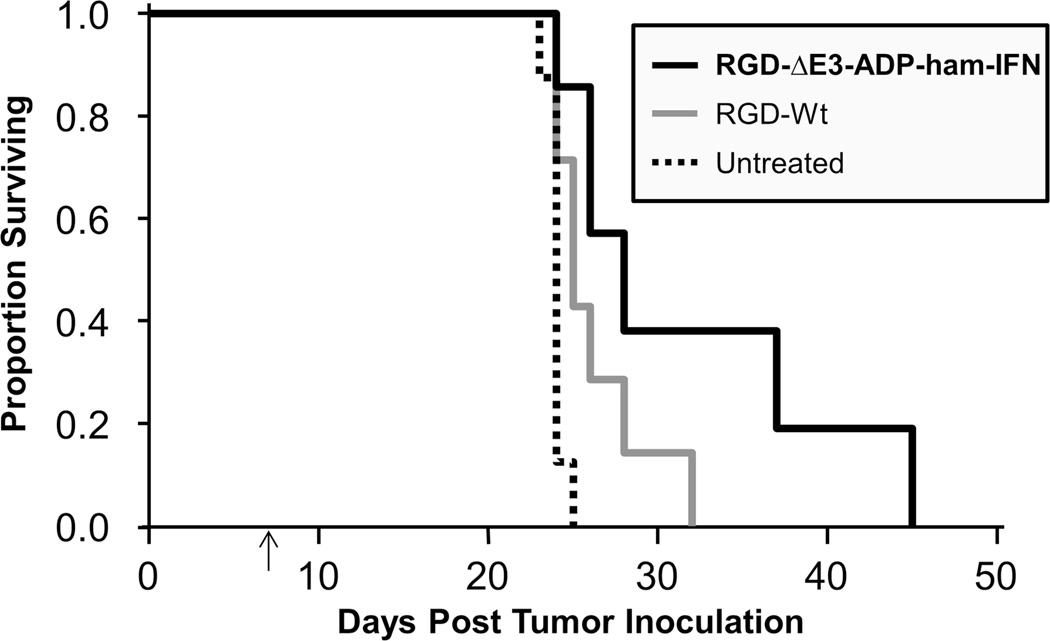
A peritoneal carcinomatosis model was established to analyze the clinical potential of our replicating IFN vector in more aggressive model. For this experiment, a single intraperitoneal injection of adenovirus was given seven days following inoculation with HP-1 cells (Figure 6).
Figure 6. Improved survival in a peritoneal carcinomatosis model.
HP-1 cells (1×106) were injected into the peritoneal cavity of Syrian hamsters on day 0. A single intraperitoneal dose of adenovirus (2×1011 vp) was administered seven days thereafter (indicated by arrow) or the animals were left untreated. Survival rate was significantly improved with the IFN-expressing virus (RGD-ΔE3-ADP-ham-IFN) compared to the untreated control (p=0.001). The wild type virus (RGD-Wt) also demonstrated a survival improvement, but it was not statistically significant. Results are shown as a proportion of surviving animals according to the Kaplan-Meier method. There were 8 animals in the untreated control group and 7 animals in the groups that received virus.
There had been only one death in the IFN group by the time all untreated animals had died (day 25). In fact, one hamster treated with the IFN-expressing oncolytic adenovirus was able to survive until day 45, which resulted in a statistically significant improvement in survival (p=0.001) when compared to the untreated animals.
A wild type adenovirus was also used as a control treatment vector in the experiment. All animals in this group were dead by day 32.
