Abstract
Numerous studies have linked severe stress to the development of major depressive disorder (MDD), and suicidal behaviors. Furthermore, recent preclinical studies from our laboratory and others have demonstrated that in rodents, chronic stress and the stress hormone cortisol has caused oxidative damage to mitochondrial function and membrane lipids in the brain. Mitochondria play a key role in synaptic neurotransmitter signaling by providing adenosine triphosphate (ATP), mediating lipid and protein synthesis, buffering intracellular calcium, and regulating apoptotic and resilience pathways. Membrane lipids are similarly essential to central nervous system (CNS) function, because cholesterol, polyunsaturated fatty acids, and sphingolipids form a lipid raft region, a special lipid region on the membrane that mediates neurotransmitter signaling through G-protein coupled receptors and ion channels. Low serum cholesterol levels, low antioxidant capacity, and abnormal early morning cortisol levels are biomarkers consistently associated with both depression and suicidal behaviors. In this review, we summarize the manner in which nutrients can protect against oxidative damage to mitochondria and lipids in the neuronal circuits associated with cognitive and affective behaviors. These nutrients include ω3 fatty acids, antioxidants (vitamin C and zinc), members of the vitamin B family (Vitamin B12 and folic acid) and magnesium. Accumulating data have shown that these nutrients can enhance neurocognitive function, and may have therapeutic benefits for depression and suicidal behaviors. A growing body of studies suggests the intriguing possibility that regular consumption of these nutrients may help prevent the onset of mood disorders and suicidal behaviors in vulnerable individuals, or significantly augment the therapeutic effect of available antidepressants. These findings have important implications for the health of both military and civilian populations.
Keywords: vitamin, oxidative stress, synaptic plasticity, lipid, suicide, zinc
INTRODUCTION
Chronic severe stress has been directly implicated in the pathogenesis of depression (Charney and Manji, 2004), and suicidal behaviors (Robinson et al., 2009). In United States (US) military populations, the suicide rate has been found to correlate with the frequency of deployment, suggesting that the prolonged exposure to stress levels may play a role (Bryan, 2010). Historically, suicide rates for active duty military personnel in the US and in other industrialized countries were lower than suicide rates for the general population—usually less than half (Rothberg et al., 1990). However, studies over the last decade have described rising suicide rates in the US military populations (Lineberry, 2009), a finding that has lent increasing urgency to both military and civilian efforts at suicide prevention.
It has been well-established that the hypothalamic-pituitary-adrenal (HPA) axis is activated during stress, with increased levels of the stress hormone cortisol. For instance, cortisol serum levels are elevated in many depressed patients, and the dexamethasone suppression test (DST) did not inhibit serum cortisol levels (Sher, 2007). Patients with Cushing’s disease, which is characterized by high cortisol levels, commonly develop depressive symptoms (Wolkowitz et al., 2001). Altered morning cortisol levels have also been noted in individuals who attempted suicide (Sher, 2007). Moreover, it is noteworthy that "depression" in the sense of a low mood is a symptom of many vitamin/mineral deficiencies (e.g. Folic acid, vitamin C, magnesium.) (Bourre, 2004). Therefore, it is important to prevent depressive episodes due to vitamin/mineral deficiency, particularly for people under high levels of stress. The role of nutrients in the treatment of depression and suicidal behaviors has been extensively studied in the civilian population (Cocchi et al., 1980, Papakostas et al., 2005, Enya et al., 2004, Li and Zhang, 2007, Sowa-Kucma et al., 2008). Whether or not the nutrients may help as a strategy for the prevention or adjunctive treatment of depression or suicidal ideation in the military and civilian population remains underexplored.
Recent preclinical and clinical studies have shown that chronic severe stress causes oxidative damage to mitochondrial function and to membrane lipids, resulting in aberrant neurotransmitter signaling and information processing in synapses and circuits mediating affective, cognitive, motoric, and neurovegetative behaviors (Shelton, 2007). We begin this review by describing the relevant evidence linking disrupted mitochondrial function, membrane lipids, and neurotransmitter signaling with depression, and suicidal behaviors. We then summarize the clinical research data demonstrating that specific nutrients that act to protect mitochondria and neurotransmitter signaling are involved in the pathogenesis and treatment of these disorders. These nutrients include ω3 fatty acids, antioxidants, B family vitamins, and magnesium. Finally, we discuss the preclinical and clinical evidence that regular use of these nutrients may have preventive effects in the treatment of depression or suicidal behavior.
STRESS AND SLEEP DEPRIVATION CAUSE OXIDATIVE DAMAGE TO MITOCHONDRIAL FUNCTION AND LIPID COMPOSITION, LEADING TO IMPAIRED NEUROTRANSMITTER SIGNALING
Stress and altered HPA axis activity have been shown to increase oxidative damage and reduce antioxidant defense (Epel, 2009, Irie et al., 2005, McIntosh and Sapolsky, 1996, Wolkowitz et al., 2008). Oxidative stress occurs when the production of oxygen-free radicals exceeds the capacity of antioxidants to neutralize them (McIntosh and Sapolsky, 1996). After chronic severe stress, in vivo glutathione (GSH, decrease of which is an indicator for oxidative stress) levels were found to be depleted, suggesting a state of oxidative stress (Madrigal et al., 2001). The study further noted that mitochondrial function was also reduced after chronic stress. Inhibiting GSH depletion by aminoguanidine (a nitric oxide synthase inhibitor) protected against the mitochondrial dysfunction induced by chronic stress (Madrigal et al., 2001). In addition, lipids in the brain are particularly vulnerable to oxidative stress, which has been found to induce lipid peroxidation, and leads to degradation of polyunsaturated fatty acids (PUFAs) (Arts et al., 2007, Virmani et al., 2005). Taken together, the evidence suggests that oxidative damage induced by chronic stress may cause mitochondrial dysfunction and reduce lipid production (including PUFAs).
Accumulating research also shows that sleep deprivation is a neurobiological stressor that causes oxidative damage in the brain (Lavie, 2009, McEwen, 2006). Other studies demonstrated that antioxidant capacity was decreased in peripheral tissues after sleep deprivation (Everson et al., 2005).Indeed, it has been proposed that one of the biological functions of sleep may be to protect against oxidative stress (Wolf et al., 2007). There is extensive and well-known literature on long-term disrupted sleep cycles as a precipitant for mood disorders (Wirz-Justice, 2006), and given the disrupted sleep cycle that many soldiers and trainees experience (e.g., during basic training, deployment, or military missions), this stressor may be of particular importance in military populations (van Liempt et al., 2006).
Oxidative stress, homocysteine (a neurotoxin for mitochondrial function) increases, and glucocorticoid receptor trafficking all affect mitochondrial function during chronic stress. High ROS (superoxide, hydrogen peroxide, and hydroxyl radical) levels damage mitochondrial function (Jou et al., 2009, Madrigal et al., 2001, Sorce and Krause, 2009). It has been shown that p66Shc is a proapoptotic protein involved in ROS production in mitochondria leading to mitochondrial damage and apoptosis under oxidative or genotoxic stress conditions such as H2O2 or UV exposure. (Calabrese V, et al., 2010). Moreover, in both acute and chronic stress animal models, the homocystenine levels were significantly increased (Black and Garbutt, 2002, de Souza et al., 2006, Taqliari et al., 2010). The molecular mechanism(s) for homocysteine increase induced by the chronic stress remains unclear. Notably, some studies have suggested a link between high homocysteine concentrations and increased risk of depression (Almeida et al., 2004, Almeida et al., 2008). Recent studies have similarly shown that the stress hormone corticosterone directly modulates mitochondrial function (Du et al., 2009). While brief increase of corticosterone enhanced mitochondrial function, high doses or long-term administration decreased levels of the glucocorticoid receptor and neuroprotective molecule B-cell lymphoma 2 (Bcl-2) in mitochondria. Similar results were found in rats exposed to a chronic stress paradigm (Du et al., 2009).
Mitochondria are key regulators of neurotransmitter signaling at dendrites and synapses. They mediate important and diverse cellular functions in the central nervous system (CNS), including adenosine triphosphate (ATP) production, synaptic protein expression, lipid synthesis, intracellular calcium buffering, resilience, and apoptosis (Quiroz et al., 2008, Zundorf and Reiser, 2011). Especially in remote axons, dendrites, and synapses, mitochondria function as a “local government” to synthesize energy ATP, lipids, and proteins; provide substrates for lipid synthesis; maintain calcium homeostasis; and modulate apoptotic pathways to determine resilience and atrophy (Quiroz et al., 2008, Zundorf and Reiser,2011). Cumulative evidence further shows that mitochondria are a key regulator of neurotransmitter signaling at the synapses (Ben-Shachar and Laifenfeld, 2004, Verstreken et al., 2005) and, in conjunction with synaptic calcium dynamics, play a very active role in regulating synaptic plasticity (Ben-Shachar and Laifenfeld, 2004, Brenner-Lavie et al., 2009, Mattson et al., 2008, Verstreken et al., 2005). For instance, mitochondrial transport is significantly increased in response to neuronal activity and is essential for enhancing synaptic strength (Mattson, 2007, Verstreken et al., 2005). In support of these findings, it has been suggested that the atrophy of hippocampal dendrites and synapses seen in response to chronic stress may be due to decreased mitochondrial function (Cook and Wellman, 2004, Pavlides et al., 2002).
Lipids are similarly particularly vulnerable to oxidative damage (Arts et al., 2007). Sixty percent of the wet weight of the mammalian brain comprises lipids. Approximately 70% of the fatty acid pool is made de novo, and 30% must be obtained through diet. Seafood, fish oils, and fortified foods are rich sources of ω–3 polyunsatuated fatty acids (ω–3 PUFAs: eicosapentaenoic acid (EPA), docosapentaenoic acid (DPAω–3), and docosahexaenoic acid (DHA)), as well as cholesterol. DHA comprises 14% of total fatty acids in the body and is concentrated in neuronal membranes and synapses (Brunner et al., 2002, Kunugi, 2001). The composition of lipids, including cholesterol and PUFAs, can be affected by both cellular function and diet. In this regard, oxidative stress has a large impact on lipid metabolism (including both cholesterol and PUFAs). Lipid peroxidation leads to oxidative lipid deterioration, which alters membrane permeability and fluidity and results in lipid degradation (Delibas et al., 2004, Engstrom et al., 2009). Lipid peroxidation can be inhibited by antioxidants such as vitamin C and vitamin E, which protect lipids from oxidation (Frank and Gupta, 2005, Mahadik et al., 2001). Notably, levels of malondialdehyde (MDA), which is a byproduct of polyunsaturated lipid degradation by ROS, were found to be significantly increased in depressed patients (Bilici et al., 2001, Khanzode et al., 2003a, Sarandol et al., 2007). Furthermore, studies have demonstrated that lower serum cholesterol and DHA levels are associated with suicide attempts (Brunner et al., 2001, Brunner et al., 2002).
The fatty acid composition of the human brain is the key to maintaining the structural and functional integrity of cellular membrane structures. Recent studies have demonstrated that one of the most important functions of cholesterol and DHA is to form lipid rafts—special, highly-ordered regions on the plasma membrane that are rich in cholesterol, DHA, and sphingolipids (Ferrer, 2009, Pani and Singh, 2009). Lipid rafts function to cluster receptors and proteins involved in signal transduction (e.g. G-protein subunits, adenylyl cyclase), aid in protein scaffolding, and facilitate internalization of G-protein coupled receptors (Ferrer, 2009, Pani and Singh, 2009). A subset of lipid rafts containing caveolin proteins (known as Caveolae) play an important role in modulating synaptic plasticity and neurite outgrowth (Ferrer, 2009, Pani and Singh, 2009). As a major component of lipid rafts, DHA regulates dopaminergic and serotoninergic neurotransmission (Kodas et al., 2002, Zimmer et al., 2000) and signal transduction (Vaidyanathan et al., 1994), and interacts with membrane-bound enzymes (Bourre et al., 1989) and ionic channels (Vreugdenhil et al., 1996). Caveolae are widely expressed in the CNS in brain microvessels, endothelial cells, astrocytes, oligodendrocytes, Schwann cells, dorsal root ganglia, and hippocampal neurons (Allen et al., 2007).
