Abstract
Cell fate decisions are integral to zonation and remodeling of the adrenal cortex. Animal models exhibiting ectopic differentiation of gonadal-like cells in the adrenal cortex can shed light on the molecular mechanisms regulating steroidogenic cell fate. In one such model, prepubertal gonadectomy (GDX) of mice triggers the formation of adrenocortical neoplasms that resemble luteinized ovarian stroma. Transcriptomic analysis and genome-wide DNA methylation mapping have identified genetic and epi-genetic markers of GDX-induced adrenocortical neoplasia. Members of the GATA transcription factor family have emerged as key regulators of cell fate in this model. Expression of Gata4 is pivotal for the accumulation of gonadal-like cells in the adrenal glands of gonadectomized mice, whereas expression of Gata6 limits the spontaneous and GDX-induced differentiation of gonadal-like cells in the adrenal cortex. Additionally, Gata6 is essential for proper development of the adrenal X-zone, a layer analogous to the fetal zone of the human adrenal cortex. The relevance of these observations to developmental signaling pathways in the adrenal cortex, to other animal models of altered adrenocortical cell fate, and to human diseases is discussed.
Keywords: Adrenal cortex, Endocrine tumor, Ferret, Gonadotropin, Hyperthecosis, Orchiectomy, Ovariectomy, Steroidogenesis
1. Adrenocortical development, zonation, and remodeling require the precise regulation of cell fate decisions
1.1. Adrenocortical development
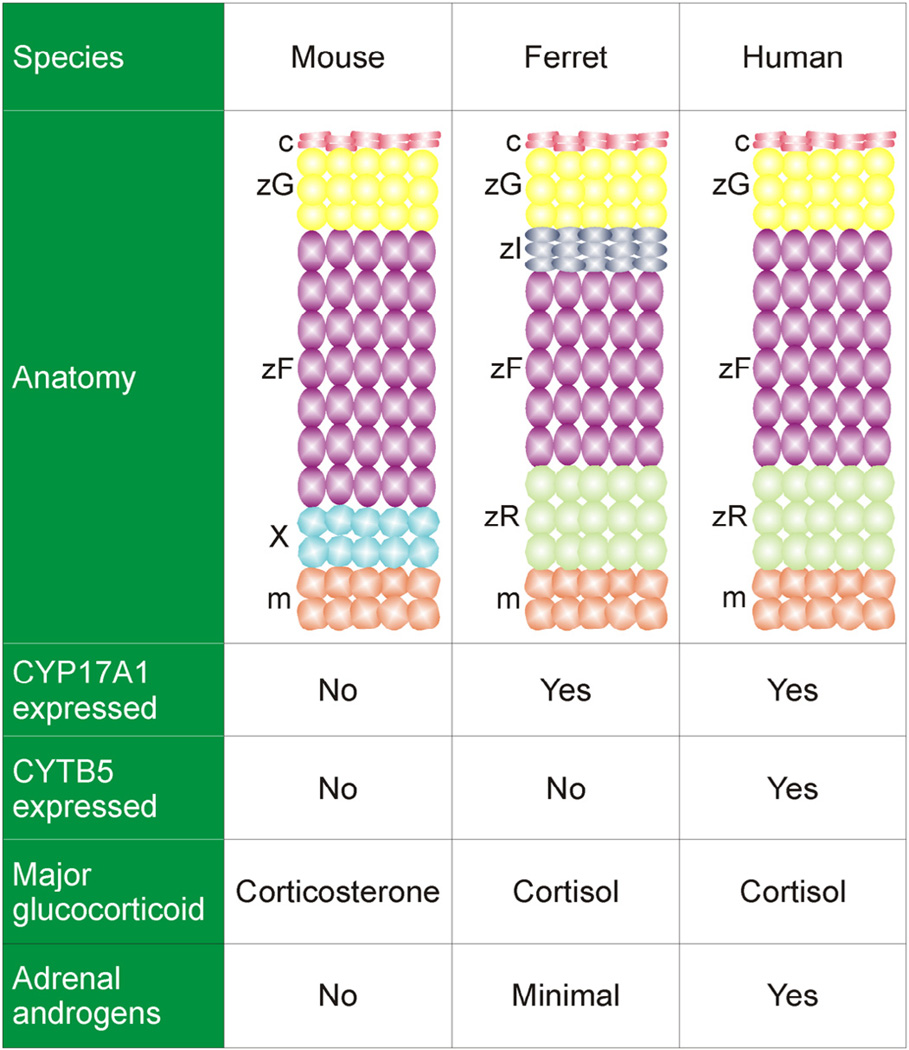
The adrenal cortex, a major site of steroid production, is composed of anatomically and functionally distinct zones (Fig. 1). In the human adrenal cortex the major zones are the zona glomerulosa (zG), zona fasciculata (zF), and zona reticularis (zR). Cells in these 3 zones synthesize mineralocorticoids, glucocorticoids, and androgens, respectively. The adrenal cortex of the mouse contains a zG and zF, but there is no discernable zR. The adrenal cortex of the young mouse contains an additional layer, the X-zone, a remnant of the fetal adrenal cortex (Morohashi and Zubair, 2011). The adrenal gland is covered by a thin capsule that serves as both a support structure and a reservoir of progenitor cells for the cortex (Simon and Hammer, 2012).
Fig. 1.
Comparative anatomy and physiology of the adrenal cortex. The mouse adrenal cortex contains a zG and zF, but there is no recognizable zR. The adrenal cortex of the young mouse contains a transient layer, the X-zone. The ferret adrenal cortex has an additional layer, the zona intermedia; the analogous layer in the rat houses stem/progenitor cells (Guasti et al., 2013b). CYP17A1 is a bifunctional enzyme with Q both the 17α-hydroxylase activity required for the synthesis of cortisol and the 17,20-lyase activity required for the synthesis of androgens. Cyp17a1 is expressed in the fetal but not postnatal adrenal cortex of the mouse, so under normal conditions the mouse adrenal secretes corticosterone as its major glucocorticoid and does not produce androgens. CYTB5 selectively enhances the 17,20-lyase activity of CYP17A1 through allosteric effects. Non-neoplastic adrenocortical cells in the ferret lack CYTB5, which may account for the low production of adrenal androgens in healthy ferrets. Abbreviations: c, capsule; m, medulla; X, X-zone; zF, zona fasciculata; zI, zona intermedia; zG, zona glomerulosa; zR, zona reticularis.
Steroidogenic cells in the adrenal glands and gonads arise from the adrenogonadal primordia (AGP), specialized cells in the urogenital ridge that coexpress the transcription factors Wilms tumor suppressor-1 (WT1) and GATA4 [reviewed in Bandiera et al. (2013)]. During em-bryogenesis, adrenal progenitor cells in the AGP upregulate steroidogenic factor-1 (Sf1, AdBP4,Nr5a1), migrate into subjacent mesenchyme, and downregulate expression of Wt1 and Gata4 (Bandiera et al., 2013). In contrast, gonadal progenitor cells in the AGP enter subjacent mesenchyme, migrate laterally, and maintain expression of Sf1, Wt1, and Gata4. The adrenal anlagen are invaded by sympathoblasts that give rise to chromaffin cells of the medulla. Subsequently, the nascent adrenal glands become enveloped by capsule cells that are derived from both surrounding mesenchyme and fetal adrenal cells that previously expressed Sf1 [reviewed in Wood et al. (2013)]. After birth the adrenal cortex partitions into discrete zones.
1.2. Adrenocortical remodeling
The adrenal cortex of the adult is a dynamic organ in which senescing cells are replaced by newly differentiated ones [reviewed in Yates et al. (2013)]. This constant turnover facilitates rapid organ remodeling in response to physiological demand for steroids. Zones can reversibly enlarge, shrink, or alter their biochemical profiles to accommodate needs. For example, in response to a low sodium or high potassium diet, the zG expands to enhance mineralocorticoid production; conversely, a high sodium diet leads to contraction of the zG [reviewed in (Yates et al. (2013)]. Similarly, adrenocorticotrophic hormone (ACTH) administration expands the zF and enhances glucocorticoid production, whereas dexamethasone administration causes contraction of this zone through apoptosis. Adrenarche in humans and certain other primates is associated with histological and functional changes in the zR, including increased expression of the gene encoding cytochrome-b5 (CYTB5), an allosteric regulator of 17,20-lyase activity of CYP17A1, and a concomitant increase in biosynthesis of the adrenal androgen dehydroepiandosterone (DHEA) (Naffin-Olivos and Auchus, 2006; Pattison et al., 2009). Adult male marmosets do not develop a functional zR, whereas female marmosets develop a functional zR in a reversible manner dependent on their social status (Pattison et al., 2009). The X-zone of the mouse normally regresses at puberty in males and during the first pregnancy in females, but a secondary X-zone can be induced in males by gonadectomy (GDX) (Hirokawa and Ishikawa, 1975).
1.3. Adrenocortical stem/progenitor cells
The adrenal cortex contains stem/progenitors cell populations that can differentiate to replace senescing cells and maintain or expand zones. In one model of adrenal zonation, the cell migration model, stem/progenitor cells in periphery of the adrenal cortex differentiate and migrate centripetally to repopulate the gland before undergoing apoptosis in the juxtamedullary region (Morley et al., 1996). Aspects of this model have been validated through lineage tracing analyses (Freedman et al., 2013; King et al., 2009; Laufer et al., 2012), but recent studies indicate that the regulation of zonation is far more complex than originally appreciated [reviewed in Pihlajoki et al. (2013b)]. It is now clear that distinct pools of stem/progenitor cells exist in the adrenal capsule, subjacent cortex, juxtamedullary region, and other sites (Table 1). Some of these pools appear to be activated only during specific developmental time frames or in response to extreme physiological demand. Adrenocortical zones can be replenished not only through centripetal but also centrifugal migration (de Joussineau et al., 2012; Sahut-Barnola et al., 2010). For example, proliferation of the stem/progenitors in the juxtamedullary region leads to centrifugal repopulation of the cortex, as is seen in secondary X-zone formation and other models (Table 1).
Table 1.
Adrenocortical stem/progenitor cell populations that contribute to steroidogenic and nonsteroidogenic cells in the mouse adrenal cortex. These progenitor populations, defined by lineage tracing analyses and related methods, are not mutually exclusive. For example, WT1+ progenitors have been shown to coexpress Gli1 and Tcf21.
| Stem/Progenitor Population | Location | Comments | References |
|---|---|---|---|
| WT1+ progenitors | Capsule | Under basal conditions, these rare AGP-like cells in the capsule can give rise to normal steroidogenic cells in the adrenal cortex. GDX triggers their differentiation into gonadal- like steroidogenic tissue. |
(Bandiera et al., 2013) |
| GLI1+ progenitors | Capsule | These cells are descendants of Sf1-expressing fetal adrenocortical cells. In response to Shh, Gli1+ progenitors migrate into the cortex, lose responsiveness to Shh, and become steroidogenic, as evidenced by expression of Sf1 and differentiation markers characteristic of the zG (Cyp11b2) or zF (Cyp11b1). |
(Huang et al., 2010; King et al., 2009; Wood et al., 2013) |
| TCF21+ progenitors | Capsule | TCF21 inhibits Sf1 expression. TCF21+ capsular cells are not descendants of the Sf1 expressing fetal adrenocortical cells; rather, they appear to arise from cells of the AGP. TCF21+ capsular cells give rise only to non-steroidogenic stromal adrenocortical cells that express collagen-1a1, desmin, and platelet derived growth factor-α but not Sf1. |
(Wood et al., 2013) |
| SHH+ progenitors | Subcapsule | These progenitors give rise to steroidogenic cells in the zF and zG but not capsule cells. | (Huang et al., 2010; King et al., 2009; Wood et al., 2013) |
| X-zone progenitors | Juxtamedullary region |
These progenitors can be activated by GDX to form a secondary X-zone in male mice. | (Hirokawa and Ishikawa, 1975) |
| Fetal adrenal-like progenitors |
Juxtamedullary region |
Normally dormant in the adult, these progenitors become activated following mutagenesis of Prkar1a to form fetal-like adrenocortical cells that express Cyp17a1 secrete cortisol, and migrate centrifugally. These same cells may be induced to differentiate into ectopic zG in response to β-catenin activation. |
(Berthon et al., 2010; de Joussineau et al., 2014; Sahut-Barnola et al., 2010) |
1.4. Signaling pathways regulating adrenocortical cell differentiation
The differentiation of adrenocortical stem/progenitor cells is regulated by a diverse group of endocrine and paracrine factors, including ACTH, angiotensin-II, and hormones traditionally associated with reproductive function, such as luteinizing hormone (LH), activin, and inhibin [reviewed in Bielinska et al. (2006)]. Developmental signaling pathways, including the sonic hedgehog (SHH), fibroblast growth factor, and Wnt/β-catenin pathways, also control cellular differentiation in the adrenal cortex (Guasti et al., 2013a, 2013b; Laufer et al., 2012; Parviainen et al., 2013). SHH is secreted by cells in the subcapsular region that express Sf1 but not the terminal enzymes required for corticoid synthesis (Ching and Vilain, 2009; Huang et al., 2010; King et al., 2009). Capsular cells, which do not express Sf1, respond to SHH by synthesizing its downstream effector GLI1. Some of these GLI+ capsule cells migrate centripetally into the cortex, lose responsiveness to SHH, and become steroidogenic, as evidenced by upregulation of Sf1 and differentiation markers characteristic of the zG (Cyp11b2) or zF (Cyp11b1). Conditional mutagenesis of Shh in steroidogenic cells results in adrenocortical hypoplasia and capsular thinning (Ching and Vilain, 2009; Huang et al., 2010; King et al., 2009). The SHH pathway is more active in the adrenal gland of the fetus than that of the adult, but the pathway can be activated in the adult in response to dexamethasone-induced adrenocortical atrophy or other experimental manipulations. Like Shh, β-catenin is expressed in subcapsular cells (Kim et al., 2008), and Wnt/β-catenin signaling maintains the undifferentiated state of adrenocortical stem/progenitor cells in this region (Berthon et al., 2012; Simon and Hammer, 2012). Targeted mutagenesis of β-catenin in SFI+ cells causes late onset adrenal hypoplasia, which is thought to be the result of stem/progenitor cell pool depletion (Kim et al., 2008).
