Abstract
Objective
Attention-deficit/hyperactivity disorder (ADHD) is a heritable neuropsychiatric disorder associated with abnormal reward processing. Limited and inconsistent data exist about the neural mechanisms underlying this abnormality. Furthermore, it is unknown whether reward processing is abnormal in unaffected siblings of participants with ADHD.
Method
We used event-related functional magnetic resonance imaging (fMRI) to investigate brain responses during reward anticipation and receipt with an adapted monetary incentive delay task in a large sample of adolescents and young adults with ADHD (n=150), their unaffected siblings (n=92), and control participants (n=108), all of the same age.
Results
Participants with ADHD showed, relative to control participants, increased responses in the anterior cingulate, anterior frontal cortex, and cerebellum during reward anticipation, and in the orbitofrontal, occipital cortex, and ventral striatum during reward receipt. Responses of unaffected siblings were increased in these regions as well, except for the cerebellum during anticipation and the orbitofrontal cortex during receipt.
Conclusion
ADHD in adolescents and young adults is associated with enhanced neural responses in frontostriatal circuitry to anticipation and receipt of reward. The findings support models emphasizing aberrant reward processing in ADHD and suggest that processing of reward is subject to familial influences. Future studies using standard monetary incentive delay task parameters have to replicate our findings.
Keywords: ADHD, reward processing, cognitive control, familiality, nucleus accumbens
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neuropsychiatric disorder affecting about 5% of children worldwide1 and is characterized by a pattern of impairing and persistent inattention and/or hyperactivity and impulsivity.2 Research on cognitive aspects of ADHD has long focused on executive functions such as working memory and response inhibition.3 However, more recent cognitive models of ADHD have indicated deficits in reward processing.4 Children with ADHD appear to be more sensitive to the positive effects of rewards on performance,5,6 make more risky decisions to obtain rewards,7 have stronger preference for immediate compared to delayed rewards,8,9 and show steeper temporal discounting compared to control participants.10,11 However, reports on behavioral measures of reward processing are inconsistent, and findings often remain unreplicated (e.g. 7,12,13). Little is known about the neural underpinnings of reward processing in particular in adolescents with ADHD. Our study aimed to investigate the neural mechanisms underlying reward processing in adolescents and young adults with ADHD, their unaffected siblings, and control participants.
Frontostriatal brain networks, including the orbitofrontal cortex, medial prefrontal cortex, and the ventral striatum (VS) play a crucial role in reward processing (for review see 14). Accordingly, studies investigating reward processing using a monetary incentive delay (MID) task have found alterations in VS signaling in both healthy populations and participants with ADHD (for review see 15). However, the manner in which VS signaling is altered is dependent on the studied population. Control participants with impulsive traits showed an increase of the striatal response to reward, whereas participants with ADHD mostly had decreased striatal responses to reward. VS responses during reward anticipation for adolescents with ADHD were observed to be lower than for control participants, but no differences were observed during reward receipt.16 However, an increased response in the same VS area during reward receipt but not during reward anticipation has been reported, as well.17 This inconsistency may be related to the small to moderate sample sizes and differences in task and study design. We aimed at resolving this discrepancy by assessing reward anticipation and reward receipt using an adaptation of the MID task in a large population of adolescents and young adults with ADHD and control participants. The MID task has been repeatedly shown to elicit a neural response in the VS to both reward anticipation and receipt (for review see 15,18-20).
ADHD has a strong genetic loading with an estimated heritability of about 80%.21 Siblings of participants with ADHD, who share on average 50% of their genetic information, have a two- to eight-fold elevated risk of ADHD relative to control participants22. Despite the high heritability of ADHD, identification of genes that contribute to the etiology of the clinical phenotype has proven challenging. The identification of endophenotypes may be helpful in unraveling the genetic component of ADHD. Endophenotypes are objective measures that represent heritable vulnerability traits associated with the disorder in the population and are thought to be intermediates on the pathway from genotype to phenotype 23. Importantly, because of their assumed heritability, it has been proposed that valid endophenotypes can be found at a higher rate in unaffected family members than in the general population 23,24. So far, two studies have investigated the familiality of behavioral measures of reward processing in the context of ADHD. These studies have reported oversensitivity to reward and abnormal preference for immediate reward in unaffected siblings 6,9. Moreover, genetic effects on reward processing in control participants have been described 25. Therefore we investigated whether neural measures of reward processing in unaffected siblings are intermediate between those of participants with ADHD and control participants, thus supporting their role as an endophenotype of ADHD.
Method
Participants
This study was approved by the local ethics committee of participating centers. Written informed consent was obtained from all participants or their legal guardians (for participants <12 years). We considered data from 571 participants of the NeuroIMAGE cohort, a large-scale cohort of families with one or more children with ADHD and control families recruited for the International Multicenter ADHD Genetics (IMAGE) study 26,27. Detailed recruitment and testing procedures for NeuroIMAGE have been described elsewhere.28
At the time of follow-up, clinical status was reassessed by a trained professional administering the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS)29 to parents and children and complemented by ADHD questionnaires (Conners' Parent and Teacher Rating Scales 30,31; see 28 for detailed diagnostic procedures). Diagnosis was based on DSM-5 criteria.2 Both unaffected siblings and control participants were free of ADHD.
The descriptive characteristics of the sample are summarized in Table 1. After applying exclusion criteria (see Supplement 1, available online) we were able to analyze 350 individuals: 150 participants with ADHD (68 predominantly inattentive, 21 predominantly hyperactive-impulsive, and 61 combined-type), 92 unaffected siblings, and 108 control participants. Age was not different between groups (Table 1), while gender was unequally distributed with a higher percentage of men with ADHD compared to the other groups (Chi2[2]=23.3; p<0.01).
