Abstract
To meet the challenge of identification of new treatments for stroke, this study was designed to evaluate a potent, nonselective opioid receptor (OR) agonist, biphalin, in comparison to subtype selective OR agonists, as a potential neuroprotective drug candidate using in vitro and in vivo models of ischemic stroke. Our in vitro approach included mouse primary neuronal cells that were challenged with glutamate and hypoxic/aglycemic (H/A) conditions. We observed that 10 nM biphalin, exerted a statistically significant neuroprotective effect after glutamate challenge, compared to all selective opioid agonists, according to lactate dehydrogenase (LDH) and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assays. Moreover, 10 nM biphalin provided superior neuroprotection after H/A-reoxygenation compared to selective opioid agonists in all cases. Our in vitro investigations were supported by in vivo studies which indicate that the nonselective opioid agonist, biphalin, achieves enhanced neuroprotective potency compared to any of the selective opioid agonists, evidenced by reduced edema and infarct ratios. Reduction of edema and infarction was accompanied by neurological improvement of the animals in two independent behavioral tests. Collectively these data strongly suggest that concurrent agonist stimulation of mu, kappa and delta ORs with biphalin is neuroprotective and superior to neuroprotection by activation of any single OR subtype.
Keywords: stroke, blood-brain barrier, opioid receptors, neuropeptide, neuroprotection
1. Introduction
Stroke is a leading cause of death and the primary cause of long-term disability in the U.S. (Roger et al., 2012). Currently, there is an unmet need for novel stroke therapeutics, since the only FDA-approved drug, t-PA, is used in only 3-5% of ischemic stroke patients (Fonarow et al., 2011; Roger et al., 2012). Ischemic stroke triggers a variety of pathogenic mechanisms which occur in minutes to days (Durukan and Tatlisumak, 2007). These include excitotoxicity, generation of free radicals, blood-brain barrier (BBB) disruption, inflammation, etc. Unfortunately, the preclinical investigative strategy of targeting a single component of the ischemic cascade for possible neuroprotection or neurorestoration has been largely unsuccessful, evidenced by the large number of clinical trials that have failed in the past decades (Moskowitz et al., 2010; Traystman, 2010).
Opioid receptors (OR) are widely distributed throughout the central and peripheral nervous systems, as well as gastrointestinal tract, which include delta, mu and kappa ORs (DOR, MOR, KOR) (Stein et al., 2003). In the 1980 and 1990’s, research focused on the potential use of high dose naloxone, a non-selective OR antagonist, for stroke neuroprotection (Hosobuchi et al., 1982). It was postulated that, independent of OR blockade, naloxone could also alter vital neuronal functions related to trans-membrane calcium flux, lipid peroxidation and antioxidative action (Faden, 1983). After continued pre-clinic and clinical evaluation, it was determined that naloxone was unsuccessful as a stroke therapeutic mainly due to aggravation of ischemic outcomes with the requirement of high dose infusions (Adams et al., 1986). Later, a KOR antagonist, nalmefene, was tested in clinic as a potential stroke neuroprotectant (Clark et al., 2000). Although, nalmefene was shown to be well tolerated (no significant CNS or cardiovascular events), it was demonstrated to be ineffective with regard to stroke neuroprotection in patients when administered after ischemia.
After the naloxone and nalmefene studies were discontinued, a growing amount of pre-clinical scientific reports have suggested that OR agonists can play a role in ischemic stroke neuroprotection. Interestingly, independent activation of OR subtypes in various stroke models has been shown to positively affect ischemic outcomes by regulating brain ionic homeostasis (DOR) (Chao et al., 2007; Chao et al., 2012), reducing ischemic striatal p-53 expression and enhancing GDNF protein levels in brain (DOR) (Borlongan et al., 2009), stimulating release of neurotrophic factors (DOR) (Tian et al., 2013), enhancing antioxidative enzyme activity (DOR) (Yang et al., 2009), inhibiting release of neuronal glutamate (KOR) (Gannon and Terrian, 1992), preservation of cerebrovascular autoregulation (KOR) (Wang et al., 2012), and enhancement of mitochondrial respiratory activity (MOR) (Feng et al., 2008). Notably, stimulation of selective OR subtypes has resulted in neuroprotection by largely non-overlapping mechanisms during both acute and prolonged ischemic injury. We therefore hypothesize that simultaneous activation of all OR subtypes, and hence independent neuroprotective pathways after stroke will lead to enhanced neuroprotection, superior to that of selective OR activation.
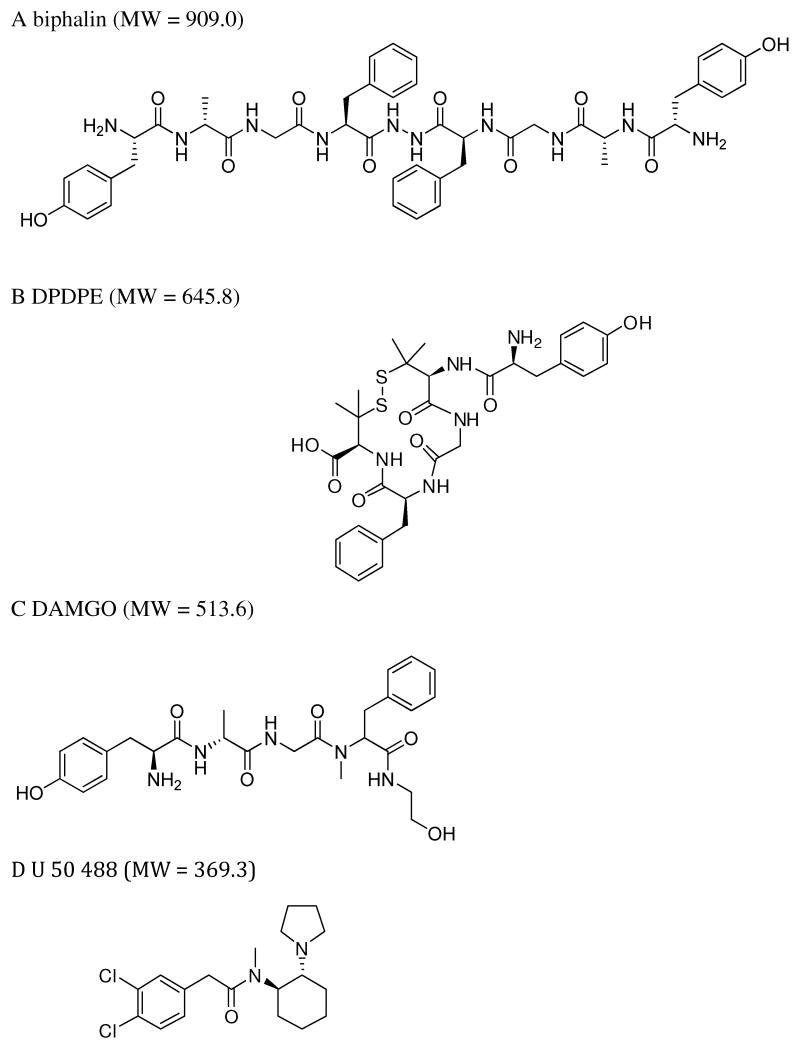
To test this hypothesis we chose to use a highly potent, non-selective OR agonist, biphalin (Fig 1A), which was first synthesized by Lipkowski et al., (1982). Biphalin exhibits high affinity to MOR and DOR and low affinity to KOR, crosses the BBB to enter CNS, and has reported serum and brain half-lives of 87 and 193 min, respectively (Horan et al., 1993). Biphalin exerts less dependence and tolerance, compared to morphine (Yamazaki et al., 2001), since these DOR and MOR-induced side effects are believed to be prevented by activation of KOR (Narita et al., 2001). To date, biphalin has been shown to be one of the most potent, peptide-based, opioid analgesics (Feliciani et al., 2013) and has recently been investigated as a potential treatment for abdominal pain associated with inflammatory bowel disease (Sobczak et al., 2014). The analgesic potency of biphalin is seven times greater than that of etorphine and three orders of magnitude greater than morphine after intracerebroventricular administration (Horan et al., 1993).
