Abstract
Purpose
To investigate the effects of immunoembolization with granulocyte–macrophage colony-stimulating factor (GM-CSF) in patients with uveal melanoma (UM) with liver-only metastasis.
Materials and Methods
In this double-blind phase II clinical trial, patients were randomized to undergo immunoembolization or bland embolization (BE). Lobar treatment was performed with GM-CSF or normal saline solution mixed with ethiodized oil followed by embolization with gelatin sponge emulsified with iodinated contrast medium. Fifty-two patients (immunoembolization, n = 25; BE, n = 27) were enrolled. Response was assessed after every two treatments. The primary endpoint was overall response rate (ORR) of liver metastases. Progression-free survival (PFS), overall survival (OS), and immunologic responses were secondary endpoints.
Results
There were five partial responses in the immunoembolization group (ORR, 21.2%; 90% confidence interval [CI], 10.3%–30.5%) and three in the BE group (ORR, 16.7%; 90% CI, 6.3%–26.9%). Stable disease was seen in 12 patients in the immunoembolization group and 19 in the BE group. OS times were 21.5 months (95% CI, 18.5–24.8 mo) with immunoembolization and 17.2 months (95% CI, 11.9–22.4 mo) with BE. The degree of proinflammatory cytokine production was more robust after immunoembolization and correlated with time to “systemic” extrahepatic progression. In the immunoembolization group, interleukin (IL)-6 levels at 1 hour (P = .001) and IL-8 levels at 18 hours after the procedure (P < .001) were significant predictors of longer systemic PFS. Moreover, a dose–response pattern was evident between posttreatment serum cytokine concentrations and systemic PFS.
Conclusions
Immunoembolization induced more robust inflammatory responses, which correlated with the delayed progression of extrahepatic systemic metastases.
Uveal melanoma (UM) is the most common primary intraocular malignant tumor in adults in the United Stated (1). As many as 50% of patients develop systemic metastases after successful treatment of the primary tumor (2). The liver is usually the first and only site of metastatic disease for a majority of patients (3). Treatment options for liver metastases include systemic chemotherapy, surgery, regional intra-arterial chemotherapy with or without embolization, and radioactive microsphere treatment (4).
The systemic chemotherapeutic regimens used in cutaneous melanoma generally are ineffective against this highly chemotherapy-resistant tumor, and only a minority of patients (<10%) are deemed eligible for treatment with surgical approaches (5). Hepatic intra-arterial chemotherapeutic infusion requires placement of implantable hepatic catheters, a technique not commonly used in the United States (6,7). Mavligit et al (8) reported a 46% response rate with the use of chemoembolization with cisplatin and polyvinyl alcohol sponge. However, these results were not consistently reproduced by others. In 2005, a phase II study consisting of 29 patients treated with chemoembolization using carmustine (9) demonstrated a median time to progression of 6.5 months. Nonetheless, despite control of hepatic metastases, progression of extrahepatic metastases was seen in two thirds of patients (9).
Therefore, a local/regional treatment that could potentially control growth of liver metastases and delay or prevent the development of systemic metastases would be an ideal treatment option. Immunoembolization with the use of granulocyte–macrophage colony-stimulating factor (GM-CSF) is a liver-directed treatment that may theoretically fulfill both goals.
The liver is the largest organ of the reticuloendothelial system and contains more than 70% of all tissue macrophages (ie, Kupffer cells). Hepatic sinusoidal lymphocytes, originally referred to as pit cells, have functions of natural killer cells. Natural killer T cells and CD3+ γδ T cells are also present in the liver (10). GM-CSF is known to stimulate macrophages and dendritic cells and increase the cytotoxicity of monocytes toward tumor cell lines through release of tumor necrosis factor (TNF). Vaccination with genetically engineered irradiated tumor cells that produce GM-CSF has been shown to induce potent, specific, and long-lasting antitumor immunity in an animal model (11). Evidence has been accumulated that GM-CSF is useful as an immunoadjuvant agent for cancer vaccines (12).
The combination of ethiodized oil with GM-CSF is intended to increase the local concentration of GM-CSF to in turn trigger stimulation of the host immune system. The rationale behind immunoembolization with GM-CSF is that tumor cells would be killed by the ischemic effect of embolization. The local immunologic reaction evoked by GM-CSF in the presence of tumor antigens would induce systemic immunity against melanoma cells and delay the development of remote systemic metastases.
In 2008, a phase I trial that used human recombinant GM-CSF for immunoembolization was reported (13). There was no maximum-tolerated, dose-limiting dose or late toxicity found at doses as high as 2,000 µg of GM-CSF, and higher doses correlated with longer systemic progression-free survival (PFS). A subsequent retrospective analysis (14) that compared immunoembolization with carmustine chemoembolization showed a significantly longer survival with immunoembolization. To further investigate the immunologic mechanism of this approach, a randomized phase II clinical trial was designed intending to further explore the efficacy of this innovative treatment.
MATERIALS AND METHODS
Patient Enrollment and Eligibility
Patients with histologically confirmed metastatic UM to the liver with no extrahepatic metastasis and at least one measurable hepatic lesion were enrolled. The total tumor volume could not exceed 50% of the liver volume. Also required were Eastern Cooperative Oncology Group performance status no higher than 1 and the following laboratory parameters: serum creatinine level no greater than 2.0 mg/dL, granulocyte count of at least 1,000/mm3, platelet count of at least 100,000/mm3, bilirubin level no greater than 2.0 mg/mL, albumin level of at least 3.0 g/dL, prothrombin time/partial thromboplastin time less than 1.5 times the normal value, and alkaline aminotransferase, alanine aminotransferase, and alkaline phosphatase levels less than 5 times the normal value.
Exclusion criteria were uncontrolled hypertension and/or uncontrolled congestive heart failure, bleeding diathesis, life expectancy of 6 months or less, pregnancy or breastfeeding, HIV infection, need for immunosuppressive therapy, and severe allergy to iodinated contrast agent or GM-CSF judged by the study physicians based on their clinical assessment. In view of technical considerations, patients with occlusion of the main portal vein, inadequate collateral flow around an occluded portal vein as determined by angiography, biliary obstruction, stent or previous biliary surgery except cholecystectomy, and arteriovenous shunt identified on arteriography of the hepatic artery were also excluded.
Study Design and Interventions
This was a double-blinded and randomized phase II study in which patients assigned to undergo BE served as controls only for immunologic outcomes. The study design was not meant and did not have adequate power to formally compare arms in terms of the primary or secondary outcomes. A prespecified minimal overall response rate (ORR) was chosen as a selection process to consider the best arm for potential future clinical trials following the “pick-the-winner” method (15,16). Eligible patients were randomly assigned to immunoembolization or bland embolization (BE), defined as embolization with ethiodized oil/saline solution emulsion and gelatin sponge (Surgifoam; Ethicon, Somerville, New Jersey) slurry without GM-CSF at a 1:1 ratio by the investigational pharmacy. Immunoembolization consisted of the same procedure as BE but with the addition of GM-CSF. Patients were stratified based on human leukocyte antigen A2 status for immunologic analysis purposes and the total volume of liver metastases (<20% vs ≥20%).
