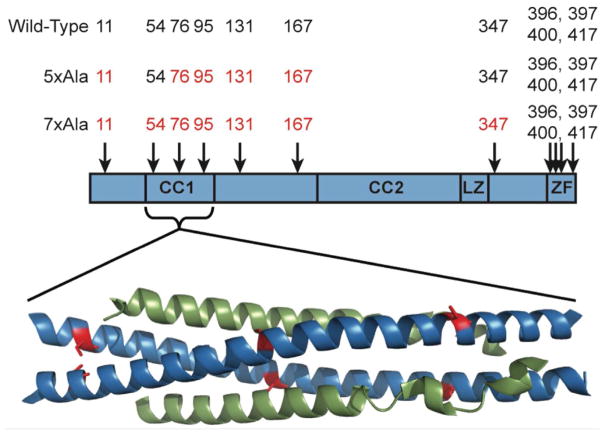
Figure 1.
Schematic representation of the domain structure of NEMO (59), showing the approximate locations of the 11 cysteine residues. CC1 and CC2 are the first and second coiled-coil regions, LZ is the leucine zipper region, and ZF is the zinc finger domain. Below the scheme is shown the X-ray co-crystal structure of NEMO(44-111) in complex with IKKβ(701-745) (30). Two molecules of NEMO(44-111) (blue) form a coiled coil that binds one molecule of IKKβ(701-745) (green) at each face. The locations of NEMO residues Cys54 (left), Cys76 (middle) and Cys95 (right) are highlighted in red.