Figure 1.
Structures of biphalin and selective OR agonists. A. Non-selective OR agonist biphalin; B. DOR selective agonist DPDPE (Tyr-D-Pen-Gly-Phe-D-Pen); C. MOR selective agonist DAMGO (Tyr-D-Ala-Gly-NMe-Phe4, Gly-ol5-enkephalin); D. KOR selective agonist U 50488.
Since past investigations have reported biphalin to play a central role in reducing neuronal cellular edema by modulating ion transporter function and expression (Yang et al., 2011a), and reduce edema in both hippocampal slices subjected to OGD and reduce cellular edema in primary neurons subjected to OGD (Yang et al., 2011b), the first aim of this study was to detect the anti-edematous efficacy of biphalin in vivo and compare it to classical, OR-selective agonists. In addition, we compared the effects of these drugs on infarction size and neurological deficit after stroke. In the present study, we have also evaluated the effect of biphalin against glutamate-induced excitotoxicity and free radical generation upon ischemia-reperfusion. Collectively, our present in vitro and in vivo experiments suggest that biphalin administration after ischemia exhibits enhanced stroke neuroprotection compared to subtype-selective OR agonists, which leads to reduced glutamate neurotoxicity and oxidative stress.
2. Results
2.1 Effect of biphalin and selective OR agonists on glutamate challenge
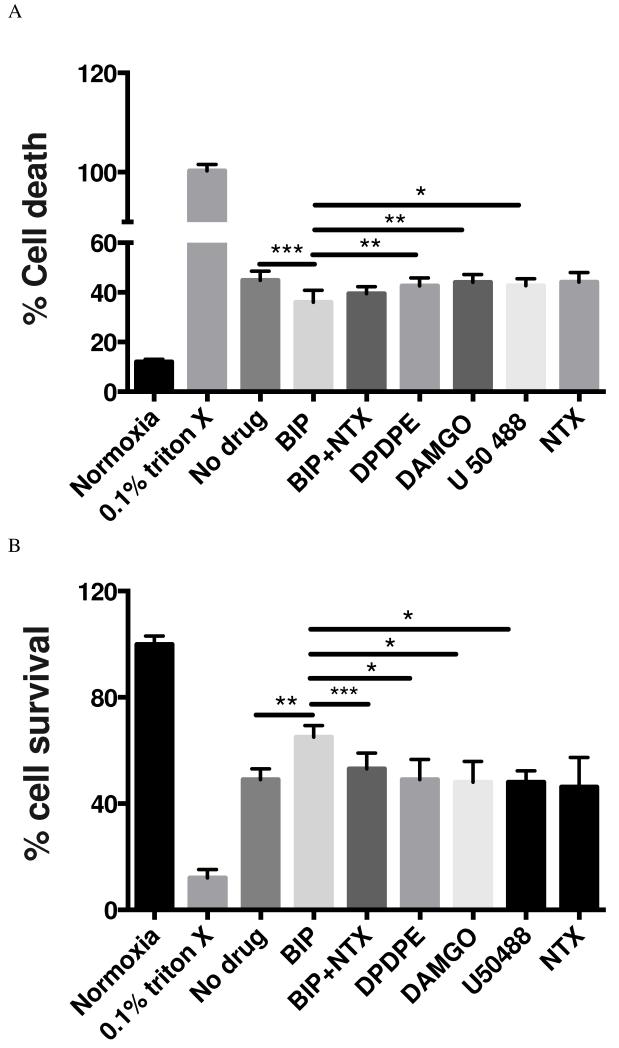
Primary mouse cortical neurons were exposed to glutamate (25 uM) for 24 hours in the presence or absence of biphalin or selective OR agonists (10 nM) and relative cytotoxicity and cell survival were assessed using LDH and MTT assays, respectively. LDH assay showed that biphalin reproducibly decreased neuronal death compared to neurons, which were subjected to glutamate challenge but were not treated with any drug (19.75% reduction in LDH release, P<0.001) (Fig 2A). This protective effect was significantly more potent than the effect of delta OR selective agent DPDPE (P<0.01), mu OR selective agent DAMGO (P<0.01) and kappa OR selective agent U50488 (P<0.05) at 10 nM concentration. Likewise, the MTT assay showed reproducible, statistically significant enhancement in neuronal survival upon biphalin treatment compared to no drug treatment (32.5 % increase in formation of formazan dye from MTA, p<0.01) and all selective OR agonists (P<0.05) (Fig 2B). Notably, non-selective OR antagonist NTX reversed the effect of biphalin in both assays (P<0.001). In either the LDH or MTT assay, no difference was observed in neurons when compared no drug treatment to NTX treatment (P>0.05).
Figure 2.
Effect of biphalin and selective OR agonists on glutamate challenge. For both graphs; *P<0.05; **P<0.01; ***P<0.001; data from 3 - 4 independent primary neuronal isolations with 2 - 3 replicate treatments per isolation. Selective agonists, DPDPE, DAMGO and U50,488 did not show any significant effect compared to no drug treatment group. All experimental groups were significantly different compared to normoxia (P<0.0001). A. LDH assay; effect of biphalin and selective OR agonists (10 nM) on glutamate exposure (25 uM) for 24 h. Compared to no drug treated group biphalin significantly decreased neuronal death (P<0.001) and effect of biphalin on neuronal death is statistically significant compared to DPDPE (P<0.01), DAMGO (P<0.01) and U50,488 (P<0.05). B. MTT assay; effect of biphalin and selective OR agonists (10 nM) on glutamate exposure (25 uM) for 24 h. Biphalin is significantly improving neuronal survival (P<0.01) compared to no drug treated group. In comparison to DPDPE (P<0.05), DAMGO (P<0.05) and U50,488 (P<0.05), biphalin showed statistical significant improvement in terms of neuronal survival. Naltrexone reversed the effect (P<0.001) of biphalin.
