Abstract
Angiogenesis in tumors is driven by multiple growth factors that activate receptor tyrosine kinases. An important driving force of angiogenesis in solid tumors is signaling through vascular endothelial growth factor (VEGF) and its receptors (VEGFRs). Angiogenesis inhibitors that target this signaling pathway are now in widespread use for the treatment of cancer. However, when used alone, inhibitors of VEGF/VEGFR signaling do not destroy all blood vessels in tumors and do not slow the growth of most human cancers. VEGF/VEGFR signaling inhibitors are, therefore, used in combination with chemotherapeutic agents or radiation therapy. Additional targets for inhibiting angiogenesis would be useful for more efficacious treatment of cancer. One promising target is the signaling pathway of hepatocyte growth factor (HGF) and its receptor (HGFR, also known as c-Met), which plays important roles in angiogenesis and tumor growth. Inhibitors of this signaling pathway have been shown to inhibit angiogenesis in multiple in vitro and in vivo models. The HGF/c-Met signaling pathway is now recognized as a promising target in cancer by inhibiting angiogenesis, tumor growth, invasion, and metastasis.
Keywords: Angiogenesis inhibitors, Endothelial cells, HGFR, Receptor tyrosine kinases, Signal transduction, Tumors, VEGF, VEGFR
INTRODUCTION
Angiogenesis, the formation of new blood vessels from the existing vasculature, contributes to many diseases, including cancer, age-related macular degeneration, diabetic retinopathy, neovascular glaucoma, psoriasis, and rheumatoid arthritis (1, 2). In solid tumors, angiogenesis is driven by multiple growth factors that act on via receptor tyrosine kinases (1, 2). An important driving force of angiogenesis in solid tumors is the signaling pathway of vascular endothelial growth factor (VEGF) and its receptors (VEGFRs) (2).
Several angiogenesis inhibitors that target the VEGF/VEGFR signaling pathway have been approved by the FDA (Food and Drug Administration) and are now used for the treatment of cancer patients (3–6). The first approved inhibitor of this signaling pathway is bevacizumab (Avastin, from Genentech), which is a monoclonal antibody against human VEGF (3). The other inhibitors are sunitinib (SU11248, from Pfizer) (4, 5) and sorafenib (BAY 43-9006, from Bayer) (6), which are small molecule compounds that inhibit phosphorylation of VEGFR and certain other receptor tyrosine kinases.
VEGF/VEGFR signaling inhibitors can block VEGF-driven angiogenesis in tumor models in mice (7, 8). These inhibitors cause regression of tumor vessels that depend on VEGF as a survival factor. However, tumor vessels that do not regress after VEGF signaling inhibition tend to become more normal (“normalization”) (8–11). Moreover, VEGF signaling inhibitors alone do not destroy all blood vessels in tumors (12). Therefore, additional targets of angiogenesis are being sought to augment the effects of VEGF inhibitors (13).
One promising target is the signaling pathway of hepatocyte growth factor (HGF, also known as scatter factor, SF) along with its receptor (HGFR, also known as c-Met). HGF, a potent mitogenic, motogenic and morphogenic factor that also plays an important role in angiogenesis and tumor growth (14–16). HGF and VEGF act synergistically on endothelial cells (16–19). HGF and c-Met are upregulated in many human cancers (20–22). Activation or upregulation of c-Met is a negative prognostic indicator in patients with various carcinomas, multiple myeloma, or glioma (23–26). For these reasons, various inhibitors of HGF/c-Met signaling pathway are being studied and developed as additional potent therapies to inhibit angiogenesis and tumor growth.