On the day of each embolization, the investigational pharmacy provided the interventional radiologist with the solution of GM-CSF or normal saline solution. For immunoembolization, 4 mL of Leukine liquid (2,000 µg GM-CSF; Bayer, Leverkusen, Germany) was used. For BE, 4 mL of normal saline solution was used instead. The solution was emulsified in ethiodized oil (Lipiodol; Guerbet, Roissy, France). The estimated amount of ethiodized oil used was calculated based on tumor volume (1 mL per centimeter of summed tumor diameters; maximum 10 mL); however, the final amount of ethiodized oil was determined based on the angiographic findings of tumors. When the emulsion had formed, the mixture was slowly injected into the hepatic artery. Afterward, gelatin sponge particles were infused until arterial stasis—ie, sluggish, pulsatile arterial flow with a “pruned-tree” appearance of branches—was achieved. Gelatin sponge (Surgifoam; Ethicon) slurry was made immediately before use in the following manner: a piece of Surgifoam (3 × 2 × 0.7 cm) was cut into tiny pieces and macerated through a stopcock with 10 mL of iodinated contrast medium. The embolization procedure was repeated every 4 weeks unless liver progression, development of extrahepatic metastases, or unacceptable toxicity was confirmed. If the tumor was confined to a single hepatic lobe, embolization was repeated to that lobe. If the liver metastases were located in both lobes, each hepatic lobe was treated alternately. Peripheral blood specimens were drawn before the embolization procedure and 1 and 18 hours after the procedure to measure GM-CSF and cytokine levels. Patients remained hospitalized overnight for hydration and symptom management with antiemetic agents, analgesic agents, antipyretic agents, and prophylactic antibiotic agents. When they had been discontinued from the study, patients were still followed every 2 months for at least 2 years for survival data collection.
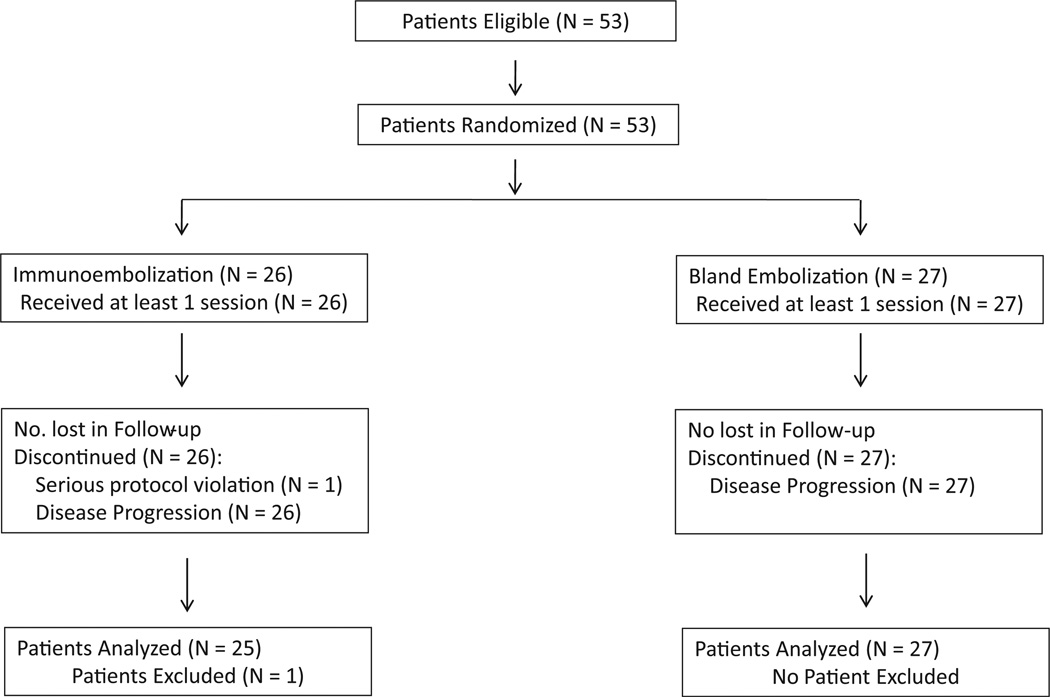
A total of 53 patients (all of white race) were enrolled between June 2005 and January 2010. One patient was removed from the study for a protocol violation. This patient was initially randomized to undergo immunoembolization, but the second embolization was mistakenly given without GM-CSF. Because that patient received both treatments, adding him to the immunoembolization arm would have been problematic in regard to the immunologic outcome. For that reason, and after contacting the regulatory agency, the patient was excluded from the study. From the remaining 52 patients, 25 received immunoembolization and 27 received BE (Fig 1). Patients’ demographic and baseline characteristics are summarized in Table 1. The total numbers of immunoembolization and BE procedures performed were 108 and 128, respectively.
Figure 1.
Study diagram per Consolidated Standards of Reporting Trials.
Table 1.
Baseline Demographic Characteristics
| Characteristic | All Patients | Bland Embolization | Immunoembolization |
|---|---|---|---|
| No. of patients | 52 (100) | 27 (52) | 25 (48) |
| Age | |||
| Median | 58 | 62 | 56 |
| Range | 28–90 | 32–90 | 28–81 |
| Sex | |||
| Male | 25 (48) | 15 (55.6) | 10 (40.0) |
| Female | 27 (52) | 12 (44.4) | 15 (60.0) |
| White race | 52 (100) | 27 (100) | 25 (100) |
| Liver involvement | |||
| < 20% | 32 (61.5) | 16 (59.3) | 16 (64.0) |
| 20%–50% | 20 (38.5) | 11 (40.7) | 9 (36.0) |
| Baseline LDH | |||
| Normal | 43 (82.5) | 22 (81.5) | 21 (84.0) |
| Elevated | 9 (17.5) | 5 (18.5) | 4 (16.0) |
| HLA-A2 status | |||
| Positive | 31 (60.0) | 17 (63.0) | 14 (56.0) |
| Negative | 21 (40.0) | 10 (37.0) | 11 (44.0) |
Note–Values in parentheses are percentages. None of the variables showed a significant difference (P ≥ .05).
HLA = human leukocyte antigen, LDH = lactate dehydrogenase.
Outcome Measurements
All patients had baseline computed tomography (CT) and magnetic resonance (MR) imaging of the liver on which measurable disease was identified. A target lesion required to be at least 10 mm in longest diameter and a maximum of six hepatic target lesions could be chosen. Clinical response was evaluated after every two embolizations based on CT of the chest, abdomen, and pelvis and abdominal MR imaging. The sum of the longest diameters of the maximum of six hepatic target lesions at baseline was compared with the sum of longest diameters after every two treatments to determine response. Response criteria were adapted from Response Evaluation Criteria In Solid Tumors (Tables E1 and E2, available online at www.jvir.org). To be categorized as a PR or complete response, changes in tumor measurements must have been confirmed by repeat assessments performed no less than 4 weeks apart.