2.2 Effects of biphalin and selective OR agonists on H/A and reoxygenation challenge
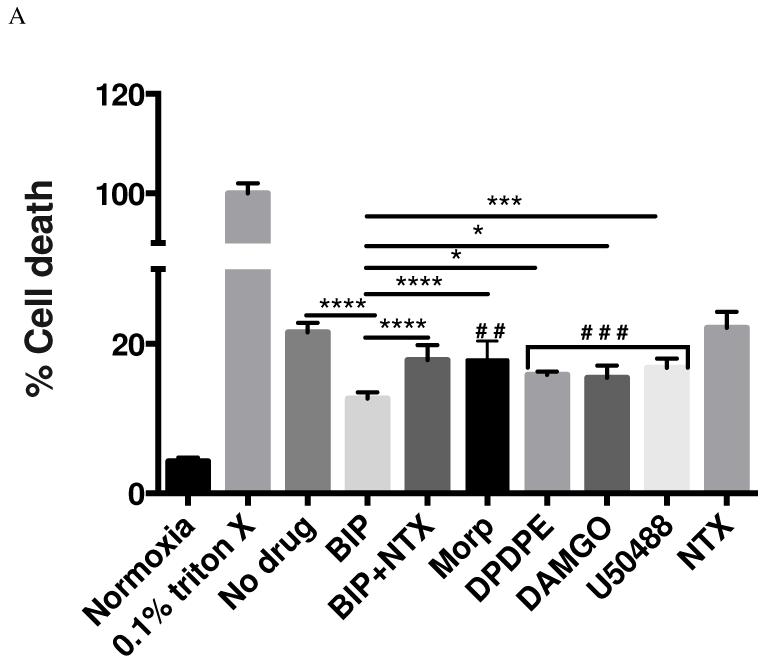
Here the effect of biphalin and selective OR agonists (10 nM) on survival of cortical neurons was measured upon exposure to 3 h H/A and 24 h reperfusion using LDH (Fig 3A) and MTT assays (Fig 3B). In the LDH assay, biphalin reproducibly decreased the cytotoxicity compared to no drug treatment (41.2% reduction, P<0.0001). The protective effect of biphalin was significantly better than that of delta, mu and kappa OR selective agonists DPDPE (P<0.05), DAMGO (P<0.05) and U50488 (P<0.001). Likewise, these results were reproduced in MTT assay. Importantly, the non-selective opioid antagonist naltrexone (NTX) reversed the effect of biphalin (P<0.0001) in both assays, demonstrating that the protective effects of this biphalin are mediated through ORs. NTX alone did not change survival of neurons measured by any of the assays.
Figure 3.
Effects of biphalin, morphine and selective OR agonists on H/A and reoxygenation challenge. For both graphs; ‘#’ compared to no drug group; ‘*’ compared to biphalin; #P<0.05; ##P<0.01; ###P<0.001;*P<0.05; **P<0.01; ***P<0.001; ****p<0.0001; data from 3 - 4 independent primary neuronal isolations with 2 - 3 replicate treatments per isolation. Compared to normoxia or 0.1% triton X, all experimental groups were significantly different (P<0.0001). A. LDH assay; effect of biphalin and selective OR agonists (10 nM) on 3 h H/A and 24 h reoxygenation. Compared to no drug treated group biphalin (P<0.0001), DPDPE (P<0.001), DAMGO (P<0.001), U50,488 (P<0.001) and Morphine (P<0.01) significantly decreased neuronal cell death. Again biphalin in comparison to DPDPE (P<0.05), DAMGO (P<0.05), U50,488 (P<0.05) and morphine (P<0.0001) showed better neuroprotection in terms of decreased neuronal death. NTX (P<0.0001) reversed the effect of biphalin. B. MTT assay; effect of biphalin, morphine and selective OR agonists (10 nM) on 3 h H/A and 24 h reoxygenation. Biphalin (P<0.0001), morphine (P<0.05), DPDPE (P<0.05), DAMGO (P<0.001) and U50.488 (P<0.001) significantly improved neuronal cell survival compared to no drug treated group. In comparison to morphine (P<0.01), DPDPE (P<0.001), DAMGO (P<0.05) and U50,488 (P<0.05), biphalin showed statistically significant improvement in terms of neuronal survival. NTX (P<0.0001) reversed the effect of biphalin.
2.3 Effects of biphalin and selective OR agonists on total ROS production
In this set of experiments, generation of ROS in neurons exposed to 3 h H/A and 24 h reperfusion in the presence or absence of OR agonists (10 nM) was assessed (Fig 4). ROS generation was reproducibly decreased when neurons were treated with biphalin (32.3% change, P<0.0001). This effect of biphalin was significantly better (P<0.01) than that of delta selective OR agonist DPDPE, yet similar to mu and kappa selective OR agonists DAMGO and U50488 (P>0.05). In this set of experiments NTX partially blocked the effect of biphalin yet this was not statistically significant.
Figure 4.
Effects of biphalin and selective OR agonists (10 nM) on total ROS production following 3 h H/A and 24 h reoxygenation in primary cortical neurons. All experimental groups were significantly different compared to normoxia (P<0.0001) or H2O2. Compared to no drug treated group, biphalin (P<0.0001), DPDPE (P<0.01), DAMGO (P<0.0001) and U50,488 (P<0.0001) decreased total ROS production. Biphalin effects on total ROS production was statistically significant in comparison to DPDPE (P<0.01). (‘#’ compared to no drug group; ‘*’ compared to biphalin; ##P<0.01; ####P<0.0001; **P<0.01; **** P<0.0001; data from 3 - 4 independent primary neuronal isolations with 2 - 3 replicate treatments per isolation).
2.4 Effect of biphalin and selective agonists on brain edema and infarct formation induced by MCAO with reperfusion
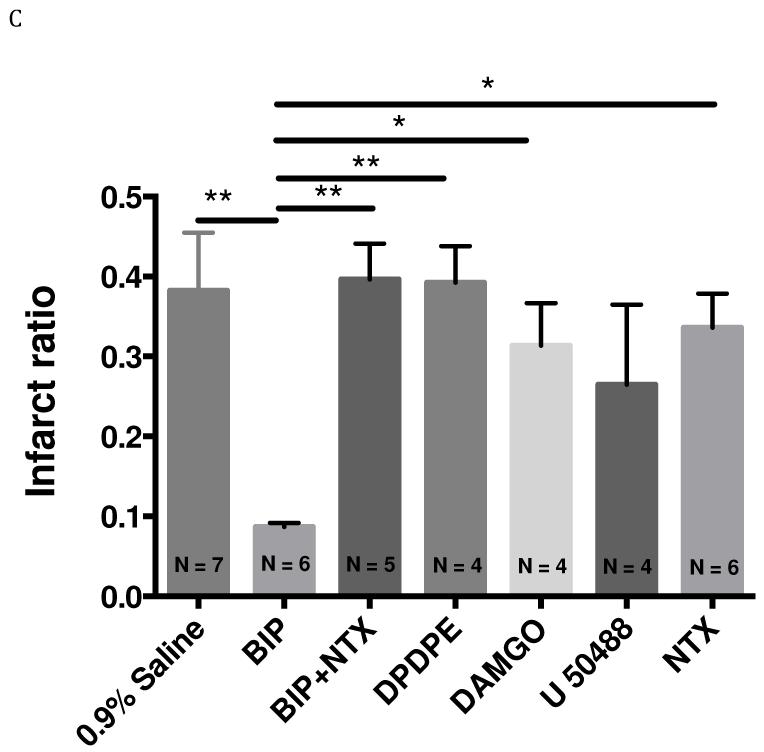
In this set of experiments the effect of OR agonists on edema formation and infarct volume after focal brain ischemia in mice were measured. All ischemic brain hemispheres showed significant swelling compared with the contralateral hemisphere in vehicle treated groups (edema ratio 0.2163), indicating significant edema formation during stroke. Biphalin and selective opioid agonists were administrated 10 min after reperfusion at a dose of 5mg/kg in saline (i.p.). Biphalin treatment resulted in a 76.4% reduction of edema ratio (P < 0.01; Fig. 5B) and 77.3% reduction of infarct ratio (P < 0.01; Fig. 5C). This effect was reversed by NTX (10 min pretreatment before the start of stroke surgery), indicating that the neuroprotective effect of biphalin was through ORs. On the contrary, none of the OR selective agonists significantly affected infarct or edema ratios, although all agents showed a trend towards neuroprotection (p > 0.05). NTX alone did not show any significant effect in decreasing edema or infarction ratios administered 10 min before stroke, at 1 mg/kg dose.
Figure 5.
