Abstract
Background
Epigenetic modifications likely control fate of hematopoietic stem cells (HSC). The chromatin modifying agents (CMA), 5-aza-2’-deoxyctidine (5azaD) and trichostatin A (TSA) have previously been shown to expand HSC from cord blood and bone marrow. Here we assessed whether CMA can also expand HSCs present in growth factor mobilized human peripheral blood (MPB).
Study Design & Methods
5azaD and TSA were sequentially added to CD34+ MPB cells in the presence of cytokines and the cells were cultured for nine days.
Results
Following culture, a 3.6 ± 0.5 fold expansion of CD34+CD90+ cells, a 10.1 ± 0.5 fold expansion of primitive colony forming unit (CFU)-mix, and a 2.2 ± 0.5 fold expansion of long-term cobble stone-area forming cells (CAFC) was observed in 5azaD/TSA expanded cells. By contrast, cells cultured in cytokines without 5azaD/TSA displayed no expansion; rather a reduction in CD34+CD90+ cells (0.7 ± 0.1 fold) and CAFCs (0.3 ± 0.1) from their initial numbers was observed. Global hypomethylation corresponding with increased transcript levels of several genes implicated in HSC self-renewal, including HOXB4, GATA2, and EZH2, was observed in 5azaD/TSA expanded MPB cells in contrast to controls. 5azaD/TSA expanded MPB cells retained in vivo hematopoietic engraftment capacity.
Conclusion
MPB CD34+ cells from donors can be expanded using 5azaD/TSA and these expanded cells retain in vivo hematopoietic reconstitution capacity. This strategy may prove to be potentially useful to augment HSCs numbers for patients who fail to mobilize.
Keywords: mobilized peripheral blood stem cells, epigenetics, transplantation
Introduction
In previous studies, we have demonstrated that epigenetic mechanisms likely play a critical role in regulating the fate of hematopoietic stem cells (HSC) derived from human bone marrow (BM) and cord blood (CB) CD34+ cells.1-4 Treatment with chromatin modifying agents (CMA), 5-aza-2’-deoxyctidine (5azaD) and trichostatin A (TSA) can alter gene expression and expand hematopoietic progenitors, including a transplantable HSC population. A large majority of HSC transplantations used for clinical therapy are currently performed using HSC derived from mobilized peripheral blood (MPB).5 Biological differences between MPB, CB, and BM derived CD34+ cells are suggested based on observed differences in gene expression profiles, patterns of cloning efficiency, and response to stimulatory cytokines.6-8
A minimum graft size of 2 × 106 CD34+ cells/kg is often cited as the number of CD34+ cells recommended because a proportion of patients would be expected to have delayed hematopoietic recovery or failure to display hematopoietic reconstitution following transplantation below this threshold.9-11 However, 15-30% of patients with multiple myeloma or lymphoma considered for high dose chemotherapy with autologous HSC rescue fail to achieve this graft size with chemotherapy or growth factor induced mobilization.12-15 Plerixafor, a CXCR-4 antagonist, can overcome failure to mobilize an adequate graft size in a significant proportion of patients, although up to 10% of patients still fail to mobilize an adequate graft after four days of apheresis.16 Developing methods to expand MPB HSC cells may provide a means to overcome failure to undergo transplantation due to suboptimal graft size.
Although there is concern that CMA may activate silenced oncogenes, these agents have been shown to prevent cancer in several animal models.17-21 Unlike other cytosine analogues such as cytarabine or gemcitabine, the sugar back-bone of 5azaD does not terminate synthesis of DNA chain at relatively low concentrations and can deplete DNMT1 without resulting in significant DNA damage.22-26 To date, there have been no unusual patterns of cytogenetic abnormality to suggest that 5azaD is capable of exacerbating chromosomal instability.27,28 Our preliminary studies on CMA expanded CB cultures have revealed no detectable cytogenetic abnormalities and no tumor formation after xenotransplantion into mice.29 Furthermore, to test genomic stability of CMA expanded grafts, we have transplanted 5azaD/TSA expanded autologous BM grafts in a non-human primate model which is currently under investigation (manuscript under preparation).29
In this study, we assessed the effects of 5azaD/TSA on expansion of MPB cells in in vitro culture and in vivo transplantation assays utilizing immunodeficient mice as a surrogate host. The MPB cells were cultured in previously determined cytokine cocktails that yielded the lowest and highest expansion of CD34+CD90+ CB cells to assess for differences in expansion based on environmental cues between MPB and CB cells.1 The objective of this study was to determine whether ex vivo epigenetic modification using 5azaD/TSA in culture could augment the numbers of transplantable HSC from a normal MPB collection.
Materials & Methods
Isolation of MPB CD34+ cells
Human umbilical cord blood (CB) were obtained following institutional guidelines as described previously.1-3 Growth factor-mobilized human MPB or bone marrow (BM) cells were obtained from healthy donors either from a commercially available source (AllCells, LLC Emeryville, Ca) or from aliquots of de-identified unused vials after the intended recipients were deceased following institutional review board guidelines. Cryopreserved human MPB mononuclear cells were rapidly thawed at 37°C and diluted in Isocove modified Dulbecco medium (IMDM; BioWhittaker, Walkersvill, MD) containing 10% heat inactivated fetal bovine serum (FBS; HyClone Laboratories, Logan, UT) and 10% ACD-A (Baxter, Deerfield, IL). The CD34+ cells were immunomagnetically enriched using magnetically activated cell sorting (MACS) CD34 progenitor kits (Miltenyi Biotech, Auburn, CA) as previously described.1-4 Purity of MPB CD34+ cells ranged between 95 - 99%.