Molecular structure of HGF and c-Met
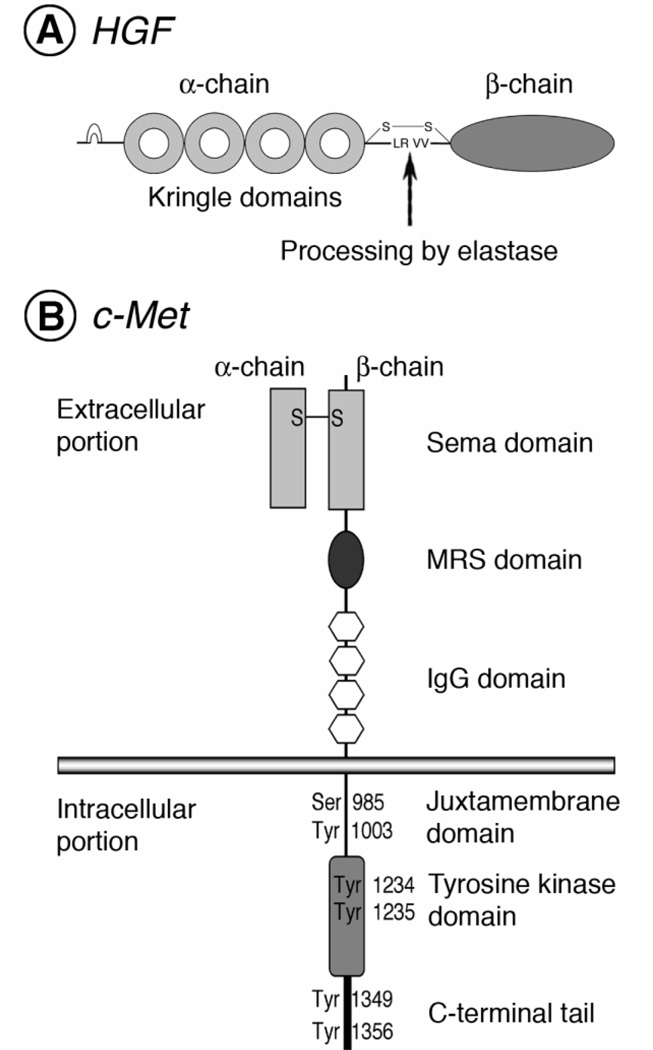
HGF is a multifunctional growth factor (20–22). It is produced as a single-chain inactive precursor protein (27, 28) (Fig. 1A). Mature active HGF is a heterodimer composed of an alpha- chain subunit (69 kDa) and a beta-chain subunit (34 kDa), which are linked by a disulfide bond (27, 29). The alpha-chain subunit contains an N-terminal hairpin domain and four kringle domains; the beta-chain subunit is a serine-protease-like domain lacking catalytic activity due to mutations in essential residues (27).
Fig. 1.
Schematic molecular structure of HGF and its receptor, c-Met. HGF (A) and c-Met (B) are initially expressed as precursor proteins and are then cleaved to mature heterodimers composed of an alpha- chain subunit and a beta-chain subunit linked by a disulfide bond. Mature HGF is a heterodimer composed of an alpha chain, which contains an N-terminal hairpin domain and four kringle domains, and a beta chain consisting of a serine-protease-like domain without enzymatic activity. Mature c-Met is composed of a glycosylated alpha subunit and a transmembrane beta subunit. The extracellular region of mature c-Met contains a Sema domain, a cysteine-rich Met-related sequence (MRS) domain, and four immunoglobulin-like structure domain. The intracellular region is composed of a juxtamembrane domain, a tyrosine kinase domain, and a C-terminal regulatory tail.
c-Met is also produced as a single-chain precursor protein (30, 31). This precursor receptor is cleaved to produce a glycosylated alpha-chain subunit (50 kDa) and a transmembrane beta- chain subunit (145 kDa), which are linked by a disulfide bond to form the mature receptor (32) (Fig. 1B).
The extracellular portion of c-Met, which is responsible for binding to HGF, contains a Sema domain (homologous to semaphorins), a cysteine-rich Met-related-sequence (MRS) domain, and four immunoglobulin-like structure (IgG domain) (32).
The intracellular portion of c-Met, which is responsible for signal transduction, is composed of a juxtamembrane domain, a tyrosine kinase domain, and a C-terminal regulatory tail (33). The juxtamembrane domain plays a key role in downregulation of the receptor (34, 35). The phosphorylation of a serine residue (Ser 985) in this domain inhibits the tyrosine kinase activity of c-Met (34). In addition, the phosphorylation of a tyrosine residue (Tyr 1003) is responsible for polyubiquitination and degradation of the receptor (35).
In the tyrosine kinase domain, two tyrosine residues (Tyr 1234 and Tyr 1235) regulate the kinase activity of c-Met (36). The other two tyrosine residues (Tyr 1349 and Tyr 1356), which are located in the C-terminal regulatory tail (the multi-substrate docking site), are the most important sites for recruiting downstream adapter molecules (37–39). Both tyrosine residues in the C-terminal tail are sufficient for the signal transduction of c-Met in vitro and in vivo (38, 40).
Expression of HGF and c-Met
HGF is expressed only by cells of mesenchymal origin (41). However, c-Met is expressed mainly by epithelial cells (41). In addition, c-Met is expressed by various other cell types including vascular endothelial cells (16), lymphatic endothelial cells (42), neural cells (43), hepatocytes (44), hematopoietic cells (45), and pericytes (46). In many tumor cells, c-Met expression is activated by HGF through an autocrine loop (47–52). The activation or upregulation of both the ligand and the receptor in tumors is a negative prognostic indicator in human cancer (23–26, 53, 54).