Effect of intraperitoneal injection of biphalin, selective OR agonists (5 mg/kg, I.P. administrated 10 min after reperfusion), non-selective OR antagonist, naltrexone, (1 mg/kg, I.P. administrated 10 min before the surgery) or vehicle on edema and infarct formation in transient MCAO (60 min occlusion and 24 h reperfusion). A. Representative TTC staining of brains from vehicle and drug -treated mice. B. Brain edema formation in vehicle and drug -treated mice. Biphalin significantly decreased edema ratio (P<0.01) compared to saline treated group. NTX reversed (P<0.05) the effect of biphalin. Compared to selective agonists DPDPE (P<0.001) and DAMGO (P<0.001), biphalin significantly reduced edema ratio. Effect of DPDPE, DAMGO and U50,488 was not statistically significant compared to saline treated group. C. Brain infarct ratio in vehicle and drug-treated mice. Biphalin significantly reduced infarct ration (P<0.01) compared to 0.9% saline treated group. Biphalin showed better neuroprotection in terms of infarct ratio reduction compared to DPDPE (P<0.01), DAMGO (P<0.05) and NTX (P<0.05). NTX reversed (P<0.01) the effect of biphalin. In terms of infarct ration reduction, DPDPE, DAMGO and U50,488 did not show a statistically significant effect compared to vehicle treated group. (*P<0.05; **P<0.01; ***P<0.001; numbers indicated in parenthesis in the figure columns donate to the number of experimental animals per group). Mean cerebral blood flow reductions ± SEM in ischemic brain for saline group 80.7 ± 1.24%, biphalin 78.5 ± 0.96%, BIP+NTX 79.7 ± 2.02 %, DPDPE 81.3 ± 2.05 %, DAMGO 79.7 ± 0.83%, U50,488 78.8 ± 1.72% and NTX 76.9 ± 2.01%.
2.5 Effects of biphalin on neurological score
Neurological score evaluation was carried out 24 h after reperfusion in experimental groups (Fig 6). Biphalin treatment (5 mg/kg, administered i.p. 10 min after reperfusion) significantly improved the neurological score of animals compared with the vehicle-treated control group (30.4% improvement; P <0.01). However, none of the OR selective agonists showed a significant effect under the same experimental conditions (5 mg/kg, administered i.p. 10 min after reperfusion).
Figure 6.
Neurological score evaluation of mice 24 h after stroke and drug treatments. Biphalin showed reduced neurological deficit in terms of neurological score compared to saline treated group (P<0.01), DPDPE (P<0.05) and DAMGO (P<0.05). NTX reversed the effect (P<0.05) of biphalin. Selective agonists DPDPE, DAMGO and U50,488 did not show a statistically significant effect when compared to saline treated group. (*: P<0.05, **P<0.01; numbers indicated in parenthesis in the figure columns donate to the number of experimental animals per group).
2.6 Effects of biphalin on locomotor activity
Locomotor activity (including: horizontal activity, total distance, number of movements, movement time, stereotypy counts, stereotypy time, and center distance) was evaluated 24 h after reperfusion in experimental animals using VersaMax Monitoring System. All of the measured parameters were decreased in vehicle-treated animals subjected to stroke in comparison to the sham-operated animals (P < 0.05). With the treatment of biphalin, all locomotor parameters were significantly improved, compared with the vehicle treated stroke group (P < 0.05). None of the animals treated with selective OR agents showed improvement in any of these parameters (Table 1).
Table 1.
Locomotor activity measurement of 24 h after stroke and drug treatments. Data represent the mean ± S.E.M. of 4-5 independent determinations; numbers indicated in parenthesis in the line of the table columns donate to the number of experimental animals per group.
| Parameters | Sham (N = 7) |
Saline (N = 7) |
Biphalin (N = 6) |
DPDPE (N = 4) |
DAMGO (N = 4) |
U50488 (N = 4) |
NTX (N = 6) |
biphalin + NTX (N = 5) |
|---|---|---|---|---|---|---|---|---|
|
Horizontal
Activity |
2762 ± 288 |
230 ± 62 φφφφ |
3035 ± 301 #### |
1044 ± 391 *** |
492 ± 131 **** |
1010 ± 497 *** |
2712 ± 419 |
609 ± 242 **** |
|
Total
Distance(CM ) |
1845 ± 208 |
100 ± 43 φφφφ |
1786 ± 276 #### |
596 ± 285 ** | 175 ± 61 *** |
347 ± 240 *** |
1251 ± 411 |
88 ± 64 **** |
|
No. of
Movements |
139 ± 21 |
10 ± 3 φφφφ |
170 ± 25 #### |
52 ± 23 ** |
32 ± 13 *** |
38 ± 25 *** |
155 ± 21 |
19 ± 6 **** |
|
Movement
Time (Seconds) |
171 ± 27 |
10 ± 4 φφφ |
202 ± 32 #### |
61 ± 25 ** |
21 ± 7 *** |
40 ± 26 *** |
120 ± 36 |
8 ± 5 **** |
|
Stereotypy
Counts |
1229 ± 113 |
114 ± 45 φφφφ |
1388 ± 170 #### |
467 ± 145 ** | 225 ± 60 **** |
563 ± 252 ** |
1297 ± 190 |
408 ± 164 *** |
|
No. of
Stereotypy |
109 ± 15 |
16 ± 3 φφ |
133 ± 6 #### |
60 ± 21 ** |
33 ± 6 *** |
68 ± 24 * |
147 ± 14 |
58 ± 19 ** |
|
Stereotypy
Time (Seconds) |
122 ± 12 |
27 ± 16 φφ |
160 ± 17 ### |
64 ± 16 ** |
39 ± 13 ** |
103 ± 36 |
172 ± 16 |
69 ± 27 * |
|
Center
Distance (CM) |
449 ± 60 |
10 ± 7 φφφ |
451 ± 83 ## |
145 ± 88 | 49 ± 31 * |
86 ± 58 * |
413 ± 204 |
42 ± 26 ** |
Compared to Sham p<0.05
p< 0.01
p<0.001
p<0.0001
Compared to Saline treated group p<0.05
p< 0.01
p<0.001
p<0.0001
Compared to Biphalin p<0.05
p< 0.01
p<0.001
p<0.0001
3. Discussion
In this study, we documented three interesting observations using a non-selective OR agonist, biphalin. First, biphalin was found to exert enhanced protective potency in primary neurons challenged in hypoxia/reoxygenation and glutamate neurotoxicity models, compared to selective OR agonists at equimolar concentrations. Second, in primary neurons, biphalin displayed significant effects at decreasing ROS generation after hypoxia/reoxygenation. Lastly, biphalin exhibited better neuroprotective effects in reducing brain edema and infarction, as well as improving neurological function after stroke in a mouse model of focal ischemia.
Stroke treatment is challenging since brain ischemia is a complex pathophysiologic process that includes temporal and spatial events that span from hours to days. With the resultant blood supply decrease in the brain after stroke, the occurrence of energy depletion triggers subsequent membrane potential loss and consequently neuronal and glial cell depolarization. Since stroke triggers several pathways leading to neuronal cell death, effective neuroprotection might require stimulation of multiple distinct pathways (Lo et al., 2003). Hence, the goal to simultaneously target several putative neuroprotective pathways by biphalin via activation of multiple OR subtypes is a rational therapeutic approach. After ischemic depolarization, excitotoxic amino acids, especially glutamate, are released in the extracellular compartment from presynaptic neuron and are believed to play a critical role in neuronal injury (Dirnagl et al., 1999). Activation of glutamate receptors leads to the attendant failure of ion homeostasis leading to cell apoptosis and necrosis (Dirnagl et al., 1999). Current stroke research also suggests that free radicals, especially during reperfusion phase, serve as important signaling molecules triggering inflammation and cell apoptosis (Durukan and Talisumak 2007).