PFS and overall survival (OS) were measured from the start of the treatment to the confirmation of progressive disease or death, respectively. PFS was further subdivided into local (ie, liver progression) or systemic (ie, extrahepatic progression) PFS.
Serum levels of interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α (Life Technologies, Grand Island, New York), and GM-CSF (R&D Systems, Minneapolis, Minnesota) were measured by commercially available enzyme-linked immunosorbent assay kits at baseline as well as at 1 hour and 18 hours after embolization. These cytokines were chosen based on previous reports on locoregional treatments of hepatic tumors (17,18). Exploratory measurements on IL-12, IL-10, and interferon-γ levels showed no treatment-related changes and were not analyzed for this study. Toxicity was assessed per National Cancer Institute Common Toxicity Criteria, version 3.0.
Statistical Considerations
The primary endpoint was ORR, including complete responses and partial responses (PRs) of the liver metastases of patients receiving immunoembolization. An ORR of 30% was selected as a surrogate of clinically meaningful effect based on our previous experience. Sample calculations were based on the optimal two-stage design to test the null (ORR ≤ 10%) versus the alternative hypothesis (ORR ≥ 30%) (19). With an actual ORR of no more than 10%, the probability of early termination was estimated to be 73.4% if fewer than two responses were seen after treating 18 patients in the first stage. For the second stage, a total of 26 patients were targeted. If the total number of responses was no more than four, the intervention was considered ineffective. This design also guaranteed less than a 10% chance of wrongly estimating the real effect of the treatment in either direction (α = 0.1). The ORR was computed by using the uniformly minimum-variance unbiased estimator, and corresponding 90% confidence intervals (CIs) were calculated by using the method of Koyama and Chen (20,21). For ethical reasons the same stopping rule was applied to the BE arm.
PFS (hepatic and systemic types) and OS were evaluated as secondary endpoints in each arm (per protocol). The estimated median survival and 95% CI was calculated by Kaplan–Meier method. As exploratory analyses, PFS and OS were also analyzed by using Cox proportional-hazards models in the whole population (N = 52) to determine predictors of these outcomes.
The average of cytokine levels from the first two treatments, or just the first value for patients treated with only one procedure as a result of progression of disease (n = 4), was used for study analysis. Mann–Whitney U test, t test, analysis of variance, and Spearman correlation were used to compare and correlate cytokines. In the immunoembolization group, robust linear regression was used to evaluate the association between averaged serum levels of cytokines and systemic PFS as well as OS. PFS and OS times and serum cytokine levels were log-transformed for analysis. Because all events were observed and no censored data were present, linear regression of log-transformed survival times was appropriate. MM-estimators were used as a robust alternative to the standard regression estimates to minimize potential influence of suspected outliers.
RESULTS
Clinical Outcomes
Of the 53 patients, there were no complete responses. With median follow-up of 19.1 months (mean, 23.5 mo; range, 2.1–70.5 mo), all patients subsequently developed hepatic progression. Six patients died without developing extrahepatic relapses, two in the BE group (7.5%) and four in the immunoembolization group (16%). Nine patients developed simultaneous hepatic progression and extrahepatic metastases, seven in the BE group (26%) and two in the immunoembolization group (8%). Only one patient remained alive at the time of manuscript preparation.
In the immunoembolization group, five patients showed PR, three of whom had at least than 20% of the liver volume compromised (Fig 2). Twelve patients showed SD and eight showed tumor progression (Table 2). The estimated ORR for the immunoembolization arm was 21.2% (90% CI, 10.3%–30.5%). The median OS was 21.5 months (95% CI, 18.5–24.8 mo). Median hepatic PFS and systemic PFS were 3.9 months (95% CI, 3.5–4.3 mo) and 10.4 months (95% CI, 7.5–13.2 mo), respectively (Table E3, available online at www.jvir.org).
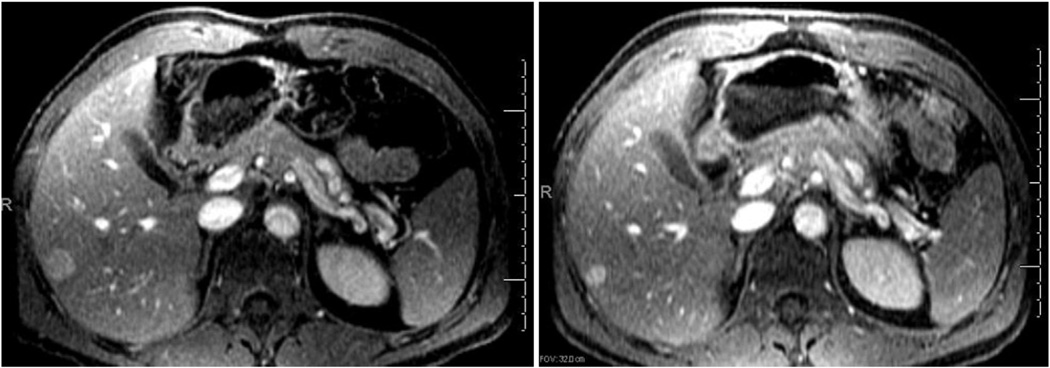
Figure 2.
Selected MR images from a patient with numerous hepatic metastases involving more than 20% of the liver at baseline (left) and 6 months after immunoembolization (right). The patient showed a PR in multiple hepatic metastases, and the largest lesion in the right hepatic lobe decreased from 1.9 × 1.8 cm to 1.2 × 1.0 cm. No progression of hepatic metastasis was seen for 13.8 months.
Table 2.
Best Radiologic Response in Hepatic Metastasis (Primary Endpoint)
| Immunoembolization | Bland Embolization | |||||
|---|---|---|---|---|---|---|
| Best Radiologic Response | All (n = 25) | < 20% (n = 16) | ≥ 20% (n = 9) | All (n = 27) | < 20% (n = 16) | ≥ 20% (n = 11) |
| CR | 0 | 0 | 0 | 0 | 0 | 0 |
| PR | 5 | 2 | 3 | 3 | 3 | 0 |
| SD | 12 | 7 | 5 | 19 | 11 | 8 |
| PD | 8 | 7 | 1 | 5 | 2 | 3 |
CR = complete response, PD = progressive disease, PR = partial response, SD = stable disease.
