Abstract
Fatty acids and consequently diet play an essential role in the formation of inflammatory mediators involved in the pathogenesis of asthma. Because intake variations of omega-6 (n-6) and omega-3 (n-3) fatty acids ultimately determine cell membrane incorporation, changes in diet have the potential to modify downstream production of inflammatory mediators derived from these compounds. It has long been hypothesized that decreasing the n-6/n-3 ratio could reduce the production of more proinflammatory mediators while increasing the formation of downstream metabolites that can serve to limit or resolve inflammation. In turn, these changes would result in improved asthma outcomes or would lower the risk for asthma incidence. This review will focus on the role of fatty acid inflammatory and resolving mediators and will summarize the clinical and epidemiologic data on how diet and obesity alter fatty acid profiles that can contribute to asthma.
Keywords: Asthma, diet, obesity, fatty acids, n-6, n-3, inflammation, resolution
Chronic airway inflammation is coordinated by a complex web of inflammatory mediators, including interleukins, adhesion molecules, inflammatory enzymes, and lipid mediators. Rigorous study in the area of lipid mediators has revealed that these mediators are produced at specific points during the processes of inflammation and resolution. Some lipid mediators promote inflammation, whereas others are made at later stages in the process and promote a return to cellular homeostasis in the resolution phase. When transition to the resolving phase from an inflammatory response to an acute injury does not occur or a state of chronic inflammation manifests, the system is overwhelmed, and negative physiologic consequences occur. Such is the case in asthma, a disease mediated by chronic airway inflammation leading to bronchoconstriction and, potentially, airway remodeling.
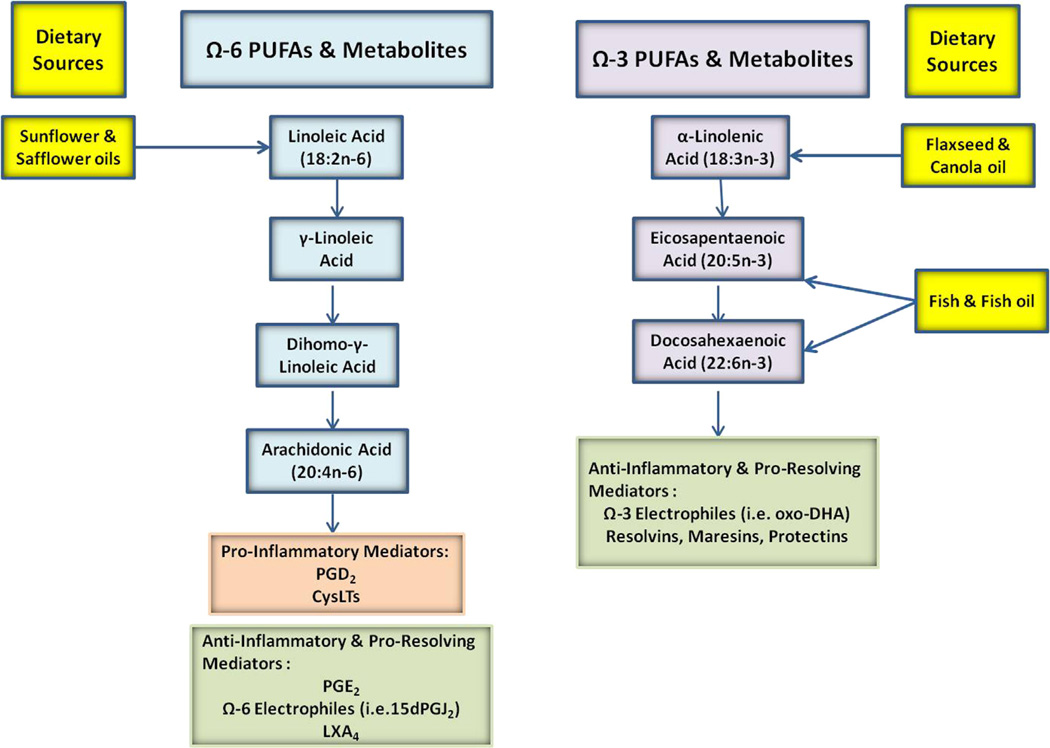
Most of the lipid mediators that regulate inflammation are metabolites derived from omega-6 (n-6) or omega-3 (n-3) fatty acids, including arachidonic acid (AA; 20:4n-6), linoleic acid (LA; 18:2n-6), eicosapentaenoic acid (EPA; 20:5n-3), and docosahexaenoic acid (DHA; 22:6n-3; Fig 1). Through enzymatic oxidation by COX, lipoxygenase (LO), cytochrome P450 (CYP) enzymes, or reactive oxygen species, oxygenated metabolites are formed, many of which possess biological actions. n-6 fatty acids are generally described as proinflammatory, and n-3 fatty acids are generally described as anti-inflammatory. In general, this is true; however, it has been realized that although a fatty acid mediator might be proinflammatory in one disease or tissue, it can be anti-inflammatory in another, as is the case for the AA-derived prostaglandin (PG) E2.
FIG 1.
Lipid mediators derived from omega-6 (Ω-6) and omega-3 (Ω-3) fatty acids.
The main AA-derived mediators of inflammation in asthma are PGs and cysteinyl leukotrienes (CysLTs).1,2 There are many other eicosanoids that have been implicated; however, their roles remain somewhat controversial compared with PGs and leukotrienes (LTs). Therefore this review will focus only on PGs and LTs as inflammatory mediators. Proresolving fatty acids are formed in response to an inflammatory event and accelerate a return to cellular homeostasis. Most of these are n-3–derived metabolites and include resolvins, protectins, and maresins. The one exception is the lipoxin family, which is derived from AA.3,4 The formation of these proresolving fatty acids requires the enzymatic activity of 5- and 15-LO, typically from 2 different cell types.4 A third category of lipid mediators are the anti-inflammatory electrophilic fatty acids. This group is derived from both n-6 and n-3 fatty acids and include metabolites that contain an α, β-unsaturated carbonyl, epoxide, or the addition of a nitro group on an alkene.5 A plethora of recent studies have shown that they have pleiotropic signaling actions that mediate inflammation by upregulating anti-inflammatory pathways and downregulating proinflammatory signaling. Lastly, the implications of fatty acid dietary intake on asthma will be discussed.