Table 1. Demographic and Behavioral Data of the NeuroIMAGE Sample.
| Participants With ADHD | Unaffected Siblings | Control Participants | |||||
|---|---|---|---|---|---|---|---|
| Demographics | n | % | n | % | n | % | Group Comparison |
| Sample | 150 | 43.2 | 92 | 26.5 | 108 | 30.3 | |
| Comorbid | 34/8 | 23/5 | 0 | 0 | 0 | 0 | |
| Male | 105 | 70 | 42 | 45.7 | 44 | 41.9 | |
| M | SD | M | SD | M | SD | ||
| Age | 17.7 | 3.0 | 18.5 | 3.8 | 17.2 | 3.0 | A=S=C |
| IQa | 97.9 | 15.3 | 99.8 | 15.6 | 107.7 | 13.9 | (A=S)<C |
| Inattentive symptomsb | 7.2 | 1.8 | 0.6 | 1.4 | 0.5 | 1.3 | A>(S=C) |
| Hyperactive/impulsive symptomsb | 6.0 | 2.4 | 0.6 | 1.0 | 0.3 | 0.8 | A>(S=C) |
|
| |||||||
| Behavior | |||||||
| Reaction times reward (ms) | 293 | 36 | 296 | 34 | 296 | 32 | |
| Reaction times neutral (ms) | 325 | 47 | 324 | 39 | 320 | 38 | |
| Difference reaction times | F(2,328)=2.7; p<0.07 A=S=C | ||||||
| Coefficient of variation reward | 0.188 | 0.104 | 0.180 | 0.053 | 0.173 | 0.061 | |
| Coefficient of variation neutral | 0.228 | 0.103 | 0.214 | 0.079 | 0.192 | 0.053 | |
| Difference coefficient of variation | 0.04 | 0.09 | 0.03 | 0.08 | 0.02 | 0.09 | F(2,275)=3.1; p<0.05 A>C;A=S;S=C |
Note: Group comparison refers to post-hoc group-wise comparisons of participants with attention-deficit/hyperactivity disorder (ADHD; A), unaffected siblings (S), and control participants (C). Ms = milliseconds.
Estimated on basis of vocabulary and block-design subtests.
Symptoms based on combination of the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) and the Children's Psychiatric Rating Scale (CPRS).
As expected in a clinical sample of participants with ADHD, the majority had a history of treatment with ADHD medication (n=114 of 150). ADHD medication consisted of treatment with methylphenidate with immediate release (MPH-IR; n=103), methylphenidate with extended release (MPH-ER; n=84), atomoxetine (n=14), and/or dextroamphetamine (n=8). All participants had discontinued use of medication for 48 hours prior to testing.
Reward Anticipation Paradigm
We used a modified version of the MID task 19,32; participants were asked to respond as quickly as possible to a target by pressing a button. Prior to this target, a cue indicated the possibility to gain a reward after a button press within a given time window. Every trial ended with a feedback screen informing about the outcome of the current trial. Depending on the participants' performance, the response window for a correct response was adapted in the next trial resulting in an expected hit rate of 33%. The experiment lasted 12 minutes and a total of € 5 could be gained. At the end of the experiment, the awarded money was paid to the participant (see Supplement 1, available online, and Figure S1 for a detailed description of the task).
Compared with the original task, our version differed on two main aspects: hit rate (33% vs. 66%) and reward magnitude (20 cents vs. $5). The rationale behind these adaptations was to increase the demands of the task with stronger task engagement as a result. Secondly, our adaptations aimed at meeting the practical constraints of our study. Considering that we limited ourselves to rewarded and neutral conditions, rewarding participants according to the original task parameters would have led to disproportional monetary rewards (approximately €80), which was a concern for us and our ethical review board.
Behavioral Measures
Behavioral outcome measures were reaction time and coefficient of variation (CV) in the rewarded and neutral conditions. Based on trials with correct responses (i.e. no premature responses [reaction time, RT<100 ms], too many [>1] or too early [i.e. before target onset] button presses or no response at all) we calculated mean reaction times. The CV was defined as the standard deviation divided by the mean. Values were log10-transformed to improve normal distribution of the data.
Image Analysis
After image acquisition, preprocessing, and initial nuisance regression (see Supplement 1, available online), statistical parametric maps were estimated for each participant with a general linear model (generalized linear model [GLM]; FSL FEAT). First-level regressors included six regressors of interest (onset times of rewarded and neutral cues, hits, and misses, each with a duration of 0 seconds) and 6 regressors of no interest. The latter regressors comprised a) onsets of rewarded and neutral targets; b) cue, target, and outcome onsets of error events; and c) a motion regressor. Error events comprised events of trials with incorrect responses. The motion regressor was inserted to control for possible movement artifacts 33. Head movements from one image to the next exceeding 0.5 mm in either the x, y, or z direction were considered movement artifacts. Onset of this error event was set to 8 seconds before the movement, and all events within this 8-second interval were discarded. To ensure we had a sufficient amount of events to model our regressors of interest, we only included participants with at least 5 events per event type (see Table S1, available online). All regressors and their temporal derivatives were convolved with a canonical hemodynamic response function (HRF). Finally, the estimated beta maps for each participant were normalized to a common space (MNI152) for group comparisons.
Group comparison was divided into two steps: identification of regions that show sensitivity to ADHD-control differences and testing of endophenotypic characteristics within these regions including participants with ADHD, unaffected siblings and control participants. The first group comparison included participants with ADHD and control participants only. We chose this approach as opposed to a more conservative approach of assessing diagnostic effects within a general linear model including all three groups in order to be maximally sensitive to diagnosis-dependent effects. An ADHD-control comparison derived from a model including the unaffected siblings would have been less sensitive, as the included siblings would increase the error variance. Alternatively, investigating only the three-group contrast would reveal only regions showing endophenotypic characteristics (for results following this latter strategy see Supplement 1 and Table S2, available online).