Investigators have suggested that neurotransmitter signaling may occur via clustering of receptors in lipid rafts or caveolae, and the effects of lipid rafts on neurotransmitter signaling have been implicated in neurological and psychiatric diseases in general (Nomura et al., 2008, Pani and Singh, 2009), and in mood disorders in particular (Brambilla et al., 2003, Shiah and Yatham, 2000, Donati et al., 2008). Traditionally, the brain systems receiving the greatest attention in neurobiological studies of mood disorders were the monoaminergic neurotransmitter systems (e.g., the serotonergic, dopaminergic, and norepinephrinergic systems) that are extensively distributed throughout the network of limbic, striatal, hippocampal, and prefrontal cortical neuronal circuits (Drevets, 2000, Manji and Duman, 2001, Nestler et al., 2002). However, recent studies show that glutamatergic synaptic plasticity may be the convergence point for the treatment of mood disorders, and alterations in this system are known to play a major role in cellular plasticity and resilience (Sanacora et al., 2008). Existing antidepressants and mood stabilizers have prominent effects on the glutamatergic system, and modulating glutamatergic, ionotropic, or metabotropic receptors results in antidepressant-like properties in animal models (Sanacora et al., 2008). The structurally dissimilar mood stabilizing agents lithium and valproate were both found to reduce synaptic expression of the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) glutamate receptor at synapses in vivo and in vitro in the hippocampus (Du et al., 2008, Du et al., 2003, Du et al., 2004). In contrast, antidepressant agents such as imipramine, lamotrigine, and riluzole enhanced surface AMPA receptor expression and phosphorylation of GluR1S845 in the hippocampus in vivo (Du et al., 2007). As a result, several glutamatergic modulators targeting various glutamate components are currently being studied in the treatment of mood disorders, including release inhibitors of glutamate, N-methyl-D-aspartate (NMDA) antagonists, AMPA throughput enhancers, and glutamate transporter enhancers (Sanacora et al., 2008). Preliminary pharmacogenetic studies have also strongly implicated glutamatergic signaling in suicidal behaviors (Lekman et al., 2008).
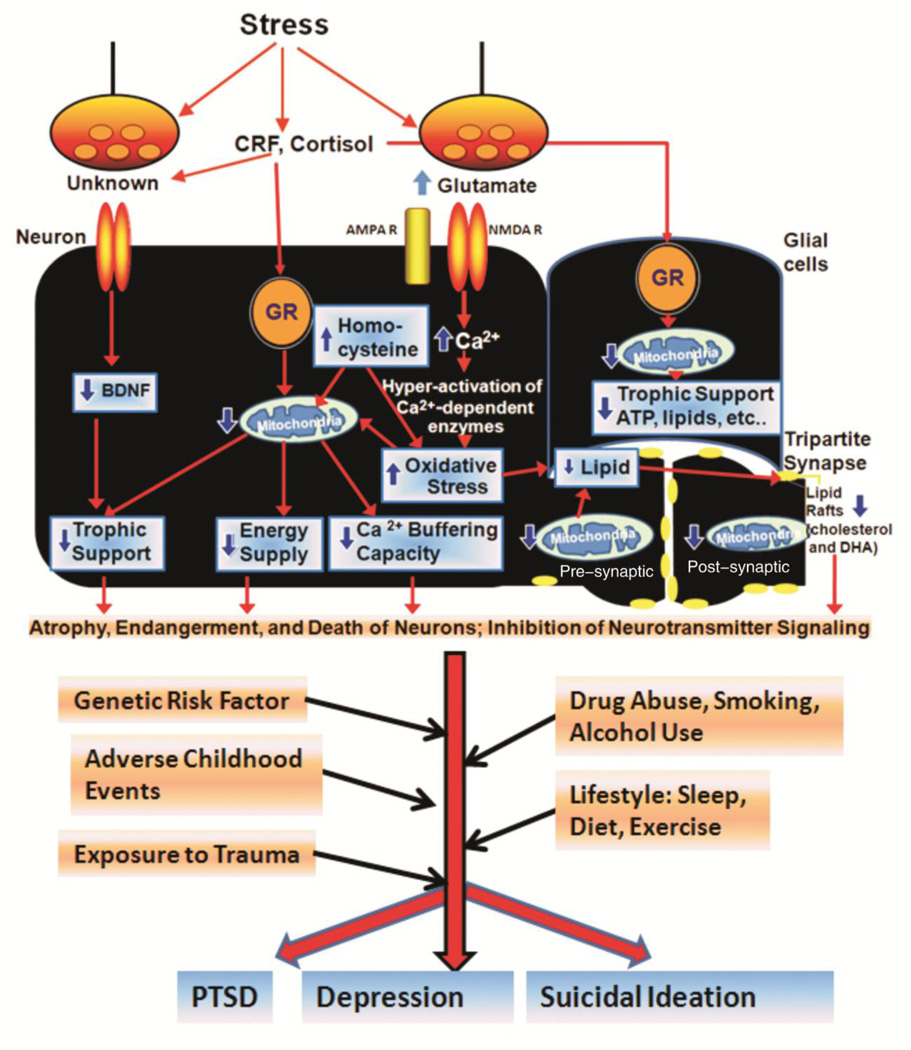
Taken together, these findings suggest that chronic stress damages mitochondrial function and subsequently changes the lipid composition in the brain. The altered lipid composition may have a large impact on the structural and functional integrity of the cellular membrane structure, ultimately leading to aberrant neurotransmitter signaling. This altered neurotransmitter signaling may in turn contribute to the pathophysiology of depression and suicidal behavior (Figure 1).
Figure 1. Stress-induced damage to mitochondrial function and neurotransmitter signaling in the pathophysiology of PTSD, depression, and suicidal ideation.
Chronic stress and sleep deprivation increase cortisol (which binds to its receptor, glucocorticoid receptor, GR) and corticotrophin releasing factor (CRF), followed by enhanced oxidative stress and higher homocysteine levels, which subsequently lead to mitochondrial dysfunction and lipid degradation in the neurons in the circuits mediating cognitive, affective, motoric, and neurovegetative functions. In addition, it was reported that chronic stress lead to down-regulation of neurotrophic factor brain-derived neurotrophic factor (BDNF) expression, which may also contribute to the chronic stress-induced neuronal damage. Chronic stress also increases intracellular glutamate levels, which may cause altered calcium signaling and oxidative stress in the neurons. Glial cells include the astrocytes, oligodendrocytes, and microglia. Tripartite synapse represents the pre-synaptic structure, the post-synaptic structure and the surrounding astrocyte as a functional unit. Astrocytes sense and regulate synaptic activity depending on intracellular Ca2+ levels. Mitochondria provide trophic support, energy, and calcium-buffering capacity in the neuronal cell body, the astrocytes, the dendrites, and the synapses. Mitochondrial dysfunction and altered lipid rafts may lead to aberrant neurotransmitter signaling, dendritic atrophy, and neuronal endangerment. This stress-induced neuronal damage interacts with genetic and environmental factors, including adverse childhood events, exposure to trauma, drug abuse, smoking, alcohol use, sleep, diet, and exercise levels to eventually precipitate mental illness in vulnerable individuals. Depending on the severity of these factors, and an individual’s personal predisposition, the course of the illness may develop towards a variety of psychiatric disorders.
CURRENT HYPOTHESES REGARDING THE ETIOLOGY OF DEPRESSION AND SUICIDAL BEHAVIORS
Many hypotheses for the pathophysiology of depression and suicidal behaviors have been proposed, and it is beyond the scope of this article to review them all. However, it is interesting to note the large and varied literature on depression implicates (but is not limited to) many different etiologies to varying degrees, including dysfunction or alterations in monoamine neurotransmitters (serotonin, norepinephrine, and dopamine) (Bourin et al., 2002, Owens, 2004, Syvalahti, 1987), oxidative stress (Maes et al.,2011), glutamatergic synaptic strength (Du et al., 2004, Du et al., 2007, Zarate and Manji, 2008), mitochondria (Rezin et al., 2008), cytokines (Maes et al., 2009b), homocysteine (Folstein et al., 2007), lipids (including cholesterol and PUFAs) (Su, 2009), neurotrophins or growth factors (Dwivedi, 2009, Molendijk et al., 2011), magnesium (Eby and Eby, 2010), zinc (Irmisch et al., 2010), cyclic AMP (cAMP) response element binding (CREB) (Czeh and Simon, 2005), and histone deacetylase (HDAC) (Covington et al., 2009). Finally, stress (Bao et al., 2008), sleep (Fang et al.,2010), altered neurotransmitter serotonin signaling (Brown and Gershon, 1993, Nordstrom and Asberg, 1992), lipids (Brunner et al., 2002, Colin et al., 2003), and neurotrophins (Dwivedi, 2009) have all been proposed as key to the pathophysiology of suicidal behavior.
As regards the hypotheses presented in this paper, several common threads from this disparate literature emerge. For instance, accumulating evidence suggests that chronic stress and sleep deprivation increase cortisol levels (Wolkowitz et al., 2001), which in turn leads to oxidative stress (Madrigal et al., 2001) and high homocysteine levels (Black and Garbutt, 2002, de Souza et al., 2006) and, subsequently, to mitochondrial damage (Madrigal et al., 2001) and lipid degradation (Arts et al., 2007) in neuronal circuits. The lipid raft region is composed of cholesterol, polyunsaturated fatty acids (including EPA, DHA), and sphingolipids and mediates neurotransmitter signaling through G-protein coupled receptors and ion channels (Pani and Singh, 2009). It has been shown that depletion of cholesterol, or EPA and DHA caused a decrease in numbers of lipid raft on the neuronal membrane, which may lead to the aberrant G-protein coupled receptor signaling (Siddiqui et al., 2007). It is note-worthy that lower serum cholesterol and DHA levels are associated with suicide attempts (Brunner et al., 2001, Brunner et al., 2002, Neaton et al., 1992). Drugs, which lower the cholesterol levels, are able to cause the depressive symptoms (Tatley and Savage, 2007). In addition, fish-intake (rich in EPA or DHA) directly protects against the onset of major depressive disorder (Weidner et.al., 1992). Moreover, chronic stress induced an inhibition to the respiratory chain in the mitochondria in the brain (Madrigal et al., 2001). Mitochondrial dysfunction caused by changes in biochemical cascade or the damage to the mitochondrial electron transport chain has been suggested to be an important pathogenic factor for the psychiatric disorders, particularly in bipolar disorders and depression (Rezin et al., 2009). Moreover, food supplements, such as B12 or folate, which protects mitochondrial functions are effective as an adjunctive therapy for the treatment of depression (Papakostas et al., 2005). All these studies imply that mitochondrial dysfunction and reduced formation of lipid raft may be involved in the etiology of depression and suicidal behavior (Figure 1). These stress-induced neuronal dysfunctions interact with the other genetic and environmental factors, including adverse childhood events, exposure to trauma, drug abuse, smoking, alcohol usage, sleep, diet, and exercise levels, to precipitate mood disorders in genetically and/or physiologically vulnerable or predisposed individuals (Figure 1). The evidence reviewed here thus provides multiple targets for the treatment of mood and anxiety disorders. Below, we review evidence supporting the ability of several nutrients that are safe and widely-available over the counter to either prevent the onset of these conditions or augment the effects of currently available therapeutics.
OXIDATIVE STRESS, CHOLESTEROL, AND ω3 FATTY ACIDS IN THE PATHOPHYSIOLOGY AND TREATMENT OF DEPRESSION AND SUICIDAL BEHAVIORS
Depression and suicidal ideation are both accompanied by decreased antioxidant levels
Antioxidants are compounds that can quench free radicals by accepting an unpaired electron. In addition to the endogenous antioxidant enzyme systems, food contains many antioxidants, including vitamin E, vitamin C, beta-carotene, leutin, α-lipoic acid, coenzyme Q10 (Co-Q10), lycopene, zeaxanthines, and selenium. Although results from large randomized trials of dietary interventions have yielded mixed results, a number of observational studies have revealed that antioxidants enhance CNS cognition and resilience, particularly in neurodegenerative and psychiatric disorders (Smith and Blumenthal, 2010). For instance, accumulating evidence has shown that MDD is associated with decreased antioxidant levels and with the induction of oxidative pathways (Ng et al., 2008, Sarandol et al., 2007). Other studies have noted that individuals with MDD have significantly lower plasma concentrations of a number of key antioxidants, including vitamin C, vitamin E, zinc, and Co-Q10 (Khanzode et al., 2003b, Maes et al., 2009a). One study suggested that lowered blood concentrations of zinc, CoQ10, vitamin E, vitamin C, and GSH might contribute to a lowered total antioxidant capacity (TAC), which was noted to be significantly lower in 57 patients with MDD than in 40 healthy volunteers (Cumurcu et al., 2009). A significant and inverse correlation was also noted between TAC and severity of depression using the Montgomery-Asberg Depression Rating Scale (MADRS) (Galecki et al., 2009). Lower antioxidant enzyme activity (e.g. glutathione peroxidase (GPX)), is another feature of depression(Ozcan et al., 2004) . It is interesting to note that MDA levels were found to be significantly higher in depressed patients, and may therefore serve as a biomarker for depression (Chang et al., 2009). Long-term stress also causes oxidative damage to DNA (Irie et al., 2005). One of the oxidative stress modifiers of DNA is 8—dehydroxyguanisine (8-OH-dG); levels of this compound were positively associated with depressive symptoms (Irie et al., 2003).