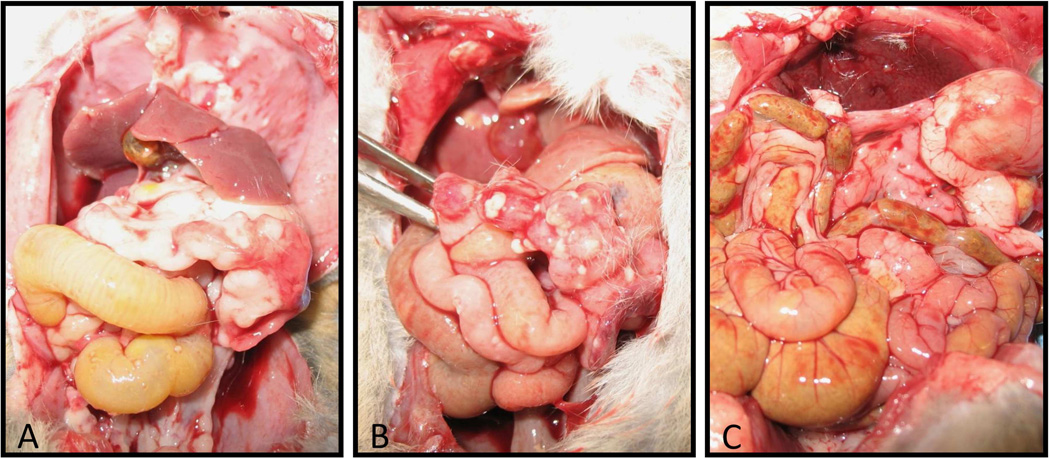
When the untreated animals were examined at necropsy, there was evidence of substantial tumor burden on the abdominal wall and all intra-abdominal organs. This was in contrast to the animals that were treated with the IFN-expressing vector which had considerably less disease (Figure 7).
Figure 7. Levels of peritoneal tumor burden following viral treatment.
A: Untreated animal examined following death at day 24 (following tumor inoculation). There is a large amount of tumor within the bowel that caused marked adhesions, as well as many deposits along the lining of the peritoneal cavity. B: Animal treated with the RGD-Wt vector following death at day 26. Compared to the untreated animal, there is only a small amount of tumor deposition along the bowel and abdominal wall. C: Animal treated with the IFN vector following death at day 28. Very small amounts of tumor deposition within the abdominal cavity.
Thus, the impressive therapeutic effect of the replicating IFN-expressing oncolytic virus was observed in a more aggressive model simulating advanced disease.
In vivo tumor suppression with an IFN-expressing virus controlled by a tumor-specific promoter
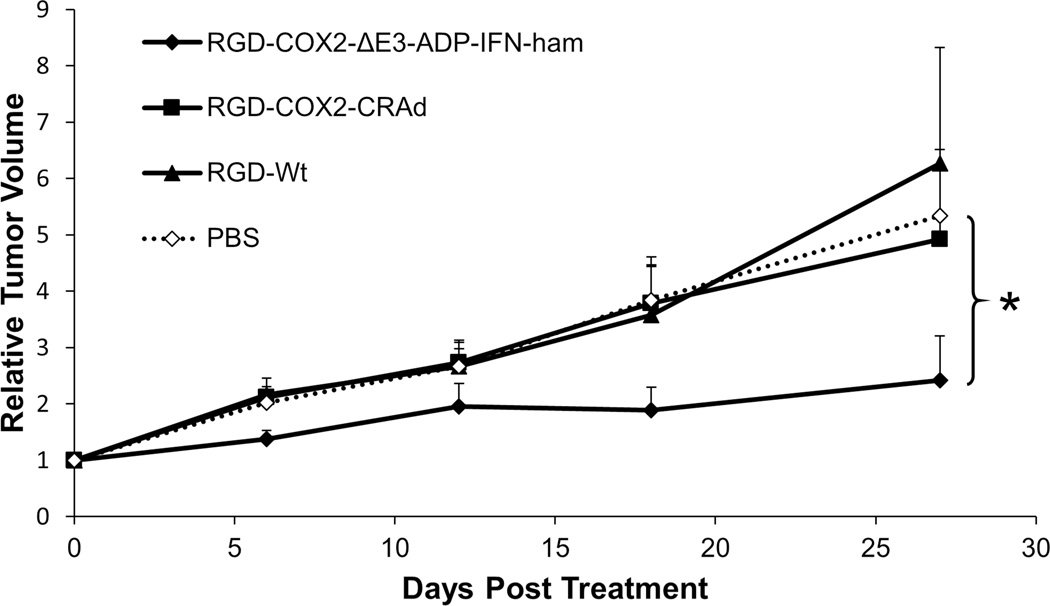
Two Cox2 promoter controlled adenoviruses (with and without IFN expression) were tested in Cox2-positive hamster pancreatic cancer tumors (Figure 8). It was clear from very early in the experiment that the Cox2-IFN virus was superior to all others. It demonstrated an almost 55% reduction in tumor growth compared to the PBS group at day 27 (p<0.05), while the two other treatment viruses (RGD-Cox2-CRAd and RGD-Wt) showed no therapeutic effect.