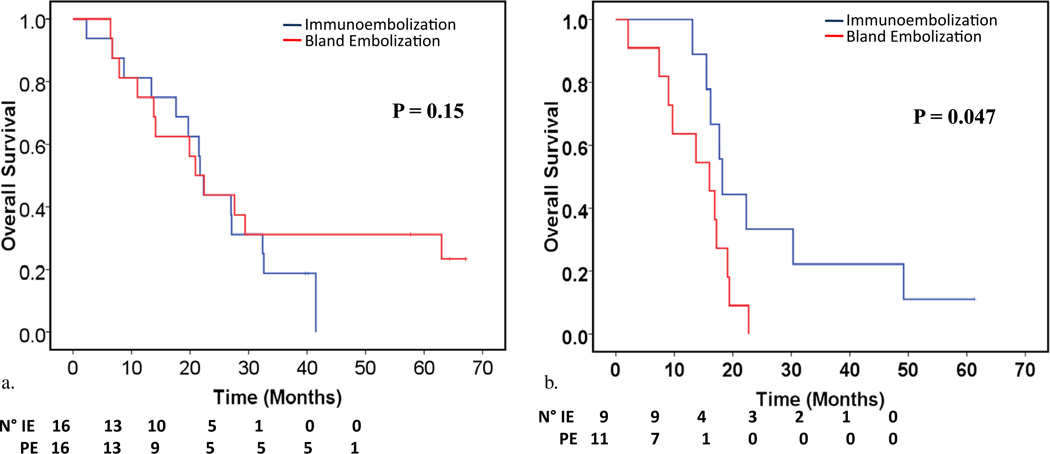
In the BE group, only three patients had a PR, all of whom had less than 20% liver involvement. Nineteen patients had SD, and five showed disease progression. The ORR for the BE arm was 16.7% (90% CI, 6.3%–26.9%). Median OS, liver PFS, and systemic PFS were 17.2 months (95% CI, 11.9–22.4 mo), 5.9 months (95% CI, 5.5–6.2 mo), and 7.1 months (95% CI, 5.0–9.1 mo), respectively. Figure 3 plots the OS of each arm by tumor burden. Patients with at least 20% liver involvement showed longer OS with immunoembolization compared with BE (P = .047), but no difference was evident in patients with lesser liver involvement.
Figure 3.
Kaplan–Meier estimates of OS rates according to tumor liver involvement: (a) less than 20% of liver volume and (b) 20%–50% of liver volume. (Available in color online at www.jvir.org.)
Exploratory analyses showed that larger liver involvement (≥20%) and increased baseline lactate dehydrogenase predicted worse OS and systemic PFS in the whole population (N = 52) after adjusting for other variables. Achieving PR in hepatic metastases was not a significant predictor of OS, but it was associated with longer systemic PFS (hazard ratio [HR] = 0.35; P = .025). Interaction terms suggested worse OS among patients with at least 20% liver involvement and who received BE (HR = 3.34; P = .012) but shorter liver PFS in patients with less than 20% liver involvement who received immunoembolization (HR = 4.26; P = .001; Table E4, available online at www.jvir.org).
Immune Response and Its Association with Clinical Outcomes
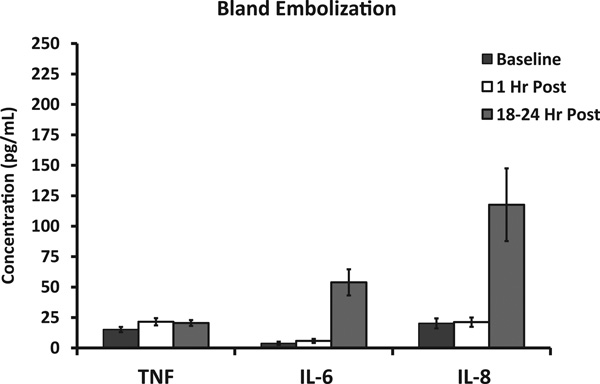
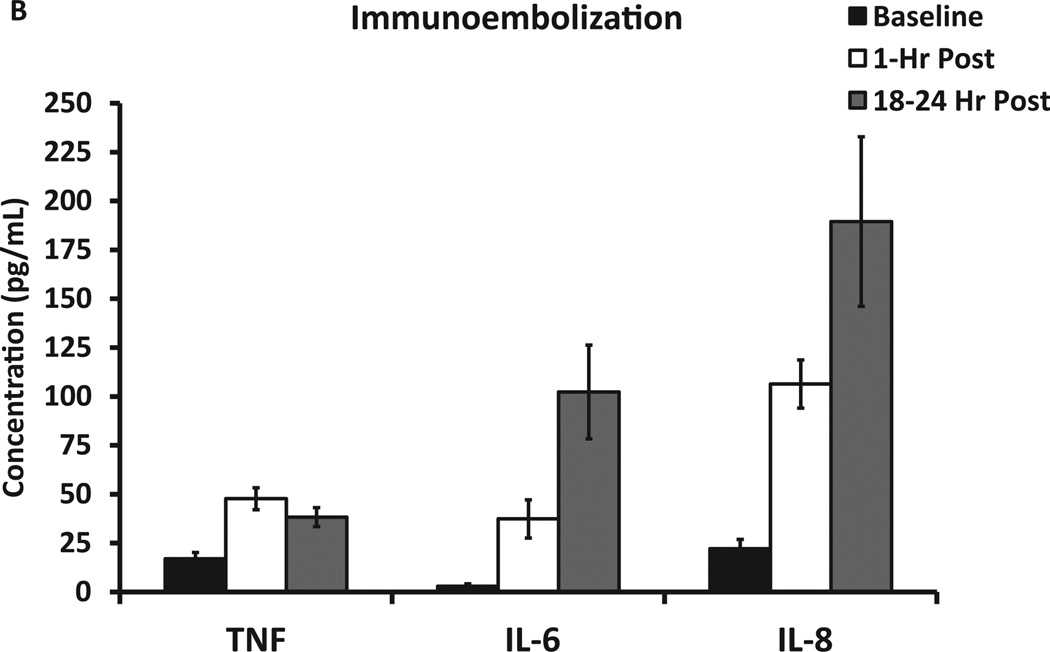
Serum GM-CSF levels peak dramatically at 1 hour after immunoembolization but tend to normalize after 18 hours. Although not significantly so, serum GM-CSF levels tended to be higher when liver tumor volume was smaller. GM-CSF levels remained low and unchanged in the BE group. Figure 4 shows the pattern of inflammation resulting from BE itself. There was a significant elevation in serum IL-6 and IL-8 levels (both P <.001) that was evident only 18 hours after the procedure. On the contrary, the addition of GM-CSF led to a rapid increment in serum TNF-α, IL-6, and IL-8 levels noticeable as early as 1 hour after treatment and statistically different compared with BE (P <.001). The ascending pattern of IL-6 and IL-8 continued even further after 18 hours (Fig 5). Overall, this suggests that immunoembolization results in a faster and more robust inflammatory response. Human leukocyte antigen A2 status did not show any significance for clinical or immunologic outcomes.
Figure 4.
Immunologic response and serum cytokine levels after embolization: IL-6, IL-8, and TNF-α serum levels at baseline and 1 hour and 18 hours after BE.
Figure 5.
