Abstract
Objectives
Compared to healthy controls, patients with fibromyalgia (FM) have more mast cells in the skin. Whether mast cells are involved in the pathogenesis of FM is unclear. We sought to determine the effects of a mast cell stabilizer (ketotifen) on FM symptoms.
Methods
Fifty-one FM subjects were randomized to daily oral ketotifen 2 mg BID (n=24) for 8 weeks or placebo (N=27). Mean age of subjects was 51.2 years (standard deviation/SD 8.4); 88% were female and 88% were white; 22% were taking concomitant opiates; and mean pressure pain sensitivity (range 0-20) was 10.0 (0.4). At study entry, the weekly average pain intensity was 6.4 (1.1) and the mean score on the Revised Fibromyalgia Impact Questionnaire (FIQR) was 66.8 (14.0).
Results
We found no statistically significant treatment group differences from baseline in either group for the two primary measures: weekly average pain intensity [ketotifen −1.3 (1.9) vs. placebo −1.5 (1.9), p=0.7]; and FIQR score [−12.1 (19.5) vs. −12.2 (18.1), p=0.9]. No secondary outcome measures (BPI pain intensity, and pressure pain sensitivity) reached statistical significance; results did not differ in the intent-to-treat and completer analyses. Other than transient sedation [6 (28.6%) vs. 1 (4.0%)], ketotifen was well tolerated.
Discussion
The study results question whether skin mast cells play a major role in the pathogenesis of FM. However, given the role of mast cells in peripheral and central nociception, and the minimal side effects of ketotifen, a randomized clinical trial using increasing doses of ketotifen may be warranted.
Keywords: Fibromyalgia, Mast Cell Stabilizer, Ketotifen, Pain
INTRODUCTION
Despite the enormous societal and personal burden of fibromyalgia (FM) (1;2), its treatment remains suboptimal. Only one-third of patients in randomized clinical trials achieve some benefit from FDA-approved drugs for FM (3-5). Poor treatment outcomes may be explained by the lack of clear understanding of the pathophysiology of FM. In the last 15 years, studies on the pathogenic mechanisms of FM have mostly focused on central sensitization, meaning the enhanced responsiveness of neurons in the central nervous system that leads to pain amplification. In previous studies, FM patients showed increased sensitivity to mechanical, thermal, and electrical stimuli (6;7).
The role of peripheral impulse activity in dynamically maintaining central sensitization has also been proposed as a mechanism for FM (8;9). For example, ongoing afferent input from peripheral sources may contribute to increased tonic nociceptive input into the spinal cord that results in augmented pain processing and central sensitization. In one study, a single lidocaine injection into a trapezius muscle tender point of FM subjects resulted in decreased mechanical hyperalgesia at the shoulder, and reduced distal secondary heat hyperalgesia in the forearm (10). Thus, reductions of impulse input from painful muscle tissue at least partially normalize distal hyperalgesia in FM patients.
Because the skin is the most extensive organ of the human body, ongoing peripheral input to maintain central sensitization may also arise from the skin. Several investigators have reported abnormalities in skin biopsies from FM patients. Kim et al reported increased expression of N-methyl-D-aspartate receptors (subtype 2D) in the skin of patients with FM vs. controls (11) . Littlejohn et al noted a reduced threshold for capsaicin-induced vasodilatation skin response in FM patients compared to healthy controls (12). The detection of interleukin-1b, interleukin-6, and tumor necrosis factor-alpha (TNF-alpha) in the skin of about 30% of FM patients suggests an inflammatory component in their FM-related pain (13). Other immunohistochemical and morphological skin changes in FM include significantly higher values of IgG deposits in the dermis and vessel walls, and a higher mean number of mast cells (14). The percentage of damaged / degranulated mast cells and the individual IgG immunofluorescence scores were correlated (14). More recently, Blanco et al reported increased number of mast cells in 100% of study participants with FM, and mast cells were increased up to 14 times compared to controls (15). With the close functional and anatomical association of mast cells with sensory nerves in the skin (16-18), increased amount of mast cell inflammatory mediators (i.e., histamine, proteases, prostaglandins and leukotrienes) and neurosensitizing molecules (i.e., TNF-alpha, monocyte chemoattractant protein-1, and interleukin-8) (19;20) may contribute to increased tonic input into the central nervous system. To date, no study has examined if the reported increased number of activated skin mast cells is clinically relevant in FM or just an epiphenomenon.
