Abstract
Chronic inflammation is recognized as a risk factor for the development of several malignancies. Local white adipose tissue (WAT) inflammation, defined by the presence of dead or dying adipocytes encircled by macrophages which form crown-like structures (CLS), occurs in the breasts (CLS-B) of most overweight and obese women. Previously, we showed that the presence of CLS-B is associated with elevated tissue levels of proinflammatory mediators and aromatase, the rate-limiting enzyme for estrogen biosynthesis. The associated increased levels of aromatase in the breast provide a plausible mechanistic link between WAT inflammation and estrogen-dependent breast cancers. Thus, breast WAT inflammation could be relevant for explaining the high incidence of estrogen-dependent tumors with aging despite diminished circulating estrogen levels after menopause. To explore this possibility, we determined whether menopause in addition to body mass index (BMI) is associated with breast WAT inflammation among 237 prospectively enrolled women. The presence of CLS-B and its severity (CLS-B/cm2) as indicators of WAT inflammation correlated with menopausal status (P=0.008 and P<0.001) and BMI (P<0.001 for both). In multivariable analyses adjusted for BMI, the postmenopausal state was independently associated with the presence (P=0.03) and severity of breast WAT inflammation (P=0.01). Mean adipocyte size increased in association with CLS-B (P<0.001). Our findings demonstrate that breast WAT inflammation, which is associated with elevated aromatase levels, is increased in association with the postmenopausal state independent of BMI. Breast WAT inflammation, a process that can potentially be targeted, may help to explain the high incidence of estrogen-dependent tumors in postmenopausal women.
Keywords: inflammation, adipose tissue, menopause, obesity, breast cancer
Introduction
Chronic inflammation is associated with the development of a growing number of epithelial malignancies (1–3). Obesity, defined as body mass index (BMI) ≥ 30, is associated with an increased risk of developing hormone receptor (HR)-positive breast cancer after menopause and a worse prognosis after breast cancer diagnosis (4–10). Obesity results in chronic, subclinical inflammation characterized by elevated circulating proinflammatory mediators (4, 11), which have been linked to the development and progression of breast cancer (12–14). Other mechanisms suggested to contribute to the obesity-cancer link include increased circulating estrogens (4, 15, 16), altered adipokine levels, and insulin resistance (12, 17).
Locally, adipocyte hypertrophy occurs in the obese fat pad leading to immune cell recruitment and inflamed white adipose tissue (WAT) (4, 18–20). This WAT inflammation, which occurs commonly in overweight and obese individuals, is histologically defined by the presence of inflammatory foci known as crown-like structures (CLS) (18). Composed of a dead or dying adipocyte encircled by macrophages, CLS were first observed in the visceral and subcutaneous fat in association with the metabolic syndrome (18, 19, 21). More recently, we demonstrated both in experimental models of obesity and in obese women with breast cancer that CLS occur in the WAT of the mammary gland and breast (CLS-B), respectively (22, 23). The association between elevated BMI and CLS-B has been confirmed in women without breast cancer who underwent reduction mammoplasty (24).
Breast WAT inflammation, manifested as CLS-B, correlates with elevated BMI, activation of NF-κB, and increased levels of proinflammatory mediators and aromatase, the rate-limiting enzyme for estrogen synthesis (22, 23). The severity of breast WAT inflammation is a better correlate to tissue levels of proinflammatory mediators and aromatase activity than is BMI (22, 23). Thus, WAT inflammation, which commonly occurs in obese individuals but has been demonstrated to occur across the spectrum of BMIs, is likely to contribute to the pathogenesis of breast cancer.
Despite substantially lower circulating levels of estrogen after menopause, somewhat paradoxically, estrogen-dependent tumors are the most common subtype of breast cancer in postmenopausal women. Similar to the obese state, postmenopausal status is associated with elevated circulating levels of C-reactive protein (CRP) and proinflammatory mediators (25, 26). Furthermore, ovariectomy sensitizes mice to WAT inflammation (27). Thus, as in obesity-associated malignancies, inflammation may provide a key link between the postmenopausal state and cancer development. However, a relationship between menopausal status and breast WAT inflammation in humans, as quantified by assessing CLS-B, has not been reported.
In the current study, we had three main objectives. First, we determined whether menopausal status, independent of BMI, contributes to breast WAT inflammation. Second, we expanded upon our previous pilot study to confirm the correlations between BMI and breast WAT inflammation, and between breast WAT inflammation and adipocyte diameter (23). Third, we investigated whether WAT inflammation of the breast is a sentinel for adipose tissue inflammation in other fat depots. We show that postmenopausal status, independent of BMI, is associated with breast WAT inflammation. Moreover, our findings confirm that the majority of obese and overweight women, and a minority of women with normal BMI, have CLS-B. Finally, we present evidence that breast WAT inflammation is a sentinel for adipose inflammation in another adipose depot. Collectively, our findings demonstrate that both obesity and the postmenopausal state independently contribute to breast WAT inflammation thereby providing a plausible explanation for the elevated risk of HR-positive breast cancer in obese postmenopausal women.
Materials and Methods
Study population and biospecimen acquisition
This study was approved by the Institutional Review Boards of Memorial Sloan Kettering Cancer Center (MSKCC) and Weill Cornell Medical College (New York, NY). Informed consent was provided by women undergoing mastectomy at MSKCC and breast WAT specimens were obtained under a standard tissue acquisition protocol. Patients underwent mastectomy for treatment of breast cancer (n=211) or to reduce risk of breast cancer (n=26). To ensure adequate tissue for analysis, patients undergoing lumpectomy were excluded. Bilateral breast WAT was obtained from a subgroup of women undergoing bilateral mastectomy. Breast and abdominal WAT were obtained from a subgroup of women undergoing mastectomy with immediate autologous flap reconstruction. Clinicopathological data (age, race, BRCA mutation status, tumor subtype, diagnoses of hypertension, diabetes, dyslipidemia, medications used, e.g., statins, breast cancer treatments including endocrine therapy and pre-operative chemotherapy) were systematically extracted from the electronic medical record (EMR) by research staff and physicians, and independent data review was carried out for quality assurance. Height and weight were prospectively recorded prior to surgery and used to calculate BMI. Standard definitions were used to categorize BMI as under- or normal weight (BMI< 25), overweight (BMI 25.0 – 29.9), or obese (BMI ≥ 30). Menopausal status was categorized as either premenopausal or postmenopausal based on National Comprehensive Cancer Network (NCCN) criteria (28). In brief, women were classified as postmenopausal if they had bilateral oophorectomy or reported permanent cessation of menses for 12 or more months in the absence of chemotherapy or endocrine therapy. All data were reviewed twice for accuracy independently by 2 physicians.