Increasing evidence supports the idea that selective OR agonists play a role in ischemic stroke neuroprotection through a variety of cellular pathways. It has been shown that DOR activation exhibits neuroprotection by stabilizing ionic hemostasis when primary neuronal cells are exposed to an acute insult (Chao et al., 2007). During prolonged stress, DOR expression was up-regulated resulting in stimulation of protein kinase C, mitogen-activated protein kinase and brain-derived neurotropic factor-tyrosine kinase B (BDNF-TrkB) signaling (Chao et al., 2012; Tian et al., 2013). DOR activation also has been shown to block Bax-related apoptosis and reduce neuronal damage (Chao et al., 2007; He et al., 2013; Yang et al., 2009). Selective KOR agonists have been shown to reduce neurotoxicity through inhibiting presynaptic glutamate release (Gannon and Terrian, 1992) and nitric oxide production (Zeynalov et al., 2006), and preserve cerebrovascular autoregulation after ischemia (Wang et al., 2012). Additionally, some studies suggest that MOR activation enhances mitochondrial respiratory activity and protects mitochondria from oxidative stress during ischemic preconditioning (Feng et al., 2008). Moreover, the MOR agonist morphine can decrease damage observed from NMDA challenge in a hippocampal slice model (Kawalec et al., 2011).
With the goal to develop an efficacious neuroprotective drug therapy for stroke, it is essential to understand the differential expression of drug targets, in this case ORs, which can vary during progression of the disease. According to in situ hybridization studies using 33P-labelled RNA probes, the most intense regional signals of MOR and KOR were observed in striatum, thalamus, hypothalamus, cerebral cortex, cerebellum and certain brainstem areas as well as the spinal cord, while the most intense signals for DOR were found in only cerebral cortex (Peckys and Landwehrmeyer, 1999). Importantly, the expression of ORs follows a different pattern during ischemia. Ting et al. found that MOR expression is increased in striatum 10 min after MCAO, and returns to basal levels at later time points (Ting et al., 1994). In another study, density of DOR was documented to decrease at 1-3 hours after MCAO, whereas MOR exhibited reduction at 6-12 hours, and KOR at 6 and 24 hours after ischemia (Boutin et al., 1999). Due to the differential patterns of OR expression and internalization in physiological and ischemic conditions, it is important to administer a selective drug at a specific time. The obvious advantage of utilizing non-selective agonist is that all ORs can be activated over an extended period of time with one drug. This notion was supported in part by our previous finding that biphalin shows a more potent effect in decreasing cellular edema formation in hippocampal slices than selective OR agonists (Yang et al., 2011b). In addition, our current findings from both in vitro and in vivo models of brain ischemia further support this hypothesis.
In the this study we chose to evaluate the protective effect of biphalin specifically in primary cortical neurons since these cell types play a vital role in processing and transmitting information in the brain, and are more vulnerable to ischemic cell death than astroglial or endothelial cells in the central nervous system (Lo et al., 2003). To confirm the neuroprotective effects of biphalin, we challenged neuronal cells with glutamate and hypoxia/aglycemia and used two independent approaches (LDH and MTT assays) to determine neuronal injury. LDH is an indicator of plasma membrane integrity and MTT is a widely used indicator of mitochondrial function, and both assays have been proved to be reliable indicators of neuronal injury (Lobner, 2000; Rashid et al., 2010). In our studies biphalin displayed a better ability to maintain integrity of plasma membrane (release of LDH), and mitochondrial function (conversion of MTT) compared to equimolar concentrations of selective OR agonists DPDPE, DAMGO and U 50 488 (Figures 2 and 3). These effects were abolished by a non-selective OR antagonist naltrexone, indicating involvement of ORs in the effects of biphalin. Our current results have good correlation with our previous work (Yang et al., 2011a; Yang et al., 2011b) and the results of Kawalec et al. (Kawalec et al., 2011). In the latter study, neuroprotective effects of biphalin were studied in organotypic hippocampal culture model of NMDA challenge, where 0.025 μM biphalin decreased the propidium iodide-detectible fraction of dying cells to 21.3%, compared to 61.9% of the vehicle treated group. In addition to neurotoxicity, during ischemic conditions, there is increased generation of ROS, which is a major cause of tissue damage. ROS causes cell death not only because of direct destruction to proteins, lipids and DNA but also disruption of the normal cellular signaling and gene regulation (Broughton et al., 2009). Our current results show that biphalin significantly reduces the levels ROS generation upon the H/A reoxygenation (Figure 4). Biphalin effects on reducing ROS generation were partially reversed by NTX, suggesting that some effects may be through non-OR mechanisms. Recently, the chemical properties of biphalin were assessed for antioxidant capacity and biphalin was found to have an antioxidant capacity 3.6 times higher than that of ascorbic acid (Garbuz et al., 2014). The high antioxidant capacity of biphalin might also contribute to its neuroprotective properties during reperfusion and is probably due to its enhanced tyramine moiety.
To evaluate the in vivo neuroprotective efficacy of biphalin, we also utilized a commonly accepted in vivo model of focal ischemia, MCAO model and compared the neuroprotective efficacy of biphalin to OR selective agonists. When we administrated all agonists at a comparable dose of 5 mg/kg 10 min after reperfusion, our results showed that biphalin reduced both edema (76.4% decrease) and infarct ratios (77.3% decrease) (Figure 5), compared to the vehicle treated mice. None of the selective OR agonists showed the improved edema and infarct ratios when administered at the same time and dose, although a trend was observed for all agonists. Correlations to functional recovery after stroke are important behavioral parameters to be tested in any pre-clinical study to increase translational relevance (Fisher et al., 2009). In our study, we evaluated the efficacy of biphalin on behavioral endpoints after stroke by utilizing two independent methods, neurological score evaluation and a separate set of blinded locomotor activity monitoring experiments, (Figure 6 and Table 1), further validating the histochemical evaluation of biphalin neuroprotection with TTC staining (Figure 5). Importantly, locomotor experiments were conducted in a blinded fashion, whereby data was collected by a researcher without prior knowledge of treatment groups and analyzed objectively by a VersaMax computerized activity monitoring system. All locomotor parameters sensitive to stroke damage significantly decreased with induction of 1 hour ischemia and 24 hours reperfusion and were significantly improved after biphalin administration. Importantly, NTX administration prior to biphalin reversed both neuroprotective and behavioral effects of biphalin.
To improve the quality of preclinical studies of purported acute stroke treatments, the Stroke Therapy Academic Industry Roundtable (STAIR) provides specific recommendations (Fisher et al., 2009). Although we have already applied several of principles in the current study, such as physiological monitoring, application of inclusion and exclusion criteria, randomization, and blinded assessment of outcome etc., our on-going and future studies plan to investigate the therapeutic time window and additional dosing to facilitate movement of the investigating drug closer to clinical application.