MEDIATORS OF INFLAMMATION
PGs and CysLTs
PGs and CysLTs are metabolites of AA.AA is cleaved from the sn-2 position of phospholipids by phospholipase A2. In the case of PGs, AA can be a substrate for either of the COX isoforms COX-1, which is constitutively expressed, or COX-2, which is upregulated in inflammation and primarily responsible for PG formation in asthmatic patients. AA is converted to PGG2 in one active site and reduced by the endoperoxide active site to PGH2. Specific synthase enzymes use PGH2 as a substrate, and the resulting products are thromboxane A2, PGI2, PGF2α, PGD2, and PGE2. PGE2 is the most abundant PG in the human body and a major metabolite in the lower respiratory tract.6,7 PGE2 has been labeled as proinflammatory because of its multiplicity of effects on the immune system, but in the respiratory system PGE2 is unique in that it has beneficial effects. Cell types that contribute to its production include airway epithelium and smooth muscle, fibroblasts, endothelial cells, and alveolar macrophages.8 PGE2 protects against bronchoconstriction, increases relaxation of airway smooth muscle, and has been shown to inhibit the release of mast cell mediators and the recruitment of inflammatory cells.6 Many, if not all, of these effects are mediated through one of 4 PGE2 prostanoid G protein–coupled receptors (GPCRs; ie, EP1-EP4).7
Although PGE2 has been exemplified as an anti-inflammatory PG, PGD2 has been shown to be proinflammatory, despite the fact that they are isomers in which the hydroxyl group and keto group are on opposite sides of the prostanoid ring. Active mast cells generate CysLTs and PGD2. PGD2 is mainly produced from mast cells that contain a hematopoietic PGD2 synthase, and it has been shown that there is a positive correlation of PGD2 concentration to asthma severity in bronchoalveolar lavage fluid.8 PGD2 acts through the thromboxane GPCR, the PGD2 receptor 1 (DP1), and the chemoattractant receptor–homologous molecule expressed on TH2 lymphocytes (CRTH2/DP2).8 The thromboxane GPCR promotes smooth muscle constriction that likely contributes to bronchoconstriction in asthmatic patients. CRTH2 activation on TH2 lymphocytes, eosinophils, and basophils results in enhanced chemotaxis and activation. CRTH2 receptor binding also induces cytokine production that might play a role in IgE activation by mast cells.8
CysLTs
CysLTs are also key mediators of asthma. LTs are derived from AA and synthesized through the 5-LO pathway in conjunction with 5-LO activating protein to catalyze the oxidation of AA to LTA4. The epoxide ring of LTA4 is opened by LTA4 hydrolase to form LTB4 or it is conjugated to glutathione by LTC4 synthase to form LTC4. LTC4 is transported out of the cell by multidrug resistance– associated protein 1. LTC4 is then subjected to extracellular metabolism to form LTD4 (loss of glutamine) and LTE4 (loss of glycine).1 These 3 LTs, LTC4, LTD4, and LTE4, comprise the CysLTs. Eosinophils and mast cells are primarily responsible for the synthesis of CysLTs in the context of asthma.1,2 Bronchoconstriction, the initiation of proinflammatory cytokine production, and airway remodeling have all been attributed to CysLTs. Additionally, CysLTs have been implicated in the trafficking and degranulation of eosinophils in the lungs, increased microvascular permeability leading to pulmonary edema, and increased mucus secretion.1,9 Unfortunately, only 50% of asthmatic patients show clinical responses to CysLT receptor agonists.9
PRORESOLVING MEDIATORS
A class of inflammation-resolving fatty acids derived from AA, EPA, and DHA exist that are dihydroxy or trihydroxy in nature. The AA-derived lipoxins and the EPA- and DHA-derived resolvins, protectins, and maresins are produced by dualenzyme reactions during acute inflammation and are proposed to mediate resolution.10 These mediators block neutrophil recruitment, promote infiltration and activation of monocytes, and induce phagocytosis and lymphatic clearance of apoptotic neutrophils by activated macrophages.10 These polyhydroxylated species require transcellular biosynthesis, the sequential actions of LOs from neighboring cells (ie, 5-LO/12-LO or 15-LO/5-LO), or they can be formed by a combination of COX-2 and cytochrome P450 or LO.10,11 The EPA-derived resolvin E1 was shown to dampen airway inflammation and airway hyperresponsiveness (AHR) in a mouse model of asthma.12 Mice administered resolvin E1 had lower eosinophil and lymphocyte recruitment, lower IL-13 and OVA-specific IgE levels, and a lower response to methacholine challenge compared with controls.12 A review by Uddin and Levy4 in 2011 summarizes the proresolving role of resolvins in pulmonary inflammation. In a study comparing patients with aspirin-intolerant asthma (AIA) with those with aspirin-tolerant asthma, Sanak et al13 reported a reduced generation of lipoxin A4 (LXA4) and aspirin-triggered LXA4 in patients with AIA.13 However, Celik et al14 found similar levels of LXA4 in both patients with AIA and those with aspirin-tolerant asthma. This difference might be attributed to a misclassification of asthmatic patients because LXA4 levels are consistently lower in patients with severe asthma, regardless of aspirin tolerance.7 The actions of these proresolving fatty acids are mediated by their binding to specific GPCRs, including CMKLR1, BLT1, ALX/FPR2, and GPR32.4 Additionally, these proresolving species have also been shown to trigger the expression of anti-inflammatory mediators, such as TGF-β and IL-10.4 Fat-1 mice15 express a Caenorhabiditis elegans desaturase that converts n-6 to n-3 fatty acids, thus increasing endogenous anti-inflammatory and proresolving n-3 metabolites. These mice additionally showed a decrease in levels of AA-derived eicosanoids, decreased allergic airway inflammation, and decreased response to methacholine challenge compared with those seen in control mice that were OVA challenged.15
ELECTROPHILIC FATTY ACIDS
Polyunsaturated fatty acid (PUFA) oxidation to an α, β-unsaturated ketone or epoxide and the addition of nitrogen dioxide (•NO2) to an alkene result in the formation of electrophilic fatty acid species. Many of these electrophilic fatty acids, such as 17-oxo-DHA, 15deoxyΔ12,14-PGJ2, and nitro-oleic acid (NO2-OA), have been structurally characterized and described as downstream metabolites of n-3 and n-6 PUFAs, but not all have been defined with regard to their biological function, despite their abundance.5,16
Electrophilic fatty acids function through the posttranslational modification of proteins and transcription factors. Multiple classes of electrophilic signaling molecules are expected to have unique patterns of downstream signaling. Electrophilic fatty acids will adduct susceptible, nucleophilic amino acid residues, such as cysteine and histidine. This adduction induces alterations in protein structure, function, and subcellular distribution.17 The targets for electrophilic fatty acid modification are thus diverse, yielding pleiotropic and sometimes reversible effects on an array of key signaling pathways. The oxo-DHA and oxo-EPA species inhibit proinflammatory cytokine and •NO production and activate Nrf2-dependent gene expression.16 Although dependent on concentration, target specificity, cell type, metabolism, and reversibility,18,19 many of these effects provide beneficial outcomes in the context of inflammation and play a potential role in asthma.20 Aside from Nrf2, other key target proteins for electrophilic fatty acids include the peroxisome proliferator activator receptor γ (PPARγ) and nuclear factor κB.5 Importantly, the endogenous production of these molecules is often a result of pro-oxidative and stress conditions, thus providing a rheostat mechanism for resolving the inflammatory environment.