HGF/c-Met signaling pathway in angiogenesis
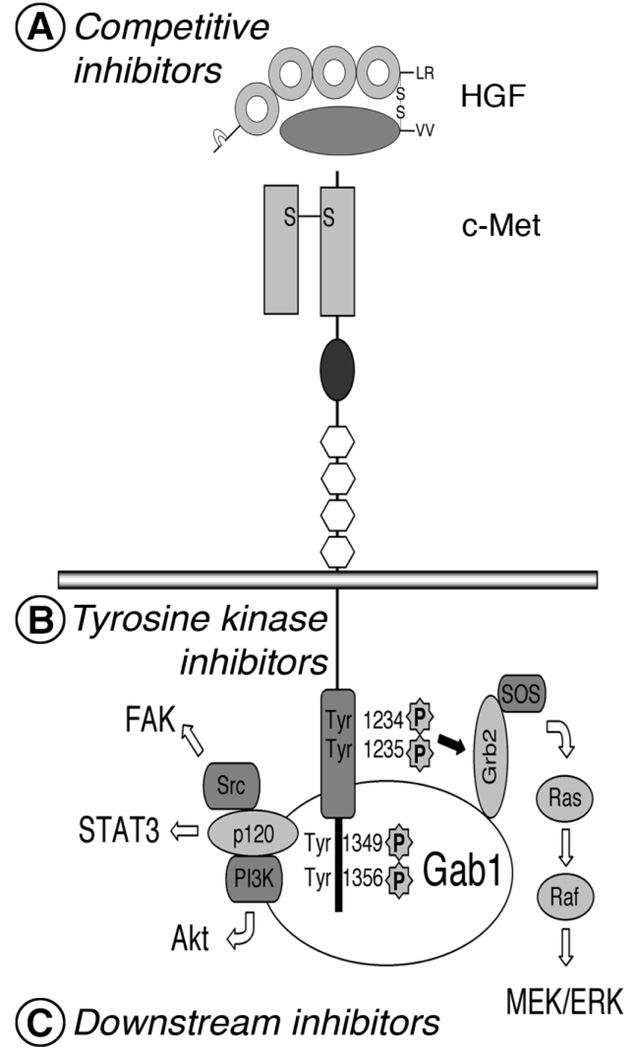
The HGF/c-Met signaling pathway plays an important role not only in embryogenesis and development but also in angiogenesis and tumor growth (15, 16, 19–22). This multifunctional signaling pathway induces mitogenesis, motogenesis, morphogenesis and angiogenesis (20–22) (Fig. 2).
Fig. 2.
Summary of the HGF/c-Met signaling pathway. HGF/c-Met signal transduction is initiated by binding of HGF to c-Met, as with other receptor tyrosine kinases. Dimerization or oligomerization of c-Met activates transphosphorylation of tyrosines (Tyr1234 and Tyr 1235) in the kinase domain followed by additional phosphorylation of other tyrosines (Tyr 1349 and Tyr 1356) in the C-terminal regulatory tail. Fully activated c-Met propagates HGF signaling in cells by recruiting and activating various adapter molecules downstream. Inhibitors of the HGF/c-Met signaling pathway, competitive inhibitors (A), tyrosine kinase inhibitors (B) or downstream inhibitors (C), target one of the molecular events of HGF/c-Met signaling activation and transduction.
On the molecular level, after ligand binding, c-Met is activated by phosphorylation of Tyr 1234 and Tyr 1235 residues, located in the tyrosine kinase domain (36). The phosphorylation of the other two tyrosines (Tyr 1349 and Tyr 1356), located in the C-terminal tail, provides a docking site for multiple substrates of downstream signal transduction such as Src, Gab1, and Grb2 (37). Therefore, HGF/c-Met signaling activates multiple signal transduction pathways including the Src/focal adhesion kinase (FAK) pathway, the p120/signal transducer and activator of transcription (STAT) 3 pathway, the phosphoinositide- 3 kinase (PI3K)/Akt pathway, and the Ras/MEK pathway (38, 39). The Src/FAK pathway regulates cell adhesion and migration (20–22). The p120/STAT3 pathway stimulates branching morphogenesis of cells (20–22). The PI3K/Akt pathway activates cell motility and cell survival (20–22). The Ras/MEK pathway mediates HGF-induced scattering and proliferation of cells (20–22). Thus, these multiple signaling pathways directly or indirectly stimulate endothelial cells: directly by motogenic or morphogenic effects and indirectly by regulation of other angiogenic factors (17–19). HGF increases expression of angiogenic mediators, including VEGF and its receptor, in endothelial cells (17).