1.5. Lineage conversion of adrenocortical cells
Fate mapping studies have shown that the functional identity of a given cell in the adrenal cortex can change in response to external cues. To illustrate this point Freedman et al. (2013) used Cyp11b2-Cre to indelibly mark zG cells and all their descendants with green fluorescent protein (GFP). By tracing the fate of GFP+ cells, these investigators demonstrated that adrenocortical zonation results from trans-differentiation of zG cells into zF cells. When zG-to-zF lineage conversion was disrupted through conditional mutagenesis of Sf1 in CYP11B2+ cells, a fully functional zF still formed, implying the existence of alternative routes for differentiation of particular cell types.
1.6. Summary and perspectives
Cell fate decisions, impacting both the differentiation of distinct pools of stem/progenitor cells and the trans-differentiation of mature steroidogenic cells, are integral to adrenocortical zonation and remodeling. The mechanisms involved are complex and redundant so as to fulfill the offsetting goals of organ homeostasis and stress adaptation.
2. GDX-induced adrenocortical neoplasia: An experimentally tractable model of altered steroidogenic cell fate
2.1. Histological features of GDX-induced adrenocortical neoplasia in the mouse
To gain insight into the factors that influence steroidogenic cell fate, we have turned to a classic model of phenotype switching wherein prepubertal GDX triggers the appearance of gonadal-like tissue in the adrenal cortex of mice (Bielinska et al., 2006). This phenomenon, termed GDX-induced adrenocortical neoplasia, is thought to reflect the metaplastic differentiation of stem/progenitor cells in the adrenal capsule and subcapsule in response to the hormonal changes that accompany GDX (↑LH, ↓inhibin, etc.). The neoplasms are composed of two principal cell types: spindle- or ovoid-shaped type A cells that have limited steroidogenic capacity, and sex steroid-producing type B cells that accumulate later within patches of type A cells (Fig. 2A,B). The formation of ectopic gonadal-like tissue from stem/progenitor cells in the adrenal gland can be viewed as an extreme example of adrenocortical remodeling in response to GDX.
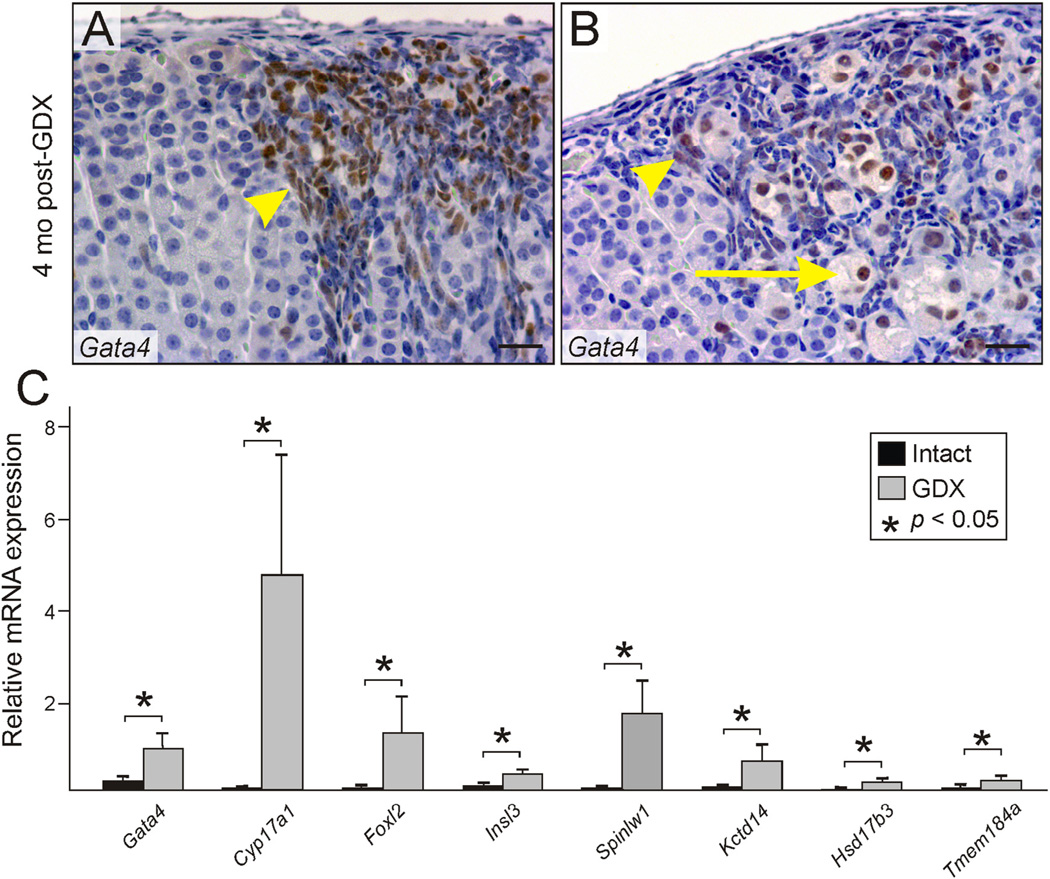
Fig. 2.
Changes in histology and gene expression associated with GDX-induced adrenocortical neoplasia. (A,B) Neoplastic cells in the adrenal cortex of a B6D2F1 female mouse that underwent prepubertal GDX 4 mo earlier. Immunoperoxidase staining for GATA4 highlights small type A cells (arrowheads) and large type B cells (arrows). Bars = 100 µm. (C) Expression of gonadal-like differentiation markers in the adrenal glands of gonadectomized vs. intact female DBA/2J mice. Whole adrenal glands from 4-mo-old gonadectomized or intact virgin female DBA/2J mice were subjected to qRT-PCR analysis. Results are normalized to β-actin mRNA levels (× 102). *P< 0.05.
2.2. Strain dependence of GDX-induced adrenocortical neoplasia
GDX-induced adrenocortical neoplasia in the mouse is strain dependent (Bielinska et al., 2006). Susceptible strains include CE/J, DBA/ 2J, and B6D2F1. Transplantation, parabiosis, and hypophysectomy experiments have established that the adrenal glands of susceptible strains of mice have an inherent predisposition to develop tumors in response to LH stimulation (Bielinska et al., 2005, 2006). Chimeric mouse studies suggest that strain susceptibility to GDX-induced neoplasia is cell-intrinsic and resides in the stem/progenitor compartment (Fig. 3). The genetic basis of strain susceptibility, however, remains unclear. Linkage analysis of crosses between susceptible (DBA/2J) and non-susceptible (C57Bl/6) mouse strains has proven that GDX-induced adrenocortical neoplasia is a complex trait influenced by multiple genetic loci, but the genes responsible for strain susceptibility have not been elucidated (Bernichtein et al., 2007). Of interest, DBA/2J and C57Bl/6 mice also differ in their sensitivity to XY male-to-female sex reversal in response to a variety of genetic perturbations, including both Y-linked and autosomal variants (Correa et al., 2012; Munger et al., 2013). C57Bl/6 mice are more susceptible to sex reversal, and transcriptomic analyses have shown that this susceptibility correlates with delayed activation of testis pathway genes and delayed repression of ovarian pathway genes. By analogy, complex regulatory networks affecting temporospatial expression of gonadal determination genes may contribute to differences in strain susceptibility to GDX-induced adrenocortical neoplasia.
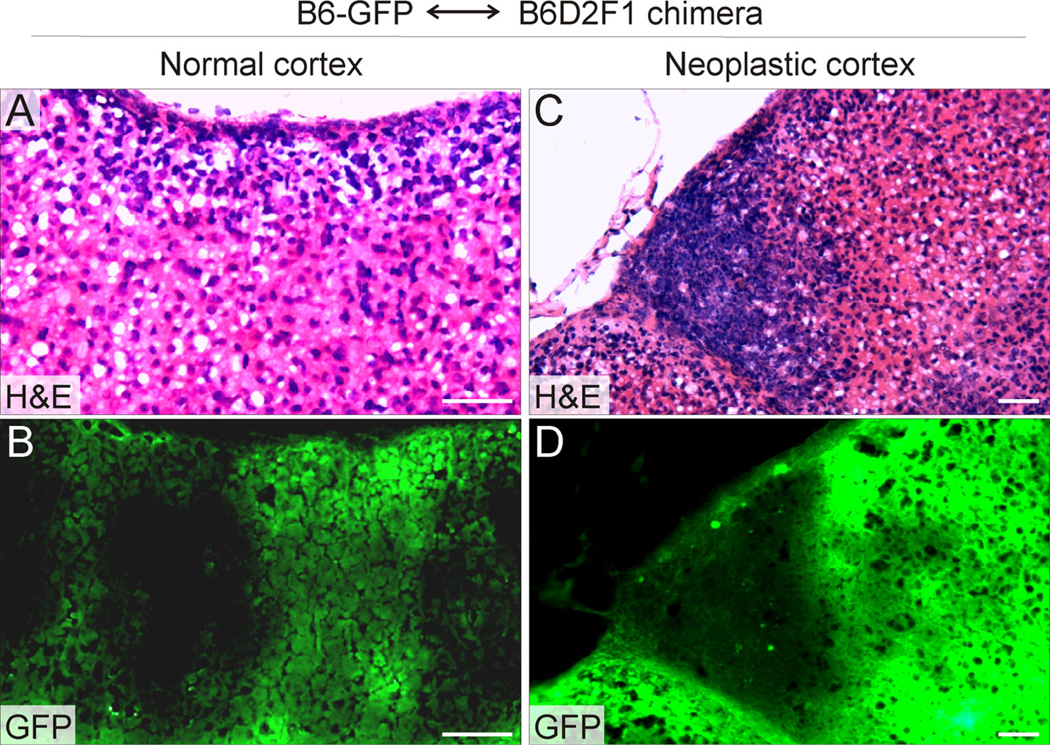
Fig. 3.
Strain susceptibility to GDX-induced adrenocortical neoplasia is cell intrinsic and resides in the stem/progenitor cell compartment. A chimeric mouse was generated by injecting Rosa26-GFP XY embryonic stem cells from a non-susceptible strain (B6) into an XY blastocyst from a susceptible strain (B6D2F1). The mouse was gonadectomized at 3 weeks of age, and adrenal glands were harvested 5 mo later. Adjacent cryosections of adrenal tissue were stained with H&E (A,C) or examined by fluorescence microscopy (B,D). Note that normal adrenocortical cells can arise from either GFP+ B6 progenitors or GFP− B6D2F1 progenitors (alternating stripes in panel B), whereas the neoplastic cells are derived exclusively from B6D2F1 progenitors. In this mouse, 4 separate tumors were identified; each was derived exclusively from B6D2F1 tissue. Bars = 50 µm.