For all cases, up to 5 paraffin blocks were prepared from WAT obtained from each surgical site (breasts +/− abdomen). For cases with ipsilateral invasive breast tumors, samples were obtained from quadrants not involved by tumor. Specimens were examined grossly and with hematoxylin and eosin (H&E) staining by a breast histopathologist (D.G.) to ensure samples were representative of normal breast tissue or abdominal subcutaneous tissue when available.
CLS analysis
Methods to detect CLS have been described previously (23). Briefly, 2 sections from each of 5 tissue blocks (5 µm thick and approximately 2 cm in diameter) were stained by H&E and for CD68, a macrophage marker (mouse monoclonal KP1 antibody; Dako; dilution 1:4,000). If fewer than 5 paraffin blocks were available, additional sections were cut from the same block at 50 µm apart. In this manner, a total of 5 sections, stained with CD68, were generated per case for CLS analysis. The CD68 stained sections were examined under light microscopy by the study pathologist (D.G.) to detect CLS in breast and abdominal WAT when available. The number of CLS in each section was counted and recorded. Following this process, the density of CLS by surface area was determined as follows. Each CD68 stained slide was digitally archived by gross photography using a Nikon COOLPIX® digital camera (Nikon Co., Tokyo, Japan). Images were stored in the tagged image file format (TIFF) and the WAT tissue area on each slide was measured using the Image J Software (NIH, Bethesda, MD). Epithelial tissue area and areas of fibrosis were excluded. The severity of WAT inflammation was quantified as CLS-B per square centimeter of WAT (CLS-B/cm2).
Adipocyte diameter
Measurement of adipocyte diameters has been described previously (23). Briefly, breast H&E sections were photographed at 20× using an Olympus BX50 microscope and MicroFire digital camera (Optronics, East Muskogee, OK). Images were stored as TIFF files and mean diameters were calculated using measurements from 30 or more individual adipocytes for each patient using the linear dimensional tool (LDT) in the Canvas 11 Software (ACD Systems International, Inc., Victoria, BC, Canada).
Biostatistics
The primary endpoints of this study were: 1) presence or absence of WAT inflammation (binary variable) defined by CLS-B or CLS in abdominal WAT when available, and 2) severity of breast WAT inflammation, defined categorically as follows and using median number of CLS-B/cm2 (0.38) as the cut-off: no inflammation (absent CLS-B), mild inflammation (≤ 0.38 CLS-B/cm2) and severe inflammation (> 0.38 CLS-B/cm2). Associations between presence of CLS-B and clinicopathologic features, including BMI (both as a continuous and categorical variable) and menopausal status, were examined using logistic regression and Fisher’s exact test where appropriate. Additional clinicopathologic features that were examined include age, race, BRCA mutation status, tumor subtypes (hormone receptor positive, HER2 amplified or triple negative), invasive or in situ carcinoma, diagnosis of hypertension, diabetes, dyslipidemia, use of nonsteroidal anti-inflammatory drugs (NSAIDs), statins, blood pressure lowering drugs, endocrine therapy and pre-operative chemotherapy. Associations between categorized number of CLS-B/cm2 and clinicopathologic features were examined using ANOVA or the non-parametric Kruskal-Wallis test for continuous variables and Fisher’s exact test or Chi-squared test for categorical variables. Among subjects with CLS-B, associations between CLS-B/cm2 (as a continuous variable) and clinicopathologic features were evaluated using Spearman’s correlation for continuous covariates and either the non-parametric Kruskal-Wallis test or Wilcoxon rank-sum test where appropriate for categorical covariates. Associations between mean adipocyte size and categorical variables including CLS-B presence, categorized CLS-B/cm2, and clinicopathologic features were evaluated using ANOVA and/or Student t-test where appropriate. The strength of the correlation between BMI and mean adipocyte size was quantified using the Spearman’s rank correlation coefficient.
For the multivariable model, covariates of interest including BMI and menopausal status were identified by the univariate analyses results in all study subjects and in subgroups defined by BMI categories and menopausal status, the Akaike information criterion (AIC) based backward variable selection procedure, and previous related studies (29). Potential confounding factors including age, use of NSAIDs, statins and pre-operative chemotherapy were also included in the final multivariable model for presence or absence of WAT inflammation (binary variable). Using a similar variable selection procedure, the following covariates including BMI, menopausal status, regular use of NSAIDs, use of endocrine therapy at the time of surgery and pre-operative chemotherapy were included in the multivariable logistic regression model for severity of breast WAT inflammation in subjects with CLS-B.
When bilateral breast tissue and abdominal subcutaneous WAT were available, the strength of correlation in CLS/cm2 between breasts or between breast and abdominal tissue were quantified using the Kendall’s rank correlation coefficient. Correlation coefficients were tested against the null hypothesis that the correlation coefficients would be 0. Results with P values less than 0.05 were considered statistically significant.
Results
Study population
From January 2011 through August 2013, we expanded our prior pilot study (n=30) leading to a total enrollment of 238 women who underwent mastectomy. Breast WAT was obtained from 237 women (only abdominal WAT was obtained from one patient) of median age 48 years (range 22 – 90, Table 1). Median BMI was 25.3 (range 17.3 – 50.0). Overall, 144/237 (61%) women were premenopausal at the time of surgery while 93/237 (39%) women were postmenopausal. Additionally, 148/237 (62%) patients had no evidence of ipsilateral invasive cancer in the breast examined for CLS-B. Thirty-four (14%) patients received preoperative chemotherapy. Fifteen (6%) patients were on tamoxifen or an aromatase inhibitor at the time of surgery. Known BRCA1 or BRCA2 gene mutations were present in 49/125 (39%) patients who were tested, reflecting the institutional catchment. Regarding co-morbid conditions, 36/237 (15%) women had hypertension and 10/237 (4%) had diabetes mellitus.