Immunologic response and serum cytokine levels after embolization: IL-6, IL-8, and TNF-α serum levels at baseline and 1 hour and 18 hours after immunoembolization.
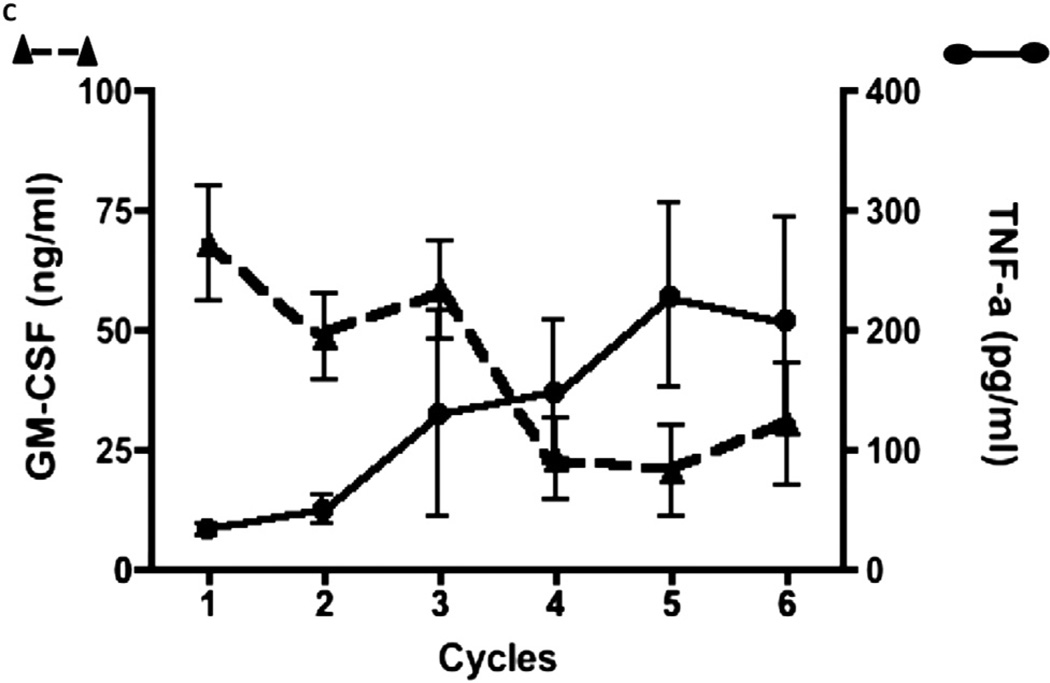
Within the immunoembolization group, no correlation was found between serum GM-CSF (at 1 h) and IL-6 (r = −0.18; P = .36) or IL-8 levels (r = 0.05; P = .79), suggesting that cytokine increases at 1 hour after treatment are more related to the inflammatory response in the liver than the direct effect of GM-CSF. Notably, the correlation between posttreatment serum GM-CSF levels and TNF-α levels changed after multiple treatments. There is no significant correlation between these two parameters for the first three treatments (r = −0.19; P = .14); however, posttreatment serum GM-CSF levels negatively correlated with TNF-α levels after the fourth treatments (r = −0.51; P <.001). Nine patients completed a total of six immunoembolization treatments, and the changes in correlation between serum levels of GM-CSF and inflammatory cytokines were investigated. There seems to be a decrease in serum GM-CSF levels after multiple immunoembolization treatments that corresponds to an increase in serum TNF-α levels (Fig 6). No similar phenomenon was observed in IL-6 or IL-8.
Figure 6.
Serum GM-CSF and TNF-α levels after multiple immunoembolizations.
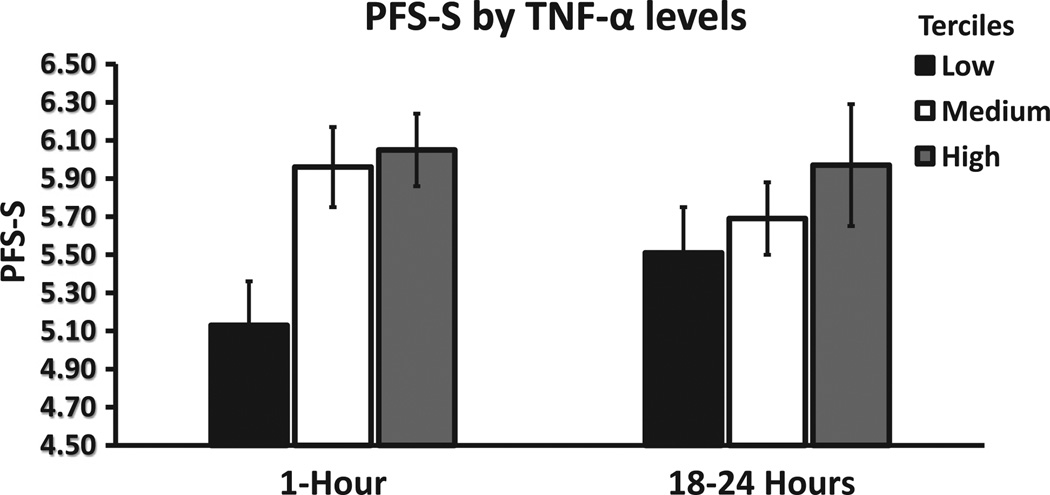
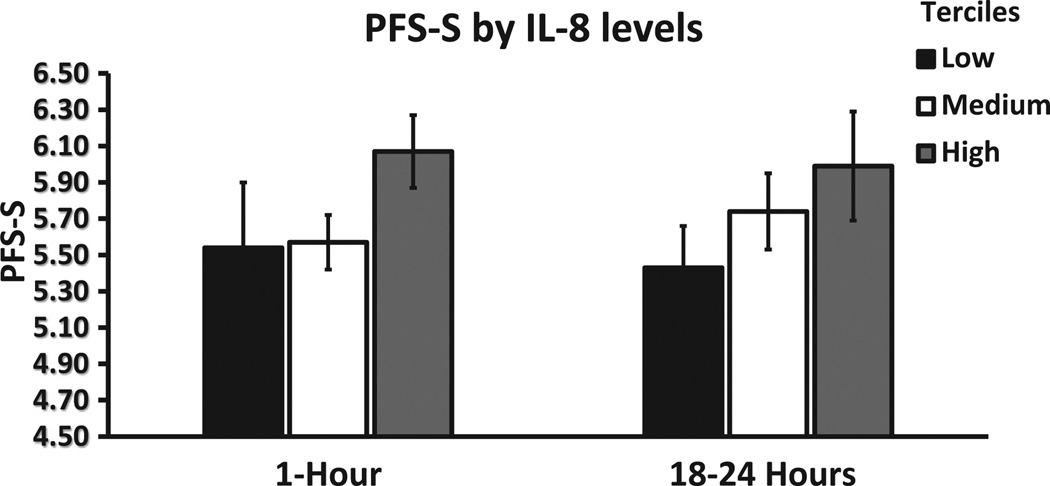
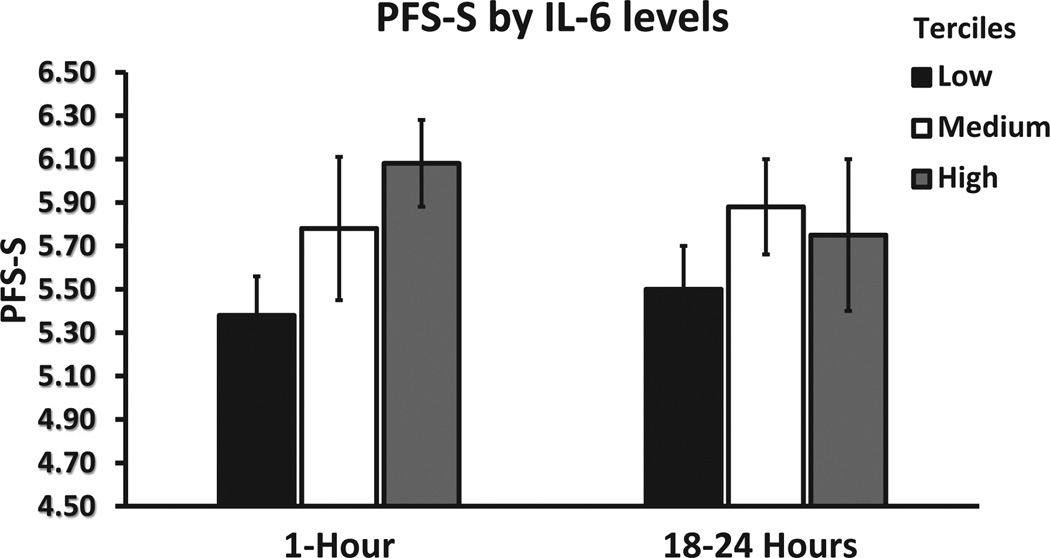
In addition, to explore the relationship between the intensity of the immunologic response after immunoembolization and the development of systemic metastases, regression analyses were undertaken. In univariate regression models, significant predictors of longer systemic PFS were higher levels of TNF-α and IL-6 at 1 hour (both P <.001), as well as higher levels of TNF-α (P = .020) and IL-8 (P = .001) at 18 hours. In the best multivariate regression model, after adjusting for all the levels of cytokines, only IL-6 levels at 1 hour (P = .001) and IL-8 levels at 18 hours after immunoembolization (P <.001) remained significant. Interestingly, a dose–response pattern becomes evident when systemic PFS is plotted as a dependent variable against cytokine concentration divided by tertiles (Figs 7–9). Similar analysis was performed for OS, but only IL-8 levels at 18 hours retained significance in the multivariate model (P = .029; Tables E5 and E6, available online at www. jvir.org).
Figure 7.
Extrahepatic (ie, systemic) PFS (PFS-S) according to TNF levels, divided by tertiles, at 1 hour and 18 hours after immunoembolization. Both variables are log-transformed (systemic PFS: 4.5 represents 2.95 months, 5.5 represents 8.05 months, and 6.5 represents 21.85 months), and error bars represent standard errors of the log-transformed mean systemic PFS in each tertile.
Figure 9.