2.3. Genetic markers of GDX-induced adrenocortical neoplasia
Expression profiling studies have shown that GDX induces the selective expression of gonadal-like markers in the adrenal glands of DBA/2J mice (Bielinska et al., 2006; Schillebeeckx et al., 2015). The list of upregulated, gonadal-like genes includes the LH receptor (Lhcgr), anti-Müllerian hormone (Amh) and its receptor (Amhr2), inhibin-α (Inha), insulin-like 3 (Insl3), the transcription factors Gata4, Wt1, and Foxl2, the serine protease inhibitor EPPIN (Spinlw1), transmembrane protein Tmem184a, potassium channel tetramerization domain containing protein Kctd14 (LOC233529), and enzymes required for sex steroid biosynthesis (Cyp17a1,Hsd17b3, and an ovarian-specific splice variant of Cyp19a1) (see Fig. 2C for examples). Some of these markers localize exclusively to type B cells (e.g., Cyp17a1, Cyp19a1) while others are found in both type A and B cells (e.g., Gata4, Foxl2). Both “male-specific” (e.g., Spinlw1) and “female-specific” (e.g., Foxl2) markers are expressed in the neoplastic cells, implying that the cells exhibit mixed characteristics of male and female gonadal somatic cells. Such indeterminate steroidogenic cell phenotypes have been reported in other experimental models (Couse et al., 2006; Heikkila et al., 2002; Val et al., 2006). Prototypical markers of adrenocortical cell differentiation, such as adrenocorticoid biosynthetic enzymes (Cyp21a1, Cyp11b1, Cyp11b2) and transcription factor Gata6 (see Section 4.1), are downregulated in the neoplastic tissue (Bielinska et al., 2006).
Along with gonadal differentiation markers, several mast cell protease genes (Cma1, Mcpt4, Mcpt6, Tpsab1, and Cpa3) are expressed in the adrenal glands of gonadectomized mice (Schillebeeckx et al., 2015), consistent with the well-documented phenomenon of mast cell infiltration of the resultant adrenocortical neoplasms (Bielinska et al., 2005; Kim et al., 1997). Whether mast cells play an essential role in GDX-induced adrenocortical tumorigenesis is unclear. Mast cells have been implicated in the pathophysiology of aldosterone-producing adenomas in humans (Cartier et al., 2005).
2.4. DNA methylation changes associated with GDX-induced adrenocortical neoplasia
In addition to genetic factors, epigenetic modifications are thought to contribute to the pathogenesis of GDX-induced adrenocortical neoplasia. Stem/progenitor cells in the mouse adrenal cortex exhibit epigenetic variability, as illustrated by studies of mice that harbor Cyp21a1 promoter-LacZ (Morley et al., 1996) or Cyp11a1 promoter-LacZ (Hu et al., 1999) transgenes. The adrenal glands of these mice contain centripetally-migrating columns of cortical cells that either do or do not express β-galactosidase, reflecting random epigenetic activation (or silencing) of the transgenes in stem/progenitor cells. Preexisting epigenetic alterations are hypothesized to affect the phenotypic plasticity of adrenocortical stem/progenitor cells, allowing some to respond to the hormonal changes associated with GDX (Bielinska et al., 2009). Epigenetic variability among stem/ progenitor cells may explain why GDX of susceptible mouse strains leads to discrete columns or wedges of proliferating neoplastic cells in the adrenal cortex (Fig. 2A) rather than widespread subcapsular cell hyperplasia seen in other experimental models (see Sections 4 and 5 and Fig. 7B).
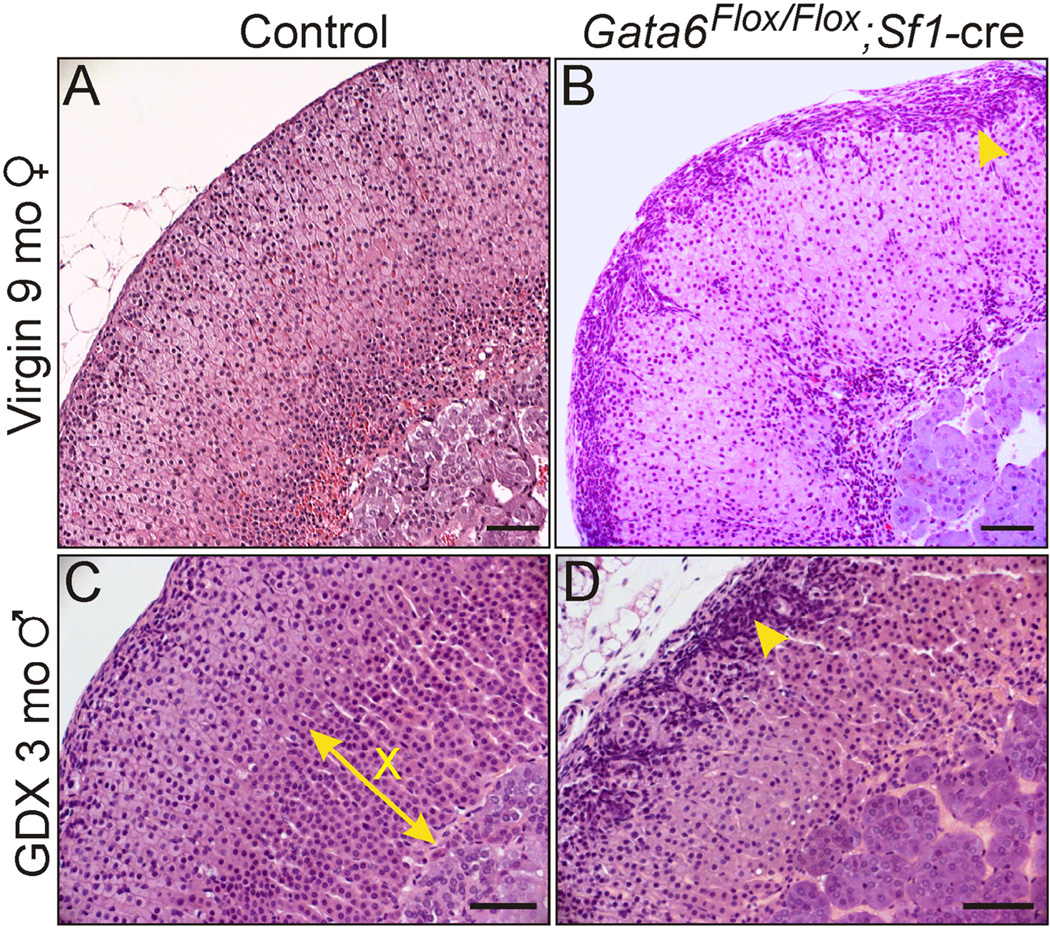
Fig. 7.
Morphological changes in the adrenal glands of Gata4Flox/Flox; Sf1-cre mice. H&E stained adrenal tissue from 9-mo-old control (A, Gata4Flox/+; Sf1-cre) and mutant (B) virgin female mice. The mutant adrenal has cytomegalic changes in the zF and subcapsular cell hyperplasia (arrowhead). H&E stained adrenal tissue from 2-mo-old control (C) and mutant (D) male mice that were gonadectomized at 3 weeks of age. The mutant adrenal exhibits subcapsular cell hyperplasia (arrowhead) and lacks a secondary X-zone (X). Bars = 100 µm.
One epigenetic modification, methylation of cytosine residues in CpG dinucleotides, has been shown to modulate progenitor cell fate in endocrine tissues (Aranda et al., 2009). For instance, conditional mutagenesis of the mouse Dnmt1 gene, which encodes the maintenance DNA methyl-transferase, causes reprogramming of pancreatic β-cells into α-cells (Dhawan et al., 2011). GDX-induced adrenocortical neoplasia may be another example of DNA methylation-regulated cell fate conversion in an endocrine tissue (Bielinska et al., 2009; Schillebeeckx et al., 2013).
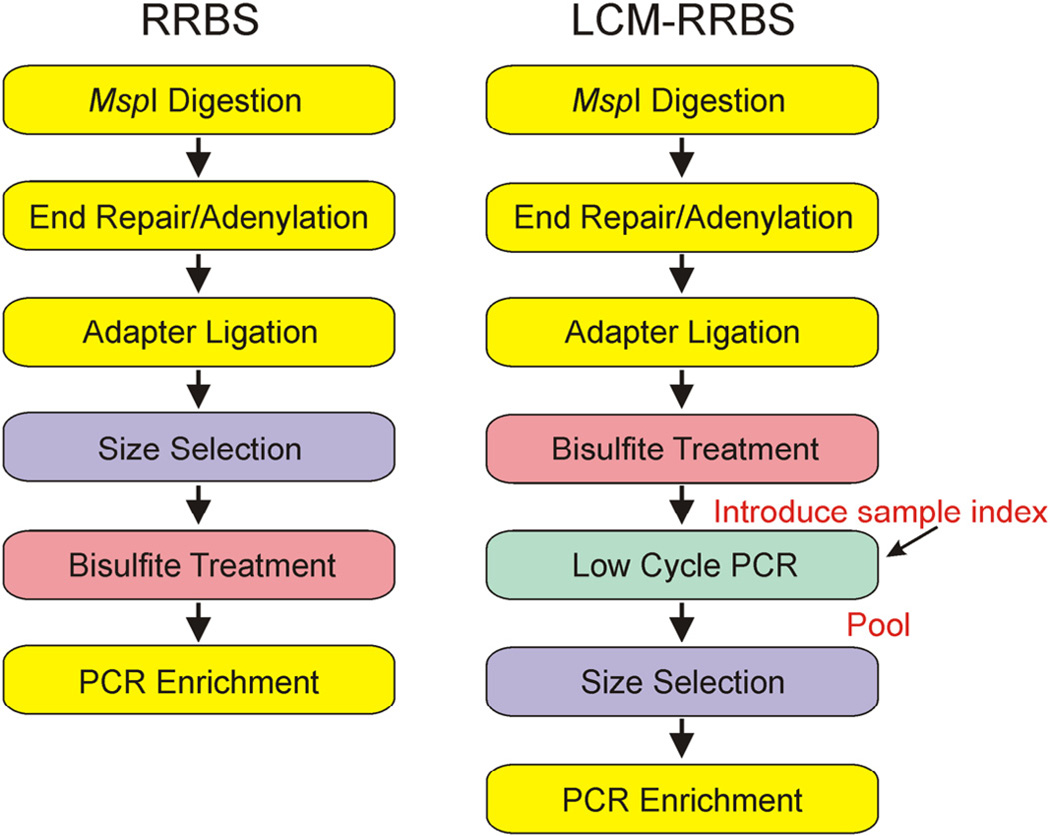
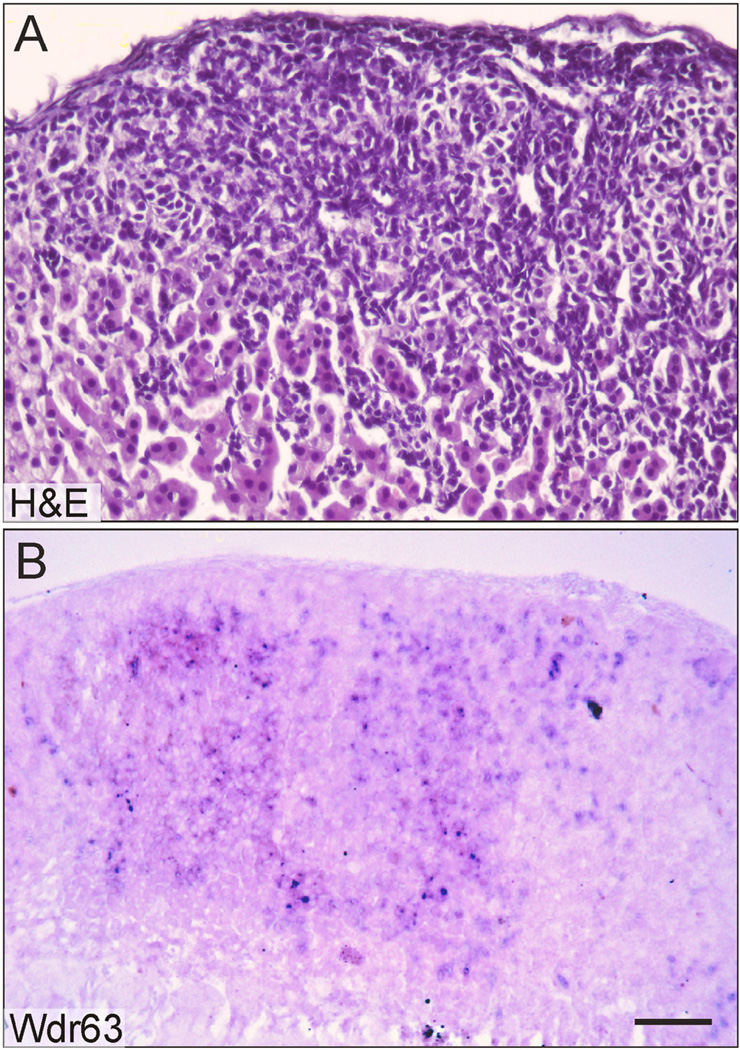
To investigate the epigenetic regulation of GDX-induced neoplasia in the mouse, we performed genome-wide DNA methylation analysis (Schillebeeckx et al., 2013). One popular method of DNA methylation mapping, reduced representation bisulfite sequencing (RRBS), lacks the sensitivity required to interrogate mouse adrenocortical neoplasms. We therefore developed an enhanced method capable of analyzing small amounts of genomic DNA (~1 ng) isolated by laser capture microdissection (LCM). A comparison of the workflows for traditional RRBS and this new technique, termed LCM–RRBS, is shown in Fig.4. Using LCM–RRBS, genes with putative roles in gonadal or adrenocortical development were found to be differentially methylated in GDX-induced adrenocortical neoplasms vs. adjacent normal tissue. For instance, Wdr63 and Tmem184a, genes previously implicated in gonadal development (Best et al., 2008; Sato et al., 2008; Svingen et al., 2007), were shown to be hypomethylated in the neoplastic cells. Conversely, Tinagl1, a gene implicated in adrenal zonation (Li et al., 2007), was found to be hypermethylated in the neoplastic tissue. In situ hybridization demonstrated that one of the hypomethylated genes, Wdr63, was expressed in GDX-induced adrenocortical neoplasms but not in adjacent normal tissue (Fig. 5).