Table 1.
Clinical characteristics of patients by CLS-B status.
| Characteristic | All Patients (n=237) |
Patients without CLS-B (n=115) |
Patients with CLS-B (n=122) |
P |
|---|---|---|---|---|
| Age, years | ||||
| Median (range) | 48 (22–90) | 47 (25–90) | 49 (22–80) | 0.38 |
| Race, n (%) | ||||
| Asian | 14 (6.0%) | 7 (6.1%) | 7 (5.8%) | |
| Black | 17 (7.3%) | 6 (5.3%) | 11 (9.2%) | |
| White | 203 (86.8%) | 101 (88.6%) | 102 (85.0%) | 0.55 |
| BMI | ||||
| Median (range) | 25.3 (17.3–50.0) | 23.1 (17.3–35.7) | 28.3 (18.4–50.0) | <0.001 |
| Menopausal status, n (%) | ||||
| Pre | 144 (60.8%) | 80 (69.6%) | 64 (52.5%) | |
| Post | 93 (39.2%) | 35 (30.4%) | 58 (47.5%) | 0.008 |
| Hypertension, n (%) | ||||
| No | 201 (84.8%) | 102 (88.7%) | 99 (81.2%) | |
| Yes | 36 (15.2%) | 13 (11.3%) | 23 (18.9%) | 0.15 |
| Diabetes, n (%) | ||||
| No | 227 (95.8%) | 112 (97.4%) | 115 (94.3%) | |
| Yes | 10 (4.2%) | 3 (2.6%) | 7 (5.7%) | 0.34 |
| NSAID use, n (%) | ||||
| No | 214 (90.3%) | 102 (88.7%) | 112 (91.8%) | |
| Yes | 23 (9.7%) | 13 (11.3%) | 10 (8.2%) | 0.51 |
| Statin use, n (%) | ||||
| No | 213 (89.9%) | 104 (90.4%) | 109 (89.3%) | |
| Yes | 24 (10.1%) | 11 (9.6%) | 13 (10.7%) | 0.83 |
| Mastectomy indication, n (%) | ||||
| Treatment | 211 (89.0%) | 105 (91.3%) | 106 (86.9%) | |
| Risk reduction | 26 (11.0%) | 10 (8.7%) | 16 (13.1%) | 0.31 |
| Estrogen receptor, n (%) | ||||
| Negative | 31 (18.7%) | 16 (19.5%) | 15 (17.9%) | |
| Positive | 135 (81.3%) | 66 (80.5%) | 69 (82.1%) | 0.84 |
| Unknown or NA | 71 (30.0%) | 33 (28.7%) | 38 (31.2%) | 0.78 |
| Progesterone receptor, n (%) | ||||
| Negative | 45 (28.3%) | 21 (26.6%) | 24 (30.0%) | |
| Positive | 114 (71.7%) | 58 (73.4%) | 56 (70.0%) | 0.73 |
| Unknown or NA | 78 (32.9%) | 36 (31.3%) | 42 (34.4%) | 0.68 |
| Preoperative hormonal therapy, n (%) | ||||
| No | 222 (93.7%) | 108 (93.9%) | 114 (93.4%) | |
| Yes | 15 (6.3%) | 7 (6.1%) | 8 (6.6%) | 1.00 |
| Preoperative chemotherapy, n (%) | ||||
| No | 203 (85.7%) | 100 (87.0%) | 103 (84.4%) | |
| Yes | 34 (14.3%) | 15 (13.0%) | 19 (15.6%) | 0.71 |
| BRCA mutation, n (%) | ||||
| No | 76 (60.8%) | 44 (67.7%) | 32 (53.3%) | |
| Yes | 49 (39.2%) | 21 (32.3%) | 28 (46.7%) | 0.14 |
| Unknown | 112 (47.3%) | 50 (43.5%) | 62 (50.8%) | 0.30 |
NSAID nonsteroidal anti-inflammatory drug; NA not applicable
BMI correlates with presence of breast WAT inflammation
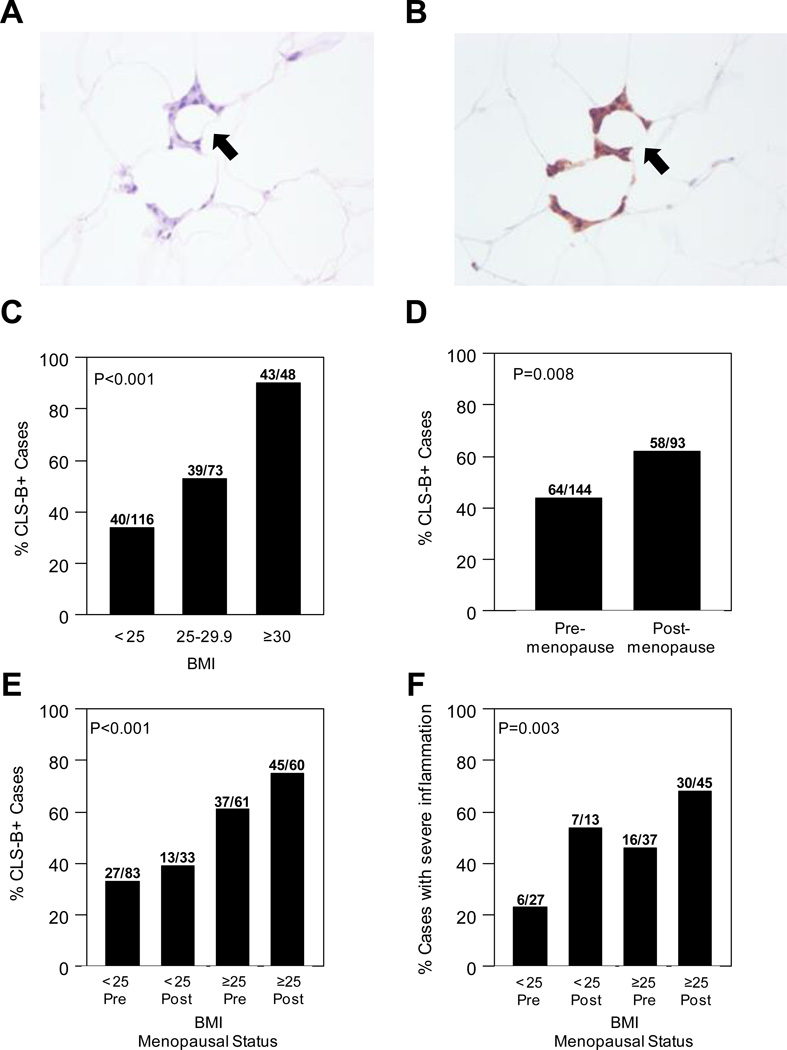
Among all study patients, 122/237 (51%) women had evidence of CLS-B (Figure 1A and B, Table 1). CLS-B were observed in 19/34 (56%) patients who received preoperative chemotherapy which is slightly higher compared to that among patients who did not receive preoperative chemotherapy (51%, P=0.71). Statistically significant differences were not found for rates of hypertension, diabetes or use of NSAIDs or statins in women with versus without CLS-B (Table 1). As in our pilot study of 30 women (23), we examined the