Figure 8. In vivo therapeutic effect of a Cox2-controlled IFN-expressing adenovirus.
Subcutaneous tumors (established with the HP-1 hamster pancreatic cancer cell line) were treated with a single intratumoral injection of adenovirus (3×1010 vp/tumor). The promoter-controlled IFN-expressing adenovirus (RGD-Cox2-ΔE3-ADP-ham-IFN) significantly outperformed the control animals treated with PBS (*p<0.05). Tumor size is shown as a relative volume compared to day 0. There were 10 tumors in each treatment group. Error bars indicate standard error of the mean.
The Cox2-IFN oncolytic adenovirus significantly outperformed the control virus lacking IFN expression as well as the wild type replication adenovirus. These observations highlighted that the insertion of the Cox2 promoter to control adenovirus replication did not detract from the anti-tumor effect of the viral construct.
Discussion
Our research group has developed a tumor-selective IFN-expressing oncolytic adenovirus which is driven by the Cox2 promoter21 and tested it in an immunocompetent Syrian hamster model. We have demonstrated the direct antitumor effect of IFN, as well as its species-specific nature. In addition, replication competent viruses demonstrated a much improved cytocidal effect as well as a greater degree of local IFN expression when compared to their non-replicating counterparts. When tested in subcutaneous tumor and peritoneal dissemination models, the IFN-expressing viruses demonstrated a profound therapeutic effect.
The coxsackie-adenovirus receptor (CAR) is the primary receptor for adenoviruses, but it is poorly expressed on pancreatic cancer cells33, 35. Therefore, modification of the viral capsid proteins has been shown to increase adenovirus infectivity36, 37. We have previously used an Ad5/Ad3 chimeric fiber that targets the Ad 3 receptor (recently identified as human desmoglein-2)38, 39 as well as a RGD (arginine-glycine-aspartate)-modified fiber which targets integrins on cell surfaces of pancreatic cancer33, 40. While the Ad5/Ad3 fiber modification is useful for improving the infectivity of conventional adenoviral vectors in human cancers, it is not optimal for hamster cell lines as they do not express the Ad3 receptor. Therefore, the RGD-based fiber modification was employed exclusively in these studies to maximize the infectivity of our viral vectors in a hamster model.
By comparing non-replicating viruses that only differed in the expression of the IFN or luciferase transgene, we were able to demonstrate the direct antitumor effect of IFN expression, which was dose dependent. Furthermore, we demonstrated that replication competent viruses expressing IFN have a much greater ability to cause cell death when compared their non-replicating counterparts. This observation is due to the fact that replication competent viruses produce significantly higher levels of IFN compared to the non-replicative controls, as well as the fact that replicating adenoviruses lyse cells as part of their replication cycle. Additionally, in the replicating vector construct, the adenovirus death protein is overexpressed and augments viral spread which subsequently improves oncolysis29, 30.
In a syngeneic tumor model, the IFN-expressing viruses proved to have the greatest anti-tumor effects, which correlated with our in vitro observations. Most remarkably, the replicating IFN-expressing virus did not just slow tumor progression, but rather caused statistically significant tumor regression.