A recent article by Reddy et al21 demonstrated that 10-NO2-OA decreased airway inflammation and AHR after methacholine challenge to the same extent as fluticasone in a mouse model using ovalbumin as the challenge. In addition, 10-NO2-OA, but not fluticasone, stimulates neutrophil apoptosis and phagocytosis. This was attributed to an increase in PPARγ activity.21 In vitro studies showed that 10-NO2-OA significantly upregulated CD36 expression by alveolar macrophages. This was in contrast to fluticasone treatment, which abolished CD36 expression.21
DIET AND ASTHMA
It has been hypothesized that variations in asthma prevalence across populations and the increase in asthma burden seen in westernized societies over past decades might be related to a combination of a progressively higher intake of n-6 fatty acids, such as LA, which is found in margarine and vegetable oils, and a lower intake of n-3, which is found in marine oils.22 This concept is largely supported by epidemiologic studies showing that populations with higher n-6 fatty acid consumption have greater asthma prevalence in contrast to those consuming average higher n-3 fatty acid diets, such as the Eskimos.23 Given that n-3 fatty acids produces eicosanoids that are less proinflammatory (PGE3 and LTB5 series) than those derived from n-6 fatty acids (PGD2 and LTB4 series) and because downstream metabolites of n-3 fatty acids have the potential to resolve inflammation, the hypothesis that n-3 fatty acid intake could improve asthma by reducing inflammation seems biologically plausible. However, the lack of consistency across observational studies and clinical trials (Table I)24–39 certainly raises the possibility that n-3 fatty acids might not be as universally effective as originally considered. Therefore it is possible that these compounds are only effective in select asthma phenotypes.
TABLE I.
Observational studies on fatty acid exposure and asthma-related outcomes
| Study design | Study population | Outcomes | Results |
|---|---|---|---|
| Case-control study, United Kingdom24 | 89 cases of asthma vs 89 community matched control subjects from local registries (mean age 43 y) | Fatty acid intake was determined by using FFQ, and erythrocyte membrane levels were determined by using mass spectrometry and odds of asthma. | n-3 fatty acids are not protective against asthma, and n-6 fatty acids are associated with lower risk of asthma. |
| Cross-sectional study, The Netherlands25 | 13,820 subjects (age 20–59 y) | Fatty acid intake was determined by using FFQ. Lung function was determined by using spirometry (FEV1 and FVC). A respiratory symptom questionnaire was used regarding reported wheeze, asthma, and COPD symptoms. | n-3 intake is not protective against COPD or asthma. High n-6 intake is associated with FEV1 decrease, notably in smokers. |
| Case-control study, Germany26 | 38 asthmatic patients with grass pollen allergy vs 19 age-matched healthy control subjects (age 18–45 y) | Lung function was measured by using spirometry, and bronchial hyperresponsiveness was measured using methacholine testing, allergen inhalation challenge, and measurement of exhaled NO. Stratification was according to low (Q25) and high (Q75) ratios of n-3 to n-6. | PUFA ratios (n-3/n-6) were less in asthmatic patients, and higher exhaled NO levels were present in Q25 asthmatic patients (or lower n-3/n-6 ratio group) compared with Q75 patients after bronchial inflammation. There was a trend toward higher bronchial hyperreactivity in Q25, as indicated by greater change in FEV1. |
| Cross-sectional study, Germany27 | 593 adults (age 20–64 y) sampled from a respiratory health survey | Fatty acid intake and metabolism were measured by using 3-d dietary survey and serum phospholipid extraction and evaluation. Lung function was measured by using FEV1 and FVC, and bronchial hyperresponsiveness was measured by using methacholine challenge. | n-3 (DHA) was associated with improved lung function in men but not women. n-6 (dihomo-γ-linolenic acid) and monosaturated fatty acid (palmitoleic acid) were negatively associated with lung function in men but not women. |
| Cross-sectional study, Greece28 | 1964 preschool children (age 24–72 mo) from nurseries and day care centers | Three-day dietary was intake measured by using a home parental dietary intake record, and asthma outcomes were measured by using the ISAAC questionnaire. | Monosaturated fatty acid intake was associated with increased risk of asthma symptoms. Magnesium intake was associated with increased wheeze, whereas vitamin C and calcium appeared to be protective. |
| Cross-sectional study, Japan29 | 452 children (age 3–6 y), including asthmatic and nonasthmatic patients | Three-day dietary intake was measured by using a home-based parental dietary intake record. Asthma case status was ascertained by means of questionnaire. | There was no association between asthma diagnosis and any type of fatty acid intake, but there was a significant association with low intake of vitamins C and E. |
| Cross-sectional study, Finland30 | 2679 children from 3 university hospitals from the Finnish Type 1 Diabetes Prediction and Prevention (DIPP) nutrition study | Dietary intake was measured during the 8th month of pregnancy by using FFQ. Asthma risk was assessed at 5 y, as determined by using the ISAAC questionnaire. | Low intake of α-linolenic acid and total PUFAs during pregnancy was associated with increased risk of asthma in offspring, whereas low intake of AA and high intake of saturated fatty acids (palmitic acid) was associated with decreased risk of asthma in offspring at 5 y. |
| Cross-sectional study, Portugal31 | 174 asthmatic patients (adults age >16 y), outpatient clinics | Dietary intake over previous 12 mo was measured by using FFQ and asthma control based on a combination of lung function (FEV1), exhaled NO levels, Asthma Control Questionnaire (ACQ) scores, and asthma quality of life (ALQ) test results. | High n-6/n-3 ratio predicted higher exhaled NO levels, whereas high n-3, α-linolenic acid, and saturated fatty acid levels were associated with lower exhaled NO levels. Higher intake of n-3 was associated with better controlled asthma. There was no association between antioxidant vitamins and minerals and controlled asthma. |
| Cross-sectional study, Germany32 | 2000 children (age 10 y) from 2 prospective birth cohort studies | Fatty acid intake was measured by using FFQ, and atopic disease was measured by using specific serum IgE concentrations with stratification by FADS1 FADS2 genotype. | There was no associations between those with atopic disease or allergic sensitization and FADS genotype or fatty acid intake. Higher margarine intake was associated with risk of asthma in homozygotes for the major allele. |