Ketotifen is a mast cell stabilizer used for the management of bronchial asthma and allergic disorders such as atopic dermatitis, allergic rhinitis/conjunctivitis, and chronic urticaria (21-25). Oral ketotifen possesses both anti-mediator and mast cell-stabilizing properties (25). In experimental and clinical conditions, ketotifen reduced antigen-induced mast cell degranulation and decreased the release of histamine, tryptase, and various prostaglandins (21;25). Ketotifen also stabilizes calcium permeability in mast cell membranes (21;26-28). Additionally, ketotifen reduces TNF-alpha levels (29;30), macrophage-derived chemokines (31), and IL-8 (32).
Given the potential role of skin mast cells in FM pathogenesis, we hypothesized that subjects receiving oral ketotifen would exhibit larger reduction in pressure hyperalgesia and greater improvement in the severity of FM symptoms than subjects who received placebo. Confirmation of the latter hypothesis would support the pathogenic role of skin mast cells in FM.
MATERIALS AND METHODS
Study Design and Procedures
We conducted a 10-week randomized, double-blind, placebo-controlled trial in which participants were randomized either to oral ketotifen or placebo (control). Randomization was stratified by use of opiates at study entry. The study had 3 phases: 1 week for screening and baseline assessment, 1 week for dose escalation, and 8 weeks for either ketotifen or placebo. Outcome assessment was performed at the end of week 1 (baseline) and week 10. During the 1-week screening phase, subjects entered their daily pain scores on a wristwatch monitor (Actiwatch). At the end of the 1-week screening period and after completing baseline assessments, eligible subjects were randomized to oral ketotifen or placebo. During the 1-week dose escalation, subjects received ketotifen 1 mg BID or an identical placebo. We used the SAS statistical package software to randomly assign subjects to the two groups by using a random number generator. Thereafter, subjects received ketotifen 2 mg BID or an identical placebo throughout the 8-week stable-dose phase. The 4 mg daily dose of oral ketotifen is the most commonly used dose for allergic skin disorders and bronchial asthma (21;22;33). Both the active medication and placebo were manufactured (Tiofarma in the Netherlands) with no difference in odor and/or appearance between the capsules containing ketotifen and the placebo.
Subjects were allowed to continue use of centrally-acting medications, including anticonvulsants, opiates, muscle relaxants, and other analgesics. To reduce co-intervention effects, subjects were instructed to stay on the same baseline medication regimen (including dose and frequency) throughout the 10-week study period. To avoid drug effects on pressure pain sensitivity, subjects were asked to avoid any as-needed medications (e.g., hydrocodone, acetaminophen, etc.) for at least 6 hours and any non-steroidal anti-inflammatory agents (NSAIDs) for 48 hours before each outcome assessment. Using a standardized side effects questionnaire medication safety assessments were conducted at the end of the 1-week escalation phase and once weekly until the week 10 outcome assessment visit.
The study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Study procedures, including written informed consent, were approved by Indiana University-Purdue University Indianapolis Institutional Review Board.
Eligibility
The inclusion criteria were as follows: (1) met the American College of Rheumatology (ACR) criteria for FM(34); (2) age range: 18-65; (3) had weekly average pain intensity score ≥ 4; and (3) on stable doses of medications for FM for at least 4 weeks.
Excluded were individuals (1) had any history of seizures; (2) had atopic dermatitis or chronic urticaria; (3) had chronic thrombocytopenia; (4) were pregnant or lactating; (5) had active psychoses; (6) had inflammatory rheumatic diseases (e.g., rheumatoid arthritis, lupus, etc.); (7) planned elective surgery; (8) had an ongoing unresolved disability claim; (9) had abnormal clinical laboratory parameters (i.e., elevated SGPT and low platelet count); or (9) currently using anti-allergy drugs (ophthalmic or oral histamine antagonist), leukotriene inhibitors (e.g., montelukast), or prednisone.