The same replication competent IFN vector was also tested in a hamster peritoneal carcinomatosis model. We believe that this model simulates the setting of advanced disease, and other groups have used a similar model in mice to demonstrate the effectiveness of intraperitoneal virus therapy41. In our experiment, there was a statistically significant increase in the survival of the animals treated with just one dose of the IFN-expressing virus. Some animals lived almost twice as long as the untreated controls and had considerably less intraabdominal tumor burden.
Cyclooxygenase 2 (Cox2) is highly expressed in pancreatic adenocarcinoma cells and many other human cancers42. In addition, there have been reports of Cox2 expression in hamster pancreatic cell lines43, and our data indicated that the Cox2 promoter placed within the adenoviral vector worked in hamster cells as intended. Therefore, the Syrian hamster model is suitable for further preclinical evaluation of the efficacy of our Cox2-controlled oncolytic viruses.
Our final in vivo investigation involved two tumor-specific conditionally replicative adenoviruses (CRAd). Here, our novel IFN-expressing CRAd was the only treatment vector to demonstrate statistically significant tumor growth suppression (Figure 8). The growth curve for the IFN-expressing virus diverges almost immediately from the other vectors (all of which lacked IFN expression) and remained so for the duration of the experiment. However, compared to the previous in vivo study without tumor-specific promoters (Figure 5), there was overall tumor progression. Reducing potential toxicities to normal organs is necessary for clinical vectors, and it has been shown that viruses with the Cox2 promoter have limited replication in normal liver tissue and have a low toxicity profile in vivo21, 33, 44. However, in our experiment, incorporation of the promoter into the adenovirus structure may have resulted in a small decrease in oncolytic potential. This observation could also be explained by the HP-1 cell line’s degree of Cox2 promoter activity (approximately 40% compared to the human A549 Cox2-positive cancer cell line).
IFN-based chemoradiation has shown great potential to treat pancreatic cancer, but the systemic delivery of IFN is limited by dose-dependent toxicities. Additionally, serum concentrations peak soon after IFN injection and the cytokine is rapidly cleared resulting in a very low percentage of IFN reaching the tumor site45, 46. In this manuscript, we presented an IFN-expressing oncolytic adenovirus driven by a tumor-specific promoter that was tested in an immunocompetent Syrian hamster model. This model’s ability to support both adenoviral replication and immune system interaction played a key role in highlighting the true potential of IFN-expressing oncolytic viruses25, 26. The model’s characteristics also helped to explain why these studies led to a much improved therapeutic effect when compared to our previous work in a mouse model, which did not allow for adenoviral replication22–24. Overall, the results were encouraging as a locally delivered IFN-expressing adenovirus had a significant impact on tumor growth in a syngeneic hamster model. Adenoviral delivery of IFN is a promising therapy for pancreatic cancer, and this work represents a key step towards clinical translation. In the future, we plan on using this same hamster model to examine the therapeutic effect of our oncolytic viruses in conjunction with chemotherapy and radiation, which represents the strongest combination of therapies for pancreatic cancers.
Acknowledgements
We thank Dr. Eiji Uchida (Nippon Medical School, Tokyo, Japan) for providing the PGHAm cell line and Dr. Aoki (National Cancer Center Research Institute, Tokyo, Japan) for allowing us to use the hamster and human IFN-alpha coding plasmids.
Grant Funding:
This project was partly supported by the following grants from National Institute of Health / National Cancer Institute: R01-CA174861-01A1 to JD, R01-CA094084 and R01-CA168448 to MY, P50-CA101955 (Pancreatic Cancer SPORE: Project #4 to MY and Developmental Project to JD), and T32CA132715 to SV/LA. Grant support was also provided by the National Institute for Minority Health Disparities: NIMHD/NIH-1P60MD003422 (SV). Additional funding was provided through a grant from the University of Minnesota-Veterans of Foreign Wars and Ladies Auxiliary (CL).