Ex vivo culture
The MPB CD34+ cells (1×105 cells/well) were cultured in IMDM containing 30% FBS supplemented with cytokines (100 ng/mL stem cell factor (SCF), 100 ng/mL FLT-3 ligand (FL), 100 ng/mL thrombopoietin (TPO) and 50 ng/mL IL-3). All cytokines were purchased from Cell Genix (Antioch, IL). The cells were incubated at 37°C in a 100%-humidified atmosphere containing 5% CO2. After an initial 16 hours of incubation, cells were exposed to 5azaD (1μM). After an additional 36 hours, the cells were washed and then equally distributed to new tissue-culture dishes in 2.5mL IMDM supplemented with 30% FBS (Hyclone Laboratories, Logan, UT, USA), TSA (5ng/mL), and cytokines (Highest yield environment/Cytokine A: 100 ng/mL SCF, 100 ng/mL FL, 100 ng/mL TPO; Lowest yield environment/Cytokine B: 100 ng/mL SCF, 100 ng/mL FL, 100 ng/mL TPO, 50ng/mL IL-3, 50ng/mL IL-6). Both 5azaD and TSA was purchased from Sigma (St Louis, MO, USA). The cytokine environments were based on previous studies for cytokine combinations yielding the highest and lowest expansion of CD34+CD90+ CB cells.1 Control cultures were incubated in identical culture conditions without the addition of 5azaD/TSA. The culture was continued for an additional seven days (total nine days) after which cultured cells were harvested. Viable cells were enumerated using the trypan blue exclusion method. Immunophenotyping was performed by flow cytometry to determine the expansion of CD34+CD90+ cells from their input numbers and in vitro clonogenic and in vivo xeno-transplantation assays were performed to determine the functional potential of CMA-expanded MPB cells. MPB cells utilized for LINE-1, PCR, and in vivo xeno transplantation studies were expanded in cytokine A (optimal environment) conditions. Fold expansion of CD34+CD90+ cells was determined by dividing the total numbers of viable cells expressing the phenotype at Day 9 by the input number of viable cells expressing the same phenotype at Day 0 as shown below.
Flow cytometric analysis
Cells were stained with the anti-human CD34 monoclonal antibody (mAb) conjugated to fluoroscein isothiocyanate (FITC) and the anti-human CD90 mAb conjugated to phycoerythrin (PE). The mAbs were purchased from Becton Dickinson PharMingen (San Diego, CA). The stained cells were analyzed on a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA) and at least 10,000 live cells were acquired per analysis.
Long Interspersed Nucleotide Element-1 (LINE-1) Assays
DNA methylation analysis was performed by EpigenDx (Worcester, MA) using quantitative pyrosequencing and the PSQ-HS96 system according to standard operating procedures.30 Long interspersed nucleotide element 1 (LINE-1) primers were used to amplify regions of interest for analysis as described previously.31 The LINE-1 assays were performed using genomic DNA to compare global methylation at four CpG sites between purified CD34+ cells derived from unmanipulated primary CB, BM and MPB samples and in MPB cells 72 hours after treatment with or without 5azaD/TSA.
RNA extraction and real-time quantitative PCR
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) from unmanipulated MPB cells and MPB cells after nine days of culture as described above (Cytokine A). Approximately 1 ug of total RNA was reverse-transcribed using a Superscript First Strand with oligo dT primers (Life Technologies, Carlsbad, CA). To quantitate the level of mRNA expression, we performed polymerase chain reaction (PCR) amplification using the 7500 Fast Sequence detector (Life Technologies). The PCR products were detected using Fast SYBR green technology (Life Technologies). GAPDH mRNA quantification was used as the internal calibrator and the standard curve method was used to determine relative mRNA quantitation. Measurements were performed in triplicate and negative controls without cDNA template were included in each assay. The primer sequences used in real-time quantitative PCR assays are shown in Table 1.
Table 1.
The primer sequences used in real-time PCR assays were as follows:
| Gene Name |
Forward Primer | Reverse Primer: |
|---|---|---|
| GAPDH | TGCACCACCAACTGCTTAGC | TCTTCTGGGTGGCAGTGATG |
| HOXB4 | ACCTCGACACCCGCTAACAAATGA | AATGGGCACGAAAGATGAGGGAGA |
| EZH2 | CAGTTTGTTGGCGGAAGCGTGTAA | AGGATGTGCACAGGCTGTATCCTT |
| GATA2 | ATTGTCAGACGACAACCACCACCT | TTCCTTCTTCATGGTCAGTGGCCT |
| PU.1 | AACGCCAAACGCACGAGTATTACC | TGAAGTTGTTCTCGGCGAAGCTCT |
Colony-forming cell assays
Colony-forming cells (CFCs) were assayed in semisolid media as previously described.1-4 Briefly, 5 × 102 cells were plated per dish in duplicate cultures containing 1mL IMDM with 1.1% methylcellulose supplemented with 30% FBS, 5 × 10−5 M 2-ME (StemCell Technologies, Vancouver, BC, Canada), 100 ng/mL SCF, 100 ng/mL FL, 50 ng/mL IL-3, 50 ng/mL IL-6, 50 ng/mL granulocyte-macrophage colony-stimulating factor, and 5 U/mL erythropoietin. All cytokines were purchased from Cell Genix. The colonies were enumerated after 14 days using standard criteria. Fold expansion was calculated by dividing the number of total CFU-mix at Day 9 by the total number of CFU-mix at Day 0.
Long-term cobblestone area-forming cell (CAFC) assays
To quantitate the number of cobblestone area-forming cells (CAFCs), primary CD34+ MPB cells and ex vivo cultured MPB cells were plated in limiting dilution onto an irradiated monolayer of the murine stromal fibroblast line, M2-10B4, as described previously.2,4 Fold expansion of CAFC was calculated by dividing the total number of CAFC at Day 9 by the total number of CAFC at Day 0.