Region Identification
To identify brain regions that showed deviant blood oxygenation level-dependent (BOLD) responses during reward anticipation and receipt, normalized contrast maps of the first-level parameter estimates were taken to second-level random effect analyses (FSL FLAME). For anticipation, we contrasted response maps for rewarded cues with response maps for neutral cues. For monetary reward receipt, we assessed the interaction of accuracy (hits vs. misses) and reward (rewarded vs. neutral trials [rewarded hits: 1; rewarded misses: -1; neutral hits: -1; neutral misses: 1]). This contrast was thought to have highest sensitivity to responses of the VS, signaling the need to adapt behavior in order to maximize reward gain and minimize punishment, commonly referred to as the reward prediction error 18. Group (ADHD vs. control) was entered as a between-subject factor in both anticipation and receipt analyses. Scan location, age, gender, comorbidity with oppositional defiant disorder (ODD)/conduct disorder (CD), and summary movement parameters (sum of all realignment parameters, and the number of movement-related error events) were added as regressors to account for effects of no interest. After initial thresholding at the voxel level (Z>2.3), statistical inference was done at a cluster level using Gaussian random field (GRF) theory-based significance testing (FSL 34; p<0.025 to correct for testing during anticipation and receipt) within a whole-brain search-space (for results of analyses restricted to a region of interest [ROI] search-space, see Supplement 1 and Table S3, available online).
Assessment of Unaffected Siblings
To subsequently examine the endophenotypic characteristics of regions identified by the procedure above, we tested the influence of familiality in each identified region using an analysis of variance (ANOVA) with group (ADHD, unaffected siblings, and control) as between-subject factor. We added scan location, age, gender, comorbidity with ODD/CD, and movement summary scores as covariates. Of note, in familial study designs that include more than one participant per family, the assumed independence of data is violated, potentially underestimating inter-individual variance in standard GLMs. The current study allowed inclusion of more than one participant with ADHD from one family. We therefore corrected for non-independence of data by adding family as a random effect. This was done in R (R 2.15.3 using the lme4 package [lme4 1.0.4 35]). All p-values were Bonferroni corrected (p<0.0083) for the number of clusters.
In addition to the regions identified by the ADHD-control comparison, we specifically examined the role of the VS using ROI analyses 18. To avoid non-independent voxel selection, we defined our ROI based on anatomical information. Each participant's anatomical magnetic resonance imaging (MRI) scan was segmented using an automatic subcortical segmentation tool (FIRST v1.2; 36). From these segmented structures, we selected the bilateral VS labelled as nucleus accumbens (NAcc), aligned them with the functional images, and extracted the mean of the parameter estimates from the contrast images for reward anticipation and receipt. We tested for endophenotypic characteristics of each of these two measures using an ANOVA with the same design as described above.
Finally, we estimated the influence of group on RT and CV difference scores (neutral RT/CV minus rewarded RT/CV).
Age, Family Gradient, and Sensitivity Analyses
Because of the wide age range of the studied sample and the divergent findings in literature including older participants, we investigated age-related effects in our sample. Therefore, we conducted two analyses. First, we divided our sample into two age groups (<18, 18+). We used this division as an additional between-subject factor, resulting in an age by diagnosis design with comorbidity with ODD/CD, scan site, gender, and motion as nuisance regressors. All reported clusters within the ROI were treated as dependent variables. Second, we added the interaction of age as a continuous measure with diagnosis to the statistical model.
We also assessed familiality of our neural measures by calculating family gradients. Results of these analyses, as well as additional post-hoc assessment of potential confounding factors such as medication use and testing at multiple sites, and main effect of task in all three diagnostic groups are reported in Supplement 1, Tables S6, S8-S11, and Figure S3, available online.
Results
Behavioral Results
Task performance is summarized in Table 1 and plotted in Figure 1. There was a main effect of cue type with faster reaction times for rewarded trials compared to neutral trials (295 ms versus 323 ms; t[335] = -19.4; p<0.001). We did not observe a significant group difference (F[2,328]=2.7; p<0.07). Regarding CV we found a main effect of cue with more variability during neutral trials (0.186 vs. 0.235; t[349]=5.9; p<0.001). We also observed a significant group effect (F[2,275] = 3.1; p<0.04). Pair-wise comparison revealed that reward-related reductions in variability were larger in participants with ADHD compared to control participants (t[236] = -2.4; p<0.02). Unaffected siblings had CV scores that were similar to those of control participants and their affected siblings.
Fig.1.
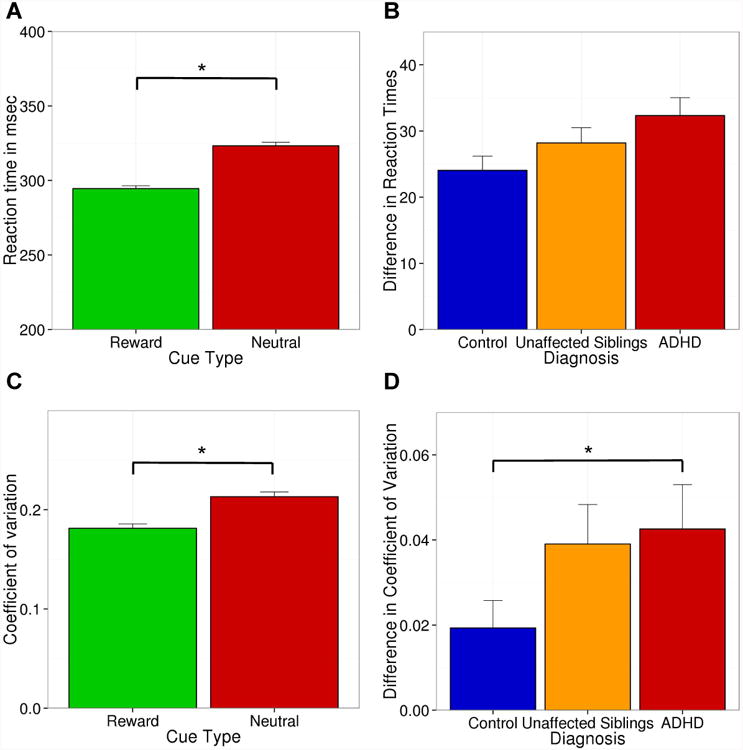
Behavioral effects of reward on reaction times (RT) and coefficient of variation (CV) across all participants (graphs A and C) and for each diagnostic group (graphs B and D). *p<0.05
General Task Effects
After family-wise error correction for multiple comparisons within the whole brain, we established a significant effect of reward anticipation for the contrast rewarded vs. neutral cue. Regions that showed response to the task manipulation included the basal ganglia including the VS, anterior cingulate cortex (ACC), insular cortex, visual cortex, and cerebellum (Figure 2). For reward receipt, we detected significant BOLD signal increases for rewarded relative to neutral hits (vs. misses) in the reward system, including the VS and frontal regions, motor cortex, and visual cortex.