Fig. 4.
Comparison of the RRBS and LCM-RRBS workflows. Genomic DNA is digested with MspI to create fragments with a 5′-CpG end. Digested fragments are blunted, adenylated, and ligated with methylated sequencing adapters. To convert the epigenetic methylation mark into a genetic mark that can be read through genomic sequencing, adapter-ligated fragments are treated with bisulfite. At this stage, converted DNA is amplified with a low-cycle PCR to introduce sample-specific indexes. Once each sample has been ‘indexed,’ samples are pooled prior to gel electrophoreses and the isolation of 40–220 base pair fragments. The purified, pooled library is PCR enriched using universal primers and sequenced on the Illumina platform to generate 50 base pair reads. By taking advantage of indexing, LCM-RRBS increases the number of samples that can be processed in parallel while reducing cost.
Fig. 5.
Expression of Wdr63, a hypomethylated gene, in GDX-induced neoplastic cells of the adrenal cortex. A female DBA/2J mouse was gonadectomized at 3 weeks of age and adrenal tissue was harvested 3 mo later. Adjacent cryosections of adrenal tissue were stained with H&E (A) or subjected to in situ hybridization using a digoxigenin-labeled Wdr63 antisense riboprobe and alkaline phosphatase conjugated anti-digoxigenin antibody (B). Note that Wdr63 is expressed in neoplastic cells but not adjacent normal adrenocortical cells. Bar = 50 µm.
2.5. Summary and perspectives
GDX-induced perturbations in the hormonal milieu cause gonadal-like cells to accumulate in the adrenal cortex of mice, and this experimental model can be harnessed to study the genetic and epigenetic factors that influence steroidogenic cell fate. Two key changes that accompany GDX-induced adrenocortical neoplasia are the upregulation of Gata4 and the reciprocal downregulation of Gata6 [reviewed in Bielinska et al. (2006)]. Evidence that these two genes directly impact tumorigenesis will be presented later (see Sections 3 and 4). Neoplastic and normal adrenocortical cells exhibit differences in DNA methylation that may reflect differences in the epigenetic fingerprints of the stem cell pools giving rise to these different cell types. Whether other epigenetic events, such as histone modification or changes in microRNA expression (Krill et al., 2013), contribute to the pathogenesis of GDX-induced adrenocortical neoplasia is unknown.
The temporospatial appearance of neoplastic cells in the adrenal cortex of gonadectomized mice suggests that type A cells may produce factors that promote differentiation of type B cells. Stromal cells of the postmenopausal ovary, which histologically and biochemically resemble type A cells, synthesize growth factor binding proteins that impact the differentiation of adjoining cells (Jabara et al., 2003). In an analogous manner, type A cells may secrete proteins that serve to insulate sex steroid-producing type B cells from the effects of growth factors that promote adrenocortical growth or differentiation. One of the genes found to be hypomethylated and upregulated in GDX-induced adrenocortical neoplasms, Igfbp6, encodes a growth factor binding protein that blocks the activity of IGF2, a known stimulator of adrenocortical cell growth (Drelon et al., 2012).
3. More than just a marker: GATA4 is a driver of GDX-induced adrenocortical neoplasia in mice
3.1. Role of Gata4 in gonadal somatic cell differentiation
Normally Gata4 is expressed in steroidogenic cells of the gonads and the fetal adrenal but not in corticoid-producing cells of the adult adrenal gland [reviewed in Viger et al. (2008)]. Putative GATA4 binding sites have been identified in the promoters and enhancers of many steroidogenic genes, and this transcription factor can act as either an activator or repressor depending on the context [reviewed in Tevosian (2014)]. Gata4−/− mice die in utero of defects in cardiac development, precluding the use of these homozygotes in studies of adrenocortical neoplasia; however, knock-in, chimera, and conditional mutagenesis studies have established that GATA4 regulates the differentiation of gonadal somatic cells, including sex steroidogenic cells, in the mouse [reviewed in Tevosian (2014)]. In humans, mutations in GATA4 and its cofactor FOG2/ZFPM2 have been linked to defects in testicular development and function (Bashamboo et al., 2014; Lourenco et al., 2011). Collectively, these studies in mice and humans suggest that GATA4 can influence the functional identity of gonadal somatic cells. By analogy, GATA4 is thought to regulate the differentiation of gonadal-like cells in the adrenal glands of gonadectomized mice.
3.2. GATA4 deficiency attenuates GDX-induced adrenocortical neoplasia in the mouse
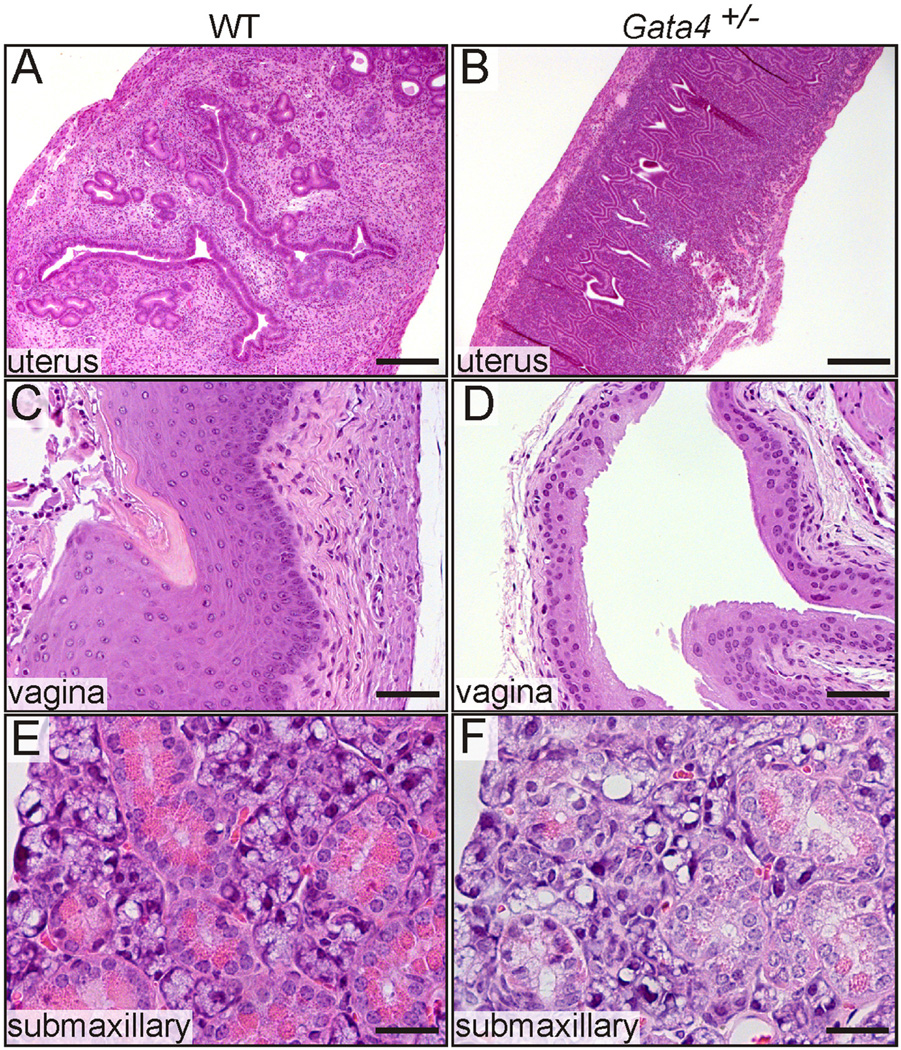
GATA4 is one of the earliest detectable markers of GDX-induced adrenocortical neoplasia and localizes to both type A and B cells (Fig. 2A,B). We used germline Gata4 haploinsufficient mice and conditional knockout (Gata4Flox/Flox; Amhr2cre/+) mice to study the role of GATA4 in GDX-induced adrenocortical neoplasia (Krachulec et al., 2012). Constitutive and acquired mutations in Gata4 were found to mitigate the accumulation of gonadal-like neoplastic cells and the expression of sex steroidogenic markers in the adrenal cortex of gonadectomized female mice. The appearance of type B cells in the adrenal cortex of wild-type but not Gata4+/− mice was associated with increased sex steroid levels in the circulation, manifested as histological changes in estrogen-responsive (uterus and vagina) and androgen-responsive (submaxillary gland) tissues (Fig. 6).
Fig. 6.
Gata4 haploinsufficiency abrogates extragonadal sex steroid production in older gonadectomized mice. Weanling B6D2F1 mice of the indicated genotypes were ovari-ectomized, and 12 mo later tissues were analyzed evidence of estrogen- orandrogen-dependent stimulation. The uteri of wild-type (WT) mice were enlarged and estrogenic (A), whereas the uteri of Gata4+/− mice were atrophic and hypoestrogenic (B). Consistent with estrogen stimulation, the vaginal epithelium of WT mice was thick and had cornified superficial layer (C). In contrast, the vaginal mucosa of Gata4+/− mice was thin (D). WT mice had evidence of ectopic androgen stimulation of the submaxillary gland, manifest as tall columnar acinar cells with basally located nuclei and abundant eosinophilic cytoplasmic granules (E). In Gata4+/− mice, the acinar cells had centrally located nuclei and only a few cytoplasmic granules, findings indicative of a lack of androgen stimulation (F). Bars = 300 µm (A,B), 75 µm (C,D), and 40 µm (E,F).
3.3. Enforced expression of Gata4 augments GDX-induced adrenocortical neoplasia in the mouse
Transgenic expression of Gata4 in the adrenal cortex using a Cyp21a1 promoter has been shown to induce adrenocortical neoplasia in a non-susceptible strain (C57Bl/6) (Chrusciel et al., 2013). Intact transgenic female mice gradually accumulate type A cells in the subcapsular cortex, and gonadectomized female and male mice develop adrenocortical neoplasms composed of type A and B cells. Based on the pattern of transgene expression in the mice and the latency of neoplasia, Chrusciel et al. suggested that the neoplastic cells are derived from the rare subcapsular stem/progenitor cells that transiently differentiate into CYP21A1+ adrenocortical cells rather than from trans-differentiation of normal CYP21A1+ zG cells.
3.4. Summary and perspectives
Loss- and gain-of-function studies have established that GATA4 directly modulates GDX-induced adrenocortical neoplasia. How GATA4 drives tumorigenesis is not fully understood. One proposed mechanism entails a feed forward signaling loop involving LHCGR and GATA4 (Vuorenoja et al., 2007). The developmental origin of GATA4+ cells in the adrenal cortex is the subject of active investigation, and recent fate mapping studies suggest that GATA4+ neoplastic cells arise from a distinctive pool of AGP-like progenitors in the adrenal capsule (see Section 5.1) (Bandiera et al., 2013).