Although the evidence to date is preliminary, levels of antioxidants, vitamins, and carotenoids have also been found to be lower in patients with a history of suicide attempts (Li et al., 2007). Investigators have speculated that lowered antioxidant capacity may impair protection against ROS, thus damaging fatty acids (Edwards et al., 1998, Maes et al., 1999). As noted above, PUFAs are particularly vulnerable to lipid peroxidation.
Lower ω3 fatty acid and serum cholesterol levels are associated with suicide attempts and MDD
Epidemiological studies have identified low fish (high in ω-3 fatty acid) consumption as a risk factor for mortality from suicide (Hibbeln and Salem, 1995, Hirayama, 1990, Sublette et al., 2006, Tanskanen et al., 2001). One study noted that frequent fish consumption (twice per week or more) significantly reduced the risk of depressive symptoms and of self-reported suicidal ideation (Tanskanen et al., 2001). A 17-year follow-up study of over 250,000 Japanese subjects showed that people who ate fish daily had a lower risk of death from suicide (Hirayama 1990). In addition, several reports indicate that lower ω3-fatty-acid levels, including lower plasma EPA, and DHA, or EPA in red blood cells, predicted greater risk of suicide attempt (Hibbeln and Salem, 1995, Huan et al., 2004, Sublette et al., 2006). Because both cholesterol and DHA are major components of the lipid raft, it is possible that reduced cholesterol and ω3-fatty-acid levels may affect the formation of lipid rafts in the CNS, and subsequently reduce neurotransmitter signaling (Czysz and Rasenick, 2013). Notably, increased formation of lipid rafts in the membrane would facilitate serotonergic (Donati et al., 2008, Renner et al., 2007), dopaminergic (Villar et al., 2009), and glutamatergic (Francesconi et al., 2009, Ponce et al., 2008) neurotransmitter signaling; all of these play important roles in the pathophysiology and treatment of psychiatric disorders. Studies have noted that low cholesterol levels are associated with increased risk of suicide (Neaton et al., 1992) and that this association shows an inverse relationship with baseline total serum cholesterol (Lester, 2002, Lindberg et al., 1992). Other studies found that individuals who attempted suicide had significantly lower cholesterol levels than controls (Atmaca et al., 2002, Boston et al., 1996, Kim et al., 2002, Kunugi et al., 1997, Maes et al., 1997a, Modai et al., 1994, Rabe-Jablonska and Poprawska, 2000, Sarchiapone et al., 2001, Takei et al., 1994). A postmortem study found that the brains of violent suicide completers had a lower grey-matter cholesterol content (Lalovic et al., 2007), and that a family history of suicidal behavior was more frequent among carriers of Smith–Lemli–Opitz syndrome, an autosomal recessive disorder characterized by abnormally low cholesterol levels (Lalovic et al., 2004).
Similarly, many studies have reported an association between low cholesterol levels and depression (Cadeddu et al., 1995, Lindberg et al., 1994, Maes et al., 1994, Morgan et al., 1993, Olusi and Fido, 1996, Suarez, 1999), including a large Finnish study involving over 29,000 men (Partonen et al., 1999). Low cholesterol levels have been found to confer increased risk of MDD (Partonen et al., 1999), and to correlate with severity of depressive symptoms in samples of elderly men (Morgan et al., 1993), middle-aged women (Horsten et al., 1997), and depressed patients (Rabe-Jablonska and Poprawska, 2000, Rafter, 2001, Steegmans et al., 2000). Studying cholesterol in depression may also help identify factors that place these patients at risk for non-response to treatment (Sonawalla 2002). Relatedly, use of cholesterol synthesis inhibitor statins (functionally HMG-CoA reductase inhibitors), which lower serum cholesterol levels, has been associated with psychiatrically adverse reactions, particularly depression and memory loss (Tatley and Savage, 2007).
The role of antioxidants and members of the vitamin B family in enhancing cognition and resilience, and in the treatment of mood disorders
Human studies as well as animal models of depression provide evidence suggesting that oxidative damage is involved in treatment resistance and in the working mechanisms of antidepressant agents (Alpert et al., 2002, Papakostas et al., 2004). For instance, one recent clinical study found that N-acetyl-cysteine (NAC), a potent antioxidant that up-regulates the glutathione pathway, significantly augmented the clinical efficacy of antidepressants and mood stabilizers in individuals with bipolar disorder (Berk et al., 2008).
Vitamin C (ascorbate) is a water-soluble vitamin that can be oxidized (dehydroascorbate). Dehydroascorbate can be recycled to ascorbate through endogenous antioxidant enzymes and glutathione. Several studies found that taking a combination of vitamin C and vitamin E supplements enhanced cognitive function in the elderly (Morris et al., 2002, Pettenuzzo et al., 2002). Vitamin C was also associated with antidepressant effects in patients with depression, secondary to adrenocorticotropic hormone (ACTH) treatment (Cocchi et al., 1980). It was effective as an adjunctive treatment to fluoxetine (Amr et al., 2013), and improved mood—as assessed by the penile-vaginal intercourse (FSI),—in healthy young adults (Brody, 2002).
Another key vitamin is folic acid, which cooperates with vitamin B12 to promote the regeneration of methionine from homocysteine. Homocysteine is toxic to mitochondrial function (Coppen and Bolander-Gouaille, 2005, Paul et al., 2004) and, as noted previously, higher homocysteine levels have been associated with depression. In addition, methionine can be converted to S-adenosylmethionine (SAMe), which is the principal methyl donor in the brain. Double-blind, clinical trials demonstrated that, when used adjunctively with standard selective serotonin reuptake inhibitors (SSRIs), SAMe had antidepressant effects in patients with MDD (Papakostas, 2009). Studies have linked lower folic acid levels to depression in elderly women (Ramos et al 2004), in a middle-aged community sample (Sachdev et al., 2005), and in male smokers (Sanchez-Villegas 2009). Vitamin B12 was similarly linked to depressive symptoms in women (Sanchez-Villegas 2009). In addition, folate depletion has been linked to disturbed metabolism of serotonergic and other biogenic amines. In studies of individuals with MDD treated with fluoxetine, low folate levels were associated with delayed onset of clinical improvement (Papakostas et al., 2005), as well as treatment resistance (Papakostas et al., 2004). Moreover, co-administration of methylfolate, a highly absorbable form of folic acid, has been found to augment the effects of SSRIs (Coppen and Bailey, 2000, Godfrey et al., 1990, Roberts et al., 2007)
Zinc is another mineral with antioxidant properties (Powell, 2000), and accumulating data suggest a relationship between low serum zinc levels and severity of depression (Maes et al., 1997b, Siwek et al.,2010). Zinc deficiency increases ROS, which could harm mitochondrial function (Corniola et al., 2008). Notably, various animal studies have demonstrated that zinc has antidepressant effects either alone or as an augmentation strategy for traditional antidepressants (Nowak et al., 2003, Sowa-Kucma et al., 2008). These effects are hypothesized to be related to zinc’s anti-oxidative properties, effects on PUFA metabolism, and neurogenesis stimulation through increased gene expression of brain-derived neurotrophic factor (BDNF) (Maes et al., 1997b, Siwek et al., 2010). A recent double-blind, placebo-controlled study of daily zinc supplementation to imipramine therapy in patients with MDD found that MADRS scores were significantly negatively correlated with serum zinc levels; furthermore, treatment-resistant patients with MDD had lower zinc concentrations than patients who were not treatment-resistant (Siwek et al., 2010).
Finally, magnesium is key to numerous enzymatic reactions involving the formation and use of ATP in energy metabolism. In addition, it is also a NMDA receptor blocker that controls calcium entry to the neurons (Eby and Eby, 2010). It is noteworthy that, in both animal studies and individuals with treatment-resistant MDD, the NMDA antagonist ketamine has rapid and long-lasting anti-depressant effects (Zarate et al., 2006). Case studies have noted that both iv (Enya et al., 2004) and oral magnesium were associated with rapid resolution of depressive symptoms secondary to various disorders, including MDD (Eby and Eby, 2006). In addition, in a double-blind randomized clinical trial, it was shown that magnesium was as effective as imipramine in treating depressive symptoms in elderly patients with Type II diabetes (Barragan-Rodriguez et al., 2008).
ω3 fatty acids in enhancing cognition and resilience, and treating mood disorders
One study found that increased fish intake, even combined with a cholesterol-lowering diet, decreased depressive symptoms (Weidner et al., 1992). Diverse studies—including epidemiological studies, case-control comparisons of blood and brain tissues, double-blind, randomized, placebo-controlled trials, and meta-analyses of these trials—have consistently indicated that low fish (high in ω-3 fatty acid) consumption or low ω3 body compositional status increases the risk of depression and other affective illnesses (Sinclair et al., 2007).
Both preclinical and clinical studies have shown that PUFA uptake enhances cognitive function (Richardson et al., 2003). Both local synthesis and uptake are thought to contribute to the brain pool of DHA; in animal studies, a DHA-and cholesterol enriched diet improved spatial learning in the Morris water-maze paradigm (Hooijmans et al., 2009). In humans, a double-blind, randomized, placebo-controlled study with one-way crossover (placebo to active treatment) reported improvements in reading, spelling, and behavior in children with developmental coordination disorder who received EPA, DHA, and γ-linolenic acid supplements (Richardson et al., 2003).
The role of ω3 fatty acids in the treatment of depression has been extensive (see Table 1 for a summary). A recent meta-analysis of ω3 fatty acid treatment trials in depression that included data from more than twelve independent studies (Table 1) showed that consistent therapeutic benefits were associated with adjunctive use of the ω3 fatty acid EPA over placebo (Martins, 2009). Indeed, most of the clinical trials using predominantly 1–2g EPA/day exhibited significant beneficial effects in depression patients (Hallahan et al., 2007, Jazayeri et al., 2008, Mischoulon et al., 2009, Nemets et al., 2002, Peet and Horrobin, 2002, Su et al., 2003, Su et al., 2008a). However, several clinical trials using DHA or fish oil enriched with DHA showed no beneficial effect for treating MDD or perinatal depression (Freeman et al., 2008, Grenyer et al., 2007, Marangell et al., 2003, Rees et al., 2008, Silvers et al., 2005). The effectiveness of ω3 fatty acids has also been evaluated in the treatment of bipolar depression, where two of three placebo-controlled, double-blind trials found a beneficial effect over placebo (Clayton et al., 2009, Stoll et al., 1999). This effect may be related to the regulation of intracellular phospholipase A2 activity (Smesny et al., 2013). In addition, ω3 fatty acid treatment was also beneficial in the treatment of schizophrenia (Nakagome et al., 2009, Peet, 2003).
Table 1.