Fig 2.
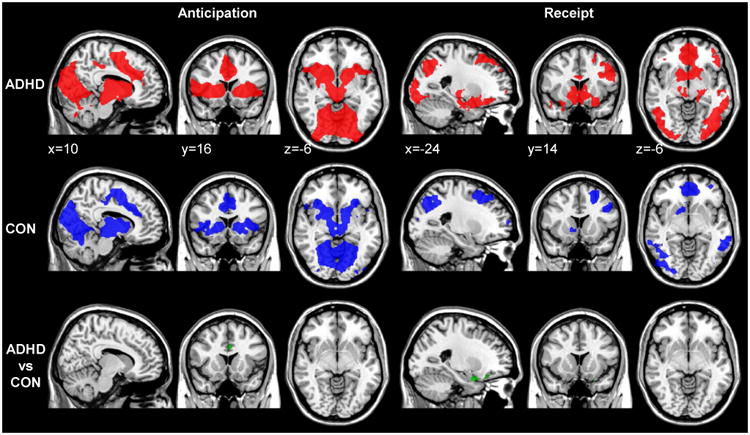
Brain responses to rewarded versus neutral cues (anticipation; left) and rewarded vs. neutral accuracy (hits versus misses; right) in participants with attention-deficit/hyperactivity disorder (ADHD; red) and control (CON) participants (blue), and the contrast ADHD vs. control participants (green). Note: All maps show Gaussian random field (GRF) theory-based cluster significance at p<0.025 within a whole-brain search-space.
Main Effects of ADHD Diagnosis
During anticipation we observed a significant main effect of ADHD (ADHD vs. control comparison) in three clusters including the ACC, frontal pole, and cerebellum (Figure 2; for results of three-groups group analysis see Supplement 1, available online). During receipt, the same comparison revealed a significant main effect of ADHD in the occipital cortex and two regions in the OFC, one of them extending into the amygdala. All significant clusters are summarized in Table 2 (post-hoc tests of the interaction that is implicitly modeled in this comparison can be found in Tables S4 and S5 and are illustrated in Figure S2, available online).
Table 2. Overview of Brain Regions Used to Test for Group Differences.
| Region | Size (Voxel) | Side | Z | X | Y | Z | Omnibus-Test | Pairwise-Comparison | |
|---|---|---|---|---|---|---|---|---|---|
| Anticipation | Frontal pole | 86 | R | 1.63 | 30 | 36 | 36 | F(2,307)=8.1; p<0.001* | (A=S)>C |
| Anterior Cingulate Cortex | 89 | L/R | 2.24 | -4 | 16 | 30 | F(2,331)=10.0; p<0.001* | (A=S)>C | |
| Cerebellum | 91 | L | 3.91 | -26 | -58 | -28 | F(2,306)=8.5; p<0.001* | A>(S=C) | |
|
| |||||||||
| ROI | Nucleus Accumbens | L/R | F(2,333)=1.0; p<0.1 | A=S=C | |||||
|
| |||||||||
| Receipt | Occipital cortex | 90 | L | 2.14 | -44 | -64 | 2 | F(2,287)=9.3; p<0.001* | (A=S)>C |
| Orbitofrontal cortex | 100 | L | 2.87 | -30 | 26 | -18 | F(2,335)=9.7; p<0.001* | (A=S)>C | |
| Orbitofrontal cortex | 127 | L | 4.64 | -22 | 4 | -24 | F(2,288)=20.0; p<0.001* | A>(S=C) | |
|
| |||||||||
| ROI | Nucleus Accumbens | L/R | F(2,332)=3.1; p<0.04* | A>C;A=S;S=C | |||||
Note: Regions comprised significant clusters (cluster p<0.025) from the attention-deficit/hyperactivity disorder (ADHD)–control comparison and one region of interest (ROI). Indicated p-values are corrected for non-independence. A = ADHD; C = control participants; L = left; R = right; S = unaffected siblings.
Familiality Analyses
Results of statistical group analysis (ADHD, unaffected siblings, control) in the significant clusters are presented in Table 2. Two different response patterns were observable. For the majority of tested regions, both participants with ADHD and unaffected siblings had increased responses relative to control participants (anticipation: ACC, frontal pole; receipt: occipital cortex, OFC). In two other regions (anticipation: cerebellum; receipt: OFC), participants with ADHD had increased brain responses relative to their unaffected siblings and control participants; the responses of unaffected siblings and control participants did not differ from each other. Group-specific mean responses are displayed in Figure 3.
Fig 3.