Development of inhibitors targeting HGF/c-Met signaling pathway
Because HGF/c-Met signaling is activated in angiogenesis and tumor growth, several strategies have been explored for inhibiting the pathway (20–22). The strategies are based on the lessons learned from studies on development of inhibitors targeting other ligands and receptor tyrosine kinases (3–5, 55). Each strategy targets one of the molecular events of HGF/c-Met activation (Fig. 2). As seen in other signal transduction pathways of receptor tyrosine kinases, HGF binds to its receptor, c-Met, on the cell surface, and then the tyrosine kinase domain of c-Met is activated by dimerization and transphosphorylation (20–22, 56). The activation of these catalytic tyrosine residues is followed by additional phosphorylation of the two tyrosines in the C-terminal regulatory tail (20–22). This fully active receptor is ready to propagate c-Met-dependent signals by recruiting and stimulating downstream signaling molecules (20–22).
One strategy for inhibiting HGF/c-Met signaling is to block the binding of HGF to c-Met (Fig. 2A). Inhibitors of HGF/c-Met binding include HGF antagonists and antibodies against HGF or c-Met (Table 1). One HGF antagonist, NK4, is a truncated form of HGF, which contains the N-terminal hairpin domain and the subsequent four kringle domains (57, 58). NK4 binds to c-Met without activating it (57). The action of NK4, which has been studied in multiple in vitro and in vivo models using different approaches of delivery, is the best-characterized competitive antagonist of HGF (57, 58). Recently, other antagonists of HGF/c-Met binding have been developed, including an uncleavable HGF (59), an N-terminal Sema domain of HGF (60), a soluble extracellular domain of c-Met (decoy Met) (61), and a recombinant splice variant of c-Met (62). In addition, an antibody against HGF or c-Met inhibits angiogenesis and tumor growth in tumor models by blocking the binding of HGF and c-Met (63–67).
Table 1.
Antagonists of HGF and c-Met binding
| Group | Compound | Developmental stage |
References |
|---|---|---|---|
| HGF/c-Met antagonists | NK4 | Preclinical | (57, 58) |
| Uncleavable HGF | Preclinical | (59) | |
| Sema | Preclinical | (60) | |
| Decoy Met | Preclinical | (61) | |
| Recombinant variant Met | Preclinical | (62) | |
| Antibodies against HGF | L2G7 (Galaxy Biotech) | Preclinical | (63) |
| AMG102 (Amgen) | Phase II | (64) | |
| Antibodies against HGF | OA-5D5 (Genentech) | Preclinical | (65) |
| CE-355621 (Pfizer) | Preclinical | (66) | |
| DN30 (Metheresis) | Preclinical | (67) | |
Another strategy is targeting phosphorylation of the tyrosine residues in the tyrosine kinase domain of c-Met (Fig. 2B, Table 2). Most common agents in this group are small molecule inhibitors of c-Met receptor tyrosine kinase. Most of them are competitive analogues of ATP, a substrate for phosphorylation of c-Met tyrosine kinase. Several small molecule c-Met inhibitors are being studied and developed (68–73). In contrast to more specific inhibition by inhibitors of HGF/c-Met binding, small molecule inhibitors may have broader specificity for targeting receptor tyrosine kinases. However, most small molecule inhibitors can be administered orally for treatment of cancer patients.
Table 2.
Inhibitors of the HGF/c-Met signaling pathway
| Group | Compound | Developmental stage |
References |
|---|---|---|---|
| Small molecule inhibitors of c-Met tyrosine kinase | SU11274 (Pfizer) | Preclinical | (68) |
| PHA665752 (Pfizer) | Preclinical | (69) | |
| PF2341066 (Pfizer) | Phase I/II | (70) | |
| XL880 (Exelixis) | Phase II | (71, 72) | |
| XL184 (Exelixis) | Phase III | (73) | |
| Downstream inhibitors | LY294002 (LC Laboratories) | Preclinical | (74) |
| PD98059 (LC Laboratories) | Preclinical | (75) | |
| PD180970 (Parke-Davis Pharmaceutical Research) | Preclinical | (76) | |
| SU6656 (Pfizer) | Preclinical | (76) | |
The third strategy is to inhibit signaling events downstream of the HGF/c-Met signaling pathway (21, 22) (Fig. 2C, Table 2). A selective PI3K inhibitor, LY294002, inhibits HGF/c-Met-induced cell motility and morphogenic changes (74). A MEK inhibitor, PD98059, prevents invasiveness of malignant tumor cells dependent on HGF/c-Met signaling (75). Src inhibitors, PD180970 and SU6656, reduce Src and STAT3 activity in lung cancer cells stimulated by HGF (76).
Conclusion and perspectives
Recently, the HGF/c-Met signaling pathway has come into the spotlight as a promising therapeutic target for inhibiting angiogenesis. Research over the past two decades has revealed that the HGF/c-Met signaling pathway plays an important role in angiogenesis and tumor growth, that this signaling pathway acts on angiogenesis synergistically with the VEGF/VEGFR signaling pathway, and that the HGF/c-Met signaling pathway promotes tumor invasion and metastasis. Inhibitors of HGF/c- Met signaling, used in combination with inhibitors of VEGF/ VEGFR signaling, should have greater efficacy in slowing angiogenesis and tumor growth and perhaps reducing tumor invasion and metastasis.