Xeno-transplantation assays
In our current studies NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, commonly called NSG mice were purchased from the Jackson Laboratories (Bar Harbor, ME) following institutional guidelines and the review board of the animal care committee. The NSG or SCID repopulation cell (SRC) assays were performed as previously described.1-4 Unmanipulated CD34+ MPB cells and CD34+ MPB cells cultured for nine days as described above, were injected through the tail vein intravenously 12 – 16 hours after a sublethal dose of total body irradiation (300 cGy). Unmanipulated primary CD34+ cells containing 50,000 CD34+CD90+ cells (Day 0) or the expanded product of CD34+ cells containing 50,000 CD34+CD90+ cells used to initiate the culture in the presence or absence of 5azaD/TSA (day 9) were injected through the tail vein in each mouse. As previously described, peripheral blood cells were analyzed by flow cytometer to detect human hematopoietic cell engraftment eight weeks after transplantation.2,4
Statistical Analysis
Results are expressed as mean ± standard error when appropriate. Statistical differences were evaluated using the student t test with significance at p of 0.05 or less.
Results
Phenotype of MPB CD34+ cells following 5azaD and TSA treatment
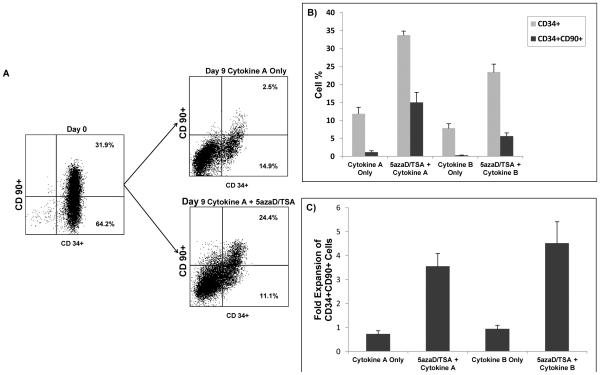
We investigated the immunophenotype of MPB CD34+ cells prior to and after treatment with cytokines and exposure to 5azaD followed by TSA in culture (Figure 1A-C). Specifically, the expansion of the subpopulation of cells coexpressing CD34+ and CD90+ was evaluated because this subpopulation serves as a reliable predictor for retention of marrow-repopulating capacity following ex vivo culture.2,32-34 After nine days of culture, cells in Cytokine A or B lacking 5azaD/TSA had progressive declines in the percentage (Figure 1B) and absolute numbers (Figure 1C) of CD34+CD90+ cells. In contrast, cells cultured with 5azaD/TSA had an expansion of CD34+CD90+ cells which was comparable between the Cytokine A and B environments and was higher in the 5azaD/TSA treated cells than in the cytokine-only conditions (Cytokine A: 3.6 ± 0.5 vs. 0.7 ± 0.1; p = 0.014, n = 5; Cytokine B: 4.5 ± 0.9 vs. 0.9 ± 0.1; p = 0.10, n = 2) (Figure 1 C).
Figure 1. Effect of chromatin modifying agents on ex vivo expansion of mobilized peripheral blood (MPB) hematopoietic stem cells.
A) Effects of 5azaD/TSA treatment on CD34 and CD90 expression following culture. Results are representative of 1 of 5 independent experiments in cytokine A condition.
B) Effect of 5azaD/TSA treatment on proportions of CD34+ and CD34+ CD90+ cells at Day 9 of culture in cytokine-only versus sequential 5azaD/TSA + cytokine environment. Cytokine A and B represent previously determined optimal and worst cytokine conditions for expansion of CD34+ CD90+ cord blood cells, respectively. A higher proportion of CD34+ CD90+ cells was observed in MPB cells cultured with 5azaD/TSA with either Cytokine A or B conditions compared to cells cultured in Cytokine A or B alone (Cytokine A: 15.0 ± 2.8% vs. 1.2 ± 0.4%, p =0.048, n = 5; Cytokine B: 5.7 ± 0.9% vs. 0.3 ± 0.03%, n = 2). Data represent mean ± standard errors of mean.
C) Effect of 5azaD/TSA on fold expansion of CD34+ CD90+ cells following nine days of culture. The fold expansion of CD34+ CD90+ cell numbers was determined by dividing the total numbers of viable cells expressing the phenotype at Day 9 by the input number of viable cells expressing the same phenotype at Day 0. A higher degree of expansion of CD34+ CD90+ cells was observed in MPB cells cultured with 5azaD/TSA with either Cytokine A or B conditions compared to cells cultured in Cytokine A or B alone (Cytokine A: 3.6 ± 0.5 vs. 0.7 ± 0.1; p = 0.014, n = 5; Cytokine B: 4.5 ± 0.9 vs. 0.9 ± 0.1; n = 2). Data represent mean ± standard errors of mean.
Global methylation and gene transcript levels in MPB cells
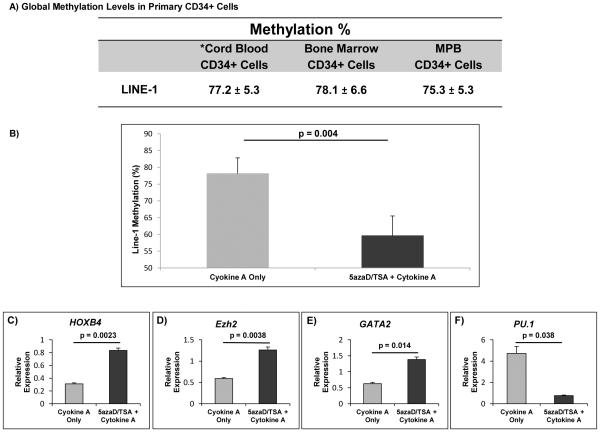
Global methylation patterns were similar between primary CD34+ cells from CB, BM, and MPB cells (Figure 2A). Methylation levels decreased significantly in the 5azaD/TSA-treated MPB CD34+ cells (53.7 ± 4.5 %) while the methylation levels remained unchanged in MPB cells cultured with cytokine A alone (74 ± 7.9 %) compared to the primary uncultured CD34+ MPB cells (75.3 ± 2.6 %) (Figure 2B).