correlation between CLS-B (presence/absence) and BMI, both as a continuous and as a categorical variable. In this expanded population, BMI correlated with CLS-B positivity (Table 1). Specifically, CLS-B were present in 43/48 (90%) obese patients, 39/73 (53%) overweight patients, and 40/116 (34%) patients with BMI < 25 (P<0.001, Figure 1C). The correlation between CLS-B positivity and BMI remained statistically significant in the multivariate model which adjusted for age, menopausal status, and use of NSAIDs or statins (P<0.001).
Figure 1. CLS-B are more common and more severe in obese and postmenopausal women.
A. H&E stained slide showing CLS-B (200×). B. Immunohistochemical staining of CD68 of same lesion shown in panel A (200×). C. Prevalence of CLS-B is higher in overweight and obese patients. D. Prevalence of CLS-B is higher in postmenopausal women. E. Overweight and obese postmenopausal women have the highest prevalence of CLS-B. F. Overweight and obese postmenopausal women have the highest prevalence of severe breast WAT inflammation (CLS-B/cm2 >0.38). Difference in prevalence of CLS-B (C, D, E) or prevalence of severe breast WAT inflammation (F) across groups were assessed using Fisher’s exact test.
BMI correlates with severity of breast WAT inflammation
The severity of breast WAT inflammation was quantified as number of CLS-B/cm2 in 233 patients who had measurable WAT area. Among these patients who also had breast WAT inflammation, median CLS-B/cm2 was 0.38 (range 0.05 – 64.44). Using this median as a cutoff, the severity of breast WAT inflammation was categorized as severe (>0.38 CLS-B/cm2) in 59/233 (25%) patients and mild (≤ 0.38 CLS-B/cm2) in 59/233 (25%) patients (Table 2). Rising BMI was associated with more severe breast WAT inflammation (P<0.001, Table 2). Specifically, among women with CLS-B, breast WAT inflammation was severe in 29/41 (71%) obese women, 17/38 (45%) overweight women, and 13/39 (33%) patients with BMI < 25 (P=0.003). Strikingly, among women with breast WAT inflammation, the severity of inflammation was greater in those with hypertension (P<0.05) or diabetes (P=0.05; Table 2). Use of NSAIDs or statins did not vary based on the severity of breast WAT inflammation. Use of preoperative hormonal therapy was highest in those with severe breast WAT inflammation but this difference was not statistically significant (P=0.08; Table 2). Use of preoperative chemotherapy was more frequent in patients who did not have severe breast WAT inflammation (P=0.03; Table 2); however, the usage of preoperative chemotherapy did not vary with the presence or absence of inflammation (Table 1). Among patients tested for BRCA mutations, BRCA mutations were found in a greater number of patients with severe breast WAT inflammation but this difference was not statistically significant (P=0.06; Table 2).
Table 2.
Clinical characteristics of patients by severity of breast white adipose tissue inflammation.