Acknowledgments
We thank Mimi Zeiger, (UCSF) for editing the manuscript. The authors also thank Barbara Sennino, Beverly Falcón, Peter Baluk, and Hiroya Hashizume for their valuable advice and discussion. This work was supported in part by a grant from the Korea Research Foundation (KRF-04-04-02-2). The research on which this review was based was supported in part by National Institutes of Health grants HL24136 and HL59157 from the National Heart, Lung, and Blood Institute, CA82923 from the National Cancer Institute and funding from AngelWorks Foundation to DMcD.
REFERENCES
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Chen H, Davis-Smyth T, Gerber HP, Nguyen TN, Peers D, Chisholm V, Hillan KJ, Schwall RH. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat. Med. 1998;4:336–340. doi: 10.1038/nm0398-336. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12:713–718. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- 4.Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JV, Booth BP, Verbois SL, Morse DE, Liang CY, Chidambaram N, Jiang JX, Tang S, Mahjoob K, Justice R, Pazdur R. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin. Cancer Res. 2007;13:1367–1373. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 5.Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, Dagher R, Justice R, Pazdur R. Food and drug administration drug approval summary: sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007;12:107–113. doi: 10.1634/theoncologist.12-1-107. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multi-kinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 7.Brekken RA, Overholser JP, Stastny VA, Waltenberger J, Minna JD, Thorpe PE. Selective inhibition of vascular endothelial growth factor (VEGF) receptor 2 (KDR/Flk-1) activity by a monoclonal anti-VEGF antibody blocks tumor growth in mice. Cancer Res. 2000;60:5117–5124. [PubMed] [Google Scholar]
- 8.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am. J. Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 11.Yao VJ, Ozawa MG, Varner AS, Kasman IM, Chanthery YH, Pasqualini R, Arap W, McDonald DM. Antiangiogenic therapy decreases integrin expression in normalized tumor blood vessels. Cancer Res. 2006;66:2639–2649. doi: 10.1158/0008-5472.CAN-05-1824. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, O'Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, Melnyk O, Ramies D, Lin M, Sandler A. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J. Clin. Oncol. 2007;25:4743–4750. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 14.Gherardi E, Stoker M. Hepatocytes and scatter factor. Nature. 1990;346:228. doi: 10.1038/346228b0. [DOI] [PubMed] [Google Scholar]
- 15.Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P, Rosen EM. Scatter factor induces blood vessel formation in vivo. Proc. Natl. Acad. Sci. U. S. A. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding S, Merkulova-Rainon T, Han ZC, Tobelem G. HGF receptor up-regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood. 2003;101:4816–4822. doi: 10.1182/blood-2002-06-1731. [DOI] [PubMed] [Google Scholar]
- 17.Wojta J, Kaun C, Breuss JM, Koshelnick Y, Beckmann R, Hattey E, Mildner M, Weninger W, Nakamura T, Tschachler E, Binder BR. Hepatocyte growth factor increases expression of vascular endothelial growth factor and plasminogen activator inhibitor-1 in human keratinocytes and the vascular endothelial growth factor receptor flk-1 in human endothelial cells. Lab. Invest. 1999;79:427–438. [PubMed] [Google Scholar]
- 18.Gerritsen ME, Tomlinson JE, Zlot C, Ziman M, Hwang S. Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells. Br. J. Pharmacol. 2003;140:595–610. doi: 10.1038/sj.bjp.0705494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang YW, Su Y, Volpert OV, Vande Woude GF. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12718–12723. doi: 10.1073/pnas.2135113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maulik G, Shrikhande A, Kijima T, Ma PC, Morrison PT, Salgia R. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev. 2002;13:41–59. doi: 10.1016/s1359-6101(01)00029-6. [DOI] [PubMed] [Google Scholar]
- 21.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 22.Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat. Rev. Drug Discov. 2008;7:504–516. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- 23.Miller CT, Lin L, Casper AM, Lim J, Thomas DG, Orringer MB, Chang AC, Chambers AF, Giordano TJ, Glover TW, Beer DG. Genomic amplification of MET with boundaries within fragile site FRA7G and upregulation of MET pathways in esophageal adenocarcinoma. Oncogene. 2006;25:409–418. doi: 10.1038/sj.onc.1209057. [DOI] [PubMed] [Google Scholar]