| Cross-sectional study, Spain33 | 638 Spanish schoolchildren (age 8–13 y) | Prevalence of current asthma was measured by using a parental questionnaire. Intake of lipids, fatty acids, and lipid-rich foods in a 3-d period was measured by using parental dietary record. | Energy from lipids, saturated fatty acids, and myristic and palmitic acids was significantly associated with current asthma, as well as butter intake. There was no association between asthma and intake of any other fatty acids, n-6/n-3 ratio, or consumption of margarine, milk products, fish, meat, eggs, or vegetable oils. |
| Cross-sectional study, Japan34 | 25,033 children (age 6–15 y) using data from the Ryukyus Child Health Study (RYUCHS) | Symptoms of asthma were defined based on diagnostic criteria from ISAAC, and dietary intake was measured by using food questionnaires for children over a 1-mo period. | Intake of n-3 and n-6 fatty acids and linolenic acid was significantly associated with increased prevalence of wheeze. There was no association between consumption of α-LA, EPA, DHA, AA, or n-3/n-6 ratio with the prevalence of wheeze. Total fat, saturated fatty acids, and monounsaturated fatty acids were not related to wheeze. |
| Cross-sectional study, Australia35 | 1,601 adults with and without known asthma (age 20–44 y) | Dietary intake was measured by using FFQ. Asthma and atopy were measured based on respiratory questionnaire results, skin prick test results, lung function (FEV1), and methacholine challenge results for bronchial hyperresponsiveness and plasma fatty acid levels. | There was no association between any plasma fatty acid levels and atopy or asthma, except dihomo cLA, an n-6 fatty acid, which was positively associated with asthma diagnosis. |
| Nested case-control study, Germany36 | 526 children (age 8–11 y) from Munich from ISAAC phase II | Plasma fatty acid levels were measured. Asthma Symptoms or diagnosis were determined based on respiratory questionnaires completed by parents. Atopy was measured by using skin prick tests, and bronchial hyperresponsiveness was measured after hypertonic saline challenge. | EPA levels were not associated with asthma or lung function, whereas linolenic levels were associated (both with n-3 fatty acids). There was a strong association between AA and asthma (n-6 fatty acid) with FEV1 decreases. LA was negatively associated with current asthma and an incremental change in FEV1. The n-6/n-3 ratio, palmitic acid, and OA had no association with asthma or lung function. |
| Longitudinal analysis37 | 20-y follow-up in a cohort of 4,162 Americans (age 18–30 y) without a history of asthma | Diet was assessed by using FFQ at 3 time points in a 20-y period. Incidents of self-reported asthma were determined based on a physician’s diagnosis and/or use of asthma medications. | Four hundred forty-six cases of incident asthma were identified in a 20-y follow-up, and n-3 fatty acid intake was inversely associated with incident asthma. |
| Cross-sectional study, United States and Canada38 | 2,112 twelfth-grade students | Lung function was determined by using a respiratory questionnaire. Dietary intake of antioxidants, micronutrients, retinol, and fatty acids were measured. | Low dietary fruit intake and n-3 fatty acid intake were associated with increased odds of respiratory symptoms (chronic bronchitis, wheeze, and asthma). Lower vitamin C intake was seen in smokers, and they had higher ORs for respiratory symptoms. |
| Cross-sectional study, Finland39 | 2441 children (age 5 y) participating in a diabetes mellitus type 1 study cohort | Dietary intake of fats in the 8th month of pregnancy was determined by using FFQ. A parental record of allergic disease was determined by using the ISACC questionnaire in offspring at 5 y. | Higher intake of total PUFAs and a-linolenic fatty acid was weakly associated with a nonsignificantly lower risk of allergic rhinitis |
COPD, Chronic obstructive pulmonary disease; EIB, exercise-induced bronchospasm; FADS, fatty acid desaturase; FFQ, food frequency questionnaire; FVC, forced vital capacity; ISAAC, International Study of Asthma and Allergies in Childhood; OR, odds ratio.
Observational studies have not consistently shown in children or adults that increased n-3 fatty acid intake is associated with improved FEV1, respiratory symptoms, or asthma control. Similarly, some studies have shown that lower n-3 fatty acid levels or decreased n-3/n-6 ratios are protective for asthma. Paradoxically, increased n-3 intake was related to higher wheeze prevalence (Table I). In addition to the residual confounders from many potential biases associated with n-3 fatty acid intake, the lack of consistency in results across observational studies might relate to many ways that fatty acid consumption is estimated; although some studies use food frequency questionnaires, others rely on plasma or cell membrane levels (Table I).
Several randomized clinical trials have been conducted to date for the treatment of asthma (Table II).40–50 As with observational studies, the use of diverse clinical end points for study outcomes, in addition to different combinations of n-3 fatty acid concentration and duration, makes it difficult to appreciate whether this intervention is useful. However, pooled estimates derived from a Cochrane meta-analysis of 9 randomized clinical trials did not show improvements in clinical outcomes, including bronchial hyperresponsiveness.51 Therefore the study authors found insufficient evidence to recommend the use of n-3 fatty acid products to improve the health of asthmatic patients. Considering that asthma is a complex syndrome comprised of diseases with different clinical phenotypes, select asthmatic patients could benefit more from taking n-3 fatty acids or, alternatively, be harmed more by being exposed to higher concentrations of n-6 fatty acids. Among atopic asthmatic patients, n-3 fatty acid supplementation has been shown to reduce exhaled nitric oxide levels and sputum eosinophil counts in asthmatic patients pre-exposed to sensitized allergens; however, these results have not been widely replicated.40 Two well-conducted randomized studies have shown that n-3 fatty acid supplementation significantly reduces the exercise-induced FEV1 decrease and reduces use of short-acting β-agonists,40–42,52 although a similar intervention was not useful among patients with mild-to-moderate asthma exercising with less intensity under ambient conditions.53
TABLE II.