| Characteristic | Breast WAT Inflammation | |||
|---|---|---|---|---|
| None (n=115) | Mild (n=59)a | Severe (n=59)b | P | |
| Age, years | ||||
| Median (range) | 47 (25–90) | 46 (22–72) | 50 (27–80) | 0.15 |
| Race, n (%) | ||||
| Asian | 7 (6.1%) | 3 (5.3%) | 4 (6.8%) | |
| Black | 6 (5.3%) | 1 (1.8%) | 9 (15.3%) | |
| White | 101 (88.6%) | 53 (93.0%) | 46 (78.0%) | 0.07 |
| BMI | ||||
| Median (range) | 23.1 (17.3–35.7) | 26.0 (20.4–40.2) | 29.8 (18.4–50.0) | <0.001 |
| Menopausal Status, n (%) | ||||
| Pre | 80 (69.6%) | 39 (66.1%) | 22 (37.3%) | |
| Post | 35 (30.4%) | 20 (33.9%) | 37 (62.7%) | <0.001 |
| Hypertension, n (%) | ||||
| No | 102 (88.7%) | 52 (88.1%) | 44 (74.6%) | |
| Yes | 13 (11.3%) | 7 (11.9%) | 15 (25.4%) | <0.05 |
| Diabetes, n (%) | ||||
| No | 112 (97.4%) | 58 (98.3%) | 53 (89.8%) | |
| Yes | 3 (2.6%) | 1 (1.7%) | 6 (10.2%) | 0.05 |
| NSAID use, n (%) | ||||
| No | 102 (88.7%) | 53 (89.8%) | 55 (93.2%) | |
| Yes | 13 (11.3%) | 6 (10.2%) | 4 (6.8%) | 0.71 |
| Statin use, n (%) | ||||
| No | 104 (90.4%) | 55 (93.2%) | 50 (84.7%) | |
| Yes | 11 (9.6%) | 4 (6.8%) | 9 (15.2%) | 0.36 |
| Mastectomy Indication, n (%) | ||||
| Treatment | 105 (91.3%) | 53 (89.8%) | 49 (83.1%) | |
| Risk Reduction | 10 (8.7%) | 6 (10.2%) | 10 (16.9%) | 0.29 |
| Estrogen receptor, n (%) | ||||
| Negative | 16 (19.5%) | 10 (23.3%) | 5 (13.5%) | |
| Positive | 66 (80.5%) | 33 (76.7%) | 32 (86.5%) | 0.55 |
| Unknown or NA | 33 (28.7%) | 16 (27.1%) | 22 (37.3%) | 0.42 |
| Progesterone receptor, n (%) | ||||
| Negative | 21 (26.6%) | 13 (31.7%) | 10 (28.6%) | |
| Positive | 58 (73.4%) | 28 (68.3%) | 25 (71.4%) | 0.82 |
| Unknown or NA | 36 (31.3%) | 18 (30.5%) | 24 (40.7%) | 0.42 |
| Preoperative hormonal therapy, n (%) | ||||
| No | 108 (93.9%) | 58 (98.3%) | 52 (88.1%) | |
| Yes | 7 (6.1%) | 1 (1.7%) | 7 (11.9%) | 0.08 |
| Preoperative chemotherapy, n (%) | ||||
| No | 100 (87.0%) | 45 (76.3%) | 55 (93.2%) | |
| Yes | 15 (13.0%) | 14 (23.7%) | 4 (6.8%) | 0.03 |
| BRCA mutation, n (%) | ||||
| No | 44 (67.7%) | 19 (63.3%) | 12 (41.4%) | |
| Yes | 21 (32.3%) | 11 (36.7%) | 17 (58.6%) | 0.06 |
| Unknown | 50 (43.5%) | 29 (49.2%) | 30 (50.9%) | 0.63 |
. CLS-B/cm2 ≤ 0.38
. CLS-B/cm2 > 0.38
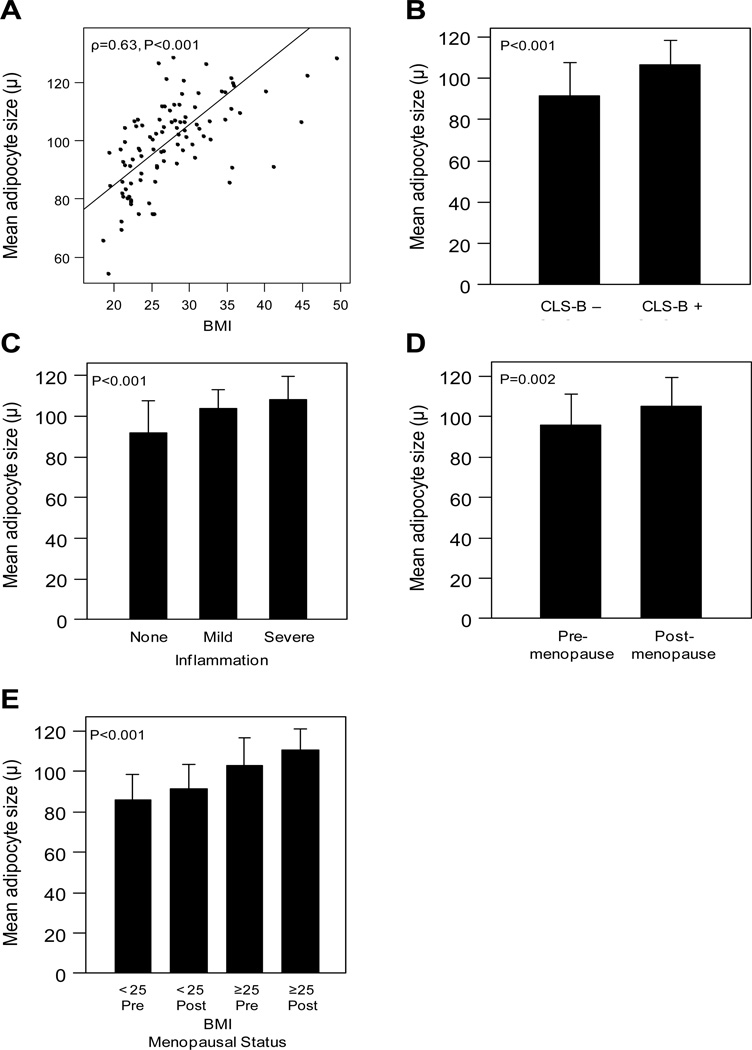
We next investigated the relationship between adipocyte size in the breast and the presence and severity of breast WAT inflammation in the first 101 patients enrolled. In this subset, we found a positive correlation between mean adipocyte size (μ) and BMI (ρ=0.63, P<0.001; Figure 2A). Mean adipocyte size was larger in patients with CLS-B (107 μ; standard deviation, s.d., 12 μ) versus those without CLS-B (92 μ, s.d. 16 μ, P<0.001, Figure 2B). Additionally, increasing severity of breast WAT inflammation was associated with larger adipocyte size (P<0.001, Figure 2C).
Figure 2. Adipocyte size correlates with BMI and WAT inflammation, and is larger in postmenopausal and obese women.
A. A moderate correlation was observed between mean adipocyte size and BMI (ρ=0.63, P<0.001, Pearson’s method). B. Patients with CLS-B have larger mean adipocyte size in the breast (P<0.001, t-test). C. Mean adipocyte size increases with more severe breast WAT inflammation (P<0.001, ANOVA). D. Mean adipocyte size is larger in postmenopausal women (P=0.002, t-test). E. Mean adipocyte size is larger in overweight and obese postmenopausal women (P<0.001, ANOVA).