In conclusion, our data confirms that the non-selective, potent OR agonist biphalin displays excellent effects at decreasing ischemic damage in both in vitro and in vivo stroke models, compared to the selective OR agonists. Interestingly, our study demonstrated enhanced neuroprotective effects of biphalin with the in vivo model of focal ischemia compared to neuronal cultures. This could also be due to the action of biphalin on astrocytes, which express a variety of excitatory amino acid transporters (EATTs). Recently it has been reported that DOR activation upregulates astrocytic EAAT 1, 2 expression and function, thereby reducing neuroexcitotoxicity (Liang et al., 2014). Another important biologic effect of biphalin to consider are the possible hypothermic effects of this agent during stroke, since both DOR and KOR activation have been shown to mediate hypothermia (Kaneko et. al., 2012; Rawls and Benamer 2011), potentially providing additional neuroprotection during ischemia. Future studies are also warranted assessing the neuroprotective window of biphalin after stroke, involvement of other brain cell types, and even the potential combination of this neuroprotective therapy with the thrombolysis drug, t-PA.
4. Experimental procedures
4.1 Animals
All studies were approved by the Institutional Animal Care and Use Committee of Texas Tech University Health Sciences Center and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). 25-30 g CD-1 male mice, obtained from Charles River Laboratories, Inc. (Wilmington, MA), were kept under standardized light and dark conditions (12 h), humidity (70%) with temperature at 22°C and free access to food and water.
4.2 Mouse Primary Cortical Neuronal Cultures
Mouse primary cortical neurons were isolated and cultured as previously described (Yang et al., 2011b) with a slight modification. In brief, cerebral cortices were isolated from E16 or E17 embryos (CD-1 mice; Charles River Laboratories) and dissected in Hank’s balanced salt solution (HBSS) without Ca2+ and Mg2+ supplemented with 10 μg/ml gentamycin. Dissected pieces of free of meninges cortices were digested in 0.25% trypsin for 10-15 min at 37°C, neutralized with trypsin inhibitor. Dissociated cell suspensions were seeded into 12 or 24 well plates (0.2 × 106 per cm2 surface area) coated with poly-L-lysine (Sigma-Aldrich, St. Louis, MO) and cultured in Neurobasal medium (Invitrogen, Carlsbad, CA, USA) supplemented with 1.3 mM L-glutamine, 25 μg/ml gentamicin, and 2% B27 (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified atmosphere of 5% CO2 in air. Medium was totally replaced with fresh Neurobasal medium after overnight incubation and refreshed every 3 days. Data presented from in vitro experiments described below involved 3 - 4 primary neuronal isolations with 2 - 3 replicates for each treatment per isolation.
4.3 Glutamate induced neurotoxicity
Day 7-8 primary cortical neurons were subjected to glutamatergic stress by incubating with 25 μM glutamate (Sigma-Aldrich, St. Louis, MO) for 24 h in plain NB medium. Control cells were maintained in plain NB medium for 24 h in the absence of glutamate treatment. All opioid agonists (10 nM) and the non-selective antagonist naltrexone (100 nM) were administrated with glutamate.
4.4 Hypoxia/aglycemia (H/A) and reoxygenation challenge
7 or 8 in vitro-day-old primary cortical neurons were exposed to H/A condition as described previously (Yang et al., 2011a). The Earle’s balanced salt solution (140 mM NaCl, 5.36 mM KCl, 0.83 mM MgSO4, 1.8 mM CaCl2, 1.02 mM NaH2 PO4, and 6.19 mM NaHCO3) without glucose was used to create an aglycemic condition. Hypoxia (1 % oxygen) was induced by placing the cells in a custom-made hypoxic polymer glove box (Coy Laboratories, Grass Lake, MI), which was infused with 95% N2 and 5% CO2 at 37°C. Biphalin and selective opioid receptor agonist (10 nM) with or without the non-selective OR antagonist naltrexone (100 nM) (Sigma-Aldrich, St. Louis, MO) were added to the Earle’s balanced salt solution at the start of H/A. After 3 h H/A, the medium was replaced by neurobasal medium containing the same concentration of the drugs noted above, and cells were returned to cell culture incubator for 24 h.
4.5 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay
MTT assay was performed as previously reported (Marutani et al., 2012) with a slight modification. In brief, 3 h before the end of experiment, 0.25 mg/ml MTT was added to the cells and incubated at 37°C for 3 h in the dark. After the incubation, the cells were washed with HBSS, followed by addition of dimethyl sulfoxyde (DMSO) to dissolve the formed blue dye and measurement of absorbance at 570 nm together with reference absorbance at 670 nm (BioTek, Winooski, VT). Cell viability is presented as ratio to control cell by the absorbance at 570 nm reading.
4.6 Lactate dehydrogenase (LDH) assay
The LDH assay was carried out using LDH Cytotoxicity Detection Kit (Roche South San Francisco, CA, USA) following the protocol recommended by manufacturer (Marutani et al., 2012). At the end of experiment, the medium was collected and centrifuged at 7000 rpm for 5 min. Neurons were lysed with 0.1% Triton-X and centrifuged at 10000 rpm for 10 min. Content of LDH was measured in both the medium and lysates. The percentage of released LDH was calculated as: LDH medium/[LDH (medium+ cell lysates)]*100. The results are presented as ratio to control cells.
4.7 Reactive oxygen species (ROS) detection
Generation of ROS was measured by using a cell-permeable dye 2,7-dichlorodihydrofluorescein diacetate (H2DCF-DA; Invitrogen, Carlsbad, CA, USA) as described previously (Lopez et al., 2006). Neurons were incubated with 10 μM CM-H2DCF-DA for 3 h at 37°C before the end of H/A or NMDA challenge. After the incubation, the medium was removed, cells were washed with PBS and fluorescent intensity was measured at 485 nm excitation and at 535 nm emission using a plate reader (BioTek, Winooski, VT).
4.8 Dose-selection and drug administration for in vivo studies
For in vitro neuroprotection experiments, we selected 10 nM final concentrations for all agonists. This concentration was chosen based on our extensive concentration-response studies assessing effects of biphalin on cellular edema following ischemia in both organotypic hippocampal slices (Kawalec et al., 2011) and primary cortical neurons (Yang et al., 2011a; Yang et al., 2011b). This effective concentration was also confirmed in preliminary concentration-response studies evaluating neuroprotective effects of biphalin in primary cortical neurons (data not shown). To achieve ~10 nM brain concentration of biphalin in our in vivo studies, 5 mg/kg IP dose of the drug was chosen. This calculation is based on previous in vivo brain and spinal cord distribution studies estimating that approximately 0.05% of injected biphalin enters the brain (Abbruscato et al., 1997). The non-selective OR antagonist, naltrexone, was administrated IP at 1 mg/kg (Haj-Mirzaian et al., 2014) 10 min before the surgery. We selected an equivalent dose (5 mg/kg) for DPDPE, DAMGO, and U-50,488 with the idea that selective agonist would have an equivalent, if not better chance to enter the ischemic brain and bind to their respective opioid receptors. Biphalin has a significantly higher molecular weight compared to all 3 specific agonists (Fig 1) (1.4× higher than DPDPE, 1.8× higher than DAMGO, and 2.5× higher than U-50,488), suggesting that molar concentration of biphalin would be 1.4 to 2.5 fold less than that of the selective agonists in the systemic circulation. In addition, biphalin would be at a disadvantage compared to the selective agonists because of its decreased plasma stability: reported plasma T1/2 for biphalin is 87 min (Horan et al, 1993), compared to > 240 min for DPDPE (Weber et al., 1992), > 200 min for DAMGO (Van Dorpe et al., 2010), and 156 min for U40,488 (Jones et al., 2010).