Clinical trials of fatty acid supplementation and asthma
| Study design | Study population | Treatment | Outcomes | Results |
|---|---|---|---|---|
| Randomized, double-blind, placebo-controlled trial, Portugal44 | 20 female patients with stable persistent asthma | DHA and EPA combination plus 10 mg of vitamin E or placebo twice daily for 2 wk | FENO was determined, with ACQ scores and FEV1 as secondary outcomes. | Short-term dietary supplementation with n-3 PUFAs was not associated with changes in exhaled NO levels, asthma control, or lung function in women with stable asthma. |
| Observational cohort analysis of randomized controlled trials, Australia43 | 516 children (age 18 mo-5 y) with a family history of asthma | Fish oil supplement (500 mg of tuna fish oil) or placebo (500 mg of Sunola oil) | Symptoms or diagnoses of asthma and allergic disease were determined at 18 mo, 3y,and 5y. | There was no association between plasma levels of n-3 or n-6 at 18 mo, 3 y, and 5 y and the prevalence of asthma or wheezing, eczema, or atopy at 5 y. |
| Double-blind, randomized controlled trial, Australia45 | 39 asthmatic children (age 8–12 y) with a history of wheeze in the last 12 mo and AHR to histamine | Fish oil (n-3 group, n = 20) or safflower/palm/olive oil (n-6 group, n = 19) supplements for 6 mo | Lung function and bronchial hyperresponsiveness were determined by using FEV1, FVC, and histamine inhalation. LPS stimulated PBMC TNF-α production. | TNF level decrease in the n-3 group trended toward significance. Diet had no effect on the severity of asthma in children or other outcome measures. |
| Parallel double-blind, randomized trial, Germany40 | 23 asthmatic patients with dust mite allergy (age 22–29 y) | PUFA-enriched blend (450 mg of EPA, 180 mg of, 60 mg of γ-LA, and 60 mg of stearidonic acid) or placebo | Three weeks of dietary supplementation and then daily challenge with low dose mite allergen was done for 2 wk after which subjects. Lung function was measured based on FEV1 and exhaled NO levels. Sputum and plasma eosinophil counts and CysLT levels were measured after allergen exposure. | Exhaled NO levels were lower in the n-3 PUFA group before and after allergen challenge compared with the placebo group. Serum eosinophil counts, eosinophilic cationic protein levels, and in vitro CysLT release are decreased in the n-3 PUFA group. |
| Randomized controlled trial, Australia46 | 616 pregnant women whose unborn children were at high risk of asthma based on family history | HDM avoidance intervention; dietary supplementation with daily n-3–rich tuna fish oil (500 mg), margarines, and cooking oils or placebo (Sunola oil) starting at 6 mo | Asthma symptoms or diagnosis or treatment were assessed by using a parental questionnaire, atopic status was determined by using skin prick testing, and serum IgE levels in offspring were measured at 18 mo. | There was a lower prevalence of wheeze in the diet intervention group, but physician-diagnosed asthma was not reduced. HDM reduction reduced use of oral steroids but did not affect outcomes. |
| Randomized, double-blind, placebo-controlled trial, Denver, Colorado47 | 43 children (age 6–14 y) with mild-to-moderate persistent asthma | Enriched n-3 fatty acid and antioxidant (3 g of EPA, 1.6 g of DHA, 3.0 g of GLA; vitamins E and C, β-carotene, taurine, zinc, copper, selenium, molybdenum, and calcium) formula vs control (same caloric amount blended with 100% high-oleic safflower oil) formula for 12-wk trial as between meal snack or at mealtime | Numbers of asthma-free days were determined, and assessment of fatty acid levels and safety outcomes was performed. Measurement of asthmatic symptoms, skin prick test responses, serum eosinophilic cationic protein levels, eosinophil counts, serum IgE levels, exhaled NO levels, and methacholine challenge test results were determined. | There were no differences in asthma-free days between the 2 groups. Higher exhaled NO levels were seen in the control group compared with the treatment group at 4, 8, and 12 wk. Higher EPA levels were found in serum and PBMC phospholipids in the treatment group. No differences were found in adverse events. |
| Randomized, double-blind crossover study, United Kingdom41 | 20 subjects, including 10 with clinically diagnosed EIB and 10 healthy control subjects recruited from university and sporting teams | Supplemental fish oil (3.2 g of EPA and 2.2 g of DHA) or placebo (olive oil) for 3 wk, washout period (normal diet) for 2 wk, and then alternative supplement for 3 wk | At all 3 phases, plasma LTB4, TNF-α, IL-1β, and urinary LTE4 and 9α levels, as well as 11β-PGF2 levels, were measured before and after exercise. Lung function was measured by using FEV1 after exercise and sputum inflammatory mediators. | There was less of a decrement in lung function after exercise and decreased inflammatory mediator levels in the n-3 PUFA diet group. |
| Double-blind crossover study, Sweden48 | 25 patients with mild-to-moderate asthma (20–54 y) | 3 wk n-3 fatty acid supplement (daily dose: 4 g of EPA and 2 g of DHA) or placebo (50:50 mix of soybean and corn oil), followed by washout period (normal diet) for 3 wk and then switch to alternative therapy for 3 wk | Fatty acid composition of serum phospholipids at baseline and after each period of treatment was measured. | n-3 diet supplementation alters PUFA ratio and the ratio of downstream oxylipins. |
| Randomized, double-blind, crossover study, United Kingdom42 | 16 patients with mild-to-moderate persistent asthma with EIB | n-3 PUFAs (3.2 g of EPA and 2.0 g of DHA) or placebo (olive oil) over 8 wk (1 wk on normal diet, 3 wk on treatment diet or placebo, 2-wk washout period, and then switched to alternative diet for 2 wk) | Pre-exercise and postexercise measurements of pulmonary function, induced sputum cell count differential, proinflammatory eicosanoid metabolite and cytokine concentrations, and eicosanoid metabolites LTB4 and LTB5 were measured from activated PMNLs. | Fish oil diet improved pulmonary function and reduced sputum proinflammatory mediators compared with placebo and normal diets. |
| Randomized, double-blind, placebo-controlled trial, Russia49 | 46 patients with mild-to-moderate atopic asthma (age 18–56 y) | Four capsules of lipid extract (50 mg of n-3 PUFAs, including DHA and EPA, and 100 mg of olive oil) or placebo (150 mg of olive oil) for 8 wk | Patient-recorded symptoms and medication use before study medication doses were determined. FEV1 was measured by using spirometry and concentration of exhaled H2O2. | Decreased daytime wheeze and exhaled H2O2 levels and increased morning PEF were seen in the lipid extract group. |
| Cross-sectional study, Denmark50 | 528 children (age 16 y) identified in registries from previous study in 1990 whose mothers had fish oil supplementation or placebo during the last 6 wk of pregnancy | Fish oil supplementation with four 1-g gelatin capsules (32% EPA, 23% DHA, and 2 mg of tocopherol) or four 1-g capsules of olive oil (control 1) or no supplement (control 2) from week 30 of pregnancy until delivery | Asthma diagnosis was determined in offspring at 16 y of age. Follow-up was extracted from the national patient registry. | Supplementation with n-3 PUFAs during late pregnancy reduced the risk of asthma and allergic asthma in offspring. |
ACQ, Asthma Control Questionnaire; CysLT, cysteinyl leukotriene; EIB, exercise-induced bronchospasm; FENO, fraction of exhaled nitric oxide; FFQ, food frequency questionnaire; FVC, forced vital capacity; HDM, house dust mite; NO, nitric oxide; PEF, peak expiratory flow; PMNL, peripheral mononuclear lymphocytes.