Breast WAT inflammation is associated with postmenopausal status
We next evaluated the rate of CLS-B positivity and the severity of breast WAT inflammation in pre- versus postmenopausal women. The postmenopausal state was associated with the presence of breast WAT inflammation (P=0.008; Table 1, Figure 1D). Specifically, CLS-B occurred in 58/93 (62%) postmenopausal women versus 64/144 (44%) premenopausal women. Additionally, the postmenopausal state was associated with more severe inflammation as evidenced by greater number of CLS-B/cm2 (P<0.001, Table 2). These associations remained statistically significant in both multivariable models for the presence/absence of CLS-B (P=0.03) and for severity of WAT inflammation in patients with CLS-B (P=0.01). When categorized by menopausal status and BMI, overweight/obese postmenopausal women were the most likely to have breast WAT inflammation (P<0.001, Figure 1E). Similarly, overweight/obese postmenopausal women had the highest incidence of severe breast WAT inflammation (P=0.003, Figure 1F).
In light of these findings, and given the association between larger adipocyte size and CLS-B positivity, we examined breast adipocyte size in pre- versus postmenopausal women. Postmenopausal women had larger mean adipocyte size (105 μ, s.d. 14 μ) than premenopausal women (96 μ, s.d. 16 μ, P=0.002; Figure 2D). When grouped by menopausal status and BMI, overweight/obese postmenopausal women had the largest mean adipocyte size in the breast (P<0.001; Figure 2E).
Given that obesity is a risk factor for the development of HR-positive breast cancer in postmenopausal women (4,5), we next examined the relationship between WAT inflammation and HR-positive breast cancer. Among women with invasive breast cancer inclusive of all BMIs and menopausal status, we did not observe an association between CLS-B and HR-positive tumors (Tables 1 and 2). When stratified by BMI and menopause status, the incidence of HR-positive, HER2-nonamplified breast cancer was highest in obese postmenopausal women (84.2%, P=0.04). Moreover, the incidence and severity of WAT inflammation were highest in obese postmenopausal women (P<0.001 for both).
WAT inflammation occurs simultaneously in both breasts and abdominal subcutaneous fat
As obesity is associated with low grade systemic inflammation characterized by elevated levels of circulating proinflammatory mediators (4, 11), we investigated whether WAT inflammation occurs simultaneously at the tissue level among different adipose depots. Bilateral breast WAT was obtained from a subset of 63 women undergoing bilateral mastectomy. In this subgroup, there was a high rate of concordant CLS-B status (positive or negative) between bilateral breasts. Specifically, 49/63 (78%) women had concordant CLS-B status between breasts while 14/63 (22%) had discordant status (Table 3). Notably, the median number of CLS-B/cm2 was greater in women who had WAT inflammation present in both breasts (0.6, range 0.2 – 51.9) versus women with WAT inflammation in one breast but not the other (0.2, range 0.1 – 0.5, P<0.001; Table 3).
Table 3.
Concordance of WAT inflammation between adipose depots.
| Sites | CLS Absent at Both Sites |
CLS Present at Both Sites |
Discordant CLS Status |
P | |
|---|---|---|---|---|---|
| Left versus Right breast | Patients, n (%) | 17 (27.0%) | 32 (50.8%) | 14 (22.2%) | |
| CLS/cm2 Median (range) | 0.6 (0.2–51.9) | 0.2 (0.1–0.5) | <0.001 | ||
| Breast versus Abdomen | Patients, n (%) | 3 (23.1%) | 7 (53.8%) | 3 (23.1%) | |
| CLS/cm2 Median (range) | 1.5 (0.4–26.7) | 0.3 (0.2–1.1) | 0.12 | ||
Abdominal subcutaneous WAT was obtained from a subset of 13 women undergoing mastectomy with immediate autologous flap reconstruction. In this subgroup 10/13 (77%) had evidence of CLS in abdominal WAT. Similar to findings in women with bilateral breast WAT, there was a high rate of concordant CLS status between breast and abdominal subcutaneous WAT. Specifically, 10/13 (77%) women had concordance in the presence or absence of CLS in breast and abdominal WAT (Table 3).
Discussion
In the current study, we demonstrate that the postmenopausal state is independently associated with breast WAT inflammation. Our findings in breast tissue are consistent with recent reports linking the postmenopausal state to systemic inflammation (25, 26). For example, circulating levels of CRP are higher in postmenopausal compared to premenopausal women (25). In a nested case-control study, Gross and colleagues demonstrated that increased circulating levels of soluble tumor necrosis factor (TNF) receptor 2 (TNF-R2), a receptor that binds TNF and other cytokines, is associated with higher risk of developing breast cancer in postmenopausal women (26). Additionally, systemic inflammation characterized by elevated levels of prostaglandin E2 (PGE2)-metabolite (PGE-M) in the urine, is associated with a greater risk of breast cancer after menopause (29, 30). Here, we provide the first evidence that postmenopausal women, compared to their premenopausal counterparts, have larger adipocytes and greater prevalence and severity of WAT inflammation in the breast itself. These findings are consistent with prior evidence that estrogen has a strong inhibitory effect on the expression of key lipogenic genes and that larger adipocytes appear to be more likely to die leading to WAT inflammation (31, 32).
Breast cancers that express the estrogen receptor (ER) and progesterone receptor (PR) represent the most common subtype of the disease including in postmenopausal women. After the ovaries cease to produce estrogen, circulating levels of estrogens drop significantly compared to the premenopausal state. Despite this relative estrogen depletion, the incidence of estrogen-dependent tumors rises with age, accounting for nearly 85% of breast cancers diagnosed in women 80 years and older (33). Given that WAT inflammation is associated with increased amounts of aromatase in the breast (23, 34), the increased presence and severity of breast WAT inflammation after menopause provides a plausible explanation for the paradoxical observation that hormone-dependent tumors occur with increasing frequency in the setting of falling circulating estrogen levels. Specifically, locally produced estrogens as a result of WAT inflammation may explain the increased incidence of estrogen-dependent tumors in postmenopausal women. The mechanisms of breast carcinogenesis could include estrogen receptor-mediated activation of multiple signaling pathways that affect cell proliferation and apoptosis (35). Genotoxic estrogen metabolites could also be important. Another possibility is that ligand-independent activation of ER-α could occur in the context of inflamed WAT. In support of the potential link between WAT inflammation and estrogen-dependent breast cancers, we found that the incidence and severity of breast WAT inflammation were highest in obese postmenopausal women, the group with the highest incidence of HR-positive breast cancer.