Figure 2. Global methylation and gene transcript levels in primary uncultured MPB CD34+ cells and after treatment with 5azaD/TSA.
Long interspersed nucleotide element-1 (LINE-1 assays) assays were performed using genomic DNA to compare global methylation of four CpG sites between purified CD34+ cells derived from unmanipulated primary cord blood, bone marrow and mobilized peripheral blood (MPB) samples and at 72 hours after treatment of MPB cells with or without 5azaD/TSA.
A) Global methylation levels were similar between unmanipulated, primary CD34+ cells from cord blood, bone marrow, and MPB samples. *The data on cord blood in part was previously published (Ref #37, reprinted with permission) and used here for comparison purposes. B) Global methylation levels decreased significantly after sequential treatment with 5azaD/TSA compared to cells cultured in cytokine-only conditions (53.7 ± 4.5% vs. 74.0 ± 7.9%, respectively; P =0.004). C-F) Relative mRNA levels in MPB cells cultured in 5azaD/TSA and Cytokine A show higher transcript levels of HOXB4, EZH2, and GATA2 in 5azaD/TSA treated cells and lower transcript levels of PU.1 compared to Cytokine A-only expanded cells. Gene transcript levels were measured using the whole culture product derived from CD34+ cells after 9 days of culture. Measurements were obtained in triplicate and a negative control (lacking the cDNA template) was included for each assay (n = 3).
We assessed expression of genes generally implicated in HSC self-renewal (HOXB4, GATA2, EZH2) and differentiation (PU.1) in expanded MPB cells initiated with CD34+ cells. We observed significantly higher transcript levels of HOXB4, GATA2, and EZH2 and lower transcript levels of PU.1 in MPB cells expanded with 5azaD/TSA compared to cells cultured with cytokine A alone (Figure 2 C-F). Higher transcript levels of EZH2 and lower transcript levels of PU.1 were observed in MPB cells exposed to 5azaD/TSA compared to unmanipulated CD34+ MPB cells (data not shown).
In vitro and in vivo functional potential of MPB cells following 5azaD and TSA treatment
To determine whether the 5azaD/TSA treated cells retained their functional capacity, we compared CFC and CAFC potential. The plating efficiencies and expansion of total CFCs between optimal cytokine-only (Cytokine A) and 5azaD/TSA plus cytokine A expanded MPB cells were similar (Table 2). The CFU-mix represents a multipotent colony forming unit. Compared to primary uncultured cells, 5azaD/TSA treated MPB cells in Cytokine A or B conditions had an expansion of CFU-mix primitive colonies which was not observed in MPB cells cultured in the cytokine-only conditions.
Table 2.
Colony forming unit potential of 5azaD/TSA expanded mobilized peripheral blood cells
| Mean number of CFU / 500 cells plated | ||||||
|---|---|---|---|---|---|---|
| Culture Condition |
CFU-GM | BFU-E | CFU-mix | Total CFU | Plating Efficiency (%) |
Fold Expansion of CFU-mix |
|
Day 0
Primary MPB |
64.0 ± 11.8 | 75.2 ± 0.2 | 2.7 ± 0.8 | 141.8 ± 10.9 | 28.4 ± 2.2 | n/a |
|
Day 9
Cytokine A |
38.2 ± 8.7 | 3.5 ± 0.8 | 0 | 41.7 ± 9.4 | 8.3 ± 1.9 | 0 |
|
Day 9
5azaD/TSA + Cytokine A |
49.2 ± 6.3 | 8.2 ± 1.1 | 3 ± 0 | 60.3 ± 6.3 | 10.4 ± 1.9 | 10.1 ± 0.5 |
|
Day 9
Cytokine B |
14.0 ± 0.7 | 1.3 ± 0.2 | 0 | 15.3 ± 0.5 | 3.1 ± 0.1 | 0 |
|
Day 9
5azaD/TSA + Cytokine B |
33.5 ± 9.2 | 6.5 ± 0.4 | 2.25 ± 0.2 | 42.3 ± 9.0 | 8.5 ± 1.8 | 13.0 ± 0.2 |
MPB = mobilized peripheral blood; CFU = colony-forming unit; BFU = burst-forming unit; 5azaD = 5-aza-2’-deoxyctidine (5azaD); TSA = trichostatin A Cytokine A and B represent cytokine conditions with the highest and lowest expansion of CD34+CD90+ cord blood cells, respectively1
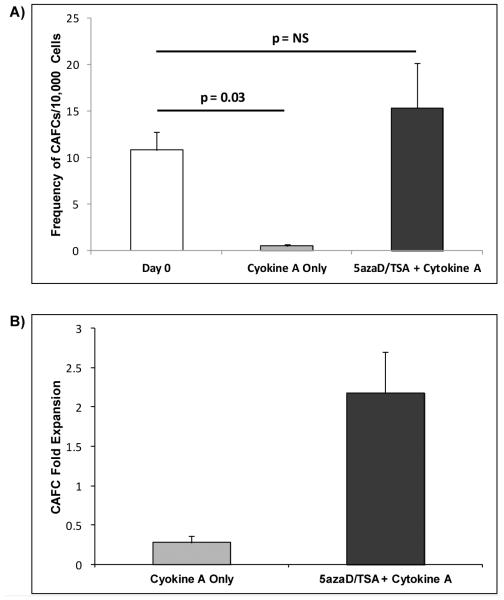
After five weeks of culture, the primary uncultured CD34+ cells had a frequency of 10.8 ± 1.9 CAFCs per 10,000 CD34+ cells plated as determined by limiting dilution assays. The cells exposed to cytokines alone in the absence of 5azaD/TSA treatment demonstrated a 20-fold reduction in CAFC frequency (Cytokine A: 0.5 ± 0.2 CAFCs per 10,000 cells, n = 3; Cytokine B: 0.6 ± 0.4 CAFCs per 10000 cells, n = 2). In contrast, cells exposed to sequential 5azaD/TSA plus cytokines maintained CAFC frequency comparable to the primary uncultured CD34+ cells (5azaD/TSA + Cytokine A: 15.4 ± 4.8 CAFCs per 10,000 cells, n = 3; 5azaD/TSA + Cytokine B: 8.8 ± 1.6 CAFCs per 10000 cells, n = 2) (Figure 3A). The increase in CAFCs in the 5azaD/TSA expanded cells resulted in an expansion of CAFC (5azaD/TSA + Cytokine A: 2.2-fold ± 0.5, n = 3; 5azaD/TSA + Cytokine B: 1.5-fold ± 0.4, n = 2) while a reduction in CAFC producing cells was observed in cells cultured in the cytokine-only environment (Cytokine A: 0.3 ± 0.1, n = 3; Cytokine B: 0.4 ± 0.3, n = 2) (Figure 3B). The increase in the absolute number of CAFC from their initial number was 7.9-fold higher in 5azaD/TSA expanded MPB cells than the MPB cells cultured in the absence of 5azaD/TSA (Figure 3B)
Figure 3. In vitro functional potential of ex vivo expanded MPB cells.