There have been a limited number of projects evaluating whether n-3 fatty acid exposure in utero could reduce the incidence of atopy and asthma in offspring. Although the majority show some degree of protection, this has not been a consistent result across all studies. Protection is thought to occur through a mechanism involving n-3 fatty acid–mediated reductions in PGE2 levels, which can subsequently decrease TH2-related cytokine and IgE levels.43,54,55
Although fish oil supplementation does not result in improved asthma control, it might be that management of overall caloric intake of saturated fats is more important. One key feature of the western diet is a chronic metabolic surplus that is low in antioxidants but high in saturated fats, leading to storage of these surplus fats and eventually obesity. It is not disputed that obesity and asthma are linked; however, this relationship has not been well defined. Obesity can be differentiated by its low-grade chronic inflammatory state and already altered immune system because of an increase in proinflammatory adipokine and leptin/adiponectin levels.56 This proinflammatory state found in obesity might be a critical player in the role of lipid mediators in asthmatic patients. A very telling study conducted by de Vries and Howie57 showed that mice fed a diet high in saturated fat and allergen challenged before having an obese phenotype (between 6–8 weeks) displayed decreased proinflammatory cytokine production by lung draining lymph node cells and decreased eotaxin and lung eosinophilia compared with control mice on an 11% fat diet. Compared with a low-fat diet, asthmatic patients randomized to a high-fat diet showed acute increased airway neutrophilia and Toll-like receptor 4 mRNA expression and reductions in FEV1 and forced vital capacity. These studies strongly suggest that a higher fat intake can lead to airway inflammatory and functional changes, which could worsen asthma symptoms.
CONJUGATED LINOLEIC ACID: THE BENEFICIAL n-6 FATTY ACID
Conjugated linoleic acid (cLA) has been touted for its favorable health implications, including its anticancer and anti-inflammatory properties and its association with reduced risk of cardiovascular events.58–60 This n-6 fatty acid is synthesized in vivo by rumen bacteria from LA and might also be created during the heat processing of animal-derived foods, including milk and meat. Meat contributes approximately 25% to 30% of cLA in a Western diet, with the remainder from dairy. Although found in meat and dairy products, nonruminants and human subjects produce cLA from the trans-isomer of OA (vaccenic acid). This cLA formation is mediated by the entero-salivary microbiome through bacterial Δ9-desaturase activity.60 Over the years, normal dietary consumption of cLA has decreased in the western diet because of changes in our consumption, some of which reflect a heart-healthy diet.
cLA was first recognized for its metabolic effects on obesity, body composition, and insulin insensitivity.26 The predominant isomer of cLA in milk and meat is cis-9, trans-11-LA. This is in contrast to the commercial preparation, which is typically derived from sunflower oil, in which proportions of the 2 main isomers (cis-9, trans-11 and trans-10, cis-12) are equal.26 Although specific effects have been attributed to individual isomers, many animal and human studies have used a combination of the 2 main isomers. In a study by MacRedmond et al,61 investigators asked whether cLA would be efficacious as a dietary supplement for overweight patients with mild asthma. In this randomized, double-blind, placebo-controlled study subjects were given 4.5 g/d cLA or placebo for 12 weeks. The average body mass index of the group was 27.9 kg/m2. Measurements at week 12 compared with initial testing showed that the group receiving cLA had significant improvements in AHR, as measured based on the results of methacholine challenge (PC20), and a significant decrease in body mass index with an associated reduction in the leptin/adiponectin ratio. Subjects also reported an increased tolerance for severe exercise. However, there were no differences in FEV1, systemic cytokine levels, or induced sputum cell counts. Much like n-3 PUFAs compete with n-6 PUFAs as substrates for enzymatic oxidation, cLA might compete with LA as a substrate for desaturases and elongases, resulting in an overall reduction in AA formation. cLA might also reduce eicosanoid formation through the transcriptional regulation of COX and LO and could mediate inflammation through PPARγ.26 All of these mechanisms and effects on body composition would benefit asthmatic patients.62
CONCLUSIONS
Fatty acids play an essential role in the development and resolution of inflammatory pathways relevant to the pathophysiology of asthma. Although dietary interventions have been largely disappointing, there is ongoing interest to determine whether specific endogenous fatty acids can be used as therapeutic agents to resolve airway inflammation in asthmatic patients and, perhaps more importantly, to determine which type of asthmatic phenotype would gain the greatest benefit.
Abbreviations used
- AA
Arachidonic acid
- AHR
Airway hyperresponsiveness
- AIA
Aspirin-intolerant asthma
- cLA
Conjugated linoleic acid
- CRTH2/DP2
Chemoattractant receptor–homologous molecule expressed on TH2 lymphocytes
- CysLT
Cysteinyl leukotriene
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- GPCR
G protein–coupled receptor
- LA
Linoleic acid
- LO
Lipoxygenase
- LT
Leukotriene
- LXA4
Lipoxin A4
- n-3
Omega-3
- n-6
Omega-6
- OA
Oleic acid
- PG
Prostaglandin
- PPARγ
Peroxisome proliferator activator receptor γ
- PUFA
Polyunsaturated fatty acid
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel SE. Arachidonic acid metabolites: mediators of inflammation in asthma. Pharmacotherapy. 1997;17(Suppl):3S–12S. [PubMed] [Google Scholar]
- 3.Levy BD. Resolvin D1 and resolvin E1 promote the resolution of allergic airway inflammation via shared and distinct molecular counter-regulatory pathways. Front Immunol. 2012;3:390. doi: 10.3389/fimmu.2012.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uddin M, Levy BD. Resolvins: natural agonists for resolution of pulmonary inflammation. Prog Lipid Res. 2011;50:75–88. doi: 10.1016/j.plipres.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schopfer F, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sastre B, del Pozo V. Role of PGE2 in asthma and nonasthmatic eosinophilic bronchitis. Mediators Inflamm. 2012;2012:645383. doi: 10.1155/2012/645383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velazquez JR, Teran LM. Aspirin-intolerant asthma: a comprehensive review of biomarkers and pathophysiology. Clin Rev Allergy Immunol. 2013;45:75–86. doi: 10.1007/s12016-012-8340-0. [DOI] [PubMed] [Google Scholar]