Clinical studies exploring the use of nutrients in treating depression and suicidal behaviors.
| Clinical trial | Design | Participants | Interventions | Outcomes | Conclusion | |
|---|---|---|---|---|---|---|
|
ω3 |
Haberka et.al. 2013a | Randomized, standard therapy control. | 52 patients with acute myocardial infarction. | n-3 PUFA 1g/day + standard therapy. | BDI STAI-S STAI-T ESQ | Significantly reduced depressive and anxiety symptoms |
| Mozurkewic h et.al. 2013b | Double blind, randomized, controlled. | 126 pregnant women at risk for depression. | EPA-rich fish oil (1060 mg EPA plus 274 mg DHA), DHA-rich fish oil (900 mg DHA plus 180 mg EPA) | BDI | No significant difference. | |
| Krawczyk et.al. 2013c | Control group, antidepressant treatment with lithium and lamotrigine. | 21 patients diagnosed with a treatment-resistant depression. | 2.2g of EPA, 700mg of DHA, 240mg of GLA, 40mg of vitamin E, primrose oil. | HDRS scale | Marked improvement in depressive symptom. | |
| Gertsik et.al. 2012d | Randomized, masked, placebo-controlled. | 42 patients diagnosed with major depression. | Two 1g capsules containing a bend of 900mg of EPA, 200mg of DHA and 100mg of other omega-3 fatty acid, twice daily plus citalopram. | HDRS | Combination therapy was more effective and demonstrated significant better HDRS score. | |
| Mischoulon et.al. 2009e | Double blind, randomized, placebo-controlled. | 35 patients with DSM- IV diagnosed major depression. Mean age: 45. | EPA 1g/day (16), or placebo (19) for 8 weeks | HDRS | EPA was superior to placebo, but the effect did not reach statistical significance because of small sample size (p=0.087). | |
| Jazayeri et.al. 2008f | Double blind, randomized, placebo-controlled. | 60 patients with DSM-IV diagnosed major depression. Age range: 20–59. | Fluoxetine 20mg/day (16), EPA 1g/day (16), or fluoxetine plus EPA (16) for 8 weeks. | HDRS | EPA 1g/day was as effective as fluoxetine. EPA plus fluoxetine was superior to either agent alone. | |
| Rees 2008g | Double blind, randomized, placebo-controlled. | 26 patients with DSM-IV diagnosed perinatal depression. Mean age: 33. | Fish oil, 6g/day (0.4g EPA/day, 1.64g DHA /day) (13), or placebo (13) for 6 weeks. | EPDS, HDRS | Fish oil (0.4g EPA/day; 2.2gDHA/day) showed no significant effect as monotherapy for perinatal depression. | |
| Su 2008h | Double-blind, randomized, placebo-controlled. | 36 pregnant women with DSM-IV diagnosed major depression. Mean age: 31 | 2.2g EPA/day plus 1.2g DHA/day (18), or placebo (18) for 8 weeks. | BDI, EPDS, HDRS-21 | The group receiving EPA and DHA as monotherapy had significantly lower depression rating scale scores than those receiving placebo | |
| Freeman 2008i | Randomized, placebo-controlled. | 59 patients with DSM-IV diagnosed perinatal depression. Mean age: 30 | 1.9g EPA and 1.9g DHA/day (28), or placebo (31) for 6 weeks. | EPDS HDRS, CGI | No significant difference between the EPA/DHA and placebo groups | |
| Mischoulon 2008j | Double-blind, randomized, placebo-controlled. | 35 patients with DSM-IV diagnosed major depression. Mean age: 42. | DHA 1g/day (14), DHA 2g/day (11), DHA 4g/day (10), for 12 weeks | HDRS | Patients receiving either 1g or 2g per day of DHA had significant increases in their HDRS scores. | |
| Hallahan 2007k | Randomized, placebo-controlled. | 49 patients with history of repeated self-harm. Mean age: 30. | EPAX 5500 (1.2g EPA/ day, 0.9 DHA/day) (22) or placebo (27) for 12 weeks. | HDRS, BDI | EPA/DHA treatment substantially reduced surrogate markers for suicidal behavior and depression score. | |
| Grenyer 2007l | Double-blind, placebo-controlled. | 83 patients with major depression, age range 18–65. | Regular antidepressants, plus 0.6g EPA/day or 2.2g DHA/day, for 16 weeks. | HDRS, BDI | Tuna fish oil (0.6g EPA/day, 2.2g DHA/day) had no significant beneficial effects. | |
| Silvers 2005m | Double-blind, placebo-controlled. | 77 patients with clinically diagnosed major depression . Mean age: 39. | Standard antidepressants plus 8g tuna fish oil (0.6g EPA, 2.4g DHA) (40), or placebo (37) for 12 weeks. | HDRS, BDI | Mood improved significantly for all patients, including those receiving placebo. Fish oil did not improve mood more than placebo. | |
| Marangell 2003n | Double-blind, placebo-controlled. | 36 patients with DSM-IV diagnosed major depression, age range 18–65. | Standard antidepressant plus DHA 2g/day (18), or placebo (17) for 6 weeks. | HDRS, MADRS | DHA 2 g/day showed no significant benefit. | |
| Su 2003o | Double-blind, placebo-controlled. | 28 patients with DSM-IV diagnosed major depression. Mean age: 37 | Antidepressants plus 9.6g ω3 fatty acid (4.4g EPA, 2.2g DHA) (11) or placebo (17) for 8 weeks. | HDRS-21 | Significantly lower HDRS score in the group receiving ω-3 fatty acids. | |
| Nemets 2002p | Double-blind, randomized, placebo-controlled, | 20 patients with DSM-IV diagnosed major depression. Age range: 28–73. | Standard antidepressant plus 2g EPA/day (10), or placebo (10) for 3 weeks | HDRS | Highly significant benefits for EPA compared to placebo. | |
| Peet and Horrobin 2002q | Double-blind, randomized, placebo-controlled, stratified by sex. | 70 depressed patients with a HDRS score >15. Mean age: 44. | Standard antidepressant plus 1g EPA/day (14), 2g EPA/day (18), 4g EPA/day (17), or placebo (19) for 12 weeks. | HDRS, MADRS, BDI | Highly significant improvement with 1g EPA/day treatment. No effect for 2gEPA/day or 4g EPA/day. | |
| Vitami n C | Amr et.al.2013r | Radomized, double-blind, placebo-controlled. | 24 pediatric patients with depression. | Fluoxetine(10–20mg/day) plus vitamin C (1000mg/day) or placebo. | CDRS, CDI, CGI. | Vitamin C may be an effective adjuvant agent in the treatment of MDD in the pediatric patients. |
| Brody 2002s | Double-blind, randomized, placebo- controlled. | 81 healthy subjects, mean age 24.4. | Vitamin C 3000mg/day (42), or placebo (39) for 2 weeks. | BDI | Vitamin C significantly reduced the BDI scores. | |
| Cocchi 1980t | Case series, depression secondary to ACTH treatment of pediatric hepatitis. | 4 cases of “idiopathic” depression (ages 5, 7, 19, and 29). | Vitamin C 50mg/kg/day, for 2 weeks | Symptom-based diagnosis | Completely recovery from psychiatric disturbance. | |
| Folate, B12, | Almeida 2010u | Double-blind, randomized, placebo-controlled. | 273 stroke survivors, Mean age:63. | Folic acid (2mg/day), vitamin B6 (25mg/day), vitamin B12 (0.5mg/day) (136), and placebo (137) for 1–10.5 years. | MINI (2006) | B-vitamins were associated with a lower hazard of depression compared to placebo. |
| Resler 2008v | Double-blind, randomized, placebo-controlled. | 27 patients with DSM-IV diagnosed major depression age range: 26–49. | Fluoxetine (20mg/day) plus folic acid (10mg/day) (14), or placebo (13), for 6 weeks. | HDRS | Folic acid significantly lowered the HDRS score as an adjunctive therapy. | |
| Coppen 2000w | Double-blind, randomized, placebo-controlled, stratified by sex. | 127—major depression (DSM III), age mean: 44. | 20mg fluoxetine/day plus 500 mcg/day folic acid (51), or 20mg fluoxetine/day plus placebo (58) for 10 weeks. | HDRS | Folic acid greatly improved the antidepressant action of fluoxetine. | |
| Godfrey 1990x | Double-blind, randomized, placebo-controlled, stratified by diagnosis. | 24 patients with DSM-III-diagnosed major depression and RBC folate<200ug/l, age range:20–70 | Standard antidepressant treatment plus 15 mg methyl-tetrahydrofolate (12), or placebo (12) for 6 months. | HDRS | Clinical and social recovery was significantly improved in those receiving methylfolate plus standard antidepressants compared with those receiving placebo. | |
| Passeri 1993y | Double-blind, randomized, placebo-controlled. | 96 patients with DSM-IIIR-diagnosed dementia, MMSE 12–23, HDRS>17 RBC folate 175–700ng/ml, age>65. | Standard antidepressant treatment plus 50 mg methyltetrahydrofolate (47), or trazodone (49) for 8 weeks. | HDRS | Methyltetrahydrofolate was as effective as trazodone in significantly reducing HDRS scores. | |
| Mg | Barragan-Rodrigues 2008z | Randomized clinical trial. | 23 elderly patients with depression, type-2 diabetes and hypomagnesemia | 50ml MgCl2 5% solution (450mg) or 50mg imipramine for 12 weeks. | Yasavage and Brink Score | Depression scores were identical for the magnesium-and imipramine-treated groups. |
| Eby 2006aa | Case series. | 4 patients with depression, hypomanic depression, or postpartum depression (ages: 23, 35, 40, 59). | Magnesium 125–300mg/day, for 4–7 days. | Symptom-based diagnosis. | Depression was reduced or patients were symptom-free after 4–7 days. | |
| Enya 2004bb | Case report. | 1 patient with Gitelman’s syndrome-related depression and hypokalemia, age: 69. | Spironolactone (25mg/day) and Magnesium Sulfate (20mEq/day, i.v. injection) for two days | Symptom-based diagnosis | Depressive symptoms disappeared after the second day of i.v. magnesium. | |
| Zn | Siwek 2009cc | Double-blind, randomized, placebo-controlled, | 60 patients with DSM-IV diagnosed unipolar depression, age range: 18–55. | Imipramine (~140mg/day) plus 25mg zinc/day (30), or plus placebo (30) for 12 weeks. | HADRS, BDI, MADRS | Zinc augmented the antidepressant effect of imipramine in treatment-resistant patients. No effect on patients who were not treatment-resistant. |
| Nowak 2003dd | Double-blind, placebo-controlled, stratified by sex. | 15 patients with DSM-IV diagnosed major depression, age range: 25–57. | Standard antidepressant plus zinc 25 mg/day (6), or placebo (9) for 6 or 12 weeks. | HDRS, BDI | Zinc supplementation significantly reduced depression rating scale scores after 6 or 12 weeks. |
HDRS: Hamilton Depression Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; BDI: Beck Depression Inventory; EPDS Edinburgh Postnatal Depression Scale. MINI: Mini-International Psychiatric Interview.
Haberka M, Mizia-Stec K, Mizia M, Gieszczyk K, Chmiel A, Sitnik-Warchulska K, G¹sior Z. Effects of n-3 polyunsaturated fatty acids on depressive symptoms, anxiety and emotional state in patients with acute myocardial infarction. Pharmacol Rep. 65(1):59–68.2013
Mozurkewich EL, Clinton CM, Chilimigras JL, Hamilton SE, Allbaugh LJ, Berman DR, Marcus SM, Romero VC, Treadwell MC, Keeton KL, Vahratian AM, Schrader RM, Ren J, Djuric Z. The Mothers, Omega-3, and Mental Health Study: a double-blind, randomized controlled trial. Am J Obstet Gynecol. Apr;208(4):313.e1–9. 2013.
Krawczyk K, Rybakowski J. Augmentation of antidepressants with unsaturated fatty acids omega-3 in drug-resistant depression. Psychiatr Pol. Jul-Aug;46(4):585-98.2012.
Gertsik L, Poland RE, Bresee C, Rapaport MH.:Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J Clin Psychopharmacol. Feb;32(1):61-4. 2012.
Mischoulon D, Papakostas GI, Dording CM, et al.: A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J Clin Psychiatry 70:1636-1644, 2009.
Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al.: Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry 42:192-198, 2008.
Rees AM, Austin MP, Parker GB: Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry 42:199-205, 2008.
Su KP, Huang SY, Chiu TH, et al.: Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 69:644-651, 2008.
Freeman MP, Davis M, Sinha P, et al.: Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J Affect Disord 110:142-148, 2008.
Mischoulon D, Best-Popescu C, Laposata M, et al.: A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharmacol 18:639-645, 2008.
Hallahan B, Hibbeln JR, Davis JM, et al.: Omega-3 fatty acid supplementation in patients with recurrent self-harm. Single-centre double-blind randomised controlled trial. Br J Psychiatry 190:118-122, 2007.
Grenyer BF, Crowe T, Meyer B, et al.: Fish oil supplementation in the treatment of major depression: a randomized double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 31:1393-1396, 2007.
Silvers KM, Woolley CC, Hamilton FC, et al.: Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids 72:211-218, 2005.
Marangell LB, Martinez JM, Zboyan HA, et al.: A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry 160:996-998, 2003
Su KP, Huang SY, Chiu CC, et al.: Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 13:267-271, 2003.
Nemets B, Stahl Z, Belmaker RH: Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 159:477-479, 2002
Peet M, Horrobin DF: A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 59:913-919, 2002.
Amr M, El-Mogy A, Shams T, et.al.:Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. Nutr J.12:12-31 2013.
Brody S: High-dose ascorbic acid increases intercourse frequency and improves mood: a randomized controlled clinical trial. Biol Psychiatry 52:371-374, 2002.
Cocchi P, Silenzi M, Calabri G, et al.: Antidepressant effect of vitamin C. Pediatrics 65:862-863, 1980.
Almeida OP, Marsh K, Alfonso H, et al.: B-vitamins reduce the long-term risk of depression after stroke: The VITATOPS-DEP trial. Ann Neurol 68:503-510. 2010.
Resler G, Lavie R, Campos J, et al.: Effect of folic acid combined with fluoxetine in patients with major depression on plasma homocysteine and vitamin B12, and serotonin levels in lymphocytes. Neuroimmunomodulation 15:145-152, 2008.