- 24.Stellrecht CM, Phillip CJ, Cervantes-Gomez F, Gandhi V. Multiple myeloma cell killing by depletion of the MET receptor tyrosine kinase. Cancer Res. 2007;67:9913–9920. doi: 10.1158/0008-5472.CAN-07-0770. [DOI] [PubMed] [Google Scholar]
- 25.Garcia S, Dales JP, Charafe-Jauffret E, Carpentier-Meunier S, Andrac-Meyer L, Jacquemier J, Andonian C, Lavaut MN, Allasia C, Bonnier P, Charpin C. Overexpression of c-Met and of the transducers PI3K, FAK and JAK in breast carcinomas correlates with shorter survival and neoangiogenesis. Int. J. Oncol. 2007;31:49–58. [PubMed] [Google Scholar]
- 26.Puri N, Ahmed S, Janamanchi V, Tretiakova M, Zumba O, Krausz T, Jagadeeswaran R, Salgia R. c-Met is a potentially new therapeutic target for treatment of human melanoma. Clin. Cancer Res. 2007;13:2246–2253. doi: 10.1158/1078-0432.CCR-06-0776. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 28.Gherardi E, Gray J, Stoker M, Perryman M, Furlong R. Purification of scatter factor, a fibroblast-derived basic protein that modulates epithelial interactions and movement. Proc. Natl. Acad. Sci. U. S. A. 1989;86:5844–5848. doi: 10.1073/pnas.86.15.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gohda E, Tsubouchi H, Nakayama H, Hirono S, Sakiyama O, Takahashi K, Miyazaki H, Hashimoto S, Daikuhara Y. Purification and partial characterization of hepatocyte growth factor from plasma of a patient with fulminant hepatic failure. J. Clin. Invest. 1988;81:414–419. doi: 10.1172/JCI113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 31.Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, Narsimhan RP, Hartmann G, Zarnegar R, Michalopoulos GK, Birchmeier W, Comoglio PM. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. Embo. J. 1991;10:2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigues GA, Naujokas MA, Park M. Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol. Cell. Biol. 1991;11:2962–2970. doi: 10.1128/mcb.11.6.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bardelli A, Ponzetto C, Comoglio PM. Identification of functional domains in the hepatocyte growth factor and its receptor by molecular engineering. J. Biotechnol. 1994;37:109–122. doi: 10.1016/0168-1656(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 34.Gandino L, Longati P, Medico E, Prat M, Comoglio PM. Phosphorylation of serine 985 negatively regulates the hepatocyte growth factor receptor kinase. J. Biol. Chem. 1994;269:1815–1820. [PubMed] [Google Scholar]
- 35.Peschard P, Ishiyama N, Lin T, Lipkowitz S, Park M. A conserved DpYR motif in the juxtamembrane domain of the Met receptor family forms an atypical c-Cbl/Cbl-b tyrosine kinase binding domain binding site required for suppression of oncogenic activation. J. Biol. Chem. 2004;279:29565–29571. doi: 10.1074/jbc.M403954200. [DOI] [PubMed] [Google Scholar]
- 36.Longati P, Bardelli A, Ponzetto C, Naldini L, Comoglio PM. Tyrosines1234–1235 are critical for activation of the tyrosine kinase encoded by the MET proto-oncogene (HGF receptor) Oncogene. 1994;9:49–57. [PubMed] [Google Scholar]
- 37.Zhu H, Naujokas MA, Fixman ED, Torossian K, Park M. Tyrosine 1356 in the carboxyl-terminal tail of the HGF/SF receptor is essential for the transduction of signals for cell motility and morphogenesis. J. Biol. Chem. 1994;269:29943–29948. [PubMed] [Google Scholar]
- 38.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio PM. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 39.Ponzetto C, Bardelli A, Maina F, Longati P, Panayotou G, Dhand R, Waterfield MD, Comoglio PM. A novel recognition motif for phosphatidylinositol 3-kinase binding mediates its association with the hepatocyte growth factor/scatter factor receptor. Mol. Cell. Biol. 1993;13:4600–4608. doi: 10.1128/mcb.13.8.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maina F, Casagranda F, Audero E, Simeone A, Comoglio PM, Klein R, Ponzetto C. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell. 1996;87:531–542. doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- 41.Zarnegar R. Regulation of HGF and HGFR gene expression. Exs. 1995;74:33–49. doi: 10.1007/978-3-0348-9070-0_3. [DOI] [PubMed] [Google Scholar]