- 8.Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D(2) pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131:1504–1512. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Misso NL, Thompson PJ. Prostaglandins and leukotrienes: mediators of inflammation and asthma. Enfield (NH): Science Publishers; 2012. [Google Scholar]
- 10.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, et al. Novel proresolving aspirin-triggered DHA pathway. Chem Biol. 2011;18:976–987. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, et al. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Sanak M, Levy BD, Clish CB, Chiang N, Gronert K, Mastalerz L, et al. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. Eur Respir J. 2000;16:44–49. doi: 10.1034/j.1399-3003.2000.16a08.x. [DOI] [PubMed] [Google Scholar]
- 14.Celik GE, Erkekol FO, Misirligil Z, Melli M. Lipoxin A4 levels in asthma: relation with disease severity and aspirin sensitivity. Clin Exp Allergy. 2007;37:1494–1501. doi: 10.1111/j.1365-2222.2007.02806.x. [DOI] [PubMed] [Google Scholar]
- 15.Bilal S, Haworth O, Wu L, Weylandt KH, Levy BD, Kang JX. Fat-1 transgenic mice with elevated omega-3 fatty acids are protected from allergic airway responses. Biochim Biophys Acta. 2011;1812:1164–1169. doi: 10.1016/j.bbadis.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batthyany C, Schopfer FJ, Baker PR, Duran R, Baker LM, Huang Y, et al. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J Biol Chem. 2006;281:20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin D, Saleh S, Liebler DC. Reversibility of covalent electrophile-protein adducts and chemical toxicity. Chem Res Toxicol. 2008;21:2361–2369. doi: 10.1021/tx800248x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudolph TK, Freeman BA. Transduction of redox signaling by electrophile-protein reactions. Sci Signal. 2009;2:re7. doi: 10.1126/scisignal.290re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudolph V, Schopfer FJ, Khoo NK, Rudolph TK, Cole MP, Woodcock SR, et al. Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction. J Biol Chem. 2009;284:1461–1473. doi: 10.1074/jbc.M802298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy AT, Lakshmi SP, Dornadula S, Pinni S, Rampa DR, Reddy RC. The nitrated Fatty Acid 10-nitro-oleate attenuates allergic airway disease. J Immunol. 2013;191:2053–2063. doi: 10.4049/jimmunol.1300730. [DOI] [PubMed] [Google Scholar]
- 22.Black PN, Sharpe S. Dietary fat and asthma: is there a connection? Eur Respir J. 1997;10:6–12. doi: 10.1183/09031936.97.10010006. [DOI] [PubMed] [Google Scholar]
- 23.Horrobin DF. Low prevalences of coronary heart disease (CHD), psoriasis, asthma and rheumatoid arthritis in Eskimos: are they caused by high dietary intake of EPA, a genetic variation of essential fatty acid (EFA) metabolism or a combination of both? Med Hypotheses. 1987;22:421–428. doi: 10.1016/0306-9877(87)90037-5. [DOI] [PubMed] [Google Scholar]
- 24.Broadfield EC, McKeever TM, Whitehurst A, Lewis SA, Lawson N, Britton J, et al. A case-control study of dietary and erythrocyte membrane fatty acids in asthma. Clin Exp Allergy. 2004;34:1232–1236. doi: 10.1111/j.1365-2222.2004.02032.x. [DOI] [PubMed] [Google Scholar]
- 25.McKeever TM, Lewis SA, Cassano PA, Ocke M, Burney P, Britton J, et al. The relation between dietary intake of individual fatty acids, FEV1 and respiratory disease in Dutch adults. Thorax. 2008;63:208–214. doi: 10.1136/thx.2007.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitz R, Rose MA, Schubert R, Beermann C, Kaufmann A, Bohles HJ, et al. Omega-3 polyunsaturated fatty acids and bronchial inflammation in grass pollen allergy after allergen challenge. Respir Med. 2010;104:1793–1798. doi: 10.1016/j.rmed.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Kompauer I, Demmelmair H, Koletzko B, Bolte G, Linseisen J, Heinrich J. Association of fatty acids in serum phospholipids with lung function and bronchial hyperresponsiveness in adults. Eur J Epidemiol. 2008;23:175–190. doi: 10.1007/s10654-007-9218-y. [DOI] [PubMed] [Google Scholar]
- 28.Emmanouil E, Manios Y, Grammatikaki E, Kondaki K, Oikonomou E, Papadopoulos N, et al. Association of nutrient intake and wheeze or asthma in a Greek pre-school population. Pediatr Allergy Immunol. 2010;21:90–95. doi: 10.1111/j.1399-3038.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K, Wada K, Sahashi Y, Tamai Y, Tsuji M, Watanabe K, et al. Associations of intake of antioxidant vitamins and fatty acids with asthma in pre-school children. Public Health Nutr. 2013;16:2040–2045. doi: 10.1017/S1368980012004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lumia M, Luukkainen P, Tapanainen H, Kaila M, Erkkola M, Uusitalo L, et al. Dietary fatty acid composition during pregnancy and the risk of asthma in the offspring. Pediatr Allergy Immunol. 2011;22:827–835. doi: 10.1111/j.1399-3038.2011.01202.x. [DOI] [PubMed] [Google Scholar]
- 31.Barros R, Moreira A, Fonseca J, Delgado L, Castel-Branco MG, Haahtela T, et al. Dietary intake of alpha-linolenic acid and low ratio of n-6:n-3 PUFA are associated with decreased exhaled NO and improved asthma control. Br J Nutr. 2011;106:441–450. doi: 10.1017/S0007114511000328. [DOI] [PubMed] [Google Scholar]
- 32.Standl M, Sausenthaler S, Lattka E, Koletzko S, Bauer CP, Wichmann HE, et al. FADS gene variants modulate the effect of dietary fatty acid intake on allergic diseases in children. Clin Exp Allergy. 2011;41:1757–1766. doi: 10.1111/j.1365-2222.2011.03833.x. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Rodriguez E, Perea JM, Jimenez AI, Rodriguez-Rodriguez P, Lopez-Sobaler AM, Ortega RM. Fat intake and asthma in Spanish school children. Eur J Clin Nutr. 2010;64:1065–1071. doi: 10.1038/ejcn.2010.127. [DOI] [PubMed] [Google Scholar]
- 34.Miyake Y, Sasaki S, Arakawa M, Tanaka K, Murakami K, Ohya Y. Fatty acid intake and asthma symptoms in Japanese children: the Ryukyus Child Health Study. Clin Exp Allergy. 2008;38:1644–1650. doi: 10.1111/j.1365-2222.2008.03074.x. [DOI] [PubMed] [Google Scholar]