We also confirmed that WAT inflammation occurs commonly in obese and overweight women, and that inflammation is present in a minority of women who are under- or normal weight. Strikingly, 90% of obese women had CLS-B, and the presence of WAT inflammation was associated with larger adipocyte size. These results are consistent with the findings in our original pilot study of 30 women (23). Although the postmenopausal state is associated with WAT inflammation independent of BMI, the addition of obesity represents a “two-hit” phenotype as obese, postmenopausal women had the highest rate and greatest severity of breast WAT inflammation. Several recent studies have suggested a link between metabolic syndrome and an increased risk of breast cancer (36–39). WAT inflammation is now recognized to be an important component of obesity-related disorders that occur as part of the metabolic syndrome including diabetes mellitus and cardiovascular diseases (20). Notably, in the current study, women with diabetes or hypertension were more likely to have severe breast WAT inflammation. Possibly, WAT inflammation in the breast itself will help to explain the link between metabolic syndrome and increased risk of breast cancer.
We previously quantified the severity of breast WAT inflammation using a CLS-B index and demonstrated that this index rises with increasing BMI and correlates more strongly with aromatase activity than does BMI (23). Here we refined our method to assay the severity of breast WAT inflammation by determining the density of CLS-B quantified as number of CLS-B/cm2. In addition to the increased prevalence, the severity of breast WAT inflammation was worse in obese and overweight women compared with lean women. Importantly, however, while the majority of obese women had breast WAT inflammation, 5/48 (10%) obese women in our study did not. Furthermore, 40/116 (34%) under- and normal weight women (BMI<25) were found to have breast WAT inflammation. Thus, the presence and severity of CLS-B are not merely surrogates of BMI. The absence of breast WAT inflammation in a minority (10%) of obese women and its presence in 34% of under- or normal weight women illustrates this and raises additional issues. First, these findings are consistent with the observation that a small minority of obese individuals, as defined by BMI, are metabolically healthy (40–42). Second, a subset of lean individuals are known to be metabolically unhealthy (43). Based on our findings, it will be worthwhile to determine in normal sized individuals if WAT inflammation is associated with metabolic abnormalities. This should enable us to better understand the contribution of WAT inflammation to the pathogenesis of breast cancer in lean as well as in obese women.
We also found evidence that WAT inflammation is systemic, occurring simultaneously in multiple fat depots including the contralateral breast and abdominal subcutaneous tissue. This observation is consistent with growing evidence that obesity is associated with subclinical, systemic low grade inflammation (4, 11, 18). The concordance of inflammatory status between tumor-bearing and tumor-free breasts demonstrates that WAT inflammation occurs independent of pre-existing neoplasia. Importantly, the concordant inflammatory status (present or absent) between the breast and abdominal subcutaneous WAT strengthens the rationale for analyzing abdominal WAT, a more clinically accessible depot, in future studies of interventions that aim to attenuate WAT inflammation. Moreover, the fact that the breast is a sentinel for adipose inflammation in other fat depots raises the intriguing possibility that this diffuse low grade inflammatory state will be associated with blood biomarkers. Future studies will be needed to determine if blood biomarkers of CLS-B can be identified.
Our study is strengthened by a prospective tissue acquisition design and tissue assessment by a histopathologist experienced in the detection and grading of WAT inflammation. To our knowledge, this is the largest study of human breast WAT to date. However, a potential limitation of this study is the inclusion of patients with cancer. Although we have attempted to control for potential confounding factors, similar studies should be carried out in individuals without cancer. Obtaining sufficient amounts of breast tissue from cancer free individuals to assess adipose inflammation poses practical limitations especially if questions concerning menopause are to be addressed. Importantly, there is no evidence that WAT inflammation occurs as a consequence of neoplasia, and, on the contrary, WAT inflammation has been well described in obese, cancer free individuals including within non-tumorous breasts (18, 19, 24). If a non-invasive blood biomarker of WAT inflammation can be developed, future studies in larger cohorts of cancer free individuals may be conducted. This will be particularly important if such a biomarker is to be evaluated for predicting risk and/or outcomes.
In conclusion, this study demonstrates that the postmenopausal state is independently associated with the presence and severity of breast WAT inflammation. We also confirm that WAT inflammation occurs in most obese women and some who are lean. Given that WAT inflammation is associated with elevated aromatase levels, our study provides a potential mechanistic explanation for the increased incidence of estrogen-dependent breast cancers in obese, postmenopausal women. WAT inflammation, manifested as CLS-B, may represent a more robust biomarker of risk than BMI and could provide a more specific pathological target that can be used for the development of effective prevention and treatment strategies. Interventions that target WAT inflammation including diet, exercise, medications, or a combination thereof, may prove useful for breast cancer prevention and treatment.
Acknowledgements
The authors thank all the patients who consented to provide tissue for this study. In addition, we thank the staff of the Clinical Trials Office and Pathology Core at Memorial Sloan Kettering Cancer Center.
Grant Support:
This work was supported by grants NIH/NCI R01CA154481 (to A.J. Dannenberg), UL1TR000457 of the Clinical and Translational Science Center at Weill Cornell Medical College (to N.M. Iyengar and X. K. Zhou), 2013 Conquer Cancer Foundation of the American Society of Clinical Oncology (ASCO) Young Investigator Award (to N.M. Iyengar), 2013 Royal Academy of Medicine in Ireland, St. Luke’s Young Investigator Award (to P.G. Morris), 2012 Expedition Inspiration (Brenda M. Williams) Young Investigator Award (to P.G. Morris), the Botwinick-Wolfensohn Foundation (in memory of Mr. and Mrs. Benjamin Botwinick; to A.J. Dannenberg), and the Breast Cancer Research Foundation (to A.J. Dannenberg and C.A. Hudis). This work was presented in part at the 2014 ASCO Annual Meeting, resulting in a 2014 Conquer Cancer Foundation of ASCO Merit Award (to N.M. Iyengar).