- 42.Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. Embo. J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung W, Castren E, Odenthal M, Vande Woude GF, Ishii T, Dienes HP, Lindholm D, Schirmacher P. Expression and functional interaction of hepatocyte growth factor-scatter factor and its receptor c-met in mammalian brain. J. Cell Biol. 1994;126:485–494. doi: 10.1083/jcb.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okano J, Shiota G, Kawasaki H. Expression of hepatocyte growth factor (HGF) and HGF receptor (c-met) proteins in liver diseases: an immunohistochemical study. Liver. 1999;19:151–159. doi: 10.1111/j.1478-3231.1999.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 45.Kmiecik TE, Keller JR, Rosen E, Vande Woude GF. Hepatocyte growth factor is a synergistic factor for the growth of hematopoietic progenitor cells. Blood. 1992;80:2454–2457. [PubMed] [Google Scholar]
- 46.Liu Y, Wilkinson FL, Kirton JP, Jeziorska M, Iizasa H, Sai Y, Nakashima E, Heagerty AM, Canfield AE, Alexander MY. Hepatocyte growth factor and c-Met expression in pericytes: implications for atherosclerotic plaque development. J. Pathol. 2007;212:12–19. doi: 10.1002/path.2155. [DOI] [PubMed] [Google Scholar]
- 47.Rahimi N, Tremblay E, McAdam L, Park M, Schwall R, Elliott B. Identification of a hepatocyte growth factor autocrine loop in a murine mammary carcinoma. Cell Growth Differ. 1996;7:263–270. [PubMed] [Google Scholar]
- 48.Anastasi S, Giordano S, Sthandier O, Gambarotta G, Maione R, Comoglio P, Amati P. A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation. J. Cell Biol. 1997;137:1057–1068. doi: 10.1083/jcb.137.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Otsuka T, Takayama H, Sharp R, Celli G, LaRochelle WJ, Bottaro DP, Ellmore N, Vieira W, Owens JW, Anver M, Merlino G. c-Met autocrine activation induces development of malignant melanoma and acquisition of the metastatic phenotype. Cancer Res. 1998;58:5157–5167. [PubMed] [Google Scholar]
- 50.Yi S, Tsao MS. Activation of hepatocyte growth factor-met autocrine loop enhances tumorigenicity in a human lung adenocarcinoma cell line. Neoplasia. 2000;2:226–234. doi: 10.1038/sj.neo.7900080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horiguchi N, Takayama H, Toyoda M, Otsuka T, Fukusato T, Merlino G, Takagi H, Mori M. Hepatocyte growth factor promotes hepatocarcinogenesis through c-Met autocrine activation and enhanced angiogenesis in transgenic mice treated with diethylnitrosamine. Oncogene. 2002;21:1791–1799. doi: 10.1038/sj.onc.1205248. [DOI] [PubMed] [Google Scholar]
- 52.Vadnais J, Nault G, Daher Z, Amraei M, Dodier Y, Nabi IR, Noel J. Autocrine activation of the hepatocyte growth factor receptor/met tyrosine kinase induces tumor cell motility by regulating pseudopodial protrusion. J. Biol. Chem. 2002;277:48342–48350. doi: 10.1074/jbc.M209481200. [DOI] [PubMed] [Google Scholar]
- 53.Peghini PL, Iwamoto M, Raffeld M, Chen YJ, Goebel SU, Serrano J, Jensen RT. Overexpression of epidermal growth factor and hepatocyte growth factor receptors in a proportion of gastrinomas correlates with aggressive growth and lower curability. Clin. Cancer Res. 2002;8:2273–2285. [PubMed] [Google Scholar]
- 54.Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro. Oncol. 2005;7:436–451. doi: 10.1215/S1152851705000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist. 2006;11:753–764. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- 56.Sheth PR, Hays JL, Elferink LA, Watowich SJ. Biochemical basis for the functional switch that regulates hepatocyte growth factor receptor tyrosine kinase activation. Biochemistry. 2008;47:4028–4038. doi: 10.1021/bi701892f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumoto K, Nakamura T. Mechanisms and significance of bifunctional NK4 in cancer treatment. Biochem. Biophys. Res. Commun. 2005;333:316–327. doi: 10.1016/j.bbrc.2005.05.131. [DOI] [PubMed] [Google Scholar]
- 58.Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T. HGF/NK4, a four-kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res. 2000;60:6737–6743. [PubMed] [Google Scholar]