A) Effects of 5azaD/TSA on frequency of CAFC. CAFC frequency was computed using minimization by regression to the cell number at which 37% of wells showed negative CAFC growth with 95% statistical precision. CAFC frequency was similar between 5azaD/TSA + Cytokine A treated MPB cells (15.4 ± 4.8) and unmanipulated CD34+ cells (10.8 ± 1.9). Based on CAFC frequency determined by limiting dilution analyses the mean number of CAFC assayable from 10,000 unmanipulated primary MPB CD34+ cells or 10,000 cells from the whole expansion culture after 9 days started with CD34+ cells was determined. Significantly lower CAFC frequency was observed in MPB cells cultured in Cytokine A alone (0.5 ± 0.2, p=0.036) compared to the unmanipulated CD34+ cells. B) Effects of 5azaD/TSA on CAFC fold expansion. The fold expansion was determined by dividing the number of total CAFC assayed from the culture product at Day 9 by the total number of CAFC assayed from primary CD34+ cells at Day 0. An expansion of CAFC was observed in 5azaD/TSA treated MPB cells (2.2 ± 0.5) while a reduction in CAFC was observed in MPB cells cultured in Cytokine A alone (0.3 ± 0.1) (p =0.1).
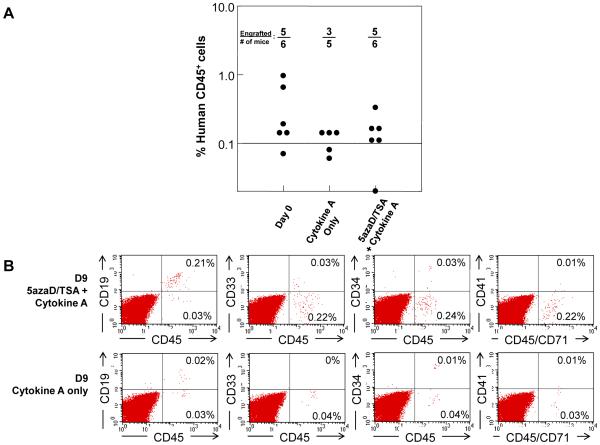
In order to examine whether the expanded MPB cells retained in vivo hematopoietic reconstitution capacity, we compared the NSG repopulating potential of equal numbers of unmanipulated MPB CD34+CD90+ cells or its expanded product after ex vivo culture. We observed that eight weeks after transplantation, hematopoietic engraftment in 5azaD/TSA-expanded CD34+ MPB cells (frequency of engraftment: 5/6) was similar to primary CD34+ MPB cells (frequency of engraftment: 5/6) (Figure 4A). In comparison, engraftment after infusing equal numbers of CD34+CD90+ cells cultured in Cytokine A in the absence of 5azaD/TSA was achieved in only 3 of 5 mice. As shown in Figure 4B 5azaD/TSA expanded cells were capable of imultilineage hematopoietic engraftment including both myeloid and lymphoid cells.
Figure 4. In vivo hematopoietic reconstitution potential of ex vivo expanded MPB cells.
A) In vivo hematopoietic reconstitution capacity of 5azaD/TSA expanded MPB cells in NSG mice. NSG mice were transplanted with CD34+ cells containing 5 × 104 CD34+CD90+ cells from uncultured CD34+ MPB cells or the product of an equal initial number of CD34+CD90+ cells after 9 days of culture in the presence of 5azaD/TSA and cytokine A or Cytokine A alone. Similar rates of engraftment were observed between equal doses of CD34+CD90+ from primary and 5azaD/TSA expanded MPB cells. B) Representative flow cytometry analysis of mice transplanted with MPB cells expanded in the presence of 5azaD/TSA or control cultures. Bone marrow cells were stained with fluorochrome conjugated antibodies against human CD45, CD19, CD33, CD34 and CD41 to determine multilineage human hematopoietic engraftment.