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: new insights into breast cancer development and progression. American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting. 2013:46–51. doi: 10.1200/EdBook_AM.2013.33.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature reviews Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England journal of medicine. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 7.Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast cancer research and treatment. 2008;111:329–342. doi: 10.1007/s10549-007-9785-3. [DOI] [PubMed] [Google Scholar]
- 8.Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer causes & control : CCC. 2002;13:325–332. doi: 10.1023/a:1015288615472. [DOI] [PubMed] [Google Scholar]
- 9.Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, et al. Effect of obesity on prognosis after early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 10.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast cancer research and treatment. 2010;2010:23. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 11.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA : the journal of the American Medical Association. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 12.Hursting SD, Digiovanni J, Dannenberg AJ, Azrad M, Leroith D, Demark-Wahnefried W, et al. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res (Phila) 2012;5:1260–1272. doi: 10.1158/1940-6207.CAPR-12-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. British journal of cancer. 2003;88:1721–1726. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. Journal of the National Cancer Institute. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 17.Iyengar NM, Morris PG, Hudis CA, Dannenberg AJ. Obesity, Inflammation, and Breast Cancer. In: Dannenberg AJ, Berger NA, editors. Obesity, Inflammation, and Cancer. New York: Springer; 2013. pp. 181–217. [Google Scholar]
- 18.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual review of physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 19.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. Journal of lipid research. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. Journal of lipid research. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X, Casbas-Hernandez P, Bigelow C, Makowski L, Joseph Jerry D, Smith Schneider S, et al. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast cancer research and treatment. 2012;131:1003–1012. doi: 10.1007/s10549-011-1789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva DC, Costa LO, Vasconcelos AA, Cerqueira JC, Fantato D, Torres DC, et al. Waist circumference and menopausal status are independent predictors of endothelial low-grade inflammation. Endocr Res. 2014;39:22–25. doi: 10.3109/07435800.2013.797431. [DOI] [PubMed] [Google Scholar]
- 26.Gross AL, Newschaffer CJ, Hoffman-Bolton J, Rifai N, Visvanathan K. Adipocytokines, inflammation, and breast cancer risk in postmenopausal women: a prospective study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22:1319–1324. doi: 10.1158/1055-9965.EPI-12-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi EK, Kim WK, Sul OJ, Park YK, Kim ES, Suh JH, et al. TNFRSF14 deficiency protects against ovariectomy-induced adipose tissue inflammation. J Endocrinol. 2014;220:25–33. doi: 10.1530/JOE-13-0341. [DOI] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology Version 3.2014: Breast Cancer. 2014 Available from: http://www.nccn.org. [Google Scholar]
- 29.Morris PG, Zhou XK, Milne GL, Goldstein D, Hawks LC, Dang CT, et al. Increased levels of urinary PGE-M, a biomarker of inflammation, occur in association with obesity, aging, and lung metastases in patients with breast cancer. Cancer Prev Res (Phila) 2013;6:428–436. doi: 10.1158/1940-6207.CAPR-12-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S, Taylor JA, Milne GL, Sandler DP. Association between Urinary Prostaglandin E2 Metabolite and Breast Cancer Risk: A Prospective, Case-Cohort Study of Postmenopausal Women. Cancer Prev Res (Phila) 2013;6:511–518. doi: 10.1158/1940-6207.CAPR-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nashi N, Kang S, Barkdull GC, Lucas J, Davidson TM. Lingual fat at autopsy. Laryngoscope. 2007;117:1467–1473. doi: 10.1097/MLG.0b013e318068b566. [DOI] [PubMed] [Google Scholar]
- 32.Birkeland KI. Early insulin therapy in type 2 diabetes? Tidsskr Nor Laegeforen. 2012;132:2151–2152. doi: 10.4045/tidsskr.12.0866. [DOI] [PubMed] [Google Scholar]
- 33.Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21:28–34. doi: 10.1200/JCO.2003.03.088. [DOI] [PubMed] [Google Scholar]
- 34.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer discovery. 2012;2:356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. The New England journal of medicine. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 36.Pothiwala P, Jain SK, Yaturu S. Metabolic syndrome and cancer. Metabolic syndrome and related disorders. 2009;7:279–288. doi: 10.1089/met.2008.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capasso I, Esposito E, Pentimalli F, Crispo A, Montella M, Grimaldi M, et al. Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer biology & therapy. 2010;10:1240–1243. doi: 10.4161/cbt.10.12.13473. [DOI] [PubMed] [Google Scholar]
- 38.Porto LA, Lora KJ, Soares JC, Costa LO. Metabolic syndrome is an independent risk factor for breast cancer. Archives of gynecology and obstetrics. 2011;284:1271–1276. doi: 10.1007/s00404-011-1837-6. [DOI] [PubMed] [Google Scholar]
- 39.Rosato V, Bosetti C, Talamini R, Levi F, Montella M, Giacosa A, et al. Metabolic syndrome and the risk of breast cancer in postmenopausal women. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:2687–2692. doi: 10.1093/annonc/mdr025. [DOI] [PubMed] [Google Scholar]
- 40.Xu XJ, Gauthier MS, Hess DT, Apovian CM, Cacicedo JM, Gokce N, et al. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. Journal of lipid research. 2012;53:792–801. doi: 10.1194/jlr.P022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, et al. Insulin-sensitive obesity. American journal of physiology Endocrinology and metabolism. 2010;299:E506–E515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 42.Denis GV, Obin MS. 'Metabolically healthy obesity': origins and implications. Molecular aspects of medicine. 2013;34:59–70. doi: 10.1016/j.mam.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deepa M, Papita M, Nazir A, Anjana RM, Ali MK, Narayan KM, et al. Lean people with dysglycemia have a worse metabolic profile than centrally obese people without dysglycemia. Diabetes technology & therapeutics. 2014;16:91–96. doi: 10.1089/dia.2013.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]