Extrahepatic (ie, systemic) PFS (PFS-S) according to IL-8 levels, divided by tertiles, at 1 hour and 18 hours after immunoembolization. Both variables are log-transformed (systemic PFS: 4.5 represents 2.95 months, 5.5 represents 8.05 months, and 6.5 represents 21.85 months), and error bars represent standard errors of the log-transformed mean systemic PFS in each tertile.
Toxicity
There were no treatment-related deaths. Late toxicity was not observed in any patient during 2 years of followup. There was no significant difference in the incidence or severity of adverse events between two groups (Table 3). The most common ones were transient increases of hepatic enzyme levels and liver pain. Two patients in the BE group and four in the immunoembolization group had delayed hospital discharge as a result of significant but transient increases of hepatic enzyme levels.
Table 3.
Adverse Events
| Immunoembolization (n = 108) | Bland Embolization (n = 128) | |||
| Adverse Event | Grade 1/2 (%) | Grade 3 (%)* | Grade 1/2 (%) | Grade 3 (%)* |
|---|---|---|---|---|
| Days 1–7† | ||||
| Cardiac | 5.6 | 0 | 4.1 | 0.5 |
| Constitutional | 3.5 | 0 | 2.7 | 0 |
| Dermal | 6.4 | 0.8 | 3.1 | 0 |
| Gastrointestinal | 9.3 | 0.3 | 3.8 | 0 |
| Increased aminotransferases | 19.9 | 2.7 | 17.3 | 1.1 |
| Hematologic | 21.1 | 0 | 15.3 | 0 |
| Musculoskeletal | 0.2 | 0 | 0.7 | 0 |
| Neurologic | 2.4 | 0 | 0 | 0 |
| Ophthalmologic | 0.8 | 0 | 1.6 | 0 |
| Abdominal pain | 51.6 | 0.8 | 38.9 | 1 |
| Pulmonary | 1.7 | 0 | 0.5 | 0 |
| Day ≥ 8† | ||||
| Cardiac | 2.4 | 0 | 0.5 | 0 |
| Constitutional | 1.7 | 0 | 2.1 | 0 |
| Dermal | 0.8 | 0.8 | 3.1 | 0 |
| Gastrointestinal | 2.9 | 0.1 | 2.0 | 0 |
| Increased aminotransferases | 12.2 | 0.3 | 9.6 | 0.1 |
| Hematologic | 11.2 | 0 | 7.4 | 0 |
| Musculoskeletal | 0 | 0 | 1.1 | 0 |
| Neurologic | 0 | 0 | 0.5 | 0 |
| Ophthalmologic | 0 | 0 | 1.6 | 0 |
| Abdominal pain | 20.1 | 0 | 26.9 | 0.5 |
| Pulmonary | 0.8 | 0.4 | 1.1 | 0 |
Note–Because adverse events were recorded in each individual procedure, they are reported as percentage over the total number of procedures performed in each arm.
No grade 4 toxicity was noted.
Adverse events recorded between days 1 and 7 are attributed to the procedure and the experimental drug. Adverse events recorded after the first week are attributed mainly to experimental drug (granulocyte–macroph age colony-stimulating factor).
DISCUSSION
The working hypothesis of the present study was that immunoembolization would induce a more robust inflammatory response, triggering a systemic immune recognition of UM and delaying the progression of extrahepatic metastasis. In that sense, some details about the immunologic response might be important.
First, there were obvious changes in the timing and magnitude of the inflammatory reaction after immunoembolization. Cytokines were measured to estimate the degree of inflammation after both types of locoregional liver treatment. Lipiodol itself might stimulate immunologic responses. However, when comparing cytokine production in both arms, it becomes evident that the addition of GM-CSF results in a much stronger response beyond the baseline levels produced by the procedure itself.