Discussion
In this study, CMAs were used to examine whether MPB cells can be expanded ex vivo without compromising its in vivo hematopoietic reconstitution potential. Our current data indicates that sequential addition of 5azaD/TSA in culture results in a 3.6-fold expansion of primitive CD34+CD90+ cells, a 10.1-fold expansion of CFU-mix, and 2.2-fold expansion of CAFC. Notably, the 5azaD/TSA expanded MPB cells retained in vivo hematopoietic reconstitution potential in NOD/SCID mice. However SRC frequency will need to be determined by a transplanting 5azaD/TSA expanded cells using a limiting dilution approach to confirm our observations here. Notably the CD34 negative fraction does not contribute to generation of CFC, CAFC or SRC. CD34+CD90+ MPB cells are 3.6-fold expanded in the presence of 5azaD/TSA in culture and retain functional potency comparable to unmanipulated MPB cells unlike control cultures. We have previously demonstrated that 5azaD/TSA expanded CD34+CD90+ cells exclusively retain in vivo repopulating potential (SRC) following transplantation but not more committed CD34+CD90− cells.2 Ex vivo expansion of HSC from MPB cells could facilitate treatment for patients with diseases such as multiple myeloma and lymphoma who are unable to mobilize an optimal graft size. Of note, bone marrow harvest is not an option for a subset of patients who fail to mobilize an optimal MPB graft following chemotherapy or radiation therapy. 5azaD is a cytosine analogue that facilitates passive demethylation by irreversibly inhibiting DNA methyl transferase.35 Trichostatin A is a histone deacetylase inhibitor that maintains acetylation of histones providing a chromatin structure favorable for gene expression.36 Previous studies in CD34+ CB and BM cells have shown these agents to work in synergy to promote proliferation and self renewal of HSC.1-4,37 Interestingly, using identical culture conditions, we observed a 3.6-fold expansion of MPB CD34+CD90+ cells which is intermediate to the 2.4-fold and 10.9-fold expansion of CD34+CD90+ cells observed in BM and CB cells, respectively.2-4 While the exact cause of the difference in expandability of CD34+CD90+ cells obtained from different biological sources is not clear, it does not appear to be related to differences in global methylation levels. The basal methylation levels of MPB cells were comparable to BM and CB cells as evidenced by LINE-1 assays in our current studies. Furthermore, 5azaD/TSA appears to be capable of resulting in a significant reduction in methylation levels of CD34+ MPB cells after culture which is comparable to what has been observed in CB cells.37 The decreased expandability of MPB CD34+ cells compared to CB cells may be related to cell intrinsic properties including ontogeny. Interestingly, in comparison to CB CD34+ cells, MPB CD34+ cells appear to be less amenable to differences in cytokine environments.1 Global methylation patterns in primary CD34+ cells were similar between the different cell sources. Interestingly a recent study has shown that adult MPB derived CD34+ cells display hypomethylation of some differentiation associated genes which are methylated in CB CD34+ cells (ontogenetically younger) reminiscent of methylation marks observed in more mature granulocytes and monocytes derived from CB. The changes observed in adult MPB CD34+ cells may be linked with ageing associated restriction of lineage potency.38 Furthermore, although several clinical trials using expanded CB cells are being studied to our knowledge currently there are no ongoing clinical trials using ex vivo expanded MPB cells. The expanded CB cells in clinical trials involving notch ligand or BM stroma based co-culture system appear to expand only short term progenitors, requiring transplantation of an unmanipulated second CB unit for successful long term blood cell engraftment .39, 40 A comparison of various ex vivo HSC expansion strategies and quantification was summarized in a recent review article.41
The expression patterns of genes implicated in HSC fate and function were compared between CD34+ MPB cells exposed to 5azaD/TSA + Cytokine A versus Cytokine A alone. The HOXB4 gene is a member of the homeobox family of genes that has increased expression in primitive HSC and HOXB4-overexpressing cells have been shown to have enhanced HSC regeneration.42, 43 We detected significantly higher transcript levels of the HOXB4 gene in 5azaD/TSA treated MPB cells compared to cells exposed to cytokines alone. The EZH2 gene encodes a Polycomb group protein involved in histone methylation and deacetylation and overexpression of EZH2 has been shown to conserve long-term repopulating potential of HSCs.44 Expression of EZH2 was also higher in 5azaD/TSA expanded MPB cells than MPB cells expanded in cytokine A alone. The GATA2 gene encodes a transcription factor expressed in HSC thought to be important for maintenance of HSC.45 Higher transcript levels of GATA2 were also observed in MPB cells exposed to 5azaD/TSA than cytokine-only MPB cells. The transcription factor PU.1 is expressed at intermediate levels in HSC and increased levels are associated with developmental fate of HSC to myeloid lineage cells.46 We observed lower transcript levels of PU.1 after culture with 5azaD/TSA compared to MPB CD34+ cells cultured in cytokine alone. The increased expression of HOXB4, EZH2, and GATA2 and decreased expression of PU.1 in CD34+ MPB cells treated with 5azaD/TSA is consistent with the roles of these genes in HSC self-renewal and differentiation.
Approximately ten thousand autologous transplantations were conducted in 2010 as standard of care for treatment of patients with diseases including multiple myeloma and lymphoma in the US.5 Theories for reduced marrow HSC reserve are based on the observations that patients treated with more myelotoxic agents have impaired mobilization and in-vivo murine models showing cytotoxic chemotherapies reducing progenitor HSC marrow cellularity and self-renewal capacity.47,48 The ex vivo expansion of MPB cells with 5azaD/TSA can potentially be applied to expand the graft size in poor mobilizers. It would be critical to examine whether using 5azaD/TSA in culture, particularly for MPB CD34+ cells exposed to chemotherapy/radiation therapy from multiple myeloma or lymphoma, can expand transplantable HSC and thus potentially facilitate optimizing a suboptimal graft for transplantation. This could potentially enable a significant proportion of patients who are unable to mobilize an adequate graft size to receive autologous transplantation. Certainly, this will need to be evaluated in future studies using CD34+ cells harvested from patients after chemo/radio therapy.
In summary, the sequential addition of 5azaD/TSA in MPB CD34+ cells leads to expansion of the primitive population of CD34+CD90+ cells which maintained in vitro clonogenic potential, including both CFU-mix colonies and primitive CAFC, as well as in vivo hematopoietic reconstitution capacity. The potential use of this expansion strategy to augment transplantable HSC ex vivo from poor mobilizers after growth factor stimulation may provide a means to allow a significant number of patients to undergo MPB transplantation as therapeutic options as well as expanding HSCs for cell based therapies such as ex vivo generation of blood products.