4. GATA6 inhibits gonadal-like differentiation in the adrenal cortex of mice
4.1. Role of GATA6 in steroidogenic cell differentiation and function in the adrenal cortex
Another GATA transcription factor implicated in steroidogenic cell fate is GATA6 (Viger et al., 2008). Gata6 is expressed in the adrenal cortex of the fetal mouse (Kiiveri et al., 2002). Postnatally, adrenal expression of Gata6 is limited to capsular and subcapsular cells (Pihlajoki et al., 2013a). Additionally, GATA6 is expressed in the zR of primates, where it is thought to regulate androgen biosynthesis (Jimenez et al., 2003; Kiiveri et al., 2002; Nakamura et al., 2007, 2009). Like GATA4, GATA6 can act as either a repressor or activator of gene expression, depending on the context (Tevosian, 2014).
4.2. GATA6 deficiency enhances the accumulation of gonadal-like cells in the adrenal cortex
Gata6−/− mice die early in gestation, so the role of this transcription factor in adrenal function cannot be ascertained from these animals [reviewed in Tevosian (2014); Viger et al. (2008)]. The impact of GATA6 deficiency on adrenal gland development and physiology has been assessed by conditionally deleting Gata6 in murine adrenocortical cells using Cre-LoxP recombination with Sf1-cre (Pihlajoki et al., 2013a). The resultant mice have a thin, cytomegalic adrenal cortex. Type A cells accumulate in the adrenal subcapsule of Gata6Flox/Flox;Sf1-cre mice (Fig. 7), and there is a concomitant upregulation of Gata4, Inha, Inhba, Inhbb, Amhr2, Tcf21 and | other gonadal-like markers. Furthermore, markers of type B cells (Lhcgr, Cyp17a1) are overexpressed in the adrenal glands of gonadectomized Gata6Flox/Flox;Sf1-cre mice. Thus, GATA6 limits the spontaneous and GDX-induced differentiation of adrenal stem/ progenitor cells into gonadal-like cells.
4.3. GATA6 deficiency is associated with impaired X-zone development
Young virgin female Gata6Flox/Flox;Sf1-cre mice lack an X-zone, and castrated male Gata6Flox/Flox;Sf1-cre mice lack a secondary X-zone (Pihlajoki et al., 2013a). Whether the absence of a primary or secondary X-zone in these animals reflects a lack of progenitor proliferation or precocious degeneration of a nascent zone is unclear. Gata6 is not expressed in the X-zone of postnatal wild-type mice, arguing that the effect of Gata6 ablation on X-zone development is either a non-cell autonomous phenomenon or that it occurs in fetal adrenal cells that coexpress Gata6 and Sf1-cre (see Section 5.5).
4.4. Summary and perspectives
Gata6Flox/Flox;Sf1-cre mice, together with the aforementioned Gata4 mouse models (Sections 3.2 and 3.3), offer genetic support of the longstanding hypotheses that GATA6 promotes adrenocortical differentiation and GATA4 enhances gonadal-like differentiation [reviewed in Bielinska et al. (2006); Simon and Hammer (2012)]. In the absence of GATA6, GATA4 dominates and stem/progenitor cells differentiate into gonadal-like cells.
Comparison with studies performed in limb bud may offer mechanistic insight into the phenotype of Gata6Flox/Flox;Sf1-cre mice (Kozhemyakina et al., 2014). Hindlimb buds express Gata6 in an anterior-posterior gradient, and conditional mutagenesis of Gata6 using Prx1-cre leads to ectopic expression of Shh and its target genes, including Gli1, in the anterior mesenchyme of hindlimb buds. The resultant mice exhibit hindlimb preaxial polydactyly. Conversely, enforced expression of Gata6 in the limb bud represses expression of Shh and results in hypomorphic limbs. Chromatin immunoprecipitation (ChIP) and related experiments demonstrate that GATA6 binds to and represses both Shh and its target gene Gli1 in limb buds. In an analogous fashion, GATA6 may repress transcription of Shh and Gli1 in the adrenal cortex. Supporting this premise, the SHH-responsive gene Gli1 is upregulated in the adrenal glands of gonadectomized Gata6Flox/Flox;Sf1-cre mice (Pihlajoki et al., 2013a).
The relationship between GATA4 and GATA6 during steroidogenic progenitor cell differentiation is reminiscent of the interplay between GATA1 and GATA2 during hematopoietic progenitor differentiation. Despite having similar zinc finger DNA binding domains, GATA1 and GATA2 function uniquely to control distinct aspects of he-matopoiesis (Bresnick et al., 2010). GATA2 is required for the formation and maintenance of hematopoietic stem cells, whereas GATA1 drives the differentiation of hematopoietic progenitors into certain blood cell lineages. GATA1 directly represses Gata2 transcription, and this involves GATA1-mediated displacement of GATA2 from chromatin. This process is termed a “GATA switch.” In hematopoietic cells, GATA switches occur at numerous genes with essential functions (Bresnick et al., 2010). By analogy, GATA4 and GATA6 may regulate distinct aspects of steroidogenic cell development. GATA6 may be crucial for maintenance of steroidogenic stem/progenitor cells, perhaps via modulation of Wnt/β-catenin signaling (see Section 5.4), while GATA4 is required for terminal differentiation of progenitors into sex steroidogenic cells. Of note, methylation mapping studies have shown that an epigenetic switch from GATA2 to GATA6 expression accompanies endometriosis in women and leads to aberrant expression of genes involved in steroidogenesis (Dyson et al., 2014). Further experiments are needed to determine whether a GATA switch operates in the adrenal cortex of gonadectomized mice.
Another intriguing feature of the Gata6Flox/Flox;Sf1-cre mouse is the absence of the X-zone. The function of the normally ephemeral X-zone remains enigmatic. The analogous zone in humans, the fetal zone, expresses CYP17A1 and CYTB5 and produces large amounts of the androgen DHEA and its sulfated form DHEA-S that are converted by the sequential actions of the liver and placenta into estrogens. Androgen production by the human fetal zone, however, is not vital for prenatal survival, as shown by studies of humans with impaired CYP17A1 17,20-lyase activity (Miller and Auchus, 2011). Heterozygous loss-of-function mutations in human GATA6 have been linked to pancreatic agenesis, cardiac malformations, and biliary tract abnormalities, but not primary adrenocortical defects (Allen et al., 2012; Bonnefond et al., 2012; Maitra et al., 2010). It is conceivable that human GATA6 haploinsufficiency is associated with a subtle adrenal phenotype such as impaired fetal zone development or reduced DHEA(-S) production in the fetus, older child, or adult. Conversely, enforced expression of Gata6 in the mouse adrenal might be predicted to cause persistence of the X-zone or ectopic expression of zR-like markers (see Section 5.3).
5. Complementary mouse models offering insight into ectopic gonadal-like differentiation and aberrant X-zone development
5.1. WT1 gain-of-function model
Through fate mapping of WT1+ cells, the Schedl laboratory has identified a long-lived progenitor population in the adrenal capsule characterized by expression of Wt1 and Gata4, markers of the AGP (Bandiera et al., 2013). Under basal conditions, these AGP-like cells give rise to normal steroidogenic cells in the adrenal cortex (Table 1). GDX activates these WT1+ progenitors and triggers their differentiation into gonadal-like steroidogenic tissue. Thus, WT1+ capsular cells represent a reserve stem/progenitor cell population with AGP-like features that can be mobilized in response to extreme physiological demand (i.e., GDX-induced hormonal changes). These WT1+ capsular cells are presumed to be the progenitors of GDX-induced adrenocortical neoplasms.
During embryogenesis Wt1 repression is necessary for proper differentiation of stem/progenitor cells into adrenocortical cells (Bandiera et al., 2013). Ectopic expression of a transcriptionally active WT1 isoform (–KTS) in SF1+ progenitors results in adrenocortical hypoplasia, accumulation of type A cells, increased adrenal expression Gata4, Gli1, and Tcf21, and contraction of the X-zone. These phenotypic features are strikingly similar to those found in Gata6Flox/ Flox;Sf1-cre mice. Using ChIP and related experiments, Schedl and colleagues have shown that WT1 directly regulates the expression of Gli1 in adrenal tissue and proposed that ectopic expression of Wt1 prevents differentiation into SF1+ adrenocortical steroidogenic cells by maintaining cells in a GLI1+ progenitor state. As noted earlier (see Section 4.4), GATA6 binds and represses Gli1 in hindlimb buds, and Gli1 is upregulated in the adrenal glands of Gata6Flox/Flox;Sf1-cre mice. Collectively, these studies suggest that Gli1 activation, either through upregulation of Wt1 or downregulation of Gata6, is pivotal for gonadal-like differentiation in the adrenal cortex.
5.2. Sf1 overexpression model
Another interesting mouse model, developed by the Lalli laboratory, harbors multiple copies of the steroidogenic factor-1 (Sf1) genetic locus, mimicking the amplification of Sf1 seen in childhood adrenocortical carcinoma (Doghman et al., 2007; Figueiredo et al., 2005). These mice develop adrenocortical neoplasms that express gonadal-like markers including Gata4. Intriguingly, genetic ablation of the SF1 target gene Vnn1, encoding the gonadal-like marker Vanin-1, has been shown to reduce the severity of neoplastic lesions in the Sf1 transgenic mice (Latre de Late et al., 2014). Vanin-1 has pantetheinase activity, which releases cysteamine in tissues and regulates the response to oxidative stress by modulating glutathione production. On the basis of these experiments, it has been proposed that alterations of intracellular redox mechanisms contribute to the pathogenesis of adrenocortical neoplasia induced by Sf1 overexpression. Whether similar mechanisms operate in GDX-induced adrenocortical neoplasia is unknown.
5.3. Prkar1a loss-of-function model
Inactivating mutations in the protein kinase A regulatory subunit gene (PRKAR1A) lead to excessive cAMP production and cause Carney complex, a syndrome associated with adrenocortical neoplasia and pituitary-independent Cushing syndrome. As shown by the Martinez laboratory, conditional mutagenesis of Prkar1a in the adrenal cortex of mice (using Akr1b7-cre) leads to disrupted stem/progenitor cell differentiation, excess cell proliferation, and impaired apoptosis in the adrenal cortex (Sahut-Barnola et al., 2010). As these mice age, a new zone composed of cells that express Cyp17a1 and secrete cortisol appears in the inner aspect of the cortex. This ectopic X-like zone is thought to arise from normally dormant stem/progenitor cells in the juxtamedullary region (de Joussineau et al., 2012; Sahut-Barnola et al., 2010). When activated, these cells proliferate, migrate centrifugally, and give rise to tumors via cAMP and mTOR dependent pathways (de Joussineau et al., 2014). The juxtamedullary stem/progenitor cells that proliferate and transform in the Prkar1a loss-of-function model may be related to the X-zone progenitors that are absent or precociously depleted from the adrenal glands of Gata6Flox/Flox;Sf1-cre mice.
5.4. Aberrant Wnt/β-catenin signaling models
The Wnt/β-catenin signaling pathway is essential for cell renewal in the adrenal cortex (Simon and Hammer, 2012), and activation of this pathway has been documented in human adrenocortical tumors (Assie et al., 2014; Tissier et al., 2005). The Val laboratory has shown that constitutive activation of β-catenin in the adrenal cortex of mice (using Akr1b7-cre) triggers the accumulation type A cells in the subcapsule and abnormal steroidogenic cells in the juxtamedullary region (Berthon et al., 2010). Frank tumors arise from the latter, suggesting that progenitors in the juxtamedullary region of the adrenal cortex can be transformed in response to β-catenin signaling. Other alterations in cell fate, such as ectopic zG formation at the expense of zF, are evident in this model.
It is tempting to speculate that GATA6 modulates Wnt/β-catenin signaling in the adrenal cortex. In pulmonary and intestinal epithelia GATA6 interacts with the Wnt/β-catenin and bone mor-phogenetic protein signaling pathways to regulate the balance between stem/progenitor cell expansion and differentiation (Beuling et al., 2011, 2012; Tian et al., 2011; Whissell et al., 2014; Zhang et al., 2008). During colorectal tumorigenesis, human GATA6 directly enhances the expression of LGR5, which interacts with R-spondins and thereby activates Wnt/β-catenin signaling (Tsuji et al., 2014). At the 2014 Adrenal Meeting Andreas Schedl reported that conditional mutagenesis of Rspo3, an R-spondin expressed in adrenal subcapsular cells, causes adrenocortical hypoplasia.