With immunoembolization, the levels of cytokines peak almost instantly after the procedure; in particular, IL-6 and IL-8 levels continued to increase hours after the procedure. These two cytokine levels did not correlate with serum GM-CSF levels measured 1 hour after the infusion, arguing against a pure pharmacologic effect of higher doses of GM-CSF on nonhepatic sites, but rather corresponding more to individual variability in immunologic reactions, especially local inflammatory responses in the liver. In that regard, even within the immunoembolization group, there seem to be cases in which a more robust immunologic response occurs, and, more importantly, these patients showed longer extrahepatic recurrence–free periods, presumably as a result of better immunologic control of circulating micrometastases. It is obviously difficult, if not impossible, to fully prove this theory; however, the fact that a dose–response relationship between at least some of the cytokine concentrations and systemic PFS could be identified may represent a strong argument in its favor. It is theoretically possible that a higher proportion of patients could have reached the minimal immunologic threshold to delay the growth of distant metastases with the addition of GM-CSF.
A potential confounding factor directly related to this point would be the effects produced by other therapies used after the patient had liver progression in terms of systemic PFS. Whereas this is theoretically feasible in other malignancies in which second- and third-line therapies are effective, UM is remarkably resistant when it has progressed outside the liver. In a recent phase II randomized clinical trial (22), PFS times with systemic treatment of metastatic UM were 15.9 weeks (95% CI, 8.4–21.1 wk) with the mitogen-activated protein kinase kinase inhibitor selumetinib and 7 weeks (95% CI, 4.3–8.4 wk) in those who received temozolomide.
Currently, there is no Food and Drug Administration –approved treatment for metastatic UM. Data from most phase II/III studies published during the past decade reveal a median survival time ranging between 9 and 11 months (23–33). In addition, reported ORRs are generally dismal. There have been encouraging results reported with radioembolization and drug-eluting beads loaded with irinotecan (34,35), but the majority of studies were single-arm studies with small number of patients, and clinical benefits of these new approaches remain to be investigated with large clinical trials.
Although the present study was small and selection bias toward patients without extrahepatic metastases was present, the results appear promising, especially considering the fact that this intervention adds essentially no further toxicity to BE and that development of extrahepatic metastases was delayed with immunoembolization compared with BE. A possible next step would be a direct comparison between immunoembolization and systemic treatment (ie, selumetinib) in patients with liver-only metastases or combination treatments with immunoembolization and systemic treatment. Considering mild toxicity, combination of immunoembolization with newer forms of immunotherapy (ie, ipilimumab) would also be a reasonable future step. Because other locoregional treatment options such as irinotecan-eluting beads or radioembolization are now available, the sequential use of these locoregional treatments would also be beneficial to patients with UM with liver-only or liver-dominant metastases, and warrants further investigation.
The present study has some limitations that have to be acknowledged. First, the total number of patients included was relatively small. Also, the 30% hepatic ORR was arbitrarily chosen as clinically meaningful based on our previous experience from phase I and chemoembolization trials. However, notably, the hepatic ORR in the immunoembolization arm was 21.2% and the null hypothesis (ie, ORR ≤ 10%) was rejected. On the contrary, in the BE arm, only three patients showed a PR, which did not satisfy the prespecified condition (ie, at least four responses were needed) to consider further investigation. This becomes even more relevant considering that most of the locoregional control is achieved through the ischemia produced by the embolization procedure itself. It is possible that the additional local inflammation produced by GM-CSF could add extra clinical benefit, especially in those cases with larger tumor volumes (ie, ≥ 20%). Moreover, the original idea of adding a comparison arm with BE was to assess immunologic endpoints, not to compare clinical outcomes. In this regard, the BE arm was added for the purpose of proof of concept, and comparisons of survival and PFS times between these two arms were not intended and were obviously underpowered. This represents a limitation in terms of the study conclusions.
The prognosis of patients with UM with hepatic metastasis is extremely poor and the OS is generally short: less than 1 year in most cases. Immunoembolization seems to be safe, easy to administer, and potentially effective. The results obtained in the present study are encouraging; however, further clinical and basic research is needed to optimize and improve the efficacy of immunoembolization.
Supplementary Material
Figure 8.
Extrahepatic (ie, systemic) PFS (PFS-S) according to IL-6 levels, divided by tertiles, at 1 hour and 18 hours after immunoembolization. Both variables are log-transformed (systemic PFS: 4.5 represents 2.95 months, 5.5 represents 8.05 months, and 6.5 represents 21.85 months), and error bars represent standard errors of the log-transformed mean systemic PFS in each tertile.
ACKNOWLEDGMENTS
This study was approved by the IRB of Thomas Jefferson University and the FDA (BB-IND 8260). It was partially supported by an NCI grant (5R21CA103250-02, PI: T.S.).
ABBREVIATIONS
- BE
bland embolization
- CI
confidence interval
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- HR
hazard ratio
- IL
interleukin
- ORR
overall response rate
- OS
overall survival
- PFS
progression-free survival
- PR
partial response
- SD
stable disease
- TNF
tumor necrosis factor
- UM
uveal melanoma
Footnotes
None of the authors have identified a conflict of interest.
Tables E1–E6 are available online at www.jvir.org.