- 59.Mazzone M, Basilico C, Cavassa S, Pennacchietti S, Risio M, Naldini L, Comoglio PM, Michieli P. An uncleavable form of pro-scatter factor suppresses tumor growth and dissemination in mice. J. Clin. Invest. 2004;114:1418–1432. doi: 10.1172/JCI22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kong-Beltran M, Stamos J, Wickramasinghe D. The Sema domain of Met is necessary for receptor dimerization and activation. Cancer Cell. 2004;6:75–84. doi: 10.1016/j.ccr.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Michieli P, Mazzone M, Basilico C, Cavassa S, Sottile A, Naldini L, Comoglio PM. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell. 2004;6:61–73. doi: 10.1016/j.ccr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 62.Tiran Z, Oren A, Hermesh C, Rotman G, Levine Z, Amitai H, Handelsman T, Beiman M, Chen A, Landesman-Milo D, Dassa L, Peres Y, Koifman C, Glezer S, Vidal-Finkelstein R, Bahat K, Pergam T, Israel C, Horev J, Tsarfaty I, Ayalon-Soffer M. A novel recombinant soluble splice variant of Met is a potent antagonist of the hepatocyte growth factor/scatter factor-Met pathway. Clin. Cancer Res. 2008;14:4612–4621. doi: 10.1158/1078-0432.CCR-08-0108. [DOI] [PubMed] [Google Scholar]
- 63.Kim KJ, Wang L, Su YC, Gillespie GY, Salhotra A, Lal B, Laterra J. Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin. Cancer Res. 2006;12:1292–1298. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- 64.Jun HT, Sun J, Rex K, Radinsky R, Kendall R, Coxon A, Burgess TL. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin. Cancer Res. 2007;13:6735–6742. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- 65.Martens T, Schmidt NO, Eckerich C, Fillbrandt R, Merchant M, Schwall R, Westphal M, Lamszus K. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin. Cancer Res. 2006;12:6144–6152. doi: 10.1158/1078-0432.CCR-05-1418. [DOI] [PubMed] [Google Scholar]
- 66.Tseng JR, Kang KW, Dandekar M, Yaghoubi S, Lee JH, Christensen JG, Muir S, Vincent PW, Michaud NR, Gambhir SS. Preclinical efficacy of the c-Met inhibitor CE-355621 in a U87 MG mouse xenograft model evaluated by 18F-FDG small-animal PET. J. Nucl. Med. 2008;49:129–134. doi: 10.2967/jnumed.106.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petrelli A, Circosta P, Granziero L, Mazzone M, Pisacane A, Fenoglio S, Comoglio PM, Giordano S. Ab-induced ectodomain shedding mediates hepatocyte growth factor receptor down-regulation and hampers biological activity. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5090–5095. doi: 10.1073/pnas.0508156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berthou S, Aebersold DM, Schmidt LS, Stroka D, Heigl C, Streit B, Stalder D, Gruber G, Liang C, Howlett AR, Candinas D, Greiner RH, Lipson KE, Zimmer Y. The Met kinase inhibitor SU11274 exhibits a selective inhibition pattern toward different receptor mutated variants. Oncogene. 2004;23:5387–5393. doi: 10.1038/sj.onc.1207691. [DOI] [PubMed] [Google Scholar]
- 69.Puri N, Khramtsov A, Ahmed S, Nallasura V, Hetzel JT, Jagadeeswaran R, Karczmar G, Salgia R. A selective small molecule inhibitor of c-Met, PHA665752, inhibits tumorigenicity and angiogenesis in mouse lung cancer xenografts. Cancer Res. 2007;67:3529–3534. doi: 10.1158/0008-5472.CAN-06-4416. [DOI] [PubMed] [Google Scholar]
- 70.Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, Yamazaki S, Alton GR, Mroczkowski B, Los G. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 2007;6:3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 71.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, Balak M, Chang WC, Yu CJ, Gazdar A, Pass H, Rusch V, Gerald W, Huang SF, Yang PC, Miller V, Ladanyi M, Yang CH, Pao W. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl. Acad. Sci. U. S. A. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srinivasan R, Choueiri TK, Vaishampayan U, Rosenberg JE, Stein MN, Logan T, Bukowski RM, Mueller T, Keer HN, Linehan WM. A phase II study of the dual MET/VEGFR2 inhibitor XL880 in patients (pts) with papillary renal carcinoma (PRC) J. Clin. Oncol. 2008;26:5103. [Google Scholar]
- 73.Salgia R, Sherman S, Hong DS, Ng CS, Frye J, Janisch L, Ratain MJ, Kurzrock R. A phase I study of XL184, a RET, VEGFR2, and MET kinase inhibitor, in patients (pts) with advanced malignancies, including pts with medullary thyroid cancer (MTC) J. Clin. Oncol. 2008;26:3522. [Google Scholar]
- 74.Maulik G, Madhiwala P, Brooks S, Ma PC, Kijima T, Tibaldi EV, Schaefer E, Parmar K, Salgia R. Activated c-Met signals through PI3K with dramatic effects on cytoskeletal functions in small cell lung cancer. J. Cell. Mol. Med. 2002;6:539–553. doi: 10.1111/j.1582-4934.2002.tb00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong AS, Roskelley CD, Pelech S, Miller D, Leung PC, Auersperg N. Progressive changes in Met-dependent signaling in a human ovarian surface epithelial model of malignant transformation. Exp. Cell Res. 2004;299:248–256. doi: 10.1016/j.yexcr.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 76.Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–4165. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]