Coppen A, Bailey J: Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord 60:121-130, 2000.
Godfrey PS, Toone BK, Carney MW, et al.: Enhancement of recovery from psychiatric illness by methylfolate. Lancet 336:392-395, 1990.
Passeri M, Cucinotta D, Abate G, et al.: Oral 5'-methyltetrahydrofolic acid in senile organic mental disorders with depression: results of a double-blind multicenter study. Aging (Milano) 5:63-71, 1993.
Barragan-Rodriguez L, Rodriguez-Moran M, Guerrero-Romero F: Efficacy and safety of oral magnesium supplementation in the treatment of depression in the elderly with type 2 diabetes: a randomized, equivalent trial. Magnes Res 21:218-223, 2008.
Eby GA, Eby KL: Rapid recovery from major depression using magnesium treatment. Med Hypotheses 67:362-370, 2006.
Enya M, Kanoh Y, Mune T, et al.: Depressive state and paresthesia dramatically improved by intravenous MgSO4 in Gitelman's syndrome. Intern Med 43:410-414, 2004.
Siwek M, Dudek D, Schlegel-Zawadzka M, et al.: Serum zinc level in depressed patients during zinc supplementation of imipramine treatment. J Affect Disord 126:447-452.
Nowak G, Szewczyk B, Wieronska JM, et al.: Antidepressant-like effects of acute and chronic treatment with zinc in forced swim test and olfactory bulbectomy model in rats. Brain Res Bull 61:159-164, 2003.
Studies have also evaluated the utility of ω3 fatty acids for preventing suicidal behaviors. In a randomized, double-blind, placebo-controlled trial of patients recruited from an emergency room who had exhibited recurrent self-harm behaviors, 2g/day of ω3 long chain fatty acids led to a 45% reduction in suicidal thinking, and a 30% reduction in depressive symptoms (Hallahan et al., 2007).
CONCLUDING REMARKS
This manuscript has summarized the existing evidence that ω3 fatty acids, antioxidants, B family vitamins, zinc and magnesium protect mitochondrial function and enhance neurotransmitter signaling in the brain. In addition, reduced ω3 fatty acids, oxidative capacity, B-12, and folic acid levels have been associated with both depression and suicidal ideation in humans. The question is the extent to which administration of these particular nutrients may exert beneficial effects on depressive symptoms or suicidal ideation. We have listed the components and side effects for the nutrients in Table 2.
Table 2.
The safety and side effects of the nutrients.
| Over-the- counter Nutrient |
Effective dose in the clinical trials |
Maximum Safe Dose for Long Term Usage* |
Side Effects* |
|---|---|---|---|
| ω3 fatty acid | 1000mg EPA/day (Jazaveri 2008) | One report says 21g/day | No side effect. |
| Vitamin C | 3000mg/day (Brody 2002) | 2000mg/day | No side effect. Large doses of vitamin C can deplete the body’s supply of copper. People with kidney stones or kidney failure and people taking ampicillin, indomethacin, alsalate, or tetracycline should consult their doctor. |
| Folic acid | 500mcg/day (Coppen 2000) | 400mcg/day | No side effect. |
| Vitamin B12 | 500mcg/day (Almaida 2010) | 3000mcg/day | Oral Vitamin B12 has no side effects. |
| Magnesium | 150–300mg/day(Eby 2006) | 350mg/day | No side effect is associated with this dose. |
| Zinc | 25mg/day (Siwek 2010) | 50mg/day under supervision | High doses of zinc affect the absorption of iron and copper. Zinc should be taken with food to avoid irritating the stomach. People with liver damage or an intestinal disorder should consult their doctor before taking supplementary zinc. |
While this is an issue of considerable importance for public health, it has particular urgency in military populations. Numerous recent studies have called for a way to address prevention and more effective treatment strategies for depression and suicidal ideation in both civilian and military populations. There appears to be a strong relationship between duration of combat exposure and the severity of mental illness (Dohrenwend, 2006), suggesting that the severity of stress may play a role. Specifically, repeated exposure to combat and multiple deployments might work as a repetitive severe stressor in this situation, in addition to being an “anticipatory stressor” (e.g, worrying about what will happen) as well as associated with physiological stressors (e.g., sleep deprivation) (Selby et al.,2010). Indeed, cumulative studies have shown that exposure to combat is a risk factor for both PTSD (Lapierre, 2008) and depression (Lapierre et al., 2007) in soldiers. Not surprisingly, injured soldiers also report more depressive and suicidal problems (McAllister, 2009). Furthermore, PTSD is strongly linked to suicidal behavior (Kessler, 2000). As reviewed above, psychological repetitive stress, traumatic experience, and sleep deprivation were all shown to cause oxidative stress and mitochondrial damage in the brain (Du et al., 2009, Jou et al., 2009, Su et al., 2008b, Zhang et al., 2006). Most of the civilian clinical trials cited in this paper cover a wide age range including that of most soldiers.
The multiple nutrients reviewed here affect stress-related damage to mitochondrial function and neurotransmitter signaling at different levels in order to power a therapeutic benefit. Specifically, 1) the antioxidants vitamin C would increase oxidative status during stress, and zinc would enhance the antioxidant effect; 2) vitamin B12 and folic acid would reduce the level of the toxic homocysteine and enhance the formation of antidepressant SAMe; 3) the essential ω3 fatty acids would support the function of lipid rafts; and 4) magnesium would facilitate mitochondrial enzyme function and block the calcium entry from NMDA receptors (Figure 2). As noted above, previous studies have shown that antidepressant drug resistance is associated with low serum levels of folic acid (Papakostas et al., 2004), vitamin B12 (Papakostas et al., 2004), magnesium (Eby and Eby, 2010) and antioxidant status (Maes et al., 2009a). Thus, these nutrients could be of considerable utility in either preventing the onset of mood disorders and suicidal behaviors in vulnerable individuals, or significantly augmenting the therapeutic effect of available drugs. Furthermore, because these nutrients are well known to be safe and reliable, implementing their use could be an easy way to protect individuals against stress-related psychiatric disorders, including PTSD, depression, and suicide attempts. Controlled clinical trials, both preventive and therapeutic, in both civilian and military populations, are warranted.
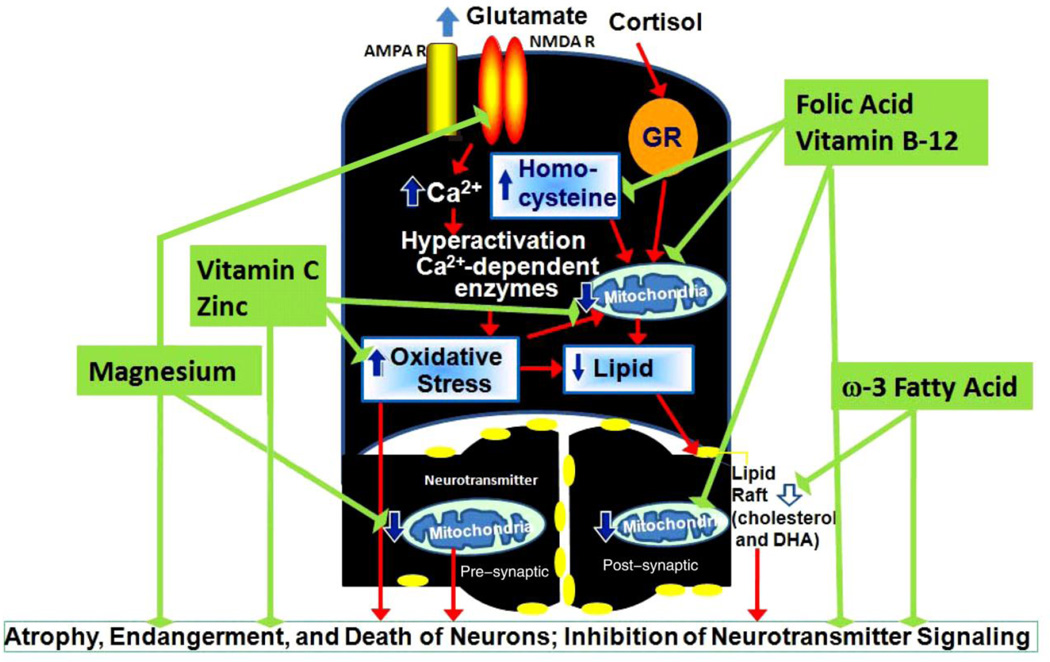
Figure 2. Nutrients protect mitochondrial function and neurotransmitter signaling against stress-induced neuronal damage.
Chronic stress causes increases of the cortisol level and the intracellular glutamate level in the brain. The high levels of cortisol may cause increased oxidative stress and higher homocy steine levels, which subsequently lead to mitochondrial dysfunction and lipid degradation. The in tra-cellular glutamate activates the N-methyl-D-aspartic acid receptor (NMDA R) and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPA R) and induces calcium influx, which leads to hyperactivation of the Ca2+ dependent enzymes and oxidative stress. The higher level of lipid oxidation may result in lipid degradation and lower levels of Eicosapntemacnioc Acid (EPA), Docosahexaenoic Acid (DHA), or other polyunsaturated fatty acid (PUFA) in the brain for the formation of the lipid rafts, followed by a decrease in the number of lipid rafts, which may affect the G-protein coupled receptor signaling at the synapses. ω3 fatty acids, vitamin C, folic acid, vitamin B12, zinc, and magnesium protect mitochondrial function and neurotransmitter signaling. In particular, ω3 fatty acids facilitate the formation of lipid rafts, which mediate neurotransmitter signaling. Vitamin C and zinc balance stress-induced oxidative stress during chronic stress situations. Vitamin B12 and folic acid reduce homocysteine levels, and enhance the formation of S-adenosylmethionine (SAMe). Magnesium is a co-enzyme for energy production in the mitochondria. It is also an NMDA antagonist that blocks excessive calcium influx and protects neurons.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support of the Yunnan High-level Professional Funding and the funding from Intramural Program of the National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services (IRP-NIMH-NIH-DHHS). We thank Dr. Husseini Manji and Dr. Jane Pearson for scientific suggestions and critical reading for this paper.
Footnotes
Publisher's Disclaimer: Disclaimer: This is a version of an unedited manuscript that has been accepted for publication. As a service to authors and researchers we are providing this version of the accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proof will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to this version also.
Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content.
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. Terms & Conditions of access and use can be found at http://www.tandfonline.com/page/terms-and-conditions
DISCLOSURE/CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose, financial or otherwise.