Primary Outcome Measures
Weekly average pain intensity
Using the Actiwatch, subjects entered their daily pain scores twice a day for one week at baseline and week 10. Subjects rated their pain intensity on a 0-to-10 numerical rating scale, with 0 = no pain sensation and 10 = the most intense pain sensation imaginable. The weekly average pain intensity was the average of the 14 pain intensity scores obtained during the 7-day assessment window. This composite measure of weekly average pain intensity had shown sensitivity to change in chronic pain intervention studies (35-37). The change in the weekly average pain intensity over time was one of the study’s primary endpoints.
Fibromyalgia Impact Questionnaire-Revised (FIQR)
FIQR is a disease-specific measure assessing a number of functioning domains related to fibromyalgia (38). The FIQR has 3 linked sets of domains: FIQ-symptom, FIQ-overall impact and FIQ-function. The FIQR has excellent correlation with the original FIQ (38;39). A higher score (score range= 0-100) indicates greater severity of global symptoms. Change in the FIQR scores from baseline (end of week 1) to week 10 was the second primary endpoint.
Secondary Outcome Measures
The (long form) Brief Pain Inventory (BPI) is a measure of pain with proven reliability and validity across different pain conditions (40;41) . The BPI rates the intensity of pain (BPI pain intensity) and interference of pain with mood, physical activity, work, social activity, relations with others, sleep, and enjoyment of life (BPI pain interference).
The Thumb Pressure Pain Sensitivity Test was used to assess pain sensitivity. The test uses a random direct scaling method to deliver stimuli of varying pressures (5 pressure levels with 3 repetitions at each level) in a random sequence (42-44). Because subjects are unaware of this random sequence, they must attend to, and rate, the stimulus-evoked sensations; therefore, the results are more meaningful (less prone to bias) (45). In this study, discrete 5-second pressure stimuli were applied to the left thumb by a 1-cm2 hard rubber probe. Using a numeric verbally anchored (0-20) pain scale (42), subjects rated the intensity of evoked pressure-pain sensations. During the screening visit, each subject completed a stair step acclimatization program in which stimulus pressures were applied to determine the individual’s pain threshold and tolerance. Based on the pain threshold and tolerance, the software generated 5 stimulus pressures. Three random sequences of these 5 stimulus pressures (total of 15 pressures) was then applied to the left thumb at baseline and week 10 visits. Higher evoked pain scores on the numeric (0-20) pain scale represent greater sensitivity to pressure pain stimuli.
We also collected data on FM-related medications, depression severity (Patient Health Questionnaire 8-item) (46-48) and body mass index at baseline.
Analysis
Based on feasibility and precision around estimates for designing future studies Julious SA recommends a minimum of 12 evaluable participants per group be considered for pilot studies (49). In our study, we over-recruited 24 subjects per group to account for a potential large dropout rate.
Treatment group comparisons were performed on baseline variables using t-tests for continuous variables and chi-square tests for categorical variables. Efficacy outcomes were analyzed with t-tests comparing ketotifen and placebo groups on change from baseline to 10 weeks. Linear mixed effects models were used for evoked pain scores, since subjects had multiple scores for varying baseline pressure stimuli. These models controlled the within-person correlation due to multiple scores per subject. Baseline pressure stimulus was also included as a covariate. The primary method of analysis was intent to treat analysis. If subjects did not complete follow-up questionnaires, the last observation carried forward (LOCF) was used to impute 10-week scores but only for 5 participants (3 subjects in the active group and 2 in the placebo). Given that the LOCF method is prone to bias (50) results from the completer analyses were also reported. Statistical analyses were performed using SAS version 9.1.