REFERENCES
- 1.Shields CL, Kaliki S, Furuta M, et al. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8033 cases. Retina. 2012;32:1363–1372. doi: 10.1097/IAE.0b013e31824d09a8. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Furuta M, Thangappan A, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989–998. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 3.Sato T, Han F, Yamamoto A. The biology and management of uveal melanoma. Curr Oncol Rep. 2008;10:431–438. doi: 10.1007/s11912-008-0066-z. [DOI] [PubMed] [Google Scholar]
- 4.Sato T. Locoregional management of hepatic metastasis from primary uveal melanoma. Semin Oncol. 2010;37(2):127–138. doi: 10.1053/j.seminoncol.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Nathan FE, Sato T, Hart E, et al. Response to combination chemotherapy of liver metastases from choroidal melanoma compared with cutaneous melanoma. Proc Am Soc Clin Oncol. 1994;13:394. [Google Scholar]
- 6.Leyvraz S, Spataro V, Bauer J, et al. Treatment of ocular melanoma metastatic to liver by hepatic arterial chemotherapy. J Clin Oncol. 1997;15(7):2589–2595. doi: 10.1200/JCO.1997.15.7.2589. [DOI] [PubMed] [Google Scholar]
- 7.Cantore M, Fiorentini G, Aitini E, et al. Intra-arterial hepatic carboplatin-based chemotherapy for ocular melanoma metastatic to the liver. Report of a phase II study. Tumori. 1994;80:37–39. doi: 10.1177/030089169408000107. [DOI] [PubMed] [Google Scholar]
- 8.Mavligit GM, Charnsangavej C, Carrasco CH, et al. Regression of ocular melanoma metastatic to the liver after hepatic arterial chemoembolization with cisplatin and polyvinyl sponge. JAMA. 1988;260:947–976. [PubMed] [Google Scholar]
- 9.Patel K, Sullivan K, Berd D, et al. Chemoembolization of the hepatic artery with BCNU for metastatic uveal melanoma: results of a phase II study. Melanoma Res. 2005;15(4):297–304. doi: 10.1097/00008390-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 10.The Human Liver Contains Multiple Population of NK cells, T cells, and CD3+CD56+ Natural T cells with Distinct Cytotoxic Activities and Th1, Th2, Nad Th0 Cytokine Secretion Patterns. The Journal of Immunology. 1999;163:2314–2321. [PubMed] [Google Scholar]
- 11.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony stimulation factor stimulated potent, specific, and long lasting antitumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dranoff G. GM-CSF-based cancer vaccines. Immunological Reviews. 2002;188:147–154. doi: 10.1034/j.1600-065x.2002.18813.x. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Eschelman DJ, Gonsalves CF, et al. Immunoembolization of malignant liver tumors, including uveal melanoma, using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2008;26(33):5436–5442. doi: 10.1200/JCO.2008.16.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto A, Chervoneva I, Sullivan KL, et al. High-dose immunoembolization: survival benefit in patients with hepatic metastases from uveal melanoma. Radiology. 2009;252(1):290–298. doi: 10.1148/radiol.2521081252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scher H, Heller G. Picking the winners in a sea of plenty. Clin Cancer Res. 2002;8(2):400–404. [PubMed] [Google Scholar]
- 16.Gray R, Manola J, Saxman S, et al. Phase II clinical trial design: methods in translational research from the Genitourinary Committee at the Eastern Cooperative Oncology Group. Clin Cancer Res. 2006;12:1966–1969. doi: 10.1158/1078-0432.CCR-05-1136. [DOI] [PubMed] [Google Scholar]
- 17.Schell S, Wessels F, Abouhamze A, et al. Pro- and Antiinflammatory cytokine production after radiofrequency ablation of unresectable hepatic tumors. J Am Coll Surg. 2002;195:774. doi: 10.1016/s1072-7515(02)01333-9. [DOI] [PubMed] [Google Scholar]
- 18.Osada Shinji, Imai Hisashi, Hiroyuki Tomita, et al. Serum cytokine levels in response to hepatic cryoablation. J Surg Oncol. 2007;95:491–498. doi: 10.1002/jso.20712. [DOI] [PubMed] [Google Scholar]
- 19.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clinical Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 20.Jung SH, Kim KM. On the estimation of the binomial probability in multistage clinical trials. Statistics in Medicine. 2004;23:881–896. doi: 10.1002/sim.1653. [DOI] [PubMed] [Google Scholar]
- 21.Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Statistics in Medicine. 2008;27:3145–3154. doi: 10.1002/sim.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvajal R, Sosman J, Quevedo J, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014;311(23):2397–2405. doi: 10.1001/jama.2014.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatia S, Moon J, Margolin KA, et al. Phase II trial of sorafenib in combination with carboplatin and paclitaxel in patients with metastatic uveal melanoma: SWOG S0512. PLoS One. 2012;7(11):e48787. doi: 10.1371/journal.pone.0048787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homsi J, Bedikian A, Papadopoulos N, et al. Phase 2 open-label study of weekly docosahexaenoic acid-paclitaxel in patients with metastatic uveal melanoma. Melanoma Res. 2010;20(6):507–510. doi: 10.1097/CMR.0b013e3283403ce9. [DOI] [PubMed] [Google Scholar]
- 25.Penel N, Delcambre C, Durando X, et al. O-Mel-Inib: a Cancéro-pôle Nord-Ouest multicenter phase II trial of high-dose imatinib mesylate in metastatic uveal melanoma. Invest New Drugs. 2008;26(6):561–565. doi: 10.1007/s10637-008-9143-2. [DOI] [PubMed] [Google Scholar]
- 26.Schmittel A, Schmidt-Hieber M, Martus P, et al. A randomized phase II trial of gemcitabine plus treosulfan versus treosulfan alone in patients with metastatic uveal melanoma. Ann Oncol. 2006;17(12):1826–1829. doi: 10.1093/annonc/mdl309. [DOI] [PubMed] [Google Scholar]
- 27.O’Neill PA, Butt M, Eswar CV, et al. A prospective single arm phase II study of dacarbazine and treosulfan as first-line therapy in metastatic uveal melanoma. Melanoma Res. 2006;16(3):245–248. doi: 10.1097/01.cmr.0000205017.38859.07. [DOI] [PubMed] [Google Scholar]
- 28.Schmittel A, Schuster R, Bechrakis N, et al. A two-cohort phase II clinical trial of gemcitabine plus treosulfan in patients with metastatic uveal melanoma. Melanoma Res. 2005;15(5):447–451. doi: 10.1097/00008390-200510000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Schmittel A, Scheulen ME, Bechrakis N, et al. Phase II trial of cisplatin, gemcitabine and treosulfan in patients with metastatic uveal melanoma. Melanoma Res. 2005;15(3):205–207. doi: 10.1097/00008390-200506000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Noter S, Rothbarth J, Pijl M, et al. Isolated hepatic perfusion with high-dose melphalan for the treatment of uveal melanoma metastases confined to the liver. Melanoma Res. 2004;14(1):67–72. doi: 10.1097/00008390-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Bedikian A, Papadopoulos N, Plager C, et al. Phase II evaluation of temozolomide in metastatic choroidal melanoma. Melanoma Res. 2003;13(3):303–306. doi: 10.1097/00008390-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Kivelä T, Suciu S, Hansson J, et al. Bleomycin, vincristine, lomustine and dacarbazine (BOLD) in combination with recombinant interferon alpha-2b for metastatic uveal melanoma. Eur J Cancer. 2003;39(8):1115–1120. doi: 10.1016/s0959-8049(03)00132-1. [DOI] [PubMed] [Google Scholar]
- 33.Pyrhönen S, Hahka-Kemppinen M, Muhonen T, et al. Chemoimmunotherapy with bleomycin, vincristine, lomustine, dacarbazine (BOLD), and human leukocyte interferon for metastatic uveal melanoma. Cancer. 2002;95(11):2366–2372. doi: 10.1002/cncr.10996. [DOI] [PubMed] [Google Scholar]
- 34.Gonsalves C, Eschelman D, Sullivan K, et al. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience. AJR Am J Roentgenol. 2011;196(2):468–473. doi: 10.2214/AJR.10.4881. [DOI] [PubMed] [Google Scholar]
- 35.Fiorentini G, Aliberti C, Del Conte A, et al. Intra-arterial hepatic chemoembolization (TACE) of liver metastases from ocular melanoma with slow-release irinotecan-eluting beads. Early results of a phase II clinical study. In Vivo. 2009;23(1):131–137. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.