References
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Merchant NB, Parikh AA, Liu EH. Adjuvant chemoradiation therapy for pancreas cancer: who really benefits? Adv Surg. 2010;44:149–164. doi: 10.1016/j.yasu.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 4.Holsti LR, Mattson K, Niiranen A, Standertskiold-Nordenstam CG, Stenman S, Sovijarvi A, et al. Enhancement of radiation effects by alpha interferon in the treatment of small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1987;13:1161–1166. doi: 10.1016/0360-3016(87)90189-1. [DOI] [PubMed] [Google Scholar]
- 5.Wadler S, Wersto R, Weinberg V, Thompson D, Schwartz EL. Interaction of fluorouracil and interferon in human colon cancer cell lines: cytotoxic and cytokinetic effects. Cancer Res. 1990;50:5735–5739. [PubMed] [Google Scholar]
- 6.Vokes EE. The promise of biochemical modulation in combined modality therapy. Semin Oncol. 1994;21:29–33. [PubMed] [Google Scholar]
- 7.Ismail A, Van Groeningen CJ, Hardcastle A, Ren Q, Aherne GW, Geoffroy F, et al. Modulation of fluorouracil cytotoxicity by interferon-alpha and -gamma. Mol Pharmacol. 1998;53:252–261. doi: 10.1124/mol.53.2.252. [DOI] [PubMed] [Google Scholar]
- 8.Morak MJ, van Koetsveld PM, Kanaar R, Hofland LJ, van Eijck CH. Type I interferons as radiosensitisers for pancreatic cancer. Eur J Cancer. 2011;47:1938–1945. doi: 10.1016/j.ejca.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Ma JH, Patrut E, Schmidt J, Knaebel HP, Buchler MW, Marten A. Synergistic effects of interferon-alpha in combination with chemoradiation on human pancreatic adenocarcinoma. World J Gastroenterol. 2005;11:1521–1528. doi: 10.3748/wjg.v11.i10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann K, Mehrle S, Schmidt J, Buchler MW, Marten A. Interferon-alpha restitutes the chemosensitivity in pancreatic cancer. Anticancer Res. 2008;28:1499–1507. [PubMed] [Google Scholar]
- 11.Antoniou G, Kountourakis P, Papadimitriou K, Vassiliou V, Papamichael D. Adjuvant therapy for resectable pancreatic adenocarcinoma: review of the current treatment approaches and future directions. Cancer Treat Rev. 2014;40:78–85. doi: 10.1016/j.ctrv.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Nukui Y, Picozzi VJ, Traverso LW. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2000;179:367–371. doi: 10.1016/s0002-9610(00)00369-x. [DOI] [PubMed] [Google Scholar]
- 13.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185:476–480. doi: 10.1016/s0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt J, Abel U, Debus J, Harig S, Hoffmann K, Herrmann T, et al. Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J Clin Oncol. 2012;30:4077–4083. doi: 10.1200/JCO.2011.38.2960. [DOI] [PubMed] [Google Scholar]
- 15.Ohashi M, Yoshida K, Kushida M, Miura Y, Ohnami S, Ikarashi Y, et al. Adenovirus-mediated interferon alpha gene transfer induces regional direct cytotoxicity and possible systemic immunity against pancreatic cancer. Br J Cancer. 2005;93:441–449. doi: 10.1038/sj.bjc.6602713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shashkova EV, Spencer JF, Wold WS, Doronin K. Targeting interferon-alpha increases antitumor efficacy and reduces hepatotoxicity of E1A-mutated spread-enhanced oncolytic adenovirus. Mol Ther. 2007;15:598–607. doi: 10.1038/sj.mt.6300064. [DOI] [PubMed] [Google Scholar]