REFERENCES
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Lautenschlager N, Flicker L, Leedman P, Vasikaran S, Gelavis A, Ludlow J. Association between homocysteine, depression, and cognitive function in community-dwelling older women from Australia. J Am Geriatr Soc. 2004;52:327–328. doi: 10.1111/j.1532-5415.2004.05202001_9.x. [DOI] [PubMed] [Google Scholar]
- Almeida OP, McCaul K, Hankey GJ, Norman P, Jamrozik K, Flicker L. Homocysteine and depression in later life. Arch Gen Psychiatry. 2008;65:1286–1294. doi: 10.1001/archpsyc.65.11.1286. [DOI] [PubMed] [Google Scholar]
- Alpert JE, Mischoulon D, Rubenstein GE, Bottonari K, Nierenberg AA, Fava M. Folinic acid (Leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry. 2002;14:33–38. doi: 10.1023/a:1015271927517. [DOI] [PubMed] [Google Scholar]
- Amr M, El-Mogy A, Shams T, Vieira K, Lakhan SE. Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: a randomized, doubleblind, placebo-controlled pilot study. Nutr J. 2013;12:31. doi: 10.1186/1475-2891-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts MJ, Grun C, de Jong RL, Voss HP, Bast A, Mueller MJ, Haenen GR. Oxidative degradation of lipids during mashing. J Agric Food Chem. 2007;55:7010–7014. doi: 10.1021/jf070505+. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Kuloglu M, Tezcan E, Ustundag B, Gecici O, Firidin B. Serum leptin and cholesterol values in suicide attempters. Neuropsychobiology. 2002;45:124–127. doi: 10.1159/000054950. [DOI] [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57:531–553. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Barragan-Rodriguez L, Rodriguez-Moran M, Guerrero-Romero F. Efficacy and safety of oral magnesium supplementation in the treatment of depression in the elderly with type 2 diabetes: a randomized, equivalent trial. Magnes Res. 2008;21:218–223. [PubMed] [Google Scholar]
- Ben-Shachar D, Laifenfeld D. Mitochondria, synaptic plasticity, and schizophrenia. Int Rev Neurobiol. 2004;59:273–296. doi: 10.1016/S0074-7742(04)59011-6. [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Bush AI. N-acetyl cysteine for depressive symptoms in bipolar disorder--a doubleblind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Boston PF, Dursun SM, Reveley MA. Cholesterol and mental disorder. Br J Psychiatry. 1996;169:682–689. doi: 10.1192/bjp.169.6.682. [DOI] [PubMed] [Google Scholar]
- Bourin M, David DJ, Jolliet P, Gardier A. [Mechanism of action of antidepressants and therapeutic perspectives] Therapie. 2002;57:385–396. [PubMed] [Google Scholar]
- Bourre JM. [The role of nutritional factors on the structure and function of the brain: an update on dietary requirements] Rev Neurol (Paris) 2004;160:767–792. doi: 10.1016/s0035-3787(04)71032-2. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr. 1989;119:1880–1892. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. 715. [DOI] [PubMed] [Google Scholar]
- Brenner-Lavie H, Klein E, Ben-Shachar D. Mitochondrial complex I as a novel target for intraneuronal DA: modulation of respiration in intact cells. Biochem Pharmacol. 2009;78:85–95. doi: 10.1016/j.bcp.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Brody S. High-dose ascorbic acid increases intercourse frequency and improves mood: a randomized controlled clinical trial. Biol Psychiatry. 2002;52:371–374. doi: 10.1016/s0006-3223(02)01329-x. [DOI] [PubMed] [Google Scholar]
- Brown AS, Gershon S. Dopamine and depression. J Neural Transm Gen Sect. 1993;91:75–109. doi: 10.1007/BF01245227. [DOI] [PubMed] [Google Scholar]
- Brunner J, Parhofer KG, Schwandt P, Bronisch T. [Cholesterol, omega-3 fatty acids, and suicide risk: empirical evidence and pathophysiological hypotheses] Fortschr Neurol Psychiatr. 2001;69:460–467. doi: 10.1055/s-2001-17564. [DOI] [PubMed] [Google Scholar]
- Brunner J, Parhofer KG, Schwandt P, Bronisch T. Cholesterol, essential fatty acids, and suicide. Pharmacopsychiatry. 2002;35:1–5. doi: 10.1055/s-2002-19834. [DOI] [PubMed] [Google Scholar]
- Bryan CJ, Cukrowicz KC, West CL, Morrow CE. Combat experience and the acquired capability for suicide. J Clin Psychol. 2010;66:1044–1056. doi: 10.1002/jclp.20703. [DOI] [PubMed] [Google Scholar]
- Cadeddu G, Fioravanti P, Antonicelli R, Gasparrini PM, Gaetti R. [Relationship between cholesterol levels and depression in the elderly] Minerva Med. 1995;86:251–256. [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Mancuso C, Lentile R, Stella AM, Butterfield DA. Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods Mol Biol. 2010;610:285–308. doi: 10.1007/978-1-60327-029-8_17. [DOI] [PubMed] [Google Scholar]
- Chang Y, Liu YP, Liu CF. The effect on serotonin and MDA levels in depressed patients with insomnia when far-infrared rays are applied to acupoints. Am J Chin Med. 2009;37:837–842. doi: 10.1142/S0192415X09007272. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004;2004:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63:1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- Cocchi P, M Silenzi, et al. Antidepressant effect of vitamin C. Pediatrics. 1980;65(4):862–863. [PubMed] [Google Scholar]
- Colin A, Reggers J, Castronovo V, Ansseau M. [Lipids, depression and suicide] Encephale. 2003;29:49–58. [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. 2000;60:121–130. doi: 10.1016/s0165-0327(00)00153-1. [DOI] [PubMed] [Google Scholar]
- Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol. 2005;19:59–65. doi: 10.1177/0269881105048899. [DOI] [PubMed] [Google Scholar]
- Corniola RS, Tassabehji NM, Hare J, Sharma G, Levenson CW. Zinc deficiency impairs neuronal precursor cell proliferation and induces apoptosis via p53- mediated mechanisms. Brain Res. 2008;1237:52–61. doi: 10.1016/j.brainres.2008.08.040. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumurcu BE, Ozyurt H, Etikan I, Demir S, Karlidag R. Total antioxidant capacity and total oxidant status in patients with major depression: impact of antidepressant treatment. Psychiatry Clin Neurosci. 2009;63:639–645. doi: 10.1111/j.1440-1819.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- Czysz AH, Rasenick MM. G-protein signaling, lipid rafts and the possible sites of action for the antidepressant effects of n-3 polyunsaturated fatty acids. CNS Neurol Disord Drug Targets. 2013;12:466–473. doi: 10.2174/1871527311312040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Simon M. [Neuroplasticity and depression] Psychiatr Hung. 2005;20:4–17. [PubMed] [Google Scholar]
- de Souza FG, Rodrigues MD, Tufik S, Nobrega JN, D'Almeida V. Acute stressor-selective effects on homocysteine metabolism and oxidative stress parameters in female rats. Pharmacol Biochem Behav. 2006;85:400–407. doi: 10.1016/j.pbb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Delibas N, Altuntas I, Sutcu R, Yonden Z, Koylu H. Effects of dietary long chain PUFAs on hippocampal lipid peroxidation and NMDA receptor subunits A and B concentration in streptozotocin-diabetic rats. Int J Neurosci. 2004;114:1353–1364. doi: 10.1080/00207450490476147. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP. Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychol Bull. 2006;132:477–495. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati RJ, Dwivedi Y, Roberts RC, Conley RR, Pandey GN, Rasenick MM. Postmortem brain tissue of depressed suicides reveals increased Gs alpha localization in lipid raft domains where it is less likely to activate adenylyl cyclase. J Neurosci. 2008;28:3042–3050. doi: 10.1523/JNEUROSCI.5713-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Du J, Creson TK, Wu LJ, Ren M, Gray NA, Falke C, Wei Y, Wang Y, Blumenthal R, Machado-Vieira R, Yuan P, Chen G, Zhuo M, Manji HK. The role of hippocampal GluR1 and GluR2 receptors in manic-like behavior. J Neurosci. 2008;28:68–79. doi: 10.1523/JNEUROSCI.3080-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Gray NA, Falke C, Yuan P, Szabo S, Manji HK. Structurally dissimilar antimanic agents modulate synaptic plasticity by regulating AMPA glutamate receptor subunit GluR1 synaptic expression. Ann N Y Acad Sci. 2003;1003:378–380. doi: 10.1196/annals.1300.031. [DOI] [PubMed] [Google Scholar]
- Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST, Einat H, Manji HK. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci. 2004;24:6578–6589. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA, Jr, Manji HK. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, Machado-Vieira R, McEwen BS, Manji HK. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci U S A. 2009;106:3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat. 2009;5:433–449. doi: 10.2147/ndt.s5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eby GA, 3rd, Eby KL. Magnesium for treatment-resistant depression: a review and hypothesis. Med Hypotheses. 2010;74:649–660. doi: 10.1016/j.mehy.2009.10.051. [DOI] [PubMed] [Google Scholar]
- Eby GA, Eby KL. Rapid recovery from major depression using magnesium treatment. Med Hypotheses. 2006;67:362–370. doi: 10.1016/j.mehy.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- Engstrom K, Saldeen AS, Yang B, Mehta JL, Saldeen T. Effect of fish oils containing different amounts of EPA, DHA, and antioxidants on plasma and brain fatty acids and brain nitric oxide synthase activity in rats. Ups J Med Sci. 2009;114:206–213. doi: 10.3109/03009730903268958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enya M, Kanoh Y, Mune T, Ishizawa M, Sarui H, Yamamoto M, Takeda N, Yasuda K, Yasujima M, Tsutaya S, Takeda J. Depressive state and paresthesia dramatically improved by intravenous MgSO4 in Gitelman's syndrome. Intern Med. 2004;43:410–414. doi: 10.2169/internalmedicine.43.410. [DOI] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Everson CA, Laatsch CD, Hogg N. Antioxidant defense responses to sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol. 2005;288:R374–R383. doi: 10.1152/ajpregu.00565.2004. [DOI] [PubMed] [Google Scholar]
- Fang BJ, Tonelli LH, Soriano JJ, Postolache TT. Disturbed sleep: linking allergic rhinitis, mood and suicidal behavior. Front Biosci (Schol Ed) 2010;2:30–46. doi: 10.2741/s44. [DOI] [PubMed] [Google Scholar]
- Ferrer I. Altered mitochondria, energy metabolism, voltage-dependent anion channel, and lipid rafts converge to exhaust neurons in Alzheimer's disease. J Bioenerg Biomembr. 2009;41:425–431. doi: 10.1007/s10863-009-9243-5. [DOI] [PubMed] [Google Scholar]
- Folstein M, Liu T, Peter I, Buell J, Arsenault L, Scott T, Qiu WW. The homocysteine hypothesis of depression. Am J Psychiatry. 2007;164:861–867. doi: 10.1176/ajp.2007.164.6.861. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci. 2009;29:3590–3602. doi: 10.1523/JNEUROSCI.5824-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank B, Gupta S. A review of antioxidants and Alzheimer's disease. Ann Clin Psychiatry. 2005;17:269–286. doi: 10.1080/10401230500296428. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Davis M, Sinha P, Wisner KL, Hibbeln JR, Gelenberg AJ. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J Affect Disord. 2008;110:142–148. doi: 10.1016/j.jad.2007.12.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galecki P, Szemraj J, Bienkiewicz M, Zboralski K, Galecka E. Oxidative stress parameters after combined fluoxetine and acetylsalicylic acid therapy in depressive patients. Hum Psychopharmacol. 2009;24:277–286. doi: 10.1002/hup.1014. [DOI] [PubMed] [Google Scholar]
- Godfrey PS, Toone BK, Carney MW, Flynn TG, Bottiglieri T, Laundy M, Chanarin I, Reynolds EH. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336:392–395. doi: 10.1016/0140-6736(90)91942-4. [DOI] [PubMed] [Google Scholar]
- Grenyer BF, Crowe T, Meyer B, Owen AJ, Grigonis-Deane EM, Caputi P, Howe PR. Fish oil supplementation in the treatment of major depression: a randomised doubleblind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1393–1396. doi: 10.1016/j.pnpbp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Hallahan B, Hibbeln JR, Davis JM, Garland MR. Omega-3 fatty acid supplementation in patients with recurrent self-harm. Single-centre double-blind randomised controlled trial. Br J Psychiatry. 2007;190:118–122. doi: 10.1192/bjp.bp.106.022707. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Salem N., Jr Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr. 1995;62:1–9. doi: 10.1093/ajcn/62.1.1. [DOI] [PubMed] [Google Scholar]
- Hirayama T. [A large scale cohort study on the effect of life styles on the risk of cancer by each site] Gan No Rinsho. 1990:233–242. Spec No: [PubMed] [Google Scholar]
- Hooijmans CR, Van der Zee CE, Dederen PJ, Brouwer KM, Reijmer YD, van Groen T, Broersen LM, Lutjohann D, Heerschap A, Kiliaan AJ. DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPswe/PS1dE9 mice. Neurobiol Dis. 2009;33:482–498. doi: 10.1016/j.nbd.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Horsten M, Wamala SP, Vingerhoets A, Orth-Gomer K. Depressive symptoms, social support, and lipid profile in healthy middle-aged women. Psychosom Med. 1997;59:521–528. doi: 10.1097/00006842-199709000-00009. [DOI] [PubMed] [Google Scholar]
- Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Irie M, Asami S, Ikeda M, Kasai H. Depressive state relates to female oxidative DNA damage via neutrophil activation. Biochem Biophys Res Commun. 2003;311:1014–1018. doi: 10.1016/j.bbrc.2003.10.105. [DOI] [PubMed] [Google Scholar]
- Irie M, Miyata M, Kasai H. Depression and possible cancer risk due to oxidative DNA damage. J Psychiatr Res. 2005;39:553–560. doi: 10.1016/j.jpsychires.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Irmisch G, Schlaefke D, Richter J. Zinc and fatty acids in depression. Neurochem Res. 2010;35:1376–1383. doi: 10.1007/s11064-010-0194-3. [DOI] [PubMed] [Google Scholar]
- Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, Jalali M, Peet M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42:192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- Jou SH, Chiu NY, Liu CS. Mitochondrial dysfunction and psychiatric disorders. Chang Gung Med J. 2009;32:370–379. [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(Suppl 5):4–12. discussion 13-14. [PubMed] [Google Scholar]
- Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003a;8:365–370. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- Khanzode SS, Khanzode SD, Dakhale GN. Serum and plasma concentration of oxidant and antioxidants in patients of Helicobacter pylori gastritis and its correlation with gastric cancer. Cancer Lett. 2003b;195:27–31. doi: 10.1016/s0304-3835(03)00147-2. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee HJ, Kim JY, Yoon DK, Choi SH, Lee MS. Low serum cholesterol is correlated to suicidality in a Korean sample. Acta Psychiatr Scand. 2002;105:141–148. doi: 10.1034/j.1600-0447.2002.10352.x. [DOI] [PubMed] [Google Scholar]
- Kodas E, Vancassel S, Lejeune B, Guilloteau D, Chalon S. Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats: critical role of developmental stage. J Lipid Res. 2002;43:1209–1219. [PubMed] [Google Scholar]
- Kunugi H. [Low serum cholesterol and suicidal behavior] Nippon Rinsho. 2001;59:1599–1604. [PubMed] [Google Scholar]
- Kunugi H, Takei N, Aoki H, Nanko S. Low serum cholesterol in suicide attempters. Biol Psychiatry. 1997;41:196–200. doi: 10.1016/S0006-3223(95)00672-9. [DOI] [PubMed] [Google Scholar]
- Lalovic A, Levy E, Luheshi G, Canetti L, Grenier E, Sequeira A, Turecki G. Cholesterol content in brains of suicide completers. Int J Neuropsychopharmacol. 2007;10:159–166. doi: 10.1017/S1461145706006663. [DOI] [PubMed] [Google Scholar]
- Lalovic A, Merkens L, Russell L, Arsenault-Lapierre G, Nowaczyk MJ, Porter FD, Steiner RD, Turecki G. Cholesterol metabolism and suicidality in Smith-Lemli-Opitz syndrome carriers. Am J Psychiatry. 2004;161:2123–2126. doi: 10.1176/appi.ajp.161.11.2123. [DOI] [PubMed] [Google Scholar]
- Lapierre CB. Deployment with combat exposure increases the risk of new-onset PTSD. Evid Based Ment Health. 2008;11:126. doi: 10.1136/ebmh.11.4.126. [DOI] [PubMed] [Google Scholar]
- Lapierre CB, Schwegler AF, Labauve BJ. Posttraumatic stress and depression symptoms in soldiers returning from combat operations in Iraq and Afghanistan. J Trauma Stress. 2007;20:933–943. doi: 10.1002/jts.20278. [DOI] [PubMed] [Google Scholar]
- Lavie L. Oxidative stress--a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51:303–312. doi: 10.1016/j.pcad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Lekman M, Paddock S, McMahon FJ. Pharmacogenetics of major depression: insights from level 1 of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial. Mol Diagn Ther. 2008;12:321–330. doi: 10.1007/BF03256297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester D. Serum cholesterol levels and suicide: a meta-analysis. Suicide Life Threat Behav. 2002;32:333–346. doi: 10.1521/suli.32.3.333.22177. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang J. Serum concentrations of antioxidant vitamins and carotenoids are low in individuals with a history of attempted suicide. Nutr Neurosci. 2007;10:51–58. doi: 10.1080/10284150701250747. [DOI] [PubMed] [Google Scholar]
- Lindberg G, Larsson G, Setterlind S, Rastam L. Serum lipids and mood in working men and women in Sweden. J Epidemiol Community Health. 1994;48:360–363. doi: 10.1136/jech.48.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg G, Rastam L, Gullberg B, Eklund GA. Low serum cholesterol concentration and short term mortality from injuries in men and women. Bmj. 1992;305:277–279. doi: 10.1136/bmj.305.6848.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lineberry TW. Suicide rates in 2009. Do the economy and wars have an effect? Minn Med. 2009;92:49–52. [PubMed] [Google Scholar]
- Madrigal JL, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, Leza JC. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology. 2001;24:420–429. doi: 10.1016/S0893-133X(00)00208-6. [DOI] [PubMed] [Google Scholar]
- Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85:275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Maes M, Delanghe J, Meltzer HY, Scharpe S, D'Hondt P, Cosyns P. Lower degree of esterification of serum cholesterol in depression: relevance for depression and suicide research. Acta Psychiatr Scand. 1994;90:252–258. doi: 10.1111/j.1600-0447.1994.tb01589.x. [DOI] [PubMed] [Google Scholar]
- Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O and NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):676–692. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Lower plasma Coenzyme Q10 in depression: a marker for treatment resistance and chronic fatigue in depression and a risk factor to cardiovascular disorder in that illness. Neuro Endocrinol Lett. 2009a;30:462–469. [PubMed] [Google Scholar]
- Maes M, Smith R, Christophe A, Vandoolaeghe E, Van Gastel A, Neels H, Demedts P, Wauters A, Meltzer HY. Lower serum high-density lipoprotein cholesterol (HDL-C) in major depression and in depressed men with serious suicidal attempts: relationship with immune-inflammatory markers. Acta Psychiatr Scand. 1997a;95:212–221. doi: 10.1111/j.1600-0447.1997.tb09622.x. [DOI] [PubMed] [Google Scholar]
- Maes M, Vandoolaeghe E, Neels H, Demedts P, Wauters A, Meltzer HY, Altamura C, Desnyder R. Lower serum zinc in major depression is a sensitive marker of treatment resistance and of the immune/inflammatory response in that illness. Biol Psychiatry. 1997b;42:349–358. doi: 10.1016/S0006-3223(96)00365-4. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M. The inflammatory and neurodegenerative (I and ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009b;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- Mahadik SP, Evans D, Lal H. Oxidative stress and role of antioxidant and omega- 3 essential fatty acid supplementation in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:463–493. doi: 10.1016/s0278-5846(00)00181-0. [DOI] [PubMed] [Google Scholar]
- Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull. 2001;35:5–49. [PubMed] [Google Scholar]
- Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003;160:996–998. doi: 10.1176/appi.ajp.160.5.996. [DOI] [PubMed] [Google Scholar]
- Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2009;28:525–542. doi: 10.1080/07315724.2009.10719785. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Mitochondrial regulation of neuronal plasticity. Neurochem Res. 2007;32:707–715. doi: 10.1007/s11064-006-9170-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW. Psychopharmacological issues in the treatment of TBI and PTSD. Clin Neuropsychol. 2009;23:1338–1367. doi: 10.1080/13854040903277289. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- McIntosh LJ, Sapolsky RM. Glucocorticoids may enhance oxygen radical-mediated neurotoxicity. Neurotoxicology. 1996;17:873–882. [PubMed] [Google Scholar]
- Mischoulon D, Papakostas GI, Dording CM, Farabaugh AH, Sonawalla SB, Agoston AM, Smith J, Beaumont EC, Dahan LE, Alpert JE, Nierenberg AA, Fava M. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J Clin Psychiatry. 2009;70:1636–1644. doi: 10.4088/JCP.08m04603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modai I, Valevski A, Dror S, Weizman A. Serum cholesterol levels and suicidal tendencies in psychiatric inpatients. J Clin Psychiatry. 1994;55:252–254. [PubMed] [Google Scholar]
- Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, Voshaar RO, Elzinga BM. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry. 2011;16(11):1088–1095. doi: 10.1038/mp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RE, Palinkas LA, Barrett-Connor EL, Wingard DL. Plasma cholesterol and depressive symptoms in older men. Lancet. 1993;341:75–79. doi: 10.1016/0140-6736(93)92556-9. [DOI] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol. 2002;59:1125–1132. doi: 10.1001/archneur.59.7.1125. [DOI] [PubMed] [Google Scholar]
- Nakagome K, Yamada T, Matsumura H, Sakaki N, Sunao M. [Clinical application of omega 3 polyunsaturated fatty acids in psychiatry] Seishin Shinkeigaku Zasshi. 2009;111:1512–1519. [PubMed] [Google Scholar]
- Neaton JD, Blackburn H, Jacobs D, Kuller L, Lee DJ, Sherwin R, Shih J, Stamler J, Wentworth D. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med. 1992;152:1490–1500. [PubMed] [Google Scholar]
- Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002;159:477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Nomura K, Castanon-Cervantes O, Davidson A, Fukuhara C. Selective serotonin reuptake inhibitors and raft inhibitors shorten the period of Period1-driven circadian bioluminescence rhythms in rat-1 fibroblasts. Life Sci. 2008;82:1169–1174. doi: 10.1016/j.lfs.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom P, Asberg M. Suicide risk and serotonin. Int Clin Psychopharmacol. 1992;6(Suppl 6):12–21. doi: 10.1097/00004850-199206006-00003. [DOI] [PubMed] [Google Scholar]
- Nowak G, Szewczyk B, Wieronska JM, Branski P, Palucha A, Pilc A, Sadlik K, Piekoszewski W. Antidepressant-like effects of acute and chronic treatment with zinc in forced swim test and olfactory bulbectomy model in rats. Brain Res Bull. 2003;61:159–164. doi: 10.1016/s0361-9230(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Olusi SO, Fido AA. Serum lipid concentrations in patients with major depressive disorder. Biol Psychiatry. 1996;40:1128–1131. doi: 10.1016/S0006-3223(95)00599-4. [DOI] [PubMed] [Google Scholar]
- Owens MJ. Selectivity of antidepressants: from the monoamine hypothesis of depression to the SSRI revolution and beyond. J Clin Psychiatry. 2004;65(Suppl 4):5–10. [PubMed] [Google Scholar]
- Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. Int Clin Psychopharmacol. 2004;19:89–95. doi: 10.1097/00004850-200403000-00006. [DOI] [PubMed] [Google Scholar]
- Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009;45:625–633. doi: 10.1016/j.ceca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostas GI. Evidence for S-adenosyl-L-methionine (SAM-e) for the treatment of major depressive disorder. J Clin Psychiatry. 2009;70(Suppl 5):18–22. doi: 10.4088/JCP.8157su1c.04. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Lebowitz BD, Mischoulon D, Ryan JL, Nierenberg AA, Bottiglieri T, Alpert JE, Rosenbaum JF, Fava M. The relationship between serum folate, vitamin B12, and homocysteine levels in major depressive disorder and the timing of improvement with fluoxetine. Int J Neuropsychopharmacol. 2005;8:523–528. doi: 10.1017/S1461145705005195. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Mischoulon D, Ryan JL, Nierenberg AA, Bottiglieri T, Rosenbaum JF, Alpert JE, Fava M. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 1: predictors of clinical response in fluoxetine-resistant depression. J Clin Psychiatry. 2004;65:1090–1095. doi: 10.4088/jcp.v65n0810. [DOI] [PubMed] [Google Scholar]
- Partonen T, Haukka J, Virtamo J, Taylor PR, Lonnqvist J. Association of low serum total cholesterol with major depression and suicide. Br J Psychiatry. 1999;175:259–262. doi: 10.1192/bjp.175.3.259. [DOI] [PubMed] [Google Scholar]
- Paul RT, McDonnell AP, Kelly CB. Folic acid: neurochemistry, metabolism and relationship to depression. Hum Psychopharmacol. 2004;19:477–488. doi: 10.1002/hup.614. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- Peet M. Eicosapentaenoic acid in the treatment of schizophrenia and depression: rationale and preliminary double-blind clinical trial results. Prostaglandins Leukot Essent Fatty Acids. 2003;69:477–485. doi: 10.1016/j.plefa.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- Pettenuzzo LF, Schuck PF, Fontella F, Wannmacher CM, Wyse AT, Dutra-Filho CS, Netto CA, Wajner M. Ascorbic acid prevents cognitive deficits caused by chronic administration of propionic acid to rats in the water maze. Pharmacol Biochem Behav. 2002;73:623–629. doi: 10.1016/s0091-3057(02)00856-0. [DOI] [PubMed] [Google Scholar]
- Ponce J, de la Ossa NP, Hurtado O, Millan M, Arenillas JF, Davalos A, Gasull T. Simvastatin reduces the association of NMDA receptors to lipid rafts: a cholesterol-mediated effect in neuroprotection. Stroke. 2008;39:1269–1275. doi: 10.1161/STROKEAHA.107.498923. [DOI] [PubMed] [Google Scholar]