RESULTS
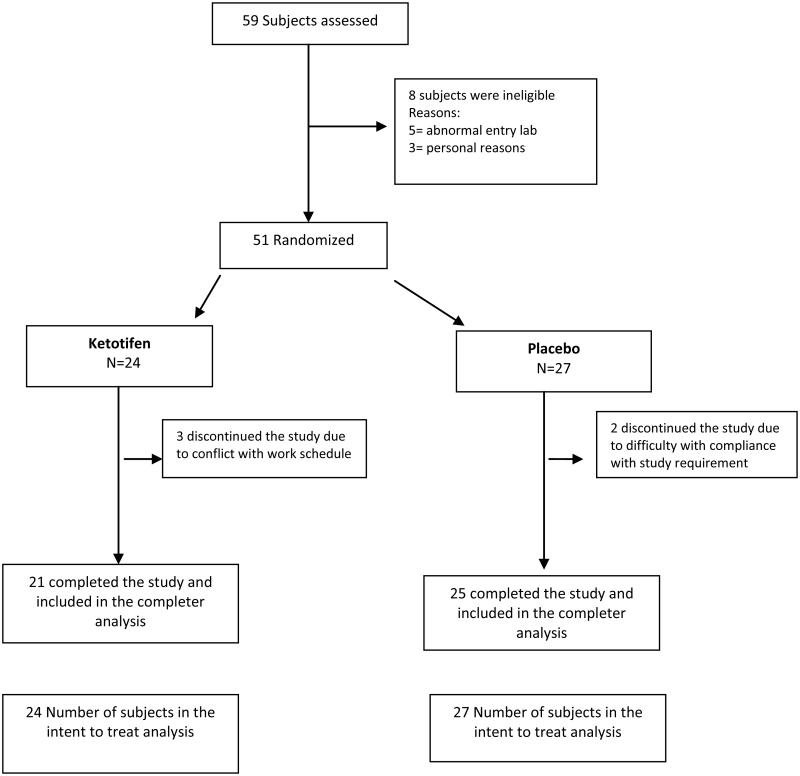
A total of 59 subjects were screened for potential participation in the study. Of these, 51 (86%) met the inclusion criteria, enrolled, and were randomized to ketotifen (n=24) or placebo (n=27). Table 1 (last column) shows the demographic characteristics of the entire cohort. Most screening failures were due to abnormal laboratory results at entry (5/8, 62%). Baseline characteristics were similar across treatment group except for race (Table 1). The active treatment group had fewer white subjects compared to the placebo group (p=0.09).
We found no statistically significant treatment group differences from baseline in either weekly average pain intensity [ketotifen −1.3 (1.9) vs. placebo −1.5 (1.9), p=0.7] or FIQR scores [−12.1 (19.5) vs. −12.2 (18.1), p=0.9] (Table 2). Further, no secondary outcome measures (BPI pain intensity, BPI pain interference, and evoked pain score) reached statistical significance (p>0.5). Results did not differ in the intent-to-treat and completer analyses. Three subjects in the ketotifen group and 2 in the placebo group discontinued the study due to either work schedule conflict or study protocol violations.
Other than transient sedation [6 (28.6%) vs. 1 (4.0%)], oral ketotifen was well tolerated (Table 4).
DISCUSSION
The goal of this randomized clinical trial was to determine if the reported increased number of skin mast cells in FM is clinically relevant or just epiphenomenon. We found that ketotifen, a well-known mast cell stabilizer, did not reduce pain sensitivity, improve clinical pain, or reduce overall FM symptom severity.
Several studies have shown that mast cells play a role in chronic pain, particularly at the visceral level (51-54). For example, the degranulation of mast cells, in close proximity to the nerves innervating the colonic mucosa, is correlated with abdominal pain in patients with irritable bowel syndrome (IBS) (51;52). Ketotifen, but not placebo, decreased abdominal pain, improved quality of life, and increased the threshold for discomfort in IBS patients with visceral hypersensitivity (55).
Despite the common elements of IBS and FM (56-58), in contrast to the IBS study (55), our null findings in this FM study may be explained by one or a combination of the following: (1) mast cells are not major players in the pathogenesis of FM; (2) mast cells play a pathogenic role, but only in a subset of FM subjects who are highly pain sensitive (similar to IBS); or (3) the degree of mast cell stabilization may have been inadequate, since we used a much lower daily dose of ketotifen than in the IBS study (4 mg vs.16 mg) (55). Unfortunately, we lacked funding to collect skin samples to directly assess the effects of ketotifen (e.g., release of histamine and tryptase) on skin mast cells. Given that a third of FM patients report sensitivity or intolerance to medications and/or chemicals (59) we opted to use the lower 4 mg daily dose, a commonly used dose for various allergic disorders (21;22;33). Finally, our null findings also may be attributed to low statistical power. To achieve 80% power and detect a moderate effect size, 64 participants are needed in each group to detect one-half standard deviation difference between groups.