4.9 Middle cerebral artery occlusion (MCAO) with reperfusion
MCAO was performed in CD-1 male mice (25-30g) as previously reported (Yang et al., 2011b) with a slight modification. In brief, surgery was performed using a Zeiss OPMI pico I surgical microscope (Carl Zeiss GmbH, Jena, Germany). Temperature was maintained at about 37°C, controlled by the thermostatic blanket (TC-1000 Temperature Controller, CWE, USA). Mice were anesthetized with 4% and maintained at 1-1.5 % isoflurane with a facemask. The laser Doppler probe (Moor Instruments, Wilmington, DE) was placed on the skull directly in the territory of the left middle cerebral artery (MCA) perfusion area to monitor the cerebral blood during the operation. A midline incision was made at the neck about 1.5 cm long. The left carotid bifurcation, external carotid artery (ECA) and common carotid artery (CCA) were isolated from the adjacent tissue. After occlusion of CCA using a micro clip, the left ECA was ligated, coagulated, and cut distally to the cranial thyroid artery. 6-0 nylon monofilament with a round tip (0.20-0.25 mm) was introduced gently up to ~8.5-9 mm to block the origin of the MCA. More than 75% blood flow decrease of the baseline was considered as a successful occlusion verified by a laser Doppler flowmetry.
After 60 min occlusion, the suture was withdrawn up to the left carotid bifurcation to restore blood flow, i.e. reperfusion. If the cerebral blood flow did not recover up to 70% of baseline within 10 min after the start of reperfusion, animals were excluded from the experimental group. Biphalin, the OR selective agonists (5 mg/kg) or saline were administered IP 10 min after reperfusion.
4.10 2, 3, 5-Triphenyltetrazolium chloride (TTC) staining and evaluation of infarct and edema ratios
TTC staining was carried out to identify viable and nonviable brain tissue (Gorgulu et al., 2000). After stroke experiments mice were anaesthetized with an overdose of isoflurane (4%) and euthanized through cervical dislocation, mouse brain was quickly removed and sectioned into 1mm thick slices by tissue chopper (McIlwain Tissue Chopper). Coronal brain slices were incubated in a 1% solution of TTC in saline at 37 °C for 5-10 min and were scanned by the scanner. Quantification of images was carried out using image analysis software (Image J 1.47, National Institutes of Health, Bethesda, MD, downloadable from (http://rsb.info.nih.gov/ij/download.html). To minimize the overestimation of infarcted area due to brain tissue swelling, we evaluated the damage of the ischemic hemisphere using the edema correction as described previously (Sydserff et al., 1996; Yang et al., 2011a). Three measurements were measured on each slice: infarct area (X) (mm2), Y: area of the infracted (ipsilateral) hemisphere slice (mm2), and Z: area of the noninfarcted (contralateral) hemisphere slices (mm2). S: brain swelling (mm2), S=Y-Z. I: corrected infarct (mm2), was calculated using the following equation I= X-S. The infarct and edema ratio results are presented as the brain average of the percentage of ipsilateral to contralateral hemispheric areas.
4.11 Neurological score evaluation
Neurologic examinations were carried out 24 hr after reperfusion (Hara et al., 1996). Mouse was held gently by tail, suspended at about 50 cm above the bench and monitored for forelimb flexion. Extension of both forelimbs straight toward the floor was considered absence of a neurologic deficit and score 0 was assigned to the animal. Score of 1, mild neurological deficit, was recorded when the animal showed forelimb flexion, i.e., the mouse flexed the forelimb contralateral to the injured hemisphere. Then the mouse was placed on a large sheet of soft plastic coated paper to make sure that the mouse can grip firmly by their claws. While holding from the tail the mouse was pushed behind the shoulder with gentle lateral pressure until the forelimb slid several inches. Score of 2, moderate neurological deficit, was recorded when the mouse showed decreased resistance to lateral push and forelimb flexion. Lastly, the mouse was placed on the floor and its movements were monitored. Score of 3, severe neurological deficit, was recorded when the animal showed the same behavior as for score 2, plus a circling movement.
4.12 Locomotor activity
Locomotor activity of experimental animals was evaluated using VersaMax animal monitors (Accuscan Instruments Inc., Columbus, OH), which allows for objective measurement of a set of behavioral parameters that have been shown previously to reflect behavioral changes associated with neurological damage induced by MCAO (Vendrame et al., 2004). We have used this system to assess the neurological deficit of mice after ischemic damage in previous studies using both permanent and transient MCAO (Yang et al., 2011a). The experimental mice were placed onto the monitoring chamber to acclimate to the environment 20 min before the experiment. Animals were monitored for five-min spans in 4 sequential cycles. All behavioral assays were carried out between 5:00 PM and 10:00 PM. Locomotor activity monitoring and data analysis was conducted without knowlege of the treatment conditions.
4.13 Statistical Analysis
All data are expressed as the mean ± S.E.M. The values were analyzed by one-way analysis of variance and post hoc analysis using Dunnett multiple comparison (Prism, version 5.0; GraphPad Software Inc., San Diego, CA). P values less than 0.05 were considered statistically significant.
Biphalin provides enhanced in vitro and in vivo ischemia-reperfusion neuroprotection
Treatment reduces neuronal damage induced by glutamate and hypoxia-reperfusion
Treatment reduces free radical damage in neurons challenged with hypoxia-reperfusion
Treatment significantly reduces brain stroke damage and improves behavioral endpoints
Biphalin efficiently reduces stroke damage compared to selective opioid agents.
Acknowledgments
This work was supported by National Institution of Health Grant RO1 NS076012 to Thomas J. Abbruscato
Nonstandard abbreviations used in the paper
- OR
Opioid receptor
- DOR
Delta Opioid receptor
- MOR
Mu Opioid Receptor
- KOR
Kappa Opioid Receptor
- NKCC
Na+, K+, 2Cl− cotransporter
- BBB
Blood-Brain Barrier
- H
Hypoxia
- A
Aglycemia
- TTC
2,3,5-Triphenyltetrazolium chloride
- ANOVA
One-way Analysis of Variance
- CCA
Common Carotid Artery
- ECA
External Carotid Artery
- MCA
Middle Cerebral Artery
- MCAO
Middle Cerebral Artery Occlusion
- tMCAO
transient MCAO
- I
ischemia
- R
reperfusion
- EBSS
Earle’s Balanced Salt Solution
- ROS
Reactive oxygen species
- H2DCF-DA
2,7-dichlorodihydrofluorescein diacetate
- PKC
protein kinase C
- SAR
structure-activity relationship
- EAAT
excitatory amino acid transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbruscato TJ, Thomas SA, Hruby VJ, Davis TP. Brain and spinal cord distribution of biphalin, correlation with opioid receptor density and a mechanism of CNS entry. J. Neurochem. 1997;69(3):1236–45. doi: 10.1046/j.1471-4159.1997.69031236.x. [DOI] [PubMed] [Google Scholar]
- Adams HP, Jr., et al. A dose-escalation study of large doses of naloxone for treatment of patients with acute cerebral ischemia. Stroke. 1986;17:404–9. doi: 10.1161/01.str.17.3.404. [DOI] [PubMed] [Google Scholar]
- Boutin H, et al. Differential time-course decreases in nonselective, mu-, delta-, and kappa-opioid receptors after focal cerebral ischemia in mice. Stroke. 1999;30:1271–7. doi: 10.1161/01.str.30.6.1271. discussion 1278. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–9. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Chao D, et al. delta-, but not mu-, opioid receptor stabilizes K(+) homeostasis by reducing Ca(2+) influx in the cortex during acute hypoxia. J Cell Physiol. 2007;212:60–7. doi: 10.1002/jcp.21000. [DOI] [PubMed] [Google Scholar]