- 17.Shashkova EV, Kuppuswamy MN, Wold WS, Doronin K. Anticancer activity of oncolytic adenovirus vector armed with IFN-alpha and ADP is enhanced by pharmacologically controlled expression of TRAIL. Cancer Gene Ther. 2008;15:61–72. doi: 10.1038/sj.cgt.7701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hara H, Kobayashi A, Yoshida K, Ohashi M, Ohnami S, Uchida E, et al. Local interferon-alpha gene therapy elicits systemic immunity in a syngeneic pancreatic cancer model in hamster. Cancer Sci. 2007;98:455–463. doi: 10.1111/j.1349-7006.2007.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benedict WF, Tao Z, Kim CS, Zhang X, Zhou JH, Adam L, et al. Intravesical Ad-IFNalpha causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFN-alpha protein. Mol Ther. 2004;10:525–532. doi: 10.1016/j.ymthe.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Hatanaka K, Suzuki K, Miura Y, Yoshida K, Ohnami S, Kitade Y, et al. Interferon-alpha and antisense K-ras RNA combination gene therapy against pancreatic cancer. J Gene Med. 2004;6:1139–1148. doi: 10.1002/jgm.602. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong L, Arrington A, Han J, Gavrikova T, Brown E, Yamamoto M, et al. Generation of a novel, cyclooxygenase-2-targeted, interferon-expressing, conditionally replicative adenovirus for pancreatic cancer therapy. Am J Surg. 2012;204:741–750. doi: 10.1016/j.amjsurg.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong L, Davydova J, Brown E, Han J, Yamamoto M, Vickers SM. Delivery of interferon alpha using a novel Cox2-controlled adenovirus for pancreatic cancer therapy. Surgery. 2012;152:114–122. doi: 10.1016/j.surg.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan SJ, Gordon FC, Gregory DW, McPhie JL, Postlethwaite R, White R, et al. Infection of mouse liver by human adenovirus type 5. J Gen Virol. 1978;40:45–61. doi: 10.1099/0022-1317-40-1-45. [DOI] [PubMed] [Google Scholar]
- 24.Ginsberg HS, Moldawer LL, Sehgal PB, Redington M, Kilian PL, Chanock RM, et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991;88:1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 26.Wold WS, Toth K. Chapter three--Syrian hamster as an animal model to study oncolytic adenoviruses and to evaluate the efficacy of antiviral compounds. Adv Cancer Res. 2012;115:69–92. doi: 10.1016/B978-0-12-398342-8.00003-3. [DOI] [PubMed] [Google Scholar]
- 27.Yanagi K, Onda M, Uchida E. Effect of angiostatin on liver metastasis of pancreatic cancer in hamsters. Jpn J Cancer Res. 2000;91:723–730. doi: 10.1111/j.1349-7006.2000.tb01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davydova J, Gavrikova T, Brown EJ, Luo X, Curiel DT, Vickers SM, et al. In vivo bioimaging tracks conditionally replicative adenoviral replication and provides an early indication of viral antitumor efficacy. Cancer Sci. 2010;101:474–481. doi: 10.1111/j.1349-7006.2009.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE, Wold WS. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- 30.Doronin K, Toth K, Kuppuswamy M, Ward P, Tollefson AE, Wold WS. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J Virol. 2000;74:6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davydova J, Le LP, Gavrikova T, Wang M, Krasnykh V, Yamamoto M. Infectivity-enhanced cyclooxygenase-2-based conditionally replicative adenoviruses for esophageal adenocarcinoma treatment. Cancer Res. 2004;64:4319–4327. doi: 10.1158/0008-5472.CAN-04-0064. [DOI] [PubMed] [Google Scholar]