In conclusion, our study shows that ketotifen at 4 mg daily was well tolerated but was not associated with significant changes on pain sensitivity and FM symptoms. The study results question the clinical significance of the previously reported increase number of skin mast cells in the pathogenesis of FM. However, given the epidemiologic and biologic link between IBS and FM (56-58), the role of mast cells in peripheral and central nociception (60), and the minimal side effects of oral ketotifen, investigators should consider conducting adequately powered randomized clinical trials using increasing doses of ketotifen for FM patients, who have limited treatment options.
Supplementary Material
Figure 1.
Flow of Participants in the Trial
ACKNOWLEDGEMENT
Funding: National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant number: R21 AR061807). The funding institution did not play any role in the collection, analysis, data interpretation, and writing of the manuscript.
Funding Support:
This study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R21 AR061807; PI: Ang)
Reference List
- (1).Annemans L, Le LK, Taieb C. Societal and patient burden of fibromyalgia syndrome. Pharmacoeconomics. 2009;27(7):547–59. doi: 10.2165/11313650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- (2).Spaeth M. Epidemiology, costs, and the economic burden of fibromyalgia. Arthritis Res Ther. 2009;11(3):117. doi: 10.1186/ar2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Crofford LJ, Rowbotham MC, Mease PJ, Russell IJ, Dworkin RH, Corbin AE, et al. Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52(4):1264–73. doi: 10.1002/art.20983. [DOI] [PubMed] [Google Scholar]
- (4).Mease PJ, Clauw DJ, Gendreau RM, Rao SG, Kranzler J, Chen W, et al. The efficacy and safety of milnacipran for treatment of fibromyalgia. a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36(2):398–409. doi: 10.3899/jrheum.080734. [DOI] [PubMed] [Google Scholar]
- (5).Arnold LM, Rosen A, Pritchett YL, D'Souza DN, Goldstein DJ, Iyengar S, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain. 2005;119(1-3):5–15. doi: 10.1016/j.pain.2005.06.031. [DOI] [PubMed] [Google Scholar]
- (6).Gracely RH, Grant MA, Giesecke T. Evoked pain measures in fibromyalgia. Best Pract Res Clin Rheumatol. 2003;17(4):593–609. doi: 10.1016/s1521-6942(03)00036-6. [DOI] [PubMed] [Google Scholar]
- (7).Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46(5):1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- (8).Mense S. The pathogenesis of muscle pain. Curr Pain Headache Rep. 2003;7(6):419–25. doi: 10.1007/s11916-003-0057-6. [DOI] [PubMed] [Google Scholar]
- (9).Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102(1-2):87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- (10).Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain. 2009;145(1-2):96–104. doi: 10.1016/j.pain.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kim SH, Jang TJ, Moon IS. Increased expression of N-methyl-D-aspartate receptor subunit 2D in the skin of patients with fibromyalgia. J Rheumatol. 2006;33(4):785–8. [PubMed] [Google Scholar]
- (12).Littlejohn GO, Weinstein C, Helme RD. Increased neurogenic inflammation in fibrositis syndrome. J Rheumatol. 1987;14(5):1022–5. [PubMed] [Google Scholar]
- (13).Salemi S, Rethage J, Wollina U, Michel BA, Gay RE, Gay S, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30(1):146–50. [PubMed] [Google Scholar]
- (14).Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia--relevant findings or epiphenomena? Scand J Rheumatol. 1997;26(4):308–13. doi: 10.3109/03009749709105321. [DOI] [PubMed] [Google Scholar]
- (15).Blanco I, Beritze N, Arguelles M, Carcaba V, Fernandez F, Janciauskiene S, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010 doi: 10.1007/s10067-010-1474-7. [DOI] [PubMed] [Google Scholar]
- (16).Skofitsch G, Savitt JM, Jacobowitz DM. Suggestive evidence for a functional unit between mast cells and substance P fibers in the rat diaphragm and mesentery. Histochemistry. 1985;82(1):5–8. doi: 10.1007/BF00502084. [DOI] [PubMed] [Google Scholar]