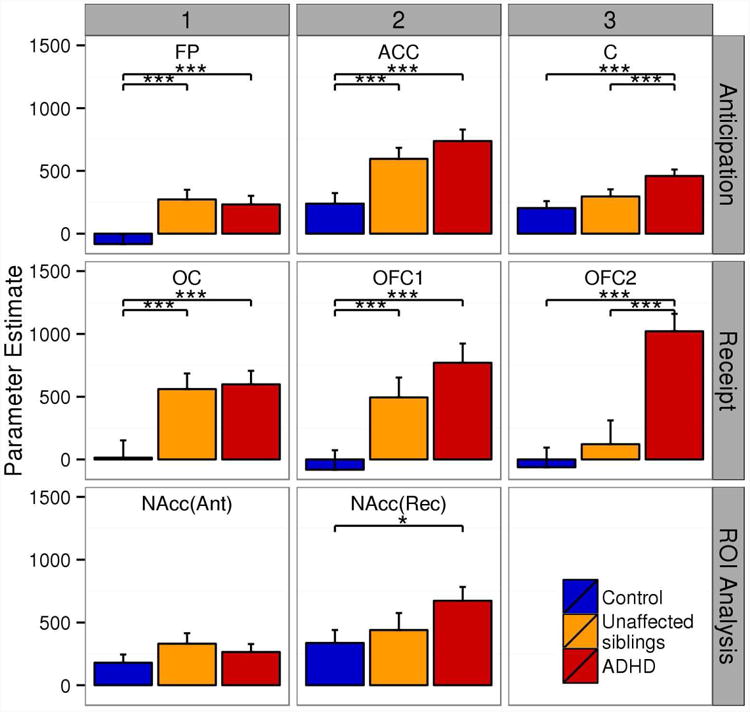
Group means for significant clusters from the attention-deficit/hyperactivity disorder (ADHD)-control comparisons and regions-of-interest (ROI; NAcc) analyses. Note: Regions are frontal pole (FP), cerebellum (C), anterior cingulate cortex (ACC), occipital cortex (OC), orbitofrontal cortex (OFC), and OFC extending into amygdala (OFC2). Error bars indicate standard error of the mean. *p<0.05 ***p<0.001.
Results of the ROI analysis are also indicated in Table 2 and Tables S10 and S11, available online. For reward anticipation and receipt, analysis of the VS revealed a main effect of task with increased BOLD response for rewarded trials compared with neutral trials (anticipation: t[257]=5.0; p<0.001; receipt: t[257]=5.8; p<0.001). For receipt, we additionally observed an ADHD effect (F[2,334]=3.2; p<0.04), with an increased BOLD response in ADHD compared to control participants (t[334]=2.44; p<0.04). Unaffected siblings did not differ from their affected siblings and the control participants. During anticipation BOLD responses were equal between all groups (F[2,336]=1.1, p<0.4).
Age Analyses
None of the brain regions (i.e. clusters from the ADHD-control comparison and NAcc during anticipation and receipt) showed a significant effect of age or interaction between age and diagnosis (see Supplement 1 and Table S7, available online).
Discussion
The aim of this study was to investigate the neural mechanisms of reward processing in ADHD and its potential as an endophenotype of ADHD. Our results revealed that ADHD is characterized by increased reward-related neural responses during anticipation and receipt as well as reduced variability of behavioral responses for rewarded cues. These findings extend previous observations of increased impact of reward on behavior in adolescent ADHD.5 Further, unaffected siblings of participants with ADHD also had increased neural responses during anticipation and during receipt, suggesting that familial factors play a role in this increased sensitivity of the reward system.
Participants with ADHD specifically displayed an increased response to reward in the frontal pole and orbitofrontal cortex. During anticipation this increased response extended to the ACC, which together with the basal ganglia forms the frontostriatal reward network. Here, the orbitofrontal cortex has been put forward as the central structure for representing the value of an expected outcome, 37 whereas the ACC has been associated with performance monitoring 38. Consequently, it might be that participants with ADHD overestimate the expected value of reward outcome. Additionally, they might recruit more resources to monitor actions or prepare a response.
Participants with ADHD also exhibited an increased response to reward receipt in the VS. VS responsiveness has been considered to code either for the hedonic value representation of reward (i.e. the amount of subjective pleasure in individual experiences) and to represent reward prediction error coding (i.e. the difference between expected and actual reward 18). This would suggest that participants with ADHD might be hypersensitive to reward because they experience receiving a reward relative to no reward as more pleasurable than control participants. Alternatively, they might be unable to correctly establish an association between a reward-predicting cue and the receipt, leading to an increased prediction error response during reward receipt relative to non-reward. A third explanation would be that participants with ADHD experience the inability to gain reward as overly aversive, which may result in a stronger signal of the brain to avoid such a situation in future 39.
A novel aspect of the current study was the assessment of familiality of the neural mechanisms of reward processing in ADHD. For most of these brain regions, the unaffected siblings exhibited increases of brain responses that made them comparable to affected participants. For two regions (cerebellum, OFC), we found that their brain responses were more similar to the control participants. Together our results suggest that unaffected siblings show part of the disorder-specific changes in neural functioning; however, these changes were present to a lesser extent. The observed changes in the neural substrate underlying reward processing in symptom-free family members of participants with ADHD suggest that familial factors (i.e. genes and/or shared environment) contribute to alterations in the reward system. Interestingly, genes such as DAT1, which can confer risk to ADHD, have been found to affect reward processing in control participants 25 and participants with ADHD 17. As a result, altered reward processing is a potential new endophenotype of ADHD. Of note, we used strict diagnostic criteria to guarantee that unaffected siblings neither exhibited subthreshold ADHD nor differed from control participants on ADHD symptom measures.
Our data replicate behavioral reports of altered reward sensitivity in adolescents with ADHD 5. However, they are in contrast with reports of decreased responsiveness of the VS in anticipation of reward in adults with ADHD,15 as well as with one study with adolescents with ADHD.16 Age may have a critical influence on the functioning of the reward system. Evidence supporting this account comes from studies showing developmental changes in neural firing of the reward system. These studies have mainly shown hypersensitivity of the reward system in adolescents. 40 Adolescence is thought to be characterized by behavioral changes such as increased risk-taking and behavioral impulsivity 41,42 putatively caused by an imbalance of basal ganglia and prefrontal cortex maturation. Moreover, impulsivity is in the healthy population associated with increases in sensitivity to reward 15. Given that healthy adolescents and participants with ADHD share increased behavioral impulsivity, we speculate that both adolescents and participants with ADHD might experience an age-related imbalance of neural development. At later ages, when regulatory functions of the prefrontal regions have matured in healthy populations 43, signaling in striatal regions may not only be normalized in participants with ADHD but may even be suppressed, resulting in a hypo-responsive reward system. However, we did not find support for this interpretation in our study since effects of age or an interaction of age by diagnosis were non-significant. This may be because our participants are mainly late adolescents and young adults. Accordingly, it might be that striatal hypo-responsiveness occurs at a later age. Another possibility is that adult ADHD is different from adolescent ADHD in terms of neural underpinnings. Longitudinal studies are needed to differentiate between these possible explanations.