- 32.Davydova J, Gavrikova T, Brown EJ, Luo X, Curiel DT, Vickers SM, et al. In vivo bioimaging tracks conditionally replicative adenoviral replication and provides an early indication of viral antitumor efficacy. Cancer Sci. 2010;101:474–481. doi: 10.1111/j.1349-7006.2009.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto M, Davydova J, Wang M, Siegal GP, Krasnykh V, Vickers SM, et al. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125:1203–1218. doi: 10.1016/s0016-5085(03)01196-x. [DOI] [PubMed] [Google Scholar]
- 34.Ono HA, Le LP, Davydova JG, Gavrikova T, Yamamoto M. Noninvasive visualization of adenovirus replication with a fluorescent reporter in the E3 region. Cancer Res. 2005;65:10154–10158. doi: 10.1158/0008-5472.CAN-05-1871. [DOI] [PubMed] [Google Scholar]
- 35.Wesseling JG, Bosma PJ, Krasnykh V, Kashentseva EA, Blackwell JL, Reynolds PN, et al. Improved gene transfer efficiency to primary and established human pancreatic carcinoma target cells via epidermal growth factor receptor and integrin-targeted adenoviral vectors. Gene Ther. 2001;8:969–976. doi: 10.1038/sj.gt.3301473. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann JK, Nettelbeck DM. Virus chimeras for gene therapy, vaccination, and oncolysis: adenoviruses and beyond. Trends Mol Med. 2012;18:365–376. doi: 10.1016/j.molmed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto M, Curiel DT. Current issues and future directions of oncolytic adenoviruses. Mol Ther. 2010;18:243–250. doi: 10.1038/mt.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Beyer I, Persson J, Song H, Li Z, Richter M, et al. A new human DSG2-transgenic mouse model for studying the tropism and pathology of human adenoviruses. J Virol. 2012;86:6286–6302. doi: 10.1128/JVI.00205-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Li ZY, Liu Y, Persson J, Beyer I, Moller T, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez PJ, Vickers SM, Ono HA, Davydova J, Takayama K, Thompson TC, et al. Optimization of conditionally replicative adenovirus for pancreatic cancer and its evaluation in an orthotopic murine xenograft model. Am J Surg. 2008;195:481–490. doi: 10.1016/j.amjsurg.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Ottolino-Perry K, Tang N, Head R, Ng C, Arulanandam R, Angarita FA, et al. Tumor vascularization is critical for oncolytic vaccinia virus treatment of peritoneal carcinomatosis. Int J Cancer. 2014;134:717–730. doi: 10.1002/ijc.28395. [DOI] [PubMed] [Google Scholar]
- 42.Wesseling JG, Yamamoto M, Adachi Y, Bosma PJ, van Wijland M, Blackwell JL, et al. Midkine and cyclooxygenase-2 promoters are promising for adenoviral vector gene delivery of pancreatic carcinoma. Cancer Gene Ther. 2001;8:990–996. doi: 10.1038/sj.cgt.7700403. [DOI] [PubMed] [Google Scholar]
- 43.Crowell PL, Schmidt CM, Yip-Schneider MT, Savage JJ, Hertzler DA, 2nd, Cummings WO. Cyclooxygenase-2 expression in hamster and human pancreatic neoplasia. Neoplasia. 2006;8:437–445. doi: 10.1593/neo.04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto M, Alemany R, Adachi Y, Grizzle WE, Curiel DT. Characterization of the cyclooxygenase-2 promoter in an adenoviral vector and its application for the mitigation of toxicity in suicide gene therapy of gastrointestinal cancers. Mol Ther. 2001;3:385–394. doi: 10.1006/mthe.2001.0275. [DOI] [PubMed] [Google Scholar]
- 45.Koeller JM. Biologic response modifiers: the interferon alfa experience. Am J Hosp Pharm. 1989;46:S11–S15. [PubMed] [Google Scholar]
- 46.Suzuki K, Aoki K, Ohnami S, Yoshida K, Kazui T, Kato N, et al. Adenovirus-mediated gene transfer of interferon alpha improves dimethylnitrosamine-induced liver cirrhosis in rat model. Gene Ther. 2003;10:765–773. doi: 10.1038/sj.gt.3301949. [DOI] [PubMed] [Google Scholar]