- 35.Woods RK, Raven JM, Walters EH, Abramson MJ, Thien FC. Fatty acid levels and risk of asthma in young adults. Thorax. 2004;59:105–110. doi: 10.1136/thorax.2003.009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolte G, Kompauer I, Fobker M, Cullen P, Keil U, Mutius E, et al. Fatty acids in serum cholesteryl esters in relation to asthma and lung function in children. Clin Exp Allergy. 2006;36:293–302. doi: 10.1111/j.1365-2222.2006.02441.x. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Xun P, Zamora D, Sood A, Liu K, Daviglus M, et al. Intakes of long-chain omega-3 (n-3) PUFAs and fish in relation to incidence of asthma among American young adults: the CARDIA study. Am J Clin Nutr. 2012;97:173–178. doi: 10.3945/ajcn.112.041145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burns JS, Dockery DW, Neas LM, Schwartz J, Coull BA, Raizenne M, et al. Low dietary nutrient intakes and respiratory health in adolescents. Chest. 2007;132:238–245. doi: 10.1378/chest.07-0038. [DOI] [PubMed] [Google Scholar]
- 39.Nwaru BI, Erkkola M, Lumia M, Kronberg-Kippila C, Ahonen S, Kaila M, et al. Maternal intake of fatty acids during pregnancy and allergies in the offspring. Br J Nutr. 2012;108:720–732. doi: 10.1017/S0007114511005940. [DOI] [PubMed] [Google Scholar]
- 40.Schubert R, Kitz R, Beermann C, Rose MA, Lieb A, Sommerer PC, et al. Effect of n-3 polyunsaturated fatty acids in asthma after low-dose allergen challenge. Int Arch Allergy Immunol. 2009;148:321–329. doi: 10.1159/000170386. [DOI] [PubMed] [Google Scholar]
- 41.Mickleborough TD, Murray RL, Ionescu AA, Lindley MR. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med. 2003;168:1181–1189. doi: 10.1164/rccm.200303-373OC. [DOI] [PubMed] [Google Scholar]
- 42.Mickleborough TD, Lindley MR, Ionescu AA, Fly AD. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129:39–49. doi: 10.1378/chest.129.1.39. [DOI] [PubMed] [Google Scholar]
- 43.Almqvist C, Garden F, Xuan W, Mihrshahi S, Leeder SR, Oddy W, et al. Omega-3 and omega-6 fatty acid exposure from early life does not affect atopy and asthma at age 5 years. J Allergy Clin Immunol. 2007;119:1438–1444. doi: 10.1016/j.jaci.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 44.Moreira A, Moreira P, Delgado L, Fonseca J, Teixeira V, Padrao P, et al. Pilot study of the effects of n-3 polyunsaturated fatty acids on exhaled nitric oxide in patients with stable asthma. J Investig Allergol Clin Immunol. 2007;17:309–313. [PubMed] [Google Scholar]
- 45.Hodge L, Salome CM, Hughes JM, Liu-Brennan D, Rimmer J, Allman M, et al. Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur Respir J. 1998;11:361–365. doi: 10.1183/09031936.98.11020361. [DOI] [PubMed] [Google Scholar]
- 46.Mihrshahi S, Peat JK, Marks GB, Mellis CM, Tovey ER, Webb K, et al. Eighteen-month outcomes of house dust mite avoidance and dietary fatty acid modification in the Childhood Asthma Prevention Study (CAPS) J Allergy Clin Immunol. 2003;111:162–168. doi: 10.1067/mai.2003.36. [DOI] [PubMed] [Google Scholar]
- 47.Covar R, Gleason M, Macomber B, Stewart L, Szefler P, Engelhardt K, et al. Impact of a novel nutritional formula on asthma control and biomarkers of allergic airway inflammation in children. Clin Exp Allergy. 2010;40:1163–1174. doi: 10.1111/j.1365-2222.2010.03523.x. [DOI] [PubMed] [Google Scholar]
- 48.Lundstrom SL, Yang J, Brannan JD, Haeggstrom JZ, Hammock BD, Nair P, et al. Lipid mediator serum profiles in asthmatics significantly shift following dietary supplementation with omega-3 fatty acids. Mol Nutr Food Res. 2013;57:1378–1389. doi: 10.1002/mnfr.201200827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emelyanov A, Fedoseev G, Krasnoschekova O, Abulimity A, Trendeleva T, Barnes PJ. Treatment of asthma with lipid extract of New Zealand green-lipped mussel: a randomised clinical trial. Eur Respir J. 2002;20:596–600. doi: 10.1183/09031936.02.02632001. [DOI] [PubMed] [Google Scholar]
- 50.Olsen SF, Osterdal ML, Salvig JD, Mortensen LM, Rytter D, Secher NJ, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167–175. doi: 10.1093/ajcn/88.1.167. [DOI] [PubMed] [Google Scholar]
- 51.Woods RK, Thien FC, Abramson MJ. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database Syst Rev. 2002;(3):CD001283. doi: 10.1002/14651858.CD001283. [DOI] [PubMed] [Google Scholar]
- 52.Mickleborough TD, Lindley MR, Montgomery GS. Effect of fish oil-derived omega-3 polyunsaturated fatty acid supplementation on exercise-induced bronchoconstriction and immune function in athletes. Phys Sportsmed. 2008;36:11–17. doi: 10.3810/psm.2008.12.7. [DOI] [PubMed] [Google Scholar]
- 53.Arm JP, Horton CE, Mencia-Huerta JM, House F, Eiser NM, Clark TJ, et al. Effect of dietary supplementation with fish oil lipids on mild asthma. Thorax. 1988;43:84–92. doi: 10.1136/thx.43.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoyos C, Almqvist C, Garden F, Xuan W, Oddy WH, Marks GB, et al. Effect of omega 3 and omega 6 fatty acid intakes from diet and supplements on plasma fatty acid levels in the first 3 years of life. Asia Pac J Clin Nutr. 2008;17:552–557. [PubMed] [Google Scholar]
- 55.Kremmyda LS, Vlachava M, Noakes PS, Diaper ND, Miles EA, Calder PC. Atopy risk in infants and children in relation to early exposure to fish, oily fish, or long-chain omega-3 fatty acids: a systematic review. Clin Rev Allergy Immunol. 2011;41:36–66. doi: 10.1007/s12016-009-8186-2. [DOI] [PubMed] [Google Scholar]
- 56.Wood LG, Gibson PG. Dietary factors lead to innate immune activation in asthma. Pharmacol Ther. 2009;123:37–53. doi: 10.1016/j.pharmthera.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 57.de Vries A, Howie SE. Diet and asthma—can you change what you or your children are by changing what you eat? Pharmacol Ther. 2009;122:78–82. doi: 10.1016/j.pharmthera.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Tricon S, Burdge GC, Kew S, Banerjee T, Russell JJ, Jones EL, et al. Opposing effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on blood lipids in healthy humans. Am J Clin Nutr. 2004;80:614–620. doi: 10.1093/ajcn/80.3.614. [DOI] [PubMed] [Google Scholar]
- 59.Zulet MA, Marti A, Parra MD, Martinez JA. Inflammation and conjugated linoleic acid: mechanisms of action and implications for human health. J Physiol Biochem. 2005;61:483–494. doi: 10.1007/BF03168454. [DOI] [PubMed] [Google Scholar]
- 60.Churruca I, Fernandez-Quintela A, Portillo MP. Conjugated linoleic acid isomers: differences in metabolism and biological effects. Biofactors. 2009;35:105–111. doi: 10.1002/biof.13. [DOI] [PubMed] [Google Scholar]
- 61.MacRedmond R, Singhera G, Attridge S, Bahzad M, Fava C, Lai Y, et al. Conjugated linoleic acid improves airway hyper-reactivity in overweight mild asthmatics. Clin Exp Allergy. 2010;40:1071–1078. doi: 10.1111/j.1365-2222.2010.03531.x. [DOI] [PubMed] [Google Scholar]
- 62.Macredmond R, Dorscheid DR. Conjugated linoleic acid (CLA): is it time to supplement asthma therapy? Pulm Pharmacol Ther. 2011;24:540–548. doi: 10.1016/j.pupt.2011.03.005. [DOI] [PubMed] [Google Scholar]