The findings reported here should be interpreted in the context of the strengths and limitations of our study. We examined reward processing in a large sample of carefully phenotyped participants using a family design. A modified version of the well-established monetary incentive delay paradigm was employed, which induced clear behavioral and neural activation effects as both whole-brain and ROI analyses indicated. Another strength of the study was that all participants were scanned while off medication. Furthermore, we were able to rule out the effects of common confounds such as medication use, gender, and comorbidity with ODD/CD.
Our task was modified compared to the traditional versions of the MID 32. Specifically, we included only one level of reward with relatively low reward magnitude and lowered hit probability. Evidence from imaging studies on reward processing in healthy participants suggests that reward processing in the striatum is dependent on both of these parameters 44. Considering that previous studies with children and adults with ADHD have reported differences in striatal responses for a high reward condition only, it may be that inconsistencies between these studies and ours might be due to differences in reward magnitude. Indeed, signals in the striatum are most robust when high rewards are at stake, while responses to lower rewards are present yet less reproducible 45. Although we cannot exclude this possibility, we argue that this is unlikely given observations that dopaminergic midbrain neurons code for the relative rather than absolute value of a reward. Indeed, reward-related responses adapt to the context in which a reward is presented 46, in the sense that they depend on a combination of the overall expected value and their variance rather than the absolute magnitude of reward value. As is the case for dopamine neurons, 46 reward-related BOLD signals should maintain their sensitivity over a large range of reward values. This argument is supported by the clear and strong reward-related responses we observed in regions that are typically associated with reward processing (see Figure 2)32.
Another aspect of the changed task parameters relates to the underlying cognitive process. Specifically, it might be that due to the infrequent hits, our task was generally experienced as too difficult, leading to frustration and surprise rather than anticipating and receiving reward. Indeed, striatal structures are known to show biphasic responses, making them capable of responding to positive (reward) as well as negative (punishment) stimulation.47 Moreover, such an account would be in line with experimental findings demonstrating increased responses in reward processing brain regions to delayed rewards 39. Yet, both reward magnitude and reward probability are coded relatively rather than absolutely. 46 This implies that the perception of a cue as rewarding or punishing depends on the context. Accordingly, our hit rate of 33% would have been experienced as frustrating only when participants were able to compare this with experimental paradigms or conditions with a higher hit rate. Secondly, we observed faster and more stable responses on rewarded trials for all participants indicating that participants were aware of the reward component of the task rather than being surprised by the infrequent hits. Nevertheless, future studies need to confirm our findings. Moreover, given that another study reporting no VS differences in the anticipatory phase 48 applied a paradigm that also differed on reward probability, it suggests that this parameter is a crucial task parameter and deserves systematic investigation in future.
Finally, our task was originally designed to investigate reward anticipation and was not optimized for assessing reward receipt processing. The amount of trials used to estimate responses during reward receipt was relatively small and, with twice as many misses as hits, unequally distributed (for details see Table S1, available online). This may have resulted in a suboptimal estimation of receipt-related effects. Nevertheless, we are confident that the receipt-related effects reported here are robust and reliable based on the size of the sample we used as well as the clear main task effect of receipt (irrespective of group) that is in line with previous reports 47. A final note of caution concerns the functional specificity of the observed effects to reward processing. Specifically, we cannot exclude the possibility that the reported hyper-activation during reward anticipation and receipt reflects non-reward specific effects of ADHD on the processing of salient events 49.
To summarize, adolescents and young adults with ADHD, compared to control participants, had increased responses of the reward system (even when relatively low rewards were at stake). Unaffected siblings of the participants with ADHD showed the same altered response to reward anticipation (frontal pole; ACC) and receipt (occipital lobe; OFC) as their affected siblings whereas no changes could be observed in the cerebellum (anticipation) and the OFC (receipt). Our findings highlight that familial factors contribute to the pathogenesis of ADHD by affecting the reward system and suggest that altered reward processing is a promising endophenotype of ADHD.
Supplementary Material
Figure S1: The monetary incentive delay task.
Figure S2: Parameter estimates of event types constituting the contrast for reward receipt. Note: ADHD = attention-deficit/hyperactivity disorder; CON = control; NAcc = Nucleus accumbens; OFC = orbitofrontal cortex; ROI = region of interest.
Figure S3. Parameter estimates of contrast for reward anticipation (left four figures) and receipt (right four figures) in brain regions showing sensitivity to attention-deficit/hyperactivity disorder (ADHD)-control differences for participants with ADHD (A) and unaffected siblings (S). Note: Regions: frontal pole (FP), anterior cingulate cortex (ACC), cerebellum (C), nucleus accumbens (NAcc), occipital cortex (OC) and orbitofrontal cortex (OFC). BOLD = blood oxygenation level dependent.