Acknowledgements
This work was supported in part by grants from the Leukemia and Lymphoma Society (White Plains, NY, USA) Translational Research Program and Institutional (Office of the Vice Chancellor for Research) ‘Areas of Excellence’ Award to NM and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000048 to SLS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors are indebted to Dr. Victor Gordeuk for his critical reading of the manuscript and helpful suggestions. We are indebted to Drs. Pablo Rubinstein and Ludy Dobrila of the New York Blood Center, New York, NY, and Dr. John Wingard and Ms. Emma H Rosenau from LifeCord Cord Blood Bank & University of Florida Health Cancer Center, Gainesville, Florida for providing CB units for research.
Footnotes
Disclosures of Interest:
The authors have no commercial, proprietary or financial interest in the products or companies described in this article.
This work was presented in part in abstract form during the 2011 American Society of Hematology Meeting
References
- 1.Araki H, Baluchamy S, Yoshinaga K, et al. Cord blood stem cell expansion is permissive to epigenetic regulation and environmental cues. Exp Hematol. 2009;37:1084–95. doi: 10.1016/j.exphem.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki H, Mahmud N, Milhem M, et al. Expansion of human umbilical cord blood SCID-repopulating cells using chromatin-modifying agents. Exp Hematol. 2006;34:1409. doi: 10.1016/j.exphem.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Araki H, Yoshinaga K, Boccuni P, et al. Chromatin-modifying agents permit human hematopoietic stem cells to undergo multiple cell divisions while retaining their repopulating potential. Blood. 2007;109:3570–8. doi: 10.1182/blood-2006-07-035287. [DOI] [PubMed] [Google Scholar]
- 4.Milhem M, Mahmud N, Lavelle D, et al. Modification of hematopoietic stem cell fate by 5aza 2'deoxycytidine and trichostatin A. Blood. 2004;103:4102–10. doi: 10.1182/blood-2003-07-2431. [DOI] [PubMed] [Google Scholar]
- 5.Center for International Blood & Marrow Transplant Research Current uses and outcomes of hematopoietic stem cell transplantation 2012. 2012 Retrieved on March 3, 2013 from http://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx. [Google Scholar]
- 6.Wang TY, Chang SJ, Chang MD, et al. Unique biological properties and application potentials of CD34+ CD38− stem cells from various sources. Taiwan J Obstet Gynecol. 2009;48:356–69. doi: 10.1016/S1028-4559(09)60324-7. [DOI] [PubMed] [Google Scholar]
- 7.Hao QL, Shah AJ, Thiemann FT, et al. A functional comparison of CD34 + CD38− cells in cord blood and bone marrow. Blood. 1995;86:3745–53. [PubMed] [Google Scholar]
- 8.Wang JC, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919–24. [PubMed] [Google Scholar]
- 9.Gordon MY, Blackett NM. Some factors determining the minimum number of cells required for successful clinical engraftment. Bone Marrow Transplant. 1995;15:659–62. [PubMed] [Google Scholar]
- 10.Glaspy JA, Shpall EJ, LeMaistre CF, et al. Peripheral blood progenitor cell mobilization using stem cell factor in combination with filgrastim in breast cancer patients. Blood. 1997;90:2939–51. [PubMed] [Google Scholar]
- 11.Shpall EJ, Champlin R, Glaspy JA. Effect of CD34+ peripheral blood progenitor cell dose on hematopoietic recovery. Biol Blood Marrow Transplant. 1998;4:84–92. doi: 10.1053/bbmt.1998.v4.pm9763111. [DOI] [PubMed] [Google Scholar]
- 12.Wuchter P, Ran D, Bruckner T, et al. Poor mobilization of hematopoietic stem cells-definitions, incidence, risk factors, and impact on outcome of autologous transplantation. Biol Blood Marrow Transplant. 2010;16:490–9. doi: 10.1016/j.bbmt.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Hosing C, Saliba RM, Ahlawat S, et al. Poor hematopoietic stem cell mobilizers: a single institution study of incidence and risk factors in patients with recurrent or relapsed lymphoma. Am J Hematol. 2009;84:335–7. doi: 10.1002/ajh.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jantunen E, Itala M, Siitonen T, et al. Blood stem cell mobilization and collection in patients with chronic lymphocytic leukaemia: a nationwide analysis. Bone Marrow Transplant. 2008;41:239–44. doi: 10.1038/sj.bmt.1705897. [DOI] [PubMed] [Google Scholar]
- 15.Pavone V, Gaudio F, Console G, et al. Poor mobilization is an independent prognostic factor in patients with malignant lymphomas treated by peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;37:719–24. doi: 10.1038/sj.bmt.1705298. [DOI] [PubMed] [Google Scholar]
- 16.DiPersio JF, Micallef IN, Stiff PJ, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27:4767–73. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 17.Laird PW, Jackson-Grusby L, Fazeli A, et al. Suppression of intestinal neoplasia by DNA hypomethylation. Cell. 1995;81:197–205. doi: 10.1016/0092-8674(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 18.Lantry LE, Zhang Z, Crist KA, et al. 5-Aza-2'-deoxycytidine is chemopreventive in a 4-(methyl-nitrosamino)-1-(3-pyridyl)-1-butanone-induced primary mouse lung tumor model. Carcinogenesis. 1999;20:343–6. doi: 10.1093/carcin/20.2.343. [DOI] [PubMed] [Google Scholar]
- 19.Carr BI, Rahbar S, Asmeron Y, et al. Carcinogenicity and haemoglobin synthesis induction by cytidine analogues. Br.J.Cancer. 1988;57:395–402. doi: 10.1038/bjc.1988.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGregor F, Muntoni A, Fleming J, et al. Molecular changes associated with oral dysplasia progression and acquisition of immortality: potential for its reversal by 5-azacytidine. Cancer Res. 2002;62:4757–66. [PubMed] [Google Scholar]
- 21.Belinsky SA, Klinge DM, Stidley CA, et al. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 2003;63:7089–93. [PubMed] [Google Scholar]