- Chao D, et al. DOR activation inhibits anoxic/ischemic Na+ influx through Na+ channels via PKC mechanisms in the cortex. Exp Neurol. 2012;236:228–39. doi: 10.1016/j.expneurol.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WM, et al. The Cervene Stroke Study Investigators Cervene (Nalmefene) in acute ischemic stroke: final results of a phase III efficacy study. Stroke. 2000;31:1234–9. doi: 10.1161/01.str.31.6.1234. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87:179–97. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Faden AI. Neuropeptides and stroke: current status and potential application. Stroke. 1983;14:169–72. doi: 10.1161/01.str.14.2.169. [DOI] [PubMed] [Google Scholar]
- Feliciani F, et al. Structure-activity relationships of biphalin analogs and their biological evaluation on opioid receptors. Mini Rev Med Chem. 2013;13:11–33. [PubMed] [Google Scholar]
- Feng Y, et al. Endomorphins and morphine limit anoxia-reoxygenation-induced brain mitochondrial dysfunction in the mouse. Life Sci. 2008;82:752–63. doi: 10.1016/j.lfs.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Fisher M, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–50. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonarow GC, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123:750–8. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- Gannon RL, Terrian DM. Kappa opioid agonists inhibit transmitter release from guinea pig hippocampal mossy fiber synaptosomes. Neurochem Res. 1992;17:741–7. doi: 10.1007/BF00969007. [DOI] [PubMed] [Google Scholar]
- Garbuz O, et al. The non-opioid receptor, antioxidant properties of morphine and the opioid peptide analog biphalin. Peptides. 2014;63C:1–3. doi: 10.1016/j.peptides.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Gorgulu A, et al. Reduction of edema and infarction by Memantine and MK-801 after focal cerebral ischaemia and reperfusion in rat. Acta Neurochir (Wien) 2000;142:1287–92. doi: 10.1007/s007010070027. [DOI] [PubMed] [Google Scholar]
- Haj-Mirzaian A, et al. Opioid/NMDA receptors blockade reverses the depressant-like behavior of foot shock stress in the mouse forced swimming test. Eur J Pharmacol. 2014;735:26–31. doi: 10.1016/j.ejphar.2014.03.053. [DOI] [PubMed] [Google Scholar]
- Hara H, et al. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J Cereb Blood Flow Metab. 1996;16:605–11. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- He X, et al. Neuroprotection against hypoxia/ischemia: delta-opioid receptor-mediated cellular/molecular events. Cell Mol Life Sci. 2013;70:2291–303. doi: 10.1007/s00018-012-1167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan PJ, et al. Antinociceptive profile of biphalin, a dimeric enkephalin analog. J Pharmacol Exp Ther. 1993;265:1446–54. [PubMed] [Google Scholar]
- Hosobuchi Y, Baskin DS, Woo SK. Reversal of induced ischemic neurologic deficit in gerbils by the opiate antagonist naloxone. Science. 1982;215:69–71. doi: 10.1126/science.6274019. [DOI] [PubMed] [Google Scholar]
- Jones DC, et al. Identification of a κ-opioid agonist as a potent and selective lead for drug development against human African trypanosomiasis. Biochem Pharmacol. 2010;80(10):1478–86. doi: 10.1016/j.bcp.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawalec M, et al. Neuroprotective potential of biphalin, multireceptor opioid peptide, against excitotoxic injury in hippocampal organotypic culture. Neurochem Res. 2011;36:2091–5. doi: 10.1007/s11064-011-0568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lobner D. Comparison of the LDH and MTT assays for quantifying cell death: validity for neuronal apoptosis? J Neurosci Methods. 2000;96:147–52. doi: 10.1016/s0165-0270(99)00193-4. [DOI] [PubMed] [Google Scholar]
- Lopez E, et al. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic Biol Med. 2006;40:940–51. doi: 10.1016/j.freeradbiomed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Marutani E, et al. A novel hydrogen sulfide-releasing N-methyl-D-aspartate receptor antagonist prevents ischemic neuronal death. J Biol Chem. 2012;287:32124–35. doi: 10.1074/jbc.M112.374124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–98. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Funada M, Suzuki T. Regulations of opioid dependence by opioid receptor types. Pharmacol Ther. 2001;89:1–15. doi: 10.1016/s0163-7258(00)00099-1. [DOI] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- Rashid M, Arumugam TV, Karamyan VT. Association of the novel non-AT1, non-AT2 angiotensin binding site with neuronal cell death. J Pharmacol Exp Ther. 2010;335:754–61. doi: 10.1124/jpet.110.171439. [DOI] [PubMed] [Google Scholar]
- Roger VL, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med. 2003;9:1003–8. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- Sydserff SG, Green AR, Cross AJ. The effect of oedema and tissue swelling on the measurement of neuroprotection; a study using chlormethiazole and permanent middle cerebral artery occlusion in rats. Neurodegeneration. 1996;5:81–5. doi: 10.1006/neur.1996.0011. [DOI] [PubMed] [Google Scholar]
- Tian X, et al. Effect of delta-opioid receptor activation on BDNF-TrkB vs. TNF-alpha in the mouse cortex exposed to prolonged hypoxia. Int J Mol Sci. 2013;14:15959–76. doi: 10.3390/ijms140815959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting P, Xu S, Krumins S. Endogenous opioid system activity following temporary focal cerebral ischemia. Acta Neurochir Suppl (Wien) 1994;60:253–6. doi: 10.1007/978-3-7091-9334-1_67. [DOI] [PubMed] [Google Scholar]
- Traystman RJ. Neuroprotection: introduction. Stroke. 2010;41:S63. doi: 10.1161/STROKEAHA.110.598557. [DOI] [PubMed] [Google Scholar]
- Van Dorpe S, et al. Analytical characterization and comparison of the blood-brain barrier permeability of eight opioid peptides. Peptides. 2010 Jul;31(7):1390–9. doi: 10.1016/j.peptides.2010.03.029. 2010. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. Salvinorin A administration after global cerebral hypoxia/ischemia preserves cerebrovascular autoregulation via kappa opioid receptor in piglets. PLoS One. 2012;7:e41724. doi: 10.1371/journal.pone.0041724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SJ, et al. Whole body and brain distribution of [3H]cyclic [D-Pen2,D-Pen5] enkephalin after intraperitoneal, intravenous, oral and subcutaneous administration. J Pharmacol Exp Ther. 1992 Dec;263(3):1308–16. [PubMed] [Google Scholar]
- Yamazaki M, et al. The opioid peptide analogue biphalin induces less physical dependence than morphine. Life Sci. 2001;69:1023–8. doi: 10.1016/s0024-3205(01)01194-8. [DOI] [PubMed] [Google Scholar]
- Yang L, et al. Characterization of neuroprotective effects of biphalin, an opioid receptor agonist, in a model of focal brain ischemia. J Pharmacol Exp Ther. 2011a;339:499–508. doi: 10.1124/jpet.111.184127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, et al. Opioid receptor agonists reduce brain edema in stroke. Brain Res. 2011b;1383:307–16. doi: 10.1016/j.brainres.2011.01.083. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. delta-Opioid receptor activation attenuates oxidative injury in the ischemic rat brain. BMC Biol. 2009;7:55. doi: 10.1186/1741-7007-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeynalov E, et al. Neuroprotective effect of selective kappa opioid receptor agonist is gender specific and linked to reduced neuronal nitric oxide. J Cereb Blood Flow Metab. 2006;26:414–20. doi: 10.1038/sj.jcbfm.9600196. [DOI] [PubMed] [Google Scholar]