Table S1: Descriptive Statistics of Receipt Events for all Included Participants
Table S2: Comparison of Significant Clusters From Attention-Deficit/Hyperactivity Disorder (ADHD)-Control Comparison Using
Table S3: Results of Statistical Testing for Voxelwise Differences Between Participants With Attention-Deficit/Hyperactivity Disorder (ADHD) and Control Participants in the Ventral Striatal Search Space
Table S4: Results of the Tukey Honest Significant Difference (HSD) Post-Hoc Test: Pairwise Comparison of Receipt Events
Table S5: Results of the Tukey Honest Significant Difference (HSD) Post-Hoc Test (Continued): Comparison of Interaction Between Receipt Events
Table S6: Frequency of Positive Family Gradients (Affected–Unaffected Family Member)
Table S7: Results of Statistical Testing for Linear Age Effects and Comparison of Adolescents and Adults With and Without Attention-Deficit/Hyperactivity Disorder (ADHD)
Table S8: Statistics for Comparison of Medication-Naïve vs. Medication Users for all Significant Clusters From Attention-Deficit/Hyperactivity Disorder (ADHD)–Control Comparison and the Nucleus Accumbens Region-of-Interest (ROI)
Table S9: Site-Specific Group Means of the Significant Clusters Gained From the Attention-Deficit/Hyperactivity Disorder (ADHD)–Control Comparison
Table S10. Group-Specific Main Effects of Task on the Blood Oxygenation Level Dependent Response in the Ventral Striatal Region of Interest
Table S11. Group-Specific Main Effects of Task on the Blood Oxygenation Level Dependent Response in the Ventral Striatal Region of Interest
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant R01MH62873 (SF), NWO Large Investment Grant 1750102007010 (JB), and grants from Radboud University Medical Center, University Medical Center Groningen and Accare, and VU University Amsterdam.
Drs. Mennes, Cools, Zwiers, Buitelaar, and Mr. von Rhein served as the statistical experts for this research.
The authors acknowledge the department of pediatrics of the VU University Medical Center for having provided the opportunity to use the mock scanner for preparation of their participants. The authors thank Paul Gaalman, BSc, technical assistant, Donders Centre for Cognitive Neuroimaging, Nijmegen, for technical magnetic resonance imaging (MRI) assistance and are grateful to all participants for their contribution.
Footnotes
Supplemental material cited in this article is available online.
Disclosure: Dr. Cools has served as a consultant to Abbott Laboratories and Pfizer. Dr. Oosterlaan has received an investigator initiated grant from Shire. Dr. Hoekstra has received advisory panel payments from Shire as well as an unrestricted research grant from Shire. Dr. Faraone has received consulting income, travel expenses, and/or research support from, and/or has served on the advisory board of Pfizer, Ironshore, Shire, Akili Interactive Labs, Alcobra, CogCubed, Impax, NeuroLifeSciences, VAYA Pharma, and Neurovance, and research support from NIH. His institution (SUNY) is seeking a patent for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he has received consulting fees or served on advisory boards or participated in continuing medical education programs sponsored by Shire, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer, and Eli Lilly and Co. He has received royalties from books published by Guilford Press (Straight Talk about Your Child's Mental Health) and Oxford University Press (Schizophrenia: The Facts). Dr. Buitelaar has served as a consultant to/member of advisory board of/speaker for Janssen Cilag BV, Eli Lilly and Co., Bristol-Myers Squibb, Shering Plough, UCB, Shire, Novartis, and Servier. Drs. Zwiers, van der Schaaf, Franke, Luman, Heslenfeld, Hartman, van Dongen, and Mennes, Mr. von Rhein, Mr. van Rooij, and Ms. Lojowska report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 3.Willcutt EG, Doyle AE, Nigg JT, et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neurosci Biobehav Rev. 2010;34:744–754. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Uebel H, Albrecht B, Asherson P, et al. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. Journal of Child Psychology and Psychiatry. 2010;51:210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groen Y, Gaastra GF, Lewis-Evans B, Tucha O. Risky Behavior in Gambling Tasks in Individuals with ADHD – A Systematic Literature Review. In: Pessiglione M, editor. PLoS ONE. Vol. 8. 2013. p. e74909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bitsakou P, Psychogiou L, Thompson M, Sonuga-Barke EJS. Delay Aversion in Attention Deficit/Hyperactivity Disorder: an empirical investigation of the broader phenotype. Neuropsychologia. 2009;47:446–456. doi: 10.1016/j.neuropsychologia.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Marco R, Miranda A, Schlotz W, et al. Delay and reward choice in ADHD: An experimental test of the role of delay aversion. Neuropsychology. 2009;23:367–380. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- 10.Scheres A, Lee A, Sumiya M. Temporal reward discounting and ADHD: task and symptom specific effects. J Neural Transm. 2008;115:221–226. doi: 10.1007/s00702-007-0813-6. [DOI] [PubMed] [Google Scholar]
- 11.Demurie E, Roeyers H, Baeyens D, Sonuga-Barke E. Common alterations in sensitivity to type but not amount of reward in ADHD and autism spectrum disorders. J Child Psychol Psychiatry. 2011;52:1164–1172. doi: 10.1111/j.1469-7610.2010.02374.x. [DOI] [PubMed] [Google Scholar]
- 12.Sjöwall D, Roth L, Lindqvist S, Thorell LB. Multiple deficits in ADHD: executive dysfunction, delay aversion, reaction time variability, and emotional deficits. Journal of Child Psychology and Psychiatry. 2012;54:619–627. doi: 10.1111/jcpp.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solanto MV, Gilbert SN, Raj A, et al. Neurocognitive functioning in AD/HD, predominantly inattentive and combined subtypes. J Abnorm Child Psychol. 2007;35:729–744. doi: 10.1007/s10802-007-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plichta MM, Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: A meta-analytic review of the fMRI literature. Neurosci Biobehav Rev. 2014;38:125–134. doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 17.Paloyelis Y, Mehta MA, Faraone SV, et al. Striatal Sensitivity During Reward Processing in Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:722–732.e9. doi: 10.1016/j.jaac.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sescousse G, Caldú X, Segura B, Dreher JC. Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:681–696. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Hoogman M, Aarts E, Zwiers M, et al. Nitric Oxide Synthase Genotype Modulation of Impulsivity and Ventral Striatal Activity in Adult ADHD Patients and Healthy Comparison Subjects. Am J Psychiatry. 2011;168:1099–1106. doi: 10.1176/appi.ajp.2011.10101446. [DOI] [PubMed] [Google Scholar]