- (17).Siebenhaar F, Magerl M, Peters EM, Hendrix S, Metz M, Maurer M. Mast cell-driven skin inflammation is impaired in the absence of sensory nerves. J Allergy Clin Immunol. 2008;121(4):955–61. doi: 10.1016/j.jaci.2007.11.013. [DOI] [PubMed] [Google Scholar]
- (18).Sugiura H, Maeda T, Uehara M. Mast cell invasion of peripheral nerve in skin lesions of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1992;176:74–6. [PubMed] [Google Scholar]
- (19).Okayama Y, Ono Y, Nakazawa T, Church MK, Mori M. Human skin mast cells produce TNF-alpha by substance P. Int Arch Allergy Immunol. 1998;117(Suppl 1):48–51. doi: 10.1159/000053571. [DOI] [PubMed] [Google Scholar]
- (20).Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Grant SM, Goa KL, Fitton A, Sorkin EM. Ketotifen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs. 1990;40(3):412–48. doi: 10.2165/00003495-199040030-00006. [DOI] [PubMed] [Google Scholar]
- (22).Kamide R, Niimura M, Ueda H, Imamura S, Yamamoto S, Yoshida H, et al. Clinical evaluation of ketotifen for chronic urticaria: multicenter double-blind comparative study with clemastine. Ann Allergy. 1989;62(4):322–5. [PubMed] [Google Scholar]
- (23).Mansfield LE, Taistra P, Santamauro J, Ting S, Andriano K. Inhibition of dermographia, histamine, and dextromethorphan skin tests by ketotifen. A possible effect on cutaneous vascular response to mediators. Ann Allergy. 1989;63(3):201–6. [PubMed] [Google Scholar]
- (24).Taskapan O, Harmanyeri Y. Ketotifen and chronic urticaria. Arch Dermatol. 1998;134(2):240–1. doi: 10.1001/archderm.134.2.240. [DOI] [PubMed] [Google Scholar]
- (25).Craps LP. Immunologic and therapeutic aspects of ketotifen. J Allergy Clin Immunol. 1985;76(2):389–93. doi: 10.1016/0091-6749(85)90659-1. Pt 2. [DOI] [PubMed] [Google Scholar]
- (26).Schoch C. In vitro inhibition of human conjunctival mast-cell degranulation by ketotifen. J Ocul Pharmacol Ther. 2003;19(1):75–81. doi: 10.1089/108076803762718132. [DOI] [PubMed] [Google Scholar]
- (27).Sharif NA, Xu SX, Miller ST, Gamache DA, Yanni JM. Characterization of the ocular antiallergic and antihistaminic effects of olopatadine (AL-4943A), a novel drug for treating ocular allergic diseases. J Pharmacol Exp Ther. 1996;278(3):1252–61. [PubMed] [Google Scholar]
- (28).Lambiase A, Micera A, Bonini S. Multiple action agents and the eye: do they really stabilize mast cells? Curr Opin Allergy Clin Immunol. 2009;9(5):454–65. doi: 10.1097/ACI.0b013e3283303ebb. [DOI] [PubMed] [Google Scholar]
- (29).Ockenga J, Rohde F, Suttmann U, Herbarth L, Ballmaier M, Schedel I. Ketotifen in HIV-infected patients: effects on body weight and release of TNF-alpha. Eur J Clin Pharmacol. 1996;50(3):167–70. doi: 10.1007/s002280050087. [DOI] [PubMed] [Google Scholar]
- (30).Galatowicz G, Ajayi Y, Stern ME, Calder VL. Ocular anti-allergic compounds selectively inhibit human mast cell cytokines in vitro and conjunctival cell infiltration in vivo. Clin Exp Allergy. 2007;37(11):1648–56. doi: 10.1111/j.1365-2222.2007.02782.x. [DOI] [PubMed] [Google Scholar]
- (31).Hung CH, Li CY, Lai YS, Hsu PC, Hua YM, Yang KD. Discrepant clinical responses and blood chemokine profiles between two non-steroidal anti-inflammatory medications for children with mild persistent asthma. Pediatr Allergy Immunol. 2005;16(4):306–9. doi: 10.1111/j.1399-3038.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- (32).Ohtani T, Aiba S, Mizuashi M, Kawamoto Y, Tagami H. Evaluation of the efficacy of antihistamines using human monocyte-derived dendritic cells stimulated with histamine. J Am Acad Dermatol. 2003;49(2):234–42. doi: 10.1067/s0190-9622(03)01478-6. [DOI] [PubMed] [Google Scholar]
- (33).Schwarzer G, Bassler D, Mitra A, Ducharme FM, Forster J. Ketotifen alone or as additional medication for long-term control of asthma and wheeze in children. Cochrane Database Syst Rev. 2004;(1):CD001384. doi: 10.1002/14651858.CD001384.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- (35).Ehde DM, Jensen MP. Feasibility of a cognitive restructuring intervention for treatment of chronic pain in persons with disabilities. Rehabilitation Psychology. 2004;49:254–8. [Google Scholar]
- (36).Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain. 2002;96(3):365–73. doi: 10.1016/S0304-3959(01)00483-3. [DOI] [PubMed] [Google Scholar]