- 22.Covey JM, D'Incalci M, Tilchen EJ, et al. Differences in DNA damage produced by incorporation of 5-aza-2'-deoxycytidine or 5,6-dihydro-5-azacytidine into DNA of mammalian cells. Cancer Res. 1986;46:5511–7. [PubMed] [Google Scholar]
- 23.Schermelleh L, Haemmer A, Spada F, et al. Dynamics of Dnmt1 interaction with the replication machinery and its role in postreplicative maintenance of DNA methylation. Nucleic Acids Res. 2007;35:4301–12. doi: 10.1093/nar/gkm432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Momparler RL, Goodman J. In vitro cytotoxic and biochemical effects of 5-aza-2'-deoxycytidine. Cancer Res. 1977;37:1636–9. [PubMed] [Google Scholar]
- 25.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 26.Halaban R, Krauthammer M, Pelizzola M, et al. Integrative analysis of epigenetic modulation in melanoma cell response to decitabine: clinical implications. PLoS One. 2009;4:e4563. doi: 10.1371/journal.pone.0004563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saunthararajah Y, Lavelle D, DeSimone J. DNA hypo-methylating agents and sickle cell disease. Br J Haematol. 2004;26:629–36. doi: 10.1111/j.1365-2141.2004.05064.x. [DOI] [PubMed] [Google Scholar]
- 28.Lubbert M, Wijermans P, Kunzmann R, et al. Cytogenetic responses in high-risk myelodysplastic syndrome following low-dose treatment with the DNA methylation inhibitor 5-aza-2'-deoxycytidine. Br J Haematol. 2001;114:349–57. doi: 10.1046/j.1365-2141.2001.02933.x. [DOI] [PubMed] [Google Scholar]
- 29.Taioli S, Petro B, Gaitonde S, et al. Evaluation of Genotoxicity of Chromatin Modifying Agents Expanded Hematopoietic Graft in a Non-Human Primate Model. Blood. 2012;120:2994. (abstract) [Google Scholar]
- 30.Yang AS, Estecio MR, Doshi K, et al. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32(3):e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirabello L, Savage SA, Korde L, et al. LINE-1 methylation is inherited in familial testicular cancer kindreds. BMC Med Genet. 2010;11:77. doi: 10.1186/1471-2350-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayani H, Lansdorp PM. Thy-1 expression is linked to functional properties of primitive hematopoietic progenitor cells from human umbilical cord blood. Blood. 1994;83:2410–7. [PubMed] [Google Scholar]
- 33.Danet GH, Lee HW, Luongo JL, et al. Dissociation between stem cell phenotype and NOD/SCID repopulating activity in human peripheral blood CD34(+) cells after ex vivo expansion. Exp Hematol. 2001;29:1465–73. doi: 10.1016/s0301-472x(01)00750-0. [DOI] [PubMed] [Google Scholar]
- 34.Dorrell C, Gan OI, Pereira DS, et al. Expansion of human cord blood CD34(+)CD38(-) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood. 2000;95:102–10. [PubMed] [Google Scholar]
- 35.Pietrobono R, Pomponi MG, Tabolacci E, et al. Quantitative analysis of DNA demethylation and transcriptional reactivation of the FMR1 gene in fragile X cells treated with 5-azadeoxycytidine. Nucleic Acids Res. 2002;30:3278–85. doi: 10.1093/nar/gkf434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung M. Inhibitors of histone deacetylase as new anticancer agents. Curr Med Chem. 2001;8:1505–11. doi: 10.2174/0929867013372058. [DOI] [PubMed] [Google Scholar]
- 37.Mahmud N, Petro B, Baluchamy S, et al. Differential effects of epigenetic modifiers on the expansion and maintenance of human cord blood stem/progenitor cells. Biol Blood Marrow Transplant. 2014;20:480–9. doi: 10.1016/j.bbmt.2013.12.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bocker MT, Hellwig I, Breiling A, et al. Genome-wide promoter DNA methylation dynamics of human hematopoietic progenitor cells during differentiation and aging. Blood. 2011;117:e182–9. doi: 10.1182/blood-2011-01-331926. [DOI] [PubMed] [Google Scholar]
- 39.Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–6. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367:2305–15. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walasek MA, van Os R, de Haan G. Hematopoietic stem cell expansion: challenges and opportunities. Ann N Y Acad Sci. 2012;1266:138–50. doi: 10.1111/j.1749-6632.2012.06549.x. [DOI] [PubMed] [Google Scholar]
- 42.Sauvageau G, Lansdorp PM, Eaves CJ, et al. Differential expression of homeobox genes in functionally distinct CD34+ subpopulations of human bone marrow cells. Proc Natl Acad Sci U S A. 1994;91:12223–7. doi: 10.1073/pnas.91.25.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonchuk J, Sauvageau G, Humphries RK. HOXB4 overexpression mediates very rapid stem cell regeneration and competitive hematopoietic repopulation. Exp Hematol. 2001;29:1125–34. doi: 10.1016/s0301-472x(01)00681-6. [DOI] [PubMed] [Google Scholar]
- 44.Kamminga LM, Bystrykh LV, de Boer A, et al. The Polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood. 2006;107:2170–9. doi: 10.1182/blood-2005-09-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai FY, Keller G, Kuo FC, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–6. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 46.DeKoter RP, Singh H. Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science. 2000;288:1439–41. doi: 10.1126/science.288.5470.1439. [DOI] [PubMed] [Google Scholar]
- 47.Ketterer N, Salles G, Moullet I, et al. Factors associated with successful mobilization of peripheral blood progenitor cells in 200 patients with lymphoid malignancies. Br J Haematol. 1998;103:235–42. doi: 10.1046/j.1365-2141.1998.00960.x. [DOI] [PubMed] [Google Scholar]
- 48.Neben S, Hellman S, Montgomery M, et al. Hematopoietic stem cell deficit of transplanted bone marrow previously exposed to cytotoxic agents. Exp Hematol. 1993;21:156–62. [PubMed] [Google Scholar]