- 20.Hermans EJ, Bos PA, Ossewaarde L, et al. Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. Neuroimage. 2010;52:277–283. doi: 10.1016/j.neuroimage.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Faraone SV, Mick E. Molecular Genetics of Attention Deficit Hyperactivity Disorder. Psychiatric Clinics of North America. 2010;33:159–180. doi: 10.1016/j.psc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faraone SV, Doyle AE. The nature and heritability of attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2001;10:299–316. [PubMed] [Google Scholar]
- 23.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 24.Rommelse NNJ, Geurts HM, Franke B, et al. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci Biobehav Rev. 2011;35:1363–1396. doi: 10.1016/j.neubiorev.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Dreher JC, Kohn P, Kolachana B, et al. Variation in dopamine genes influences responsivity of the human reward system. Proceedings of the National Academy of Sciences. 2009;106:617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brookes K, Xu X, Chen W, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006;11:934–953. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- 27.Rommelse N, Altink M, Martin ME, et al. Neuropsychological measures probably facilitate heritability research of ADHD. Archives of Clinical Neuropsychology. 2008;23:579–591. doi: 10.1016/j.acn.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Rhein D, Mennes M, van Ewijk H, et al. The NeuroIMAGE study: a prospective phenotypic, cognitive, genetic and MRI study in children with attention-deficit/hyperactivity disorder. Design and descriptives. European child and adolescent psychiatry. 2014 doi: 10.1007/s00787-014-0573-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Conners CK, Erhardt D, Epstein JN, et al. Self-ratings of ADHD symptoms in adults I: Factor structure and normative data. J Atten Disord. 1999;3:141–151. [Google Scholar]
- 31.Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- 32.Knutson B, Fong GW, Adams CM, et al. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 33.Keulers EHH, Goulas A, Jolles J, Stiers P. Maturation of task-induced brain activation and long range functional connectivity in adolescence revealed by multivariate pattern classification. Neuroimage. 2012;60:1250–1265. doi: 10.1016/j.neuroimage.2011.12.079. [DOI] [PubMed] [Google Scholar]
- 34.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 35.Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. 2013 http://lme4.r-forge.r-project.org/
- 36.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Doherty J, Kringelbach ML, Rolls ET, et al. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 38.Kennerley SW, Walton ME, Behrens TEJ, et al. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 39.Lemiere J, Danckaerts M, Van Hecke W, et al. Brain activation to cues predicting inescapable delay in adolescent Attention Deficit/Hyperactivity Disorder: an fMRI pilot study. Brain Res. 2012;1450:57–66. doi: 10.1016/j.brainres.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 40.Galvan A. Adolescent development of the reward system. Front Hum Neurosci. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnett JJ. Adolescent storm and stress, reconsidered. American Psychological Association. 1999;54:317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- 43.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yacubian J, Gläscher J, Schroeder K, et al. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu CC, Samanez-Larkin GR, Katovich K, Knutson B. Affective traits link to reliable neural markers of incentive anticipation. Neuroimage. 2014;84:279–289. doi: 10.1016/j.neuroimage.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paloyelis Y, Asherson P, Kuntsi J. Are ADHD symptoms associated with delay aversion or choice impulsivity? A general population study. J Am Acad Child Adolesc Psychiatry. 2009;48:837–846. doi: 10.1097/CHI.0b013e3181ab8c97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zink CF, Pagnoni G, Martin ME, et al. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23:8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: The monetary incentive delay task.
Figure S2: Parameter estimates of event types constituting the contrast for reward receipt. Note: ADHD = attention-deficit/hyperactivity disorder; CON = control; NAcc = Nucleus accumbens; OFC = orbitofrontal cortex; ROI = region of interest.
Figure S3. Parameter estimates of contrast for reward anticipation (left four figures) and receipt (right four figures) in brain regions showing sensitivity to attention-deficit/hyperactivity disorder (ADHD)-control differences for participants with ADHD (A) and unaffected siblings (S). Note: Regions: frontal pole (FP), anterior cingulate cortex (ACC), cerebellum (C), nucleus accumbens (NAcc), occipital cortex (OC) and orbitofrontal cortex (OFC). BOLD = blood oxygenation level dependent.
Table S1: Descriptive Statistics of Receipt Events for all Included Participants
Table S2: Comparison of Significant Clusters From Attention-Deficit/Hyperactivity Disorder (ADHD)-Control Comparison Using
Table S3: Results of Statistical Testing for Voxelwise Differences Between Participants With Attention-Deficit/Hyperactivity Disorder (ADHD) and Control Participants in the Ventral Striatal Search Space
Table S4: Results of the Tukey Honest Significant Difference (HSD) Post-Hoc Test: Pairwise Comparison of Receipt Events
Table S5: Results of the Tukey Honest Significant Difference (HSD) Post-Hoc Test (Continued): Comparison of Interaction Between Receipt Events
Table S6: Frequency of Positive Family Gradients (Affected–Unaffected Family Member)
Table S7: Results of Statistical Testing for Linear Age Effects and Comparison of Adolescents and Adults With and Without Attention-Deficit/Hyperactivity Disorder (ADHD)
Table S8: Statistics for Comparison of Medication-Naïve vs. Medication Users for all Significant Clusters From Attention-Deficit/Hyperactivity Disorder (ADHD)–Control Comparison and the Nucleus Accumbens Region-of-Interest (ROI)
Table S9: Site-Specific Group Means of the Significant Clusters Gained From the Attention-Deficit/Hyperactivity Disorder (ADHD)–Control Comparison
Table S10. Group-Specific Main Effects of Task on the Blood Oxygenation Level Dependent Response in the Ventral Striatal Region of Interest
Table S11. Group-Specific Main Effects of Task on the Blood Oxygenation Level Dependent Response in the Ventral Striatal Region of Interest