- (37).Jensen MP, Hanley MA, Engel JM, Romano JM, Barber J, Cardenas DD, et al. Hypnotic analgesia for chronic pain in persons with disabilities: a case series. Int J Clin Exp Hypn. 2005;53(2):198–228. doi: 10.1080/00207140590927545. [DOI] [PubMed] [Google Scholar]
- (38).Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther. 2009;11(4):R120. doi: 10.1186/ar2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23(5):S154–S162. Suppl 39. [PubMed] [Google Scholar]
- (40).Arnold LM, Lu Y, Crofford LJ, Wohlreich M, Detke MJ, Iyengar S, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50(9):2974–84. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- (41).Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–7. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- (42).Gracely RH, Dubner R, McGrath PA. Narcotic analgesia: fentanyl reduces the intensity but not the unpleasantness of painful tooth pulp sensations. Science. 1979;203(4386):1261–3. doi: 10.1126/science.424753. [DOI] [PubMed] [Google Scholar]
- (43).Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, et al. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144(1-2):95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- (44).Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105(3):403–13. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- (45).Gracely RH. Studies of Experimental Human Pain. In: McMahon S, Koltzenburg M, editors. Wall and Melzack's Textbook of Pain. 5th Elsevier, Health Sciences Division, Churchill Livingstone; Philadelphia, PA: 2005. pp. 267–89. [Google Scholar]
- (46).Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatric annals. 2002;32(9):1–7. [Google Scholar]
- (47).Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1-3):163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- (48).Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–59. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- (49).Julious S. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Statist. 2005;4:287–91. [Google Scholar]
- (50).Molnar FJ, Hutton BF, Fergusson D. Does analysis using "last observation carried forward" introduce bias in dementia research?(1488-2329 (Electronic)) doi: 10.1503/cmaj.080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Barbara G, Wang B, Stanghellini V, de GR, Cremon C, Di NG, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132(1):26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- (52).Barbara G, Stanghellini V, de GR, Cremon C, Cottrell GS, Santini D, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- (53).Gao G, Ouyang A, Kaufman MP, Yu S. ERK1/2 signaling pathway in mast cell activation-induced sensitization of esophageal nodose C-fiber neurons. Dis Esophagus. 2011;24(3):194–203. doi: 10.1111/j.1442-2050.2010.01127.x. [DOI] [PubMed] [Google Scholar]
- (54).Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology. 2007;69(4):34–40. doi: 10.1016/j.urology.2006.08.1109. Suppl. [DOI] [PubMed] [Google Scholar]
- (55).Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der HS, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59(9):1213–21. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- (56).Riedl A, Schmidtmann M, Stengel A, Goebel M, Wisser AS, Klapp BF, et al. Somatic comorbidities of irritable bowel syndrome: a systematic analysis. J Psychosom Res. 2008;64(6):573–82. doi: 10.1016/j.jpsychores.2008.02.021. [DOI] [PubMed] [Google Scholar]
- (57).Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol. 2007;13(27):3699–704. doi: 10.3748/wjg.v13.i27.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Kurland JE, Coyle WJ, Winkler A, Zable E. Prevalence of irritable bowel syndrome and depression in fibromyalgia. Dig Dis Sci. 2006;51(3):454–60. doi: 10.1007/s10620-006-3154-7. [DOI] [PubMed] [Google Scholar]
- (59).Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160(2):221–7. doi: 10.1001/archinte.160.2.221. [DOI] [PubMed] [Google Scholar]
- (60).Heron A, Dubayle D. A focus on mast cells and pain. J Neuroimmunol. 2013;264(1-2):1–7. doi: 10.1016/j.jneuroim.2013.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.