- Powell SR. The antioxidant properties of zinc. J Nutr. 2000;130:1447S–1454S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- Quiroz JA, Gray NA, Kato T, Manji HK. Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:2551–2565. doi: 10.1038/sj.npp.1301671. [DOI] [PubMed] [Google Scholar]
- Rabe-Jablonska J, Poprawska I. Levels of serum total cholesterol and LDL-cholesterol in patients with major depression in acute period and remission. Med Sci Monit. 2000;6:539–547. [PubMed] [Google Scholar]
- Rafter D. Biochemical markers of anxiety and depression. Psychiatry Res. 2001;103:93–96. doi: 10.1016/s0165-1781(01)00251-7. [DOI] [PubMed] [Google Scholar]
- Ramos MI, Allen LH, Haan MN, Green R, Miller JW. Plasma folate concentrations are associated with depressive symptoms in elderly Latina women despite folic acid fortification. Am J Clin Nutr. 2004;80:1024–1028. doi: 10.1093/ajcn/80.4.1024. [DOI] [PubMed] [Google Scholar]
- Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42:199–205. doi: 10.1080/00048670701827267. [DOI] [PubMed] [Google Scholar]
- Renner U, Glebov K, Lang T, Papusheva E, Balakrishnan S, Keller B, Richter DW, Jahn R, Ponimaskin E. Localization of the mouse 5-hydroxytryptamine(1A) receptor in lipid microdomains depends on its palmitoylation and is involved in receptor-mediated signaling. Mol Pharmacol. 2007;72:502–513. doi: 10.1124/mol.107.037085. [DOI] [PubMed] [Google Scholar]
- Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction and psychiatric disorders. Neurochem Res. 2009;34:1021–1029. doi: 10.1007/s11064-008-9865-8. [DOI] [PubMed] [Google Scholar]
- Rezin GT, Cardoso MR, Goncalves CL, Scaini G, Fraga DB, Riegel RE, Comim CM, Quevedo J, Streck EL. Inhibition of mitochondrial respiratory chain in brain of rats subjected to an experimental model of depression. Neurochem Int. 2008;53:395–400. doi: 10.1016/j.neuint.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Cyhlarova E, Ross MA. Omega-3 and omega-6 fatty acid concentrations in red blood cell membranes relate to schizotypal traits in healthy adults. Prostaglandins Leukot Essent Fatty Acids. 2003;69:461–466. doi: 10.1016/j.plefa.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Roberts SH, Bedson E, Hughes D, Lloyd K, Menkes DB, Moat S, Pirmohamed M, Slegg G, Thome J, Tranter R, Whitaker R, Wilkinson C, Russell I. Folate augmentation of treatment - evaluation for depression (FolATED): protocol of a randomised controlled trial. BMC Psychiatry. 2007;7:65. doi: 10.1186/1471-244X-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ME, Teyhen DS, Wu SS, Dugan JL, Wright AC, Childs JD, Yang G, George SZ. Mental health symptoms in combat medic training: a longitudinal examination. Mil Med. 2009;174:572–577. doi: 10.7205/milmed-d-02-4108. [DOI] [PubMed] [Google Scholar]
- Rothberg JM, Fagan J, Shaw J. Suicide in United States Army personnel, 1985 – 1986. Mil Med. 1990;155:452–456. [PubMed] [Google Scholar]
- Sachdev PS, Parslow RA, Lux O, Salonikas C, Wen W, Naidoo D, Christensen H, Jorm AF. Relationship of homocysteine, folic acid and vitamin B12 with depression in a middle-aged community sample. Psychol Med. 2005;35:529–538. doi: 10.1017/s0033291704003721. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martinez-Gonzalez MA. Association between folate, vitamin B(6) and vitamin B(12) intake and depression in the SUN cohort study. J Hum Nutr Diet. 2009;22:122–133. doi: 10.1111/j.1365-277X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- Sarchiapone M, Camardese G, Roy A, Della Casa S, Satta MA, Gonzalez B, Berman J, De Risio S. Cholesterol and serotonin indices in depressed and suicidal patients. J Affect Disord. 2001;62:217–219. doi: 10.1016/s0165-0327(99)00200-1. [DOI] [PubMed] [Google Scholar]
- Selby EA, Anestis MD, Bender TW, Ribeiro JD, Nock MK, Rudd MD, Bryan CJ, Lim IC, Baker MT, Gutierrez PM, Joiner TE., Jr Overcoming the fear of lethal injury: evaluating suicidal behavior in the military through the lens of the Interpersonal- Psychological Theory of Suicide. Clin Psychol Rev. 2010;30:298–307. doi: 10.1016/j.cpr.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC. The molecular neurobiology of depression. Psychiatr Clin North Am. 2007;30:1–11. doi: 10.1016/j.psc.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher L. The role of the hypothalamic-pituitary-adrenal axis dysfunction in the pathophysiology of alcohol misuse and suicidal behavior in adolescents. Int J Adolesc Med Health. 2007;19:3–9. doi: 10.1515/ijamh.2007.19.1.3. [DOI] [PubMed] [Google Scholar]
- Shiah IS, Yatham LN. Serotonin in mania and in the mechanism of action of mood stabilizers: a review of clinical studies. Bipolar Disord. 2000;2:77–92. doi: 10.1034/j.1399-5618.2000.020201.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui RA, Harvey KA, Zaloga GP, Stillwell W. Modulation of lipid rafts by Omega-3 fatty acids in inflammation and cancer: implications for use of lipids during nutrition support. Nutr Clin Pract. 2007;22:74–88. doi: 10.1177/011542650702200174. [DOI] [PubMed] [Google Scholar]
- Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids. 2005;72:211–218. doi: 10.1016/j.plefa.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Sinclair AJ, Begg D, Mathai M, Weisinger RS. Omega 3 fatty acids and the brain: review of studies in depression. Asia Pac J Clin Nutr. 2007;16(Suppl 1):391–397. [PubMed] [Google Scholar]
- Siwek M, Dudek D, Schlegel-Zawadzka M, Morawska A, Piekoszewski W, Opoka W, Zieba A, Pilc A, Popik P, Nowak G. Serum zinc level in depressed patients during zinc supplementation of imipramine treatment. J Affect Disord. 2010;126:447–452. doi: 10.1016/j.jad.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Smesny S, Milleit B, Hipler UC, Milleit C, Schafer MR, Klier CM, Holub M, Holzer I, Berger GE, Otto M, Nenadic I, Berk M, McGorry PD, Sauer H, Amminger GP. Omega-3 fatty acid supplementation changes intracellular phospholipase A activity and membrane fatty acid profiles in individuals at ultra-high risk for psychosis. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.7. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA. Diet and neurocognition: review of evidence and methodological considerations. Curr Aging Sci. 2010;3:57–66. doi: 10.2174/1874609811003010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawalla SB, Papakostas GI, Petersen TJ, Yeung AS, Smith MM, Sickinger AH, Gordon J, Israel JA, Tedlow JR, Lamon-Fava S, Fava M. Elevated cholesterol levels associated with nonresponse to fluoxetine treatment in major depressive disorder. Psychosomatics. 2002;43:310–316. doi: 10.1176/appi.psy.43.4.310. [DOI] [PubMed] [Google Scholar]
- Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- Sowa-Kucma M, Legutko B, Szewczyk B, Novak K, Znojek P, Poleszak E, Papp M, Pilc A, Nowak G. Antidepressant-like activity of zinc: further behavioral and molecular evidence. J Neural Transm. 2008;115:1621–1628. doi: 10.1007/s00702-008-0115-7. [DOI] [PubMed] [Google Scholar]
- Steegmans PH, Hoes AW, Bak AA, van der Does E, Grobbee DE. Higher prevalence of depressive symptoms in middle-aged men with low serum cholesterol levels. Psychosom Med. 2000;62:205–211. doi: 10.1097/00006842-200003000-00009. [DOI] [PubMed] [Google Scholar]
- Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- Su KP. Biological mechanism of antidepressant effect of omega-3 fatty acids: how does fish oil act as a 'mind-body interface? Neurosignals. 2009;17:144–152. doi: 10.1159/000198167. [DOI] [PubMed] [Google Scholar]
- Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13:267–271. doi: 10.1016/s0924-977x(03)00032-4. [DOI] [PubMed] [Google Scholar]
- Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC, Pariante CM. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008a;69:644–651. doi: 10.4088/jcp.v69n0418. [DOI] [PubMed] [Google Scholar]
- Su YA, Wu J, Zhang L, Zhang Q, Su DM, He P, Wang BD, Li H, Webster MJ, Rennert OM, Ursano RJ. Dysregulated mitochondrial genes and networks with drug targets in postmortem brain of patients with posttraumatic stress disorder (PTSD) revealed by human mitochondria-focused cDNA microarrays. Int J Biol Sci. 2008b;4:223–235. doi: 10.7150/ijbs.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez EC. Relations of trait depression and anxiety to low lipid and lipoprotein concentrations in healthy young adult women. Psychosom Med. 1999;61:273–279. doi: 10.1097/00006842-199905000-00004. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- Syvalahti E. Monoaminergic mechanisms in affective disorders. Med Biol. 1987;65:89–96. [PubMed] [Google Scholar]
- Tagliari B, dos Santos TM, Cunha AA, Lima DD, Delwing D, Sitta A, Vargas CR, Dalmaz C, Wyse AT. Chronic variable stress induces oxidative stress and decreases butyrylcholinesterase activity in blood of rats. J Neural Transm. 2010;117:1067–1076. doi: 10.1007/s00702-010-0445-0. [DOI] [PubMed] [Google Scholar]
- Takei N, Kunugi H, Nanko S, Aoki H, Iyo R, Kazamatsuri H. Low serum cholesterol and suicide attempts. Br J Psychiatry. 1994;164:702–703. doi: 10.1192/s0007125000034632. [DOI] [PubMed] [Google Scholar]
- Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Viinamaki H. Fish consumption, depression, and suicidality in a general population. Arch Gen Psychiatry. 2001;58:512–513. doi: 10.1001/archpsyc.58.5.512. [DOI] [PubMed] [Google Scholar]
- Tatley M, Savage R. Psychiatric adverse reactions with statins, fibrates and ezetimibe: implications for the use of lipid-lowering agents. Drug Saf. 2007;30:195–201. doi: 10.2165/00002018-200730030-00003. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan VV, Rao KV, Sastry PS. Regulation of diacylglycerol kinase in rat brain membranes by docosahexaenoic acid. Neurosci Lett. 1994;179:171–174. doi: 10.1016/0304-3940(94)90961-x. [DOI] [PubMed] [Google Scholar]
- van Liempt S, Vermetten E, Geuze E, Westenberg HG. Pharmacotherapy for disordered sleep in post-traumatic stress disorder: a systematic review. Int Clin Psychopharmacol. 2006;21:193–202. doi: 10.1097/00004850-200607000-00001. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Villar VA, Jones JE, Armando I, Palmes-Saloma C, Yu P, Pascua AM, Keever L, Arnaldo FB, Wang Z, Luo Y, Felder RA, Jose PA. G protein-coupled receptor kinase 4 (GRK4) regulates the phosphorylation and function of the dopamine D3 receptor. J Biol Chem. 2009;284:21425–21434. doi: 10.1074/jbc.M109.003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virmani A, Gaetani F, Binienda Z. Effects of metabolic modifiers such as carnitines, coenzyme Q10, and PUFAs against different forms of neurotoxic insults: metabolic inhibitors, MPTP, and methamphetamine. Ann N Y Acad Sci. 2005;1053:183–191. doi: 10.1196/annals.1344.016. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M, Bruehl C, Voskuyl RA, Kang JX, Leaf A, Wadman WJ. Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc Natl Acad Sci U S A. 1996;93:12559–12563. doi: 10.1073/pnas.93.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner G, Connor SL, Hollis JF, Connor WE. Improvements in hostility and depression in relation to dietary change and cholesterol lowering. The Family Heart Study. Ann Intern Med. 1992;117:820–823. doi: 10.7326/0003-4819-117-10-820. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A. Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol. 2006;21(Suppl 1):S11–S15. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- Wolf J, Lewicka J, Narkiewicz K. Obstructive sleep apnea: an update on mechanisms and cardiovascular consequences. Nutr Metab Cardiovasc Dis. 2007;17:233–240. doi: 10.1016/j.numecd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Mellon S. When blue turns to grey: do stress and depression accelerate cell aging? World J Biol Psychiatry. 2008;9:2–5. doi: 10.1080/15622970701875601. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2:115–143. doi: 10.3109/15622970109026799. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Manji HK. The role of AMPA receptor modulation in the treatment of neuropsychiatric diseases. Exp Neurol. 2008;211:7–10. doi: 10.1016/j.expneurol.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou R, Li X, Ursano RJ, Li H. Stress-induced change of mitochondria membrane potential regulated by genomic and non-genomic GR signaling: a possible mechanism for hippocampus atrophy in PTSD. Med Hypotheses. 2006;66:1205–1208. doi: 10.1016/j.mehy.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Zimmer L, Delpal S, Guilloteau D, Aioun J, Durand G, Chalon S. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci Lett. 2000;284:25–28. doi: 10.1016/s0304-3940(00)00950-2. [DOI] [PubMed] [Google Scholar]
- Zundorf G, Reiser G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid Redox Signal. 2011;14(7):1275–1288. doi: 10.1089/ars.2010.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]