5.5. Aberrant SUMOylation models
SUMO (small ubiquitin-like modifier) proteins function as post-translational modifiers, and SUMOylation represses the transcriptional activity of Sf1 by affecting its DNA binding. The Ingraham laboratory has characterized mice in which the endogenous Sf1 gene of the mouse has been replaced with a mutant lacking a key SUMOylation site (Lee et al., 2011). These mice have perturbed cell fate specification in steroidogenic tissues, including ectopic expression of gonadal markers (e.g., Sox9, Amhr2) in the adrenal glands and adrenocortical markers (e.g.,Akr1b7, Cyp21a1) in the testis. The mutant mice also exhibit persistence of the X-zone. In another model, mice with a global increase in SUMOylation due to deficiency of the deSUMOylase Senp2 exhibit impaired cardiogenesis due in part to repression of the Gata6 gene by the Polycomb repressor complex (Kang et al., 2010). Altogether, these studies suggest a possible link between changes in SUMOylation, expression of Gata6, and regulation of the X-zone.
5.6. Inhibin loss-of-function model
Gonadectomized Inha−/− mice develop subcapsular cell hyperplasia and juxtamedullary tumors that express Gata4 and other gonadal-like markers. This model has been studied extensively by the Hammer laboratory (Beuschlein et al., 2003; Looyenga and Hammer, 2007; Looyenga et al., 2010). Enforced expression of LH enhances adrenocortical neoplasia in these mice. Loss of inhibin leads to increased availability of the TGF-β type III receptor betaglycan and enhanced TGF-β2 signaling in the adrenal glands of these animals.
5.7. Other models of altered adrenocortical cell fate
A mouse model of MCM4 deficiency is associated with the progressive accumulation of type A cells (Hughes et al., 2012). Overexpression of Igf2 in the adrenal cortex of mice (using Akr1b7-cre) leads to the accumulation of subcapsular type A cells that express Gli1 and Tcf21 (Drelon et al., 2012). When expressed under the control of the Inha promoter, SV40 large T-antigen elicits gonadal-like tumors in the adrenal glands of gonadectomized transgenic mice (Rahman and Huhtaniemi, 2001). These tumors arise at the medulla boundary, although type A cells are also evident in the subcapsular region of these transgenic mice (Bielinska et al., 2006).
5.8. Summary and perspectives
Juxtamedullary changes (expansion, persistence, or loss of an X-like zone) and subcapsular cell hyperplasia (type A cells) are recurring themes in the aforementioned mouse models. The juxtamedullary changes are thought to reflect effects on stem cells in this region (Table 1). Subcapsular cell hyperplasia is presumed to result from a misspecification of capsular or subcapsular stem/ progenitor cells. Rather than differentiating to enter the steroidogenic lineage as GATA6+/GLI1- cells, these progenitors instead express Gata4 and retain Gli1 expression [reviewed in Yates et al. (2013)]. Circumstantial evidence from Cyp21a1 promoter-Gata4 transgenic mice (Chrusciel et al., 2013) and conditional knockout mice generated using Akr1b7-cre (Berthon et al., 2010; Drelon et al., 2012; Sahut-Barnola et al., 2010) suggests that type A cells are derived from differentiating stem cells that transiently activate Cyp21a1 or Akr1b7 expression before the adrenocortical steroidogenic program is squelched by GATA4 upregulation (Yates et al., 2013). GATA4 and GATA6 interact with many of the key signaling pathways (SHH, Wnt/ β-catenin, and cAMP) implicated in adrenocortical zonation, remodeling and function, which may account for the frequent dysregulation of these two GATA factors in the various mouse models of ectopic gonadal-like differentiation and aberrant X-zone development.
6. More than just an oddity of mice: Relevance of GDX-induced adrenocortical neoplasia to diseases affecting humans and companion animals
It is easy to dismiss GDX-induced adrenocortical neoplasia and related models of heterotopic gonadal-like differentiation as mere idiosyncrasies of mice that have little relevance to human disease, but this view may be a shortsighted. As will be summarized later, diseases with analogous features have been reported in humans and other species.
6.1. GDX-induced adrenal tumors in domesticated animals
GDX-induced adrenocortical neoplasia is a well documented phenomenon in not only mice but also hamsters, ferrets, goats, and other domesticated species (Beuschlein et al., 2012; Bielinska et al., 2009). Castration of male Angora goats, which enhances mohair production, is associated with a striking increase in the incidence of adrenocortical adenomas (12% vs. 0%, P < 0.001) (Altman et al., 1969). GDX-induced adrenocortical neoplasia is a major cause of morbidity in the domestic ferret, affecting up to 20% of these companion animals. The neoplastic cells that accumulate in the adrenal glands of gonadectomized ferrets express gonadal-like markers (e.g. Lhcgr, Gata4, Inha, Foxl2) and secrete sex steroids rather than corticoids (Bielinska et al., 2006; Schillebeeckx et al., 2015; Schoemaker et al., 2002). Ferret adrenocortical tumors express CYTB5, which enhances the 17,20-lyase activity of CYP17A1 and favors the production of androgens over cortisol (Fig. 1) (Wagner et al., 2008).
In ferrets the ectopic production of sex steroids by neoplastic adrenocortical tissue causes a syndrome known as adrenal-associated endocrinopathy (AAE), characterized by alopecia, vulvar enlargement, and stranguria (Bielinska et al., 2006). As in mice, the chronic elevation in circulating LH that follows GDX is thought to be essential for neoplastic transformation of the ferret adrenal cortex (Bielinska et al., 2006). Inhibition of LH secretion with gonadotropin-releasing hormone agonists can ameliorate signs of AAE (Wagner et al., 2001; Zeeland et al., 2014). Another effective treatment for AAE is GonaCon, a gonadotropin-releasing hormone vaccine originally developed to reduce the fertility of wildlife species (Miller et al., 2013). Treatment of young, gonadectomized ferrets with GonaCon reduces the incidence of adrenocortical neoplasia later in life.
6.2. Gonadotropin-dependent adrenocortical tumors in humans
The human adrenal cortex constitutively expresses low levels of LHCGR, and this receptor has been shown to be functionally active in the adrenal during pregnancy and other high gonadotropin states (Bernichtein et al., 2008). Therefore, it has been proposed that adrenal responsiveness to LH, influenced by modifier genes, may contribute to adrenocortical tumorigenesis in humans (Bernichtein et al., 2008). Although rare, benign adrenocortical neoplasms with histological features resembling luteinized ovarian stroma, termed “thecal metaplasia,” have been reported in postmenopausal women (Fidler, 1977; Wong and Warner, 1971) and men with acquired testicular atrophy (Romberger and Wong, 1989).
6.3. Postmenopausal ovarian hyperthecosis
This condition is characterized by the accumulation of luteinized thecal cells within the ovarian stroma separate from follicles (Nagamani et al., 1999). Like ferrets with GDX-induced adrenocortical neoplasia, women with postmenopausal ovarian hyperthecosis manifest signs and symptoms of androgen excess that can be alleviated by pharmacologic suppression of LH secretion (Beuschlein et al., 2012; Nagamani et al., 1999; Vollaard et al., 2011). Ovarian hyperthecosis occurs in only a minority of postmenopausal women, suggesting that genetic or epigenetic modifiers impact the development of this disease, as is true of GDX-induced adrenocortical neoplasms in the mouse. In one noteworthy case a postmenopausal woman simultaneously developed ovarian hyperthecosis and a benign adrenocortical tumor (Marcondes et al., 2008). The inconsistent nomenclature used to describe the histopathology of the climacteric ovary has muddled our understanding of postmenopausal hyperthecosis and related disorders such as “ovarian Leydig cell hyperplasia” (Mehta et al., 2014) and “thecomatosis” (Staats et al., 2008).
6.4. Testicular adrenal rest tumors
Leydig cells in the adult testis can arise from different populations of stem/progenitor cells, including undifferentiated mesenchymal cells in the testicular interstitium, vascular progenitors, and peritubular cells (Davidoff et al., 2004; Landreh et al., 2014; Mendis-Handagama and Ariyaratne, 2001). Men with disrupted adrenocortical function due to CYP21A1 deficiency develop neoplastic nodules of hormonally-active adrenocortical tissue in the testis (testicular adrenal rest tumors, TARTs), thought to arise from one of these reservoirs of pluripotential stem/ progenitor cells (Reisch et al., 2013; Val et al., 2006). Thus, TARTs can be viewed as the testicular counterpart of GDX-induced adrenocortical neoplasms. At the 2014 Adrenal Meeting Sergei Tevosian reported that Gata4/Gata6 double knockout mice generated with Sf1-cre exhibit severe adrenal hypoplasia; female double knockout mice die from adrenocortical insufficiency, whereas their male counterparts survive owing to heterotopic glucocorticoid production by TART-like cells.
Like a tritone chord substitution in the jazz standard “Cast Your Fate to the Wind,” the pluripotency of stem/progenitor cells in steroidogenic tissues is a double-edged sword. Reharmonization with a tritone substitution imparts movement to the bass line, but creates tension. Stem/progenitor cell pluripotency facilitates stress adaptation, but creates ectopic foci of steroidogenesis.
7. A twist of fate
The phenomenon of GDX-induced adrenocortical neoplasia in inbred mice was first identified 75 years ago by George Woolley and collaborators, and over the ensuing decades many articles were published on this topic. By the turn of the century, however, this classic model had fallen out of favor, though references to it lingered in veterinary medicine textbooks. As fate would have it, a pet ferret owned by the investigator who discovered GATA4 developed a symptomatic adrenocortical neoplasm that overexpressed this transcription factor (Peterson et al., 2004), and this observation rekindled interest in the inbred mouse model of GDX-induced adrenocortical neoplasia. As highlighted in this review article, the rejuvenated classic model has now combined with genetically-engineered models to yield valuable insights into the regulation of steroidogenic cell differentiation.
Acknowledgements
We thank Rosie the star-crossed ferret. Grant support: NIH (DK52574, DK075618, and DA025744), AHA (13GRNT16850031), Sigrid Jusélius Foundation, the Academy of Finland, DAAD (German Academic Exchange Service) fellowship D/12/40505, and CIMO (Centre for International Mobility Finland) fellowship TM-13-8769.
Footnotes
Disclosure summary: The authors have nothing to disclose.
References
- Allen HL, Flanagan SE, Shaw-Smith C, De Franco E, Akerman I, Caswell R, et al. GATA6 haploinsufficiency causes pancreatic agenesis in humans. Nat. Genet. 2012;44:20–22. doi: 10.1038/ng.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman NH, Streett CS, Terner JY. Castration and its relationship to tumors of the adrenal gland in the goat. Am. J. Vet. Res. 1969;30:583–589. [PubMed] [Google Scholar]
- Aranda P, Agirre X, Ballestar E, Andreu EJ, Roman-Gomez J, Prieto I, et al. Epigenetic signatures associated with different levels of differentiation potential in human stem cells. PLoS ONE. 2009;4:e7809. doi: 10.1371/journal.pone.0007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assie G, Letouze E, Fassnacht M, Jouinot A, Luscap W, Barreau O, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 2014;46:607–612. doi: 10.1038/ng.2953. [DOI] [PubMed] [Google Scholar]
- Bandiera R, Vidal VP, Motamedi FJ, Clarkson M, Sahut-Barnola I, von Gise A, et al. WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland. Dev. Cell. 2013;27:5–18. doi: 10.1016/j.devcel.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashamboo A, Brauner R, Bignon-Topalovic J, Lortat-Jacob S, Karageorgou V, Lourenco D, et al. Mutations in the FOG2/ZFPM2 gene are associated with anomalies of human testis determination. Hum. Mol. Genet. 2014;23:3657–3665. doi: 10.1093/hmg/ddu074. [DOI] [PubMed] [Google Scholar]
- Bernichtein S, Petretto E, Jamieson S, Goel A, Aitman TJ, Mangion JM, et al. Adrenal gland tumorigenesis after gonadectomy in mice is a complex genetic trait driven by epistatic loci. Endocrinology. 2007;149:651–661. doi: 10.1210/en.2007-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernichtein S, Alevizaki M, Huhtaniemi I. Is the adrenal cortex a target for gonadotropins? Trends Endocrinol. Metab. 2008;19:231–238. doi: 10.1016/j.tem.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Berthon A, Sahut-Barnola I, Lambert-Langlais S, de Joussineau C, Damon-Soubeyrand C, Louiset E, et al. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum. Mol. Genet. 2010;19:1561–1576. doi: 10.1093/hmg/ddq029. [DOI] [PubMed] [Google Scholar]
- Berthon A, Martinez A, Bertherat J, Val P. Wnt/beta-catenin signalling in adrenal physiology and tumour development. Mol. Cell. Endocrinol. 2012;351:87–95. doi: 10.1016/j.mce.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Best D, Sahlender DA, Walther N, Peden AA, Adams IR. Sdmg1 is a conserved transmembrane protein associated with germ cell sex determination and germline-soma interactions in mice. Development. 2008;135:1415–1425. doi: 10.1242/dev.019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuling E, Baffour-Awuah NY, Stapleton KA, Aronson BE, Noah TK, Shroyer NF, et al. GATA factors regulate proliferation, differentiation, and gene expression in small intestine of mature mice. Gastroenterology. 2011;140:1219–1229. doi: 10.1053/j.gastro.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuling E, Aronson BE, Tran LM, Stapleton KA, Ter Horst EN, Vissers LA, et al. GATA6 is required for proliferation, migration, secretory cell maturation, and gene expression in the mature mouse colon. Mol. Cell. Biol. 2012;32:3392–3402. doi: 10.1128/MCB.00070-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuschlein F, Looyenga BD, Bleasdale SE, Mutch C, Bavers DL, Parlow AF, et al. Activin induces x-zone apoptosis that inhibits luteinizing hormone-dependent adrenocortical tumor formation in inhibin-deficient mice. Mol. Cell. Biol. 2003;23:3951–3964. doi: 10.1128/MCB.23.11.3951-3964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuschlein F, Galac S, Wilson DB. Animal models of adrenocortical tumorigenesis. Mol. Cell. Endocrinol. 2012;351:78–86. doi: 10.1016/j.mce.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M, Genova E, Boime I, Parviainen H, Kiiveri S, Leppäluoto J, et al. Gonadotropin-induced adrenocortical neoplasia in NU/J nude mice. Endocrinology. 2005;146:3975–3984. doi: 10.1210/en.2004-1643. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Kiiveri S, Parviainen H, Mannisto S, Heikinheimo M, Wilson DB. Gonadectomy-induced adrenocortical neoplasia in the domestic ferret (Mustela putorius furo) and laboratory mouse. Vet. Pathol. 2006;43:97–117. doi: 10.1354/vp.43-2-97. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Parviainen H, Kiiveri S, Heikinheimo M, Wilson DB. Review paper: origin and molecular pathology of adrenocortical neoplasms. Vet. Pathol. 2009;46:194–210. doi: 10.1354/vp.46-2-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefond A, Sand O, Guerin B, Durand E, De Graeve F, Huyvaert M, et al. GATA6 inactivating mutations are associated with heart defects and, inconsistently, with pancreatic agenesis and diabetes. Diabetologia. 2012;55:2845–2847. doi: 10.1007/s00125-012-2645-7. [DOI] [PubMed] [Google Scholar]
- Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S. GATA switches as developmental drivers. J. Biol. Chem. 2010;285:31087–31093. doi: 10.1074/jbc.R110.159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier D, Jegou S, Parmentier F, Lihrmann I, Louiset E, Kuhn JM, et al. Expression profile of serotonin4 (5-HT4) receptors in adrenocortical aldosterone-producing adenomas. Eur. J. Endocrinol. 2005;153:939–947. doi: 10.1530/eje.1.02051. [DOI] [PubMed] [Google Scholar]
- Ching S, Vilain E. Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. Genesis. 2009;47:628–637. doi: 10.1002/dvg.20532. [DOI] [PubMed] [Google Scholar]
- Chrusciel M, Vuorenoja S, Mohanty B, Rivero-Muller A, Li X, Toppari J, et al. Transgenic GATA-4 expression induces adrenocortical tumorigenesis in C57Bl/6 mice. J. Cell Sci. 2013;126:1845–1857. doi: 10.1242/jcs.119347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa SM, Washburn LL, Kahlon RS, Musson MC, Bouma GJ, Eicher EM, et al. Sex reversal in C57BL/6J XY mice caused by increased expression of ovarian genes and insufficient activation of the testis determining pathway. PLoS Genet. 2012;8:e1002569. doi: 10.1371/journal.pgen.1002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Rodriguez KF, Johnson JA, Poirier D, Korach KS. The intraovarian actions of estrogen receptor-alpha are necessary to repress the formation of morphological and functional Leydig-like cells in the female gonad. Endocrinology. 2006;147:3666–3678. doi: 10.1210/en.2006-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Joussineau C, Sahut-Barnola I, Levy I, Saloustros E, Val P, Stratakis CA, et al. The cAMP pathway and the control of adrenocortical development and growth. Mol. Cell. Endocrinol. 2012;351:28–36. doi: 10.1016/j.mce.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Joussineau C, Sahut-Barnola I, Tissier F, Dumontet T, Drelon C, Batisse-Lignier M, et al. mTOR pathway is activated by PKA in adrenocortical cells and participates in vivo to apoptosis resistance in primary pigmented nodular adrenocortical disease (PPNAD) Hum. Mol. Genet. 2014;23:5418–5428. doi: 10.1093/hmg/ddu265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Muller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J. Cell Biol. 2004;167:935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan S, Georgia S, Tschen SI, Fan G, Bhushan A. Pancreatic beta cell identity is maintained by DNA methylation-mediated repression of Arx. Dev. Cell. 2011;20:419–429. doi: 10.1016/j.devcel.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doghman M, Karpova T, Rodrigues GA, Arhatte M, De MJ, Cavalli LR, et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol. Endocrinol. 2007;21:2968–2987. doi: 10.1210/me.2007-0120. [DOI] [PubMed] [Google Scholar]
- Drelon C, Berthon A, Ragazzon B, Tissier F, Bandiera R, Sahut-Barnola I, et al. Analysis of the role of Igf2 in adrenal tumour development in transgenic mouse models. PLoS ONE. 2012;7:e44171. doi: 10.1371/journal.pone.0044171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson MT, Roqueiro D, Monsivais D, Ercan CM, Pavone ME, Brooks DC, et al. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014;10:e1004158. doi: 10.1371/journal.pgen.1004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler WJ. Ovarian thecal metaplasia in adrenal glands. Am. J. Clin. Pathol. 1977;67:318–323. doi: 10.1093/ajcp/67.4.318. [DOI] [PubMed] [Google Scholar]
- Figueiredo BC, Cavalli LR, Pianovski MA, Lalli E, Sandrini R, Ribeiro RC, et al. Amplification of the steroidogenic factor 1 gene in childhood adrenocortical tumors. J. Clin. Endocrinol. Metab. 2005;90:615–619. doi: 10.1210/jc.2004-0942. [DOI] [PubMed] [Google Scholar]
- Freedman BD, Kempna PB, Carlone DL, Shah MS, Guagliardo NA, Barrett PQ, et al. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev. Cell. 2013;26:666–673. doi: 10.1016/j.devcel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasti L, Candy Sze WC, McKay T, Grose R, King PJ. FGF signalling through Fgfr2 isoform IIIb regulates adrenal cortex development. Mol. Cell. Endocrinol. 2013a;371:182–188. doi: 10.1016/j.mce.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasti L, Cavlan D, Cogger K, Banu Z, Shakur A, Latif S, et al. Dlk1 upregulates Gli1 expression in male rat adrenal capsule cells through the activation of beta1 integrin and ERK1-2. Endocrinology. 2013b;154:4675–4684. doi: 10.1210/en.2013-1211. [DOI] [PubMed] [Google Scholar]
- Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Ishikawa H. Electron microscopic observations on the castration-induced X zone in the adrenal cortex of male mice. Cell Tissue Res. 1975;162:119–130. doi: 10.1007/BF00223267. [DOI] [PubMed] [Google Scholar]
- Hu MC, Chou SJ, Huang YY, Hsu NC, Li H, Chung BC. Tissue-specific, hormonal, and developmental regulation of SCC-LacZ expression in transgenic mice leads to adrenocortical zone characterization. Endocrinology. 1999;140:5609–5618. doi: 10.1210/endo.140.12.7177. [DOI] [PubMed] [Google Scholar]
- Huang CC, Miyagawa S, Matsumaru D, Parker KL, Yao HH. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010;151:1119–1128. doi: 10.1210/en.2009-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CR, Guasti L, Meimaridou E, Chuang CH, Schimenti JC, King PJ, et al. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J. Clin. Invest. 2012;122:814–820. doi: 10.1172/JCI60224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabara S, Christenson LK, Wang CY, McAllister JM, Javitt NB, Dunaif A, et al. Stromal cells of the human postmenopausal ovary display a distinctive biochemical and molecular phenotype. J. Clin. Endocrinol. Metab. 2003;88:484–492. doi: 10.1210/jc.2002-021274. [DOI] [PubMed] [Google Scholar]
- Jimenez P, Saner K, Mayhew B, Rainey WE. GATA-6 is expressed in the human adrenal and regulates transcription of genes required for adrenal androgen biosynthesis. Endocrinology. 2003;144:4285–4288. doi: 10.1210/en.2003-0472. [DOI] [PubMed] [Google Scholar]
- Kang X, Qi Y, Zuo Y, Wang Q, Zou Y, Schwartz RJ, et al. SUMO-specific protease 2 is essential for suppression of polycomb group protein-mediated gene silencing during embryonic development. Mol. Cell. 2010;38:191–201. doi: 10.1016/j.molcel.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiiveri S, Liu J, Westerholm-Ormio M, Narita N, Wilson DB, Voutilainen R, et al. Differential expression of GATA-4 and GATA-6 in fetal and adult mouse and human adrenal tissue. Endocrinology. 2002;143:3136–3143. doi: 10.1210/endo.143.8.8939. [DOI] [PubMed] [Google Scholar]
- Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, et al. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135:2593–2602. doi: 10.1242/dev.021493. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kubota H, Kiuchi Y, Doi K, Saegusa J. Subcapsular cell hyperplasia and mast cell infiltration in the adrenal cortex of mice: comparative study in 7 inbred strains. Exp. Anim. 1997;46:303–306. doi: 10.1538/expanim.46.303. [DOI] [PubMed] [Google Scholar]
- King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc. Natl. Acad. Sci. U.S.A. 2009;106:21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemyakina E, Ionescu A, Lassar AB. GATA6 is a crucial regulator of Shh in the limb bud. PLoS Genet. 2014;10:e1004072. doi: 10.1371/journal.pgen.1004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krachulec J, Vetter M, Schrade A, Löbs AK, Bielinska M, Cochran R, et al. GATA4 is a critical regulator of gonadectomy-induced adrenocortical tumorigenesis in mice. Endocrinology. 2012;153:2599–2611. doi: 10.1210/en.2011-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krill KT, Gurdziel K, Heaton JH, Simon DP, Hammer GD. Dicer deficiency reveals microRNAs predicted to control gene expression in the developing adrenal cortex. Mol. Endocrinol. 2013;27:754–768. doi: 10.1210/me.2012-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreh L, Spinnler K, Schubert K, Hakkinen MR, Auriola S, Poutanen M, et al. Human testicular peritubular cells host putative stem Leydig cells with steroidogenic capacity. J. Clin. Endocrinol. Metab. 2014;99:E1227–E1235. doi: 10.1210/jc.2013-4199. [DOI] [PubMed] [Google Scholar]
- Latre de Late P, Wakil AE, Jarjat M, de Krijger RR, Heckert LL, Naquet P, et al. Vanin-1 inactivation antagonizes the development of adrenocortical neoplasia in Sf-1 transgenic mice. Endocrinology. 2014;155:16. doi: 10.1210/en.2014-1088. [DOI] [PubMed] [Google Scholar]
- Laufer E, Kesper D, Vortkamp A, King P. Sonic hedgehog signaling during adrenal development. Mol. Cell. Endocrinol. 2012;351:19–27. doi: 10.1016/j.mce.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FY, Faivre EJ, Suzawa M, Lontok E, Ebert D, Cai F, et al. Eliminating SF-1 (NR5A1) sumoylation in vivo results in ectopic hedgehog signaling and disruption of endocrine development. Dev. Cell. 2011;21:315–327. doi: 10.1016/j.devcel.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Mukai K, Suzuki T, Suzuki R, Yamashita S, Mitani F, et al. Adrenocortical zonation factor 1 is a novel matricellular protein promoting integrin-mediated adhesion of adrenocortical and vascular smooth muscle cells. FEBS J. 2007;274:2506–2522. doi: 10.1111/j.1742-4658.2007.05786.x. [DOI] [PubMed] [Google Scholar]
- Looyenga BD, Hammer GD. Genetic removal of Smad3 from inhibin-null mice attenuates tumor progression by uncoupling extracellular mitogenic signals from the cell cycle machinery. Mol. Endocrinol. 2007;21:18. doi: 10.1210/me.2006-0402. [DOI] [PubMed] [Google Scholar]
- Looyenga BD, Wiater E, Vale W, Hammer GD. Inhibin-A antagonizes TGFβ2 signaling by down-regulating cell surface expression of the TGFβ coreceptor betaglycan. Mol. Endocrinol. 2010;24:608–620. doi: 10.1210/me.2008-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco D, Brauner R, Rybczynska M, Nihoul-Fekete C, McElreavey K, Bashamboo A. Loss-of-function mutation in GATA4 causes anomalies of human testicular development. Proc. Natl. Acad. Sci. U.S.A. 2011;108:1597–1602. doi: 10.1073/pnas.1010257108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra M, Koenig SN, Srivastava D, Garg V. Identification of GATA6 sequence variants in patients with congenital heart defects. Pediatr. Res. 2010;68:281–285. doi: 10.1203/PDR.0b013e3181ed17e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes JA, Curi DD, Matsuzaki CN, Barcellos CR, Rocha MP, Hayashida SA, et al. Ovarian hyperthecosis in the context of an adrenal incidentaloma in a postmenopausal woman. Arq. Bras. Endocrinol. Metabol. 2008;52:1184–1188. doi: 10.1590/s0004-27302008000700016. [DOI] [PubMed] [Google Scholar]
- Mehta JM, Miller JL, Cannon AJ, Mardekian SK, Kenyon LC, Jabbour SA. Ovarian Leydig cell hyperplasia: an unusual case of virilization in a postmenopausal woman. Case Rep Endocrinol. 2014;2014:762745. doi: 10.1155/2014/762745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendis-Handagama SM, Ariyaratne HB. Differentiation of the adult Leydig cell population in the postnatal testis. Biol. Reprod. 2001;65:660–671. doi: 10.1095/biolreprod65.3.660. [DOI] [PubMed] [Google Scholar]
- Miller LA, Fagerstone KA, Wagner RA, Finkler M. Use of a GnRH vaccine, GonaCon, for prevention and treatment of adrenocortical disease (ACD) in domestic ferrets. Vaccine. 2013;31:4619–4623. doi: 10.1016/j.vaccine.2013.07.035. [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley SD, Viard I, Chung BC, Ikeda Y, Parker KL, Mullins JJ. Variegated expression of a mouse steroid 21-hydroxylase/beta-galactosidase transgene suggests centripetal migration of adrenocortical cells. Mol. Endocrinol. 1996;10:585–598. doi: 10.1210/mend.10.5.8732689. [DOI] [PubMed] [Google Scholar]
- Morohashi K, Zubair M. The fetal and adult adrenal cortex. Mol. Cell. Endocrinol. 2011;336:193–197. doi: 10.1016/j.mce.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Munger SC, Natarajan A, Looger LL, Ohler U, Capel B. Fine time course expression analysis identifies cascades of activation and repression and maps a putative regulator of mammalian sex determination. PLoS Genet. 2013;9:e1003630. doi: 10.1371/journal.pgen.1003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naffin-Olivos JL, Auchus RJ. Human cytochrome b5 requires residues E48 and E49 to stimulate the 17,20-lyase activity of cytochrome P450c17. Biochemistry. 2006;45:755–762. doi: 10.1021/bi051623y. [DOI] [PubMed] [Google Scholar]
- Nagamani M, Osuampke C, Kelver ME. Increased bioactive luteinizing hormone levels and bio/immuno ratio in women with hyperthecosis of the ovaries: possible role of hyperinsulinemia. J. Clin. Endocrinol. Metab. 1999;84:1685–1689. doi: 10.1210/jcem.84.5.5698. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Suzuki T, Sasano H. Transcription factor GATA-6 in the human adrenocortex: association with adrenal development and aging. Endocr. J. 2007;54:783–789. doi: 10.1507/endocrj.k07e-001. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Xing Y, Sasano H, Rainey WE. The mediator complex subunit 1 enhances transcription of genes needed for adrenal androgen production. Endocrinology. 2009;150:4145–4153. doi: 10.1210/en.2009-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parviainen H, Schrade A, Kiiveri S, Prunskaite-Hyyrylainen R, Haglund C, Vainio S, et al. Expression of Wnt and TGF-β pathway components and key adrenal transcription factors in adrenocortical tumors: association to carcinoma aggressiveness. Pathol. Res. Pract. 2013;209:503–509. doi: 10.1016/j.prp.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison JC, Abbott DH, Saltzman W, Conley AJ, Bird IM. Plasticity of the zona reticularis in the adult marmoset adrenal cortex: voyages of discovery in the New World. J. Endocrinol. 2009;203:313–326. doi: 10.1677/JOE-08-0554. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Kiupel M, Bielinska M, Kiiveri S, Heikinheimo M, Capen CC, et al. Transcription factor GATA-4 is a marker of anaplasia in adrenocortical neoplasms of the domestic ferret (Mustela putorius furo) Vet. Pathol. 2004;41:446–449. doi: 10.1354/vp.41-4-446. [DOI] [PubMed] [Google Scholar]
- Pihlajoki M, Gretzinger E, Cochran R, Kyrönlahti A, Schrade A, Hiller T, et al. Conditional mutagenesis of Gata6 in SF1-positive cells causes gonadal-like differentiation in the adrenal cortex of mice. Endocrinology. 2013a;154:1754–1767. doi: 10.1210/en.2012-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajoki M, Heikinheimo M, Wilson DB. Never underestimate the complexity of remodeling. Endocrinology. 2013b;154:4446–4449. doi: 10.1210/en.2013-1982. [DOI] [PubMed] [Google Scholar]
- Rahman NA, Huhtaniemi IT. Ovarian tumorigenesis in mice transgenic for murine inhibin alpha subunit promoter-driven Simian Virus 40 T-antigen: ontogeny, functional characteristics, and endocrine effects. Biol. Reprod. 2001;64:1122–1130. doi: 10.1095/biolreprod64.4.1122. [DOI] [PubMed] [Google Scholar]
- Reisch N, Rottenkolber M, Greifenstein A, Krone N, Schmidt H, Reincke M, et al. Testicular adrenal rest tumors develop independently of long-term disease control: a longitudinal analysis of 50 adult men with congenital adrenal hyperplasia due to classic 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2013;98:E1820–E1826. doi: 10.1210/jc.2012-3181. [DOI] [PubMed] [Google Scholar]
- Romberger CF, Wong TW. Thecal metaplasia in the adrenal gland of a man with acquired bilateral testicular atrophy. Arch. Pathol. Lab. Med. 1989;113:1071–1075. [PubMed] [Google Scholar]
- Sahut-Barnola I, de Joussineau C, Val P, Lambert-Langlais S, Damon C, Lefrancois-Martinez AM, et al. Cushing’s syndrome and fetal features resurgence in adrenal cortex-specific Prkar1a knockout mice. PLoS Genet. 2010;6:e1000980. doi: 10.1371/journal.pgen.1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Baba T, Zubair M, Miyabayashi K, Toyama Y, Maekawa M, et al. Importance of forkhead transcription factor Fkhl18 for development of testicular vasculature. Mol. Reprod. Dev. 2008;75:1361–1371. doi: 10.1002/mrd.20888. [DOI] [PubMed] [Google Scholar]
- Schillebeeckx M, Schrade A, Löbs AK, Pihlajoki M, Wilson DB, Mitra RD. Laser capture microdissection-reduced representation bisulfite sequencing (LCM-RRBS) maps changes in DNA methylation associated with gonadectomy-induced adrenocortical neoplasia in the mouse. Nucleic Acids Res. 2013;41:e116. doi: 10.1093/nar/gkt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillebeeckx M, Pihlajoki M, Gretzinger E, Yang W, Thol F, Hiller T, et al. Novel markers of gonadectomy-induced adrenocortical neoplasia. Mol. Cell. Endocrinol. 2015;399:122–130. doi: 10.1016/j.mce.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker NJ, Teerds KJ, Mol JA, Lumeij JT, Thijssen JH, Rijnberk A. The role of luteinizing hormone in the pathogenesis of hyperadrenocorticism in neutered ferrets. Mol. Cell. Endocrinol. 2002;197:117–125. doi: 10.1016/s0303-7207(02)00285-x. [DOI] [PubMed] [Google Scholar]
- Simon DP, Hammer GD. Adrenocortical stem and progenitor cells: implications for adrenocortical carcinoma. Mol. Cell. Endocrinol. 2012;351:2–11. doi: 10.1016/j.mce.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staats PN, McCluggage WG, Clement PB, Young RH. Luteinized thecomas (thecomatosis) of the type typically associated with sclerosing peritonitis: a clinical, histopathologic, and immunohistochemical analysis of 27 cases. Am. J. Surg. Pathol. 2008;32:1273–1290. doi: 10.1097/PAS.0b013e3181666a5f. [DOI] [PubMed] [Google Scholar]
- Svingen T, Beverdam A, Bernard P, McClive P, Harley VR, Sinclair AH, et al. Sex-specific expression of a novel gene Tmem184a during mouse testis differentiation. Reproduction. 2007;133:983–989. doi: 10.1530/REP-06-0379. [DOI] [PubMed] [Google Scholar]
- Tevosian S. Transgenic mouse models in the study of reproduction: insight into GATA protein function. Reproduction. 2014;148:R1–R14. doi: 10.1530/REP-14-0086. [DOI] [PubMed] [Google Scholar]
- Tian Y, Zhang Y, Hurd L, Hannenhalli S, Liu F, Lu MM, et al. Regulation of lung endoderm progenitor cell behavior by miR302/367. Development. 2011;138:1235–1245. doi: 10.1242/dev.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagnere AM, et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65:7622–7627. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Kawasaki Y, Furukawa S, Taniue K, Hayashi T, Okuno M, et al. The miR-363-GATA6-Lgr5 pathway is critical for colorectal tumourigenesis. Nat Commun. 2014;5:3150. doi: 10.1038/ncomms4150. [DOI] [PubMed] [Google Scholar]
- Val P, Jeays-Ward K, Swain A. Identification of a novel population of adrenal-like cells in the mammalian testis. Dev. Biol. 2006;299:250–256. doi: 10.1016/j.ydbio.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol. Endocrinol. 2008;22:781–798. doi: 10.1210/me.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollaard ES, van Beek AP, Verburg FA, Roos A, Land JA. Gonadotropin-releasing hormone agonist treatment in postmenopausal women with hyperandrogenism of ovarian origin. J. Clin. Endocrinol. Metab. 2011;96:1197–1201. doi: 10.1210/jc.2010-1991. [DOI] [PubMed] [Google Scholar]
- Vuorenoja S, Rivero-Muller A, Kiiveri S, Bielinska M, Heikinheimo M, Wilson DB, et al. Adrenocortical tumorigenesis, luteinizing hormone receptor and transcription factors GATA-4 and GATA-6. Mol. Cell. Endocrinol. 2007;269:38–45. doi: 10.1016/j.mce.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Wagner RA, Bailey EM, Schneider JF, Oliver JW. Leuprolide acetate treatment of adrenocortical disease in ferrets. J. Am. Vet. Med. Assoc. 2001;218:1272–1274. doi: 10.2460/javma.2001.218.1272. [DOI] [PubMed] [Google Scholar]
- Wagner S, Kiupel M, Peterson RA, Heikinheimo M, Wilson DB. Cytochrome b5 expression in gonadectomy-induced adrenocortical neoplasms of the domestic ferret (Mustela putorius furo) Vet. Pathol. 2008;45:439–442. doi: 10.1354/vp.45-4-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whissell G, Montagni E, Martinelli P, Hernando-Momblona X, Sevillano M, Jung P, et al. The transcription factor GATA6 enables self-renewal of colon adenoma stem cells by repressing BMP gene expression. Nat. Cell Biol. 2014;16:695–707. doi: 10.1038/ncb2992. [DOI] [PubMed] [Google Scholar]
- Wong TW, Warner NE. Ovarian thecal metaplasia in the adrenal gland. Arch. Pathol. 1971;92:319–328. [PubMed] [Google Scholar]
- Wood MA, Acharya A, Finco I, Swonger JM, Elston MJ, Tallquist MD, et al. Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development. 2013;140:4522–4532. doi: 10.1242/dev.092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates R, Katugampola H, Cavlan D, Cogger K, Meimaridou E, Hughes C, et al. Adrenocortical development, maintenance, and disease. Curr. Top. Dev. Biol. 2013;106:239–312. doi: 10.1016/B978-0-12-416021-7.00007-9. [DOI] [PubMed] [Google Scholar]
- Zeeland YR, Pabon M, Roest J, Schoemaker NJ. Use of a GnRH agonist implant as alternative for surgical neutering in pet ferrets. Vet. Rec. 2014;175:66. doi: 10.1136/vr.102389. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, et al. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat. Genet. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]